Abstract
Psychotic disorders are highly debilitating and constitute a major public health burden. Identifying markers of psychosis risk and resilience is a necessary step toward understanding etiology and informing prevention and treatment efforts in Clinical High Risk (CHR) for psychosis individuals. In this context, it is important to consider that neural risk markers have been particularly useful in identifying mechanistic determinants along with predicting clinical outcomes. Notably, despite a growing body of supportive literature outside of the CHR area and the promise of recent findings identifying potential neural markers, the current work on resilience markers has received little attention. The present review provides a brief overview of brain-based risk markers with a focus on predicting symptom course. Next, the review turns to protective markers, examining research from non-psychiatric and schizophrenia fields to build an understanding of framing, priorities, and potential, applying these ideas to contextualizing a small but informative body of resiliency-relevant CHR research. Four domains (neurocognition, emotion regulation, allostatic load, and sensory and sensorimotor function) were identified and discussed in terms of behavioral and neural markers. Taken together, the literature suggests significant predictive value for brain-based markers for individuals at CHR for psychosis, and the limited but compelling resiliency work highlights the critical importance of expanding this promising area of inquiry.
Keywords: resilience, biomarker, clinical high risk for psychosis, neuroimaging, magnetic resonance imaging, schizophrenia
1. Introduction
Psychotic disorders comprise a massive public health cost (1), and are highly debilitating, characterized by a chronic course and substantial diversity in clinical manifestations (2, 3). A sizeable portion of individuals that go on to be diagnosed with a psychotic disorder experience a “prodromal” phase prior to illness onset (4-6). Research on youth at Clinical High Risk (CHR) for developing psychosis thus aims to identify individuals at high risk of transitioning. CHR individuals can present with attenuated psychotic symptoms, short limited psychotic symptoms, or genetic risk accompanied by functional or cognitive decline (5, 7). The approach is valuable in informing and implementing prevention and intervention treatments at the individual and public health level, and also provides insight into the pathogenesis of the illness (allowing insight prior to the onset of many confounding factors associated with illness onset such as antipsychotic use and lifestyle changes).
Identifying markers that aid in the identification, prediction of clinical course, and treatment efficacy of CHR individuals is critical from a prevention and intervention standpoint (8, 9). Despite the prominence of brain research in CHR samples (10), brain-based risk and protective markers have not been recently reviewed. In addition to denoting risk and resiliency, neural markers have the strong potential to aid in identifying mechanistic determinants and inform conceptual understanding (11). Investigating protective neural markers occurring with simultaneous exposure to heightened risk factors for illness could help identify those with the greatest need for care and provide clues for potential targets for treatment and preventive efforts (12-14).
We offer an overview of brain-based risk and protective markers in the CHR literature to give perspective on where the field currently stands along with future directions. Possible resiliency factors will receive the most discussion, as the research has thus far been very limited in this regard. The first part of the review will center around briefly summarizing recent reviews and discussion surrounding CHR risk markers. The second part of the review will discuss resiliency markers, with special attention to cognitive, behavioral, and biological markers related to brain abnormalities. Taken together, the review aims to critically discuss neurological neural marker work in CHR populations and offer synthesis and future directions on understanding resiliency and protective factors.
2. Risk markers
Relative to protective factors, neural markers of risk have been studied much more extensively (10). Oftentimes, risk markers have been identified through comparisons including non-clinical, non-help seeking comparison groups (15). For example, a voxel-based meta-analysis of CHR neural markers found reductions in CHR gray matter volume compared to controls in right superior temporal gyrus, left precuneus, left medial frontal gyrus, right middle frontal gyrus, bilateral parahippocampal/hippocampal regions and bilateral anterior cingulate (16). Another quantitative review using activation likelihood estimation (ALE) found that compared to controls, CHR showed dysfunction in right inferior parietal lobule, left medial frontal gyrus, left superior temporal gyrus, and superior frontal gyrus (17). These reviews have lent the field substantial insight and clarified key candidate pathogenic mechanisms; however, there are limitations to establishing risk markers through comparisons with control groups that are often non-psychiatric and non-help seeking. Given the exceedingly high incidence of co-morbid diagnoses in CHR groups (4, 5, 18), it is unclear whether neural markers assessed in this regard are capturing general risk for co-morbid psychopathology, rather than risk for psychosis specifically.
Alternatively, recent reviews have focused in on neural markers predicting transition to a psychotic disorder (19-22). One meta-analysis found decreased prefrontal, cingulate, insular and cerebellar gray matter volume in those that transitioned, along with reduced activation in prefrontal cortex, reduced neuronal density, and increased membrane turnover in frontal and cingulate cortex (20). A qualitative review, in turn, identified progressive gray matter loss, altered white matter integrity, and anomalous functional connectivity as solid predictors of transition (19). To build on the literature on transition outcomes and neural markers, which has been essential to our understanding of CHR manifestations, a valuable future direction will be to expand from focusing on transition to mapping neural markers to symptomatology independently of transition outcomes; this focus has been less prevalent in the literature. Increasingly tracking neural markers, symptomatology and functional outcomes regardless of transition status will be beneficial in determining degrees of risk and vulnerability, possibly aiding in parsing out heterogeneity in psychosis symptom presentation. Table 1/Figure 1 provide an overview of literature tracking neural markers and attenuated symptomatology.
Table 1.
Sampling of clinical High Risk (CHR) for psychosis investigations denoting risk markers and relationships with clinical outcomes.
| Imaging modality |
Utility | Neural marker predicting illness course in CHR |
|---|---|---|
| A) Structural MRI (gray matter) | Measures gray matter features, indexing factors such as neural structure, development, environmental and genetic influences | Decreased prefrontal, cingulate, insular and cerebellar gray matter volume (20) |
| Baseline cortical thickness in right lateral and medial temporal cortex and left insular cortex (125); insular volume (126) | ||
| Inversion of left ventral posterior hippocampus (96) and reduced parahippocampal volume (127) | ||
| Baseline pituitary volumes (128) | ||
| Gray matter reduction in right superior frontal, middle frontal, and medial orbitofrontal (129) | ||
| Reduced baseline volume in frontal, temporal, posterior, and cingulate regions (130) | ||
| B) Structural MRI (white matter) | Measures white matter features, indexing factors such as myelination, which can inform understanding of efficiency of signal transmission among regions and neural networks | Increases in thalamo-motor tract Fractional Anisotropy (FA) (131) |
| Reductions in frontal FA (132) | ||
| Increases in inferior fronto-occipital fasciculus, anterior thalamic radiation, superior longitudinal fasciculus, corticospinal tract FA (133) | ||
| C) Functional MRI (resting state) | Estimates how regions within a network relate intrinsically/on a “trait” level (without the addition of a specific task or behavior) | Dissimilar functional network organization (134) |
| Aberrant structural covariance in salience, executive control, auditory, and motor networks (135) | ||
| Reduced activation in prefrontal cortex (20) | ||
| Reduced insula connectivity (136) | ||
| Altered midbrain-prefrontal connectivity (137) | ||
| Altered cingulate topological features (138) | ||
| Thalamic dysconnectivity (139) | ||
| Altered connectivity in dorsal anterior cingulate cortex, midcingulate cortex, supplementary motor area, and mesial superior frontal gyrus (140) | ||
| Cerebello-thalamo-cortical hyperconnectivity (141) | ||
| D) Functional MRI (task-based) | Estimates brain activity co-occurring with certain behaviors and cognition | Increased activation in bilateral prefrontal cortex, brainstem (midbrain/basilar pons), left hippocampus, and greater midbrain-prefrontal cortex connectivity during verbal fluency task (137) |
| Greater activation in superior temporal gyrus, caudate, and left inferior frontal gyrus during language processing task (142) | ||
| Less activation in prefrontal cortex, precuneus and temporal lobes during theory of mind task (143) | ||
| E) Magnetic Resonance Spectroscopy (MRS) | Measures metabolite concentration changes indexing alterations in neurochemical mechanisms | Reduced neuronal density, and increased membrane turnover in frontal and cingulate cortex (20) |
| Lower medial prefrontal GABA levels (144) | ||
| Higher striatal glutamate levels (145, 146) | ||
| Lower thalamic glutamate levels (147) | ||
| F) Arterial Spin Labeling (ASL) | Indexes cerebral blood flow, providing regional and temporal specificity to neural markers | Increased hippocampal resting Cerebral Blood Flow (rCBF) (148) |
| Increased pallidum rCBF (149) | ||
| Increased striatum rCBF (150) |
Figure 1.
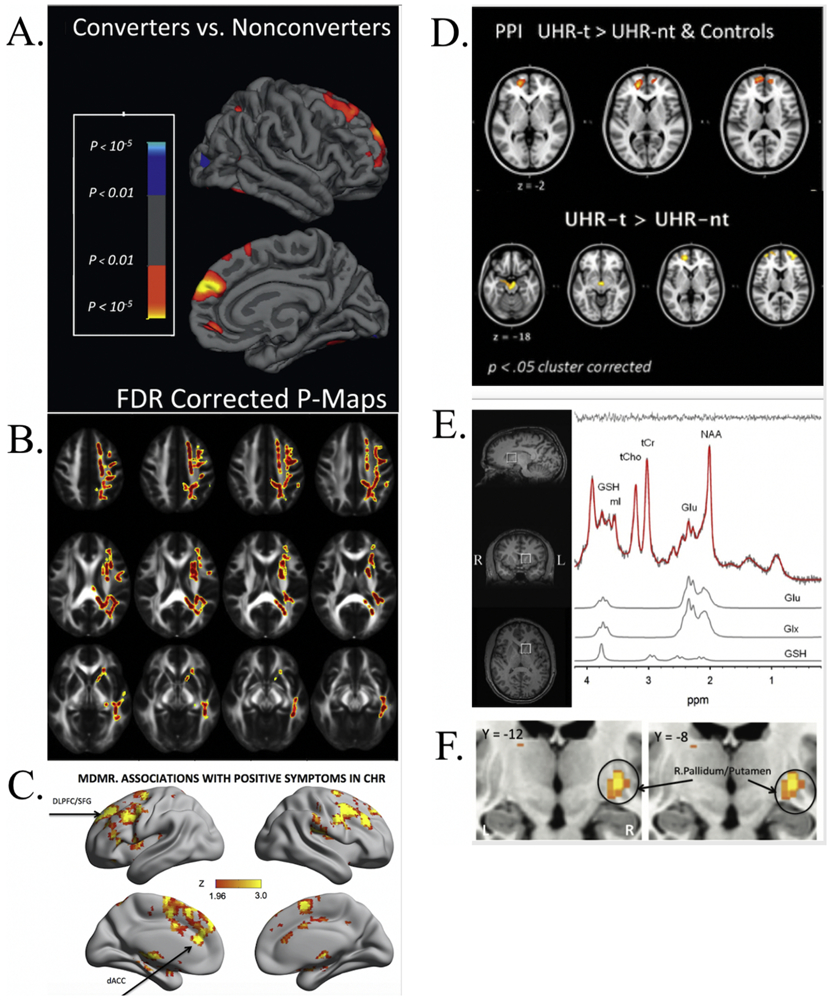
Select studies tracking neural risk markers and attenuated symptomatology per imaging modality according to Table 1. A) Rates of change in cortical thickness between converting and non-converting CHR in right superior frontal, middle frontal, and medial orbitofrontal regions (warmer colors denote cortical thinning in converters), False Discovery Rate (FDR) corrected (129); B) Univariate voxelwise analysis denoting longitudinal associations between fractional anisotropy (FA) and negative symptoms within CHR (warmer colors denote a positive association between negative symptoms and FA) (133); C) Regions whose intrinsic functional connectivity associated with positive symptoms in CHR individuals (warm colors indicate strength of association between pattern of whole-brain connections and positive symptom severity), including dorsal anterior cingulate cortex, midcingulate cortex, supplementary motor area, and mesial superior frontal gyrus, Gaussian random field-corrected for multiple comparisons (140); D) Statistical Parametric Maps (SPMs) of psychophysiological interaction (PPI) analysis with a midbrain seed region depicting prefrontal regions with greater activation during verbal fluency in CHR that transitioned versus those that did not transition and control participants (top), and SPMs showing regions of increased activation during verbal fluency in CHR that transitioned versus those that did not (bottom), applied cluster correction (137); E) Proton-Magnetic Resonance Spectroscopy (1H-MRS) spectrum in the left striatum, including voxel location (left) and representative spectrum with metabolite fits for glutamate, glutamate+glutamine, and glutathione (right); within CHR individuals, striatal glutathione (marker of oxidative stress in glutamatergic system) positively correlated with grandiosity (146); F) Basal ganglia regions with greater resting cerebral blood flow (rCBF) being associated with increased positive symptoms, Family Wise Error (FWE) corrected (149).
3. Contextualizing protective factors/resiliency markers in CHR
Among those exposed to illness risk factors, potential protective or resiliency factors can be characterized as biomarkers that relate to the development of healthier outcomes or illness remission (12-14). Within CHR research, identifying protective factors is a nascent complex and multifaceted undertaking. A recent systematic review of 20 years of CHR literature identified risk and protective factors using empirical criteria (36). Only higher attenuated positive symptoms, lower global functioning, and higher negative symptoms showed evidence for association with transition to psychosis (36). The review highlights the highly heterogeneous nature of CHR research, and calls for expanding the focus of risk and resilience beyond predicting transition to a psychotic disorder. This is especially relevant given that lifetime presence of CHR criteria maintains vulnerability across a variety of domains, regardless of conversion status (4, 5, 18). There is much to be gained from conceptualizing resilience outside of dichotomizing converters versus non-converters (which has the setback of treating non-converters as “error variance”, failing to account for psychosis risk). A growing field focusing on CHR persistence (symptoms that remain constant/do not increase in severity) and remission allows us to undertake a much-needed dimensional perspective that has potential to shed light on protective markers for psychosis spectrum symptomatology. Recent investigations undertaking such approach have yielded insights into types of biomarkers. Given the relative paucity of CHR research on protective markers, this section will first borrow from a strong body of research from non-psychiatric populations to highlight viable resiliency markers. In addition, the broader schizophrenia spectrum disorders literature will be incorporated, as some resiliency work has already been undertaken within the psychotic disorder literature, thus facilitating more direct comparisons between psychosis spectrum disorders and CHR individuals. Then, CHR investigations that have yielded insight into resiliency markers will be discussed. To make the best use of this smaller body of resilience research, the section will incorporate discussion of behavioral resiliency markers as well, with the primary focus remaining on brain-based resiliency research.
3.a.ia. Demographic and behavioral resilience markers.
Investigations of resilience in the face of exposure to adversity have identified demographic factors such as gender, age, race, and education, along with environmental factors including trauma exposure/life stressors, income change, social support, and parenting resources, and individual differences such as cognitive function and perceived efficacy (13, 23, 24). Findings have also characterized resilience as a complex and perhaps elusive construct, which can be achieved through a milieu of pathways (12, 14).
Within schizophrenia, general efforts to define resilience have spanned use of self-report resiliency scales, along with outcomes relating to better illness course and functioning (25, 26). For example, studies have characterized self-esteem, positive self-appraisals, and positive mood experiences as protective factors (27-29). Relationships between internalized stigma and global functioning have been found to be mediated by self-reported resilience (30). Other studies have focused on internal and external protective factors impacting duration of untreated psychosis and symptomatology, including psychological, social, and community resources, as well as problem solving, behavioral strategies (31), and personality characteristics (32). Research explicitly focusing on operationalizing resilience in CHR populations has been relatively sparse, though studies have reported favorable symptom profiles for those that self-report higher resilience, along with lower self-reported resilience in CHR converters (33, 34). Higher psychosocial functioning at baseline was found to predict remission (6), along with lower attenuated positive symptoms, and use of antipsychotics and anxiolytics (35).
As noted earlier, non-psychiatric and schizophrenia studies were used to classify main domains that impact or could be impacted by resilience. First, general imaging findings related to resilience in CHR will be discussed. Then, developed domains, including cognition, emotion regulation, allostatic load, and sensory/sensorimotor will be discussed in behavioral terms to frame ensuing discussion of corresponding neural markers (Table 2/Figure 2).
Table 2.
Clinical High Risk (CHR) for psychosis investigations of putative neural protective/resilience markers
| Domain | Finding |
|---|---|
| A) Overall/General | Smaller brain surface area (37). |
| Larger cortical surface area within the left hemisphere (38). | |
| B) Neurocognition and executive function | Greater volume, thickness, and surface area among frontal regions, including cingulate (38). |
| C) Emotion regulation | P300 amplitude differences not found (53). Increased amygdala and prefrontal activation, as well as stronger negative amygdala-prefrontal functional connectivity during emotional faces task (54). |
| D) Allostatic load | Increase in activation in anterior cingulate and right parahippocampal gyrus (77). |
| E) Sensory and sensorimotor | Decreases in left hippocampal rCBF (98). Increase in white matter integrity of the corpus callosum (116). |
| Higher between-network connectivity (among language, dorsal attention, cerebellar, sensorimotor, and salience networks) and more typical modular connectome organization (115). | |
| Greater volumes in temporal, parietal cortex, corpus callosum regions (38). | |
| Greater auditory mismatch negativity amplitude (118). | |
| Greater P300 novel amplitude (119). |
Figure 2.
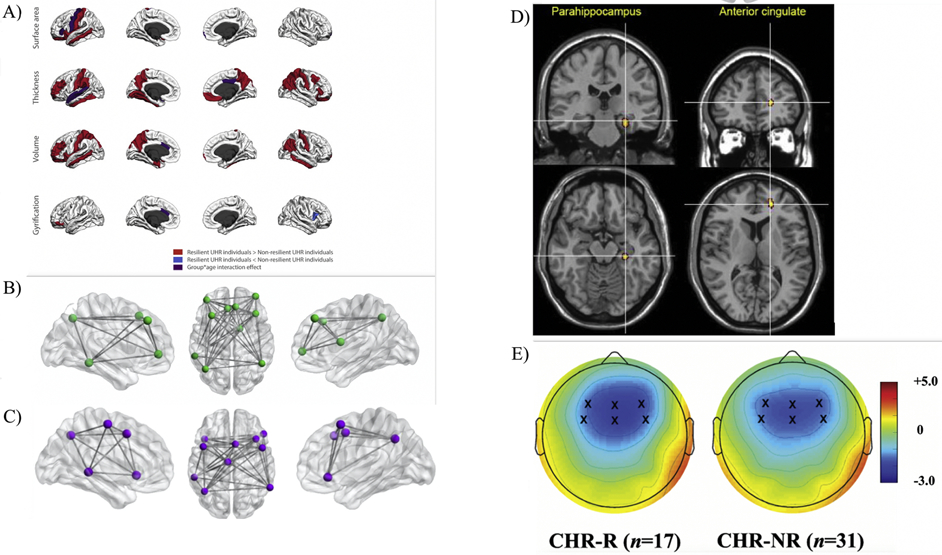
Select studies corresponding to Table 2, depicting neural resiliency markers and attenuated symptomatology per domain. A) Cortical morphology differences between CHR showing resilient outcomes with regards to symptomatology. Red depicts regions in which resilient CHR had higher values, blue depicts regions in which non-resilient CHR had higher values, and purple depicts regions that showed a group by age interaction effect (38); B) Regions constituting the Central Executive Network (CEN) (50); C) Regions constituting the Emotion Regulation Network (ERN) (50); D) CHR whose symptoms improved exhibited a longitudinal increase in activation (regions depicted in warm colors) in anterior cingulate and right parahippocampal gyrus during a working memory task, Family Wise Error (FWE) corrected (77); E) Two-dimensional topographic maps of mismatch negativity event related potential component. Frontocentral electrodes are marked with an x within the maps (118).
3.a.ib. General CHR imaging literature touching on resilience.
There are several noteworthy findings in CHR samples that include neural markers and touch on resilience, but do not fit into the above referenced four domains identified by the outside literature survey. For example, cortical abnormalities have served as candidates for CHR protective factors (37). One study found smaller brain surface area in CHR with persistent/deteriorating symptomatology relative to CHR with partially/fully remitted symptomatology (37). Larger cortical surface area within the left hemisphere has also been observed in CHR individuals that go on to exhibit resilient outcomes (38); the observed specificity to surface area poses number of cellular columns as a putative protective factor (39).
3. b. Neurocognition resilience markers.
Intact neurocognition and executive function (EF; partially due to EF associations with greater self-control/regulation, which constitute robust protective factors) have specifically been associated positively with dispositional resilience in vulnerable populations (40, 41). A phenomenon deemed “skin-deep resilience” has been identified, whereby ostensibly resilient outcomes in the face of adversity relate to self-control/executive function , (42) while co-occurring with ensuing risk-factors for long-term adverse health outcomes—suggesting that resilience can take its toll on physical health (43-45). Non-psychiatric studies highlight the critical importance of both considering executive function as a protective factor, and measuring both risk and resilience in tandem, as they can interrelate in meaningful ways. Studies have found relations between increased self-reported resilience and decreased cognitive impairment across populations including individuals with schizophrenia and non-psychiatric groups (46). Within psychotic disorder populations, efforts to identify brain-based biotypes independent of clinical features have shown that intact executive function and overall cognitive performance co-occurs with less severe manifestations of clinical psychosis (2). Studies clustering psychotic disorder groups according to clinical features have found consistent results with regards to cognitive function and symptom severity, highlighting the importance of cognitive function to conceptualizing protective factors in psychosis spectrum populations (3).
With regards to CHR populations, promising evidence has pointed to better cognitive performance at baseline among CHR individuals whose symptoms went on to remit (47); semantic fluency significantly improved in remitters over two years, while declining in non-remitters (47). Another investigation found groups with symptoms that remitted or remained constant improved significantly over time on social cognition (theory of mind and social perception) (48). Given promising preliminary evidence, future studies could benefit from considering cognitive performance over time as a protective factor within CHR groups, especially in executive function domains.
3.b.ia. Neural biomarkers related to neurocognition and executive function.
Non-psychiatric neuroscience studies aid in illuminating relations between executive neural networks and resilience. For example, a recent investigation observed a relationship between higher neighborhood murder rate and greater cardiometabolic risk. However, in youth with greater central executive network (CEN) resting state connectivity, the relation between greater neighborhood murder rate and increased cardiometabolic risk was not present; the moderation suggests CEN connectivity could serve as a protective biomarker for at-risk populations (49). The CEN facilitates self-control and inhibition as well as relating to reappraisal and suppression (50).
In terms of neural markers related to executive function and neurocognition among schizophrenia studies, reduced resting state connectivity in prefrontal networks was found to confer greater risk for the illness (51). With regards to structural imaging and prefrontal cortical folding, one investigation found that specific cortical folding patterns within the orbitofrontal cortex could serve as protective factors given their decreased incidence in first episode psychosis subjects relative to control participants (52). Among CHR investigations, high-risk individuals classified as resilient (due to symptom improvement over a 6 year period) had larger baseline frontal volumes, along with greater cortical surface area and thickness in frontal regions (38). With regards to event-related potentials, however, P300 amplitude, a late cognitive component indexing working memory updates of change and attention, was not found to predict symptom remission in CHR (53). Especially given current CHR evidence, further studies would be valuable in determining the possible protective role of neural executive neurocognitive function and structure in CHR individuals.
3.c. Emotion regulation resilience markers.
Non-psychiatric studies have long highlighted emotion regulation—the ability to efficiently modulate emotions, and effectively process negative/disruptive emotions—as a pathway to resilience in the face of adversity (12, 54, 55). Schizophrenia investigations also have found effective emotion regulation to relate to reduced stress sensitivity (56), less severe hallucinations (57) and persecutory delusions (58), as well as to better psychosocial and overall clinical outcomes (59, 60). Nonetheless, a recent meta-analysis of individuals with psychotic disorders found that while maladaptive emotion regulation strategies were associated with positive symptom severity, this was not the case with use of adaptive emotion regulation strategies (61). Thus, it is possible that adaptive emotion regulation serves as a protective factor prior to illness onset, rather than after illness onset.
Although the literature on CHR and resilient/protective outcomes stemming from emotion regulation is quite limited, there is some promising evidence from non-clinical psychosis investigations. A study found that youth that used reappraisal reported less distress from psychotic experiences (62). The study also found reappraisal to mediate the relationship between personality characteristics and psychotic experiences (62). More peripherally, cognitive therapy, which has a strong emphasis on reappraisal skills (63), has been shown to reduce both positive and negative symptomatology in CHR and first-episode psychosis populations (64-66). Though it seems that maladaptive emotion regulation is likely linked to symptomatology in CHR (60, 67-69), it is unclear whether at this stage implementing adaptive emotion regulation interventions and treatment goals would definitively serve as a protective factor; this is a valuable future direction.
3.c.ia. Neural biomarkers related to emotion regulation.
In non-psychiatric studies, modulating amygdala reactivity and recruiting prefrontal control regions has been associated with effective emotion regulation among youth exposed to risk factors such as adverse childhood experiences and maltreatment (54, 70-72); in these individuals, reduced amygdala activation and recruitment of frontal regions during regulation of negative affect has been interpreted as efficient/adaptive emotion regulation (70). Similarly, schizophrenia studies indicate resilience to self-stigma related to functional neural correlates of emotion regulation (54, 73). Reduced symptomatology has also been linked to normalization of prefrontal-limbic activation due to cognitive therapy involving reappraisal training (74, 75).
Within CHR populations, CHR individuals that remitted performed with greater accuracy during an emotional faces task at baseline (76). During the emotional faces task, CHR that remitted, similar to control participants, showed increased amygdala and prefrontal activation, as well as stronger negative amygdala-prefrontal functional connectivity (76). Another investigation tracked CHR symptomatology longitudinally, finding that clinical symptomatologic and functional improvement related to a longitudinal increase in activation in the anterior cingulate and right parahippocampal gyrus (54); change in anterior cingulate response was directly correlated with improvement in functioning (77). Future investigations will be needed in order to understand the extent toward which emotion regulation could relate to these neural presentations.
3.d. Allostatic load resilience markers.
Allostatic load refers to consequences of chronic exposure to increasing/fluctuating neural/neuroendocrine responses due to chronic stressful environmental challenges (78). Within non-psychiatric populations, youth under conditions of high cumulative socioeconomic status (SES)-related risk who displayed resilient outcomes of high psychosocial competence, also displayed indicators of presumed high allostatic load compared to youth at low SES-related risk (42). These results are consistent with another investigation of youth from disadvantaged neighborhoods (79).
In regards to allostatic load and HPA axis function, elevated diurnal cortisol due to chronic stress exposure in individuals at-risk or diagnosed with a psychotic disorder has been associated with a loss of ability to respond adaptively to acute stress, resulting in a blunted cortisol response to acute psychosocial stress (80-82). On the other hand, in healthy or at-risk individuals exhibiting a resilience biomarker, “normal” diurnal cortisol is associated with a preserved capacity for response to acute stress (80-82). Consistent with this notion, an investigation comparing cortisol levels in CHR remission and psychotic transition groups found subjects in the remission group had lower baseline cortisol relative to CHR that converted (83). Given that lower stress exposure has been directly associated with less severe positive symptoms (84), allostatic load remains an essential consideration to models of resilience and protective factors.
3.d.ia. Neural biomarkers related to allostatic load.
In addition to being intricately associated with risk and resilience to psychiatric disorders (85, 86), allostatic load is liable to impact widespread neural systems subserving stress sensitivity (87). Protective or resiliency factors relating to allostatic load could involve capitalizing on developmental brain plasticity. Regions with greater plasticity, or with protracted developmental trajectories, which are sensitive to stress exposure and involved in the stress response could be particularly relevant targets (e.g. hippocampal/medial temporal regions, along with certain prefrontal regions) (88-93). In psychotic disorder populations, findings have been limited, though indicators of greater allostatic load have been linked with reduced overall cortical thickness and white matter integrity (94, 95).
Among CHR studies, there is some intriguing preliminary evidence pointing toward future targets for resiliency. For example, compared with CHR individuals whose symptomatology improved or stayed the same, our group found significantly greater hippocampal shape inversion in CHR individuals with deteriorating symptomatology, along with a trend level difference in CHR individuals who showed increasingly impaired tolerance to normal stress at follow up (96). Another longitudinal investigation found that CHR that remitted did not exhibit decline in hippocampal CA1 volume over time (97). A study comparing groups based on changes in symptomatology over time found that compared to CHR with partially/fully remitted symptoms, 12- to17-year-olds at CHR with persistent/increased symptoms showed smaller surface area in rostral anterior cingulate, lateral and medial prefrontal regions, and parahippocampal gyrus. Longitudinal decreases in hippocampal rCBF have also been observed in CHR remitters (98). Future investigations examining relations between clinical symptomatology, impaired stress tolerance, and allostatic load will aid understanding of protective factors.
3.d. Sensory, motor, sensorimotor function resilience markers.
Aberrant sensory integration and motor functioning serve as indices of vulnerability across psychiatric conditions (99, 100). Neurological soft signs, which index facets of aberrant sensory and motor processing (101), are particularly robustly observed in psychosis spectrum populations (99, 101-104). Within psychotic disorder individuals, a meta-analytic synthesis suggests symptom remission and functional outcomes relate to reductions in sensorimotor dysfunction (102, 105), suggesting intact sensorimotor function could serve as a protective factor in psychotic disorder individuals.
CHR investigations exploring protective factors surrounding sensory and motor functioning have been particularly scarce. One investigation did not observe differences in motor or perceptual disturbances between CHR remission, persistence and progression groups (106). While indexes of sensorimotor function such as neurological soft signs have been found to relate to symptomatology in CHR (101), these have not been centrally explored in the context of remission or protective factors. Notably, a study clustered CHR individuals into motor performance profiles (including dyskinesias, psychomotor slowing, and neurological soft signs) (107). The cluster that was characterized by healthy motor performance exhibited less impaired cognitive function and negative symptomatology compared to clusters with impaired motor performance (107). Given the promising preliminary evidence and the key role of motor abnormalities in the pathogenesis of psychotic disorders, exploring sensory and motor function in the context of CHR persistence/remission and resilience will be a valuable future direction.
3.d.ia. Neural biomarkers related to sensory, motor, and sensorimotor function.
Sensory and motor symptoms have long been conceptualized as transdiagnostic markers of neural vulnerability (108-111). However, research explicitly aiming to identify resilience markers within the sensory/sensorimotor domain has been sparse. One investigation observed altered left hemisphere occipitotemporal connectivity in patients with childhood-onset schizophrenia and unaffected siblings. For unaffected siblings, the aberrant connectivity had normalized by mid-adolescence, indicating a possible sensory processing resilience endophenotype (112). With regards to sensory processing, neural auditory information processing assessed with event-related potential data has been associated with both functional outcomes and negative symptoms in psychotic disorder individuals (113). In terms of motor function, striatal sub-regional shape abnormalities have been observed in childhood-onset schizophrenia relative to healthy volunteers. These striatal shape abnormalities were partially shared by unaffected siblings, suggesting a resilience endophenotype (114).
Within the CHR literature, a study using functional connectivity to predict symptom progression outcomes in CHR individuals found that higher between-network connectivity (among language, dorsal attention, cerebellar, sensorimotor, and salience networks) and more typical modular connectome organization predicted improvement in clinical outcomes and symptomatology (115). Another study explored resilience structural neural biomarkers defined by CHR outcome 6 years later (38). CHR individuals classified as resilient showed greater volumes at baseline in temporal, parietal cortex, and corpus callosum, regions underlying auditory processing, sensory and sensorimotor integration (38); these differences remained stable over time. More specifically, and consistent with above gray matter findings, a study showed positive symptom improvement over time correlated with an increase in white matter integrity of the corpus callosum (116); findings in corpus callosum across gray and white matter metrics could underlie more integrated cognitive and sensorimotor processing, serving as a protective marker (110, 117). Other studies explored sensory processing using event-related potential data. Auditory mismatch negativity amplitude, which relates to preattentive auditory processing, significantly predicted both remission from CHR symptomatology and general functioning at 6 years follow up (118). Reduced P300 novel amplitude, indexing impaired salience processing, was also recently found in CHR that did not remit, compared to those that remitted (119). As it stands, the literature would benefit from more longitudinal investigations integrating across multiple imaging modalities are warranted, which would help to solidify our understanding of possible sensory/sensorimotor resilience markers.
4. Conclusion
Decades of CHR research have yielded a wealth of fruitful information on neural risk markers, which have been summarized across several reviews (10, 16, 17, 19-22). Future investigations will benefit from tracking attenuated symptomatology along neural markers despite conversion status. To frame an emergent literature and highlight a promising area of focus, behavioral and brain-based resiliency markers were conceptualized across four domains, reviewed among available CHR studies on symptom remission and persistence. Domains of executive function and neurocognition, emotion regulation, allostatic load, sensory and sensorimotor function were delineated with the aid of existing non-psychiatric and psychotic disorder literature. Although neural markers may not appear as widely scalable/translatable as behavior, they are critical tools to validating scalable behavioral markers, and necessary for spearheading efforts to parse out etiological and pathophysiological heterogeneity in psychotic disorders (2). As a consequence, neural markers also hold immense promise for precision medicine approaches to treatment and prevention (120, 121). As such, future resiliency research would benefit from integrating behavioral and neural markers when envisioning CHR resilience profiles.
In addition to these summary points, we hope to frame future directions in light of what was reviewed. CHR risk marker research has often centered on predicting conversion to a psychotic disorder (36). While these efforts are necessary, the present review proposes that to maximize progress in the field, the scope ought to be broadened from considering conversion as the unique determinant, to tracking attenuated positive symptomatology over time, and dedicating greater consideration to those whose symptoms remit, versus those whose symptoms persist or progress (along with greater attention to other outcomes such as functioning and neurocognition). Comparisons based on conversion status can limit samples sizes, and suffer from limitations related to false-negatives (5, 7). Further, psychosis symptoms can be debilitating irrespective of conversion status (122, 123). Critically, as highlighted throughout the review, resilient-appearing outcomes can belie risk-conferring components due to factors such as wear-and-tear of biological and neurological systems in light of exceedingly high demands. Conversely, poor outcomes signaling risk factor exposure could contain underlying protective/resilient components that preserve the integrity and foundations of biological/neural systems under increased demands (51, 124). Thus, to understand risk, it is necessary to understand resilience, as it is necessary to grasp and identify existing protective factors in order to conceptualize risk. Future work incorporating multimodal brain-based risk and resiliency markers simultaneously is necessary to continue to develop our ability to embrace the complexity and considerable promise in this area. To this end, well-powered studies and large-scale consortia such as Computerized Assessment for Psychosis Risk (CAPER) and Multisite Assessment of Psychosis-Risk (MAP) should carefully consider these issues when rising to meet this need.
Acknowledgements
Summary figures were created to synthesize the discussed literature. Figures from the discussed studies were also added to ease visualization of the authors’ work. The manuscript will abide by copyright permission guidelines for images used in the figures.
Financial Disclosures
This work was supported by NIMH grants F31MH119776 (TV), F31MH119720 (AE), R01MH112613 (LE), R01MH118545 (LE), R01MH120091 (LE), 1R01MH112545 (VM), R01120088 (VM), R01MH116039 (VM), MH119677 (VM), MH110374 (VM). The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. (2017): Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 390:1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, et al. (2016): Identification of distinct psychosis biotypes using brain-based biomarkers. American Journal of Psychiatry. 173:373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickinson D, Pratt DN, Giangrande EJ, Grunnagle M, Orel J, Weinberger DR, et al. (2018): Attacking heterogeneity in schizophrenia by deriving clinical subgroups from widely available symptom data. Schizophrenia bulletin. 44:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon AE, Borgwardt S, Riecher-Rössler A, Velthorst E, de Haan L, Fusar-Poli P (2013): Moving beyond transition outcomes: meta-analysis of remission rates in individuals at high clinical risk for psychosis. Psychiatry research. 209:266–272. [DOI] [PubMed] [Google Scholar]
- 5.Beck K, Andreou C, Studerus E, Heitz U, Ittig S, Leanza L, et al. (2019): Clinical and functional long-term outcome of patients at clinical high risk (CHR) for psychosis without transition to psychosis: a systematic review. Schizophrenia research. 210:39–47. [DOI] [PubMed] [Google Scholar]
- 6.Beck K, Studerus E, Andreou C, Egloff L, Leanza L, Simon AE, et al. (2019): Clinical and functional ultra-long-term outcome of patients with a clinical high risk (CHR) for psychosis. European Psychiatry. 62:30–37. [DOI] [PubMed] [Google Scholar]
- 7.Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, et al. (2016): An individualized risk calculator for research in prodromal psychosis. American Journal of Psychiatry. 173:980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goff DC, Romero K, Paul J, Perez-Rodriguez MM, Crandall D, Potkin SG (2016): Biomarkers for drug development in early psychosis: current issues and promising directions. European Neuropsychopharmacology. 26:923–937. [DOI] [PubMed] [Google Scholar]
- 9.Weickert CS, Weickert TW, Pillai A, Buckley PF (2013): Biomarkers in schizophrenia: a brief conceptual consideration. Disease markers. 35:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niznikiewicz MA (2019): Neurobiological approaches to the study of clinical and genetic high risk for developing psychosis. Psychiatry research. [DOI] [PubMed] [Google Scholar]
- 11.Keshavan MS, Clementz BA, Pearlson GD, Sweeney JA, Tamminga CA (2013): Reimagining psychoses: an agnostic approach to diagnosis. Schizophrenia research. 146:10–16. [DOI] [PubMed] [Google Scholar]
- 12.Bonnano G (2004): Loss, trauma and human resilience: Conceptual and empirical connections and separateness. American Psychologist. 59:20–28. [DOI] [PubMed] [Google Scholar]
- 13.Bonanno GA, Galea S, Bucciarelli A, Vlahov D (2007): What predicts psychological resilience after disaster? The role of demographics, resources, and life stress. Journal of consulting and clinical psychology. 75:671. [DOI] [PubMed] [Google Scholar]
- 14.Bonanno GA (2005): Resilience in the face of potential trauma. Current directions in psychological science. 14:135–138. [Google Scholar]
- 15.Millman ZB, Gold JM, Mittal VA, Schiffman J (2019): The critical need for help-seeking controls in clinical high-risk research. Clinical Psychological Science. 7:1171–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fusar-Poli P, Borgwardt S, Crescini A, Deste G, Kempton MJ, Lawrie S, et al. (2011): Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neuroscience & Biobehavioral Reviews. 35:1175–1185. [DOI] [PubMed] [Google Scholar]
- 17.Dutt A, Tseng H-H, Fonville L, Drakesmith M, Su L, Evans J, et al. (2015): Exploring neural dysfunction in ‘clinical high risk’for psychosis: a quantitative review of fMRI studies. Journal of psychiatric research. 61:122–134. [DOI] [PubMed] [Google Scholar]
- 18.Michel C, Ruhrmann S, Schimmelmann BG, Klosterkötter J, Schultze-Lutter F (2018): Course of clinical high-risk states for psychosis beyond conversion. European archives of psychiatry and clinical neuroscience. 268:39–48. [DOI] [PubMed] [Google Scholar]
- 19.Chung Y, Cannon TD (2015): Brain imaging during the transition from psychosis prodrome to schizophrenia. The Journal of nervous and mental disease. 203:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz R, Drewe J, et al. (2010): Neuroimaging predictors of transition to psychosis—a systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews. 34:1207–1222. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt A, Cappucciati M, Radua J, Rutigliano G, Rocchetti M, Dell’Osso L, et al. (2017): Improving prognostic accuracy in subjects at clinical high risk for psychosis: systematic review of predictive models and meta-analytical sequential testing simulation. Schizophrenia bulletin. 43:375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egerton A, Borgwardt SJ, Tognin S, Howes OD, McGuire P, Allen P (2011): An overview of functional, structural and neurochemical imaging studies in individuals with a clinical high risk for psychosis. Neuropsychiatry. 1:477. [Google Scholar]
- 23.Masten AS, Hubbard JJ, Gest SD, Tellegen A, Garmezy N, Ramirez M (1999): Competence in the context of adversity: Pathways to resilience and maladaptation from childhood to late adolescence. Development and psychopathology. 11:143–169. [DOI] [PubMed] [Google Scholar]
- 24.Masten AS, Best KM, Garmezy N (1990): Resilience and development: Contributions from the study of children who overcome adversity. Development and psychopathology. 2:425–444. [Google Scholar]
- 25.Bozikas VP, Parlapani E, Holeva V, Skemperi E, Bargiota SI, Kirla D, et al. (2016): Resilience in patients with recent diagnosis of a schizophrenia spectrum disorder. The Journal of nervous and mental disease. 204:578–584. [DOI] [PubMed] [Google Scholar]
- 26.Mizuno Y, Wartelsteiner F, Frajo-Apor B (2016): Resilience research in schizophrenia: A review of recent developments. Current opinion in psychiatry. 29:218–223. [DOI] [PubMed] [Google Scholar]
- 27.van Zelst C, Van Nierop M, Oorschot M, Myin-Germeys I, van Os J, Delespaul P (2014): Stereotype awareness, self-esteem and psychopathology in people with psychosis. PloS one. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson J, Gooding PA, Wood AM, Taylor PJ, Pratt D, Tarrier N (2010): Resilience to suicidal ideation in psychosis: Positive self-appraisals buffer the impact of hopelessness. Behaviour research and therapy. 48:883–889. [DOI] [PubMed] [Google Scholar]
- 29.Palmer BW, Martin AS, Depp CA, Glorioso DK, Jeste DV (2014): Wellness within illness: happiness in schizophrenia. Schizophrenia research. 159:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galderisi S, Rossi A, Rocca P, Bertolino A, Mucci A, Bucci P, et al. (2014): The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry. 13:275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mo’tamedi H, Rezaiemaram P, Aguilar-Vafaie ME, Tavallaie A, Azimian M, Shemshadi H (2014): The relationship between family resiliency factors and caregiver-perceived duration of untreated psychosis in persons with first-episode psychosis. Psychiatry research. 219:497–505. [DOI] [PubMed] [Google Scholar]
- 32.Boyette L-L, van Dam D, Meijer C, Velthorst E, Cahn W, de Haan L, et al. (2014): Personality compensates for impaired quality of life and social functioning in patients with psychotic disorders who experienced traumatic events. Schizophrenia bulletin. 40:1356–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim KR, Song YY, Park JY, Lee EH, Lee M, Lee SY, et al. (2013): The relationship between psychosocial functioning and resilience and negative symptoms in individuals at ultra-high risk for psychosis. Australian & New Zealand Journal of Psychiatry. 47:762–771. [DOI] [PubMed] [Google Scholar]
- 34.Marulanda S, Addington J (2016): Resilience in individuals at clinical high risk for psychosis. Early intervention in psychiatry. 10:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee TY, Kim SN, Correll CU, Byun MS, Kim E, Jang JH, et al. (2014): Symptomatic and functional remission of subjects at clinical high risk for psychosis: a 2-year naturalistic observational study. Schizophrenia research. 156:266–271. [DOI] [PubMed] [Google Scholar]
- 36.Oliver D, Reilly TJ, Baccaredda Boy O, Petros N, Davies C, Borgwardt S, et al. (2020): What causes the onset of psychosis in individuals at clinical high risk? A meta-analysis of risk and protective factors. Schizophrenia bulletin. 46:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung Y, Allswede D, Addington J, Bearden CE, Cadenhead K, Cornblatt B, et al. (2019): Cortical abnormalities in youth at clinical high-risk for psychosis: Findings from the NAPLS2 cohort. NeuroImage: Clinical. 23:101862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Wit S, Wierenga LM, Oranje B, Ziermans TB, Schothorst PF, van Engeland H, et al. (2016): Brain development in adolescents at ultra-high risk for psychosis: Longitudinal changes related to resilience. NeuroImage: Clinical. 12:542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wierenga LM, Langen M, Oranje B, Durston S (2014): Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 87:120–126. [DOI] [PubMed] [Google Scholar]
- 40.Taylor ZE, Ruiz Y (2019): Executive function, dispositional resilience, and cognitive engagement in Latinx children of migrant farmworkers. Children and Youth Services Review. 100:57–63. [Google Scholar]
- 41.Masten AS, Herbers JE, Desjardins CD, Cutuli J, McCormick CM, Sapienza JK, et al. (2012): Executive function skills and school success in young children experiencing homelessness. Educational Researcher. 41:375–384. [Google Scholar]
- 42.Brody GH, Yu T, Chen E, Miller GE, Kogan SM, Beach SR (2013): Is resilience only skin deep? Rural African Americans’ socioeconomic status–related risk and competence in preadolescence and psychological adjustment and allostatic load at age 19. Psychological science. 24:1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen E, Miller GE (2013): Socioeconomic status and health: mediating and moderating factors. Annual Review of Clinical Psychology. 9:723–749. [DOI] [PubMed] [Google Scholar]
- 44.Chen E, Shalowitz MU, Story RE, Hayen R, Leigh AK, Hoffer LC, et al. (2019): The costs of high self-control in Black and Latino youth with asthma: Divergence of mental health and inflammatory profiles. Brain, behavior, and immunity. 80:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller GE, Yu T, Chen E, Brody GH (2015): Self-control forecasts better psychosocial outcomes but faster epigenetic aging in low-SES youth. Proceedings of the National Academy of Sciences. 112:10325–10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng M, Pan Y, Zhou L, Chen X, Liu C, Huang X, et al. (2018): Resilience and cognitive function in patients with schizophrenia and bipolar disorder, and healthy controls. Frontiers in psychiatry. 9:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee TY, Shin YS, Shin NY, Kim SN, Jang JH, Kang D-H, et al. (2014): Neurocognitive function as a possible marker for remission from clinical high risk for psychosis. Schizophrenia research. 153:48–53. [DOI] [PubMed] [Google Scholar]
- 48.Shakeel M, Lu L, Cannon T, Cadenhead K, Cornblatt B, McGlashan T, et al. (2019): Longitudinal changes in social cognition in individuals at clinical high risk for psychosis: An outcome based analysis. Schizophrenia research. 204:334–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller GE, Chen E, Armstrong CC, Carroll AL, Ozturk S, Rydland KJ, et al. (2018): Functional connectivity in central executive network protects youth against cardiometabolic risks linked with neighborhood violence. Proceedings of the National Academy of Sciences. 115:12063–12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nusslock R, Brody GH, Armstrong CC, Carroll AL, Sweet LH, Yu T, et al. (2019): Higher peripheral inflammatory signaling associated with lower resting-state functional brain connectivity in emotion regulation and central executive networks. Biological psychiatry. 86:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ganella EP, Seguin C, Bartholomeusz CF, Whittle S, Bousman C, Wannan CM, et al. (2018): Risk and resilience brain networks in treatment-resistant schizophrenia. Schizophrenia research. 193:284–292. [DOI] [PubMed] [Google Scholar]
- 52.Bartholomeusz CF, Whittle SL, Montague A, Ansell B, McGorry PD, Velakoulis D, et al. (2013): Sulcogyral patterns and morphological abnormalities of the orbitofrontal cortex in psychosis. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 44:168–177. [DOI] [PubMed] [Google Scholar]
- 53.Kim M, Lee TY, Lee S, Kim SN, Kwon JS (2015): Auditory P300 as a predictor of short-term prognosis in subjects at clinical high risk for psychosis. Schizophrenia research. 165:138–144. [DOI] [PubMed] [Google Scholar]
- 54.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. (2014): Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral cortex. 24:2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonanno GA (2013): Meaning making, adversity, and regulatory flexibility. Memory. 21:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lincoln TM, Hartmann M, Kother U, Moritz S (2015): Dealing with feeling: Specific emotion regulation skills predict responses to stress in psychosis. Psychiatry research. 228:216–222. [DOI] [PubMed] [Google Scholar]
- 57.Badcock JC, Paulik G, Maybery MT (2011): The role of emotion regulation in auditory hallucinations. Psychiatry research. 185:303–308. [DOI] [PubMed] [Google Scholar]
- 58.Westermann S, Lincoln TM (2011): Emotion regulation difficulties are relevant to persecutory ideation. Psychology and Psychotherapy: Theory, Research and Practice. 84:273–287. [DOI] [PubMed] [Google Scholar]
- 59.Perry Y, Henry JD, Grisham JR (2011): The habitual use of emotion regulation strategies in schizophrenia. British Journal of Clinical Psychology. 50:217–222. [DOI] [PubMed] [Google Scholar]
- 60.Chapman HC, Visser KF, Mittal VA, Gibb BE, Coles ME, Strauss GP (2020): Emotion regulation across the psychosis continuum. Development and psychopathology. 32:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ludwig L, Werner D, Lincoln TM (2019): The relevance of cognitive emotion regulation to psychotic symptoms-a systematic review and meta-analysis. Clinical psychology review. 101746. [DOI] [PubMed] [Google Scholar]
- 62.Shi J, Yao Y, Zhan C, Mao Z, Yin F, Zhao X (2018): The relationship between big five personality traits and psychotic experience in a large non-clinical youth sample: the mediating role of emotion regulation. Frontiers in psychiatry. 9:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morrison AP, Barratt S (2010): What are the components of CBT for psychosis? A Delphi study. Schizophrenia Bulletin. 36:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Addington J, Epstein I, Liu L, French P, Boydell KM, Zipursky RB (2011): A randomized controlled trial of cognitive behavioral therapy for individuals at clinical high risk of psychosis. Schizophrenia research. 125:54–61. [DOI] [PubMed] [Google Scholar]
- 65.Morrison AP, French P, Walford L, Lewis SW, Kilcommons A, Green J, et al. (2004): Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomised controlled trial. The British Journal of Psychiatry. 185:291–297. [DOI] [PubMed] [Google Scholar]
- 66.Phillips LJ, McGorry PD, Yuen HP, Ward J, Donovan K, Kelly D, et al. (2007): Medium term follow-up of a randomized controlled trial of interventions for young people at ultra high risk of psychosis. Schizophrenia Research. 96:25–33. [DOI] [PubMed] [Google Scholar]
- 67.Osborne KJ, Willroth EC, DeVylder JE, Mittal VA, Hilimire MR (2017): Investigating the association between emotion regulation and distress in adults with psychotic-like experiences. Psychiatry research. 256:66–70. [DOI] [PubMed] [Google Scholar]
- 68.Lincoln TM, Sundag J, Schlier B, Karow A (2018): The relevance of emotion regulation in explaining why social exclusion triggers paranoia in individuals at clinical high risk of psychosis. Schizophrenia bulletin. 44:757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ered A, Gibson LE, Maxwell SD, Cooper S, Ellman LM (2017): Coping as a mediator of stress and psychotic-like experiences. European Psychiatry. 43:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schweizer S, Walsh ND, Stretton J, Dunn VJ, Goodyer IM, Dalgleish T (2016): Enhanced emotion regulation capacity and its neural substrates in those exposed to moderate childhood adversity. Social cognitive and affective neuroscience. 11:272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodman AM, Jenness JL, Weissman DG, Pine DS, McLaughlin KA (2019): Neurobiological markers of resilience to depression following childhood maltreatment: The role of neural circuits supporting the cognitive control of emotion. Biological psychiatry. 86:464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN (2008): Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 59:1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raij TT, Korkeila J, Joutsenniemi K, Saarni SI, Riekki TJ (2014): Association of stigma resistance with emotion regulation—functional magnetic resonance imaging and neuropsychological findings. Comprehensive psychiatry. 55:727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edwards BG, Barch DM, Braver TS (2010): Improving prefrontal cortex function in schizophrenia through focused training of cognitive control. Frontiers in human neuroscience. 4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goldin PR, Gross JJ (2010): Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 10:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gee D (2015): Amygdala-Prefrontal Function and Clinical Course among Adolescents and Young Adults at Clinical High Risk for Psychosis: UCLA. [Google Scholar]
- 77.Fusar-Poli P, Broome MR, Woolley JB, Johns L, Tabraham P, Bramon E, et al. (2011): Altered brain function directly related to structural abnormalities in people at ultra high risk of psychosis: longitudinal VBM-fMRI study. Journal of psychiatric research. 45:190–198. [DOI] [PubMed] [Google Scholar]
- 78.McEwen BS, Stellar E (1993): Stress and the individual: mechanisms leading to disease. Archives of internal medicine. 153:2093–2101. [PubMed] [Google Scholar]
- 79.Chen E, Miller GE, Brody GH, Lei M (2015): Neighborhood poverty, college attendance, and diverging profiles of substance use and allostatic load in rural African American youth. Clinical Psychological Science. 3:675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shah JL, Malla AK (2015): Much ado about much: stress, dynamic biomarkers and HPA axis dysregulation along the trajectory to psychosis. Schizophrenia research. 162:253–260. [DOI] [PubMed] [Google Scholar]
- 81.Pruessner M, Bechard-Evans L, Boekestyn L, Iyer SN, Pruessner JC, Malla AK (2013): Attenuated cortisol response to acute psychosocial stress in individuals at ultra-high risk for psychosis. Schizophrenia research. 146:79–86. [DOI] [PubMed] [Google Scholar]
- 82.Walker E, Mittal V, Tessner K (2008): Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 4:189–216. [DOI] [PubMed] [Google Scholar]
- 83.Walker EF, Trotman HD, Pearce BD, Addington J, Cadenhead KS, Cornblatt BA, et al. (2013): Cortisol levels and risk for psychosis: initial findings from the North American prodrome longitudinal study. Biological psychiatry. 74:410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pruessner M, Iyer SN, Faridi K, Joober R, Malla AK (2011): Stress and protective factors in individuals at ultra-high risk for psychosis, first episode psychosis and healthy controls. Schizophrenia research. 129:29–35. [DOI] [PubMed] [Google Scholar]
- 85.Howell BR, Sanchez MM (2011): Understanding behavioral effects of early life stress using the reactive scope and allostatic load models. Development and psychopathology. 23:1001–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McEwen BS (2004): Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Sciences. 1032:1–7. [DOI] [PubMed] [Google Scholar]
- 87.Beauchaine TP, Neuhaus E, Zalewski M, Crowell SE, Potapova N (2011): The effects of allostatic load on neural systems subserving motivation, mood regulation, and social affiliation. Development and Psychopathology. 23:975–999. [DOI] [PubMed] [Google Scholar]
- 88.Bennett EL, Diamond MC, Krech D, Rosenzweig MR (1964): Chemical and anatomical plasticity of brain. Science. 146:610–619. [DOI] [PubMed] [Google Scholar]
- 89.McEwen BS (2016): In pursuit of resilience: stress, epigenetics, and brain plasticity. Annals of the New York Academy of Sciences. 1373:56–64. [DOI] [PubMed] [Google Scholar]
- 90.McEwen BS, Gianaros PJ (2011): Stress-and allostasis-induced brain plasticity. Annual review of medicine. 62:431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McEwen BS (1999): Stress and hippocampal plasticity. Annual review of neuroscience. 22:105–122. [DOI] [PubMed] [Google Scholar]
- 92.Kuipers SD, Trentani A, Den Boer J, Ter Horst G (2003): Molecular correlates of impaired prefrontal plasticity in response to chronic stress. Journal of neurochemistry. 85:1312–1323. [DOI] [PubMed] [Google Scholar]
- 93.Katz M, Liu C, Schaer M, Parker KJ, Ottet M-C, Epps A, et al. (2009): Prefrontal plasticity and stress inoculation-induced resilience. Developmental neuroscience. 31:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chiappelli J, Kochunov P, Savransky A, Fisseha F, Wisner K, Du X, et al. (2017): Allostatic load and reduced cortical thickness in schizophrenia. Psychoneuroendocrinology. 77:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nugent KL, Chiappelli J, Sampath H, Rowland LM, Thangavelu K, Davis B, et al. (2015): Prolonged Cortisol Reactivity to Stress and White Matter in Schizophrenia. Psychosomatic medicine. 77:733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dean DJ, Orr JM, Bernard JA, Gupta T, Pelletier-Baldelli A, Carol EE, et al. (2016): Hippocampal shape abnormalities predict symptom progression in neuroleptic-free youth at ultrahigh risk for psychosis. Schizophrenia bulletin. 42:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ho NF, Holt DJ, Cheung M, Iglesias JE, Goh A, Wang M, et al. (2017): Progressive decline in hippocampal CA1 volume in individuals at ultra-high-risk for psychosis who do not remit: findings from the longitudinal youth at risk study. Neuropsychopharmacology. 42:1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Allen P, Chaddock CA, Egerton A, Howes OD, Bonoldi I, Zelaya F, et al. (2016): Resting hyperperfusion of the hippocampus, midbrain, and basal ganglia in people at high risk for psychosis. American Journal of Psychiatry. 173:392–399. [DOI] [PubMed] [Google Scholar]
- 99.Mayoral M, Merchán-Naranjo J, Rapado M, Leiva M, Moreno C, Giráldez M, et al. (2010): Neurological soft signs in juvenile patients with Asperger syndrome, early-onset psychosis, and healthy controls. Early intervention in psychiatry. 4:283–290. [DOI] [PubMed] [Google Scholar]
- 100.Ahmed AS (2007): Post-traumatic stress disorder, resilience and vulnerability. Advances in Psychiatric Treatment. 13:369–375. [Google Scholar]
- 101.Mittal VA, Dean DJ, Bernard JA, Orr JM, Pelletier-Baldelli A, Carol EE, et al. (2014): Neurological soft signs predict abnormal cerebellar-thalamic tract development and negative symptoms in adolescents at high risk for psychosis: a longitudinal perspective. Schizophrenia bulletin. 40:1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bachmann S, Degen C, Geider FJ, Schröder J (2014): Neurological soft signs in the clinical course of schizophrenia: results of a meta-analysis. Frontiers in psychiatry. 5:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Behere RV (2013): Dorsolateral prefrontal lobe volume and neurological soft signs as predictors of clinical social and functional outcome in schizophrenia: A longitudinal study. Indian journal of psychiatry. 55:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dimitri-Valente G, Rigucci S, Manfredi G, Girardi P, Ferracuti S (2012): Neurological soft signs: meaning and relevance along the course of psychiatric illness. An objective and rapid screening for psychosis? Rivista dipsichiatria. 47:465–478. [DOI] [PubMed] [Google Scholar]
- 105.Emsley R, Oosthuizen P, Niehaus D, Koen L (2007): Changing the course of schizophrenia-predictors of treatment outcome revisited. South African Journal of Psychiatry. 13. [Google Scholar]
- 106.Ziermans TB, Schothorst PF, Sprong M, van Engeland H (2011): Transition and remission in adolescents at ultra-high risk for psychosis. Schizophrenia research. 126:58–64. [DOI] [PubMed] [Google Scholar]
- 107.Dean DJ, Walther S, Bernard JA, Mittal VA (2018): Motor clusters reveal differences in risk for psychosis, cognitive functioning, and thalamocortical connectivity: evidence for vulnerability subtypes. Clinical psychological science. 6:721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Levit-Binnun N, Davidovitch M, Golland Y (2013): Sensory and motor secondary symptoms as indicators of brain vulnerability. Journal of Neurodevelopmental Disorders. 5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kaufmann T, Skåtun KC, Alnæ D, Doan NT, Duff EP, Tønnesen S, et al. (2015): Disintegration of sensorimotor brain networks in schizophrenia. Schizophrenia bulletin. 41:1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Berman RA, Gotts SJ, McAdams HM, Greenstein D, Lalonde F, Clasen L, et al. (2016): Disrupted sensorimotor and social–cognitive networks underlie symptoms in childhood-onset schizophrenia. Brain. 139:276–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shergill SS, White TP, Joyce DW, Bays PM, Wolpert DM, Frith CD (2014): Functional magnetic resonance imaging of impaired sensory prediction in schizophrenia. JAMA psychiatry. 71:28–35. [DOI] [PubMed] [Google Scholar]
- 112.Zalesky A, Pantelis C, Cropley V, Fornito A, Cocchi L, McAdams H, et al. (2015): Delayed development of brain connectivity in adolescents with schizophrenia and their unaffected siblings. JAMA psychiatry. 72:900–908. [DOI] [PubMed] [Google Scholar]
- 113.Thomas ML, Green MF, Hellemann G, Sugar CA, Tarasenko M, Calkins ME, et al. (2017): Modeling deficits from early auditory information processing to psychosocial functioning in schizophrenia. JAMA psychiatry. 74:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chakravarty MM, Rapoport JL, Giedd JN, Raznahan A, Shaw P, Collins DL, et al. (2015): Striatal shape abnormalities as novel neurodevelopmental endophenotypes in schizophrenia: a longitudinal study. Human brain mapping. 36:1458–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Collin G, Nieto-Castanon A, Shenton ME, Pasternak O, Kelly S, Keshavan MS, et al. (2019): Brain functional connectivity data enhance prediction of clinical outcome in youth at risk for psychosis. NeuroImage: Clinical. 102108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Katagiri N, Pantelis C, Nemoto T, Zalesky A, Hori M, Shimoji K, et al. (2015): A longitudinal study investigating sub-threshold symptoms and white matter changes in individuals with an ‘at risk mental state’(ARMS). Schizophrenia research. 162:7–13. [DOI] [PubMed] [Google Scholar]
- 117.Pulvermüller F, Garagnani M (2014): From sensorimotor learning to memory cells in prefrontal and temporal association cortex: a neurocomputational study of disembodiment. Cortex. 57:1–21. [DOI] [PubMed] [Google Scholar]
- 118.Kim M, Lee TH, Yoon YB, Lee TY, Kwon JS (2018): Predicting remission in subjects at clinical high risk for psychosis using mismatch negativity. Schizophrenia bulletin. 44:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tang Y, Wang J, Zhang T, Xu L, Qian Z, Cui H, et al. (2019): P300 as an index of transition to psychosis and of remission: Data from a clinical high risk for psychosis study and review of literature. Schizophrenia research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tognin S, van Hell HH, Merritt K, Winter-van Rossum I, Bossong MG, Kempton MJ, et al. (2020): Towards precision medicine in psychosis: benefits and challenges of multimodal multicenter studies—PSYSCAN: translating neuroimaging findings from research into clinical practice. Schizophrenia bulletin. 46:432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Quinlan EB, Banaschewski T, Barker GJ, Bokde AL, Bromberg U, Büchel C, et al. (2019): Identifying biological markers for improved precision medicine in psychiatry. Molecular psychiatry .1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rutigliano G, Valmaggia L, Landi P, Frascarelli M, Cappucciati M, Sear V, et al. (2016): Persistence or recurrence of non-psychotic comorbid mental disorders associated with 6-year poor functional outcomes in patients at ultra high risk for psychosis. Journal of affective disorders. 203:101–110. [DOI] [PubMed] [Google Scholar]
- 123.Schlosser DA, Jacobson S, Chen Q, Sugar CA, Niendam TA, Li G, et al. (2012): Recovery from an at-risk state: clinical and functional outcomes of putatively prodromal youth who do not develop psychosis. Schizophrenia bulletin. 38:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lo C-YZ, Su T-W, Huang C-C, Hung C-C, Chen W-L, Lan T-H, et al. (2015): Randomization and resilience of brain functional networks as systems-level endophenotypes of schizophrenia. Proceedings of the National Academy of Sciences. 112:9123–9128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tognin S, Pettersson-Yeo W, Valli I, Hutton C, Woolley J, Allen P, et al. (2014): Using structural neuroimaging to make quantitative predictions of symptom progression in individuals at ultra-high risk for psychosis. Frontiers in psychiatry. 4:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Takahashi T, Wood SJ, Yung AR, Phillips LJ, Soulsby B, McGorry PD, et al. (2009): Insular cortex gray matter changes in individuals at ultra-high-risk of developing psychosis. Schizophrenia Research. 111:94–102. [DOI] [PubMed] [Google Scholar]
- 127.Mechelli A, Riecher-Rössler A, Meisenzahl EM, Tognin S, Wood SJ, Borgwardt SJ, et al. (2011): Neuroanatomical abnormalities that predate the onset of psychosis: a multicenter study. Archives of general psychiatry. 68:489–495. [DOI] [PubMed] [Google Scholar]
- 128.Saunders TS, Mondelli V, Cullen AE (2019): Pituitary volume in individuals at elevated risk for psychosis: A systematic review and meta-analysis. Schizophrenia research. 213:23–31. [DOI] [PubMed] [Google Scholar]
- 129.Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TG, et al. (2015): Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biological psychiatry. 77:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cropley VL, Lin A, Nelson B, Reniers RL, Yung AR, Bartholomeusz CF, et al. (2016): Baseline grey matter volume of non-transitioned “ultra high risk” for psychosis individuals with and without attenuated psychotic symptoms at long-term follow-up. Schizophrenia research. 173:152–158. [DOI] [PubMed] [Google Scholar]
- 131.Bernard JA, Orr JM, Mittal VA (2017): Cerebello-thalamo-cortical networks predict positive symptom progression in individuals at ultra-high risk for psychosis. NeuroImage: Clinical. 14:622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Carletti F, Woolley JB, Bhattacharyya S, Perez-Iglesias R, Fusar Poli P, Valmaggia L, et al. (2012): Alterations in white matter evident before the onset of psychosis. Schizophrenia bulletin. 38:1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Krakauer K, Nordentoft M, Glenthøj B, Raghava J, Nordholm D, Randers L, et al. (2018): White matter maturation during 12 months in individuals at ultra-high-risk for psychosis. Acta Psychiatrica Scandinavica. 137:65–78. [DOI] [PubMed] [Google Scholar]
- 134.Collin G, Seidman LJ, Keshavan MS, Stone WS, Qi Z, Zhang T, et al. (2018): Functional connectome organization predicts conversion to psychosis in clinical high-risk youth from the SHARP program. Molecular psychiatry. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Heinze K, Reniers RL, Nelson B, Yung AR, Lin A, Harrison BJ, et al. (2015): Discrete alterations of brain network structural covariance in individuals at ultra-high risk for psychosis. Biological psychiatry. 77:989–996. [DOI] [PubMed] [Google Scholar]
- 136.Wang C, Ji F, Hong Z, Poh J, Krishnan R, Lee J, et al. (2016): Disrupted salience network functional connectivity and white-matter microstructure in persons at risk for psychosis: findings from the LYRIKS study. Psychological medicine. 46:2771–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Allen P, Luigjes J, Howes OD, Egerton A, Hirao K, Valli I, et al. (2012): Transition to psychosis associated with prefrontal and subcortical dysfunction in ultra high-risk individuals. Schizophrenia bulletin. 38:1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lord L-D, Allen P, Expert P, Howes O, Broome M, Lambiotte R, et al. (2012): Functional brain networks before the onset of psychosis: a prospective fMRI study with graph theoretical analysis. NeuroImage: Clinical. 1:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, et al. (2015): Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA psychiatry. 72:882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Colibazzi T, Yang Z, Horga G, Yan C-G, Corcoran CM, Klahr K, et al. (2017): Aberrant temporal connectivity in persons at clinical high risk for psychosis. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2:696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cao H, Chén OY, Chung Y, Forsyth JK, McEwen SC, Gee DG, et al. (2018): Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nature communications. 9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sabb FW, van Erp TG, Hardt ME, Dapretto M, Caplan R, Cannon TD, et al. (2010): Language network dysfunction as a predictor of outcome in youth at clinical high risk for psychosis. Schizophrenia research. 116:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Marjoram D, Job DE, Whalley HC, Gountouna V-E, McIntosh AM, Simonotto E, et al. (2006): A visual joke fMRI investigation into Theory of Mind and enhanced risk of schizophrenia. Neuroimage. 31:1850–1858. [DOI] [PubMed] [Google Scholar]
- 144.Modinos G, Şimşek F, Horder J, Bossong M, Bonoldi I, Azis M, et al. (2018): Cortical GABA in subjects at ultra-high risk of psychosis: relationship to negative prodromal symptoms. International Journal of Neuropsychopharmacology. 21:114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.de la Fuente-Sandoval C, León-Ortiz P, Azcárraga M, Favila R, Stephano S, Graff-Guerrero A (2013): Striatal glutamate and the conversion to psychosis: a prospective 1H-MRS imaging study. International Journal of Neuropsychopharmacology. 16:471–475. [DOI] [PubMed] [Google Scholar]
- 146.Demro C, Rowland L, Wijtenburg SA, Waltz J, Gold J, Kline E, et al. (2017): Glutamatergic metabolites among adolescents at risk for psychosis. Psychiatry research. 257:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Egerton A, Stone JM, Chaddock CA, Barker GJ, Bonoldi I, Howard RM, et al. (2014): Relationship between brain glutamate levels and clinical outcome in individuals at ultra high risk of psychosis. Neuropsychopharmacology. 39:2891–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Modinos G, Şimşek F, Azis M, Bossong M, Bonoldi I, Samson C, et al. (2018): Prefrontal GABA levels, hippocampal resting perfusion and the risk of psychosis. Neuropsychopharmacology. 43:2652–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Allen P, Azis M, Modinos G, Bossong MG, Bonoldi I, Samson C, et al. (2018): Increased resting hippocampal and basal ganglia perfusion in people at ultra high risk for psychosis: replication in a second cohort. Schizophrenia bulletin. 44:1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kindler J, Schultze-Lutter F, Hauf M, Dierks T, Federspiel A, Walther S, et al. (2018): Increased striatal and reduced prefrontal cerebral blood flow in clinical high risk for psychosis. Schizophrenia bulletin. 44:182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]


