Abstract
The white-throated sparrow (Zonotrichia albicollis) offers unique opportunities to understand the adaptive value of supergenes, particularly their role in alternative phenotypes. In this species, alternative plumage morphs segregate with a nonrecombining segment of chromosome 2, which has been called a ‘supergene’. The species mates disassortatively with respect to the supergene; that is, each breeding pair consists of one individual with it and one without it. This species has therefore been called the “bird with four sexes”. The supergene segregates with a behavioral phenotype; birds with it are more aggressive and less parental than birds without it. Here, we review our efforts to identify the genes inside the supergene that are responsible for the behavioral polymorphism. The gene ESR1, which encodes estrogen receptor α, differs between the morphs and predicts both territorial and parental behavior. Variation in the regulatory regions of ESR1 causes an imbalance in expression of the two alleles, and the degree to which this imbalance favors the supergene allele predicts territorial singing. In heterozygotes, knockdown of ESR1 causes a phenotypic switch, from more aggressive to less aggressive. We recently showed that another gene important for social behavior, vasoactive intestinal peptide (VIP), is differentially expressed between the morphs and predicts territorial singing. We hypothesize that ESR1 and VIP contribute to behavior in a coordinated way and could represent co-adapted alleles. Because the supergene contains more than 1,000 individual genes, this species provides rich possibilities for discovering alleles that work together to mediate life-history trade-offs and maximize the fitness of alternative complex phenotypes.
Keywords: alternative phenotypes, coadaptation, estrogen receptor alpha, inversion polymorphism, life-history strategy, parental behavior, social behavior, song, territorial aggression, vasoactive intestinal peptide
The sparrow with four sexes
Plate 8 of Audubon’s Birds of America features two white-throated sparrows (Zonotrichia albicollis). One has black and white stripes on its crown and a clear white throat; the other has brown and tan stripes and a streaked throat. In his painting, Audubon labeled them male and female, respectively. Field guides also labeled them as such, or as adult and juvenile, until the early 1960s. Working with hundreds of specimens, Lowther (1961) discovered that Audubon and the field guides alike had been incorrect. Both male and female white-throated sparrows occur in two color morphs (Fig. 1A), now known as white-striped (WS) and tan-striped (TS), that are fixed throughout an individual’s lifetime. It is easy to understand why Audubon associated the coloration with male and female; almost every breeding pair consists of a WS bird and a TS bird (Lowther, 1961; Tuttle et al., 2016). WS-WS pairs and TS-TS pairs each constitute less than 1% of the breeding pairs in a population (Tuttle et al., 2016). This disassortative mating system, so far unique among birds, means that each bird can mate with only 25% of the population. Thus, the species has earned the nickname ‘the bird with four sexes’ (Campagna, 2016).
Figure 1.
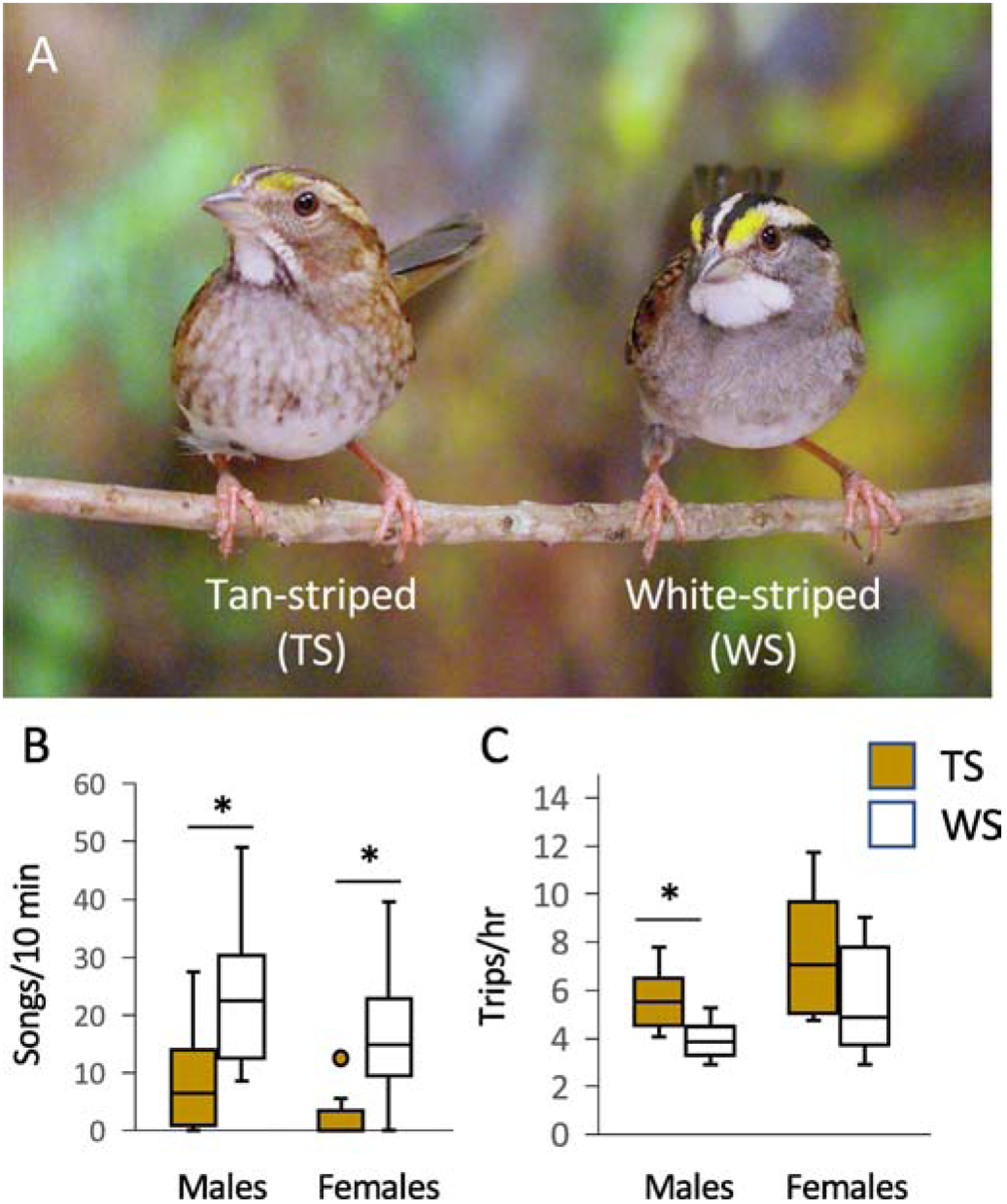
Polymorphism in white-throated sparrows. (A) Males and females occur in two plumage morphs, tan-striped (TS) and white-striped (WS). (B) WS birds of both sexes respond to simulated territorial intrusions with more vocal aggression (song rate is plotted here) than do TS birds. (C) TS males provision young in the nest at a higher rate (trips per hr) than do WS males. Photo by Jennifer Merritt. Data are replotted from Horton et al. (2014a) and Zinzow-Kramer et al. (2015).
Apart from this extraordinary mating strategy, white-throated sparrows are fairly ordinary North American sparrows (see Falls & Kopachena, 2020 for details on their migration patterns, breeding habits, and parental behavior). Briefly, they are migratory, wintering in the southeastern U.S. and breeding in the boreal forests of the northeastern U.S. and Canada. While overwintering, they travel in flocks. During the spring, breeding pairs defend multipurpose territories. Territorial defense is performed by both sexes and includes singing, flying at the intruder, and sometimes attacks. Breeding pairs are socially but not sexually monogamous; up to 40% of the young in a particular nest are fathered by a male other than the social mate, suggesting that extra-pair copulation is common. Although all nest-building and incubation are done by the female, both parents provision young in the nest.
Several of the above behaviors are performed at higher rates in one morph than the other. For example, WS birds of both sexes respond to simulated territorial intrusions (STIs) with higher song rates than do TS birds (Fig. 1B; Horton et al., 2014a). Although TS males are often robust singers, TS females rarely sing. WS males engage in more territorial intrusions and extra-pair copulations, whereas TS males are more likely to stay within their own territories and mate-guard (Tuttle, 2003). TS birds provision nestlings more often than do their WS counterparts; this effect is more often replicated in males than in females (Kopachena & Falls, 1993; Horton et al., 2014a; Fig. 1C). Overall, WS birds seem to invest more heavily in mate-seeking and intrasexual competition, whereas TS birds employ a more parental life-history strategy (Maney, 2008; Maney et al., 2015; Tuttle et al., 2003). Despite the different strategies, the morphs enjoy comparable lifetime reproductive success (Grunst et al., 2017; Tuttle et al., 2016), suggesting that the strategies are complimentary rather than competitive.
Many of the well-studied cases of alternative phenotypes in vertebrates are connected to variation in reproductive endocrine physiology (reviewed by Hau, 2007; Knapp, 2004; Miles et al., 2007; Rhen and Crews, 2002). Hormones are obvious candidates for mediating alternative phenotypes because of their often antagonistic effects on social behaviors (Finch & Rose, 1995; Gross, 1996; McGlothlin & Ketterson, 2008; Nijhout, 2003; Sinervo & Svensson, 2002). Even in species without alternative phenotypes, there is substantial evidence that trade-offs between parenting and territorial aggression are mediated by steroid hormones such as testosterone and estradiol (E2) (Ketterson & Nolan, 1994; McGlothlin et al., 2007; Wingfield et al., 1990). Across taxa, circulating androgens have been associated with increased intrasexual competition manifested as aggression or mating effort, whereas low levels have been associated with increased parenting effort (see Archer, 2006; Hau, 2007 for review). In songbirds, testosterone levels peak during periods of mating and territorial defense, then decrease during the parental phase of the breeding season (Wingfield et al., 1990). Disruptive selection that drives the sequestration of parental and territorial behavior into alternative phenotypes may thus act on genes in the steroid hormone pathway (Maney, 2008).
The behavioral polymorphism in white-throated sparrows certainly fits with a model that involves reproductive hormones. All of the known morph differences in social behavior in this species appear only during the breeding season, when plasma levels of testosterone and E2 are relatively high. In winter, when the gonads are regressed and plasma testosterone and E2 are much lower, dominance rank and aggression within social groups are unrelated to morph (reviewed by Maney & Goodson, 2011). The seasonal nature of the behavioral polymorphism points to these steroids as possible mediators. In fact, plasma testosterone and E2 are higher in WS than TS birds during breeding (Horton et al., 2014a; Spinney et al., 2006; Swett & Breuner, 2009). When plasma levels of these steroids are experimentally equalized in laboratory-housed birds, however, morph differences in singing and other aggressive behaviors persist (Maney et al., 2009; Merritt et al., 2018). Thus, the behavioral differences are not caused simply by differences in hormone levels. Testosterone and E2 appear to interact with other factors that are likely genetically differentiated between the morphs (Maney, 2008; Maney et al., 2015).
The genetic basis for the plumage polymorphism in this species was originally discovered half a century ago. In what would be the first demonstration of a chromosomal polymorphism in birds, Thorneycroft (1966; 1975) showed that whereas TS birds have two copies of a submetacentric version of chromosome 2, meaning that the two arms are of unequal length, WS birds have at least one copy of a rearranged, metacentric homolog with the centromere in the center. This work demonstrated that the WS phenotype is inherited as a dominant trait linked to the metacentric version of the chromosome. Thorneycroft suspected that the polymorphism came about via a chromosomal inversion. Thomas et al. (2008) confirmed that the metacentric arrangement, now known as ZAL2m, contains at least two inversions relative to the submetacentric version, ZAL2 (Fig. 2A, B). The rearranged region is one of the largest of its kind, harboring more than a thousand genes (Thomas et al., 2008; Sun et al., 2018).
Figure 2.
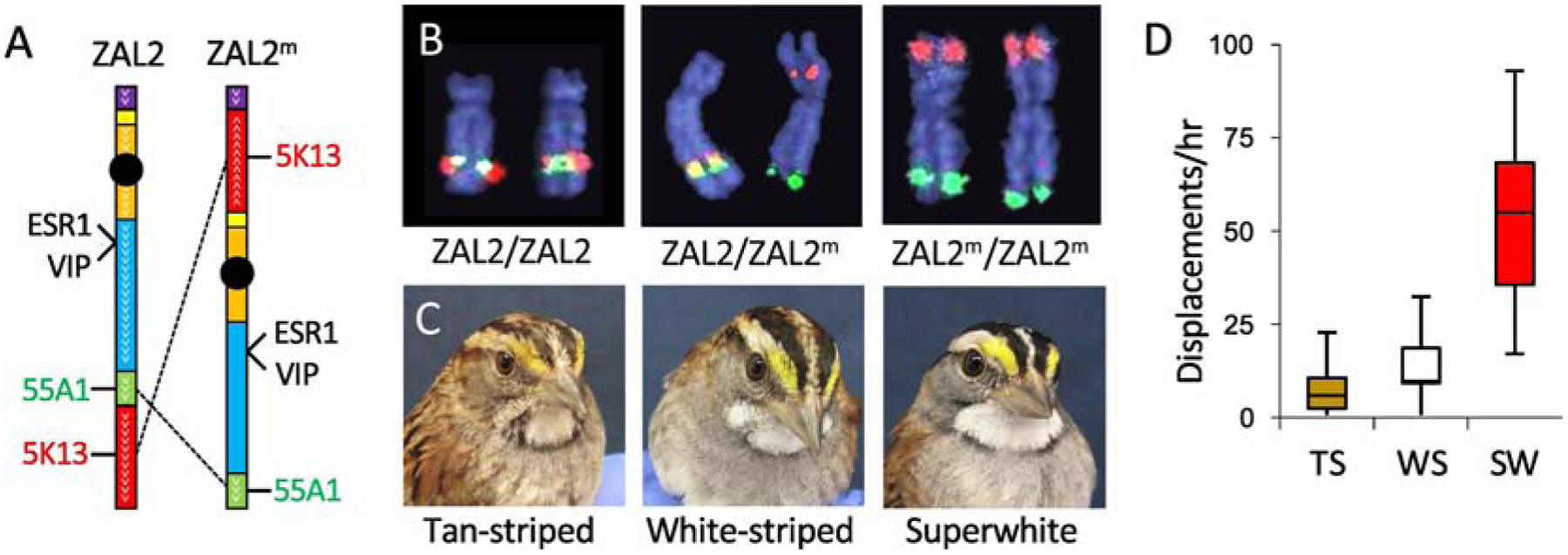
The white-striped phenotype in white-throated sparrows is linked to a rearrangement of chromosome 2. Note that we follow conventional nomenclature for avian chromosomes, numbering them from largest to smallest (Ladjali-Mohammedi et al., 1999). Chromosome 2 in white-throated sparrows corresponds to chromosome 3 in chickens (Thomas et al., 2008). (A) Zebra finch BAC clones 55A1 and 5K13 both map to the long arm of ZAL2, but because of a series of inversions, they map to opposite arms of ZAL2m. (B) Fluorescent in situ hybridization shows the locations of the two clones (red and green) on ZAL2 and ZAL2m. Tan-striped (TS) birds have two copies of ZAL2 and white-striped (WS) birds have one copy of ZAL2 and one of ZAL2m. ZAL2m/ZAL2m homozygotes, or superwhite (SW) birds, are rare. The three birds shown in (C) are hatch-year females; at this age, the plumage is usually duller than in adults (compare to Fig. 1A). Nonetheless, this SW bird showed striking bright plumage even as a hatch year female. This bird also showed high levels of aggression (D), performing more displacements in one-on-one behavioral trials than TS or WS birds matched with same-morph opponents. Map of chromosome two (A) redrawn from Thomas et al. (2008). Photos in (B) and (C) reprinted from Horton et al. (2013) with permission. Data in (D) redrawn from Horton et al. (2013).
Nearly all birds of the WS morph are ZAL2/ZAL2m heterozygotes; ZAL2m/ZAL2m homozygotes are rare. Such homozygotes can result only from WS-WS matings, which are largely prevented by the disassortative mating system. Given the known rate of WS-WS pairings, and based on the genotyping of thousands of wild birds, ZAL2m/ZAL2m homozygotes occur at about the expected frequency (1/500 birds; Horton et al., 2013; Tuttle et al., 2016). Only a single individual of that genotype has been behaviorally characterized (Horton et al., 2013). The phenotype of this bird was an exaggerated version of the WS morph, both with respect to plumage and behavior (Fig. 2C, D); that is, it was extremely aggressive and sang at an unusually high rate. Thus, alleles on ZAL2m may affect aggressive behavior in a dosage-dependent manner.
Clearly, the ZAL2/2m rearrangement has captured alleles that affect not only plumage but also a suite of behaviors. These alleles, particularly those that affect behavior, are likely numerous and work together in complex ways. Because they are always inherited together, identifying causal alleles is a challenging task. The many layers of biological organization between genotype and phenotype impose additional challenges. Below, we describe our efforts to leverage what was already known about social behavior in songbirds to identify the changes in genetic sequence that have driven the evolution of alternative behavioral phenotypes, which in this case constitute alternative life-history strategies, in this interesting species.
The ZAL2m rearrangement is a special kind of supergene
When a group of alleles is inherited together and collectively controls a complex, adaptive phenotype, it is called a ‘supergene’ (reviewed by Schwander et al., 2014; Thompson & Jiggins, 2014). The co-inheritance, which is key to this concept, is caused by tight linkage disequilibrium. Recombination within supergenes is suppressed, often because of inversions. After an inversion occurs, the affected haplotype can no longer easily recombine with its counterpart, due to the disruption of synapsis between homologous sequences. Rare recombination events between the inverted and non-inverted haplotypes would be highly detrimental, resulting in massive deletions and duplications likely including the centromeres. Thus, the alleles inside the inversion cannot be exchanged for others in the usual way, and are co-inherited.
Inversions clearly disrupt gene flow, and can even disrupt gene function if breakpoints occur within genes. Nonetheless, not only are inversions commonly maintained in populations but they also can spread to fixation. Since their discovery almost a century ago (Sturvesant, 1921), many researchers have speculated about their adaptive significance. Dobzhansky (1950) hypothesized that inversions are adaptive when they bind together alleles that function well together. In other words, individuals with a particular allele of one gene would do best if they also had a particular allele of another. Such co-adaptation could arise for two proteins that interact directly with each other. Imagine, for example, a hormone with two isoforms A and B, each of which interacts optimally with the corresponding receptor isoforms A and B, respectively. Individuals with the A allele of both hormone and receptor would do better than those with a mismatched set. Alternatively, such a relationship between alleles could arise if they each independently influence traits that are best inherited together, such as a preference for a particular food and an ability to better digest that food. Capturing co-adapted alleles together inside an inversion helps ensure that they stay together, benefiting both the individual and the allele.
Kirkpatrick and Barton (2006) challenged Dobzhansky’s view, arguing that inversions can be adaptive even in the absence of co-adaptation. They noted that if an inversion contains a set of alleles that are adapted to the local environment, suppression of recombination would preserve overall fitness by protecting that segment of the genome from introgression of nonadapted alleles. Inversions with distribution patterns suggestive of local adaptation have been found in Anopheles mosquitos (Ayala et al., 2017), Drosophila (Anderson et al., 2005), Arabidopsis (Fransz et al., 2016), and even humans (Stefansson et al., 2005). In the yellow monkey flower (Mimulus guttatus), which has been called the ―poster child for the local adaptation hypothesis‖ (Kirkpatrick, 2010), an inversion polymorphism underlies phenotypic variation between two ecotypes (Lowry & Willis, 2010). The annual form thrives in hot, dry habitats whereas the perennial form is adapted to cooler, wetter environments. The traits that differ between the ecotypes, and which co-segregate with the inversion, not only confer advantages in each local environment but also result in reproductive isolation between the two ecotypes—one ecotype flowers before the other. The two are not completely isolated, however, and they can interbreed (Lowry & Willis, 2010). Nonetheless, evidence from other model organisms suggests that inversion polymorphisms can ultimately lead to enough reproductive isolation to drive speciation (reviewed by Hoffmann & Rieseberg, 2008; Mérot et al., 2020a; Wellenreuther & Bernatchez, 2018).
The ZAL2/ZAL2m rearrangement in white-throated sparrows meets the definition of a supergene because it co-segregates with complex phenotypes that are stably maintained and because recombination is strongly suppressed within it. Analyses of gene flow between the haplotypes have shown high values for the fixation index (FST) inside the rearrangement, indicating a significant degree of isolation between the ZAL2 and ZAL2m haplotypes (Fig. 3A; Huynh et al., 2011; Thomas et al., 2008; Tuttle et al., 2016; Sun et al., 2018). Unlike many supergenes, however, the adaptive significance of ZAL2/ZAL2m is clearly not local adaptation and the supergene is not leading to speciation. Birds of both genotypes are always found together in the same population—and almost always within the same breeding pair. Rather than facilitating adaptation to variable local habitats, this polymorphism resembles nascent sex chromosomes (Tuttle et al., 2016; Sun et al., 2018). Sex chromosomes are, in fact, popular examples of supergenes and often originate as inversion polymorphisms (Hoffmann & Rieseberg, 2008; Schwander et al., 2014; Thompson & Jiggins, 2014; Wellenreuther & Bernatchez, 2018). In birds and mammals, sex is determined by the non-recombining sex chromosomes W (in female birds) and Y (in male mammals), each of which is nearly always in a state of heterozygosity. In white-throated sparrows, because of disassortative mating, approximately half of the offspring are heterozygous for the non-recombining variant ZAL2m and the other half are homozygous for the recombining homolog ZAL2. Thus, not only do white-throated sparrows have four effective sexes, but they are also evolving a new system of heteromorphic chromosomes that look in many respects like sex chromosomes (Thompson & Jiggins, 2014; Tuttle et al., 2016).
Figure 3.
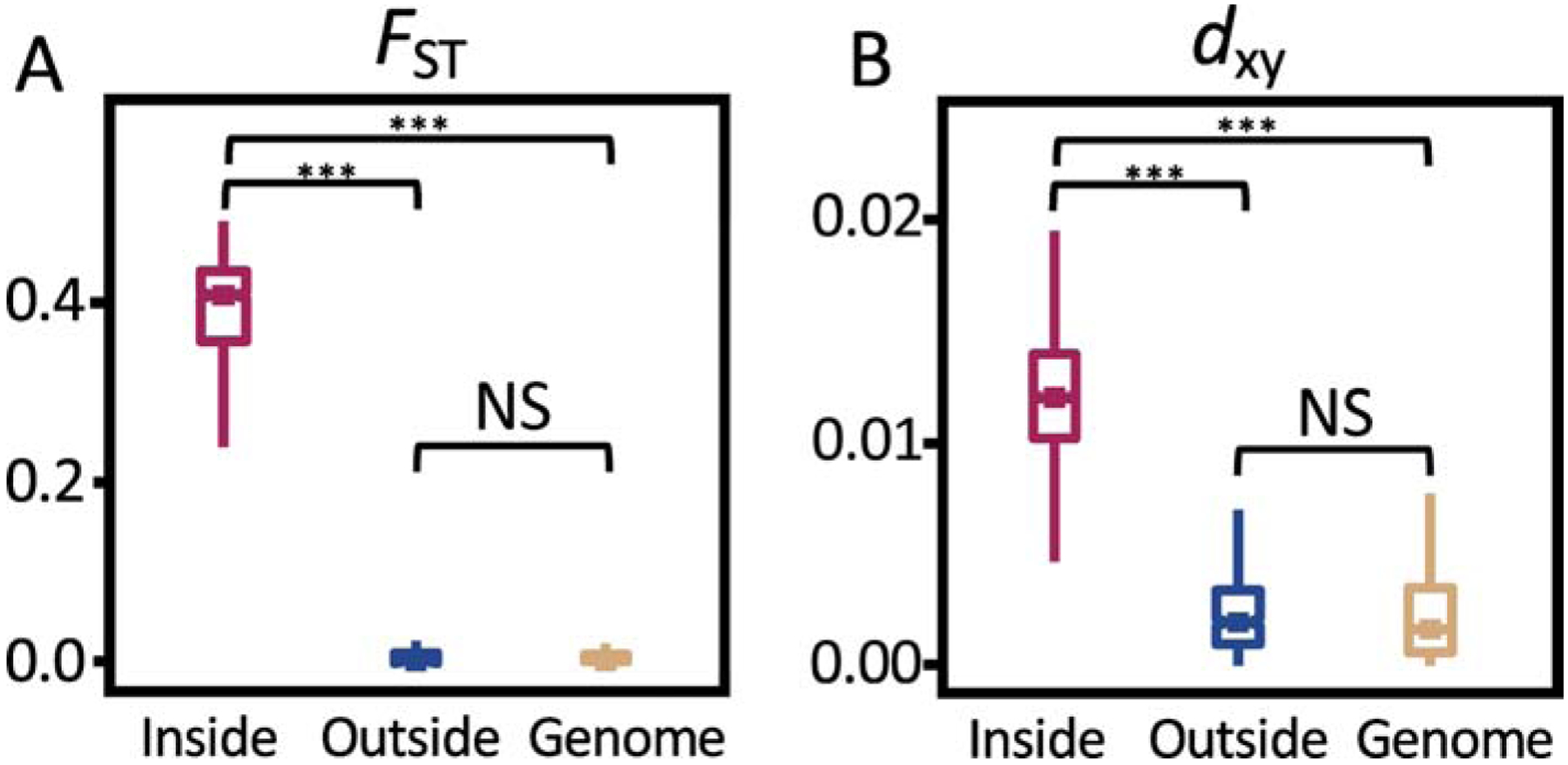
Genetic divergence between the ZAL2 and ZAL2m chromosomes in the white-throated sparrow. Fixation index (FST) shown in (A) indicates a high degree of population differentiation (suppression of recombination) between the two chromosomes inside the rearrangement. Pairwise nucleotide divergence (dxy) shown in (B) indicates significant genetic differentiation between the two chromosomes within the rearrangement compared with the rest of the genome. FST and dxy were measured in 10-kb non-overlapping windows and were significantly higher in scaffolds within the rearrangement than in those outside it (Mann–Whitney U test, ***P < 0.001; NS, not significant). Reprinted from Sun et al. (2018) with permission.
The key to the evolution of ZAL2m is its near-constant state of heterozygosity. Inversions suppress recombination most effectively in heterozygotes; the inverted segment is free to recombine normally in individuals with two copies, but in heterozygotes, successful recombination requires a much rarer event, such as a double crossover or gene conversion (see Hoffmann & Rieseberg, 2008; Kirkpatrick & Barton, 2006; Wellenreuther & Bernatchez, 2018). The scarcity of ZAL2m/ZAL2m homozygotes means that the ZAL2m chromosome is largely devoid of recombination, and effectively undergoes asexual reproduction. As a consequence, de novo mutations can accumulate on ZAL2m independently of ZAL2. The ZAL2m sequence has, in fact, diverged (Fig. 3B) such that the two haplotypes are now 1–2% different from each other (Huynh et al. 2011; Sun et al., 2018). ZAL2m does not, however, show strong signatures of genetic degeneration. Recent analyses have revealed only a slight increase in nonsynonymous polymorphisms (Tuttle et al., 2016), and low incidence of pseudogenization and repetitive sequences, the classic markers of degeneration (Davis et al., 2011; Sun et al., 2018). Although it is not degenerating, ZAL2m is clearly differentiating in ways that can, and do, affect behavior.
Connecting genotype to phenotype
The genetic variation that underlies phenotypic variation can occur in coding sequences or regulatory sequences, which may affect either protein structure or levels of gene expression, respectively. For most of the 20th century, researchers were interested primarily in the effects of mutations in coding regions. These mutations were an obvious place to begin investigation because coding regions govern the sequence of amino acids that build proteins. Non-synonymous mutations, or mutations that cause an amino acid substitution in the encoded protein, can result in the elongation or truncation of proteins or alter protein folding. These changes ultimately alter protein function, which may lead to changes in downstream phenotypes.
Investigating the effects of mutations on protein function is often insufficient for understanding changes in phenotype, however. Although mutations in coding regions do cause phenotypic divergence in some cases, particularly for rapidly evolving gene families such as odorant receptors and G-protein coupled receptors (Bendesky & Bargmann, 2011; Niepoth & Bendesky, 2020), they explain only a portion of the diversity found in nature. Empirical evidence is accumulating that mutations in non-coding, regulatory regions, outside the coding sequence, are a major source of phenotypic divergence (Merritt, 2019; Wittkopp & Kalay, 2012; Wray, 2007). Mutations in regulatory elements such as promoters and enhancers can increase or decrease transcription of that gene as well as affect the distribution of its expression in the body or the brain. Such changes are thought to be important contributors to phenotypic divergence in morphological characters because they allow the level of gene expression to be fine-tuned to the developmental stage, tissue, or cell type (Carroll, 2008; c.f. Hoekstra & Coyne, 2007).
Below, we describe our recent explorations of candidate genes that are (1) captured inside the ZAL2/ZAL2m rearrangement, (2) implicated in the social behaviors that differ between the morphs, and (3) differentially expressed between the morphs. We offer support for the hypothesis that in white-throated sparrows, the ZAL2/ZAL2m arrangement harbors a collection of alleles beneficial to the WS strategy, characterized by increased territorial aggression and lower parental effort, whereas the ZAL2 harbors alleles that favor the inverse TS strategy.
Estrogen receptor α
Accumulating evidence that the morph differences in social behavior depend on reproductive hormones (Maney & Goodson, 2011; Maney et al., 2009) suggests that these behavioral differences may depend on differentiation of hormone signaling pathways. One of the genes within this pathway, and also inside the ZAL2m/ZAL2 rearrangement, is ESR1, the gene that encodes estrogen receptor α (ERα). The ERα protein binds estrogens, particularly E2, a major metabolite of testosterone that can be synthesized de novo in the brain (reviewed by Heimovics et al., 2018). When bound to E2, ERα modulates gene expression through binding to DNA at estrogen response elements. More recent evidence suggests that ERα can also drive rapid changes in membrane excitability and intracellular cascades through actions as a membrane-associated receptor (reviewed by Heimovics et al., 2018). Thus ERα is poised to coordinate biological systems through multiple mechanisms.
The first studies on the ERα-knockout mouse demonstrated a causal role for this gene in facilitating aggression and reducing parental care in both sexes (Ogawa et al., 1998a, Ogawa et al., 1998b). Even fine-tuning the level of ESR1 expression in individual nuclei in the brain can drive territorial aggression and inhibit prosocial behavior in mice and voles (Trainor et al., 2006; Stetzik et al., 2018). In songbirds, ERα expression in the hypothalamus and ventral telencephalon predicts singing during STIs (Rosvall et al., 2012). Thus, ESR1 became one of the top candidate genes in the ZAL2m rearrangement to mediate the life-history tradeoff between the morphs (Thomas et al., 2008).
The coding region of ESR1 on the ZAL2m allele contains two fixed differences, relative to ZAL2, that result in amino acid substitutions. Neither change has occurred in a critical region of the receptor, and neither is expected to alter E2 or DNA binding (Horton et al., 2014b). Rather than one allele conferring a change in protein function, it may be a change in the degree of expression of ESR1 that drives morph differences in behavior. During the breeding season, expression levels of ESR1 depend on morph in almost every region of the brain in which it has been measured (Horton et al., 2014b). In contrast, we could detect no morph differences in the expression of two steroid-related genes not located inside the supergene, aromatase and androgen receptor (Grogan et al., 2019). The regions showing morph differences in ESR1 expression include nuclei within the social behavior network as well as vocal control nuclei (Horton et al., 2014b). In most of the regions in which a morph difference has been detected, TS birds have higher ESR1 expression than WS birds. For example, in the rostral portion of the medial preoptic area (rPOM), TS birds have higher ESR1 expression than WS birds (Fig. 4A). This expression positively predicts the rate of nestling provisioning even when controlling for morph and plasma levels of testosterone and E2 (Fig. 4B), suggesting that it may be causal for morph differences in parental provisioning (Horton et al., 2014b).
Figure 4.
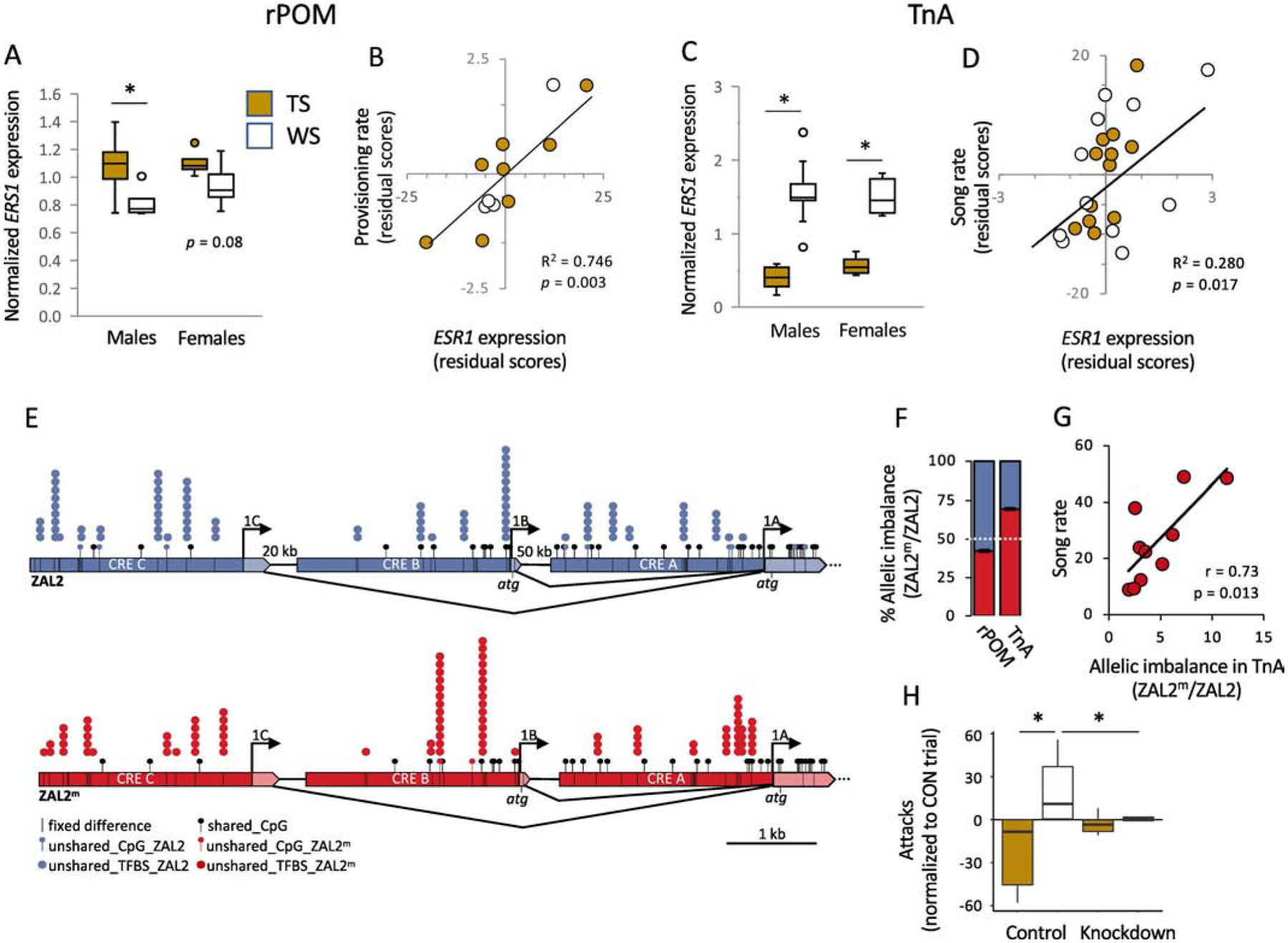
Expression of ESR1 mediates morph differences in behavior in white-throated sparrows. (A) Expression of ESR1 mRNA in the rostral portion of the medial preoptic area (rPOM) differs by morph and (B) predicts parental provisioning behavior (male data are shown). Provisioning rate is the number of trips made to the nest to feed nestlings per hour on post-hatch day seven during the first nest of the season. (C) ESR1 expression in nucleus taeniae of the amygdala (TnA) differs by morph and (D) predicts the number of songs produced by males in response to a 10-min simulated territorial intrusion early in the breeding season. mRNA expression in (A – D) was measured using in situ hybridization. In (A) and (C), values are normalized to the series mean within sex. Scatterplots in (B) and (D) show residual scores after controlling for the effects of morph, plasma testosterone, and plasma estradiol (E2). (E) ESR1 is alternatively spliced. Dark blue or red regions are cis-regulatory elements (CREs); transcribed regions are light colors. Black lines within CREs represent 42 fixed differences distinguishing ZAL2 from ZAL2m. Lollipops represent CpG sites. Stacked circles represent transcription factors that are expressed in TnA and for which a binding site is disrupted by a fixed difference. (F) Allelic imbalance in rPOM and TnA of free-living heterozygous (WS) adults during the breeding season. In the bar graphs, each column represents the relative expression of ZAL2 (blue) and ZAL2m (red). The white dashed line represents a null ratio of 0.5. (G) Behavioral responses of free-living adult males to STI were predicted by the degree of allelic imbalance in TnA. (H) ESR1 knockdown eliminated the morph difference in E2-induced aggression in laboratory-housed birds. Data show the extent to which an oral dose of E2 increased attacks directed toward a conspecific, compared with baseline. * p < 0.05. Data are replotted from Horton et al. (2014b) and Merritt et al. (2020).
One region intersecting both of the neural circuits mentioned above is the nucleus taeniae of the amygdala (TnA), also known as the ventromedial arcopallium, which is considered to be functionally similar to the medial amygdala in mammals because of its gene expression, connectivity, and function (Mello et al., 2019; Cheng et al., 1999; Reiner et al., 2004). In this region is the largest known morph difference in ESR1 expression; WS birds have much higher expression than TS birds do (Fig. 4C) and this expression is associated with singing rate during STIs (Fig. 4D) (Horton et al., 2014b). In fact, ESR1 expression in TnA predicts STI-induced singing independently of morph, plasma testosterone, or plasma E2, suggesting that morph differences in ESR1 expression in this region may mediate morph differences in territorial singing.
It is possible that morph differences in ESR1 expression (Fig. 4A, 4C), as well as the associations between that expression and behavior (Fig. 4B, 4D), could be caused by the morph differences in behavior (Fig. 1B, 1C). Effects of behavior on hormone secretion are well-established (Wingfield et al., 1990; 2020), and expression of genes in steroid pathways could also be affected. We suspect this scenario is unlikely, however, because ESR1 is differentially expressed in TnA even during the non-breeding season, when the birds are not engaging in territorial aggression (Grogan et al., 2019). Furthermore, differential expression can be detected in nestlings, long before the development of territorial or parental behavior. At posthatch day 7, approximately 2 days before natural fledging, morph differences in ESR1 are detectable in rPOM and TnA of nestlings; this effect is most pronounced in males (Grogan et al., 2019). These results demonstrate that morph differences in ESR1 expression precede the emergence of the morph difference in aggression, making it unlikely that the former is causal for the latter.
Instead, our work implicates genetic divergence between the ESR1 alleles, particularly in regulatory regions, as the cause of the expression difference between morphs. Fixed differences between ZAL2 and ZAL2m, including single nucleotide polymorphisms, insertions, and deletions, are found throughout the non-coding areas of ESR1. These areas include regions upstream of the start site, called cis-regulatory regions, which are likely to regulate transcription of the gene. The three cis-regulatory elements (CREs) located 2 kb upstream of the transcription start sites of the gene collectively show 0.7% sequence divergence between ZAL2 and ZAL2m (Fig. 4E) (Merritt et al., 2020; Sun et al., 2018). The ZAL2m and ZAL2 alleles of ESR1 are, in fact, under different transcriptional control, as evidenced by allelic imbalance--that is, significantly different expression of the two alleles—in the brains of WS birds. The impact of this divergence can be seen even in in vitro reporter assays, in which a regulatory region of ESR1 was inserted upstream of a reporter gene. In an avian cell line, a regulatory region of the ZAL2m allele consistently drove greater expression of the reporter gene than did the homologous region of ZAL2 (Merritt et al., 2020). This result showed clearly that the genetic variation in ESR1 regulatory regions significantly impacts the level of expression even in vitro, in the absence of other differential regulatory mechanisms, such as transcription factor binding or chromatin accessibility, that may be operating in vivo.
In the brain, the direction of allelic imbalance depends on brain region. In rPOM, where ESR1 is expressed at a higher level in TS than WS birds (Fig. 4A), the ZAL2 allele is expressed at a higher rate than ZAL2m (Fig. 4F) (Merritt et al., 2020). In contrast, ESR1 is expressed at a higher level in WS than TS birds in TnA (Fig. 4C), and accordingly, the ZAL2m allele is overexpressed in that region (Fig. 4F). These patterns are detectable in nestlings as well, suggesting that they are not driven by engaging in territorial behavior (Merritt et al., 2020). Region-to-region variation in allelic imbalance, that is, which allele is expressed more, could be caused by a number of factors. First, the fixed differences in the cis-regulatory regions are predicted to disrupt the binding of nearly 300 transcription factors (Merritt et al., 2020). The subset of available transcription factors varies locally; thus, transcription of each allele can potentially be regulated independently by multiple mechanisms from region to region (Fig. 4E) (Merritt et al., 2020). Second, local variation in allelic imbalance could be driven by epigenetic factors, for example DNA methylation. We found that the ESR1 CREs are methylated at higher levels on ZAL2 than on ZAL2m (Merritt et al., 2020). This differential methylation was not, however, driven by the degree of methylation at the same CpG sites on each allele. Instead, we found that the higher methylation of the ZAL2 in TnA is driven primarily by sites located only on ZAL2 (Fig. 4E) (Merritt et al., 2020). Like the expression of the AVPR1A gene in Microtus voles (Okhovat et al., 2015; Okhovat et al., 2017), the expression of ESR1 in white-throated sparrows appears to be regulated by a combination of genetic and epigenetic factors. It is possible that the higher number of influential CpG sites on ZAL2, compared with ZAL2m, plays a key role in phenotypic plasticity (see Okhovat et al., 2018).
Together, the above results on ESR1 lead us to two main conclusions. First, the fact that the two alleles are expressed at different levels, both in vitro and in vivo, tells us that the regions of the ESR1 gene that modulate its own expression are meaningfully differentiated from each other. Second, the local environment in each of these brain regions brings a unique compliment of factors, such as transcription factor availability, chromatin accessibility, and DNA methylation, that interact with this cis-regulatory variation in different ways (Sun et al., 2020). The end result is that in some brain regions, expression of the ZAL2m allele is higher, and in others, the ZAL2 allele. This remarkable plasticity may at least partly explain how the genetic differentiation within the supergene is able to impact multiple behaviors, for example territoriality and parental behavior, in different directions.
In this section we have seen that fixed differences between the ZAL2 and ZAL2m alleles of ESR1 (Fig. 4E) are likely causing the two alleles to be expressed to different degrees particularly in TnA (Fig. 4F). Remarkably, the degree of allelic imbalance favoring ZAL2m significantly predicts territorial singing in response to STI (Fig. 4G). In other words, the greater the expression of the supergene allele, the more aggressive the bird. We next sought to show causal evidence that variation in ESR1 expression explains morph differences in behavior. To do so, we used antisense oligonucleotides to knock down ESR1 expression in TnA in laboratory-housed birds (Merritt et al., 2020). We then measured the degree to which an oral, bolus dose of E2 increased aggression toward a conspecific. In birds receiving control (scrambled) oligonucleotides, E2 facilitated aggression in the WS but not the TS birds, confirming that the WS birds are normally more sensitive than TS to the effects of exogenous E2 on aggression (see Merritt et al., 2018). In the birds receiving ESR1 knockdown, E2-induced aggression did not depend on morph – all birds behaved like TS birds (Fig. 4H). Furthermore, in the control animals, the degree of ESR1 expression in TnA predicted the degree of aggression even when controlling for morph, replicating our finding in free-living birds (Fig. 4D). Overall, this series of studies represents the first causal evidence a specific gene within a supergene contributing to differentiation of an associated behavioral phenotype.
Vasoactive intestinal peptide
We next turned our attention to a different gene inside the ZAL2m/ZAL2 rearrangement, VIP, which encodes vasoactive intestinal peptide. This 28-amino acid polypeptide, which is highly conserved among vertebrates, was named after its role in regulating gastrointestinal blood flow and vasodilation (reviewed by Klimaschewski, 1997; Lee & Berezin, 1984). It has since been found to be widespread throughout the vertebrate brain and critical in the regulation of social behavior (Kingsbury, 2016; Kingsbury & Wilson, 2015). In the hypothalamus of the chicken (Gallus domesticus), VIP is expressed in two notable cell populations, one of which includes the anterior hypothalamus (AH) and the other the infundibular nucleus (INF) (Kuenzel et al., 1997). The expression of VIP in both of these regions is particularly relevant to the morph differences in behavior in white-throated sparrows because in songbirds, VIP in AH is associated with aggression while VIP in INF is associated with parental behavior (reviewed by Kingsbury & Wilson, 2016).
To explore the potential role of VIP expression in territorial aggression in white-throated sparrows, we used in situ hybridization to label VIP mRNA in AH in free-living birds during the breeding season (Horton et al., 2020). The results were striking – regardless of sex or stage of the breeding season (territory establishment and feeding nestlings), VIP in AH was expressed at higher levels in WS birds than in TS birds (Fig. 5A, 5B). Furthermore, this expression significantly predicted territorial singing in response to STI in both sexes (Fig. 5C, 5D). We therefore have strong, if correlational, evidence that morph differences in VIP expression in AH may play a role in the behavioral phenotypes in this species. Our group has not yet manipulated VIP expression in AH to test for a causal role in aggression; nonetheless, there is already strong evidence of such in songbirds. Working with violet-eared waxbills (Uraeginthus granatina) and zebra finches (Taeniopygia guttata), Goodson et al. (2012) showed that antisense knockdown of VIP expression in AH significantly reduced aggressive behaviors, such as displacements, in both species. We therefore predict that knocking down VIP in AH of WS white-throated sparrows is likely to reduce aggression, making the phenotype more TS-like. This result would place VIP in the same category as ESR1: a gene that mediates the effect of the ZAL2m/ZAL2 on aggression in this species.
Figure 5.
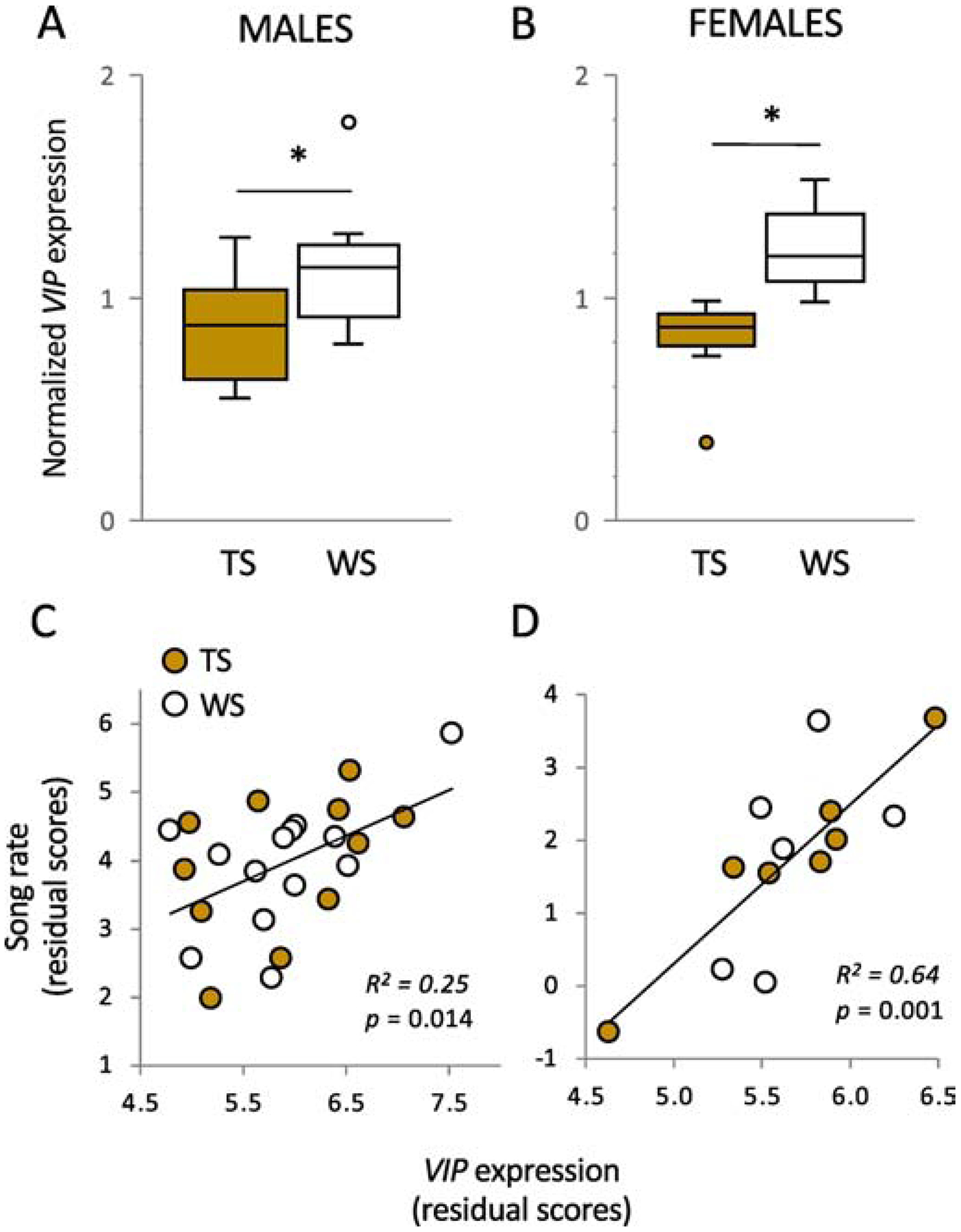
Expression of VIP mRNA in the anterior hypothalamus differs between the morphs (A, B) and predicts territorial singing (C, D) in white-throated sparrows. mRNA expression was measured using in situ hybridization. In (A) and (B), values are normalized to the series mean. (C) and (D) show residual scores after controlling for the effects of morph and plasma steroid hormones (testosterone for males, estradiol for females). Song rate is the number of songs produced by the resident in response to a 10-min simulated territorial intrusion. All data are from birds sampled early in the breeding season. *p < 0.05. Data replotted from Horton et al. (2020).
In another of its diverse roles in vertebrates, and specifically in birds, VIP secreted from the median eminence has long been known to be a prolactin releasing factor (reviewed by Smiley, 2019, Kosonsiriluk et al 2008; Kulick et al., 2005). VIP administration increases levels of circulating prolactin in multiple taxa including domestic turkeys (Meleagris gallopavo) (El Halawani et al., 1990), bantam hens (Gallus gallus) (Macnamee et al., 1986), and in several songbird species, including the white-crowned sparrow (Zonotrichia leucophrys) (Maney et al., 1999). Prolactin is well-known to be associated with parenting behavior, in both sexes, across vertebrates (reviewed by Smiley, 2019; Schradin & Anzenberger, 1999). Importantly, prolactin has been associated with provisioning behaviors in both male and female songbirds (reviewed by Lynn, 2016; Smiley & Adkin-Regan 2016). Because TS male white-throated sparrows provision young at higher rates than WS males (Fig. 1C), we hypothesized that TS males would have higher levels of VIP in INF, the cell population that controls prolactin release. Our in situ hybridization study showed that, in fact, TS males do have higher VIP expression than WS males in this region (Horton et al., 2020). This morph difference was not significant in females; however, we could not detect a morph difference in provisioning rate in these females (Fig. 1C).
We are currently investigating the ZAL2m and ZAL2 gene sequences of VIP to identify the variation that could drive morph differences in expression. While there are no non-synonymous substitutions in VIP, there is a high degree of fixed genetic variation in both the 5’ upstream and intergenic regions of the gene (Sun et al., 2018). As noted above for ESR1, such variation may affect expression by altering transcription factor binding sites or numbers of CpG sites. It is also possible that the epigenetic environment could be modified such that differential DNA methylation may occur in the absence of fixed sequence differences. Thus, for VIP, it will be important to compare DNA methylation of ZAL2m and ZAL2 at shared, as well as unshared, sites (Merritt et al., 2020). Methylation of the cis-regulatory region of a gene has most often been associated with decreased gene expression; however, the precise role of DNA methylation in the regulation of gene expression is dynamic. It depends on the region and tissue in which it occurs (Suzuki & Bird, 2008), meaning that we cannot necessarily predict expression from the degree of DNA methylation. We are currently investigating the relationships between variation in methylation of the VIP gene and expression of VIP.
Inversions and complex alternative phenotypes: Back to Dobzhansky?
Social behaviors are complex and polygenic. Thus, we should expect that understanding their genetic basis will require careful consideration of multiple genes and how they are regulated. The ZAL2m supergene in white-throated sparrows has captured variant alleles of ESR1 and VIP that seem to confer high levels of expression in TnA and AH, respectively (Figs. 4C, 5A, 5B; Horton et al., 2014b; 2020). Existing evidence, from this species and other songbirds, suggests that the increased expression of each of these two genes, in these respective regions, contributes to a territorial, aggressive phenotype (Goodson et al., 2012; Merritt et al., 2020). Under conditions in which a such a phenotype is adaptive, individuals with the ZAL2m alleles of both ESR1 and VIP will have a selective advantage and natural selection will favor the linkage between them. From the alleles’ perspective, the linkage to each other promotes their probability of being maintained in the population. Further, all of the other alleles linked to these two will also be maintained due to genetic hitchhiking (Barton, 2000). Similarly, the ZAL2 allele seems to confer high levels of ESR1 and VIP expression in POM and INF, respectively (Horton et al., 2014b; 2020), which may result in increased parental behavior (Fig. 1C; Horton et al., 2014a; Smiley, 2019). At least with respect to these two genes, each of the two haplotypes has captured alleles that contribute either to heightened aggression (the ZAL2m allele of ESR1 and VIP) or to increased parental provisioning (the ZAL2 allele of ESR1 and VIP).
This segregation of territorial vs. parental phenotypes should remind us of life-history trade-offs proposed by Trivers (1972; see also Ketterson & Nolan, 1992; Maney, 2008). The two morphs of white-throated sparrows lie at either end of a continuum, with investment in resource defense and mating success at one end and investment in current offspring at the other. The morphs thus exemplify a classic trade-off between investing in territoriality and mating effort versus parental care (Maney, 2008). The responsible genes in such systems are subject to antagonistic selection (Mérot et al., 2020b; Zajitschek & Connallon, 2018), under which certain alleles are beneficial to only one of two (or sometimes more) alternative life-history strategies. Inversion-based supergenes often mediate this segregation of beneficial alleles (Wellenreuther & Bernatchez, 2018). Inversions on sex chromosomes, for example, can capture sex-determining genes together with alleles that benefit the life-history strategy of a particular sex; over time, other alleles benefiting that strategy can accumulate within the nonrecombining region (Charlesworth et al., 2005; Rice, 1987; Rubenstein et al., 2019). In the case of the white-throated sparrow, alleles that benefit the WS strategy are expected to accumulate on ZAL2m (Thomas et al., 2008).
This view of the evolution of ZAL2/ZAL2m fits well with Dobzhanky’s ideas about coadaptation (Dobzhansky, 1970). Inside the rearrangement, ZAL2m alleles may interact to maximize the fitness of the WS strategy. These interactions could, as noted above, be limited to independent effects of multiple genes on the same phenotype, e.g. aggression. It is also possible that the gene products of ZAL2m alleles are adapted to interact directly with each other. To show evidence of such, it is necessary to look for variation in the coding regions of at least two genes, both inside the rearrangement. As a transcription factor, ERα interacts with many other proteins. As noted above, ESR1 contains two non-synonymous fixed differences that result in amino acid substitutions. Both of these changes are located within disordered areas of the protein; Ala552Thr is found in the C-terminus of the ligand-binding domain and Val73Ile occurs within the N-terminal activation function1 (AF1) domain (Horton et al., 2014b). The AF1 domain is important for E2-independent transactivation via interactions with the TATA binding protein (TBP); the TBP gene has also been captured within the ZAL2m/ZAL2 supergene and harbors four nonsynonymous changes. Other genes inside the supergene include NCOA7 and GREB1, which contain six and 25 missense mutations, respectively. Both protein products interact directly with ERα as transcriptional co-activators (Lazennec et al., 1997; Mohammed et al., 2013). Any of these genes could be co-adapted with ESR1.
Most of what we know about the predicted functions of each domain of ERα is based on its actions as a nuclear transcription factor. Given the growing appreciation for ERα as a membrane-associated receptor (Meitzen & Mermelstein, 2011), it is possible that one or both of the two coding region polymorphisms affect ERα function in the membrane. Relatively little is known about the unstructured regions in the context of membrane-associated actions because it is difficult to capture flexible regions of proteins using x-ray crystallography or electron microscopy. New insight into the rapid effects of E2 in songbirds (Heimovics et al., 2018; Merritt et al., 2018; 2020; Fig. 4H) warrants a reconsideration of these polymorphisms and their potential effects on membrane signaling. The gene GRM1, which is inside the supergene, encodes a glutamate receptor that interacts with ERα situated in cell membranes (Dewing et al., 2007). With three nonsynonymous mutations, GRM1 represents yet another contender for possible co-adaptation with ESR1. Overall, the list of genes that interact with or are co-expressed with ESR1 is extensive. Many of these genes are inside ZAL2/ZAL2m and correlated with both morph and singing (Zinzow-Kramer et al., 2015). These genes, like others inside the supergene, offer abundant opportunities to identify co-adapted alleles and understand their evolutionary trajectories.
Any analysis of a supergene must acknowledge that, for most genes, location matters. Until the end of the 20th century, it was thought that eukaryotic genes are randomly distributed in the genome (reviewed by Hurst et al., 2004). Today, we know that gene order is not random. Genes with related functions form physical clusters along chromosomes and are subject to co-regulation (Al-Shahrour, 2010; Hurst et al., 2004; Michalak, 2008). Thus, physical proximity could provide insight about functional relationships among ZAL2/ZAL2m genes. The genes ESR1 and VIP are in fact part of a gene cluster, or synteny block, that is conserved across most vertebrate classes. These two genes are located together, separated by only one gene, in many vertebrate taxa including rodents, marsupials, whales, birds, turtles, crocodiles, and primates (Ensembl release 100; Kondo et al., 2010; Yates et al., 2020). The close proximity of the two genes not only suggests related functions, but also provides a mechanism by which ESR1 and VIP could be tightly linked in other species, even in the absence of a structural rearrangement. Inside this cluster, ESR1 and VIP are always separated by just one gene, MYCT1, which encodes the transcriptional activator MYC Target 1. In white-throated sparrows, expression of MYCT1 in TnA depends on morph and is positively correlated with territorial singing (Zinzow-Kramer et al., 2015). The function of this gene is not well-understood outside of its role in cancer; we currently do not know whether it has functional relevance to territoriality or whether its pattern of expression could instead be a side effect of its location between ESR1 and VIP (Hurst et al., 2004).
Coregulation of neighboring genes is accomplished via a variety of mechanisms. Cis-regulatory elements, such as enhancers, can directly affect transcription of neighboring genes, histone modifications can co-suppress linked genes, and tertiary structures can bring genes even closer together (Hurst et al., 2004; Liao & Zhang, 2008). Despite their close proximity to each other, however, we currently have no evidence that ESR1 and VIP are co-regulated in white-throated sparrows. In fact, their expression is anticorrelated in our samples from free-living birds in breeding condition. Using ESR1 data from Horton et al. (2014a) and VIP data from Horton et al. (2020), we can see that ESR1 and VIP expression are negatively correlated in both AH (R = −0.240, p = 0.033) and TnA (R = −0.376, p = 0.001) (Fig. 6). These correlations may be driven by the fact that each gene is differentially expressed, however. In AH, ESR1 expression is higher in TS birds (F1,78 = 4.787; p = 0.032) and VIP expression is higher in WS birds (F1,78 = 39.03; p <0.001). In TnA, ESR1 expression is higher in WS birds (F1,78 = 150.144, p < 0.001) and VIP expression is higher in TS birds (F1,78 = 10.374, p = 0.002). When morph is controlled in the model, the correlations are no longer significant (AH: R = −0.131, p = 0.254; TnA: R = −0.194, p = 0.09), suggesting that they are an artifact of the morph differences themselves, not evidence of coregulation. The fact that the direction of the morph difference in expression changes from region to region, in both genes, strongly suggests that cis-regulatory variation in these genes interacts in complex ways with the local transcriptional environment (Merritt et al., 2020). It is entirely possible that in a different brain region or tissue, VIP and ESR1 could be co-regulated, perhaps by testosterone. Our future research will focus on the interplay between these two genes as well as others, such as coactivators, gonadotropin receptors, and serotonin receptors, also inside the supergene.
Figure 6.
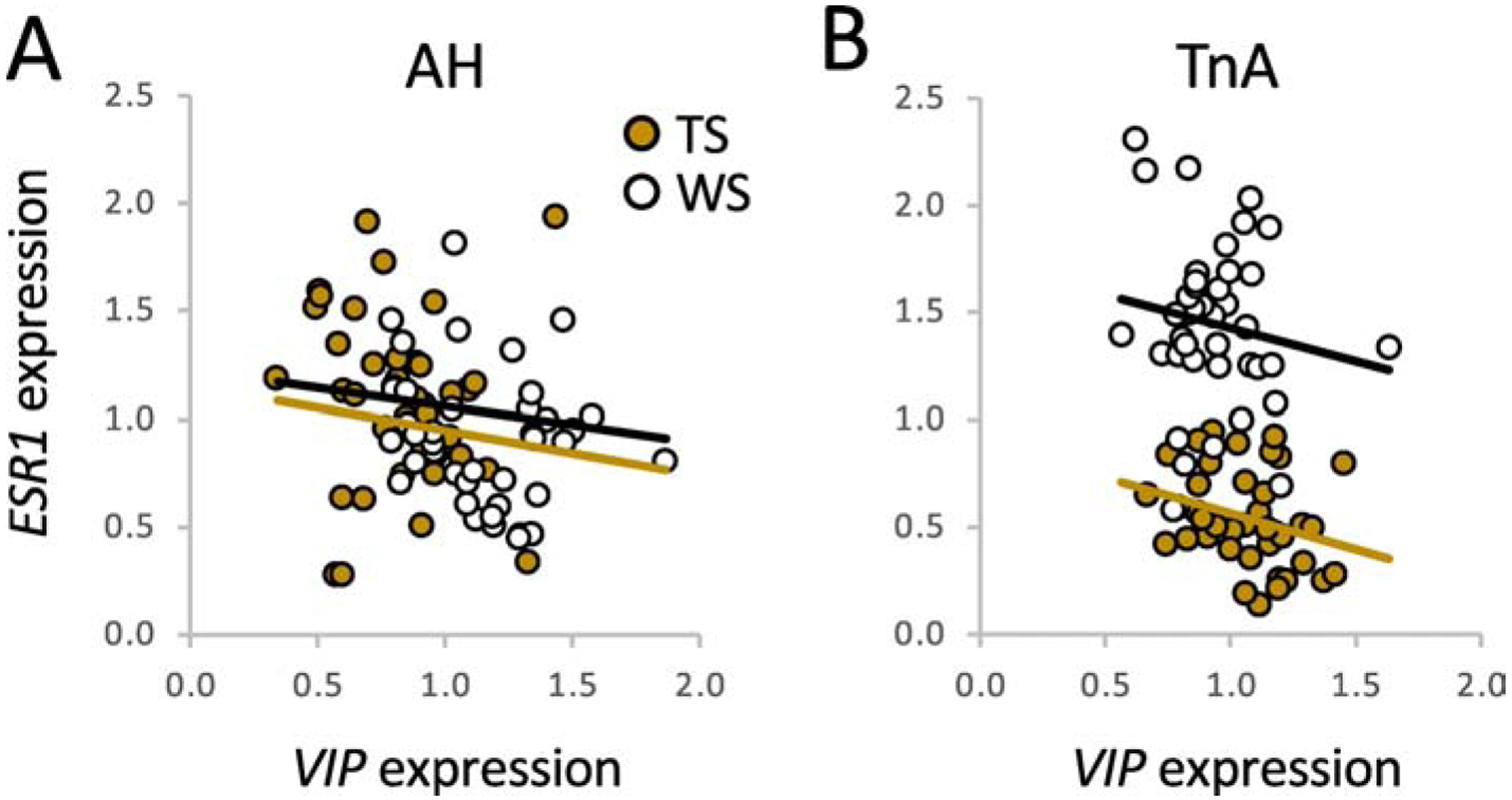
Negative correlations between ESR1 and VIP expression in the anterior hypothalamus (A) and nucleus taeniae (B) of free-living white-throated sparrows (n = 79). mRNA was labeled using in situ hybridization in alternate brain sections (see Horton et al., 2014b; 2020). The analysis includes birds of both sexes, sampled throughout the breeding season. Data are normalized to the series mean for each gene in each region. Correlations are significant when morphs are pooled but nonsignificant within morph. Trendlines are shown for each morph separately.
Supergenes: diverse phenotypes, similar challenges
The white-throated sparrow is not the only species with a supergene linked to life-history strategies. In a shorebird called the ruff (Philomachus pugnax), an inversion polymorphism underlies a complex mating system with three different male morphs: territorial males with showy plumage, satellite males, which form temporary alliances with territorial males, and female-like faeders, which sneak copulations (Küpper et al., 2016; Lamichhaney et al., 2016). Whereas the territorial males are homozygous for the standard arrangement of chromosome 11, the other two morphs have a single copy of an inversion containing 125 genes. Satellite males have one particular haplotype of the inversion, whereas faeders have another; both haplotypes are homozygous lethal. The inversion contains several genes involved in steroid hormone metabolism, such as SDR42E1 and HSD17B2, leading its discoverers to speculate that the behavioral morphs may be at least partly hormone-dependent (Küpper et al., 2016; Lamichhaney et al., 2016). To date, however, no genes have been causally linked to behavioral phenotypes.
In the seaweed fly (Coelopa frigida), all populations are polymorphic for a large inversion (~1,000 genes) that has suppressed recombination, resulting in substantial differentiation between the haplotypes. This polymorphism mediates a life-history trade-off in that one haplotype favors faster larval development and the other reproductive output (Mérot et al., 2018; 2020b). Similarly, in rainbow trout (Oncorhynchus mykiss), alternative reproductive phenotypes segregate with a large inversion that mediates a trade-off between early maturation and later fecundity (Pearse et al., 2014; 2019). Both of these systems elegantly illustrate how supergenes, working under antagonistic selection, can drive the evolution of alternative strategies that maximize opposite ends of a life-history continuum.
Two of the most fascinating examples of supergenes are associated with colony structure in eusocial ants. In both alpine silver ants (Formica selysi) and fire ants (Solenopsis invicta), an inversion polymorphism segregates with the number of queens per colony, e.g., one queen or multiple queens (Purcell et al., 2014; Wang et al., 2013). Remarkably, the inversions in the two species evolved independently and do not share any genes (Purcell et al., 2014). Although the social structures and genotypic systems of these two species differ slightly from each other, the striking convergence of their genomic architecture demonstrates that inversion polymorphisms represent a general genetic mechanism in the evolution of complex phenotypes (Rubenstein et al., 2019). Because the genes inside these inversions are essentially nonoverlapping in the face of a convergent phenotype, these ant species also demonstrate the highly polygenic nature of such phenotypes.
Inversion polymorphisms clearly mediate a large number of spectacular phenotypes across a diverse array of species. Next steps should, clearly, include identifying the causal genes inside these inversions and determining the mechanisms by which those genes drive alternative life-history strategies (Wellenreuther & Bernatchez, 2018). Taking these steps has been difficult, however, because of the tight linkage that characterizes all supergenes. Individuals with a particular supergene allele typically have all of the supergene alleles, rendering useless the standard methods of identifying causal loci. Further, the most interesting supergene-mediated phenotypes are found in non-model organisms, for which fewer genetic resources are available and technologies such as CRISPR can lag behind if the species does not breed in captivity. Definitive experimental evidence of a causal gene, for any of these remarkable phenotypes, is vanishingly rare. The white-throated sparrow has proven to be a tractable model for asking these questions partly because genomic resources for songbirds are relatively well-developed (Mello & Clayton, 2015). More importantly, we have been able to leverage decades of research on the hormonal control of territoriality and parental behavior to identify strong candidate genes; it was because we already knew that territorial aggression depends on androgen- and estrogen-signaling pathways (e.g., Maney et al., 2009; Soma et al., 2000; Wingfield, 1984) that we considered and were ultimately able to confirm ERα as a causal gene. Our work shows that even in the age of Big Data, a hypothesis-driven approach can be quite powerful. We expect that other causal genes, including those contributing to plumage, will eventually be identified using similar strategies.
As genomic resources for non-model species continue to expand, our ability to understand causal genotype-phenotype connections, particularly in ecological settings, will grow exponentially (Bengston et al., 2018; O’Connell & Hofmann, 2011; Kratochwil & Meyer, 2015). Identifying the genes that drive supergene-mediated phenotypes will become commonplace. Many examples of coadapted alleles will be discovered, lending experimental support to a century-old hypothesis about the adaptive significance of inversions. We believe that the white-throated sparrow will continue to be a key player in these discoveries, not only because the ZAL2/ZAL2m supergene is well-characterized but also because this species has another large chromosomal rearrangement known, as ZAL3/ZAL3a (Thorneycroft 1966; 1975), which has captured nearly 1,000 genes (N. Baran, unpublished). To date, however, no associated phenotype has been characterized. We expect that this extraordinary species will continue to fascinate and surprise us—and, as it did for Audubon, challenge our assumptions.
Acknowledgments
We thank Katie Grogan, Harris Jeong, Clifton McKee, Christina Michael, Justin Michaud, Eric Ortlund, Sandra Shirk, Dan Sun, Jim Thomas, Emily Young, and Wendy Zinzow-Kramer for their contributions to this work.
Funding: This work was supported by NIH Grant 1R01MH082833 to D.L.M and S.V.Y., NSF Grant IOS-1627789 to D.L.M and S.V.Y., and NIH Grant 1F31MH114509 to J.R.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Shahrour F, Minguez P, Marqués-Bonet T, Gazave E, Navarro A, & Dopazo J (2010). Selection upon genome architecture: conservation of functional neighborhoods with changing genes. PLoS Computational Biology, 6(10), e1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AR, Hoffmann AA, McKechnie SW, Umina PA, & Weeks AR (2005). The latitudinal cline in the In (3R) Payne inversion polymorphism has shifted in the last 20 years in Australian Drosophila melanogaster populations. Molecular Ecology, 14(3), 851–858. [DOI] [PubMed] [Google Scholar]
- Archer J (2006). Testosterone and human aggression: an evaluation of the challenge hypothesis. Neuroscience & Biobehavioral Reviews, 30(3), 319–345. [DOI] [PubMed] [Google Scholar]
- Ayala D, Acevedo P, Pombi M, Dia I, Boccolini D, Costantini C, … & Fontenille D (2017). Chromosome inversions and ecological plasticity in the main African malaria mosquitoes. Evolution, 71(3), 686–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton NH (2000). Genetic hitchhiking. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 355(1403), 1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendesky A, & Bargmann CI (2011). Genetic contributions to behavioural diversity at the gene–environment interface. Nature Reviews Genetics, 12(12), 809–820. [DOI] [PubMed] [Google Scholar]
- Campagna L (2016). Supergenes: the genomic architecture of a bird with four sexes. Current Biology, 26(3), R105–R107. [DOI] [PubMed] [Google Scholar]
- Carroll SB (2008). Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell, 134(1), 25–36. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B, & Marais G (2005). Steps in the evolution of heteromorphic sex chromosomes. Heredity, 95(2), 118–128. [DOI] [PubMed] [Google Scholar]
- Cheng MF, Chaiken M, Zuo M, & Miller H (1999). Nucleus taenia of the amygdala of birds: anatomical and functional studies in ring doves (Streptopelia risoria) and European starlings (Sturnus vulgaris). Brain, Behavior and Evolution, 53(5–6), 243–270. [DOI] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, & Micevych P (2007). Membrane estrogen receptor-α interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. Journal of Neuroscience, 27(35), 9294–9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T 1970. Genetics of the evolutionary process. New York: Columbia University Press. [Google Scholar]
- Dobzhansky T (1950). Genetics of natural populations. XIX. Origin of heterosis through natural selection in populations of Drosophila pseudoobscura. Genetics, 35(3), 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Halawani ME, Silsby JL, & Mauro LJ (1990). Vasoactive intestinal peptide is a hypothalamic prolactin-releasing neuropeptide in the turkey (Meleagris gallopavo). General and Comparative Endocrinology, 78(1), 66–73. [DOI] [PubMed] [Google Scholar]
- Falls JB & Kopachena JG (2020). White-throated Sparrow (Zonotrichia albicollis), version 1.0 In Birds of the World (Poole AF, Editor). Cornell Lab of Ornithology, Ithaca, NY, USA. [Google Scholar]
- Finch CE & Rose MR (1995). Hormones and the physiological architecture of life history evolution. The Quarterly Review of Biology, 70(1), 1–52. [DOI] [PubMed] [Google Scholar]
- Fransz P, Linc G, Lee CR, Aflitos SA, Lasky JR, Toomajian C, … & Kuzak M (2016). Molecular, genetic and evolutionary analysis of a paracentric inversion in Arabidopsis thaliana. The Plant Journal, 88(2), 159–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kelly AM, Kingsbury MA, & Thompson RR (2012). An aggression-specific cell type in the anterior hypothalamus of finches. Proceedings of the National Academy of Sciences, 109(34), 13847–13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan KE, Horton BM, Hu Y, & Maney DL (2019). A chromosomal inversion predicts the expression of sex steroid-related genes in a species with alternative behavioral phenotypes. Molecular and Cellular Endocrinology, 495: 110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross MR (1996). Alternative reproductive strategies and tactics: diversity within sexes. Trends in Ecology & Evolution, 11(2), 92–98. [DOI] [PubMed] [Google Scholar]
- Grunst AS, Grunst ML, Rathbun NA, Hubbard JK, Safran RJ, Gonser RA, & Tuttle EM (2017). Disruptive selection on plumage coloration across genetically determined morphs. Animal Behaviour, 124, 97–108. [Google Scholar]
- Hau M (2007). Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. Bioessays 29, 133–144. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Merritt JR, Jalabert C, Ma C, Maney DL, & Soma KK (2018). Rapid effects of 17 β-estradiol on aggressive behavior in songbirds: environmental and genetic influences. Hormones & Behavior, 104, 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra HE & Coyne JA (2007). The locus of evolution: evo devo and the genetics of adaptation. Evolution: International Journal of Organic Evolution, 61(5), 995–1016. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, & Rieseberg LH (2008). Revisiting the impact of inversions in evolution: from population genetic markers to drivers of adaptive shifts and speciation? Annual Review of Ecology, Evolution, and Systematics, 39, 21–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton BM, Michael CM, Prichard MR, & Maney DL (2020). Vasoactive intestinal peptide as a mediator of the effects of a supergene on social behavior. Proceedings of the Royal Society B, 287, 20200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton BM, Moore IT, & Maney DL (2014a). New insights into the hormonal and behavioural correlates of polymorphism in white-throated sparrows. Animal Behaviour, 93, 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton BM, Hudson WH, Ortlund EA, Shirk S, Thomas JW, Young ER, Zinzow-Kramer WM, & Maney DL (2014b). Estrogen receptor α polymorphism in a species with alternative behavioral phenotypes. Proceedings of the National Academy of Sciences, 111, 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton BM, Hu Y, Martin CL, Bunke BP, Matthews BS, Moore IT, Thomas JW, & Maney DL (2013). Behavioral characterization of a white-throated sparrow homozygous for the ZAL2m chromosomal arrangement. Behavior Genetics, 43, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton BM, Hauber ME, & Maney DL (2012). Morph matters: Aggression bias in a polymorphic sparrow. PLoS ONE, 7, e48705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst LD, Pál C, & Lercher MJ (2004). The evolutionary dynamics of eukaryotic gene order. Nature Reviews Genetics, 5(4), 299–310. [DOI] [PubMed] [Google Scholar]
- Huynh LY, Maney DL, & Thomas JW (2011). Chromosome-wide linkage disequilibrium caused by an inversion polymorphism in the white-throated sparrow. Heredity, 106, 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh LY, Maney DL, & Thomas JW (2010). Contrasting population genetic patterns within the white-throated sparrow genome (Zonotrichia albicollis). BMC Genetics, 11, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterson ED, & Nolan V Jr (1992). Hormones and life histories: an integrative approach. The American Naturalist, 140, S33–S62. [DOI] [PubMed] [Google Scholar]
- Kingsbury MA (2015). New perspectives on vasoactive intestinal polypeptide as a widespread modulator of social behavior. Current Opinions in Behavioral Science, 6, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury MA, & Wilson LC (2016). The role of VIP in social behavior: neural hotspots for the modulation of affiliation, aggression, and parental care. Integrative and Comparative Biology. 56(6), 1238–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick M (2010). How and why chromosome inversions evolve. PLoS Biol, 8(9), e1000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick M, & Barton N (2006). Chromosome inversions, local adaptation and speciation. Genetics, 173(1), 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimaschewski L (1997). VIP – a ‘Very Important Peptide’ in the sympathetic nervous system? Anatomy and Embryology, 196(4), 269–277. [DOI] [PubMed] [Google Scholar]
- Knapp R (2003). Endocrine mediation of vertebrate male alternative reproductive tactics: the next generation of studies. Integrative and Comparative Biology, 43(5), 658–668. [DOI] [PubMed] [Google Scholar]
- Kondo M, Maruoka T, Otsuka N, Kasamatsu J, Fugo K, Hanzawa N, & Kasahara M (2010). Comparative genomic analysis of mammalian NKG2D ligand family genes provides insights into their origin and evolution. Immunogenetics, 62(7), 441–450. [DOI] [PubMed] [Google Scholar]
- Kopachena JG, & Falls JB (1993). Re-evaluation of morph-specific variations in parental behavior of the white-throated sparrow. The Wilson Bulletin, 105, 48–59. [Google Scholar]
- Kosonsiriluk S, Sartsoongnoen N, Chaiyachet O. a., Prakobsaeng N, Songserm T, Rozenboim I, … Chaiseha Y (2008). Vasoactive intestinal peptide and its role in continuous and seasonal reproduction in birds. General and Comparative Endocrinology, 159(1), 88–97. [DOI] [PubMed] [Google Scholar]
- Kratochwil CF, & Meyer A (2015). Closing the genotype–phenotype gap: emerging technologies for evolutionary genetics in ecological model vertebrate systems. BioEssays, 37(2), 213–226. [DOI] [PubMed] [Google Scholar]
- Kuenzel WJ, McCune SK, Talbot RT, Sharp PJ, & Hill JM (1997). Sites of gene expression for vasoactive intestinal polypeptide throughout the brain of the chick (Gallus domesticus). Comparative Neurology, 381(1), 101–118. [DOI] [PubMed] [Google Scholar]
- Kulick RS, Chaiseha Y, Kang SW, Rozenboim I, & El Halawani ME (2005). The relative importance of vasoactive intestinal peptide and peptide histidine isoleucine as physiological regulators of prolactin in the domestic turkey. General and Comparative Endocrinology, 142(3), 267–273. [DOI] [PubMed] [Google Scholar]
- Küpper C, Stocks M, Risse JE, Dos Remedios N, Farrell LL, McRae SB, … & Kitaysky AS (2016). A supergene determines highly divergent male reproductive morphs in the ruff. Nature Genetics, 48(1), 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladjali-Mohammedi K, Bitgood JJ, Tixier-Boichard M, & De Leon FP (1999). International system for standardized avian karyotypes (ISSAK): standardized banded karyotypes of the domestic fowl (Gallus domesticus). Cytogenetic and Genome Research, 86(3–4), 271–276. [DOI] [PubMed] [Google Scholar]
- Lamichhaney S, Fan G, Widemo F, Gunnarsson U, Thalmann DS, Hoeppner MP, … & Chen W (2016). Structural genomic changes underlie alternative reproductive strategies in the ruff (Philomachus pugnax). Nature Genetics, 48(1), 84–88. [DOI] [PubMed] [Google Scholar]
- Lazennec G, Ediger TR, Petz LN, Nardulli AM, & Katzenellenbogen BS (1997). Mechanistic aspects of estrogen receptor activation probed with constitutively active estrogen receptors: correlations with DNA and coregulator interactions and receptor conformational changes. Molecular Endocrinology, 11(9), 1375–1386. [DOI] [PubMed] [Google Scholar]
- Lee T, Saito A, & Berezin I (1984). Vasoactive intestinal polypeptide-like substance: the potential transmitter for cerebral vasodilation. Science, 224(4651), 898–901. [DOI] [PubMed] [Google Scholar]
- Liao BY, & Zhang J (2008). Coexpression of linked genes in mammalian genomes is generally disadvantageous. Molecular Biology and Evolution, 25(8), 1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry DB, & Willis JH (2010). A widespread chromosomal inversion polymorphism contributes to a major life-history transition, local adaptation, and reproductive isolation. PLoS Biology, 8(9), 2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowther JK (1961). Polymorphism in the white-throated sparrow, Zonotrichia albicollis (Gmelin). Canadian Journal of Zoology, 39(3), 281–292. [Google Scholar]
- Lynn SE (2016). Endocrine and neuroendocrine regulation of fathering behavior in birds. Hormones and Behavior, 77, 237–248. [DOI] [PubMed] [Google Scholar]
- Macnamee MC, Sharp PJ, Lea RW, Sterling RJ, & Harvey S (1986). Evidence that vasoactive intestinal polypeptide is a physiological prolactin-releasing factor in the bantam hen. General and Comparative Endocrinology, 62(3), 470–478. [DOI] [PubMed] [Google Scholar]
- Maney DL (2017). Polymorphisms in sex steroid receptors: From gene sequence to behavior. Frontiers in Neuroendocrinology, 47, 47–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL (2008). Endocrine and genomic architecture of life history trade-offs in an avian model of social behavior. General and Comparative Endocrinology, 157, 275–282. [DOI] [PubMed] [Google Scholar]
- Maney DL & Goodson JL (2011). Neurogenomic mechanisms of aggression in songbirds. Advances in Genetics, 75, 83–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL, Horton BM, & Zinzow-Kramer WM (2015). Estrogen receptor alpha as a mediator of life-history trade-offs. Integrative and Comparative Biology, 55, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL, Lange HS, Raees MQ, Reid AE, & Sanford SE (2009). Behavioral phenotypes persist after gonadal steroid manipulation in white-throated sparrows. Hormones & Behavior, 55, 113–120. [DOI] [PubMed] [Google Scholar]
- Maney DL, Schoech SJ, Sharp PJ, & Wingfield JC (1999). Effects of vasoactive intestinal peptide on plasma prolactin in passerines. General and Comparative Endocrinology, 113(3), 323–330. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Jawor JM, & Ketterson ED (2007). Natural variation in a testosterone-mediated trade-off between mating effort and parental effort. The American Naturalist, 170(6), 864–875. [DOI] [PubMed] [Google Scholar]
- Meitzen J, & Mermelstein PG (2011). Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. Journal of Chemical Neuroanatomy, 42(4), 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, & Clayton DF (2015). The opportunities and challenges of large-scale molecular approaches to songbird neurobiology. Neuroscience & Biobehavioral Reviews, 50, 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Kaser T, Buckner AA, Wirthlin M, & Lovell PV (2019). Molecular architecture of the zebra finch arcopallium. Journal of Comparative Neurology, 527(15), 2512–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mérot C, Oomen RA, Tigano A, & Wellenreuther M (2020a). A roadmap for understanding the evolutionary significance of structural genomic variation. Trends in Ecology & Evolution, 35, 561–572. [DOI] [PubMed] [Google Scholar]
- Mérot C, Llaurens V, Normandeau E, Bernatchez L, & Wellenreuther M (2020b). Balancing selection via life-history trade-offs maintains an inversion polymorphism in a seaweed fly. Nature Communications, 11(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mérot C, Berdan EL, Babin C, Normandeau E, Wellenreuther M, & Bernatchez L (2018). Intercontinental karyotype–environment parallelism supports a role for a chromosomal inversion in local adaptation in a seaweed fly. Proceedings of the Royal Society B, 285(1881), 20180519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt JR (2019). Evolution of Behavior: Genotype to Phenotype In Choe JC (Ed.), Encyclopedia of Animal Behavior, (2nd ed.). (Vol. 2 234–242). Academic Press. [Google Scholar]
- Merritt JR, Grogan KE, Zinzow-Kramer WM, Sun D, Ortlund EA, Yi SV, & Maney DL (2020). A supergene-linked estrogen receptor drives alternative phenotypes in a polymorphic songbird. Proceedings of the National Academy of Sciences, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt JR, Davis MT, Jalabert C, Libecap TJ, Williams DR, Soma KK, & Maney DL (2018). Rapid effects of estradiol on aggression depend on genotype in a species with an estrogen receptor polymorphism. Hormones and Behavior, 98, 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak P (2008). Coexpression, coregulation, and cofunctionality of neighboring genes in eukaryotic genomes. Genomics, 91(3), 243–248. [DOI] [PubMed] [Google Scholar]
- Miles DB, Sinervo B, Hazard LC, Svensson EI, & Costa D (2007). Relating endocrinology, physiology and behaviour using species with alternative mating strategies. Functional Ecology 21, 653–665. [Google Scholar]
- Mohammed H, D’Santos C, Serandour AA, Ali HR, Brown GD, Atkins A, … & Zwart W (2013). Endogenous purification reveals GREB1 as a key estrogen receptor regulatory factor. Cell Reports, 3, 342e349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niepoth N & Bendesky A (2020). How natural genetic variation shapes behavior. Annual Review of Genomics and Human Genetics, in press. [DOI] [PubMed] [Google Scholar]
- Nijhout HF (2003). Development and evolution of adaptive polyphenisms. Evolution & Development, 5(1), 9–18. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, & Hofmann HA (2011). Genes, hormones, and circuits: an integrative approach to study the evolution of social behavior. Frontiers in Neuroendocrinology, 32(3), 320–335. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, & Pfaff DW (1998a). Roles of estrogen receptor-α gene expression in reproduction-related behaviors in female mice. Endocrinology, 139(12), 5070–5081. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Washburn TF, Taylor J, Lubahn DB, Korach KS, & Pfaff DW (1998b). Modifications of testosterone-dependent behaviors by estrogen receptor-α gene disruption in male mice. Endocrinology, 139(12), 5058–5069. [DOI] [PubMed] [Google Scholar]
- Okhovat M, Chen IC, Dehghani Z, Zheng DJ, Ikpatt JE, Momoh H, & Phelps SM (2018). Genetic variation in the developmental regulation of cortical avpr1a among prairie voles. Genes, Brain and Behavior, 17(1), 36–48. [DOI] [PubMed] [Google Scholar]
- Okhovat M, Maguire SM, & Phelps SM (2017). Methylation of avpr1a in the cortex of wild prairie voles: effects of CpG position and polymorphism. Royal Society Open Science, 4(1), 160646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okhovat M, Berrio A, Wallace G, Ophir AG, & Phelps SM (2015). Sexual fidelity trade-offs promote regulatory variation in the prairie vole brain. Science, 350(6266), 1371–1374. [DOI] [PubMed] [Google Scholar]
- Pearse DE, Barson NJ, Nome T, Gao G, Campbell MA, Abadía-Cardoso A, … & Moen T (2019). Sex-dependent dominance maintains migration supergene in rainbow trout. Nature Ecology & Evolution, 3(12), 1731–1742. [DOI] [PubMed] [Google Scholar]
- Pearse DE, Miller MR, Abadía-Cardoso A, & Garza JC (2014). Rapid parallel evolution of standing variation in a single, complex, genomic region is associated with life history in steelhead/rainbow trout. Proceedings of the Royal Society B: Biological Sciences, 281(1783), 20140012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell J, Brelsford A, Wurm Y, Perrin N, & Chapuisat M (2014). Convergent genetic architecture underlies social organization in ants. Current Biology, 24(22), 2728–2732. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, … & Wild M (2004). Revised nomenclature for avian telencephalon and some related brainstem nuclei. Journal of Comparative Neurology, 473(3), 377–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen T & Crews D (2002). Variation in reproductive behaviour within a sex: neural systems and endocrine activation. Journal of Neuroendocrinology 14, 517–531. [DOI] [PubMed] [Google Scholar]
- Rice WR (1987). The accumulation of sexually antagonistic genes as a selective agent promoting the evolution of reduced recombination between primitive sex-chromosomes. Evolution 41, 911–914. [DOI] [PubMed] [Google Scholar]
- Rosvall KA, Bergeon Burns CM, Barske J, Goodson JL, Schlinger BA, Sengelaub DR, & Ketterson ED (2012). Neural sensitivity to sex steroids predicts individual differences in aggression: implications for behavioural evolution. Proceedings of the Royal Society B: Biological Sciences, 279(1742), 3547–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinervo B, Svensson E (2002). Correlational selection and the evolution of genomic architecture. Heredity 89, 329–338. [DOI] [PubMed] [Google Scholar]
- Smiley KO (2019). Prolactin and avian parental care: New insights and unanswered questions. Hormones and Behavior, 111, 114–130. [DOI] [PubMed] [Google Scholar]
- Smiley KO, & Adkins-Regan E (2016). Relationship between prolactin, reproductive experience, and parental care in a biparental songbird, the zebra finch (Taeniopygia guttata). General and Comparative Endocrinology, 232, 17–24. [DOI] [PubMed] [Google Scholar]
- Smiley KO, & Adkins-Regan E (2018). Lowering prolactin reduces post-hatch parental care in male and female zebra finches (Taeniopygia guttata). Hormones and Behavior, 98, 103–114. [DOI] [PubMed] [Google Scholar]
- Soma KK, Tramontin AD, & Wingfield JC (2000). Oestrogen regulates male aggression in the non–breeding season. Proceedings of the Royal Society of London. Series B: Biological Sciences, 267(1448), 1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinney LH, Bentley GE, & Hau M (2006). Endocrine correlates of alternative phenotypes in the white-throated sparrow (Zonotrichia albicollis). Hormones and Behavior, 50, 762–771. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Helgason A, Thorleifsson G, Steinthorsdottir V, Masson G, Barnard J, … & Desnica N (2005). A common inversion under selection in Europeans. Nature Genetics, 37(2), 129–137. [DOI] [PubMed] [Google Scholar]
- Stetzik L, Ganshevsky D, Lende MN, Roache LE, Musatov S, & Cushing BS (2018). Inhibiting ERα expression in the medial amygdala increases prosocial behavior in male meadow voles (Microtus pennsylvanicus). Behavioural Brain Research, 351, 42–48. [DOI] [PubMed] [Google Scholar]
- Sturtevant AH (1921). A case of rearrangement of genes in Drosophila. Proceedings of the National Academy of Sciences of the United States of America, 7(8), 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Huh I, Zinzow-Kramer WM, Maney DL, and Yi SV (2018). Rapid regulatory evolution of a non-recombining autosome linked to divergent behavioral phenotypes. Proceedings of the National Academy of Sciences, 115, 2794–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Layman T, Jeong H, Chatterjee P, Grogan KE, Merritt JR, Maney DL, Yi SV Genome-wide variation in DNA methylation linked to developmental stage and chromosomal suppression of recombination in white-throated sparrows. bioRxiv, 2020.07.24.220582, 10.1101/2020.07.24.220582 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki MM, & Bird A (2008). DNA methylation landscapes: provocative insights from epigenomics. Nature Reviews Genetics, 9(6), 465–476. [DOI] [PubMed] [Google Scholar]
- Swett MB, & Breuner CW (2009). Plasma testosterone correlates with morph type across breeding substages in male white-throated sparrows. Physiological and Biochemical Zoology, 82, 572–579. [DOI] [PubMed] [Google Scholar]
- Thomas JW, Cáceres M, Lowman JJ, Morehouse CB, Short ME, Baldwin EL, Maney DL, & Martin CL (2008). The chromosomal polymorphism linked to variation in social behavior in the white-throated sparrow (Zonotrichia albicollis) is a complex rearrangement and suppressor of recombination. Genetics, 179, 1455–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneycroft HB (1975). A cytogenetic study of the white-throated sparrow, Zonotrichia albicollis (Gmelin). Evolution, 611–621. [DOI] [PubMed] [Google Scholar]
- Thorneycroft HB (1966). Chromosomal polymorphism in the white-throated sparrow, Zonotrichia albicollis (Gmelin). Science, 154(3756), 1571–1572. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Greiwe KM, & Nelson RJ (2006). Individual differences in estrogen receptor α in select brain nuclei are associated with individual differences in aggression. Hormones and Behavior, 50(2), 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers RL (1972). Parental investment and sexual selection In: Campbell B (Ed.), Sexual Selection and the Descent of Man. Aldine, Chicago, pp. 139–179. [Google Scholar]
- Tuttle EM (2003). Alternative reproductive strategies in the white-throated sparrow: behavioral and genetic evidence. Behavioral Ecology, 14(3), 425–432. [Google Scholar]
- Tuttle EM, Bergland AO, Korody ML, Brewer MS, Newhouse DJ, Minx P, … & Gonser RA (2016). Divergence and functional degradation of a sex chromosome-like supergene. Current Biology, 26(3), 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wurm Y, Nipitwattanaphon M, Riba-Grognuz O, Huang YC, Shoemaker D, & Keller L (2013). A Y-like social chromosome causes alternative colony organization in fire ants. Nature, 493(7434), 664–668. [DOI] [PubMed] [Google Scholar]
- Wellenreuther M, & Bernatchez L (2018). Eco-evolutionary genomics of chromosomal inversions. Trends in Ecology & Evolution, 33(6), 427–440. [DOI] [PubMed] [Google Scholar]
- Wills QF, Livak KJ, Tipping AJ, Enver T, Goldson AJ, Sexton DW, & Holmes C (2013). Single-cell gene expression analysis reveals genetic associations masked in whole-tissue experiments. Nature Biotechnology, 31(8), 748–752. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM Jr, & Ball GF (1990). The” challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. The American Naturalist, 136(6), 829–846. [Google Scholar]
- Wingfield JC (1984). Androgens and mating systems: testosterone-induced polygyny in normally monogamous birds. The Auk, 101(4), 665–671. [Google Scholar]
- Wittkopp PJ, & Kalay G (2012). Cis-regulatory elements: molecular mechanisms and evolutionary processes underlying divergence. Nature Reviews Genetics, 13(1), 59–69. [DOI] [PubMed] [Google Scholar]
- Wray GA (2007). The evolutionary significance of cis-regulatory mutations. Nature Reviews Genetics, 8(3), 206–216. [DOI] [PubMed] [Google Scholar]
- Yates AD, Achuthan P, Akanni W, Allen J, Allen J, Alvarez-Jarreta J, … & Bhai J (2020). Ensembl 2020. Nucleic Acids Research, 48(D1), D682–D688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajitschek F, & Connallon T (2018). Antagonistic pleiotropy in species with separate sexes, and the maintenance of genetic variation in life-history traits and fitness. Evolution, 72(6), 1306–1316. [DOI] [PubMed] [Google Scholar]
- Zinzow-Kramer WM, Horton BM, McKee CD, Michaud JM, Tharp GK, Thomas JW, Tuttle EM, Yi SV, & Maney DL (2015). Genes located in a chromosomal inversion are correlated with territorial song in white-throated sparrows. Genes, Brain, and Behavior, 14, 641–654. [DOI] [PMC free article] [PubMed] [Google Scholar]


