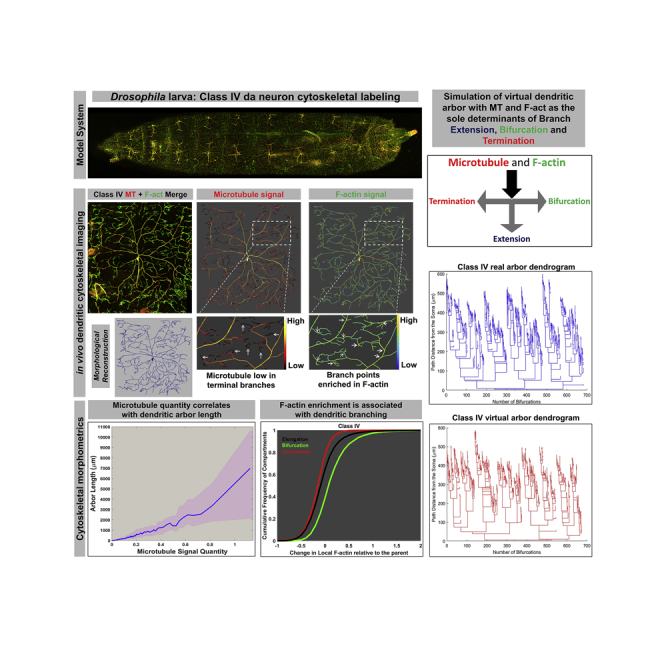
Summary
Microtubules (MTs) and F-actin (F-act) have long been recognized as key regulators of dendritic morphology. Nevertheless, precisely ascertaining their distinct influences on dendritic trees have been hampered until now by the lack of direct, arbor-wide cytoskeletal quantification. We pair live confocal imaging of fluorescently labeled dendritic arborization (da) neurons in Drosophila larvae with complete multi-signal neural tracing to separately measure MTs and F-act. We demonstrate that dendritic arbor length is highly interrelated with local MT quantity, whereas local F-act enrichment is associated with dendritic branching. Computational simulation of arbor structure solely constrained by experimentally observed subcellular distributions of these cytoskeletal components generated synthetic morphological and molecular patterns statistically equivalent to those of real da neurons, corroborating the efficacy of local MT and F-act in describing dendritic architecture. The analysis and modeling outcomes hold true for the simplest (class I), most complex (class IV), and genetically altered (Formin3 overexpression) da neuron types.
Subject Areas: Biological Sciences, Molecular Biology, Neuroscience, Cell Biology
Graphical Abstract
Highlights
-
•
Local microtubule quantity is highly interrelated with arbor length
-
•
Bifurcations are strongly associated with enrichment in local F-actin
-
•
A model based on local microtubule and F-actin reproduces dendritic morphology
-
•
The above findings are robust across cell types and genetic alterations
Biological Sciences; Molecular Biology; Neuroscience; Cell Biology
Introduction
Nervous systems comprise numerous neuronal subtypes, each with its own axonal-dendritic morphology and field of innervation. The specification and dynamic modification of subtype-specific dendritic architecture not only dictate how distinct neuron subtypes form functional connections with other neurons but also directly influence their computational properties (Lazarewicz et al., 2002; Ledda and Paratcha, 2017). The broad significance of dendritic form in neural function is further underscored by the wide spectrum of neurological and neurocognitive disorders that have been linked to disruptions in these processes (Franker and Hoogenraad, 2013). A vital challenge in understanding neuronal architecture is to elucidate how dendritic branches are regulated to elongate, bifurcate, and terminate, leading to their unique tree geometry, and how these regulatory rules vary to yield the striking observed diversity in structure, function, and connectivity (Lefebvre et al., 2015). Drosophila larval sensory dendritic arborization (da) neurons have proven to be an exceptionally powerful model for investigating these questions, revealing mechanistic underpinnings of numerous structural dynamics including tiling, remodeling, outgrowth, and subtype-specific branching (Jan and Jan, 2010; Nanda et al., 2017).
A combination of intrinsic genetic programming, extrinsic cues, electrophysiological activity, and proximity to other cells (neuronal and non-neuronal) sculpt mature dendritic shape and maintain arbor stability, as well as plasticity (Lefebvre et al., 2015). Each of these regulatory processes has been demonstrated to impact dendritic architecture by ultimately converging on the cytoskeleton (Franker and Hoogenraad, 2013; Jan and Jan, 2010; Ledda and Paratcha, 2017; Lefebvre et al., 2015; Nanda et al., 2017). Furthermore, the acquisition, maintenance, and modulation of diverse dendritic morphologies are largely mediated by organization and dynamics of F-actin (F-act) and microtubule (MT) cytoskeletal components that provide the underlying scaffold and fiber tracks for intracellular trafficking critical to arbor development (Coles and Bradke, 2015; Franker and Hoogenraad, 2013; Kapitein et al., 2010; Kapitein and Hoogenraad, 2015). Numerous studies have demonstrated that diverse dendritic morphologies emerge via complex growth mechanisms modulated by intrinsic signaling involving combinatorial transcription factor regulatory codes that contribute to both neuronal subtype identity and morphological diversity (Corty et al., 2016; Das et al., 2017a; de la Torre-Ubieta and Bonni, 2011; Franker and Hoogenraad, 2013; Grueber et al., 2003; Iyer et al., 2013a, 2013b; Li et al., 2004; Santiago and Bashaw, 2014; Sugimura et al., 2004; Ye et al., 2011). These transcription factor-mediated programs converge on a diverse set of cellular pathways to drive dendritic arbor diversity, many of which ultimately impact cytoskeletal architecture as a terminal mediator of arbor shape (Das et al., 2017a, 2017b; Ferreira et al., 2014; Franker and Hoogenraad, 2013; Hattori et al., 2013; Iyer et al., 2012; Jinushi-Nakao et al., 2007; Nagel et al., 2012).
Neuronal MTs form a quasi-continuous core within the dendritic tree (Bray and Bunge, 1981). MTs result from the polymerization of α- and β-tubulin heterodimers on a structural template of γ-tubulin. The polarized protofilaments are then laterally arranged into a hollow configuration. MTs exhibit dynamic instability, i.e., an instant alteration between tubulin assembly and disassembly (Howard and Hyman, 2009). This switching can be regulated by the binding or hydrolysis of GTP at the growing end of the αβ heterodimers, where the GTP-tubulin lattice promotes polymerization and GDP-tubulin lattice promotes disassembly (Alushin et al., 2014; Heald and Nogales, 2002). Unlike axons, whose MTs have rapidly polymerizing (“plus”) ends which are uniformly oriented in the anterograde direction (Heidemann et al., 1981), dendrites can have mixed MT polarity, and MTs remain significantly dynamic in mature dendritic trees. Specifically, in the fruit fly system, polymerizing MTs exist in both proximal and distal dendrites, with a dominance of retrograde polymerization in proximal and anterograde polymerization in the distal (nascent) branches. Local regulation through nucleation has also been proposed as a mechanism for dendritic MT polymerization in growing neurons without MT organizing centers (Sánchez-Huertas et al., 2016). Neurite diameter is also influenced by MT stability and organization (Stephan et al., 2015).
F-act is also a polarized structure, consisting of ATP-bound globular actin primarily polymerizing at the barbed end (Coles and Bradke, 2015). Neurons employ F-act polymerization to drive plasma membrane protrusion, and actin-filled post-synaptic filipodia are involved in synaptogenesis (Ritzenthaler et al., 2000). F-act polymerization is rate controlled by nucleators such as Arp2/3 complex, which binds to monomeric actin to facilitate polymerization (Konietzny et al., 2017) and enables F-act polymer branching (Smith et al., 2013), and the formin family, which acts downstream of Rho-GTPases to nucleate unbranched F-act (Konietzny et al., 2017). In addition to their recognized role in actin nucleation and polymerization (Breitsprecher and Goode, 2013; Chesarone et al., 2010; Kawabata Galbraith and Kengaku, 2019), formins have been also implicated in MT stabilization through formin-homology-domain-induced acetylation (Thurston et al., 2012). A single molecule, Formin3, was shown to simultaneously regulate the stability and organization of the MT and F-act cytoskeletons, thereby impacting dendritic architecture (Das et al., 2017b). Moreover, inward actin waves facilitated by actin-depolymerizing factor (ADF)/cofilin allow MTs to protrude, stabilizing initial branch growth (Flynn et al., 2012). Recent fluorescence nanoscopic observations also revealed a periodic, ring-like, membrane-attached actin-spectrin scaffold mostly compartmentalizing axonal membrane but also found in dendrites (Unsain et al., 2018).
Although numerous upstream regulatory pathways have been discovered for MTs and F-act, their distinct roles in defining overall dendritic structure are still actively debated. Studies across various experimental systems have revealed varying effects of dynamic cytoskeletal events such as stabilization and polymerization. While MT stabilization has been shown to yield branching complexity in newly formed dendrites (Yau et al., 2014), the presence of stable MTs is limited only to the primary branches in the complex neurons, compared to their arbor-wide presence in the simpler Drosophila larval neurons (Jinushi-Nakao et al., 2007). Similarly, polymerizing MTs have been observed in growing branches (Kollins et al., 2009) and, at the same time, MT polymerization away from bifurcations and terminals restricts overall branching (Yalgin et al., 2015). While actin polymerization has been shown to drive neurite outgrowth (Chia et al., 2016), contrasting experiments have indicated that actin waves at growth cones do not initiate elongation and neurite outgrowth can occur without F-act at neurite tips (Mortal et al., 2017). Since the local densities of MTs and F-act have never been quantified arbor wide in large numbers of neurons, the multifarious relation between arbor architecture and cytoskeletal composition remains incompletely understood.
Here, we deploy the recently developed multi-modal confocal image stack tracing of multi-fluorescent neurons (Nanda et al., 2018a) to reconstruct a representative sample of mature da neuron morphologies containing arbor-wide local quantifications of MT and F-act concentrations. We test whether the distributions of MT and F-act in mature arbors can provide topological and developmental insights into the emergent dendritic structure. First, we devise innovative analyses of these enhanced data uncovering a doubly dissociated effect of MTs and F-act on dendritic architecture: local MT quantity predicts downstream dendritic length (for this and subsequent non-trivial terms, see Glossary in Supplemental Information) and thereby overall arbor size; conversely, tree complexity is associated with changes in local F-act quantity, as bifurcating regions are enriched in F-act. We then show that local cytoskeletal composition is sufficient in constraining complete arbor morphology by designing a generative model that computationally simulates tree structure purely based on local MT and local F-act concentrations measured from real neurons. The analytical observations, as well as computer simulation results, remain robust across functionally distinct da neuron subclasses and genetic alteration.
Results
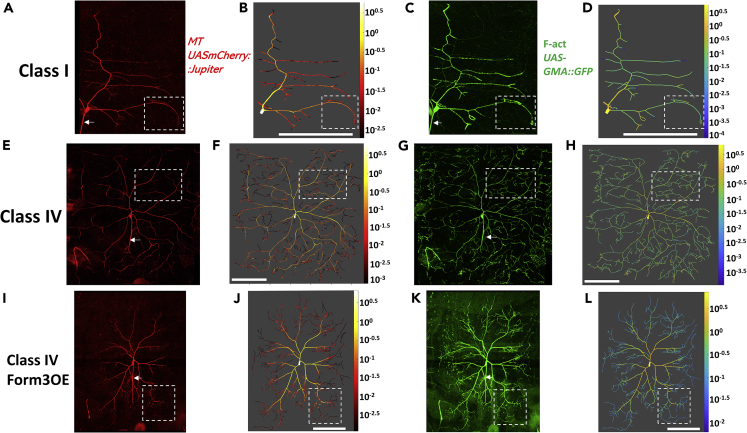
Class I and class IV neurons represent the two extremes in larval da neuron dendritic morphology with respect to arbor size and complexity (Iyer et al., 2013a). Class I neurons exhibit the simplest arbors and are the smallest of da neuron subclasses (Figures 1A–1D). Conversely, class IV neurons are space filling and constitute the most complex and largest da neurons (Figures 1E–1H). Here, we focused on the dorsal class I da neuron subtype E (ddaE) and the dorsal class IV da neuron subtype C (ddaC). Moreover, we considered genetic perturbation of cytoskeletal regulation by examining class IV (ddaC) neurons overexpressing Formin3 (Form3OE), which also differ significantly from the corresponding wild-type (WT) cells in both size and complexity (Figures 1I–1L). Formin3 overexpression was selected for genetic perturbation based on prior analyses (Das et al., 2017b) demonstrating a molecular role in differentially regulating dendritic F-act and MT cytoskeletal organization. All three neuron groups were subjected to live, in vivo imaging using genetically encoded, multi-fluorescent transgenic reporter strains to visualize F-act and MT distributions (Das et al., 2017a). All neurons were imaged during the third instar larva stage, when the da neurons acquire their mature shapes. Cytoskeletal organization in control or mutant genetic backgrounds was assessed by using class I- or class IV-specific GAL4 to label, respectively, stable MTs via the mCherry-tagged MT-associated protein Jupiter (Cabernard and Doe, 2009; Das et al., 2017a; Weiner et al., 2016) (Figures 1A, 1E, 1I, S1A, S1E, S1I, and S2) and F-act by a green fluorescent protein (GFP)-tagged moesin actin-binding domain (Figures 1C, 1G, 1K, S1C, S1G, and S1K) which has been previously validated and extensively utilized as a marker of F-act (Anderson et al., 2005; Das et al., 2017a; Dutta et al., 2002; Jinushi-Nakao et al., 2007; Lee et al., 2011; Nagel et al., 2012). Basic morphological reconstructions of these images consisted of vectorized tree structures composed of numerous, uniformly sampled, connected compartments (See Glossary in Supplemental Information for details). Enhanced multi-signal reconstructions were then created from the image stacks starting from the basic reconstruction templates by quantifying MT (Figures 1B, 1F, 1J, S1B, S1F, and S1J) and F-act (Figures 1D, 1H, 1L, S1D, S1H, and S1L) intensities in every compartment. MT intensities displayed a similar tapering pattern in all three neuron groups (Figures 1B, 1F, 1J, S1B, S1F, and S1J), generally decreasing with path distance from soma (negative Pearson correlation R coefficient for local MT quantity against path distance in all three neuron groups: −0.28 for class I, −0.36 for class IV wild type, and −0.52 for class IV Form3OE) and centrifugal branch order (Pearson coefficients for MT against centrifugal branch order: −0.22 for class I, −0.37 for class IV wild type, and −0.43 for class IV Form3OE). F-act intensities demonstrate weaker tapering tendency in class I and class IV WT neurons (Pearson coefficient for local F-act quantity against path distance: −0.21 for class I, −0.14 for class IV WT, and −0.57 for class IV Form3OE; for F-act against centrifugal branch order: −0.15 for class I, −0.09 for class IV WT, and −0.48 for class IV Form3OE), particularly in class IV WT neurons that had several terminal branches with high F-act (Figures 1D, 1H, 1L, S1D, S1H, and S1L).
Figure 1.
Confocal Images and Multi-Signal Reconstructions of da Neurons
Class I wild-type (A–D), class IV wild-type (E–H), and class IV mutant Form3OE (I–L) neurons are analyzed in this study. Microtubule (A, E, and I) and F-actin (C, G, and K) signals from confocal image stacks are traced together to produce multi-signal reconstructions, allowing the arbor-wide quantification and graphic rendering of microtubule (B, F, J) and F-actin (D, H, and L) signal (heatmaps represent cytoskeletal quantity, calculated by multiplying the local intensity by the compartment thickness: see Transparent Methods for a detailed description of microtubule and F-actin quantification). Axons (identified with arrows in A, C, E, G, I, and K), only partially visible, are not traced and analyzed in this study. White scale bars (B, D, F, H, J, and L), 100 μm. Figure S1 shows magnified versions of the areas “demarcated” by the dotted lines (A–L). See also Figures S2 and S3.
Morphometric and Cytoskeletal Differentiation among Neuron Classes
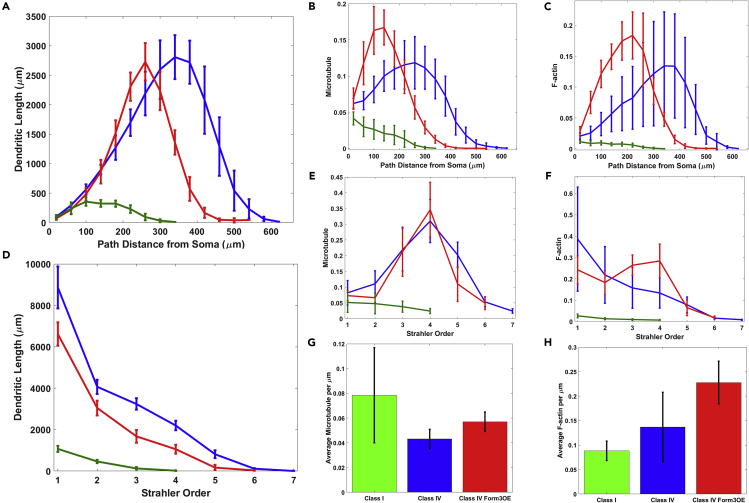
As expected, quantitative morphometry demonstrated substantial differences among the three neuronal groups in overall size and morphology (Figures 2A and 2D). Class IV WT neurons were clearly the largest in total dendritic length (19,305 ± 1510 μm, N = 10) and had the largest spanning field (height 727 ± 63 μm, width 593 ± 32 μm). Genetically altered class IV Form3OE neurons were smaller (total length 12,591 ± 985 μm, height 627 ± 44 μm, width 545 ± 32 μm, N = 9), and class I neurons were by far the smallest in length and spanning field (total length 1680 ± 166 μm, height 260 ± 21 μm, width 221 ± 21 μm, N = 19). Class IV WT neurons also had the most complex dendritic arbors as measured by the number of branch terminals (683 ± 44.7), followed by class IV Form3OE (365 ± 29.9) and the least complex class I (26 ± 4.8). Correspondingly, the maximum Strahler (centripetal) orders (Vormberg et al., 2017; see Glossary in Supplemental Information for definition) for the three groups were 7, 6, and 4, respectively (Figure 2D). These results are consistent with previous analyses of da neurons (Grueber et al., 2002; Nanda et al., 2018b).
Figure 2.
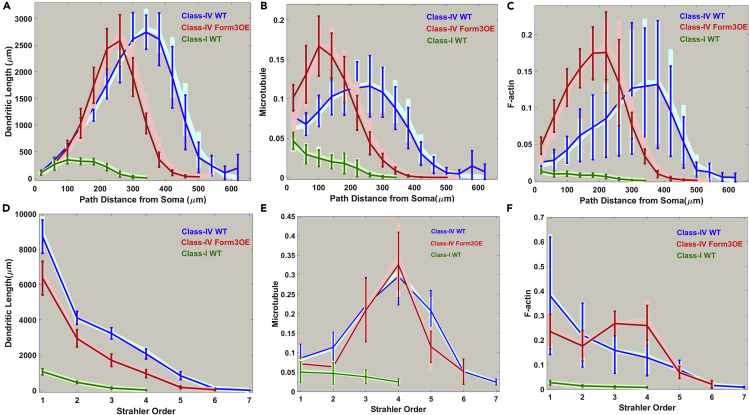
Morphological and Cytoskeletal Analysis of Three Groups of da Neurons from the Fruit Fly Larva
Distributions of dendritic length (A and D), microtubule (B and E), and F-actin (C and F) is examined as functions of path distance from soma (A–C) and Strahler order (D–F) for class I (green), class IV (blue), and mutant Class IV (red) neurons. Here, dendritic length (in Y axis) represents the total sum of dendrite length within each 40-μm path distance bin and at each Strahler order for every neuron and then averaged across neurons in each group. Similar to length, microtubule and F-actin quantities (computed as a product of signal intensity by compartment thickness: see Transparent Methods for details) are summed for each path distance bin and Strahler order and then averaged across neurons within each group. In (B), (C), (E), and (F), the Y axes were scaled so as to obtain unitary area under the curve for class IV WT (blue lines), i.e., all cytoskeletal quantities in these four plots are represented relative to class IV WT. Average microtubule and F-act quantities (per unit length) for each Strahler order and from each path distance bin are calculated by dividing the total values from (B) and (C) by the corresponding values from (A) and by dividing the total values from (E) and (F) by the corresponding values from (D). Average microtubule (G) and F-actin (H) quantities are also reported per unit of length (μm), i.e., total MT or F-actin in a neuron is divided by the total length (in μm) of that neuron. Computed values from individual neurons are then averaged across all neurons within a group. Error bars indicate standard deviation within groups. See also Figures S3 and S10 and Tables S1–S3.
We computed local MT and F-act quantity for each dendritic compartment from the multi-signal reconstructions as the product of the average signal intensity within that compartment and the local dendrite width (see Transparent Methods for details). The morphological patterns were also reflected in overall intensity distributions for both MT (Figures 2B and 2E) and F-act (Figures 2C and 2F). Notably, on average, class I had higher MT and lower F-act signal levels (per unit length) than class IV WT and class IV Form3OE, while Form3OE increased the average local quantities of both MT and F-act in class IV neurons (Figures 2G and 2H).
Interestingly, the local quantities of MT and F-act paralleled each other in the dendrites near the soma but not farther out in the dendritic trees. Specifically, in the 20% most proximal dendrites, MT and F-act intensities were highly correlated (Pearson R: 0.98 for class I, 0.88 for class IV, and 0.89 for class IV Form3OE), whereas the 20% most distal locations displayed drastically reduced correlation (R = 0.46, 0.32, and 0.68, respectively, for class I, class IV, and class IV Form3OE) (Table S1 and Figure S3).
Tree asymmetry can be quantified as the average over all bifurcations of the normalized difference between the number of terminals (topological asymmetry) or the downstream arbor lengths (length asymmetry) stemming from each of the two children of a branch point. This metric ranges between 0 for a perfectly symmetric tree and 1 for a fully asymmetric tree (Van Pelt et al., 1992) and can often discriminate among neuron types or even between dendritic arbors within the same neurons, as in the apical and basal trees of pyramidal cells (Samsonovich and Ascoli, 2005). An alternative measure of dendritic asymmetry, caulescence, captures the degree to which a neuron has a main dendritic path or trunk (Brown et al., 2008) and weighs the imbalance of each bifurcation by the size of its sub-tree, thus emphasizing branches closer to the soma (see Transparent Methods for mathematical definitions of asymmetry and caulescence). While tree asymmetry did not vary greatly among the three groups of neurons considered here, there were substantial differences in tree caulescence. Class I neurons displayed the clearest main path as reflected by their topological caulescence (0.579 ± 0.091) and even more by length caulescence (0.631 ± 0.107). Class IV dendrites grow out in all directions and hence did not show an evident main path (topological caulescence: 0.470 ± 0.080, length caulescence: 0.450 ± 0.067). Length and topological caulescences are significantly different between class I and class IV WT (p = 0.00005 and p = 0.0036), as well as between class I and class IV Form3OE (p = 0.0018 and p = 0.01) (Table S2).
Local Microtubule Quantity Is the Best Cytoskeletal Predictor of Arbor Length
Total arbor length is a strongly discriminant descriptor of dendritic architecture and quantifies tree size by accounting for both overall span and branching complexity (Brown et al., 2008). Arbor length can be measured not only at the soma, where it captures the total dendritic elongation summed over the whole neuron, but also at any dendritic position considered as the root of the downstream sub-tree (Figure S4). Sub-tree arbor length is known to correlate with several geometric characteristics measured at the local root, including path distance from the soma, centrifugal branch order, and branch thickness (Donohue and Ascoli, 2005; Nanda et al., 2018b; Samsonovich and Ascoli, 2005). Therefore, these parameters have long been considered numerical proxies of underlying molecular processes that actually shape dendritic architecture (Burke et al., 1992; Donohue and Ascoli, 2008). The neuron-wide measurement of local MT and F-act quantities allowed us to test directly how the two major cytoskeletal mediators of dendritic growth compare with local geometric parameters in predicting downstream sub-tree length across the entire dendritic arbor. Path distance from the soma, centrifugal branch order, branch thickness, and downstream arbor length were calculated at every dendritic location in all neurons.
Among all local geometric and cytoskeletal molecular parameters analyzed, MT quantity had the highest correlation with arbor length (Pearson's R coefficients, Table 1). MT quantity captured most arbor length variance (measured by the R-squared values) in class IV Form3OE (77.4%) followed by class IV WT (62.4%) and then class I (44.9%). Higher quantities of MT co-occurred with greater arbor length and, when MT ran out, dendritic branches tended to terminate (Figures 1, S1, and S4). Correspondingly, the correlation between MT quantity and arbor length was also considerably reduced in terminal branches (Table S1), where it only accounted for negligible amounts of variance (6.8% for class I, 2.6% for class IV, and 0.04% for class IV Form3OE). This is also consistent with the known greater dynamic plasticity of terminal branches, which can continuously elongate or retract in mature arbors. Interestingly, MT quantity alone had a higher correlation with arbor length than when combined with F-act quantity (Table 1). Among geometric parameters, branch diameter had the highest correlations with both MT quantity and arbor length (Table 1). Remarkably, these findings directly confirm early theoretical claims (Hillman, 1979; Burke et al., 1992). It should be noted that diameter measurement is limited by the resolution of light microscopy, which for the imaging system utilized in the current study is 311 nm (see Transparent Methods in Supplemental Information for calculation details). To estimate the maximum extent to which the correlation between arbor length and diameter could improve by eliminating the measurement error due to the resolution limit, we generated a surrogate set of diameter values by moving the measured values toward the regression line by a factor sampled from a uniform distribution with an upper bound of 311 nm (see Methods for details). Except for class I, the resulting increased correlation still remained less predictive of arbor length than local MT (Pearson correlation coefficients between surrogate diameter and arbor length: 0.73 for class I, 0.60 for class IV, and 0.67 for Form3OE). While this simulation provides an upper bound on the effect of the error, neurite thickness measured from electron microscopy will allow for a more accurate estimation.
Table 1.
The Correlation of Dendritic Arbor Length with Morphological and Cytoskeletal Parameters
| Cell Type | MT Quantity | F-act Quantity | MT + F-act Quantity | Path Distance from Soma | Branch Order | Diameter |
|---|---|---|---|---|---|---|
| Class I | 0.67 | 0.55 | 0.62 | −0.47 | −0.33 | 0.64 |
| Class IV | 0.79 | 0.43 | 0.63 | −0.45 | −0.42 | 0.49 |
| Class IV Form3OE | 0.88 | 0.76 | 0.82 | −0.47 | −0.39 | 0.61 |
Pearson correlation coefficients (R-values) of arbor length against morphological and cytoskeletal parameters at the resolution of single (2 μm long) compartments. Microtubule quantity is the best predictor of arbor length for all analyzed cell groups. See also Table S1 and S4 and Figures S4, S7, and S8.
Bifurcations Are Strongly Associated with Local F-Actin Enrichment
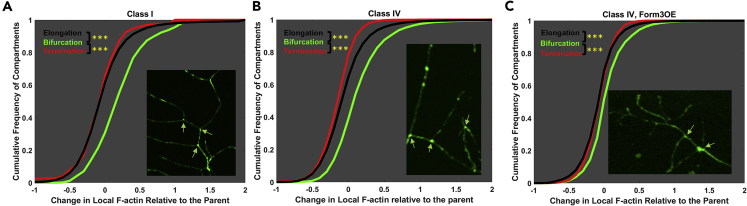
Relative to MTs, F-act quantity displayed weaker tapering tendency (except in class IV Form3OE) and lower correlation with arbor length (Figures 1 and 2 and Table 1). Instead, we observed definitive patterns in the relative change of F-act between adjacent locations. Specifically, a systematic punctate expression of F-act was qualitatively apparent at dendritic bifurcations (Figure 3). To quantify this phenomenon, we computed the difference in F-act at every dendritic compartment relative to its parent compartment (ΔF), where a ΔF value of −1 represented complete elimination of F-act (from parent to the current), a ΔF value of 0 denoted no change, a ΔF value of +1 denoted doubling of F-act quantity (see Transparent Methods in Supplemental Information for detail). We then separated all compartments based on their topological behaviors: elongations, bifurcations, and terminations. We found that most bifurcating compartments (74% in class I, 68% in class IV, and 60% in class IV Form3OE) showed F-act enrichment, i.e., an increase in F-act relative to their parent compartments. The cumulative ΔF frequency was significantly right shifted for bifurcations relative to elongations and terminations in all three neuron groups (Figures 3 and S5). The median ΔF for bifurcations was 0.201, 0.121, and 0.041 in class I, class IV, and class IV Form3OE neurons, respectively. The corresponding median ΔF for elongations was −0.024, −0.044, −0.033, respectively, indicating a tendency of F-act to decrease along non-bifurcating branches. Only 24% of class I, 25% of class IV, and 31% of class IV Form3OE bifurcations displayed a ΔF value lower than the respective elongation medians.
Figure 3.
Cumulative Frequency of the Fold Change in Local F-actin Concentration Relative to the Parent Compartment for Bifurcating (Green), Elongating (Black), and Terminating (Red) Compartments
A value of 0 indicates unchanged F-actin concentration; a value of −1 indicates complete F-actin disappearance (100% decrease relative to parent compartment); and a value of +1 indicates doubling F-actin concentration (100% increase relative to parent compartment). Class I (A), class IV (B), and mutant class IV Form3OE (C) distributions are shown separately. In all three cases, the bifurcating line is clearly right shifted relative to the terminating and elongating lines. The arrowheads in the inset confocal images point to representative branch points. Triple asterisks indicate significantly different comparisons with p < 0.001, Bonferroni-corrected 1-tail t test. See also Figures S5–S9.
To test whether F-act enrichment could be due to the enlargement of branch points, we repeated the analysis using signal intensity instead of F-act quantity, effectively normalizing the influence of dendritic diameter. This demonstrated equivalent or slightly more pronounced F-act enrichments, with median ΔF of 0.26, 0.15, and 0.07 at branch points in class I, class IV WT, and class IV Form3OE neurons, respectively. Correspondingly, only 19% of class I, 21% of class IV WT, and 27% of class IV Form3OE bifurcations displayed a ΔF intensity value lower than their respective elongation medians. Thus, F-act enrichment at bifurcations cannot be explained by branch point thickness. Furthermore, the levels of MT enrichments at branch points were modest when compared to those of F-act. Class IV WT did not show any overall MT enrichment (median ΔM at branch points was 0). Class I showed relatively lower levels of MT enrichment (median ΔM of 0.101 at branch points, compared to ΔF of 0.201); class IV Form3OE, which had the smallest levels of F-act enrichment, had similar levels of MT enrichment (ΔM of 0.043 and ΔF of 0.041). These values can be explained simply by the correlation of MT and F-act signals.
To test whether F-act enrichment occurs preferentially or exclusively in newly formed branches, we analyzed enrichment as a function of Strahler order. Because all newly formed (terminal) branches have a Strahler order of 1, higher Strahler order branches must have formed earlier in the arbor development. We observed that more than half of all branch points across all Strahler orders, except the maximum order corresponding to the soma, were F-act enriched (Figures S6A–S6C). Moreover, F-act enrichment moderately decreased with Strahler order in class I (R = −0.17, p < 0.05) and class IV Form3OE (R = −0.11, p < 0.05) neurons (Figures S6D–S6F). While enrichment in higher Strahler orders suggests stable F-act enrichments in older branches, the greater level of enrichment in lower Strahler orders may reflect a dual mechanism of transient and stable enrichment in newly formed branches. The comprehensive arbor-wide quantification in the current study thus extends previous sparse observations of this phenomenon to mature arbors.
The average bifurcation angle (the local angle created at the branch point between the two bifurcating branches) was highest for class I neurons (98 ± 6.1°) followed by class IV WT (92.8 ± 1.65°) and class IV Form3OE (90.09 ± 1.12°). We tested if local bifurcation angle was correlated with MT, F-act, or their local changes. While class I WT neurons demonstrated no such correlations, class IV WT neurons only showed very low negative correlation between local MT and bifurcation angle (R = −0.03, p < 0.05). Class IV Form3OE neurons demonstrated low correlations between bifurcation angle and all four cytoskeletal parameters (Pearson R: MT = −0.08, F-act = −0.14, ΔM = 0.04, ΔF = 0.11).
Distinct Effects of Microtubules and F-Actin on Dendritic Growth and Branching
Our quantitative analyses suggest a stark double dissociation between the two major mechanisms to establish dendritic architecture, namely branch elongation and bifurcation, and distinct cytoskeletal determinants, respectively, MT quantity and F-act enrichment. The smaller levels of residual association of branching with MT and arbor length with F-act can be attributed to the overall correlation between these two cytoskeletal quantities.
Correspondingly, an in-depth inspection of separate topological events (occurrence of terminating, elongating, or bifurcating dendrites, see Glossary in the Supplemental Information for definition) as a combined function of local MT quantity and relative F-act change (Figures S7A–S7C) revealed almost entirely complementary cytoskeletal compositions underlying bifurcations (F-act enrichment independent of MT quantity) and terminations (strong decline or absence of MT). Only in Form3OE class IV neurons did elongation-rich and termination-rich regions partially overlap (Figure S7C). We also found converging evidence at the dendritic branch level. Class I neurons had a relatively high ratio of MT to F-act quantities (0.89 ± 0.32) and displayed greater average branch length (33.57 ± 5.11 μm), i.e., a reduced number of bifurcations relative to total elongation. Class IV neurons (both WT and Form3OE), on the contrary, had shorter branches on average (WT: 14.2 ± 1.0 μm, Form3OE: 17.3 ± 0.7 μm) and lower MT to F-act ratios (WT: 0.38 ± 0.14, Form3OE: 0.25 ± 0.04) (Table S3). In other words, the ratio between arbor size and complexity paralleled the ratio between MT and F-act quantities. The proportion of arbor lacking detectable MT across neuronal groups was also negatively correlated with average branch length (R = −0.998, p < 0.001) (Figure S8).
While F-act also correlates with arbor length and MT also tends to enrich at class I and class IV Form3OE branch points, both of these observations can be attributed to the correlation between MT and F-act. The differential associations of MT with arbor length and of F-act with branching are least specific in class IV Form3OE neurons: in this group, F-act correlates relatively strongly with arbor length (though not as much as MT) and the branch point enrichment of MT is equivalent to that of F-act. This could be explained by the higher correlation between local MT and F-act in Form3OE neurons (R = 0.86) as compared to class IV WT neurons (R = 0.65).
Taken together, these interactions may provide a complete description of two fundamental aspects of dendritic arbor topology: size (total length) and complexity (number of branches). Considering this evidence, we proceeded to test whether local MT and F-act measurements could be sufficient to reproduce the arbor-wide morphological and cytoskeletal properties for all three neuron groups.
Computational Model of Arbor Generation Based on Local Microtubule and F-Actin
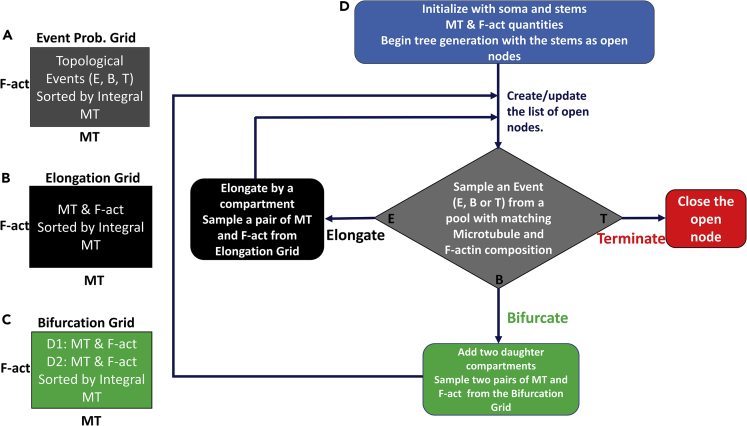
Previous studies have shown that neural arbors of various cell types (Ascoli et al., 2001; Donohue and Ascoli, 2008), including da neuron arbors (Nanda et al., 2018b), can be simulated by computational models constrained by local geometry (path distance, branch order, and branch thickness). Our analysis indicated that local cytoskeletal components had greater influence over arbor length and branching probability than previously used geometric parameters. To test the efficacy of local MT and F-act quantities in reproducing overall tree architecture, we designed a computational model of dendritic morphology purely driven by local intensities of MT and F-act measured from the reconstructed dendritic trees (Figure 4). We directly tested whether local branching behaviors (extensions, branching, etc.) can be statistically reproduced by two local cytoskeletal constraints, MT and F-act quantities, and whether the simulated structures match the topology of real neurons.
Figure 4.
Description of the Generative Model of MT- and F-act-Driven Virtual Dendritic Arbor Generation
(A–D) Stochastic sampling is carried out from three data grids (A–C) binned by local microtubule (MT) and F-actin (F-act) quantities. The grids are used to sample (A) branch topology (elongation, bifurcation, or termination), as well as the cytoskeletal compositions for the child compartments of elongations (B) and bifurcations (C). All data are sampled from the bin that best matches the MT and F-act composition of the active compartment, with a bias for higher integral microtubule values (see Methods for definition). The tree generation process (D) starts with the initial MT and F-act concentration of the soma and the dendritic stem as active compartments. For each active compartment, branch topology sampling determines whether to elongate (adding one new compartment), bifurcate (adding two new compartments), or terminate (ending the branch). If a node elongates or bifurcates, the cytoskeletal composition of the newly created compartment(s) is sampled from the corresponding data grids. The process is repeated for all newly added nodes, until all branches terminate. The model described here is employed for all three neurons types in this study.
The model consisted of two stochastic processes iterated over all generated compartments starting from the dendritic roots stemming from the soma: sampling of topological events (elongation, bifurcation, and termination) and sampling of cytoskeletal composition. Thus, in these simulations, local MT and F-act quantities determined not only the branching behavior (whether to elongate, bifurcate, or terminate) in that dendritic location but also the cytoskeletal composition of the child compartment(s), which in turn determined the branching behavior of their child compartment(s), with the process continuing until all branches terminate. The first stochastic process sampled one of three possible topological events from the corresponding data grid (Figure 4A). Sampling an elongation event added one child compartment; sampling a bifurcation event added two child compartments; sampling a termination added no compartment and closed the active compartment. The second process, determining the cytoskeletal composition in the child compartments, was further subdivided into two sampling processes, for elongation (Figure 4B) and for bifurcation (Figure 4C). The elongation grid contained the cytoskeletal composition of all compartments whose parents were not bifurcating. The bifurcation grid contained the cytoskeletal composition of all bifurcation children. Depending on whether a compartment elongated or bifurcated, one (for elongations) or two (for bifurcations) pairs of MT and F-act quantities were sampled and assigned to the newly added dendritic compartment(s). All three grids (see Glossary in Supplemental Information) are two-dimensional matrices whose entries correspond to small ranges of MT and F-act values. The sampling processes were all similarly constrained by MT and F-act composition of the active compartment, ordered by the weighted MT integral from the soma to the compartment's parent (see Transparent Methods in Supplemental Information for mathematical definition). The simulations ran until all compartments terminated (Figure 4D), and the number of simulated neurons was matched to the number of real neurons for every cell group. Note that this generative model only describes arbor topology and branch length (i.e., “dendrograms”), as well as cytoskeletal distributions, but not the three-dimensional geometry of dendritic trees (bifurcation angles and branch meandering). Both local parameters were essential to the model as high MT predicted larger downstream arbor length and enriched F-act separated bifurcation events from elongations and terminations.
Simulated Neurons Reproduce Morphology and Cytoskeletal Distributions across Neuron Groups
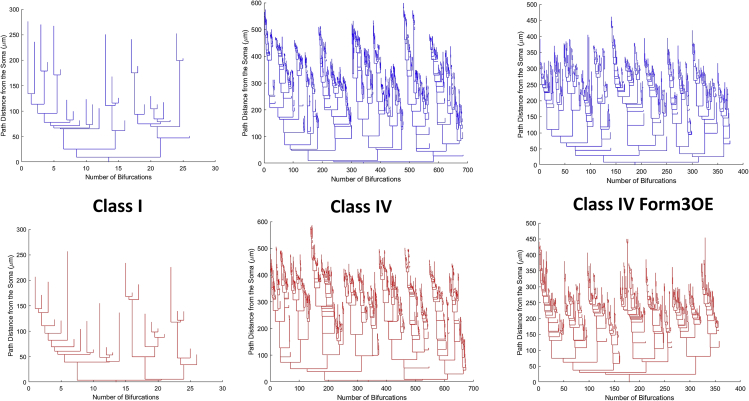
Dendrograms visually represent the size and complexity of neurons while disregarding other aspects of arbor shape such as angles. The x axis of the dendrograms represents the number of bifurcations and terminations. The Y axis represents path distance from the soma. Dendrograms are sorted so as to place the larger downstream sub-tree to the left of each bifurcation. The dendrograms generated by the computational simulations were visually and qualitatively equivalent (as if sampled from the same group) with the real neuron dendrograms for each of the three neuron groups (Figure 5). Moreover, the simulated neurons of class I, class IV, and class IV Form3OE quantitatively reproduced all measured morphometric characteristics of their real counterparts, namely total dendritic length, number of terminal tips, topological and length asymmetry, and topological and length caulescence. Thus, relative to the model-generated class I dendrograms, model-generated class IV dendrograms were much longer and more complex but had lower topological and length caulescence (Table S2). The model-generated class IV Form3OE dendrograms had generally intermediate values between the two WT subclasses in these measures. In all quantified characteristics, the model fully reproduced the class-specific morphometrics in terms of group average and variance. None of the 18 two-tailed t-tests (6 measurements for each of 3 classes, Table S2) comparing real and simulated neurons yielded statistically significant differences (p > 0.5 even with no multiple testing correction).
Figure 5.
Dendrograms of Real and Virtual Dendritic Arbors
Representative dendrograms of real (top, blue) and simulated (bottom, red) class I (left), WT class IV (middle), and mutant class IV Form3OE (right) neurons demonstrate the distinct size and complexity of the three neuron types. X axis represents the number of bifurcations and terminations; y axis represents path distance from the soma. Dendrograms are sorted so that the larger sub-tree at each bifurcation is on the left. See also Tables S1–S3.
Next, we compared real and simulated data by examining the distributions of dendritic length, MTs, and F-act as functions of path distance from soma and Strahler order. In all six cases, the class-specific patterns of the simulated neurons were qualitatively and quantitatively almost identical to those of their real equivalents (Figure 6). Thus, the model was able to capture not only the overall tree properties of class I, class IV, and class IV Form3OE but also their rise or decline across path distance and branch order.
Figure 6.
Comparison of Real and Emergent Cytoskeletal Morphometrics
(A–F) Emergent distributions of dendritic length (A and D), total microtubule (B and E), and F-actin (C and F) quantity (see Transparent Methods) by path distance (A–C) and Strahler order (D–F) of simulated class I (green), class IV (blue), and class IV Form3OE (red) neurons. Error bars indicate standard deviation. Corresponding distributions of real neurons are overlaid in lighter shades of blue, red, and green. See also Figure S10 and Tables S1–S3.
Furthermore, simulated neurons exhibited similar relations between cytoskeletal composition and morphology as real neurons. Model-generated arbor length was highly correlated to local MT quantity (Table S4) but substantially less so in terminating branches (Table S1), and F-act enrichment was strongly associated with bifurcating compartments (Figure S9). Interestingly, the negligible correlation of arbor length with local MT in terminal dendritic branches did not significantly impact the similarity of real and simulated morphologies. This is because most of the terminal branches lacked detectable MT and, although the terminal branch lengths varied stochastically irrespective of MT quantity, such variability occurred within a relatively small range of arbor length when compared to overall arbor size.
Just as in real neurons, the correlation between MT and F-act quantities also decreased with path distance in synthetic neurons (Table S1 and Figure S10). Finally, the computational model also reproduced the class-specific branch length (overall as well as separately for bifurcating and terminating branches) and the ratio between MT and F-act quantities (Table S3), thus replicating in the simulated neurons the parallel between the arbor size-to-complexity relation and the MT-to-F-act relation observed in real cells.
Discussion
MTs and F-act have long been implicated in neurostructural plasticity; however, their exact roles have not been fully elucidated due to the lack of their simultaneous quantification across the entirety of the neural arbor. With the use of the newly developed multi-signal reconstruction system (Nanda et al., 2018a), we quantified the arbor-wide MT and F-act distributions from live images of fruit fly sensory neurons. Analysis of cytoskeletal distribution revealed the association of local MT with dendritic elongation (arbor size) and of local F-act with dendritic branching (arbor complexity). These two distinct links of MTs and F-act with complementary aspects of dendritic morphology were consistent across structurally and functionally different cell types, as well as a genetic alteration affecting cytoskeletal organization.
The striking structural difference in dendritic morphology between WT class I and class IV da neurons is reflected in their substantially different functional roles and efferent patterns within the larval sensory system. Class I neurons participate in proprioceptive functions (Cheng et al., 2010; He et al., 2019; Hughes and Thomas, 2007; Vaadia et al., 2019), whereas class IV neurons are involved in polymodal nociception (Hwang et al., 2007; Lopez-Bellido et al., 2019; Tracey et al., 2003), chemosensation (Himmel et al., 2019), and photoreception (Xiang et al., 2010). Previous studies have also shown that genetic manipulations that alter overall morphology do not necessarily affect the size to complexity ratio of class IV neurons. In contrast, class I neurons drastically change their size to complexity ratio when genetically altered (Iyer et al., 2013a). Despite these structural and functional differentiations, the architectural rules revealed in this study remained consistent across the two da class extremes, quantitatively relating local cytoskeletal composition to the immediate branching behavior (bifurcation, elongation, or termination) on the one hand and to overall morphology (arbor length and asymmetry) on the other hand. Intriguingly, class-specific dendritic architectures for either class I or class IV WT neurons could be computationally simulated with high fidelity by solely considering their different distribution patterns of local cytoskeletal compositions for F-act and MTs. Even genetic alterations in the mutant class IV did not alter the fundamental relationships among local cytoskeletal composition, branching events, and overall arbor shape. The reduction in F-act enrichment at branch points for class IV Form3OE neurons relative to the WT was reflected in their relative reduction of overall branching complexity as well. We also observed across the three groups of neurons that the distribution patterns of MTs were similar to those of F-act in proximal but not distal dendrites, which could be attributable to the constraints of transcription and transport: tubulin and actin monomers are produced at the soma and then diffuse or are actively transported to the proximal branches first, followed by distal branches.
Most morphological characteristics analyzed in this study were statistically different between the three neuron groups. The proposed computational model, although simple, fully reproduced the group-by-group morphological and cytoskeletal properties observed in real neurons while demonstrating the correct relationships between cytoskeletal composition and arbor structure and vice versa. The morphological features captured by the model included total length and overall complexity, distribution of dendritic branches, asymmetry of length and branching, and prominence of main dendritic path. Cytoskeletal properties included arbor-wide distributions of MTs and F-act, correlation of MTs with arbor length, and association of branching with F-act enrichment. Therefore, the analysis and modeling results jointly suggest that the diverse dendritic morphologies across all investigated cell groups shared similar underlying cytoskeletal mechanisms.
Consistent with the model, local increases in the quantity of stable MTs through Formin3 overexpression further improved the correlation between MT quantity and arbor length. Increased F-act signal by the same genetic alteration nearly doubled local F-act quantity but actually reduced the enrichment levels at bifurcations. This suggests that enrichment events are more topology specific in WT neurons, occurring when new branching events take place, while the tree-wide increase of F-act in Form3OE decreases the difference between bifurcating and elongating compartments. As a combined result, Formin3 overexpression substantially lowered total dendritic length and arbor complexity in class IV neurons, yielding a reduction in total F-act and MT quantities despite the local increases of both cytoskeletal components. These observations corroborate the notion that class IV neurons are evolutionarily optimized to fill a large amount of space through branching and elongation, maximizing the use of available resources (Grueber et al., 2002). Disrupting the distribution of cytoskeletal resources could thus interfere with its space-filling mechanisms (Das et al., 2017a; Nanda et al., 2018b).
The consistent correlation observed between MT quantity and arbor length quantitatively confirms after forty years a seminal prediction based on early electron microscopy evidence (Hillman, 1979): namely, that the number of MT polymers progressively decreases along the dendrites with distance from the soma and approaches zero near the terminal tips. Specifically, this decrease was posited to be gradual within a branch, as some MTs stop polymerizing, and discontinuous at bifurcations, where the MT polymers would partition between the two child dendrites. While the above conceptual model was originally formulated relying on sparse evidence from individual dendritic branches, it proved to remain consistent with more recent electron microscopic data demonstrating lack of MT in terminal branches in contrast to the MT “backbone” of primary dendrites (Schneider-Mizell et al., 2016). Our multi-signal dendritic reconstruction and analysis now quantitatively corroborates these earlier observations at the level of entire arbors and across neuron groups. Those pioneering ideas along with initial ultrastructural observations that dendritic branch thickness closely corresponds with the number of MT polymers at that location (Hillman et al., 1980) inspired a successful series of early computational simulations in which larger and smaller branch diameters increased the probability of bifurcating and terminating, respectively (Donohue and Ascoli, 2008; Schneider et al., 2012). Another resource-driven phenomenological computer model posited the diffusion or active transport of somatically produced tubulin to the neurite tips or growth cones where it would polymerize to extend the existing MT structure. The dynamic competition for tubulin among outgrowing branches can simulate simple as well as complex arbor morphologies (Hjorth et al., 2014). Other computational models finessed the influence of anterograde MT movement in tree generation by reducing the likelihood of creating new branches with path distance from soma or branch order (Donohue and Ascoli, 2008; Nanda et al., 2018b; Samsonovich and Ascoli, 2005; Van Pelt and Uylings, 2002). The current study is unprecedented in driving the computational model directly by the experimentally recorded local cytoskeletal resources. While we observe that dendritic terminals mostly lacked visible MTs, it is important to note that the MT marker Jupiter used in this study tags stable MTs. Therefore, dynamic MTs and tubulin monomers might be present in the terminal dendritic regions.
While local MT quantity predicted downstream arbor length, the local change in F-act constrained branching events. Enrichment of F-act near dendritic bifurcations may be linked to satellite Golgi outposts that have also been observed at the dendritic branch points where MTs are nucleated and membrane proteins are produced (Ori-McKenney et al., 2012). Higher F-act levels may be important to maintain the presence of the Golgi apparatus at branch points since actin is involved in regulating Golgi structure and function (Gu et al., 2001). Interestingly, the differences in F-act enrichment between terminating and elongating dendrites were much more modest. This may be because the reconstructions are from static image stacks, providing a momentary snapshot of a structurally dynamic arbor. Hence, the compartments annotated as terminating could either be elongating or retracting. This interpretation is consistent with previous experimental observations that terminal branches remain dynamic even when the primary branches become stable (Lee et al., 2011).
While the local cytoskeletal composition of mature arbors was surprisingly predictive of overall dendritic size and complexity, this study did not model the geometric shape of the arbors, which results from a combination of several factors including stem orientation (Samsonovich and Ascoli, 2003) and branch angles (Bielza et al., 2014), self-avoidance (Kidd and Condron, 2007; Sundararajan et al., 2019), and tiling (Grueber et al., 2002). Dendritic pruning (Herzmann et al., 2018) and underlying developmental processes such as the polymerization, depolymerization, bundling, severing of MTs or F-act, traffics of cargos, and stoichiometry of MTs and F-act are also not accounted for in the model. Time-lapse imaging of dendritic morphology with multiple fluorescent proteins tagging the orientation of MT and F-act polymerization will be informative for future studies to investigate further arbor plasticity and the underlying subcellular changes that drive it. We have recently developed an enhanced reconstruction system to annotate temporal changes in neuronal structure simultaneously with subcellular changes (Nanda et al., 2018a). Time-varying reconstructions with local polymerization information (rate and direction) may foster a deeper understanding of potential developmental mechanisms. Future studies should attempt to directly alter MT or actin stability by using cytoskeletal effectors such as drugs (Otto et al., 1979), increase in temperature (Spedden et al., 2013), and mechanical force to observe the effects on overall neuron morphology. The sufficiency of newly hypothesized rules of growth can then be tested by implementing a computational simulation constrained by the enhanced acquired data to verify whether it also reproduces dynamical phenomena such as scaling, retraction, and branch thickening. However, in order to become viable for use in developmental models, time-lapse reconstructions require the careful registration of image stacks, which is still exceedingly challenging at the level of entire dendritic arbors in vivo (Rada et al., 2018; Sheintuch et al., 2017).
Limitations of the Study
The static images used in this study do not allow a direct assessment of the more dynamic short timescale developmental events. Additionally, the resolution limit of confocal light microscopy restricts the reliability of neurite width measurements. This study focuses on a single invertebrate species, leaving the open question of whether the conclusions can be generalized to vertebrate nervous systems.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Resource Availability
Lead Contact
Further information and request of resources should be directed to Giorgio A. Ascoli (ascoli@gmu.edu).
Materials Availability
All unique/stable reagents generated in this study are available from the Cox Lab without restriction. Requests for these reagents can be submitted to Daniel N. Cox (dcox18@gsu.edu).
Data and Code Availability
All image stacks, data, analysis and modeling code are available at Mendeley.com (DOI: https://doi.org/10.17632/v3ncxmj6fn.1). Neuronal reconstructions are available at NeuroMorpho.Org (Ascoli and Cox archives).
Acknowledgments
The authors thank Mahan Mollajafar, Anna Lulushi, Akhil Goel, and Alisha Compton for helping with neuron tracings. This study receivied funding support from National Institutes of Health (NIH) R01 NS39600 and NS086082 and BRAIN Initiative Cell Census Network (BICCN) U01 MH114829.
Author Contributions
S.N. and G.A.A. originally designed the paper and conceived the model with D.N.C. providing early ideas on experimental data. S.B. conducted all wet lab experiments and multi-signal neuronal image acquisition with D.N.C's supervision. S.N. reconstructed the neurons and wrote all analysis and simulation software. S.N. and G.A.A. analyzed the data and devised the generative algorithm of dendritic arbor morphology. S.N. created the figures and tables by incorporating feedback from all co-authors. S.N. and G.A.A. wrote the manuscript with D.N.C. and S.B. providing crucial feedback and edits. D.N.C. and G.A.A. are responsible for funding acquisition and project management.
Declaration of Interests
The authors declare no competing interests.
Published: December 18, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101865.
Supplemental Information
References
- Alushin G.M., Lander G.C., Kellogg E.H., Zhang R., Baker D., Nogales E. High-Resolution microtubule structures reveal the structural transitions in αβ-tubulin upon GTP hydrolysis. Cell. 2014;157:1117–1129. doi: 10.1016/j.cell.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R., Li Y., Resseguie M., Brenman J.E. Calcium/Calmodulin dependent protein kinase II alters structural plasticity and cytoskeletal dynamics in Drosophila. J. Neurosci. 2005;25:8878–8888. doi: 10.1523/JNEUROSCI.2005-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli G.A., Krichmar J.L., Scorcioni R., Nasuto S.J., Senft S.L., Krichmar G.L. Computer generation and quantitative morphometric analysis of virtual neurons. Anat. Embryol. (Berl). 2001;204:283–301. doi: 10.1007/s004290100201. [DOI] [PubMed] [Google Scholar]
- Bielza C., Benavides-Piccione R., López-Cruz P., Larrañaga P., Defelipe J. Branching angles of pyramidal cell dendrites follow common geometrical design principles in different cortical areas. Sci. Rep. 2014;4:5909. doi: 10.1038/srep05909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D., Bunge M.B. Serial analysis of microtubules in cultured rat sensory axons. J. Neurocytol. 1981;10:589–605. doi: 10.1007/BF01262592. [DOI] [PubMed] [Google Scholar]
- Breitsprecher D., Goode B.L. Formins at a glance. J. Cell Sci. 2013;126:1–7. doi: 10.1242/jcs.107250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K.M., Gillette T.A., Ascoli G.A. Quantifying neuronal size: summing up trees and splitting the branch difference. Semin. Cell Dev. Biol. 2008;19:485–493. doi: 10.1016/j.semcdb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R.E., Marks W.B., Ulfhake B. A parsimonious description of motoneuron dendritic morphology using computer simulation. J. Neurosci. 1992;12:2403–2416. doi: 10.1523/JNEUROSCI.12-06-02403.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabernard C., Doe C.Q. Apical/basal spindle orientation is required for neuroblast homeostasis and neuronal differentiation in Drosophila. Dev. Cell. 2009;17:134–141. doi: 10.1016/j.devcel.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Cheng L.E., Song W., Looger L.L., Jan L.Y., Jan Y.N. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron. 2010;67:373–380. doi: 10.1016/j.neuron.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesarone M.A., Dupage A.G., Goode B.L. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat. Rev. Mol. Cell Biol. 2010;11:62–74. doi: 10.1038/nrm2816. [DOI] [PubMed] [Google Scholar]
- Chia J.X., Efimova N., Svitkina T.M. Neurite outgrowth is driven by actin polymerization even in the presence of actin polymerization inhibitors. Mol. Biol. Cell. 2016;27:3695–3704. doi: 10.1091/mbc.E16-04-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles C.H., Bradke F. Coordinating neuronal actin-microtubule dynamics. Curr. Biol. 2015;25:R677–R691. doi: 10.1016/j.cub.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Corty M.M., Tam J., Grueber W.B. Dendritic diversification through transcription-factor mediated suppression of alternative morphologies. Development. 2016;143:1351–1362. doi: 10.1242/dev.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R., Bhattacharjee S., Patel A.A., Harris J.M., Bhattacharya S., Letcher J.M., Clark S.G., Nanda S., Iyer E.P.R., Ascoli G.A., Cox D.N. Dendritic cytoskeletal architecture is modulated by combinatorial transcriptional regulation in Drosophila melanogaster. Genetics. 2017;207:1401–1421. doi: 10.1534/genetics.117.300393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R., Letcher J.M., Harris J.M., Foldi I., Nanda S., Bobo H.M., Mihály J., Ascoli G.A., Cox D.N. Formin3 regulates dendritic architecture via microtubule stabilization and is required for somatosensory nociceptive behavior. bioRxiv. 2017:227348. doi: 10.1101/227348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre-Ubieta L., Bonni A. Transcriptional regulation of neuronal polarity and morphogenesis in the mammalian brain. Neuron. 2011;72:22–40. doi: 10.1016/j.neuron.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue D.E., Ascoli G.A. A comparative computer simulation of dendritic morphology. Plos Comput. Biol. 2008;4:e1000089. doi: 10.1371/journal.pcbi.1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue D.E., Ascoli G.A. Local diameter fully constrains dendritic size in basal but not apical trees of CA1 pyramidal neurons. J. Comput. Neurosci. 2005;19:223–238. doi: 10.1007/s10827-005-1850-5. [DOI] [PubMed] [Google Scholar]
- Dutta D., Bloor J.W., Ruiz-Gomez M., VijayRaghavan K., Kiehart D.P. Real-time imaging of morphogenetic movements in Drosophila using Gal4-UAS-driven expression of GFP fused to the actin-binding domain of moesin. Genesis. 2002;34:146–151. doi: 10.1002/gene.10113. [DOI] [PubMed] [Google Scholar]
- Ferreira T., Ou Y., Li S., Giniger E., van Meyel D.J. Dendrite architecture organized by transcriptional control of the F-actin nucleator Spire. Development. 2014;141:650–660. doi: 10.1242/dev.099655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn K.C., Hellal F., Neukirchen D., Jacob S., Tahirovic S., Dupraz S., Stern S., Garvalov B.K., Gurniak C., Shaw A.E. ADF/Cofilin-Mediated actin retrograde flow directs neurite formation in the developing brain. Neuron. 2012;76:1091–1107. doi: 10.1016/j.neuron.2012.09.038. [DOI] [PubMed] [Google Scholar]
- Franker M., Hoogenraad C. Microtubule-based transport – basic mechanisms, traffic rules and role in neurological pathogenesis. J. Cell Sci. 2013;126:2319–2329. doi: 10.1242/jcs.115030. [DOI] [PubMed] [Google Scholar]
- Grueber W.B., Jan L.Y., Jan Y.N. Different levels of the homeodomain protein cut regulate distinct dendrite branching patterns of Drosophila multidendritic neurons. Cell. 2003;112:805–818. doi: 10.1016/s0092-8674(03)00160-0. [DOI] [PubMed] [Google Scholar]
- Grueber W.B., Jan L.Y., Jan Y.N. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- Gu F., Crump C.M., Thomas G. Trans-Golgi network sorting. Cell Mol. Life Sci. 2001;58:1067–1084. doi: 10.1007/PL00000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y., Usui T., Satoh D., Moriyama S., Shimono K., Itoh T., Shirahige K., Uemura T. Sensory-neuron subtype-specific transcriptional programs controlling dendrite morphogenesis: genome-wide analysis of abrupt and knot/collier. Dev. Cell. 2013;27:530–544. doi: 10.1016/j.devcel.2013.10.024. [DOI] [PubMed] [Google Scholar]
- He L., Gulyanon S., Mihovilovic Skanata M., Karagyozov D., Heckscher E.S., Krieg M., Tsechpenakis G., Gershow M., Tracey W.D. Direction selectivity in Drosophila proprioceptors requires the mechanosensory channel Tmc. Curr. Biol. 2019;29:945–956. doi: 10.1016/j.cub.2019.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R., Nogales E. Microtubule dynamics. J. Cell Sci. 2002;115:3–4. doi: 10.1242/jcs.115.1.3. [DOI] [PubMed] [Google Scholar]
- Heidemann S.R., Landers J.M., Hamborg M.A. Polarity orientation of axonal microtubules. J. Cell Biol. 1981;91:661–665. doi: 10.1083/jcb.91.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzmann S., Götzelmann I., Reekers L.F., Rumpf S. Spatial regulation of microtubule disruption during dendrite pruning in Drosophila. Dev. 2018;145:dev156950. doi: 10.1242/dev.156950. [DOI] [PubMed] [Google Scholar]
- Hillman D. Neuronal shape parameters and substructures as a basis of neuronal form. In: Schmitt FO W.F., editor. The Neurosciences, Fourth Study Program. MIT Press; Cambridge, MA: 1979. pp. 477–498. [Google Scholar]
- Hillman D.E., Llinás R., Chujo M., Crank R. Direct analysis of nervous system ultrastructure by computer control of STEM. Scanning Electron. Microsc. 1980;(Pt 1):125–135. [PubMed] [Google Scholar]
- Himmel N.J., Letcher J.M., Sakurai A., Gray T.R., Benson M.N., Cox D.N. Drosophila menthol sensitivity and the Precambrian origins of transient receptor potential-dependent chemosensation. Philos. Trans. R. Soc. B Biol. Sci. 2019;374:20190369. doi: 10.1098/rstb.2019.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth J.J., Van Pelt J., Mansvelder H.D., Van Ooyen A. Competitive dynamics during resource-driven neurite outgrowth. PLoS One. 2014;9:e86741. doi: 10.1371/journal.pone.0086741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J., Hyman A.A. Growth, fluctuation and switching at microtubule plus ends. Nat. Rev. Mol. Cell Biol. 2009;10:569–574. doi: 10.1038/nrm2713. [DOI] [PubMed] [Google Scholar]
- Hughes C.L., Thomas J.B. A sensory feedback circuit coordinates muscle activity in Drosophila. Mol. Cell. Neurosci. 2007;35:383–396. doi: 10.1016/j.mcn.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang R.Y., Zhong L., Xu Y., Johnson T., Zhang F., Tracey W.D. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr. Biol. 2007;17:2105–2116. doi: 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer E.P.R., Iyer S.C., Sullivan L., Wang D., Meduri R., Graybeal L.L., Cox D.N. Functional genomic analyses of two morphologically distinct classes of Drosophila sensory neurons: post-mitotic roles of transcription factors in dendritic patterning. PLoS One. 2013;8:e72434. doi: 10.1371/journal.pone.0072434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S.C., Iyer E.P.R., Meduri R., Rubaharan M., Kuntimaddi A. Cut, via CrebA, Transcriptionally regulates the COPII secretory pathway to direct dendrite development in Drosophila. J. Cell Sci. 2013;126:4732–4745. doi: 10.1242/jcs.131144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S.C., Wang D., Iyer E.P.R., Trunnell S.A., Meduri R., Shinwari R., Sulkowski M.J., Cox D.N. The RhoGEF trio functions in sculpting class specific dendrite morphogenesis in Drosophila sensory neurons. PLoS One. 2012;7:e33634. doi: 10.1371/journal.pone.0033634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y.-N., Jan L.Y. Branching out: mechanisms of dendritic arborization. Nat. Rev. Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinushi-Nakao S., Arvind R., Amikura R., Kinameri E., Liu A.W., Moore A.W. Knot/Collier and cut control different aspects of dendrite cytoskeleton and synergize to define final arbor shape. Neuron. 2007;56:963–978. doi: 10.1016/j.neuron.2007.10.031. [DOI] [PubMed] [Google Scholar]
- Kapitein L.C., Hoogenraad C.C. Building the neuronal microtubule cytoskeleton. Neuron. 2015;87:492–506. doi: 10.1016/j.neuron.2015.05.046. [DOI] [PubMed] [Google Scholar]
- Kapitein L.C., Schlager M.A., Kuijpers M., Wulf P.S., van Spronsen M., MacKintosh F.C., Hoogenraad C.C. Mixed microtubules steer dynein-driven cargo transport in dendrites. Curr. Biol. 2010;20:290–299. doi: 10.1016/j.cub.2009.12.052. [DOI] [PubMed] [Google Scholar]
- Kawabata Galbraith K., Kengaku M. Multiple roles of the actin and microtubule-regulating formins in the developing brain. Neurosci. Res. 2019;138:59–69. doi: 10.1016/j.neures.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Kidd T., Condron B. Avoiding the SCAMs. Neuron. 2007;54:350–352. doi: 10.1016/j.neuron.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Kollins K.M., Bell R.L., Butts M., Withers G.S. Dendrites differ from axons in patterns of microtubule stability and polymerization during development. Neural Dev. 2009;4:26. doi: 10.1186/1749-8104-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konietzny A., Bär J., Mikhaylova M. Dendritic actin cytoskeleton: structure, functions, and regulations. Front. Cell. Neurosci. 2017;11:147. doi: 10.3389/fncel.2017.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarewicz M.T., Migliore M., Ascoli G.A. A new bursting model of CA3 pyramidal cell physiology suggests multiple locations for spike initiation. BioSystems. 2002;67:129–137. doi: 10.1016/s0303-2647(02)00071-0. [DOI] [PubMed] [Google Scholar]
- Ledda F., Paratcha G. Mechanisms regulating dendritic arbor patterning. Cell. Mol. Life Sci. 2017;74:4511–4537. doi: 10.1007/s00018-017-2588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.B., Bagley J.A., Lee H.Y., Jan L.Y., Jan Y.N. Pathogenic polyglutamine proteins cause dendrite defects associated with specific actin cytoskeletal alterations in Drosophila. Proc. Natl. Acad. Sci. U S A. 2011;108:16795–16800. doi: 10.1073/pnas.1113573108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre J.L., Sanes J.R., Kay J.N. Development of dendritic form and function. Annu. Rev. Cell Dev. Biol. 2015;31:741–777. doi: 10.1146/annurev-cellbio-100913-013020. [DOI] [PubMed] [Google Scholar]
- Li W., Wang F., Menut L., Gao F.B. BTB/POZ-zinc finger protein abrupt suppresses dendritic branching in a neuronal subtype-specific and dosage-dependent manner. Neuron. 2004;43:823–834. doi: 10.1016/j.neuron.2004.08.040. [DOI] [PubMed] [Google Scholar]
- Lopez-Bellido R., Himmel N.J., Gutstein H.B., Cox D.N., Galko M.J. An assay for chemical nociception in Drosophila larvae. Philos. Trans. R. Soc. B Biol. Sci. 2019;374:20190282. doi: 10.1098/rstb.2019.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortal S., Iseppon F., Perissinotto A., D’Este E., Cojoc D., Napolitano L.M.R., Torre V. Actin waves do not Boost neurite outgrowth in the early stages of neuron maturation. Front. Cell Neurosci. 2017;11:402. doi: 10.3389/fncel.2017.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel J.C., Delandre C., Zhang Y., Förstner F., Moore A.W., Tavosanis G. Fascin controls neuronal class-specific dendrite arbor morphology. Development. 2012;139:2999–3009. doi: 10.1242/dev.077800. [DOI] [PubMed] [Google Scholar]
- Nanda S., Chen H., Das R., Bhattacharjee S., Cuntz H., Torben-Nielsen B., Peng H., Cox D.N., Schutter E. De, Ascoli G.A. Design and implementation of multi-signal and time-varying neural reconstructions. Sci. Data. 2018;5:170207. doi: 10.1038/sdata.2017.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda S., Das R., Bhattacharjee S., Cox D.N., Ascoli G.A. Morphological determinants of dendritic arborization neurons in Drosophila larva. Brain Struct. Funct. 2018;223:1107–1120. doi: 10.1007/s00429-017-1541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda S., Das R., Cox D.N., Ascoli G.A. Structural plasticity in dendrites: developmental neurogenetics, morphological reconstructions, and computational modeling. In: Petrosini Laura., editor. Vol. 30. Springer Press, Contemporary 753 Clinical Neuroscience Series: Cham; 2017. pp. 1–34. (Neurobiological and Psychological Aspects of Brain Recovery). [Google Scholar]
- Ori-McKenney K.M., Jan L.Y., Jan Y.N. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron. 2012;76:921–930. doi: 10.1016/j.neuron.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto A.M., Zumbe A., Gibson L., Kubler A.M., Jimenez de Asua L. Cytoskeleton-disrupting drugs enchance effect of growth factors and hormones on initiation of DNA synthesis. Proc. Natl. Acad. Sci. U S A. 1979;76:6435–6438. doi: 10.1073/pnas.76.12.6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada L., Kilic B., Erdil E., Ramiro-Cortés Y., Israely I., Unay D., Cetin M., Argunsah A.Ö. Tracking-assisted detection of dendritic spines in time-lapse microscopic images. Neuroscience. 2018;394:189–205. doi: 10.1016/j.neuroscience.2018.10.022. [DOI] [PubMed] [Google Scholar]
- Ritzenthaler S., Suzuki E., Chiba A. Postsynaptic filopodia in muscle cells interact with innervating motoneuron axons. Nat. Neurosci. 2000;3:1012–1017. doi: 10.1038/79833. [DOI] [PubMed] [Google Scholar]
- Samsonovich A.V., Ascoli G.A. Statistical determinants of dendritic morphology in hippocampal pyramidal neurons: a hidden Markov model. Hippocampus. 2005;15:166–183. doi: 10.1002/hipo.20041. [DOI] [PubMed] [Google Scholar]
- Samsonovich A.V., Ascoli G.A. Statistical morphological analysis of hippocampal principal neurons indicates cell-specific repulsion of dendrites from their own cell. J. Neurosci. Res. 2003;71:173–187. doi: 10.1002/jnr.10475. [DOI] [PubMed] [Google Scholar]
- Sánchez-Huertas C., Freixo F., Viais R., Lacasa C., Soriano E., Lüders J. Non-centrosomal nucleation mediated by augmin organizes microtubules in post-mitotic neurons and controls axonal microtubule polarity. Nat. Commun. 2016;7:12187. doi: 10.1038/ncomms12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago C., Bashaw G.J. Transcription factors and effectors that regulate neuronal morphology. Development. 2014;141:4667–4680. doi: 10.1242/dev.110817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Mizell C.M., Gerhard S., Longair M., Kazimiers T., Li F., Zwart M.F., Champion A., Midgley F.M., Fetter R.D., Saalfeld S., Cardona A. Quantitative neuroanatomy for connectomics in Drosophila. Elife. 2016;5:e12059. doi: 10.7554/eLife.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C.J., Bezaire M., Soltesz I. Toward a full-scale computational model of the rat dentate gyrus. Front. Neural Circuits. 2012;6:83. doi: 10.3389/fncir.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheintuch L., Rubin A., Brande-Eilat N., Geva N., Sadeh N., Pinchasof O., Ziv Y. Tracking the same neurons across multiple days in Ca2+ imaging data. Cell Rep. 2017;21:1102–1115. doi: 10.1016/j.celrep.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B.A., Daugherty-Clarke K., Goode B.L., Gelles J. Pathway of actin filament branch formation by Arp2/3 complex revealed by single-molecule imaging. Proc. Natl. Acad. Sci. U S A. 2013;110:1285–1290. doi: 10.1073/pnas.1211164110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spedden E., Kaplan D.L., Staii C. Temperature response of the neuronal cytoskeleton mapped via atomic force and fluorescence microscopy. Phys. Biol. 2013;10:056002. doi: 10.1088/1478-3975/10/5/056002. [DOI] [PubMed] [Google Scholar]
- Stephan R., Goellner B., Moreno E., Frank C.A., Hugenschmidt T., Genoud C., Aberle H., Pielage J. Hierarchical microtubule organization controls axon caliber and transport and determines synaptic structure and stability. Dev. Cell. 2015;33:5–21. doi: 10.1016/j.devcel.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura K., Satoh D., Estes P., Crews S., Uemura T. Development of morphological diversity of dendrites in Drosophila by the BTB-Zinc finger protein Abrupt. Neuron. 2004;43:809–822. doi: 10.1016/j.neuron.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Sundararajan L., Smith C.J., Watson J.D., Millis B.A., Tyska M.J., Miller D.M. Actin assembly and non-muscle myosin activity drive dendrite retraction in an UNC-6/Netrin dependent self-avoidance response. PLoS Genet. 2019;15:e1008228. doi: 10.1371/journal.pgen.1008228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston S.F., Kulacz W.A., Shaikh S., Lee J.M., Copeland J.W. The ability to induce microtubule acetylation is a general feature of Formin proteins. PLoS One. 2012;7:e48041. doi: 10.1371/journal.pone.0048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey W.D., Wilson R.I., Laurent G., Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Unsain N., Stefani F.D., Cáceres A. The actin/spectrin membrane-associated periodic skeleton in neurons. Front. Synaptic Neurosci. 2018;10:10. doi: 10.3389/fnsyn.2018.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaadia R.D., Li W., Voleti V., Singhania A., Hillman E.M.C., Grueber W.B. Characterization of proprioceptive system dynamics in behaving Drosophila larvae using high-speed volumetric microscopy. Curr. Biol. 2019;29:935–944.e4. doi: 10.1016/j.cub.2019.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pelt J., Uylings H.B.M. Branching rates and growth functions in the outgrowth of dendritic branching patterns. Netw. Comp Neural. 2002;13:261–281. doi: 10.1088/0954-898x/13/3/302. [DOI] [PubMed] [Google Scholar]
- Van Pelt J., Uylings H.B.M., Verwer R.W.H., Pentney R.J., Woldenberg M.J. Tree asymmetry-A sensitive and practical measure for binary topological trees. Bull. Math. Biol. 1992;54:759–784. doi: 10.1007/BF02459929. [DOI] [PubMed] [Google Scholar]
- Vormberg A., Effenberger F., Muellerleile J., Cuntz H. Universal features of dendrites through centripetal branch ordering. Plos Comput. Biol. 2017;13:e1005615. doi: 10.1371/journal.pcbi.1005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A.T., Lanz M.C., Goetschius D.J., Hancock W.O., Rolls M.M. Kinesin-2 and Apc function at dendrite branch points to resolve microtubule collisions. Cytoskeleton. 2016;73:35–44. doi: 10.1002/cm.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Yuan Q., Vogt N., Looger L.L., Jan L.Y., Jan Y.N. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalgin C., Ebrahimi S., Delandre C., Yoong L.F., Akimoto S., Tran H., Amikura R., Spokony R., Torben-Nielsen B., White K.P., Moore A.W. Centrosomin represses dendrite branching by orienting microtubule nucleation. Nat. Neurosci. 2015;18:1437–1445. doi: 10.1038/nn.4099. [DOI] [PubMed] [Google Scholar]
- Yau K.W., vanBeuningen S.F.B., Cunha-Ferreira I., Cloin B.M.C., vanBattum E.Y., Will L., Schätzle P., Tas R.P., vanKrugten J., Katrukha E.A. Microtubule minus-end binding protein CAMSAP2 controls axon specification and dendrite development. Neuron. 2014;82:1058–1073. doi: 10.1016/j.neuron.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Ye B., Kim J.H., Yang L., McLachlan I., Younger S., Jan L.Y., Jan Y.N. Differential regulation of dendritic and axonal development by the novel Krüppel-like factor Dar1. J. Neurosci. 2011;31:3309–3319. doi: 10.1523/JNEUROSCI.6307-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All image stacks, data, analysis and modeling code are available at Mendeley.com (DOI: https://doi.org/10.17632/v3ncxmj6fn.1). Neuronal reconstructions are available at NeuroMorpho.Org (Ascoli and Cox archives).