Abstract
Background
Multiple Sclerosis (MS) and Migraine are comorbid neurologic conditions. Migraine prevalence is three times higher in the MS clinic population compared to the general population, and patients with MS and migraine are more symptomatic than patients with MS without migraine.
Objective
We sought to conduct a pilot feasibility and acceptability study of the RELAXaHEAD app in MS-Migraine patients and to assess whether there was any change in migraine disability and MS pain-related disability.
Methods
Randomized controlled study of patients with MS-migraine ages 18–80 years with 4+ headache days/ month who were willing to engage in smartphone based behavioral therapy. Half received the RELAXaHEAD app with progressive muscle relaxation (PMR) and the other half received the app without the PMR. Data was collected for 90 days on measures of recruitment, retention, engagement, and adherence to RELAXaHEAD. Preliminary data was also collected on migraine disability (MIDAS) and MS pain (PES).
Results
Sixty-two subjects with MS-migraine were enrolled in the study (34 in PMR arm, 28 in monitored usual care arm). On average, during the 90 days, participants played the PMR on average 1.8 times per week, and for 12.9 minutes on days it was played. Forty-one percent (14/34) of the participants played the PMR two or more times weekly on average. Data was entered into the daily diaries, on average, 49% (44/90) of the days. There were major challenges in reaching subjects in follow-up for the efficacy data, and there was no significant change in migraine disability (MIDAS) scores or MS Pain (PES) scores from baseline to the endpoints. During the six-month follow-up, most patients felt either positively or neutral about the relaxation therapy.
Conclusion
There was interest in scalable accessible forms of behavioral therapy to treat migraine and MS-related pain in patients with MS and comorbid migraine. Similar to prior studies, a significant minority were willing to practice the PMR at least twice weekly. In the societal shift from telephone to more text and internet-based interactions, follow up was challenging, but those reached indicated that they appreciated the PMR and would recommend it to others. Future work should focus on engagement and efficacy.
Keywords: Smartphone application, behavioral therapy, Progressive muscle relaxation therapy, Electronic diary, Multiple Sclerosis Pain, Migraine
1. INTRODUCTION
Patients with Multiple Sclerosis (MS) suffer from significant pain, depression, and anxiety,1 and continue to seek additional therapies beyond available and effective pharmacological therapies.2 One cause of pain in patients with MS is migraine. Migraine is the second most disabling condition per the World Health Organization (WHO),3 and a prior study showed that migraine prevalence is three times higher in the MS clinic population compared to the general population.4 Moreover, MS patients with migraine are more symptomatic than MS patients without migraine,4 and patients with both MS and migraine (MS-Migraine) had higher scores for fatigue (fatigue severity scale), depression (PHQ9) and anxiety (PHQ). In addition, these patients were more prone to experiencing new or worsening neurologic symptoms compared to MS patients without migraines.4
Prior research has shown that Progressive Muscle Relaxation (PMR) therapy is an effective treatment for patients with either MS or migraine.5,6 PMR is a simple, easily taught,7,8 safe,5 but under-utilized mind-body intervention.9,10 Traditionally, psychologists train patients in the use of PMR. However, physicians have difficulty finding providers, and patients have difficulty accessing and paying for behavioral treatments for chronic conditions such as for migraine or MS.11–14
A review of digital and remote communication technologies as a tool for MS management15 showed that of 28 eHealth solutions, 14 were web-based, and 11 were app based. The MS eHealth solutions to date mostly support disease monitoring, self-management, treatment, and rehabilitation. A minority may also offer patient advice and education. There have also been studies examining physical activity related to Nintendo16 and whether gamification might improve engagement in some remotely delivered MS therapies. However, to the best of our knowledge, there has not been smartphone based behavioral therapy for the prevention of MS pain and migraine.
New smartphone based interventions such as the RELAXaHEAD application (app)17 have been developed as an alternative to the traditional in-office based behavioral therapy. In a previous single arm study of patients with migraine (without Multiple Sclerosis) receiving care at an academic tertiary care hospital’s neurology department, RELAXaHEAD treatment was associated with a 50% reduction in headache days after two months of treatment compared to after one month of treatment in those who used it two or more times a week.18
The primary aim of this study was to conduct a pilot feasibility and acceptability study of the RELAX approach in MS-migraine patients who visit an MS Center. A secondary aim was to assess whether there was any change in migraine disability and MS pain related disability. We hypothesized that compared with baseline, there would be trends in the RELAX (PMR) arm towards decreased disability and improvements in MS pain related quality of life.
2. METHODS
This study was approved by our medical center’s Institutional Review Board (IRB). It was registered on ClinicalTrials.gov [NCT03183791].
2.1. Study Population and Recruitment
Patients who presented to the medical center’s Comprehensive Multiple Sclerosis Centers and various MS-related events were pre-screened for the study by Research Assistants (RAs) using EPIC,19 an Electronic Medical Record (EMR) system. RAs screened patient charts for the following criteria: age 18–80, prior diagnosis, or problem listed as “migraine” and or “headache. They had to have been diagnosed with MS by a healthcare provider, and all types of MS diagnoses were included in the study. Screened patients, thought to be potentially eligible, were approached by RAs either in-person at the MS Centers, or via phone after their appointment has ended. The RA asked patients questions to determine eligibility (see Table 1).
Table 1:
Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 4+ headache days a month | Cognitive deficits or other physical problem with the potential to interfere with behavioral therapy |
| Meets migraine criteria per International Classification of Headache Disorders (ICHD)-3b,46 | Opioid or barbiturate use 10+ days a month; Alcohol or other substance abuse |
| Speaks English | Have done PMR, cognitive behavioral therapy, biofeedback, or other relaxation therapy for migraine in the past year |
| Owns a smartphone | Does not own a smartphone |
| Willing to use a smartphone application for migraine treatment | Unable or unwilling to follow a treatment program that relies on written and audio file material |
RAs also recruited participants through MS events in NYC; individuals interested in the study provided their contact information to and were contacted by the RA to determine potential study eligibility per the same criteria.
All eligible persons interested in participating attended in-person enrollment sessions at the medical center. RAs obtained informed consent and randomized participants to the PMR or monitored usual care (MUC) condition. RAs collected headache history and baseline data via REDCap:20 demographics, medical history, medication usage, headache and MS history, psychiatric screens, and previous behavioral therapy for migraine (Table 2). RAs created a de-identified study participant account on the RELAXaHEAD online portal, downloaded the app onto participants’ smartphones, and conducted the sessions as described below. Those in the PMR group received the full version of the RELAXaHEAD app with PMR, while those in the MUC group received the same RELAXaHEAD app without PMR. During the informed consent process, the RA notified the participant that if s/he were to be randomized to the control group, s/he could receive the PMR after the study ended.
Table 2:
MS-Migraine Participant Demographics, Headache Characteristics, Prior Healthcare and Intervention Methods
| Participant Information | PMR Treatment Arm (n=34) | Control Arm (n=28) | p-value | Total Participants (n=62) |
|---|---|---|---|---|
| Sex, n(%) | 0.897 | |||
| Male | 4 (12%) | 3 (11%) | 7 (11%) | |
| Female | 30 (88%) | 25 (89%) | 55 (89%) | |
| Current Age (years) | 0.319 | |||
| mean ± sd (min-max) median [IQR], n | 38.2 ± 10.4 (22–69), 38 [30.75 – 45.25], n=34 | 41.2 ± 13 (21–73) 39 [31 – 50], n=27 | 39.5 ± 11.6 (21–73) 38 [31 – 47], n=61 | |
| Ethnicity, n(%) | 0.414 | |||
| Hispanic or Latino | 10 (29%) | 11 (39%) | 21 (34%) | |
| Not Hispanic or Latino | 24 (71%) | 17 (61%) | 41 (66%) | |
| Race, n(%) | 0.581 | |||
| White | 17 (49%) | 11 (41%) | 28 (45%) | |
| Black/African American | 10 (29%) | 9 (33%) | 19 (31%) | |
| other races | 7 (20%) | 7 (26%) | 14 (23%) | |
| unknown | 1 (3%) | 0 | 1 (2%) | |
| Past Medical History: | ||||
| Overlapping Pain Conditions, n(%) | ||||
| Chronic back pain | 19 (56%) | 16 (57%) | 0.921 | 35 (56%) |
| Arthritis | 5 (15%) | 6 (21%) | 0.523 | 11 (18%) |
| Fibromyalgia | 1 (3%) | 2 (7%) | 0.585 | 3 (5%) |
| Irritable bowel syndrome | 4 (12%) | 6 (21%) | 0.326 | 10 (16%) |
| Self Reported Psych History, n(%) | ||||
| Anxiety | 21 (62%) | 11 (39%) | 0.078 | 32 (52%) |
| Depression | 19 (56%) | 16 (57%) | 0.921 | 35 (56%) |
| Medication Usage: | ||||
| Prior medication usage, n(%) | ||||
| Migraine Preventive Medications | 23 (68%) | 12 (43%) | 0.050 | 35 (56%) |
| Botox (botulinum toxin) | 3 (9%) | 6 (21%) | 0.277 | 9 (15%) |
| Opioids | 13 (38%) | 9 (32%) | 0.618 | 22 (35%) |
| Triptans | 14 (41%) | 9 (32%) | 0.464 | 23 (37%) |
| Migraine Abortive Medications | 34 (100%) | 27 (96%) | 0.452 | 61 (98%) |
| Positive Family History of Headache | 19 (56%) | 14 (50%) | 0.644 | 33 (53%) |
| Headache Characteristicsa | ||||
| Age to first have headaches regularly | 18.6 ± 9.7 (3–43), 16 [12.5 – 25], n=33 | 22 ± 12.8 (6–58) 20 [12 – 32], n=27 | 0.245 | 20.1 ± 11.3 (3–58) 16.5 [12 – 29.5], n=60 |
| Average number of Headache Days/month | 13 ± 8.3 (5–30), 9.5 [6 – 17.75], n=34 | 12.7 ± 8.8 (5–32) 8 [6 – 17.25], n=28 | 0.885 | 12.9 ± 8.4 (5–32) 9 [6 –17.25], n=62 |
| Average pain intensity (0–10 pain scale) | 7.1 ± 1.6 (3–10), 7 [6 – 8], n=34 | 6.9 ± 1.5 (4–9) 7 [5.25 – 8], n=28 | 0.559 | 7 ± 1.5 (3–10) 7 [6 – 8], n=62 |
| MIDAS (Sum of the first 5 questions) | 42.9 ± 36.9 (2–140), 30 [17.25 – 59.25], n=34 | 34.6 ± 42.9 (0–177) 14.5 [6 – 62.75], n=28 | 0.416 | 39.2 ± 39.6 (0–177) 26.5 [8.5 – 62.25], n=62 |
| Little or no disability (0–5) | 2 (6%) | 6 (21%) | 8 (13%) | |
| Mild disability (6–10) | 2 (6%) | 6 (21%) | 8 (13%) | |
| Moderate disability (11–20) | 6 (18%) | 4 (14%) | 10 (16%) | |
| Severe disability (21+) | 24 (71%) | 12 (43%) | 36 (58%) | |
| MS Characteristics a | ||||
| Age to first develop symptoms of MS | 26.8 ± 10 (8–50), 27 [18 – 32.5], n=33 | 27.9 ± 9.1 (14–52) 27 [21 – 31], n=28 | 0.658 | 27.3 ± 9.6 (8–52) 27 [19.5 – 32], n=61 |
| Age of MS diagnosis | 31 ± 10.2 (13–50), 31 [21 – 39.25], n=34 | 31.8 ± 10.1 (16–56) 30 [24.25 – 39], n=28 | 0.782 | 31.4 ± 10.1 (13–56) 30.5 [23.5 – 39], n=62 |
| MOS PES Scores | 19 ± 10.2, 19, (6–28) | 16 ± 7, 18, (6–28), n=27 | 0.198 | 17 ± 7, 19, (6–28), n=61 |
| MS Symptoms, n(%) | ||||
| fatigue | 33 (97%) | 26 (93%) | 0.585 | 59 (95%) |
| weakness | 31 (91%) | 27 (96%) | 0.620 | 58 (94%) |
| numbness | 30 (88%) | 25 (89%) | 0.999 | 55 (89%) |
| cognitive decline/brain fog | 23 (68%) | 22 (79%) | 0.337 | 45 (73%) |
| difficulty walking | 25 (74%) | 20 (71%) | 0.854 | 45 (73%) |
| bowel issues | 14 (41%) | 14 (50%) | 0.487 | 28 (45%) |
| bladder issues | 21 (62%) | 18 (64%) | 0.838 | 39 (63%) |
| speech difficulty | l9 (56%) | 21 (75%) | 0.117 | 40 (65%) |
| depression | 26 (76%) | 18 (64%) | 0.293 | 44 (71%) |
| emotional changes | 25 (74%) | 21 (75%) | 0.895 | 46 (74%) |
| Psychiatric Screens a | ||||
| PROMIS Depression (sum) | 53.2 ± 9.4 (33.5–70.3), 55.1 [46.5 – 59.5], n=27 | 48.7 ± 10.5 (40–68.1) 42.7 [40 – 59.2], n=24 | 0.115 | 51.1 ± 10.1 (33.5–70.3) 49.7 [41.1 – 59.5], n=51 |
| PROMIS Anxiety (sum) | 54.2 ± 9.8 (34–70), 56.6 [50.7 – 60.1], n=27 | 47.7 ± 10.5 (36.7–70.7) 46.6 [38.1 – 52.8], n=24 | 0.025 | 51.1 ± 10.6 (34.3–70.7) 50.7 [40.2 – 60.1], n=51 |
| Previously visit to the Emergency Department for headaches, n(%) | 0.612 | |||
| No visits to the ED | 19 (56%) | 16 (57%) | 35 (56%) | |
| 1 visit | 5 (15%) | 2 (7%) | 7 (11%) | |
| 2 visits | 5 (15%) | 3 (11%) | 8 (13%) | |
| 3 or more visits | 5 (15%) | 7 (25%) | 12 (19%) | |
| Previous behavioral therapy for migraine, n(%) | ||||
| Combined | 6 (18%) | 1 (4%) | 0.116 | 7 (11%) |
| Cognitive Behavioral Therapy | 5 (15%) | 1 (4%) | 0.336 | 6 (10%) |
| Biofeedback | 1 (3%) | 0 | 0.653 | 1 (2%) |
| Progressive Muscle Relaxation | 3 (9%) | 0 | 0.272 | 3 (5%) |
PMR Group: RAs discussed clinical efficacy and application of PMR therapy and demonstrated how to use the RELAXaHEAD application. Participants completed a 15-minute long PMR session during the enrollment session. Participants were asked to complete the daily headache diary and perform one 15-minute PMR session and one 5-minute PMR session per day.
MUC Group: RAs demonstrated how to use the RELAXaHEAD application. Participants were asked to complete the daily headache diary.
All participants received Amazon gift cards for participation ($25 for the initial enrollment and then $1/day for data usage for up to 90 days).
2.2. Study Intervention
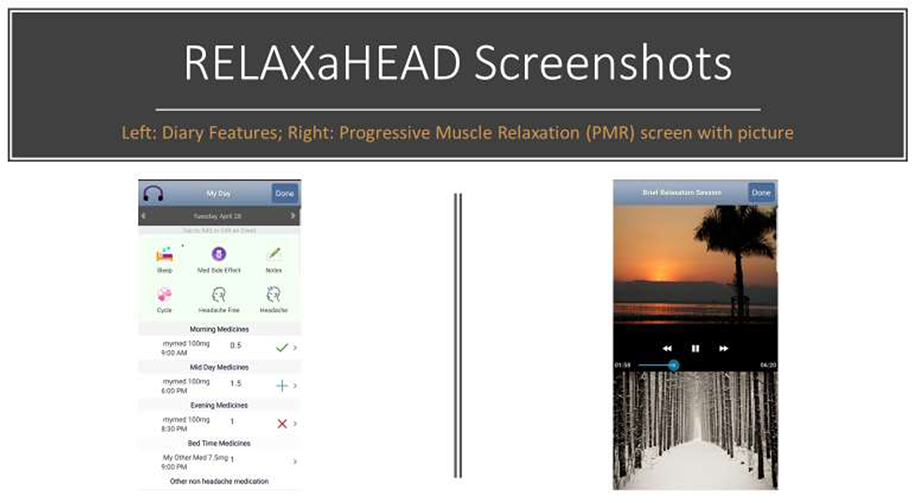
The RELAXaHEAD app, developed in partnership between NYU Langone Health and IRODY,21 was developed using an iterative approach and was beta tested with both patient and headache specialist input.17 The app has the same PMR audio files created by a psychologist used in the Stress Management in Living with Epilepsy (SMILE) study.22 The RELAXaHEAD app, used in multiple studies to assess headaches and PMR18,23, contains a headache diary, which includes features for tracking headache characteristics, headache medications, and sleep, as well as tracking medication side effects and menstrual cycles. There is also a notes section for free text notes. [Figure 1]
Figure 1:
RELAXaHEAD application
Protocol Changes:
In the original grant proposal we had planned to contact participants via telephone to complete follow-up surveys. Because of difficulties in reaching participants in other studies we had been conducting, we added email correspondence to the protocol.
Similarly, in an effort to improve compliance compared to what we had learned in prior pilot RELAXaHEAD work, compliance phone calls were instituted if the participant was missing 3 days or more of application use.
2.3. Measures
Primary Outcomes
Our primary outcomes were related to feasibility and acceptability. We collected data on measures of recruitment, retention, engagement, and adherence to RELAXaHEAD: the application tracked daily diary entries as well as PMR frequency and length of sessions. Follow-up calls were done at 48–72 hours, 1 month, 2 months, 3 months, and 6 months. During these calls, participants were asked if they enjoyed the PMR sessions, faced any obstacles, and the likelihood of recommending the app to others.
Secondary Outcomes
We had two efficacy outcomes: 1. Migraine disability using the Migraine Disability Assessment Scale (MIDAS).24, a validated 5 item questionnaire that has internal consistency and test-retest reliability. It was developed to assess headache-related disability to improve migraine care. Questions ask about prior activity limitations over the past 3 months. 2. We also assessed for the Medical Outcomes Study (MOS) Pain Effects Scale (PES). The PES scale measures how pain and other symptoms associated with MS have affected mood, ability to walk or move around, sleep, normal work (both outside and at home), recreational activities, and enjoyment of life over the past 4 weeks. Patients are asked to rank whether MS-related symptoms inferred not at all, a little, moderately, quite a bit, or to an extreme degree with each aspect of their lives.25 This survey is part of the Multiple Sclerosis Quality of Life Inventory (MSQLI).
2.4. Statistical Analyses
Sample size
As indicated by Kraemer and colleagues,26 our pilot sample size was based on the pragmatics of recruitment and the requisites for examining feasibility. A priori, our target N was 60 (30 in each group).
Analysis plans
We used block randomization27 with random block sizes of 4 to 6 to assign participants in 1:1 ratio to the two intervention groups: RELAX and MUC. The block randomization was done by a statistician unrelated to the study team. We generated descriptive statistics for all variables using means (standard deviations) and medians (IQR) for continuous variables, and frequencies (proportions) for categorical variables. We assessed the balance between intervention and control arms by comparing summary statistics (means and proportions as defined above) in each study arm using univariate tests (t-test for continuous variables and chi-squared test for binary or categorical variables) within two-tailed significance level of 0.05. (These tests were not adjusted for multiple testing and are used as exploration tools).
As previously stated, our primary focus was to examine feasibility, specifically, recruitment, retention, engagement and adherence to RELAXaHEAD. We report descriptive statistics on use of the headache diary and of the PMR. We explored migraine disability outcomes between participants assigned to the PMR and to the control arm by comparing the intra-individual change in MIDAS score from baseline to 6-month follow-up in the PMR and in the MUC group using a t-test (for participants enrolled after 15 May 2018). We also explored MS pain outcomes by comparing intra-individual changes in MS POES.
3. RESULTS
As shown in supplemental table 1, there were 2,827 people who presented to our MS Center between November 1, 2017 and September 19, 2018. Of these patients, 600 were screened in Epic as having “headache” as a prior diagnosis or problem in their problem list. Additionally, 8 patients were recruited from the Brooklyn MS Center and 10 patients at MS related events around the city in the Spring of 2018. A total of 62 patients had a confirmed migraine diagnosis based on the comprehensive survey questions and met study inclusion criteria. There were 34 in the intervention arm and 28 in the control arm. Of note, the most common reasons for ineligibility was no headaches currently (16%) and not enough headaches (10%). Of those contacted, 13% of patients stated that they were not interested in participating in the study; the most common reason was that they did not have time and/or they had too many other time commitments.
As seen in Table 2, of the 62 participants, 55 (89%) were female. Mean age was 39±11. Headaches developed on average at 20±11 years. MS symptoms developed at 27±10 years and MS was diagnosed at 31±10 years. Mean number of headache days/month was 13±8. Average headache intensity on numeric rating pain scale was 7±2. Migraine Disability Assessment (MIDAS) scores averaged 39±39. Patients previously used the following for headache management: triptans 37% (23/62), opioids 35% (22/62), oral preventive medications 47% (29/62), botulinum toxin 15% (9/62). Top comorbid conditions were: chronic back pain (56%), anxiety (52%), depression (56%). Prevalent MS symptoms: fatigue (95%), weakness (94%), numbness (89%), emotional changes (74%), depression (71%), cognitive decline (73%), difficulty walking (73%), speech (65%), bladder (63%), bowel (45%).
3.1. Quantitative Results
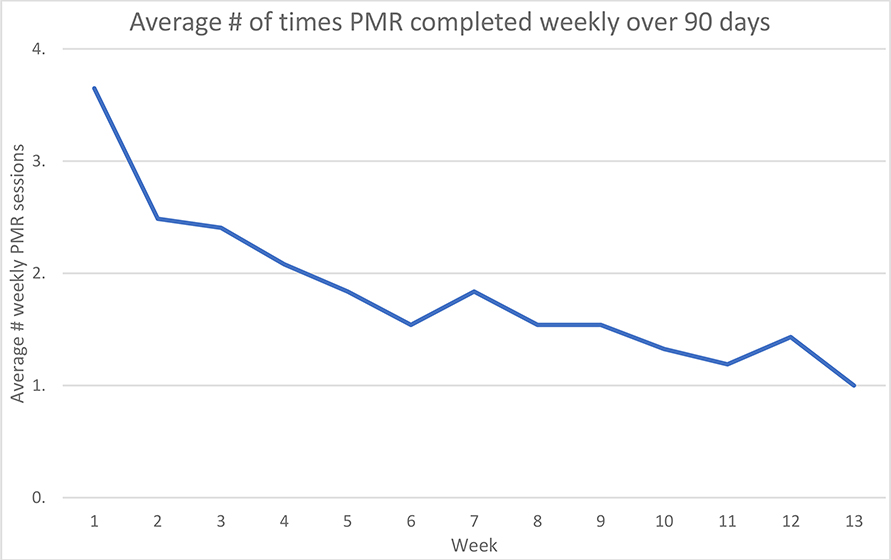
On average, in the 90-day period of the study, participants played the PMR 1.8 times per week. Forty-one percent (14/34) of the participants played the PMR two or more times weekly on average. PMR was played for an average of 12.9 minutes per day on days it was used. Baseline data comparing the high users (2+ days/week) versus low users (less than 2 days/week) can be found in the Supplemental Table 2. There were no statistically significant differences except for baseline PROMIS anxiety scores (high users 59.1 ± 5.2 (n=9) vs low users 50.2±10.1 (n=18), P=0.0208). For all study participants, data was entered into the daily diaries on average 49% (44/90) of the days. Control groups entered data on average 59% (53/90) of days, and PMR group 39% (35/90). Figure 1 shows attrition over the first 90-day period by week.
MIDAS scores for all participants at baseline averaged 42.9+/−36.9, which falls within the Severe Disability range (scores of 21+) (Table 3). Out of the total 62 participants, 44 participants (71%) completed the MIDAS questionnaire both at baseline and at study end (6-month follow up). The average at baseline was 41+/−44, and the average at the 6-month follow up was 29+/−43. The control group (n=21) scored at baseline 35+/−46 and at study end 16+/−25. The PMR group (n=23) averaged 46+/−42 at baseline and 41+/−52 at study end. (Table 3) MOS PES scores at baseline (n=61) averaged 17+/−7 (Table 4). Out of the 61 total participants, 32 (52%) participants completed both the MOS PES for baseline and at 3 months. The average was 16+/−7 at baseline, 12+/−6 at 3 months. For control subjects (n=18) baseline averaged 15+/−7, 3 months 12+/−6. For PMR subjects (n=14) baseline averaged 18+/−5, and 3 months 12+/−5. (Table 4)
Table 3:
MIDAS scores for participants (n=44) who completed both the MIDAS questionnaire at baseline and at 6-month follow up:
| MIDAS scores | All participants (n=44) | PMR (n=23) | Control (n=21) | p-value |
|---|---|---|---|---|
| Initial Questionnaire Avg, St. Dev, Median, (Min-Max) | 41+/− 44, 25, (0–177) | 46+/−42, 28, (2–140) | 35+/−46, 14, (0–177) | 0.4117 |
| 6 Month Follow Up Avg, St. Dev, Median, (Min-Max) | 29+/−43, 9.5, (0–181) | 41+/−52, 19, (0–181) | 16+/−25, 7, (0–106) | 0.0519 |
| Difference | −8 +/−43, −4, (−177,100) | −4 +/−49, −4,(−100,79) | −19.5 +/−50,−2, (−177,59) | 0.3363 |
Table 4:
MOS PES scores for 32 (32/62, 52%) participants who completed both baseline and 3 - month follow up.
| MOS PES scores | All participants (n=32) | PMR (n=14) | Control (n=18) | p-value |
|---|---|---|---|---|
| Initial Questionnaire Avg, St. Dev, Median, (Min-Max) | 16+/−7, 17, (6–25) | 18+/−5, 18, 6–25 | 15+/−7, 16, (6–25) | 0.1855 |
| 3-month Follow-Up Avg, St. Dev, Median, (Min-Max) | 12+/−6, 11.5, (5–24) | 12+/−5, 11, (5–20) | 12+/−6, 12, (5–24) | 1 |
| Difference | −2 +/−5,−0.5, (−12,10) | −3 +/−5,−2 (−10,3) | −0.4 +/− 5, 0, (−11,10) | 0.1491 |
3.2. Qualitative Results
Participants were asked 3 follow up questions: 1) What do you think of the relaxation therapy? 2) What obstacle(s) have you encountered in doing the therapy as recommended? 3) Would you recommend the therapy to others? RAs transcribed their answers into the follow-up questionnaires via REDcap. As the study progressed, the number of participants reached decreased at each follow-up time, and some subjects failed to answer all questions during each follow-up. Baseline characteristics comparing those who were reached at the one-month follow-up compared to those who were not reached for the one-month follow-up can be found in Supplemental Table 3. There were no statistically significant differences except for gender (81.6% of females completed the 1 month follow up versus 100% of females did not complete the 1 month follow up, P=0.0256). Of the 34 PMR subjects, the number reached at 48–72 hours, 1 month, 2 months, 3 months, and 6 months were as follows; 29 (85%), 15 (44%), 9 (26%), 11 (32%), 10 (29%). (Table 5) Two participants (6%) were unable to be reached at any follow up date.
Table 5:
Number of MS Patients in Intervention Arm with Qualitative Responses in Follow Up Call
| Follow Up Call | Number of Patients Reached |
|---|---|
| 48 – 72 Hour | 29 (85.3%) |
| 1 Month | 15 (44.1%) |
| 2 Months | 9 (26.5%) |
| 3 Months | 11 (32.4%) |
| 6 Months | 9 (26.5%) |
The responses to “What do you think of the relaxation therapy?” were categorized into: Positive, Neutral/Unsure/Mixed, Negative, and N/A- infrequent use of the app. During the first follow up, out of 29 responses, 20 responded positively (69%), 7 (24%) Neutral/Unsure/Mixed, and one (3%) negatively. At the 6 months follow up, 5/9 (56%) responded positively, 4/9 (44%) responded neutral/unsure/mixed. (Table 6)
Table 6:
Qualitative Responses to Follow-up Questions
| Follow Up Question | 48–72 hrs | 1mth | 2mth | 3mth | 6mth | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| What do you think of the relaxation therapy? | Total Over All Follow Ups | |||||||||||
| N/A- has not used the app often | 1/29 | 3% | 2/15 | 13% | 1/9 | 11% | 0 | 0% | 0 | 0% | 4/73 | 5% |
| Positive | 20/29 | 69% | 9/15 | 60% | 4/9 | 44% | 10/11 | 91% | 5/9 | 56% | 48/73 | 66% |
| Neutral/Unsure/Mixed Feelings | 7/29 | 24% | 3/15 | 20% | 2/9 | 22% | 1/11 | 9% | 4/9 | 44% | 17/73 | 22% |
| Negative | 1/29 | 3% | 1/15 | 7% | 2/9 | 22% | 0 | 0% | 0 | 0% | 4/73 | 4% |
| Total Participants Reached (n=34) | 29/34 | (85%) | 15/34 | (44%) | 9/34 | (26%) | 11/34 | (32%) | 9/34 | (26%) | 73/170 | 43% |
| What obstacle(s) have you encountered in doing the therapy as recommended? | Total Over All Follow Ups | |||||||||||
| App difficulties | 3/29 | 10% | 2/15 | 13% | 0 | 0% | 1/10 | 10% | 1/10 | 10% | 7 | 10% |
| Boring | 1/29 | 3% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 1% |
| External factors interfere | 1/29 | 3% | 2/15 | 13% | 2/9 | 22% | 0 | 0% | 1/10 | 10% | 6 | 9% |
| Falls asleep with therapy | 2/29 | 7% | 1/15 | 7% | 1/9 | 11% | 0 | 0% | 0 | 0% | 4 | 6% |
| Forgets to do the PMR | 1/29 | 3% | 5/15 | 33% | 1/9 | 11% | 1/10 | 10% | 0 | 0% | 8 | 11% |
| Not relaxing or uncomfortable | 1/29 | 3% | 1/15 | 7% | 0 | 0% | 0 | 0% | 1/10 | 10% | 3 | 4% |
| N/A | 1/29 | 3% | 0 | 0% | 0 | 0% | 0 | 0% | 1/10 | 10% | 2 | 3% |
| None | 12/29 | 41% | 3/15 | 20% | 3/9 | 33% | 4/10 | 40% | 3/10 | 30% | 25 | 36% |
| Scheduling/Routine | 1/29 | 3% | 0 | 0% | 1/9 | 11% | 0 | 0% | 1/10 | 10% | 3 | 4% |
| Time commitment | 5/29 | 17% | 1/15 | 7% | 1/9 | 11% | 1/10 | 10% | 0 | 0% | 8 | 11% |
| Wifi | 1/29 | 3% | 0 | 0% | 0 | 0% | 2/10 | 20% | 0 | 0% | 3 | 4% |
| Dislikes Length of Session | 0 | 0% | 0 | 0% | 0 | 0% | 1/10 | 10% | 0 | 0% | 1 | 1% |
| Recordings are repetitive | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 1/10 | 10% | 1 | 1% |
| Too emotional to use the app | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 1/10 | 10% | 1 | 1% |
| Total Participants Reached (n=34) | 29/34 | (85%) | 15/34 | (44%) | 9/34 | (26%) | 10/34 | (29%) | 10/34 | (29%) | 73/170 | 43% |
| Would you recommend the therapy to others? | Total Over All Follow Ups | |||||||||||
| Yes | 22/28 | 79% | 14/15 | 93% | 8/9 | 89% | 9/10 | 90% | 8/8 | 100% | 61/70 | 87% |
| No | 0 | 0% | 0 | 0% | 0 | 0% | 1/10 | 10% | 0 | 0% | 1/70 | 1% |
| Unsure/Neutral | 2/28 | 7% | 1/15 | 7% | 1/9 | 11% | 0 | 0% | 0 | 0% | 4/70 | 6% |
| N/A - has not done therapy long enough | 4/28 | 14% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 4/70 | 6% |
| Total Participants Reached (n=34) | 28/34 | (82%) | 15/34 | (44%) | 9/34 | (26%) | 10/34 | (29%) | 8/34 | (24%) | 70/170 | (41%) |
Given N=34 and 5 follow up interview time points, there were 170 possible responses over the study period. We were able to obtain 73 (43%) responses to “What do you think of the relaxation therapy.” Results and representative quotes from the 73 recorded responses are presented in Table 7.
Table 7:
Representative Responses to Follow Up Questions
| Follow Up Responses | Representative Quotes |
|---|---|
| What do you think of the relaxation therapy? | |
| N/A- infrequent or no use of the app | Haven’t been able to use app because of eviction and had to change address. |
| Positive | “It’s really good, it relaxes me and puts me to sleep |
| it’s cool. it’s similar to mindfulness, really relaxing, I like it | |
| I like it; it’s good to stop for a few minutes and not focus on everything. Easier to make it through the short one | |
| I like the relaxation sessions; it helps with the pain and with my anxiety. | |
| Neutral/Unsure/Mixed | It helps calm nerves but doesn’t help headaches. |
| Relaxing but hard to relax when something more internal. | |
| It’s nice doesn’t know if it helped much but enjoyable. | |
| I like it a lot; but other kinds of exercise give me better results. | |
| It’s ok. Not for me. Not really helpful, but it is okay. | |
| Negative | Have a lot going, so still get stress out even if used it. |
| What obstacle have you encountered in doing the therapy as recommended? | |
| App difficulties | Sometimes, would forget the sleep time and can’t tell the exact time fall asleep. |
| Boring | She finds it a little boring. |
| External factors interfere | Holidays interfered with ability to keep up with therapy. |
| Falls asleep with therapy | Difficult to find the time and sometimes feels asleep. |
| Forgets to do the PMR | Forgetfulness. |
| Not relaxing/uncomfortable | Uncomfortable to sit for that length of time. |
| N/A | N/A hadn’t started yet. |
| Routine/Schedule | Hard to create that routine on a daily basis. |
| Time commitment | A little hard to do it some days time-wise. |
| Wifi | None (sometimes internet connectivity is an issue) |
| Dislikes Length of Session | Do not have patience to do full 15 minutes, and sometimes sitting for 15 minutes is not always so comfortable. |
| Recordings are repetitive | Variation in the recordings would be great; it gets very repetitive. |
| Too emotional to use the app | Too emotional to use the application right now. |
| Would you recommend the therapy to others? | |
| Yes | Yes, would recommend to others. Do it for other pains too. |
| No | No. |
| Unsure/Neutral | Not sure. Right now, neutral. |
| N/A - has not done therapy long enough | Too early to tell. |
For the follow-up question, “What obstacle(s) have you encountered in doing the therapy as recommended” responses were categorized into 12 obstacles as well as the option for no obstacles or N/A if they had not been using the app. Over the 5 follow up sessions, 36% responded no obstacles. Most commonly reported obstacles were Forgets to do PMR (11%), App Difficulties (10%), Time commitment (11%) and External Factors Interfere (9%). Full responses and examples available in Table 6 and Table 7.
In the MS PMR group, few participants noted migraine/MS-specific barriers to using the RELAXaHEAD app in their follow ups. One follow up answer was, “If she has a migraine, it is hard for her to tense certain parts of her body, and those muscles won’t fire”, and another participant answered “She has been going through a lot of pain and forgets about the PMR until she has a migraine and then cannot do it while she has it.” One person mentioned tensing muscles as an obstacle but did not specify if it was during an attack. Another participant mentioned forgetting to do the PMR due to MS.
For the question, “Would you recommend the therapy to others?”, responses were categorized into Yes, No, Unsure/Neutral, and N/A- has not done therapy long enough. In the first 48–72 hour follow up, 22/28 (79%) responded Yes, 2/28 (7%) Unsure/Neutral, and 4/28 (14%) responded N/A has not done therapy long enough. At the last follow up, all of the eight respondents answered yes. In total, overall follow-ups, 61/70 (87%) responded “Yes”, 1/70 (1%) responded “No”, 4/70 (6%) were Neutral/Unsure, and 4/70 were N/A- has not done therapy long enough. (Table 6)
4. DISCUSSION
In this population of people with severe migraine disability who have had headaches for an average of over 20 years and an MS diagnosis for an average of over 10 years, we had several key findings. First, there was significant interest in a nonpharmacologic based intervention. Second, for a significant minority of participants, the RELAXaHEAD app was feasible and acceptable; 41% did the PMR at least two days a week. However, there was difficulty reaching participants throughout the study period and there were engagement issues with the use of a mobile health technology in people with MS. Understanding these issues might be helpful for developing new lines of research.
There was significant interest in a smartphone based behavioral trial for MS pain and migraine. This is especially important as patients with MS and migraine face the challenge of polypharmacy (5+ drugs);28 many patients suffer from side effects due to their multiple medications in addition to their usual symptoms.29 The option for MS patients with migraine, about half of whom had been on a migraine preventative medication, to participate in evidence-based behavioral therapy to treat their migraines and other pain was well received as designated by the recruitment statistics indicating a significant level of interest in the study.
As this was primarily a feasibility/acceptability study, we discuss considerations regarding difficulty around data collection and challenges in engagement, as well as how our engagement compares to that of the greater body of mHealth literature. Based on the low follow-up response rates (and thus limited outcome data collected), we do not focus on efficacy.
Difficulty collecting outcome data at study period intervals
Participants did not answer their phones during the follow-up data assessments. This has become an increasing issue in the conduct of clinical research. Attrition and nonresponse can be a particular disadvantage of longitudinal survey studies, and is greatly affected by study design features like time between data collection, modes of contact (i.e. telephone, email, in-person), and established rules for when participants should be contacted within the study period.30 We conducted focus groups with participants from other RELAXaHEAD studies which revealed that participants want varied methods of communication with the study team.31 Some said it is difficult to participate in study follow-up and compliance phone calls, and that they prefer to choose from among various options e.g. phone calls, text, email for contact with the study team. Thus, methods of contacting the participants should be varied and tailored to patient preferences.
This was a study of behavioral therapy-not pharmacologic therapy
Behavioral therapy takes a lot of patient effort, and there are numerous issues with adherence to behavioral therapy in general. [We refer readers to two reviews by our team for a more in-depth discussion of adherence to behavioral therapy for headache/migraine32,33 and methods to improve adherence.] In the case of MS, it appears that MS patients have better adherence to medications than behavioral interventions. In a retrospective review of adherence via electronic health records from 2004 to 2013, most MS patients (82%) had greater than 80% adherence to their MS medications34. By comparison, published research examining MS patients participating in behavioral interventions to control and manage common mental health difficulties show drop-out rates ranging from 25% to 75%.35
Type of therapy-low touch, no therapist and thus no therapeutic alliance
This was a low touch smartphone-based study. In a telephone-based CBT study for MS, while 75% of participants appreciated the convenience of telephone delivery, 46% reported missing face-to-face contact.36 In prior work, the therapeutic alliance has been found to be highly beneficial, also accounting for why education sessions (as opposed to CBT treatment sessions) were also found to be beneficial in improving study outcomes.36 In our study, there was just one in-person enrollment session and we did not have therapists interacting with the subjects. Thus, there was no therapeutic alliance.
Level of individual commitment (smartphone versus in-person sessions)
The level of effort required to enroll in the RELAXaHEAD study is minimal. In a smartphone based single arm open label study assessing the MS TeleCoach to increase physical activity levels to improve fatigue in MS, 75 patients were recruited and 57 (76%) completed the study.37 One of most frequently reported reasons for drop out was lack of motivation.37
In a smartphone-based study of MS patients assessing the feasibility of remote active testing using smartphone and smartwatch technology, there was 70% adherence to active tests (adherence defined as proportion of weeks with at least 3 days of completed testing).38 The likelihood of study discontinuation decreased throughout the year: 50% of the drop outs were within the first 4 months, and 75% were by 7.25 months. 38 While future work should target adherence, adherence in this study is not inferior to those of other smartphone-based pain studies. For example, a prior smartphone-based pain study had only 1 in 7 participants provide data on most days in a 6 months period.39
Finally, people who agree to participate in a smartphone study may not have the same level of commitment as those who agree to an in-person based behavioral study. Another study showed that baseline engagement may be a predictor of completion. In a prior study in which treatment adherence was ~80%, the authors stated that study participants were already “highly activated at baseline” meaning they were already engaged in many self-management strategies at the time of enrollment into the study.36
Daily practice is a significant time commitment
We asked participants to complete daily exercises as opposed to weekly or biweekly sessions, and we were able to track compliance in real-time. Prior behavioral studies may record whether homework was done, but often a) do not report completion of homework assignments or b) are unable to determine if the homework was done as instructed on a scheduled basis or whether answers were recorded right before the behavioral therapy session.40,41
In the CBT for MS pain, fatigue and depression versus MS education study, adherence was higher in the educational arm as opposed to the CBT arm. The authors theorized that those in the CBT arm had more homework demands such as daily symptom monitoring and skills practice, and thus this could have affected treatment adherence.36 Similarly, in our study, participants were asked to do these tasks daily, and thus the greater practice demand may have affected adherence. In our study, those who only had to complete the daily diary entered data on average 59% (53/90) of days whereas those who were expected to complete the daily diary and practice PMR did it on average 39% (35/90) of days.
Of note, prior behavioral studies may record whether homework was done, but often a) do not report completion of homework assignments or b) are unable to determine if the homework was done as instructed on a scheduled basis or whether answers were recorded right before the behavioral therapy session.40,41 We asked participants to complete daily exercises as opposed to weekly or biweekly sessions, and we were able to track compliance in real-time.
Furthermore, RELAXaHEAD focus group participants31 stated that while the RELAXaHEAD app sought to deliver behavioral therapy in a time efficient manner (method would decrease the amount of time spent traveling to and attending in-person treatments), they still had other competing time commitments and had difficulty finding time in the day. Many participants expressed that the PMR therapy was only made a priority on days that their headache attacks were severe, even though the therapy is introduced as a preventative intervention for daily use to reduce the frequency and severity of headache attacks. Thus, the expected level of effort may have been too burdensome for some participants to do on a regular basis.
Strengths
Strengths of the study were that participants were required to meet only once in person for the enrollment session; all follow-up data was collected via phone or email. When patients were recruited into the study, they often expressed that they did not have the time to meet multiple times in-person but were happy to answer phone calls and emails. Further, we were able to recruit an ethnically diverse patient population. (Table 1)
Limitations
Most participants recruited from the study received care at MS Centers in one medical system in the greater New York City region. We did not randomize study subjects based on factors such as migraine disability. Unfortunately, while the baseline average MIDAS scores indicate that both groups were severely disabled, the PMR group’s MIDAS score was still significantly higher than the MUC baseline MIDAS score. In the future, block randomization by MIDAS might help to prevent this between group asymmetry. As discussed above, we had significant difficulty reaching participants over time, and this has become a known issue with longitudinal surveys; research has found that even in large population based longitudinal studies, overall nonresponse and nonresponse due to refusals have increased over time.42 Further assessments of how to have continued engagement in taking survey assessments need to be done.
Future Work
Future work should examine other behavioral modalities for MS related pain. In a single center RCT examining telephone-based CBT to telephoned based education to address three common symptoms in MS, both groups were responders (>50% reduction in 1+ symptoms-fatigue, pain interference or depression severity) and these responses were maintained at both 6 months and 12 months. A single center RCT of mindfulness based cognitive therapy (MBCT), CBT and usual care using videoconferencing technology is already underway.43 Future work should examine whether self-efficacy and locus of control might predict those who might engage more with such behavioral interventions.36 The work should also target improved mHealth engagement. A mixed methods study of 12 patients with MS explored MS specific needs for MS health solutions, perceived barriers to adaptation and motivators for adaptation of mHealth tools for MS. Participants stated desired mHealth features as follows: (1) activity tracking, (2) incentives for completing tasks and objectives, (3) customizable goal setting, (4) optional sociability, and (5) game-like attitude among others.44 Essentially, researchers found similar results to the results of our RELAXaHEAD focus group results with non-MS migraine subjects.31 With better engagement, perhaps using the power of behavioral economics e.g. gamification,45 better follow-up data may be collected to better determine efficacy.
Importantly, future work should capitalize on the role of technology in bringing patients with MS pain together to enable more social support during the process of acquiring behavioral therapy skills. We found in focus groups conducted of our participants that they would like a social platform to engage with others. Other researchers drew similar conclusions as the authors of a mixed methods paper on MS and mHealth solutions said, “Engaging with others with MS was easier for participants with MS because they felt less conscious about their limitations; however, it also served as a reminder of the uncertain progression of the condition. Most participants preferred to avoid discussion of MS and staying away from health-related topics. This aversion should be kept in mind when designing…interventions that include socialization features.”44
5. CONCLUSIONS
There is interest in scalable accessible forms of behavioral therapy to treat migraine and MS related pain in patients with MS and comorbid migraine. Similar to prior studies, a significant minority were willing to practice the PMR at least twice weekly. Follow up was challenging but those reached indicated that they appreciated the PMR and would recommend it to others. Future work should focus on engagement and should more critically look at efficacy.
Supplementary Material
Figure 2:
PMR attrition by week over initial 90-day period
Highlights.
There is significant interest for smartphone based behavioral therapy trials.
Patients will use smartphone-based behavioral therapies in a time limited amount.
Future research should investigate best practices for maintaining engagement.
ACKNOWLEDGEMENTS
The authors would like to thank the MS clinicians in the NYU Langone Multiple Sclerosis Center, especially Drs. Balcer, Krupp, Charvet and Kister. Without pilot funding from the National Multiple Sclerosis Society and the NYU CTSI, this study could not have been possible. The authors would also like to acknowledge the following individuals who helped with patient recruitment: Christopher Kasianko; Saima Usmani; Alexandra Gewirtz; Zoe Weiss; Adama Jalloh
The authors would also like to thank Drs. Richard Lipton and Scott Powers for their ongoing help with the RELAXaHEAD studies, and Ms. Elizabath Pirraglia for her help with the tables. This work was supported by the National Multiple Sclerosis Society; the Doris Duke Charitable Foundation [Fund to Retain Clinical Scientists]; New York University Clinical Translational Science Institute (NYU CTSI); the National Center for Complementary and Integrative Health (NCCIH) [K23 AT009706–01].
Funding: This work was supported by the National Multiple Sclerosis Society; the Doris Duke Charitable Foundation [Fund to Retain Clinical Scientists]; New York University Clinical Translational Science Institute (NYU CTSI) [UL1TR001445]; the National Center for Complementary and Integrative Health (NCCIH) [K23 AT009706-01].
Footnotes
Declaration of Interest form:
Dr. Mia Minen:
The NYU School of Medicine maintains a financial disclosure process to assess individual and institutional interests that may be related to the research.
Dr. Mia Minen, a research study doctor, contributed to developing intellectual property being used in this study that is co-owned by NYU and IRODY. If the research is successful, NYU and IRODY may benefit from the outcome.
The NYU Langone Conflicts of Interest Office (CIMU) has reviewed the researcher’s and NYU’s financial interests and approved a written plan to monitor these interests for the duration of the study.
The NYU School of Medicine Institutional Review Board was informed of the CIMU determination prior to approving the study. If you would like more information, please ask the researchers, the study coordinator, or the CIMU at 212–404-4026.
Kathryn B. Schaubhut reports no conflicts of interest.
Kaitlyn Morio reports no conflicts of interest.
Declarations of Interest: Dr. Mia Minen contributed to developing intellectual property being used in this study that is co-owned by NYU and IRODY. If the research is successful, NYU and IRODY may benefit from the outcome. Kathryn Schaubhut and Kaitlyn Morio report no conflicts of interest.
Clinical trial registration: NCT03183791; ClinicalTrial.gov
Data availability statement: De-identified participant data will be made available upon reasonable request per NYU Langone Health data sharing policy.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Amtmann D, Askew RL, Kim J, et al. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol. 2015;60(1):81–90. doi: 10.1037/rep0000027 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motl RW, Mowry EM, Ehde DM, et al. Wellness and multiple sclerosis: The national MS society establishes a wellness research working group and research priorities. Mult Scler. 2017:1352458516687404. doi: 10.1177/1352458516687404 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet Neurology. 2017;16(11):877–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kister I, Caminero AB, Monteith TS, et al. Migraine is comorbid with multiple sclerosis and associated with a more symptomatic MS course. J Headache Pain. 2010;11(5):417–425. doi: 10.1007/s10194-010-0237-9 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell J, Penzien D, Wall E. Evidence-based Guidelines for Migraine Headache: Behavioral and Physical Treatments. 2000.
- 6.Gray RN, Goslin RE, McCrory DC, Eberlein K, Tulsky J, Hasselblad V. Drug treatments for the prevention of migraine headache. Rockville (MD): Center for Clinical Health Policy Research, Duke University; 1999. https://www.ncbi.nlm.nih.gov/books/NBK45457/. [PubMed] [Google Scholar]
- 7.Gustafson R Treating insomnia with a self-administered muscle relaxation training program: A follow-up. Psychol Rep. 1992;70(1):124–126. doi: 10.2466/pr0.1992.70.1.124 [doi]. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Kang J, Wang P, Zeng H. Self-relaxation training can improve sleep quality and cognitive functions in the older: A one-year randomized controlled trial. J Clin Nurs. 2013;22(9–10):1270–1280. doi: 10.1111/jocn.12096 [doi]. [DOI] [PubMed] [Google Scholar]
- 9.Bertisch SM, Wells RE, Smith MT, McCarthy EP. Use of relaxation techniques and complementary and alternative medicine by american adults with insomnia symptoms: Results from a national survey. J Clin Sleep Med. 2012;8(6):681–691. doi: 10.5664/jcsm.2264 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells RE, Bertisch SM, Buettner C, Phillips RS, McCarthy EP. Complementary and alternative medicine use among adults with migraines/severe headaches. Headache. 2011;51(7):1087–1097. doi: 10.1111/j.1526-4610.2011.01917.x [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst MM, O’Brien HL, Powers SW. Cognitive-behavioral therapy: How medical providers can increase patient and family openness and access to evidence-based multimodal therapy for pediatric migraine. Headache. 2015;55(10):1382–1396. doi: 10.1111/head.12605 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minen M, Shome A, Halpern A, et al. A migraine management training program for primary care providers: An overview of a survey and pilot study findings, lessons learned, and considerations for further research. Headache. 2016;56(4):725–740. doi: 10.1111/head.12803 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrasik F Behavioral treatment of headaches: Extending the reach. Neurol Sci. 2012;33 Suppl 1:S127–30. doi: 10.1007/s10072-012-1073-2 [doi]. [DOI] [PubMed] [Google Scholar]
- 14.Cooper CL, Hind D, Parry GD, et al. Computerised cognitive behavioural therapy for the treatment of depression in people with multiple sclerosis: External pilot trial. Trials. 2011;12:259–6215–12–259. doi: 10.1186/1745-6215-12-259 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marziniak M, Brichetto G, Feys P, Meyding-Lamade U, Vernon K, Meuth SG. The use of digital and remote communication technologies as a tool for multiple sclerosis management: Narrative review. JMIR Rehabil Assist Technol. 2018;5(1):e5. doi: 10.2196/rehab.7805 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pau M, Coghe G, Corona F, Leban B, Marrosu MG, Cocco E. Effectiveness and limitations of unsupervised home-based balance rehabilitation with nintendo wii in people with multiple sclerosis. Biomed Res Int. 2015;2015:916478. doi: 10.1155/2015/916478 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minen MT, Jalloh A, Ortega E, Powers SW, Sevick MA, Lipton RB. User design and experience preferences in a novel smartphone application for migraine management: A think aloud study of the RELAXaHEAD application. Pain Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minen MT, Adhikari S, Seng EK, et al. Smartphone-based migraine behavioral therapy: A single-arm study with assessment of mental health predictors. Nature Digital Medicine. 2019;46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.software&services. Epic Web site. https://www.epic.com/software#PatientEngagement Accessed 6/15, 2020.
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.irody.com Web site. Accessed March 21, 2018.
- 22.Haut SR, Lipton RB, Cornes S, et al. Behavioral interventions as a treatment for epilepsy: A multicenter randomized controlled trial. Neurology. 2018;90(11):e963–e970. doi: 10.1212/WNL.0000000000005109 [doi]. [DOI] [PubMed] [Google Scholar]
- 23.Usmani S, Balcer L, Galetta S, Minen MT. Feasibility of smartphone-delivered progressive muscle relaxation (PMR) in persistent post-traumatic headache (PPTH) patients. Journal of Neurotrauma. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the migraine disability assessment (MIDAS) questionnaire to assess headache-related disability. Neurology. 2001;56(6 Suppl 1):S20–8. [DOI] [PubMed] [Google Scholar]
- 25.Hadjimichael O, Kerns R, Rizzo M, Cutter G, Vollmer T. Persistent pain and uncomfortable sensations in persons with multiple sclerosis. Pain. 2007(127):35. [DOI] [PubMed] [Google Scholar]
- 26.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45(5):626–629. doi: 10.1016/j.jpsychires.2010.10.008 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altman DG, Bland JM. How to randomise. BMJ. 1999;319(7211):703–704. doi: 10.1136/bmj.319.7211.703 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frahm N, Hecker M, Zettl UK. Multi-drug use among patients with multiple sclerosis: A cross-sectional study of associations to clinicodemographic factors. Sci Rep. 2019;9(1):3743–019–40283–5. doi: 10.1038/s41598-019-40283-5 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subei AM, Ontaneda D. Risk mitigation strategies for adverse reactions associated with the disease-modifying drugs in multiple sclerosis. CNS Drugs. 2015;29(9):759–771. doi: 10.1007/s40263-015-0277-4 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams D, Brick JM. Trends in U.S. face-to-face household survey nonresponse and level of effort. Journal of Survey Statistics and Methodology. 2017;6(2). [Google Scholar]
- 31.Minen M, Morio K, Schaubhut K, Powers S, Lipton R, Seng E. Focus group findings on the migraine patient experience during research studies and ideas for future investigations. Cephalalgia. December 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gewirtz A, Minen M. Adherence to behavioral therapy for migraine: Knowledge to date, mechanisms for assessing adherence, and methods for improving adherence. Curr Pain Headache Rep. 2019;23(1):3–019–0739–3. doi: 10.1007/s11916-019-0739-3 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuzawa Y, Lee YSC, Fraser F, et al. Barriers to behavioral treatment adherence for headache: An examination of attitudes, beliefs, and psychiatric factors. Headache. 2019;59(1):19–31. doi: 10.1111/head.13429 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hao J, Pitcavage J, Jones JB, Hoegerl C, Graham J. Measuring adherence and outcomes in the treatment of patients with multiple sclerosis. J Am Osteopath Assoc. 2017;117(12):737–747. doi: 10.7556/jaoa.2017.145 [doi]. [DOI] [PubMed] [Google Scholar]
- 35.Heesen C, Bruce J, Gearing R, et al. Adherence to behavioural interventions in multiple sclerosis: Follow-up meeting report (AD@MS-2). Mult Scler J Exp Transl Clin. 2015;1:2055217315585333-Dec. doi: 10.1177/2055217315585333 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehde DM, Elzea JL, Verrall AM, Gibbons LE, Smith AE, Amtmann D. Efficacy of a telephone-delivered self-management intervention for persons with multiple sclerosis: A randomized controlled trial with a one-year follow-up. Arch Phys Med Rehabil. 2015;96(11):1945–58.e2. doi: 10.1016/j.apmr.2015.07.015 [doi]. [DOI] [PubMed] [Google Scholar]
- 37.D’hooghe M, Van Gassen G, Kos D, et al. Improving fatigue in multiple sclerosis by smartphone-supported energy management: The MS TeleCoach feasibility study. Mult Scler Relat Disord. 2018;22:90–96. doi: S2211–0348(18)30115–9 [pii]. [DOI] [PubMed] [Google Scholar]
- 38.Bove R, White CC, Giovannoni G, et al. Evaluating more naturalistic outcome measures: A 1-year smartphone study in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2015;2(6):e162. doi: 10.1212/NXI.0000000000000162 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Druce KL, McBeth J, van der Veer SN, et al. Recruitment and ongoing engagement in a UK smartphone study examining the association between weather and pain: Cohort study. JMIR Mhealth Uhealth. 2017;5(11):e168. doi: 10.2196/mhealth.8162 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haut SR, Lipton RB, Cornes S, et al. Behavioral interventions as a treatment for epilepsy: A multicenter randomized controlled trial. Neurology. 2018;90(11):e963–e970. doi: 10.1212/WNL.0000000000005109 [doi]. [DOI] [PubMed] [Google Scholar]
- 41.Powers SW, Kashikar-Zuck SM, Allen JR, et al. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: A randomized clinical trial. JAMA. 2013;310(24):2622–2630. doi: 10.1001/jama.2013.282533 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams D, Brick JM. Trends in face-to-face household survey nonresponse and level of effort. Journal of Survey Statistics and Methodology. 2018;6:186–211. [Google Scholar]
- 43.Ehde DM, Alschuler KN, Day MA, et al. Mindfulness-based cognitive therapy and cognitive behavioral therapy for chronic pain in multiple sclerosis: A randomized controlled trial protocol. Trials. 2019;20(1):774–019–3761–1. doi: 10.1186/s13063-019-3761-1 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giunti G, Kool J, Rivera Romero O, Dorronzoro Zubiete E. Exploring the specific needs of persons with multiple sclerosis for mHealth solutions for physical activity: Mixed-methods study. JMIR Mhealth Uhealth. 2018;6(2):e37. doi: 10.2196/mhealth.8996 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel MS, Chang S, Volpp KG. Improving health care by gamifying it. Harvard Business Review. 2019:6/15/2020. [Google Scholar]
- 46.Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808. doi: 10.1177/0333102413485658 [doi]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.