Abstract
We examined whether a key psychological trait—resilience, defined as one’s ability to recover quickly from difficulties—contributes to the frail phenotype in patients with cirrhosis. Included were 300 adult patients with cirrhosis who underwent outpatient physical frailty testing using the Liver Frailty Index and resilience testing using the Connor-Davidson Resilience Scale (CD-RISC). The Liver Frailty Index was categorized as robust, prefrail-robust, prefrail-frail, and frail; CD-RISC was categorized using population norms as: least, less, more, and most resilient. Linear regression was used to assess factors associated with frailty (by the Liver Frailty Index per 0.1 unit change). Among the most resilient, only 10% were frail; among the least resilient, 29% were frail. In univariable analysis, resilience was strongly associated with the Liver Frailty Index (coef=−0.13 per point increase; 95% CI, −0.20–−0.60; p<0.001) and remained significantly associated with frailty in multivariable adjustment (coef= −0.13, 95% CI −0.19– −0.07; p<0.001). Low resilience is strongly associated with the frail phenotype in patients with cirrhosis. Given that resilience is modifiable, our data suggest that effective interventions to mitigate frailty should include strategies to build resilience in patients with low baseline resilience.
Keywords: psychological resilience, physical frailty, Liver Frailty Index
1. INTRODUCTION
Frailty has commonly been defined as a distinct biologic state of decreased physiologic reserve and increased vulnerability to health stressors that predisposes individuals to adverse health outcomes.1 As originally conceptualized in the field of geriatrics, frailty is a multi-dimensional construct that encompasses physical as well as psychological factors. A number of instruments have been developed in the field of geriatrics to operationalize the multi-dimensionality of this construct; however, in patients with cirrhosis, instruments used to measure frailty have largely focused on the physical aspects of frailty.2 Our team developed the Liver Frailty Index from a cohort of patients with cirrhosis awaiting liver transplantation that includes hand grip strength, chair stands, and balance testing.3 While we have demonstrated that the Liver Frailty Index accurately captures the construct of physical frailty, little is known of its association with psychological contributors to health outcomes.
One psychological construct that has been recognized to be associated with frailty in non-cirrhotic populations is resilience.4–8 Resilience is a modifiable quality that describes an individual’s capability to thrive in the face of adversity.9 There are three requirements for resilience: 1) significant adversity/risk, 2) presence of assets or resources to offset the effects of the adversity, and 3) positive adaptation or the avoidance of a negative outcome.10 Given the dynamic course of illness that patients with cirrhosis experience, we hypothesized that psychological resilience would be associated with frailty. In this study, we aimed to evaluate the association between frailty, as measured by the Liver Frailty Index in patients with cirrhosis, and psychological resilience.
2. MATERIALS AND METHODS
2.1. Participants
Patients with cirrhosis awaiting liver transplantation who were seen in the outpatient UCSF Liver Transplant Clinics from November 19, 2018 to August 13, 2019 were eligible to participate in this study. We excluded non-English speakers from the study because of unavailability of the Connor-Davidson Resilience Scale (CD-RISC)9 in other languages.
2.2. Study procedures
At enrollment, all patients underwent an objective measurement of physical frailty with these 3 tests:
Grip strength1: measured in kilograms using a hand dynamometer in the subject’s dominant hand. The average of 3 trials was used in calculation.
Timed chair stands11: measured as the number of seconds it takes for the subject to complete 5 chair stands with the subject’s arms folded across the chest.
Balance testing11: measured as the number of seconds that the subject can balance in 3 positions (feet placed side-to-side, semi-tandem, and tandem) for a maximum of 10 seconds each.
From these 3 physical tests of frailty, the Liver Frailty Index was calculated using the calculator available at http://liverfrailtyindex.ucsf.edu.
The classifications of frailty were determined by using previously established Liver Frailty Index cutoffs with robust defined as Liver Frailty Index <3.2, prefrail-robust defined as Liver Frailty Index between 3.2 and 3.7, prefrail-frail defined as Liver Frailty Index between 3.8 and 4.4, and frail defined as Liver Frailty Index ≥ 4.5.3
At enrollment, all patients also underwent measurement of psychological resilience using the CD-RISC. The CD-RISC consists of 25 items scored on a 5-point scale. Each item was a statement that patients scored from not true at all (0), rarely true (1), sometimes true (2), often true (3), or true nearly all the time (4). Some examples of these statements are:
I believe I can achieve my goals, even if there are obstacles.
Under pressure, I stay focused and think clearly.
I can make unpopular or difficult decisions that affect other people, if it is necessary.
The sum score was calculated, with a higher score indicating higher resilience. CD-RISC was categorized using US general population norms as: least (≤73), less (74–82), more (83–90), and most (≥91) resilient.9 Permission to use the scale was granted from Drs. Connor and Davidson.
2.3. Statistical Analysis
Categorical variables were presented as percentages while continuous variables were presented as medians and interquartile ranges (IQR). Variables were compared by resilience using Wilcoxon rank-sum and chi-square tests respectively. The association between resilience and frailty was assessed using linear regression. Univariable linear regression was used to assess the association between all covariates and frailty. All covariates with p<0.2 were included in the multivariate models. In backward selection, variables not reaching significance p<0.05 were removed to develop the final multivariable model.
This study was approved by the University of California, San Francisco Institutional Review Board.
3. RESULTS
3.1. Baseline characteristics (Table 1)
Table 1.
Characteristics of the 300 patients with cirrhosis included in this study.
| Characteristics | All n=300 | By Resilience Quartile | p-value | Test of trend p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Least resilient n=86 (29%) | Less resilient n=69 (23%) | More resilient n=63 (21%) | Most resilient n=82 (27%) | |||||
| Age, years | 62 (56–67) | 60 (52–66) | 63 (55–68) | 62 (57–65) | 64 (60–68) | 0.01 | 0.004 | |
| Female | 98 (33%) | 21 (24%) | 24 (35%) | 23 (37%) | 30 (37%) | 0.28 | 0.09 | |
| Race/Ethnicity | Non-Hispanic White | 169 (56%) | 39 (45%) | 47 (68%) | 37 (59%) | 46 (56%) | 0.04 | 0.53 |
| Black | 15 (5%) | 6 (7%) | 2 (3%) | 6 (10%) | 1 (1%) | |||
| Hispanic | 77 (26%) | 26 (30%) | 17 (25%) | 14 (22%) | 20 (24%) | |||
| Asian | 31 (10%) | 14 (16%) | 2 (3%) | 4 (6%) | 11 (13%) | |||
| Other | 8 (3%) | 1 (1%) | 1 (1%) | 2 (3%) | 4 (5%) | |||
| Body mass index, kg/m2 | 29 (25–34) | 28 (25–33) | 30 (26–36) | 29 (25–34) | 30 (26–34) | 0.32 | 0.20 | |
| Etiology of liver disease | Chronic hepatitis C | 105 (35%) | 28 (33%) | 24 (35%) | 28 (44%) | 25 (30%) | 0.05 | 0.91 |
| Alcohol | 82 (27%) | 26 (30%) | 18 (26%) | 17 (27%) | 21 (26%) | |||
| Non-alcoholic steatohepatitis | 60 (20%) | 10 (12%) | 17 (25%) | 10 (16%) | 23 (28%) | |||
| Autoimmune/cholestatic | 18 (6%) | 6 (7%) | 4 (6%) | 6 (10%) | 2 (2%) | |||
| Other | 35 (12%) | 16 (19%) | 6 (9%) | 2 (3%) | 11 (13%) | |||
| HCC | 117 (39%) | 28 (33%) | 29 (42%) | 24 (38%) | 36 (44%) | 0.46 | 0.19 | |
| Hypertension | 142 (47%) | 34 (40%) | 33 (48%) | 37 (59%) | 38 (46%) | 0.14 | 0.23 | |
| Diabetes | 105 (35%) | 28 (33%) | 21 (30%) | 26 (41%) | 30 (37%) | 0.57 | 0.37 | |
| MELDNa | 16 (11–19) | 17 (13–21) | 15 (11–19) | 15 (11–19) | 15 (11–19) | 0.18 | 0.10 | |
| Total bilirubin, mg/dL | 1.8 (1.0–2.9) | 1.8 (1.1–3.0) | 1.6 (1.0–2.8) | 1.7 (1.1–2.6) | 1.8 (0.8–3.1) | 0.97 | 0.79 | |
| Creatinine, mg/dL | 0.9 (0.7–1.1) | 0.9 (0.7–1.4) | 0.9 (0.7–1.1) | 0.9 (0.7–1.1) | 0.9 (0.7–1.1) | 0.26 | 0.12 | |
| INR | 1.3 (1.2–1.5) | 1.3 (1.2–1.5) | 1.3 (1.2–1.5) | 1.3 (1.2–1.4) | 1.4 (1.2–1.6) | 0.88 | 0.56 | |
| Albumin, g/dL | 3.4 (2.9–3.8) | 3.4 (2.9–3.7) | 3.4 (2.9–3.9) | 3.4 (2.9–3.7) | 3.5 (2.8–3.9) | 0.52 | 0.42 | |
| Dialysis | 12 (4%) | 5 (6%) | 1 (1%) | 3 (5%) | 3 (4%) | 0.57 | 0.68 | |
| History of anxiety or depression | 120 (40%) | 46 (53%) | 27 (39%) | 19 (30%) | 28 (34%) | 0.02 | 0.006 | |
| Ascites on exam | 61 (20%) | 19 (22%) | 14 (20%) | 10 (16%) | 18 (22%) | 0.78 | 0.84 | |
| Hepatic encephalopathy on exam | 35 (12%) | 13 (15%) | 5 (7%) | 7 (11%) | 10 (12%) | 0.50 | 0.71 | |
Median (interquartile range) or %
A total of 300 patients with cirrhosis were included in this study. In this cohort, 33% were female, median age was 62 years, median MELDNa was 16, and 15% were frail.
Of these 300 patients, median CD-RISC score was 81 (IQR 72–92). 29%, 23%, 21%, and 27% were least, less, more, and most resilient (Figure 1). Baseline characteristics that were similar by resilience categories included sex, MELDNa, ascites, and hepatic encephalopathy. Notably, age differed by resilience category, with older age being associated with more resilience (test of trend p=0.004). In addition, the proportion with a history of anxiety or depression decreased as resilience increased (test of trend p=0.006).
Figure 1.
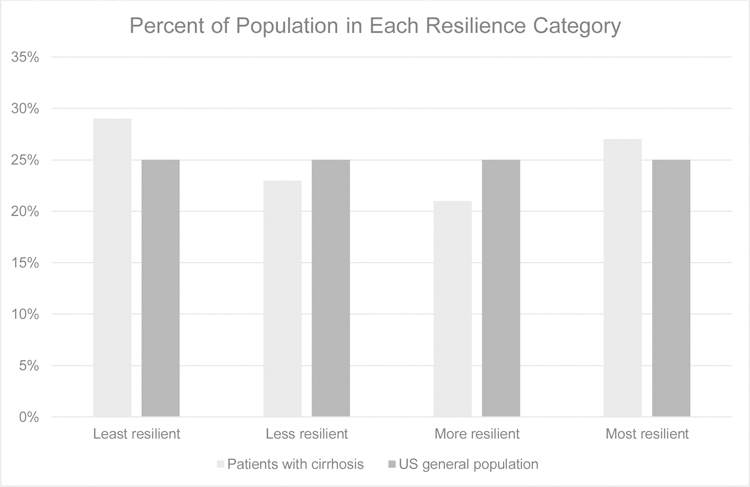
Proportion of resilience scores distributed in patients with cirrhosis compared to the US general population. These differences are not significant.
3.2. Resilience and frailty (Table 2)
Table 2.
Frailty by Resilience
| Characteristics | All n=300 | By Resilience Quartile | p-value | Test of trend p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Least resilient n=86 | Less resilient n=69 | More resilient n=63 | Most resilient n=82 | |||||
| Liver Frailty Index | 3.8 (3.2–4.3) | 4.0 (3.5–4.8) | 3.7 (3.1–4.3) | 3.6 (3.1–4.0) | 3.6 (3.1–4.2) | 0.001 | 0.001 | |
| Liver Frailty Index Category | Robust | 79 (26%) | 14 (16%) | 21 (30%) | 22 (35%) | 22 (27%) | 0.002 | 0.001 |
| Prefrail-robust | 75 (25%) | 17 (20%) | 16 (23%) | 15 (24%) | 27 (33%) | |||
| Prefrail-frail | 101 (34%) | 30 (35%) | 24 (35%) | 22 (35%) | 25 (30%) | |||
| Frail | 45 (15%) | 25 (29%) | 8 (12%) | 4 (6%) | 8 (10%) | |||
Median (interquartile range) or %
Median Liver Frailty Index scores were significantly lower (i.e., were less frail) as resilience increased (test of trend p=0.001). Patients categorized as least resilient had the highest rates of frailty: 29% were frail compared to 12%, 6%, and 10% in less, more, and most resilient groups, respectively. In patients categorized as most resilient, 27% were robust and among the least resilient, only 16% were robust.
In univariable analysis, factors associated with Liver Frailty Index per 0.1 unit with a p<0.2 were: resilience (coef.= −0.13 per point; p<0.001), Asian race (coef.= −3.44; p=0.04), NASH (coef.= 2.48; p=0.09), HCC (coef.= −2.23; p=0.03), MELDNa (coef.= 0.47; p<0.001), albumin (coef.= −3.67; p<0.001), ascites (coef.= 4.57; p<0.001), hepatic encephalopathy (coef.= 6.57; p<0.001), diabetes (coef.= 1.56; p=0.14), coronary artery disease (coef.= 4.68; p=0.06), stroke (coef.= 8.90; p=0.08), and a history of anxiety or depression (coef.= 1.80; p=0.08). In multivariable analysis, resilience (coef.= −0.13; p<0.001) remained significantly associated with Liver Frailty Index (Table 3).
Table 3.
Univariable and multivariable models to assess associations between the following co-variables and frailty, as measured by the Liver Frailty Index (per 0.1 unit).
| Coefficients for the association with frailty (95% CI) p-value |
|||
|---|---|---|---|
| Univariable Model | Multivariable Model | ||
| CD-RISC score, per point | −0.13 (−0.20–−0.06) p<0.001 |
−0.13 (−0.19–−0.07) p<0.001 |
|
| MELDNa, per point | 0.47 (0.30–0.64) p<0.001 |
0.43 (0.27–0.60) p<0.001 |
|
| Hepatic Encephalopathy | 6.57 (3.59–9.54) p<0.001 |
6.55 (3.79–9.32) p<0.001 |
|
| Etiology | Alcohol (ref) | — | — |
| HCV | 1.19 (−1.32–3.69) p=0.35 |
3.53 (1.23–5.83) p=0.003 |
|
| NASH | 2.48 (−0.40–5.37) p=0.09 |
3.39 (0.78–6.00) p=0.01 |
|
| Cholestatic | −2.67 (−7.08–1.75) p=0.24 |
−0.20 (−4.20–3.79) p=0.92 |
|
| Other | 0.34 (−3.09–3.76) p=0.85 |
2.37 (−0.87–5.62) p=0.15 |
|
| Race | White (ref) | — | — |
| Black | 0.89 (−3.66–5.44) p=0.70 |
0.16 (−3.94–4.26) p=0.94 |
|
| Hispanic | 1.77 (−0.55–4.09) p=0.14 |
0.97 (−1.10–3.04) p=0.36 |
|
| Asian | −3.44 (−6.74–−0.13) p=0.04 |
−4.82 (−7.97–−1.67) p=0.003 |
|
| Other | 2.66 (−3.46–8.77) p=0.39 |
2.16 (−3.33–7.66) p=0.44 |
|
| Coronary artery disease | 4.68 (−0.13–9.49) p=0.06 |
5.92 (1.57–10.26) p=0.01 |
|
| Stroke | 8.90 (−0.95–18.76) p=0.08 |
10.93 (2.13–19.74) p=0.02 |
|
Variables evaluated for inclusion in the multivariable models were (because of p-value<0.2 in univariable analysis): race, etiology, HCC, MELDNa, albumin, ascites, hepatic encephalopathy, diabetes, coronary artery disease, stroke, and a history of anxiety or depression. Only variables associated with a p-value<0.05 were retained in the final model.
4. DISCUSSION
In this study of 300 patients with cirrhosis, we investigated the relationship between physical frailty and resilience, the psychological ability to bounce back from stressors.9 As hypothesized, we found a strong association between low resilience and frailty. Additionally, we observed that two characteristics were strongly associated with resilience: older age, which was associated with higher resilience and a history of anxiety or depression, which was associated with lower resilience. This interesting association between older age and higher resilience may be due to selection bias, as clinicians may have been more likely to consider an older patient for liver transplantation only if s/he displayed resilience (to balance the increased risk of transplantation in older adults). Notably, neither liver disease severity (e.g., MELDNa, ascites, or hepatic encephalopathy) nor presence of comorbidities (e.g., hypertension, diabetes) were associated with resilience.
When interpreting these results, it is essential to first understand how resilience in patients with cirrhosis compares with the general population. The median CD-RISC score of the general population in the U.S. is 82 with an interquartile range of 73–90.9 The distribution of resilience scores in our cohort is similar to that of the US general population. While this cross-sectional study was not designed to determine causality, the distribution of resilience in our cohort is similar to that of the US population, suggesting low resilience may lead to frailty and not the reverse. If frailty led to a decrease in resilience, we would expect the resilience of our cohort to be lower than that of the US general population given the disproportionate prevalence of frailty in patients with cirrhosis as compared to the general population. Because of the strong association between resilience and a history of anxiety or depression, we also compared the prevalence of these mental health disorders in our cohort versus the US general population. In the US general population, lifetime prevalence of any anxiety disorder, mood disorder, or any mental health disorder is 31.0%, 21.4%, and 47.4%, respectively.12 Again, the rate of anxiety or depression is similar to that of the US general population, underscoring the likelihood of resilience influencing frailty.
How might low resilience lead to frailty? We offer a possible mechanism for this pathway. Complications of liver disease such as ascites or hepatic encephalopathy may prevent patients with cirrhosis from sustaining the same level of physical activities as they had previously engaged in. A patient with low resilience may be less likely to engage in the physical and nutritional activities that are essential to maintaining muscle mass and cardiovascular fitness. On the other hand, a patient who is more resilient may be more likely to overcome the physical and mental barriers to achieving adequate exercise and nutrition. How the presence of mental health conditions, including anxiety and depression, mediate the relationship between resilience and frailty is unknown, particularly in light of the known association between frailty and depression,13 but presents an important area for future study. Additionally, the impact of low resilience on patient reported outcomes, such as health-related quality of life, and mortality is another important area for future study.
Resilience is emerging as a predictor for health outcomes, with low resilience being associated with poor outcomes, quality of life, and physical functioning in older adult, orthopedic surgery, chronic pain, and rheumatic disease patients.4,14–17 While the impact of resilience on liver transplant outcomes must be further elucidated in additional studies, we offer potential applications for this construct in clinical practice. First, low resilience should be considered a risk factor for liver transplantation, identifying patients who may need additional support to succeed on the waitlist or after surgery. Of particular relevance to transplant patients, resilience has been associated with better treatment adherence,18 suggesting a role for increased medication support in those with low resilience. Second, resilience as a risk factor may be modifiable. Prior studies in populations with other conditions including cancer, diabetes, fibromyalgia, and older adults in general have demonstrated that resilience can improve with targeted interventions such as mindfulness and/or cognitive behavioral therapy.19–24 Though research on whether resilience training impacts physiologic health outcomes is limited, improvements in HDL cholesterol, fasting blood glucose, and bodily pain in patients with diabetes and chronic pain have been reported with such interventions.21,25 Lastly, given the association between resilience and mental health disorders, treatment of mental health disorders may be incorporated into strategies to simultaneously improve resilience.9,26
If low resilience does indeed lead to physical frailty, as we have postulated, then our data raise the possibility that improving resilience can improve physical frailty. Prehabilitation, the process of enhancing physiologic reserve and functional capacity prior to surgery,2 has long been proposed for patients awaiting solid organ transplantation27 and recently incorporated into society guidelines for improving frailty in solid organ transplant patients.28 However, the efficacy of such programs—which have focused largely on exercise and nutritional interventions—have been modest to date. The data that we have presented in this paper suggest that such programs should also incorporate strategies to increase resilience. Such strategies exist, but have yet to be applied to patients with cirrhosis.
We acknowledge the following limitations to our study. First, because we did not have access to the CD-RISC scale in other languages, we limited our study to patients who spoke English. Resilience – and psychological contributors to frailty in general – is undoubtedly influenced by cultural factors29,30 and the assessment of resilience likely differs in different languages. Second, we only ascertained whether a patient had any history of anxiety or depression, not whether their anxiety or depression has been resolved or controlled. This decision was made to dichotomize the variable given the complexity of mental health disorders. Third, the resilience measure was determined by self-reported data and there could be social desirability bias. However, the CD-RISC has been validated as a reliable measure of resilience.9
Despite these limitations, this study is the first to highlight the close relationship between resilience and frailty in patients with cirrhosis and to expand upon the construct of frailty beyond physical frailty alone. As the hepatology community embarks upon efforts to develop prehabilitation programs to mitigate frailty in patients with cirrhosis, our data raise the possibility that resilience building techniques could be a vital component of such programs. Future studies are needed to evaluate methods to improve resilience in order to reduce liver transplant waitlist mortality.
Acknowledgments
Financial support: This study was funded by NIH K23AG048337 (Lai), NIH R01AG059183 (Lai). These funding agencies played no role in the analysis of the data or the preparation of this manuscript.
Abbreviations:
- CD-RISC
Connor-Davidson Resilience Scale
- CI
confidence interval
- IQR
interquartile range
- MELDNa
Model for End-Stage Liver Disease
- NASH
nonalcoholic steatohepatitis
- UCSF
University of California, San Francisco
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 2.Lai JC, Sonnenday CJ, Tapper EB, et al. Frailty in liver transplantation: An expert opinion statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Am J Transplant. 2019;19(7):1896–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology. 2017;66(2):564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rebagliati GA, Sciumè L, Iannello P, et al. Frailty and resilience in an older population. The role of resilience during rehabilitation after orthopedic surgery in geriatric patients with multiple comorbidities. Funct Neurol. 2016;31(3):171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gijzel SMW, van de Leemput IA, Scheffer M, et al. Dynamical Resilience Indicators in Time Series of Self-Rated Health Correspond to Frailty Levels in Older Adults. J Gerontol A Biol Sci Med Sci. 2017;72(7):991–996. [DOI] [PubMed] [Google Scholar]
- 6.Martin C, Hinkley N, Stockman K, et al. Resilience, health perceptions, (QOL), stressors, and hospital admissions-Observations from the real world of clinical care of unstable health journeys in Monash Watch (MW), Victoria, Australia. J Eval Clin Pract. 2018;24(6):1310–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hale M, Shah S, Clegg A. Frailty, inequality and resilience. Clin Med (Lond). 2019;19(3):219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freitag S, Schmidt S. Psychosocial Correlates of Frailty in Older Adults. Geriatrics (Basel). 2016;1(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18(2):76–82. [DOI] [PubMed] [Google Scholar]
- 10.Windle G What is resilience? A review and concept analysis Vol 21 Reviews in Clinical Gerontology: Cambridge University Press; 2010. [Google Scholar]
- 11.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessler RC, Aguilar-Gaxiola S, Alonso J, et al. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc. 2009;18(1):23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cron DC, Friedman JF, Winder GS, et al. Depression and Frailty in Patients With End-Stage Liver Disease Referred for Transplant Evaluation. Am J Transplant. 2016;16(6):1805–1811. [DOI] [PubMed] [Google Scholar]
- 14.Wright LJ, Zautra AJ, Going S. Adaptation to early knee osteoarthritis: the role of risk, resilience, and disease severity on pain and physical functioning. Ann Behav Med. 2008;36(1):70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartone PT, Valdes JJ, Sandvik A. Psychological hardiness predicts cardiovascular health. Psychol Health Med. 2016;21(6):743–749. [DOI] [PubMed] [Google Scholar]
- 16.Evers AW, Zautra A, Thieme K. Stress and resilience in rheumatic diseases: a review and glimpse into the future. Nat Rev Rheumatol. 2011;7(7):409–415. [DOI] [PubMed] [Google Scholar]
- 17.Johnson AJ, Terry E, Bartley EJ, et al. Resilience factors may buffer cellular aging in individuals with and without chronic knee pain. Mol Pain. 2019;15:1744806919842962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noghan N, Akaberi A, Pournamdarian S, et al. Resilience and therapeutic regimen compliance in patients undergoing hemodialysis in hospitals of Hamedan, Iran. Electron Physician. 2018;10(5):6853–6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cejudo J, García-Castillo FJ, Luna P, et al. Using a Mindfulness-Based Intervention to Promote Subjective Well-Being, Trait Emotional Intelligence, Mental Health, and Resilience in Women With Fibromyalgia. Front Psychol. 2019;10:2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith JL, Hanni AA. Effects of a Savoring Intervention on Resilience and Well-Being of Older Adults. J Appl Gerontol. 2019;38(1):137–152. [DOI] [PubMed] [Google Scholar]
- 21.Steinhardt MA, Brown SA, Dubois SK, et al. A resilience intervention in African-American adults with type 2 diabetes. Am J Health Behav. 2015;39(4):507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Victoria Cerezo M, Ortiz-Tallo M, Cardenal V, et al. Positive psychology group intervention for breast cancer patients: a randomised trial. Psychol Rep. 2014;115(1):44–64. [DOI] [PubMed] [Google Scholar]
- 23.Loprinzi CE, Prasad K, Schroeder DR, et al. Stress Management and Resilience Training (SMART) program to decrease stress and enhance resilience among breast cancer survivors: a pilot randomized clinical trial. Clin Breast Cancer. 2011;11(6):364–368. [DOI] [PubMed] [Google Scholar]
- 24.Treichler EBH, Glorioso D, Lee EE, et al. A pragmatic trial of a group intervention in senior housing communities to increase resilience. Int Psychogeriatr. 2020;32(2):173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hausmann LR, Parks A, Youk AO, et al. Reduction of bodily pain in response to an online positive activities intervention. J Pain. 2014;15(5):560–567. [DOI] [PubMed] [Google Scholar]
- 26.Davidson J, Baldwin DS, Stein DJ, et al. Effects of venlafaxine extended release on resilience in posttraumatic stress disorder: an item analysis of the Connor-Davidson Resilience Scale. Int Clin Psychopharmacol. 2008;23(5):299–303. [DOI] [PubMed] [Google Scholar]
- 27.Mathur S, Janaudis-Ferreira T, Wickerson L, et al. Meeting report: consensus recommendations for a research agenda in exercise in solid organ transplantation. Am J Transplant. 2014;14(10):2235–2245. [DOI] [PubMed] [Google Scholar]
- 28.Kobashigawa J, Dadhania D, Bhorade S, et al. Report from the American Society of Transplantation on frailty in solid organ transplantation. Am J Transplant. 2019;19(4):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ungar M Resilience and Culture: The Diversity of Protective Processes and Positive Adaptation. Vol 11: Springer, Dordrecht; 2015. [Google Scholar]
- 30.Kumpfer KL. Factors and processes contributing to resilience: The positive resilience framework In: Johnson Ga. Resilience and development: Positive Life Adaptions. New York: Kluwer Academic/Plenum Publishers; 1999. [Google Scholar]


