Abstract
Background and aims
Crohn’s Disease (CD) is a chronic gastrointestinal disease resulting from the dysfunctional interplay between genetic susceptibility, the immune system and commensal intestinal microbiota. Emerging evidence suggests that treatment by suppression of the immune response and replacement of the microbiota through Fecal Microbiota Transplantation (FMT) is a promising approach for the treatment of CD.
Methods
We obtained stool metagenomes from CD patients in remission and assessed gut microbiome composition before and after FMT at the species and strain levels. Longitudinal follow-up evaluation allowed us to identify the gain, loss, and strain replacement of specific species, and link these events to the maintenance of remission in CD.
Results
We find that FMT had a significant long-term effect on patient microbial compositions, though this was primarily driven by the engraftment of donor species which remained at low abundance. 38% of FMT-driven changes were strain replacements, emphasizing the importance of detailed profiling methods such as metagenomics. Several instances of long-term coexistence between donor and patient strains were also observed. Engraftment of some Actinobacteria, and engraftment or loss of Proteobacteria, were related to better disease outcomes in CD patients who received FMT, while transmission of Bacteroidetes was deleterious.
Conclusion
Our results suggest clades that may be beneficial to transmit/eliminate through FMT, and thus provide criteria that may help identify personalized FMT donors to more effectively maintain remission in CD patients. The framework established here creates a foundation for future studies centered around the application of FMT and defined microbial communities as a therapeutic approach for treating CD.
Keywords: Crohn’s Disease (CD), Fecal Microbiota Transplantation (FMT), relapse, metagenomes
Lay summary:
This manuscript represents the first study to use metagenomics to investigate the effects of fecal microbiota transplant (FMT) in Crohn’s disease (CD). This approach enables far greater resolution than previously available, revealing strain- and species-level differences induced by FMT.
Introduction
Crohn’s Disease is one type of inflammatory bowel disease (IBD), which causes chronic relapsing and remitting inflammation of the gastrointestinal tract. The incidence and prevalence of CD is increasing worldwide1. While infrequently fatal, CD has a profound influence on quality of life. The pathogenesis of CD is complex, involving the interplay between the immune system and a dynamic and dysbiotic microbial community2,3. Inflammation in CD results from a dysregulated immune response which is accompanied by community-wide changes in the commensal microbiome. This is most readily seen in a reduction in community diversity during disease activity, largely brought on by a loss of obligate anaerobes such as Faecalibacterium prausnitzii and Roseburia intestinalis. Many of these species are considered to be beneficial producers of anti-inflammatory compounds such as short-chain fatty acids, in particular butyrate. Concurrent with this loss, an increase is observed in facultative anaerobes which are opportunistic pathogens, such as Escherichia coli and Haemophilus parainfluenzae. Akkermansia muciniphila has also been observed to bloom during CD activity, and is hypothesized to contribute to the degradation of the mucous layer in the gut triggering a larger immune response.
Management of CD currently revolves around treating symptoms, by calming the overactive immune system with immunosuppressants. Frontline treatments include corticosteroids, aminosalicylates, and biologics. Long-term use of these therapies come with significant side effects for patients, and can lead to complications including infections4 and cancers5. Furthermore, primary non-response or secondary loss of response occurs frequently in patients treated with biologics such as anti-TNF agents and vedolizumab6. Given the immune-microbiota feedback loop in CD, therapeutic approaches that additionally target the microbiota, such as Fecal Microbiota Transplant (FMT), are gaining interest7–9. FMT has already been used successfully to treat other microbially-linked diseases, notably infections of recurrent Clostridium difficile in hospitals worldwide10–12, including among IBD patients7,8,13. Microbiome modifications are particularly attractive since, rather than solely treating current symptoms, they have the potential to reduce the risk of relapse long after treatment14.
The effectiveness of FMT has not been extensively studied in IBD. Most FMT studies have been done in patients with ulcerative colitis (UC), where the results have been mixed, with some studies finding that FMT is beneficial15–17, and at least one observing no effect18. One recent pilot study in CD followed 17 patients for 24 weeks after FMT or sham transplantation19, measuring microbial community changes by 16S rRNA gene profiling. This study aimed to jointly treat immune dysfunction and microbial dysbiosis by performing FMT after inducing clinical remission using corticosteroids and performing a colon cleansing with polyethylene glycol to facilitate donor microbiota engraftment. FMT was found to significantly improve clinically-relevant disease severity metrics, including C-reactive protein levels and Crohn’s Disease Endoscopic Index of Severity. Greater donor microbiota engraftment was also associated with a reduction in the rate of relapse. In particular, two patients who were identified as having “failed FMT”s based on their 6-week similarity to the donor microbial composition eventually relapsed.
Here, from metagenomic data generated from the aforementioned pilot study19, we first characterize changes in microbiome composition over the 26 week study period, and identify changes induced by FMT both at the species and strain levels which was not possible with 16S rRNA gene sequencing data. We then quantify disease activity-induced changes in patients that eventually relapsed. Finally, we link FMT-induced changes with disease outcome, and identify microbial clades that are both beneficial and antagonistic to the probability of relapse.
Results
Study overview
We performed deep shotgun metagenomics to analyze 115 samples, originally collected in Sokol et al19, for changes in the microbiota (Fig. 1a). Among these are samples from 8 patients who received FMTs from 5 donors (each patient received stool from one donor, and samples from two donors were administered to more than one patient), and 9 patients who received sham transplantation. In total, 7 patients relapsed (2 from the FMT group, and 5 in sham; difference is not significant), though the two patients which relapsed in the FMT group both showed an absence of donor microbiota engraftment19. Samples were taxonomically profiled using MetaPhlAn2, and principal coordinate analysis of these species abundance Bray-Curtis dissimilarities indicates the major patient differences are age and gender (Fig. S1), and FMT effects are not apparent in the first two axes of variation (Fig. 1b). Patient profiles tended to be consistent over time (Fig. 1c, Fig. S4–5). Unlike many western gut datasets, we found that Actinobacteria and Firmicutes were the most prevalent, while Bacteroidetes is considerably less prevalent (Fig. S2). Bifidobacterium and Faecalibacterium make up the most abundant genera (Fig. 1c, Fig. S3).
Figure 1. FMT study design.
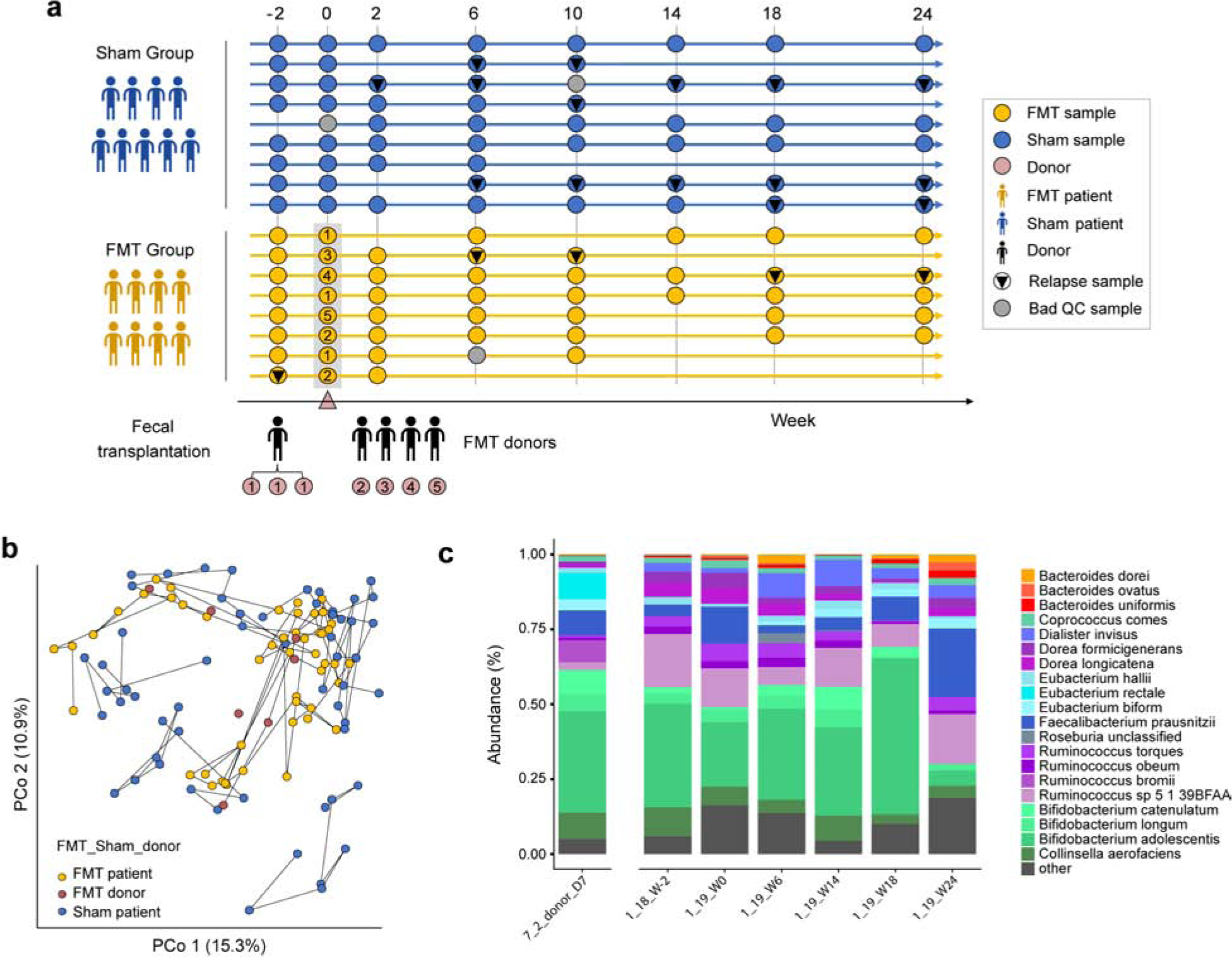
(a) 115 samples were collected from 9 sham patients (blue), 8 FMT patients (yellow), and 5 donors (numbered 1–5, light brown) over 24 weeks. Relapse (black triangles), and samples that failed QC (grey dots) are also marked. At week 0, FMT patients received fecal transplantation and sham patients received sham transplantation19. (b) Principal coordinates analysis of Bray-Curtis dissimilarities from species-level MetaPhlAn2 profiles. Lines connect samples from the same patient/donor. Separate samples were obtained at different times for the donor with 3 patients (7_2). (c) Barplot of species abundances over time in one FMT patient (1_19) and their donor (7_2_D7).
FMT induces long-term changes in patients’ microbiota
We found that the diversity of species composing the communities (alpha diversity) transiently increases following FMT (Fig. 2a), as previously observed with 16S data19, but observed no effect beyond 14 weeks with metagenomics data. Sorensen similarity between patient and donor communities showed a significant shift towards the donor community following FMT, which was persistent across the timeframe of the study. This shift is visible, but not as significant in Bray-Curtis similarities, indicating that the changes observed with the Sorensen similarity were driven by donor species which successfully colonize the patient, but remain at low abundance.
Figure 2. FMT alters the patient’s microbiome.
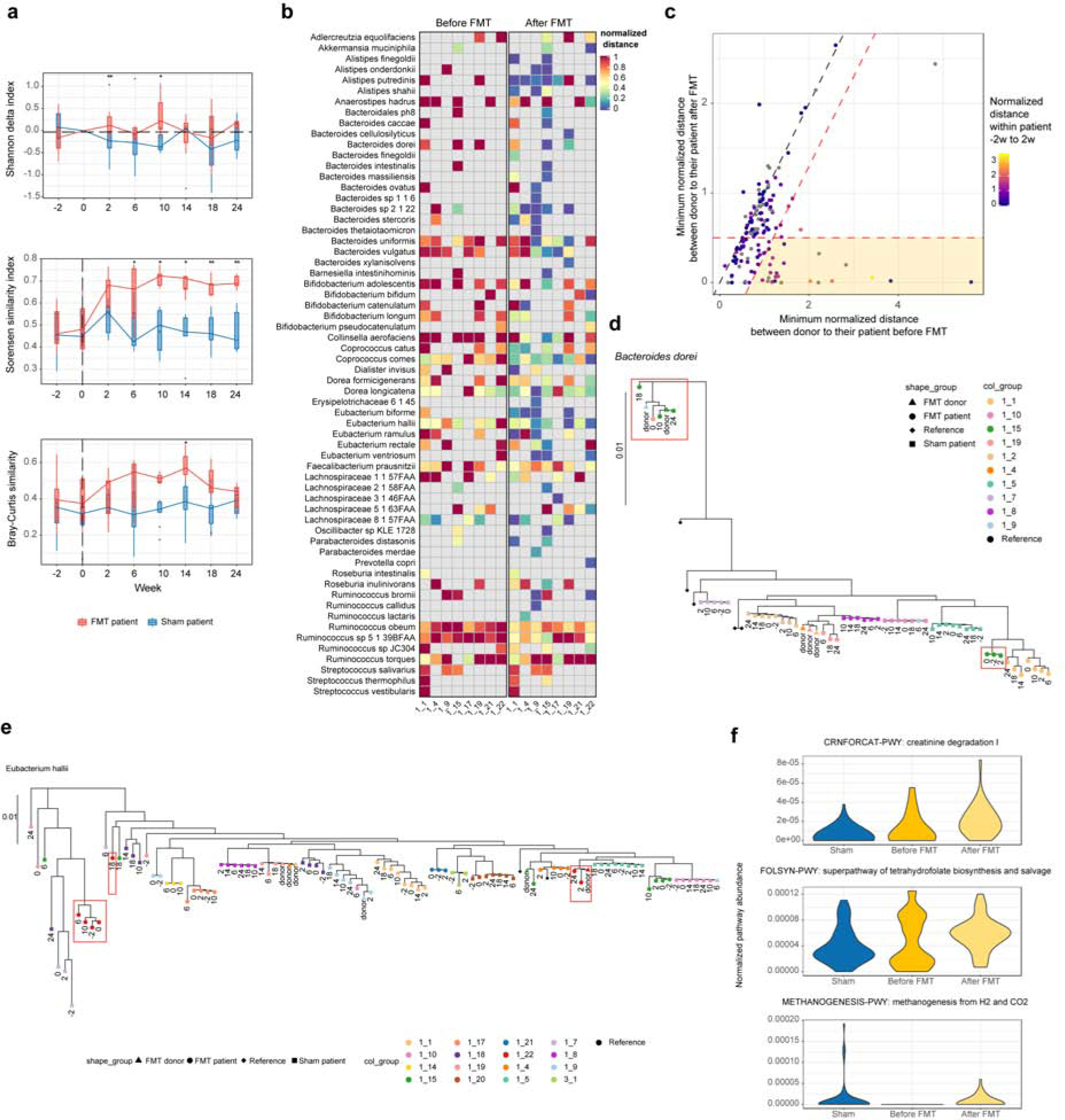
(a) Difference in species-level Shannon index from week 0 (top) shows a transient increase in diversity after FMT, but not in sham. Species-level Sorensen index (middle) shows successful and persistent engraftment of donor species in FMT patients, which is less apparent for Bray-Curtis similarity (bottom). *p-value<=0.05; **p-value<=0.001. (b) Heatmap of the minimum normalized Kimura 2-parameter distance (Methods) between donor and patient before FMT (−2w, 0w; left) and after FMT (2–24w; right). (c) Scatter plot of the minimum normalized Kimura 2-parameter distance between patient and their donor before and after FMT. Each point represents a patient/species pair. Points are colored by the distance between patient’s week −2 to week 2 strains (grey indicates a missing measurement). The 1:1 line (dashed black) indicates no changes in FMT. Thresholds for defining the top engraftment events (Table S1) are in red (Methods). 17% (23/143) of points lie in the highlighted region. (d) Phylogenetic tree of B. dorei, one of the strongest engraftment events from (c), shows a successful engraftment from donor to patient. Color indicates subject and numbers indicate sample collection time in weeks relative to FMT. (e) Phylogenetic tree of E. halii, showing long-term coexistence of donor and original patient strains. (f) Community-level potential for the top three MetaCyc pathways associated with FMT (all associations in Table S2; the model included age, sex, relapse, FMT (Methods)).
At the strain level (Methods), we found further evidence of replacement of patient strains with donor strains (Fig. 2b). Specifically, the dominant strain haplotype in patients frequently shifted towards the donor’s haplotype after FMT (Fig. 2c), as is exemplified by Bacteroides dorei (Fig. 2d). Engraftment rates varied between patients, with three patients exhibiting >40% of species transmitted. These included both 1_9 and 1_17, for which 6 and 5 species engrafted, respectively (out of 12 and 11 species, respectively, with strain profiles). While both of these patients were previously identified as failed transplantations and did not show a large shift in community taxonomic composition towards their donors19, our results show clear evidence of partial transmission by strain replacement and/or coexistence. One other patient, 1_15, showed engraftment by 8 of 19 species. We found that some species engrafted more frequently than others, including Alistipes putredinis and the butyrate producer Coprococcus comes (Fig. 2b). Others were more resistant such as the Ruminococci. For example, R. torques, a known IBD-related species3, was prevalent but did not engraft in any patient. F. prausnitzii, considered a beneficial species20, did not show clear evidence of engraftment in spite of its prevalence in both donors and patients (Fig. S4–5).
Among the clearest engraftment events (Table S1), we observed numerous instances where the donor strain took more than two weeks to engraft (low donor-patient and within-patient distances). These included instances of long-term coexistence of donor and patient strains, such as Eubacterium halii (Fig. 2e), where the dominant haplotype at weeks 2 and 24 in patient 1_22 matched that of the donor, while weeks 6 and 10 matched the patient’s original strain. Despite its low abundance in this study, we note numerous examples of engraftment of Bacteroidetes species (Table S1), a phylum considered the most stable within-individuals21. Finally, we observed one clear engraftment event of Dialister invisus. Although typically considered an oral species, long-term engraftment by FMT shows that this species has successfully colonized the patient’s GI tract.
We observed changes in the microbial community’s functional potential after FMT (Table S2). These included an increase in a creatinine degradation pathway (Fig. 2f). Creatinine, a uremic toxin generated in muscles and primarily cleared through the kidney, is partially metabolized by the gut microbiota under normal conditions22. Increased creatinine degradation by the gut bacteria may therefore contribute to lower serum creatinine.
Relapse-associated changes
We observed a decrease in community diversity at the first time point after relapse (Fig. 3a), with a recovery by the next time point. This is consistent with a loss/reduction of commensal microbial community members during CD disease activity, particularly due to the oxygenated environment of the inflamed colon. Consistent with this, we also observed a depletion in community potential for anaerobic energy metabolism (FDR = 0.045, Fig. 3b). Other functional differences included depletion of the NAD biosynthesis and tRNA charging pathways, two core metabolic functions (all results in Table S2).
Figure 3. Relapse-associated metagenomic features.
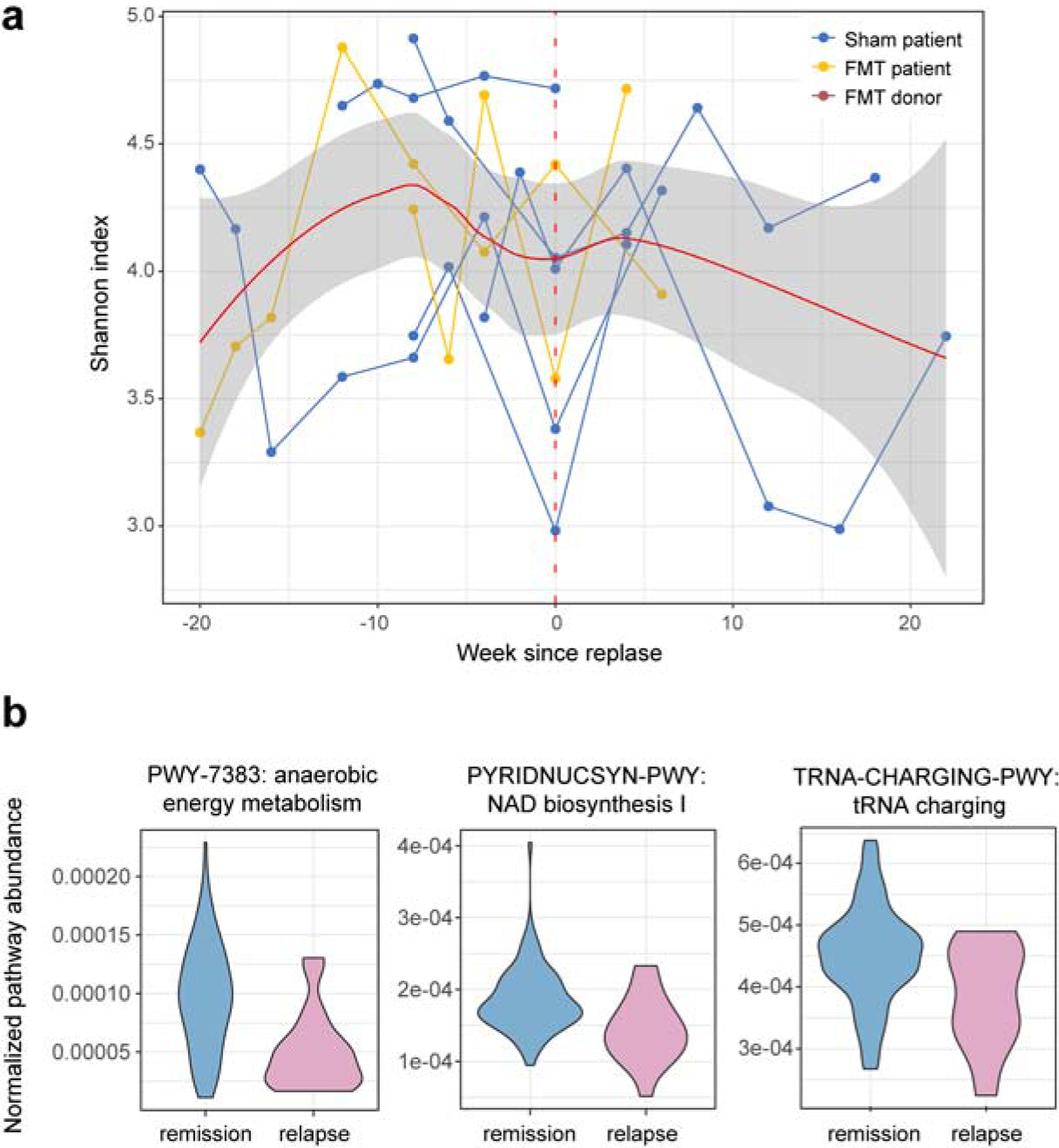
(a) Shannon index over time for all patients who relapsed, synchronized to the first relapse time point, shows a decrease in diversity at the onset of disease activity. (b) Community-level potential for the top three MetaCyc pathways associated with relapse (all associations in Table S2; the model included age, sex, relapse, FMT (Methods)).
Changes in FMT that affect relapse
We next assessed FMT-related changes that may affect the probability of relapse. From a joint analysis of species and strain profiles, we first categorized each species/patient pair based on the evidence for species gain/loss/strain replacement in FMT (Methods; Fig. 4a). We found evidence for 143 total engraftment events (88 gains and 55 replacements), with few losses (18 total, i.e. patient did not carry the species after FMT, but did before). This amounts to 20 changes per patient, on average, with a minimum of 12, implying that even the microbiomes of patients classified as “FMT failure” were profoundly impacted by the procedure (14 changes for 1_17 and 27 for 1_9; Fig. 4b, right). Losses were also observed in the sham group (20 total, or 2.5 losses per patient; one sham patient did not have a pre-FMT sample, so is not counted here), likely due to the bowel cleansing procedure prior to FMT. The majority of engraftment events in FMT patients were for Firmicutes species, though Bacteroidetes had the most gain events total (Fig. 4b, left), exemplified by Barnesiella intestinihominis with 3 gain events (Fig. 4c). This rate was observed despite the low relative abundance of Bacteroidetes species in this study, echoing the strain-level results above and implying a much greater rate of engraftment for Bacteroidetes than for Firmicutes (Fig. 4b, left). Strain replacements were not limited to low-abundance species, with 25 of the 55 replacements for species with >1% mean relative abundance before and after FMT (Table S3), primarily for Firmicutes (15 events) and Actinobacteria (8 events).
Figure 4. Joint analysis of strain and abundance levels reveals relapse-associated changes in FMT.
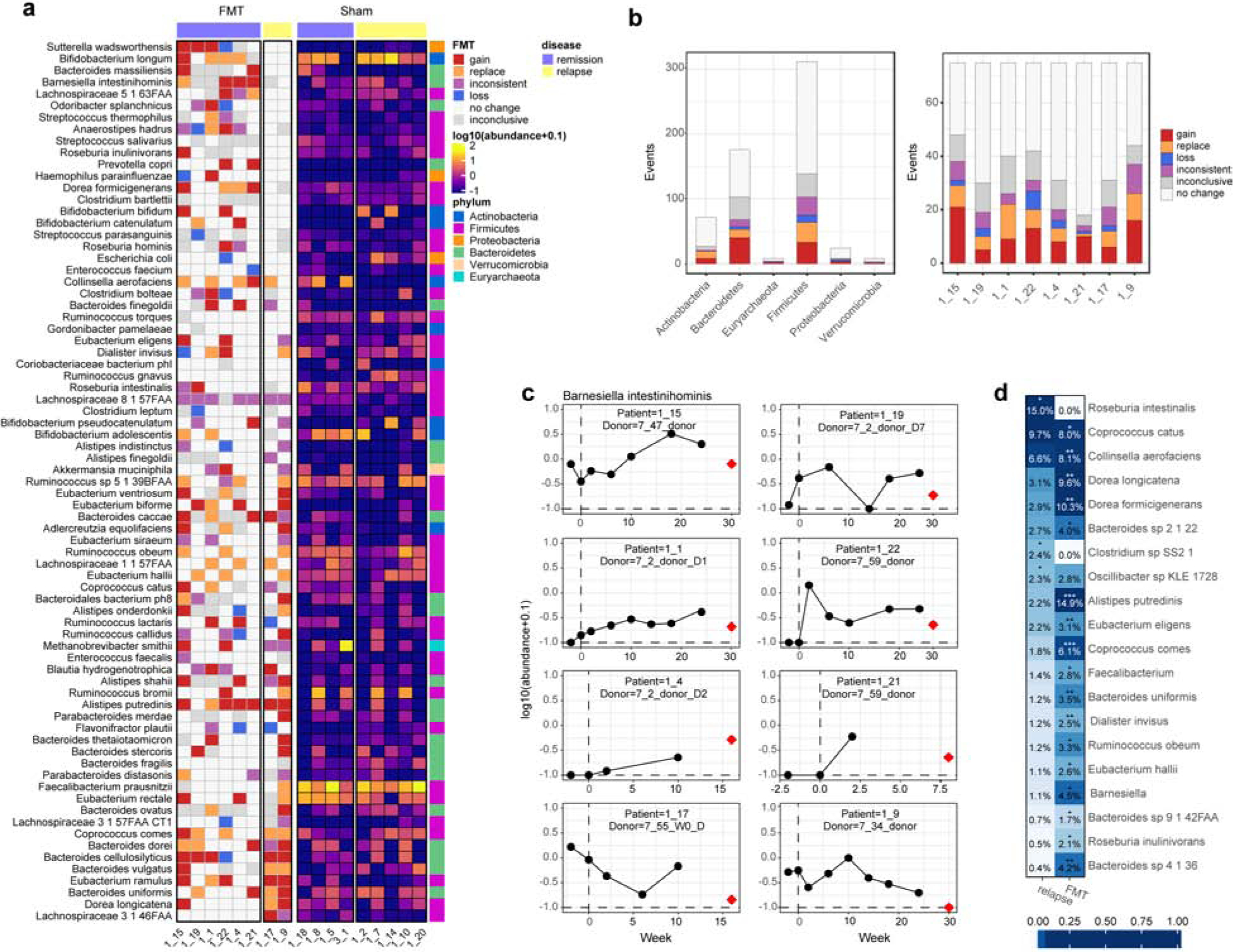
(a) Engraftment events in FMT (left) and species abundances in sham (right), ordered by the number of events in patients which eventually relapsed. Phyla are non-randomly distributed in this ranking, with Firmicutes providing apparent benefits, while Bacteroidetes shows no benefit (or harmful). (b) Number of engraftment events per phylum in each category from (a). Despite the study’s general underrepresentation of Bacteroidetes, these accounted for almost half of engraftment events. (c) Abundance of Barnesiella intestinihominis over time in all FMT patients, with abundance in the matched donor shown in red. (d) PERMANOVA detects strain-level associations with FMT and relapse (20 species with p < 0.05 shown; all results in Table S3).
To connect engraftment in FMT with probability of remission maintenance, we scored each species based on the number of engraftment events in patients that maintained remission versus those that ultimately relapsed (Fig. 4a). We found that the phyla are non-randomly distributed within this ranking. In particular, engraftment of Proteobacteria (Wilcoxon test p = 0.028) and Bacteroidetes (p = 0.032) are associated with the likelihood of relapse. High-ranking Proteobacteria in this list included Sutterella wadsworthensis, Haemophilus parainfluenzae, and Escherichia coli, all of which included losses from patients that did not relapse, consistent with these species being antagonists23. However, there were three instances of patients gaining S. wadsworthensis in FMT. The majority of Bacteroidetes species transmitted frequently in patients who relapsed, thus these largely had a negative influence on maintenance of remission. However, the top species in this ranking included Bacteroides massiliensis, which was gained by two patients in FMT and did not have any abundance in any of the 5 patients that relapsed in sham (Fig. 4a). Prevotella copri is also high in the ranking. The Prevotella genus tends to be irregularly distributed within western populations, with low prevalence but high abundance when present, making it unusual how frequently it was transmitted here. In non-western populations with lower IBD incidence, these also tend to be more prevalent. Also, none of the sham patients carried P. copri. Actinobacteria were marginally significantly non-randomly distributed (p = 0.041). The highest-ranking species, Bifidobacterium longum, is highly abundant and prevalent in this study, and was gained once and replaced with the donor strain three times in FMT patients that did not relapse. The donors for the two FMT patients that relapsed did not carry B. longum (Fig. S4). Notably, despite its prevalence and abundance in both donors and recipients in this study, we observed only a single instance of engraftment of Faecalibacterium prausnitzii. This engraftment was not associated with a beneficial outcome as this patient eventually relapsed. Therefore, this commonly-cited beneficial butyrate-producer which is heavily depleted in CD activity24.
Performing strain-level PERMANOVA tests, we found that Roseburia intestinalis has the strongest association with probability of relapse (Fig. 4d; PERMANOVA R2 = 0.15, p = 0.042; Table S4). However, this species transmitted poorly in FMT, with only one clear engraftment event, and was not significantly associated with FMT at the strain level (p = 1). Species with significant strain-level associations with FMT included Alistipes putredinis (p < 0.001), two Dorea species (D. longicatena p = 0.003 and D. formicigenerans p = 0.002), and two Coprococci (C. cactus p = 0.013 and C. comes p < 0.001). All of these species are depleted in IBD disease activity3,25.
Discussion
In this study, we examined whether changes to the gut microbial community in FMT were associated with disease activity in CD. This is the first study to use metagenomics to obtain species- and strain-resolution profiling for FMT in IBD. We found that species-level community composition experienced a transient increase in diversity following FMT. Long-term compositional changes, however, were primarily driven by the acquisition of donor species which remained at low abundance, and the abundance distribution of donor species only weakly carried over to patients. 55 instances of strain replacements were also observed, accounting for 38% of engraftment events (143 total). Many of these occurred in patients which were previously classified as FMT failures, showing that broader profiling methods such as 16S miss a non-negligible fraction of the differences occurring in FMT. We further found multiple instances of long-term coexistence of donor and patient strains, up to the final follow-up 24 weeks after FMT.
Despite the additional evidence for post-FMT microbiome shifts, total replacement of the microbiota was not achieved in any patient, even with bowel cleansing performed prior to FMT. Consistent with this, FMT to treat recurrent infection by C. difficile7 has a reduced impact on microbiome composition in IBD patients compared to non-IBD patients. This indicates that either the existing microbial communities of IBD patients are more resilient to change, or the host environment continues to actively select for the original community. Future studies should investigate how to increase the efficiency of engraftment in IBD specifically, as this may be necessary to obtain larger effects on disease activity. Engraftment potential is likely dependent on the unoccupied ecological niches in the patient pre-FMT, e.g. for B. longum26. The bowel cleansing prior to FMT here was intended to “reset” the microbiome and clear existing ecological niches, though this was not sufficient to obtain complete replacement. Other methods may include repeated rounds of FMT17,27 or complementation with targeted dietary interventions28,29.
Whole-microbiome replacement may not be necessary if we can determine a subset of microbes that reduces the probability of relapse. Given the complex interplay between the host and microbiome in IBD, it is unlikely that single species will have such an effect, though some sub-communities of microbes may collectively confer protective benefits. We found a significant association between engraftment of three phyla, Actinobacteria, Proteobacteria, and Bacteroidetes and future disease relapse. Engraftment of Actinobacteria and engraftment or loss of Proteobacteria positively impacted disease outcome. Proteobacteria contains several clades that bloom in the inflamed gut23, and species which were affected in FMT here included S. wadsworthensis, H. parainfluenzae, and E. coli. Changes to this phylum, particularly loss of species, may therefore confer some benefit. Engraftment of Bacteroidetes negatively impacted disease outcome. Despite its general low abundance in this study, we found evidence of widespread Bacteroidetes engraftment. This phylum contains species with persistent inter-individual variation, and thus constitutes an individual’s personal core microbiome21. We observe that the two FMT patients that eventually relapsed primarily received Bacteroidetes species from their donors, suggesting that widespread replacement of Bacteroidetes species may not be beneficial. These species, therefore may provide a “donor compatibility” signature that could be used to help predict whether a patient’s response to FMT will be pro- or anti-inflammatory11. Larger studies will be needed to confirm this, though evidence from a previous study with 38 patients indicated that overall relatedness of the original recipient community with the donor community is not predictive of relapse7. Still, given the large inter-personal differences in microbiomes, it may be possible to identify criteria by which patients can be carefully matched with donors in order to elicit a more precise set of changes through FMT.
In summary, our results show that subsets of the engrafted microbiome have a measurable impact on disease activity after FMT, both positive and negative. These effects appear to be patient-specific, suggesting that some donors (i.e. so-called “super-donors”) can more effectively reduce disease activity30. The small number of study participants limits the resolution at which disease-associated features can be identified, and these results will need to be validated in larger studies. Future studies should aim to use high-resolution measurement techniques such as metagenomics, as our results show that strain replacement was pervasive in FMT, and the effects of FMT were more profound than originally measured. We further found that the post-FMT perturbation to the patient’s microbiome settles into its long-term state starting from the 14-week time point. Future studies may therefore benefit from focusing their sampling before this point to more efficiently allocate samples. The framework established here creates a foundation for future studies centered around FMT as a therapeutic approach for treating CD.
Methods
Sample collection, preparation and sequencing
Stool samples were collected as described in Sokol, et al19. Nucleic acid was extracted using the AllPrep 96 PowerFecal DNA/RNA kit from QIAGEN (custom product #1114341). This method pairs bead-beating on a Tissuelyser II (QIAGEN) with a 96 well AllPrep protocol. Purified DNA was stored at −20°C. Illumina sequencing libraries were prepared from 2ng of input DNA using the Nextera XT DNA Library Preparation kit (Illumina) according to the manufacturer’s recommended protocol. Prior to sequencing, libraries were pooled by collecting equal volumes of each library. Insert sizes and concentrations for each pooled library were determined using an Agilent Bioanalyzer DNA 1000 kit (Agilent Technologies) prior to sequencing on an Illumina NovaSeq 6000 with 151bp paired-end reads to yield ~10 million paired end reads per sample. Data was analyzed using the Broad Picard Pipeline which includes de-multiplexing and data aggregation (https://broadinstitute.github.io/picard).
Profile generation
Quality control for metagenomic shotgun sequencing data was performed with KneadData v0.7.2, with additional adapter detection and trimming at a minimum overlap of 5 bp by Trim Galore!. Three of 115 samples were excluded due to low read count. Taxonomic profiles were generated using MetaPhlAn v2.7.731. Functional profiles were generated with HUMAnN2 v0.11.232, providing gene family level (here, 90% similarity) quantifications of microbial genes that are further stratified by contributing organisms. The gene families were further mapped to MetaCyc pathways33.
Strain analysis
Strain SNP haplotypes were generated using StrainPhlAn34 with preset “relaxed_parameters3” settings. Strain-level changes in FMT (Fig. 2b) were characterized from the dominant haplotype sequences in donors and patients. Briefly, Kimura 2-parameter distances (using the ape R package) were first normalized within species by dividing by the mean distance between all sample pairs for that species. We then calculated the minimum distance from each donor to their matched patient before FMT (time points: −2w, 0w) and after FMT (time points: 2w, 6w, 10w, 14w, 18w, 24w), with missing values excluded. Species are only included if at least one such measurement was possible for the species. A successful engraftment event was defined as events where: the after-FMT donor-patient distance was 0.25 lower than before FMT, the before-FMT distance was greater than 0.5, and the after-FMT distance was smaller than 0.5. We applied the same thresholds to the sham group, and found that this results in a 7% false positive rate (Fig. S6).
Phylogenetic trees (Fig. 2d–e) were generated based on the StrainPhlAn SNP haplotypes using the phangorn R package35, using the Jukes and Cantor (JC69) model. Briefly, an initial tree was constructed using Unweighted Pair Group Method with Arithmetic Mean (UPGMA) hierarchical clustering. The tree was then optimized using maximum likelihood, by iterative optimization of edge lengths, base frequencies and topology. Visualizations were generated with the ggtree R package.
Differential abundance testing
Differential abundance testing was performed with MaAsLin236 v1.0.0, using arc-sin square root transformation of abundances. The model included sex, age, whether FMT had occurred (1 after week 0 in FMT patients, 0 otherwise), and relapse, with patient as the random effect. P-values are from Wald tests, and multiple hypothesis correction was performed with the Benjamini-Hochberg false discovery rate method.
Engraftment event identification and quantification
We built an integrated view of changes in FMT by categorizing each species/patient pair based on the evidence of changes in both the abundance and strain profiles. MetaPhlAn2 abundance profiles were first filtered to the set of species with prevalence >10% (abundance > 0.001), and low abundance values (<0.0001) were clamped to 0. StrainPhlAn profiles were limited to species for which a MetaPhlAn2 profile exists. Let b be the abundance of a species before FMT, let a be the abundance after FMT, let d be the donor abundance, and sb and sa be the normalized Kimura 2-parameter distance of the donor strain to the patient’s strain before and after FMT, respectively. Filtered abundances and strain measurements were then grouped into five categories:
Gain: b = 0, a > 0, d > 0
Loss: b > 0, a = 0
Replace: a > 0, b > 0, sb - sa > 0.25
Inconclusive: a > 0, b > 0, sb or sa not measured
- No change:
- b > 0, b > 0, sb - sa <= 0.25
- b = 0, a = 0, sb and sa not measured
- Inconsistent:
- b = 0, a > 0, d = 0
- b = 0, a = 0, sb or sa is measured
Species were sorted according to the association between their changes in FMT with whether or not they relapsed. For this, a species score was defined as:
Here, νx,i denotes a score associated with the engraftment of species x in patient i as determined above, and is 1 for a gain or loss, 0.5 for a replacement, −1 for no change, and 0 otherwise. pi denotes the patient weight, and is 1 for non-relapsing FMT patients and −2.5 for FMT patients that eventually relapsed (weights chosen such that relapse and non-relapse have equal total weight).
PERMANOVA
PERMANOVA was performed with the adonis function in the R package vegan (version 2.5–6). Dissimilarity matrices were obtained from the Kimura 2-parameter distance between dominant haplotypes returned by StrainPhlAn for each species. When a species did not have sufficient coverage for haplotype calling in StrainPhlAn, its dissimilarity with all samples which had sufficient coverage was imputed with the 90th percentile of the strain dissimilarity matrix. Samples with the species were therefore considered to be very different from samples without the species. Variables were introduced in the model one at a time. The R2 statistic should thus be considered the maximum variance explainable by that measure. Repeat measures were accounted for as in Lloyd-Price et al., 20193. Specifically, permutations of time-varying features (FMT and relapse) were performed within-patient, and permutations of patient-specific features (sex and age) were permuted between patients and samples were relabeled with the patient’s permuted metadata.
Data availability
The datasets generated during and/or analyzed during the current study are available in the Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra) repository, under BioProject PRJNA625520.
Supplementary Material
What you need to know:
Background and Context
Crohn’s Disease is one type of inflammatory bowel disease (IBD), which causes chronic relapsing and remitting inflammation of the gastrointestinal tract that greatly affect patients’ quality of life. Evidence suggests that concurrent treatment of both suppression of the immune response and replacement of the microbiota through Fecal Microbiota Transplantation (FMT) is a promising approach for the treatment of CD.
New Findings
The authors analyze metagenomic data from CD patients receiving FMT while in remission, and assessed the gut microbiome composition before and after FMT at the species and strain levels.
They identified strain replacement events, profiled bacterial community trajectories after FMT, and identified FMT-induced changes in phyla in which impacted future disease relapse.
Limitations
The small number of study participants limits the resolution at which disease-associated features can be identified, and these results will need to be validated in larger studies.
Impact
Identification of bacterial clades whose transmission or elimination through FMT is beneficial provides criteria that may help identify personalized FMT donors to more effectively maintain remission in CD patients.
Acknowledgements
We thank Luke Besse for project management and making data available through the SRA and the Broad Institute Genomics Platform for sample processing and sequencing data generation. We also thank Heather Kang for editorial assistance and Melanie Schirmer for helpful discussions.
Grant support
The clinical trial was supported by a grant from Programme Hospitalier de Recherche Clinique - PHRC PHRCR-13-029 (Ministère de la Santé), Assistance Publique - Hôpitaux de Paris (CRC16), Fondation de France (fond Inkermann), and Association Francois Aupetit.
H.S. received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme (ERC-2016-StG-71577). R.J.X. received funding from Center for the Study of Inflammatory Bowel Disease (CSIBD, P30DK043351), The Crohn’s & Colitis Foundation, and NIH (AT009708).
Disclosures
R.J.X. is a consultant to Novartis and Nestle.
H.S. received consultant, board membership or lecture fees from Carenity, Abbvie, Astellas, Danone, Ferring, Mayoly Spindler, MSD, Novartis, Roche, Tillots, Enterome, Maat, BiomX, Biose, Novartis, and Takeda. He is also a co-founder of Exeliom bioscience. L.B. received consulting fees from Janssen, Pfizer and Takeda; lecture fees from Abbvie, Janssen, MSD, Ferring Pharmaceuticals, Mayoly-Spendler, and Takeda; research support from Abbott, Ferring Pharmaceuticals, Hospira-Pfizer, Janssen, MSD, Takeda and Tillots. P.S. received consulting/lecture fees or grant funding from: Takeda, Abbvie, Merck-MSD, Biocodex, Janssen, Amgen, Astellas, Pfizer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaplan GG, Ng SC. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology. 2017. February;152(2):313–21.e2. [DOI] [PubMed] [Google Scholar]
- 2.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011. June 15;474(7351):307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TW, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019. May;569(7758):655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Risk of Serious and Opportunistic Infections Associated With Treatment of Inflammatory Bowel Diseases. Gastroenterology. 2018. August;155(2):337–46.e10. [DOI] [PubMed] [Google Scholar]
- 5.Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015. April 9;372(15):1441–52. [DOI] [PubMed] [Google Scholar]
- 6.Ananthakrishnan AN, Luo C, Yajnik V, Khalili H, Garber JJ, Stevens BW, et al. Gut Microbiome Function Predicts Response to Anti-integrin Biologic Therapy in Inflammatory Bowel Diseases. Cell Host Microbe. 2017. May 10;21(5):603–10.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanna S, Vazquez-Baeza Y, González A, Weiss S, Schmidt B, Muñiz-Pedrogo DA, et al. Changes in microbial ecology after fecal microbiota transplantation for recurrent C. difficile infection affected by underlying inflammatory bowel disease. Microbiome. 2017. May 15;5(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirten RP, Grinspan A, Fu S-C, Luo Y, Suarez-Farinas M, Rowland J, et al. Microbial Engraftment and Efficacy of Fecal Microbiota Transplant for Clostridium Difficile in Patients With and Without Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019. May 4;25(6):969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plichta DR, Graham DB, Subramanian S, Xavier RJ. Therapeutic Opportunities in Inflammatory Bowel Disease: Mechanistic Dissection of Host-Microbiome Relationships. Cell. 2019. August 22;178(5):1041–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015. January 8;517(7533):205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pamer EG. Fecal microbiota transplantation: effectiveness, complexities, and lingering concerns. Mucosal Immunol. 2014. March;7(2):210–4. [DOI] [PubMed] [Google Scholar]
- 12.Staley C, Kaiser T, Vaughn BP, Graiziger C, Hamilton MJ, Kabage AJ, et al. Durable Long-Term Bacterial Engraftment following Encapsulated Fecal Microbiota Transplantation To Treat Clostridium difficile Infection. MBio [Internet]. 2019. July 23;10(4). Available from: 10.1128/mBio.01586-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer M, Kao D, Kelly C, Kuchipudi A, Jafri S-M, Blumenkehl M, et al. Fecal Microbiota Transplantation is Safe and Efficacious for Recurrent or Refractory Clostridium difficile Infection in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016. October 1;22(10):2402–9. [DOI] [PubMed] [Google Scholar]
- 14.Kim SG, Becattini S, Moody TU, Shliaha PV, Littmann ER, Seok R, et al. Microbiota-derived lantibiotic restores resistance against vancomycin-resistant Enterococcus. Nature. 2019. August;572(7771):665–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology. 2015. July;149(1):102–9.e6. [DOI] [PubMed] [Google Scholar]
- 16.Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017. March 25;389(10075):1218–28. [DOI] [PubMed] [Google Scholar]
- 17.Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA. 2019. January 15;321(2):156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossen NG, Fuentes S, van der Spek MJ, Tijssen JG, Hartman JHA, Duflou A, et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology. 2015. July;149(1):110–8.e4. [DOI] [PubMed] [Google Scholar]
- 19.Sokol H, Landman C, Seksik P, Berard L, Montil M, Nion-Larmurier I, et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: a pilot randomized controlled study. Microbiome. 2020. February 3;8(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux J-J, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008. October 28;105(43):16731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd-Price J, Mahurkar A, Rahnavard G, Crabtree J, Orvis J, Hall AB, et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature. 2017. October 5;550(7674):61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target. Am J Kidney Dis. 2016. March;67(3):483–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macfarlane GT, Blackett KL, Nakayama T, Steed H, Macfarlane S. The gut microbiota in inflammatory bowel disease. Curr Pharm Des. 2009;15(13):1528–36. [DOI] [PubMed] [Google Scholar]
- 24.Hall AB, Yassour M, Sauk J, Garner A, Jiang X, Arthur T, et al. A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med. 2017. November 28;9(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagao-Kitamoto H, Kamada N. Host-microbial Cross-talk in Inflammatory Bowel Disease. Immune Netw. 2017. Feb;17(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maldonado-Gómez MX, Martínez I, Bottacini F, O’Callaghan A, Ventura M, van Sinderen D, et al. Stable Engraftment of Bifidobacterium longum AH1206 in the Human Gut Depends on Individualized Features of the Resident Microbiome. Cell Host Microbe. 2016. October 12;20(4):515–26. [DOI] [PubMed] [Google Scholar]
- 27.Paramsothy S, Paramsothy R, Rubin DT, Kamm MA, Kaakoush NO, Mitchell HM, et al. Faecal Microbiota Transplantation for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis [Internet]. Vol. 11, Journal of Crohn’s and Colitis. 2017. p. 1180–99. Available from: 10.1093/ecco-jcc/jjx063 [DOI] [PubMed] [Google Scholar]
- 28.Kearney SM, Gibbons SM, Erdman SE, Alm EJ. Orthogonal Dietary Niche Enables Reversible Engraftment of a Gut Bacterial Commensal. Cell Rep. 2018. August 14;24(7):1842–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shepherd ES, DeLoache WC, Pruss KM, Whitaker WR, Sonnenburg JL. An exclusive metabolic niche enables strain engraftment in the gut microbiota. Nature. 2018. May;557(7705):434–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson BC, Vatanen T, Cutfield WS, O’Sullivan JM. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front Cell Infect Microbiol. 2019. January 21;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segata N, Waldron L, Ballarini A, Narasimhan V, Jousson O, Huttenhower C. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat Methods. 2012. June 10;9(8):811–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franzosa EA, McIver LJ, Rahnavard G, Thompson LR, Schirmer M, Weingart G, et al. Species-level functional profiling of metagenomes and metatranscriptomes. Nat Methods. 2018. November;15(11):962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caspi R, Billington R, Fulcher CA, Keseler IM, Kothari A, Krummenacker M, et al. The MetaCyc database of metabolic pathways and enzymes. Nucleic Acids Res. 2018. January 4;46(D1):D633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Truong DT, Tett A, Pasolli E, Huttenhower C, Segata N. Microbial strain-level population structure and genetic diversity from metagenomes. Genome Res. 2017. April;27(4):626–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schliep KP. phangorn: phylogenetic analysis in R. Bioinformatics. 2011. February 15;27(4):592–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012. April 16;13(9):R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available in the Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra) repository, under BioProject PRJNA625520.


