Abstract
Mast Cell Leukemia (MCL) is the rarest form of systemic mastocytosis, a rare group of neoplastic disease that result from clonal proliferation of mast cells and their accumulation in one or more organ systems. The diagnosis of MCL is made by meeting the World Health Organization (WHO) 2017 criteria. MCL is further subclassified as leukemic or aleukemic based on presence or absence of circulating mast cells in the peripheral blood and acute versus chronic based on presence or absence of findings indicative of impaired organ function due to mast cell infiltration.
A 64-year-old Hispanic male presented with myalgia, diarrhea, urticarial rash, back pain, and fulminant disseminated intravascular coagulation. Bone marrow examination, supplemented by immunohistochemistry results, fulfilled the WHO criteria for the diagnosis of MCL. To the best of our knowledge, this is the first documented case of aleukemic acute MCL in a Hispanic patient.
Keywords: Mast cell leukemia, Acute, Chronic, Aleukemic, Leukemic, Hispanic, Therapy
1. Introduction
Mastocytosis is a neoplastic disease due to a clonal proliferation of mast cells that accumulate in one or more organ systems. Two main variants are recognized: cutaneous mastocytosis (CM), where the disease manifestation is limited to the skin, and systemic mastocytosis (SM), in which the mast cell proliferation is systemic and the mast cells accumulate in internal tissues and organs, such as the bone marrow, liver, spleen and gastrointestinal tract. SM is a rare disease, with an approximate incidence rate of 13 cases per 100,000 individuals [1]. It is associated with a wide variety of signs and symptoms, which can be explained by mediator release and mast cell infiltration. The main symptomatology includes, but is not limited to, hypotension, rash, pruritus, diarrhea, hyperacidity, musculoskeletal pain, tachycardia, and fever. SM is diagnosed by demonstrating mast cell infiltration in at least one extracutaneous organ, with the bone marrow being the most commonly involved organ and the most commonly used to establish diagnosis [2]. Its diagnosis requires fulfilling the major diagnostic criterion and at least one of the four minor criteria, or three minor criteria. The major criterion is the presence of diffuse involvement or of multifocal compact mast cell aggregates of greater than 15 mast cells clustered together identified in at least one internal organ or in the bone marrow. The minor criteria include: (a) a greater than 25% immature or atypical mast cell infiltration in the bone marrow or other extracutaneous organs, (b) an abnormal mast cell immunophenotype (CD25 positive with or without CD2), (c) the presence of activating mutation in the KIT gene at codon 816, and (d) an elevated baseline serum tryptase level of greater than 20 ng/mL [3].
SM is subdivided into five main variants based on the mast cell burden (“B” findings) and the presence/absence of organ impairment secondary to mast cell infiltration (“C” findings) (Table 1) [4,5]. Among the different variants of SM, MCL is considered the rarest occurring in less than 1% of SM cases and carries the worst prognosis [2,6]. By definition, MCL must meet the World Health Organization (WHO) 2017 criteria for SM and is marked by diffuse bone marrow infiltration of immature or atypical mast cells accounting for at least 20% of all nucleated cells in the bone marrow (BM) aspirate smear and at least 10% in the peripheral blood smear (PBS). If mast cells account for less than 10% of the PBS, then the diagnosis is that of MCL, aleukemic variant. The aleukemic variant appears to be the most common of the two variants [7], [8], [9], [10], [11]. Progression from aleukemic MCL to leukemic MCL has been reported to occur [12]. Moreover, MCL is divided between an acute variant (67–93% of cases) and the less common chronic variant based on the presence or absence of “C” findings [2,7,13,14]. The latter has been associated with a more indolent course at disease outset, but overall similar prognosis [7,8,14].
Table 1.
B- and C-findings as per WHO 2017 Criteria for SM[3].
| B findings |
| 1 High mast cell burden (shown on bone marrow biopsy): > 30% infiltration of cellularity by mast cells (focal, dense aggregates) and serum total tryptase > 200 ng / mL |
| 1 Signs of dysplasia or myeloproliferation in non–mast cell lineage(s), but criteria are not met for definitive diagnosis of an associated hematological neoplasm, with normal or only slightly abnormal blood counts |
| 1 Hepatomegaly without impairment of liver function, palpable splenomegaly without hypersplenism and / or lymphadenopathy on palpation or imaging |
| C findings |
| 1 Bone marrow dysfunction caused by neoplastic mast cell infiltration, manifested by ≥ 1 cytopenia: absolute neutrophil count < 1.0 × 109 / L, hemoglobin level < 10 g/dL, and / or platelet count < 100 × 109 / L |
| 1 Palpable hepatomegaly with impairment of liver function, ascites and/or portal hypertension |
| 1 Skeletal involvement, with large osteolytic lesions with or without pathological fractures (pathological fractures caused by osteoporosis do not qualify as a C finding) |
| 1 Palpable splenomegaly with hypersplenism |
| 1 Malabsorption with weight loss due to gastrointestinal mast cell infiltrates |
2. Case report
The patient was a 64-year-old male, with a past medical history of hypertension and gout, who was admitted to our institution with a 2-week history back pain, myalgias, diarrhea, conjunctival erythema, decreased urine output, and diffuse maculopapular rash precipitated by naproxen intake. He was initially treated with intramuscular glucocorticoids at an outside facility, which gave relief to his rash and diarrhea. However, the persistence of the other symptoms prompted the patient present to our institution. He denied recent weight loss, abnormal bleeding, fever, and recent travel. Vitals obtained on admission included a normal BP of 138/80 mm Hg, tachycardia of 120 /min, respiratory rate of 20 /min, and a temperature of 36.9 C. Laboratory work-up on admission showed mild anemia (hemoglobin, 12.7 g/dl; Hematocrit, 36%), thrombocytopenia (platelets, 28 × 109/L), normal white blood cell count (WBC, 7.08 × 109/L; normal differential count: Neutrophils 66%, Eosinophils 5%, Basophils ~1% Monocytes 3%, Lymphocytes 25%; no circulating mast cells), thrombin time was >60 s, elevated fibrin-degradation products (D-dimer 9.41 FEU, fibrinogen 253 mg/dL), a slight prolonged prothrombin time of 24.9 (INR 2.1). Renal insufficiency was demonstrated by a BUN of 61 mg/dL, creatinine of 2.2 mg/dL, and GFR of 30 (ref >60 mL/min). LDH was elevated at 1834 U/L. Imaging revealed a 0.8 cm hypodensity in the right iliac fossa, suspicious for an abscess. Disseminated intravascular coagulation (DIC), secondary to sepsis from the possible abscess, was the initial impression. The patient was empirically treated for sepsis and provided supportive measures for DIC. Given the size of the right iliac fossa lesion, attempts to perform percutaneous drainage failed. Because of the worsening anemia and thrombocytopenia which raised the possibility of a hematologic malignancy, a bone marrow examination with biopsy and aspirate smear was performed. Two days later, the patient experienced a significantly worsening anemia, developed hypoxia and right-sided pleural effusion, and less than a week after the admission he went into cardiac arrest and expired.
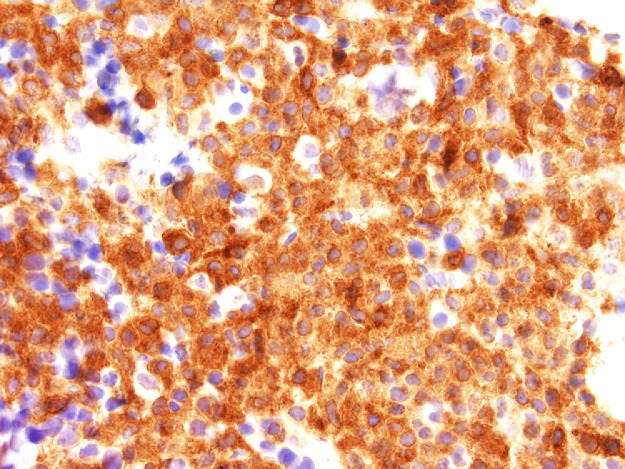
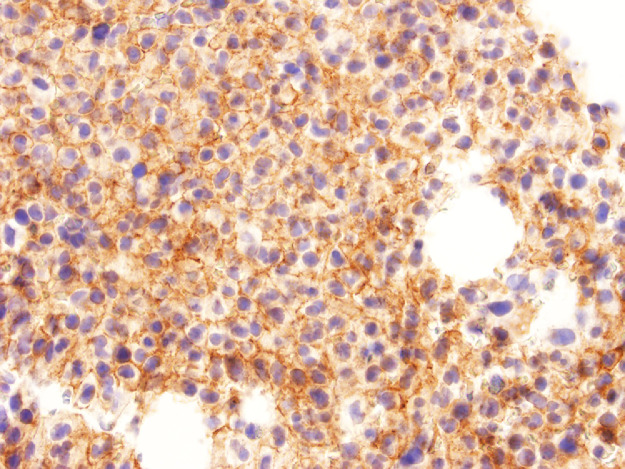
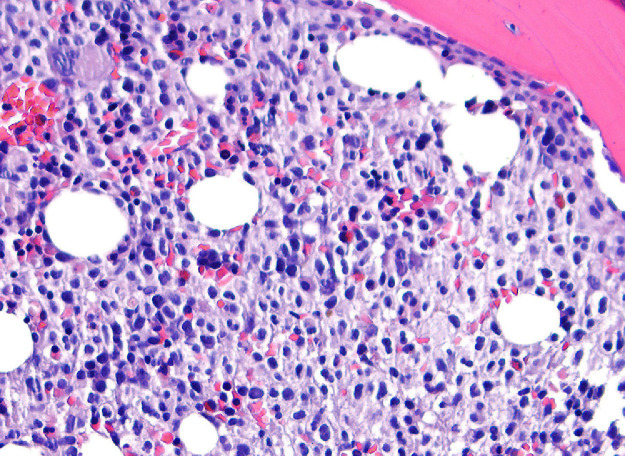
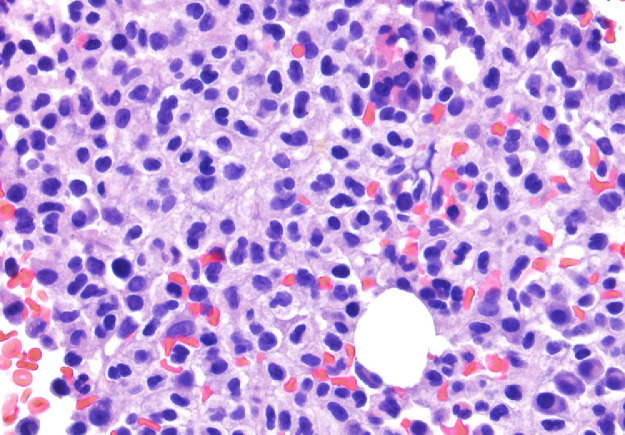
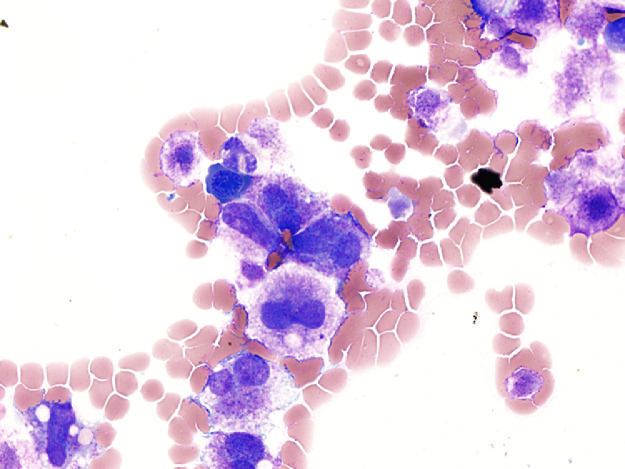
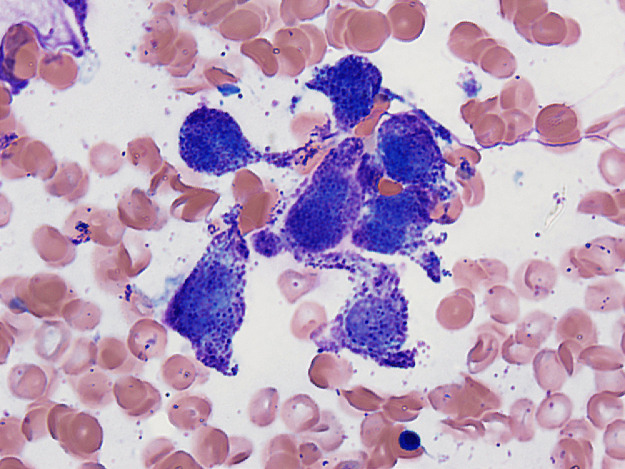
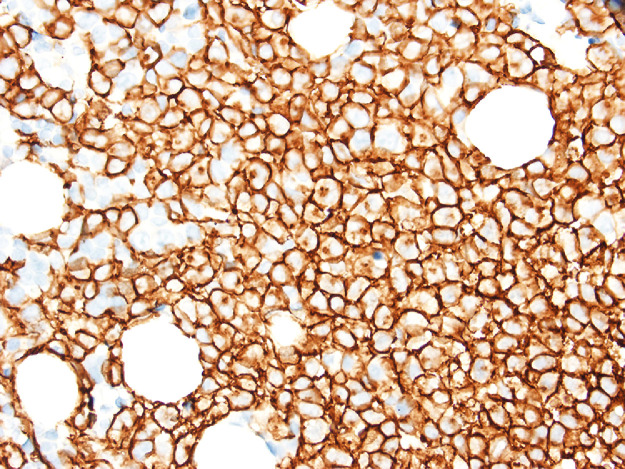
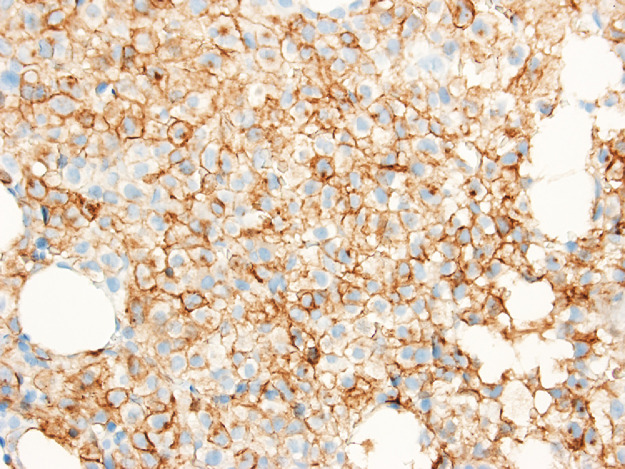
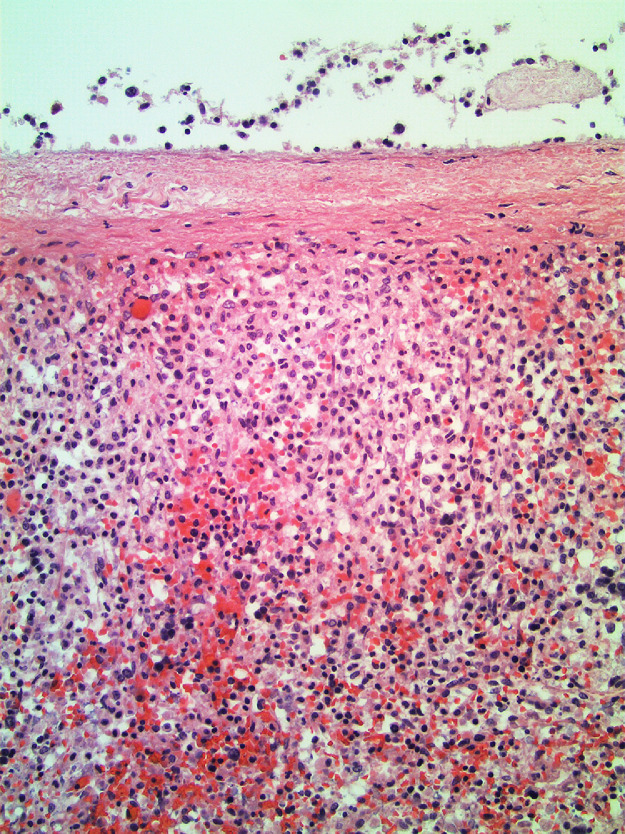
The bone marrow examination demonstrated a diffuse infiltration of the marrow cavity by a compact proliferation of abnormal mast cells occupying >70% of the intertrabecular marrow space (Fig. 1a, b). The mast cells, which accounted for at least 40% of the bone marrow aspirate smear differential count, included mostly promastocytes with a majority of the cells displaying classical nuclear bilobation and a round cytoplasm (Fig. 1c) with some spindle shaped immature forms also noted (Fig. 1d). Immunochemistry demonstrated a strong expression of CD 117 (KIT), Tryptase, CD25, and CD2 (Fig. 2a–d). KIT mutation was not detected by target NGS testing of KIT exons 8, 9, 11, 13, and 17. The karyotype analysis was normal (46, XY).
Fig. 2b.
Bone marrow biopsy immunohistochemistry showing diffuse immunoreactivity of the neoplastic mast cells for mast cell tryptase (x400).
Fig. 2c.
Bone marrow biopsy immunohistochemistry showing diffuse immunoreactivity of the neoplastic mast cells for CD25 (x400).
Fig. 1a.
Bone marrow biopsy showing marrow cavity diffusely infiltrated by a compact sheet-like proliferation of neoplastic mast cells (Low magnification, X200).
Fig. 1b.
Bone marrow biopsy showing marrow cavity diffusely infiltrated by a compact sheet-like proliferation of neoplastic mast cells. (High magnification, x400).
Fig. 1c.
Bone marrow aspirate smear showing promastocytes (immature cells with bilobated nuclei and cytoplasms packed with metachromatic mast cell granules).
Fig. 1d.
Bone marrow aspirate smear showing a cluster of spindle shaped abnormal mast cells. Neoplastic mast cells accounted for at least 40% of the differential count.
Fig. 2a.
Bone marrow biopsy immunohistochemistry showing diffuse immunoreactivity of the neoplastic mast cells for CD117 (x400).
Fig. 2d.
Bone marrow biopsy immunohistochemistry showing diffuse immunoreactivity of the neoplastic mast cells for CD2 (x400).
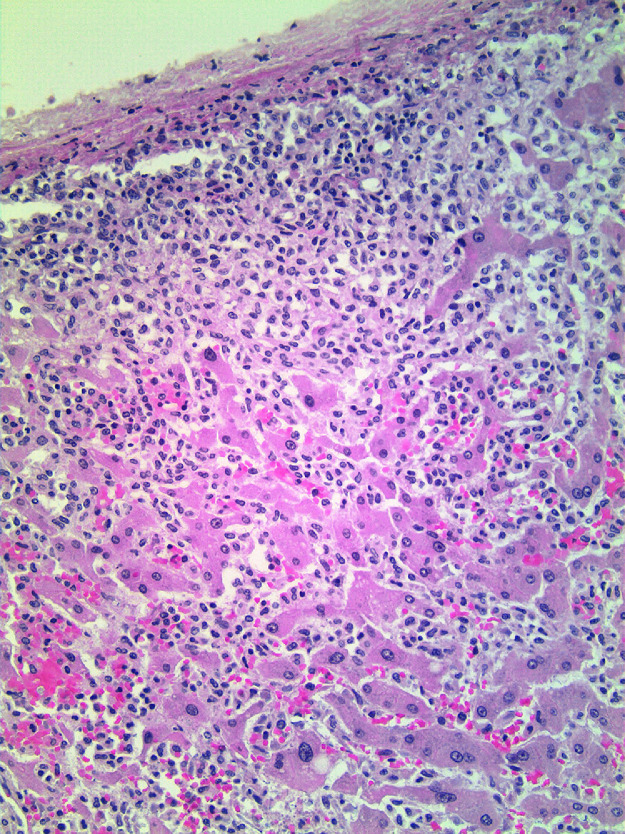
Autopsy demonstrated splenomegaly with a spleen weight of 1570 gm (normal: 150–200 gm) displaying a diffuse infiltration of mast cells (Fig. 3), and hepatomegaly with a liver weight of 3100 gm (normal: 1200–2000 gm) also showing diffuse infiltration of mast cells (Fig. 4). Additionally, a non-neoplastic gastric ulcer and a hematoma over the right iliac fossa were also documented. Combining these findings together, the patient fulfilled the major criterion and two minor criteria for the diagnosis of SM, which was then further classified as acute MCL (“C” findings were present; massive organ infiltration was also documented at autopsy), aleukemic variant (peripheral blood smear showing an absence of circulating mast cells). The DIC was considered to be secondary to MCL.
Fig. 3.
Spleen from autopsy showing subcapsular and diffuse infiltration by neoplastic mast cells as well as focal extramedullary hematopoiesis, a frequent occurrence in pathologically enlarge spleens (x200).
Fig. 4.
Liver from autopsy showing diffuse infiltration by neoplastic mast cells. Note subcapsular and intrasinusoidal mast cells location (x200).
3. Discussion
MCL is a rare and aggressive variant of SM. It is characterized by the leukemic spread of mast cells with multiple organ involvement such as the gastrointestinal tract, liver, spleen, and peritoneum. In the majority of patients skin lesions, as seen in other types of SM, are absent [3]. However, skin rashes as seen in our patient are reported to occur in MCL [8]. Most of MCL patients present with cytopenia, hepatic malfunction, hypersplenism, malabsorption, osteolytic lesions, and weight loss [3]. These are known as “C” findings, and are present in 90% of acute MCL cases; the majority of patients have hepatosplenomegaly with most common lab findings being cytopenia [6,14]. Constitutional symptoms such as weight loss, fatigue, and night sweats are also common [3,8,15]. Additionally, a third of cases also present with gastrointestinal symptoms, specifically diarrhea. A strong association with peptic ulcer disease has been reported in some studies[16]. Bleeding, hemolysis, or disseminated intravascular coagulation, which was the case for our patient, can also occur.
As shown in our case, immunophenotyping is essential in identification of abnormal mast cells. Mast cells, both in neoplastic and in reactive conditions, express tryptase and KIT (CD117) as well as certain myeloid antigens, including CD13 and CD33. Tryptase, CD117, CD25 and CD2 (the latter two antigens are not expressed in normal mast cells), are the key markers used for the identification of abnormal mast cells present in SM. CD117 is sensitive, but not specific for mast cells, while tryptase is less sensitive, but much more specific. CD25 is more useful than CD2 which is less constantly expressed [17]. MCL shows an immunophenotypic reactivity similar to the other types of SM, however, a third of MCL cases have been noted to have a double negative CD2/CD25 immunophenotype [2]. A variable proportion of MCL cases may express CD30 [18].
The KIT D816V mutation is the most common finding in SM being present in 80–90% of cases and its main phenotypic driver. In aggressive SM and in MCL, several additional mutations shared with other myeloid neoplasms (e.g. CMML) may also occur e.g. SRF2, ASXL1, RUNX1, TET2, CBL, K/N-RAS, and EZH2, which have been shown to lead to poorer outcomes [19,20]. In contrast with other types of SM, only 40–67% of MCL cases carry the typical KIT D816V mutation [3,13,[19], [20], [21]]. A proportion of these “negative” cases may carry a codon 816 KIT mutation other than D816V or a non-codon 816 KIT mutation[3,19]. In MCL, these atypical KIT mutations have a combined estimated frequency of 20%; wild-type KIT occurs in 7–11% of cases [7,20,25]. Our case fell into the latter category.
From a clinicopathologic standpoint, the differential diagnosis of MCL includes tryptase-positive AML and myelomastocytic leukemia (MML), an advanced myeloid neoplasm with partial mast cell differentiation [14]. Both can be ruled out using the WHO 2017 criteria. The former commonly presents with at least 20% myeloblasts which show weak tryptase expression. BM biopsy usually demonstrates less than 10% mast cells lacking a compact diffuse proliferative pattern. Additionally, the mast cells do not show CD25 expression. MML can present with mast cells accounting for greater than 10% on the BM aspirate differential. Leukemic mast cells in MML are usually immature or are recognized as metachromatic blasts with very low expression of tryptase. However, the proliferation is largely myeloblastic, with blasts often expressing CD34, lacking CD25 [14]. In both disease entities, a KIT mutation is usually not detected.
While the goal of therapy in indolent variants of SM has traditionally been symptomatic relief by using e.g. antihistamines, mast cell stabilizers, leukotriene inhibitors, proton pump inhibitors, and NSAIDs, in MCL (and also in aggressive systemic mastocytosis) reversal of C-findings and their organ damage-related symptomatology represents the current treatment strategy [21]. Treatment options of MCL cases range from tyrosine kinase inhibitors (TKI), chemotherapy, and allogeneic stem cell transplant [21,22]. A study by Ustun et al. only demonstrated a 17% 3-year overall survival for patients hematopoietic stem cell transplant [22]. The most promising therapeutic option are the TKIs. Midostaurin, the only currently FDA approved medication for MCL and aggressive systemic mastocytosis, is a TKI that is shown to have activity against KIT D816V resulting decreased C-findings [23]. An open-label midostaurin multicenter study of “advanced systemic mastocytosis” patients which included 16 aggressive systemic mastocytosis, 57 systemic mastocytosis with an associated hematologic neoplasm, 16 MCL showed an overall 50% response rate to this drug. Although for all patients the median overall survival was 28.7 months, among the 16 patients with mast-cell leukemia, the median overall survival was 9.4 months [23]. Imatinib and nilotinib show activity against wild-type KIT. However, activity against KIT D816V has been limited. However, rare patients with atypical KIT mutations have responded to those drugs [24].Avapritinib, a class 1 TKI with activity against PDFR-alpha, is a new drug currently undergoing clinical trials. It shows promise with a response rate up to 75% for those who did not respond to midostaurin [25].
As to the best of our knowledge, this is one of the first documented cases of MCL in a Hispanic patient. Epidemiologic information on MCL is hard to find which is understandable in view of the rarity of the disease. However, patient ethnicity was mentioned in two studies one from the United States (US) and one from France, respectively [6,26]. The US study by Jain et al. of 13 MCL cases, showed that twelve patients were Caucasians and one African-American. [6] In the French study by Lavielle et al. of the 51 MCL cases, ethnicity was stated for 21 cases, 18 Caucasians and three Asians. [26] Although several clinical trials for the treatment of MCL have been performed, ethnic data were not made available possibly due to the overall low number of accrued patients. Thus, no conclusion in relation to disease ethnic prevalence is possible at this time.
Declaration of Competing Interest
None of the authors have any conflict of interest to declare.
Acknowledgments
The authors wish to acknowledge Jude Abadie. PhD for reviewing the patient's clinical pathology results.
Contributor Information
Gian M. Galura, Email: Gian.Galura@ttuhsc.edu.
Attilio Orazi, Email: Attilio.Orazi@ttuhsc.edu.
References
- 1.Matsunaga T., Mitsui E., Jin L. An autopsy case of mast cell leukemia. Pathol. Int. 2019;69(2):122‐124. doi: 10.1111/pin.12753. [DOI] [PubMed] [Google Scholar]
- 2.Costopoulos M., Uzunov M., Bories D. Acute mast cell leukemia: a rare but highly aggressive hematopoietic neoplasm. Diagn. Cytopathol. 2018;46(7):639‐641. doi: 10.1002/dc.23965. [DOI] [PubMed] [Google Scholar]
- 3.Horny H.P., Akin C., Arber D.A. Mastocytosis. In: Swerdlow S, Campo E, Harris N, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid tissues, Updated 4th edition. IARC Press; Lyon, France: 2017. [Google Scholar]
- 4.Hoffman R., Benz E., Silberstein L. 6th ed. Elsevier Health Sciences; London: 2013. Hematology; p. 1099. [Google Scholar]
- 5.Valent P., Akin C., Hartmann K. Advances in the classification and treatment of mastocytosis: current status and outlook toward the future. Cancer Res. 2017;77(6):1261‐1270. doi: 10.1158/0008-5472.CAN-16-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain P., Wang S., Patel K.P. Mast cell leukemia (MCL): clinico-pathologic and molecular features and survival outcome. Leuk. Res. 2017;59:105–109. doi: 10.1016/j.leukres.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leguit R., Hebeda K., Kremer M. The spectrum of aggressive mastocytosis: a workshop report and literature review. Pathobiology. 2020;87(1):2‐19. doi: 10.1159/000504099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valent P., Sotlar K., Sperr W.R. Chronic mast cell leukemia: a novel leukemia-variant with distinct morphological and clinical features. Leuk. Res. 2015;39(Janury 1):1–5. doi: 10.1016/j.leukres.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain P., Wang S., Kantarjian H. Aleukemic Mast Cell Leukemia (aMCL) - clinical characteristics and outcomes. Blood. 2016;128(22):4255. doi: 10.1182/blood.v128.22.4255.4255. [DOI] [Google Scholar]
- 10.Suh M.C., Ham J.Y., Park T.I. Highly aggressive de novo aleukemic variant of mast cell leukemia without KIT D816V mutation. Ann. Lab. Med. 2017;37(November 6):547–549. doi: 10.3343/alm.2017.37.6.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noack F., Sotlar K., Notter M. Aleukemic mast cell leukemia with abnormal immunophenotype and c-kit mutation D816V. Leuk. Lymphoma. 2004;45(November 11):2295–2302. doi: 10.1080/10428190412331272695. [DOI] [PubMed] [Google Scholar]
- 12.Patel S.S., Dorfman D.M. Leukemic-phase progression of aleukemic mast cell leukemia. Blood. 2018;131(May 21):2406. doi: 10.1182/blood-2018-02-831388. PMID: 29794078. [DOI] [PubMed] [Google Scholar]
- 13.Wang R.C., Ward D., Dunn P., Chang C.C. Acute mast cell leukemia associated with t(4;5)(q21;q33) Hum. Pathol. 2017;67:198–204. doi: 10.1016/j.humpath.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Valent P., Sotlar K., Sperr W.R. Refined diagnostic criteria and classification of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): a consensus proposal. Ann. Oncol. 2014;25(September 9):1691–1700. doi: 10.1093/annonc/mdu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jawhar M., Schwaab J., Meggendorfer M., Naumann N., Horny H.P., Sotlar K. The clinical and molecular diversity of mast cell leukemia with or without associated hematologic neoplasm. Haematologica. 2017;102(6):1035–1043. doi: 10.3324/haematol.2017.163964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Travis W.D., Li C.Y., Hoagland H.C. Mast cell leukemia: report of a case and review of the literature. Mayo Clin. Proc. 1986;61(December 12):957–966. doi: 10.1016/s0025-6196(12)62636-6. [DOI] [PubMed] [Google Scholar]
- 17.Chiu A., Orazi A. Mastocytosis and related disorders. Semin. Diagn. Pathol. 2012;29(1):19‐30. doi: 10.1053/j.semdp.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Sotlar K., Cerny-Reiterer S., Petat-Dutter K. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod. Pathol. 2011;24(April 4):585–595. doi: 10.1038/modpathol.2010.224. [DOI] [PubMed] [Google Scholar]
- 19.Reiter A., George T.I., Gotlib J.R. New Developments in Diagnosis, Prognostication, and Treatment of Advanced Systemic Mastocytosis. Blood. 2020;135(16):1365–1376. doi: 10.1182/blood.2019000932. [DOI] [PubMed] [Google Scholar]
- 20.Jawhar M., Schwaab J., Schnittger S., Sotlar K., Horny H.P., Metzgeroth G. Molecular profiling of myeloid progenitor cells in multi-mutated advanced systemic mastocytosis identifies KIT D816V as a distinct and late event. Leukemia. 2015;29(5):1115–1122. doi: 10.1038/leu.2015.4. [DOI] [PubMed] [Google Scholar]
- 21.Gilreath J.A., Tchertanov L., Deininger M.W. Novel approaches to treating advanced systemic mastocytosis. Clin. Pharmacol. 2019;11:77–92. doi: 10.2147/CPAA.S206615. Published 2019 Jul 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ustun C., Reiter A., Scott B.L. Hematopoietic stem-cell transplantation for advanced systemic mastocytosis. J. Clin. Oncol. 2014;32(29):3264‐3274. doi: 10.1200/JCO.2014.55.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gotlib J., Kluin-Nelemans H.C., George T.I. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N. Engl. J. Med. 2016;374(26):2530‐2541. doi: 10.1056/NEJMoa1513098. [DOI] [PubMed] [Google Scholar]
- 24.Mital A., Piskorz A., Lewandowski K. A case of mast cell leukaemia with exon 9 KIT mutation and good response to imatinib. Eur. J. Haematol. 2011;86(June 6):531–535. doi: 10.1111/j.1600-0609.2011.01598.x. [DOI] [PubMed] [Google Scholar]
- 25.Apsel Winger B., Cortopassi W.A., Garrido Ruiz D. ATP-competitive inhibitors midostaurin and avapritinib have distinct resistance profiles in Exon 17-Mutant KIT. Cancer Res. 2019;79(August 16):4283–4292. doi: 10.1158/0008-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Georgin-Lavialle S., Lhermitte L., Dubreuil P. Mast cell leukemia. Blood. 2013;121(8):1285‐1295. doi: 10.1182/blood-2012-07-442400. [DOI] [PubMed] [Google Scholar]