Abstract
The phytocannabinoid Δ9-tetrahydrocannabinol (THC) was isolated and synthesized in the 1960s. Since then, two synthetic cannabinoids (SCBs) targeting the cannabinoid 1 (CB1R) and 2 (CB2R) receptors were approved for medical use based on clinical safety and efficacy data: dronabinol (synthetic THC) and nabilone (synthetic THC analog). To probe the function of the endocannabinoid system further, hundreds of investigational compounds were developed; in particular, agonists with (1) greater CB1/2R affinity relative to THC and (2) full CB1/2R agonist activity. This pharmacological profile may pose greater risks for misuse and adverse effects relative to THC, and these SCBs proliferated in retail markets as legal alternatives to cannabis (e.g., novel psychoactive substances [NPS], “Spice,” “K2”). These SCBs were largely outlawed in the U.S., but blanket policies that placed all SCB chemicals into restrictive control categories impeded research progress into novel mechanisms for SCB therapeutic development. There is a concerted effort to develop new, therapeutically useful SCBs that target novel pharmacological mechanisms. This review highlights the potential therapeutic efficacy and safety considerations for unique SCBs, including CB1R partial and full agonists, peripherally-restricted CB1R agonists, selective CB2R agonists, selective CB1R antagonists/inverse agonists, CB1R allosteric modulators, endocannabinoid-degrading enzyme inhibitors, and cannabidiol. We propose promising directions for SCB research that may optimize therapeutic efficacy and diminish potential for adverse events, for example, peripherally-restricted CB1R antagonists/inverse agonists and biased CB1/2R agonists. Together, these strategies could lead to the discovery of new, therapeutically useful SCBs with reduced negative public health impact.
Keywords: cannabis, cannabinoids, synthetic cannabinoids, cannabinoid receptor, Δ9-tetrahydrocannabinol, cannabidiol
Graphical Abstract
1. INTRODUCTION
The cannabis plant was widely adopted for use in Western medicine in the 19th century, with over 100 scientific articles published on the therapeutic value of cannabis at that time (Zuardi, 2006). Now approximately 32,000 scientific reports on the health effects of cannabis are available through the National Center for Biotechnology Information, and legalization of cannabis for medicinal purposes is widespread. The health conditions for which cannabis is approved vary by jurisdiction, but often include symptoms associated with cancer and its treatment, epilepsy, severe and chronic pain, and symptoms associated with human immunodeficiency virus / acquired immunodeficiency syndrome (HIV/AIDS) (Bonn-Miller et al., 2014). Much of the therapeutic potential of cannabis stems from the direct physiological effects of individual chemical entities that are unique to the cannabis plant (i.e. phytocannabinoids).
In the 1960s, the psychoactive phytocannabinoid Δ9-tetrahydrocannabinol (THC) was isolated from the Cannabis sativa plant and synthesized in the laboratory (Gaoni and Mechoulam, 1964). This work catalyzed ligand screening efforts and ultimately identified two G protein-coupled cannabinoid receptors: the cannabinoid type 1 (CB1) and type 2 (CB2) receptors (for review, (Davidson et al., 2017)). THC was identified as a partial agonist at the CB1 and CB2 receptors (Griffin et al., 2000; Iwamura et al., 2001; Mauler et al., 2002), and subsequent drug development work resulted in the regulatory approval of synthetic formulations of THC: (1) dronabinol (Marinol, Syndros), a synthetic THC medication and (2) nabilone (Cesamet), a synthetic THC-analog medication. These approvals were based on extensive randomized controlled trials (RCTs) assessing safety, tolerability, and efficacy (Borgelt et al., 2013) and highlighted the clinical utility of CB1/2 receptor agonists.
To probe function of the endocannabinoid system further, medicinal chemists synthesized a plethora of novel, investigational CB1/2 receptor agonists. These compounds were originally developed for research purposes as investigational compounds to probe the function of CB1 versus CB2 receptors; however, progress in this area was impeded by these ligands exhibiting poor selectivity between the CB1 and CB2 receptors (Castaneto et al., 2014). Many of these agonists exhibited (1) greater CB1/2R affinity relative to THC or (2) full agonist activity at the CB1/2R (versus the partial agonist actions of THC) (for review, (Castaneto et al., 2014)). As a result, activity of these compounds in the central nervous system (CNS) may pose greater risks for misuse and/or development of a substance use disorder relative to THC. The methods for synthesizing these highly-potent CB1/2 receptor agonists were published and utilized by clandestine chemists to produce compounds for commercial SCB products (Baumann et al., 2014). These novel psychoactive substances (NPS) were first marketed under trade names such as “Spice” or “K2” and sold as legal alternatives to cannabis on the Internet, in head shops, and in convenience stores. The sale of these SCB products circumvented drug laws and regulations by labeling such products as “not for human consumption,” (Vandrey et al., 2012). In these preparations, synthetic, high-affinity, high-efficacy CB1/2 receptor agonists are commonly sprayed onto dried, shredded plant material. Acute exposure to these products is associated with a number of serious adverse events including symptoms of acute psychosis, seizures, major cardiovascular events, liver and kidney toxicity, and brain hemorrhage that required emergency treatment for a number of users (Castaneto et al., 2014; Castaneto et al., 2015; Gummin et al., 2017; Rodgman et al., 2011). In response to widespread misuse and adverse events associated with the use of these products, the U.S. Drug Enforcement Administration passed the Synthetic Drug Abuse Prevention Act in July 2012, which classified several of these ligands as Schedule I substances; this legislation is continually updated to include novel, high-affinity CB1 receptor agonists (DEA, 2011) and similar regulation has occurred in many other countries.
Despite the presence of an illicit market for the non-medicinal use of high-affinity CB1/2 receptor agonists, there is a concerted effort to develop new, therapeutically useful SCBs that target novel pharmacological mechanisms. However, research into these novel SCB pharmacological mechanisms is challenged by blanket policies that place all SCB chemicals (as well as cannabis) into the most restrictive drug control categories. It is important to limit SCB regulatory control only to compounds that are identified in the illicit market as misused substances (e.g., high-affinity, high-efficacy CB1/2 receptor agonists) so that SCB therapeutic development around novel, diverse pharmacological mechanisms can continue without major research restrictions. For example, novel strategies to stimulate CB1/2 receptors without the serious adverse events associated with full CB1/2 receptor agonism in the CNS are under development, including peripherally-restricted CB1 receptor agonists, selective CB2 receptor agonists, CB1 receptor positive allosteric modulators, and functionally-biased CB1/2 receptor agonists. Conversely, strategies to block constitutive or complete activity of the CB1 receptor using antagonists, inverse agonists, and negative allosteric modulators were developed and show therapeutic promise as well. Lastly, several non-CB receptor SCBs (e.g., endocannabinoid-degrading enzyme inhibitors) are in clinical development for a range of indications. A concise review of these SCBs with respect to their pharmacological targets, therapeutic potential, and potential for misuse and toxicity is needed to inform SCB regulatory control.
The purpose of this review is to summarize both the clinical utility and adverse effects of SCBs, with emphasis on the pharmacological profiles that distinguish unique categories of SCBs (summarized in Table 1). The review will provide a brief overview of the endocannabinoid system, history of cannabinoid isolation and synthesis, and pharmacology of THC, which characterizes the first generation of medically-approved SCBs. We then discuss the pharmacology and toxicity associated with illicit SCBs, that is, high-affinity, high-efficacy CB1/2 receptor agonists. Next, we review clinical studies and registered clinical trials to describe the current status of potential SCB therapeutics, characterizing mechanisms of action (i.e., receptor pharmacology), therapeutic potential, and abuse liability, toxicity, and safety concerns. We conclude with a discussion of promising approaches to novel SCB therapeutic drug development not yet evaluated in clinical studies.
Table 1:
Overview of medically-approved and potential synthetic cannabinoid therapeutics
| Name | Primary Mechanism of Action | Investigated Therapeutic Indications | Approved Medical Use | Registered Clinical Trials | Citations |
|---|---|---|---|---|---|
| Dronabinol | CB1/2 Receptor Agonist | HIV/AIDS-induced anorexia | Yes | Marinol® (dronabinol) capsules [package insert] (2017) AbbVie Inc. SYNDROS (dronabinol) oral solution, CX [package insert] (2016) Insys Therapeutics, Inc. |
|
| Chemotherapy-induced nausea and vomiting | Yes | ||||
| Neuropathic back pain | No | NCT02460692 | |||
| Cannabis use disorder | No | Budney et al., 2007; Haney et al., 2004; Herrmann et al., 2016; Lintzeris et al., 2019; Trigo et al., 2018; Vandrey et al., 2013 | |||
| Post-traumatic stress disorder | No | NCT03008005 | |||
| Alzheimer’s disease | No | NCT02792257 | |||
| Nabilone | Chemotherapy-induced nausea and vomiting | Yes | Cesamet® (nabilone) capsules for oral administration [package insert] (2013) Meda Pharmaceuticals | ||
| Acute pain in inflammatory bowel disease | No | NCT03422861 | |||
| Sleep disorders in post-traumatic stress disorder | No | Jetly et al., 2015 | |||
| Parkinson’s disease | No | Sieradzan et al., 2001 | |||
| Alzheimer’s disease | No | NCT02351882 | |||
| Nabiximols | Multiple sclerosis (MS) related spasticity | Yes* | Sativex® Oromucosal Spray. Prescribing Information. http://sativex.co.uk/static/documents/pi_uk.pdf | ||
| Pain associated with MS and cancer | Yes** | Sativex® Product Monograph. https://pdf.hres.ca/dpd_pm/00016162.PDF | |||
| Cannabis use disorder | No | Allsop et al., 2014 | |||
| Ajulemic Acid (JBT-101) | Selective CB2 Receptor Agonist | Chronic neuropathic pain | No | Karst et al., 2003 | |
| Dermatomyositis | No | NCT03813160; NCT02466243 | |||
| Lupus | No | NCT03093402 | |||
| Diffuse cutaneous systemic sclerosis | No | NCT03398837; NCT02465437 | |||
| GW842166 | Molar extraction-related pain | No | Ostenfeld et al., 2011 | ||
| Osteoarthritis-related pain | No | NCT00479427 | |||
| AZD1940 | Peripherally-Restricted CB1 Receptor Agonist | Molar extraction-related pain | No | Kalliomaki et al., 2013 | |
| Rimonabant (SR141716) | Selective CB1 Receptor Inverse Agonist | Obesity | No*** | (Boesten et al., 2012; Pi-Sunyer et al., 2006 | |
| Smoking cessation | No | Robinson et al., 2018 | |||
| Cannabis Use Disorder | No | Huestis et al., 2007; Huestis et al., 2001 | |||
| Neurocognition in schizophrenia | No | Boggs et al., 2012 | |||
| Taranabant (MK-0364) | Smoking cessation | No | Morrison et al., 2010 | ||
| Obesity/weight loss | No | Aronne et al., 2010; Kipnes et al., 2010; Proietto et al., 2010; Wadden et al., 2010 | |||
| Surinabant (SR147778) | Smoking cessation | No | Tonstad and Aubin, 2012 | ||
| Cannabis use disorder | No | Klumpers et al., 2013 | |||
| Tetrahydrocanna bivarin (THCV) | CB1 Receptor Antagonist/CB2 Receptor Partial Agonist | Obesity-associated glucose intolerance | No**** | Jadoon et al., 2016 | |
| Subjective/cognitive effects of cannabis | No**** | Englund et al., 2016 | |||
| Lipoxin A4 (LXA4) | CB1 Receptor Positive Allosteric Modulator | Infantile eczema | No | Wu et al., 2013 | |
| Gingivitis | No | NCT02342691 | |||
| Pregnenolone | CB1 Receptor Negative Allosteric Modulator | Schizophrenia | No | Ritsner et al., 2014; Kreinin et al., 2017; Kardashev et al., 2018 | |
| Bipolar disorder | No | NCT01409096 | Brown et al., 2014 | ||
| Cannabis use disorder | No | NCT03717272; NCT02439814 | |||
| Tobacco withdrawal | No | NCT00900900 | |||
| Concurrent bipolar/alcohol use disorders | No | NCT02582905 | |||
| PF-04457845 | FAAH Inhibitor | Cannabis use disorder | No | NCT03386487 | |
| Tourette syndrome | No | NCT02134080 | |||
| JNJ-42165279 | Social anxiety disorder | No | NCT02432703 | ||
| Autism spectrum disorder | No | NCT03664232 | |||
| Anxious distress in major depressive disorder | No | NCT02498392 | |||
| ASP3652 | Chronic prostatitis/chronic pelvic pain syndrome | No | Wagenlehner et al., 2017 | ||
| URB597 (KD-4103) | Schizophrenia | No | NCT00916201 | ||
| ABX-1431 | MAGL Inhibitor | Tourette syndrome | No | NCT03625453 | Jiang and van der Stelt, 2018 |
| Pain | No | NCT03138421; NCT02929264; NCT03447756 | |||
| Cannabidiol (CBD) | 5-HT1A receptor agonist, CB1 receptor antagonist or NAM, CB2 receptor inverse agonist,TRPV1 agonist, FAAH/anandamide transporter inhibitor, PPARy receptor agonist, GPR55 receptor antagonist | Lennox-Gastaut syndrome | Yes**** | Devinsky et al., 2017; Epidiolex® (cannabidiol) oral solution [package insert] (2018) Greenwich Biosciences, Inc | |
| Dravet syndrome | Yes**** | Devinsky et al., 2018; Epidiolex® (cannabidiol) oral solution [package insert] (2018) Greenwich Biosciences, Inc | |||
| Treatment-resistant epilepsy | No | NCT03808935 | |||
| Schizophrenia | No | NCT02926859 | Leweke et al., 2012; McGuire et al., 2018; Boggs et al., 2018 | ||
| Substance use disorders | No | Hurd et al., 2019; Morgan et al., 2013; Hindocha et al., 2018b | |||
| Anxiety disorders | No | NCT03549819; NCT04086342 | Bergamaschi et al., 2011; Crippa et al., 2011; Fusar-Poli et al., 2009; Linares et al., 2019; Masataka, 2019 | ||
| Parkinson’s disease | No | NCT03582137 | Chagas et al., 2014; Lotan et al., 2014; Sieradzan et al., 2001 | ||
| Huntington’s disease | No | Consroe et al., 1991 | |||
| Chronic pain | No | NCT03984565, NCT03215940 | Notcutt et al., 2004; Wade et al., 2003 |
Nabiximols is approved to treat MS-related spasticity in Canada, United Kingdom, and several other European countries.
Nabiximols is approved to treat pain associated with MS and cancer in Canada.
Rimonabant was withdrawn from the market worldwide in 2008 due to increased depression and suicide ideation in a small percentage of participants.
Plant-derived cannabinoid was employed.
2. A PRIMER ON THE ENDOCANNABINOID SYSTEM: MODULATION BY CANNABIS SATIVA L- ISOLATED AND SYNTHETIC CANNABINOIDS
2.1. ENDOCANNABINOID SYSTEM
A primer on the endocannabinoid system is warranted to understand the pharmacological targets of medically-approved SCBs, illicit SCBs, and potential SCB therapeutics. A simplified overview of the endocannabinoid system includes (1) endogenous cannabinoid agonists, or lipid molecules that are physiological ligands for cannabinoids receptors, (2) cannabinoid receptors that are stimulated by endogenous cannabinoid agonists, and (3) enzymes that degrade endogenous cannabinoid agonists (for review, (Mechoulam and Parker, 2013)). Two endogenous cannabinoid agonists were isolated from brain and peripheral tissues, respectively, and extensively investigated: N-arachidonoylethanolamine (anandamide) and 2-arachidonoylglycerol (2-AG) (Devane et al., 1992). In the central nervous system, 2-AG and anandamide are synthesized locally in the postsynaptic neuron and act presynaptically on cannabinoid receptors, a retrograde synaptic messaging mechanism that is unlike classical neurotransmitters (e.g., dopamine, serotonin (5-HT)), which are stored in vesicles and released from presynaptic terminals (Howlett et al., 2002).
The actions of endogenous cannabinoid agonists are mediated, in part, by two G protein-coupled receptors (GPCRs): the CB1 and CB2 receptors (for reviews, (Di Marzo and Piscitelli, 2015; Pertwee, 1997)). Agonism of the Gi/o-linked CB1 or CB2 receptor results in adenylate cyclase inhibition, which decreases accumulation of intracellular cyclic adenosine monophosphate (cAMP) concentrations, transactivates rectifying potassium channels, and inhibits N-type and P/Q-type voltage-gated calcium channels (Fraguas-Sanchez et al., 2016; Lu and Mackie, 2016; Pertwee et al., 2010). The CB1 receptor is richly expressed in the central nervous system (CNS) as determined via quantitative autoradiography, in situ hybridization, and immunocytochemistry studies (Mackie, 2005). Areas in the CNS with high expression of CB1 receptors include the cerebellum, hippocampus, basal ganglia, and olfactory bulb, whereas CB1 expression is lower in the cerebral cortex, hypothalamus, thalamus, amygdala, septum, brainstem, and dorsal horn of the spinal cord (Mackie, 2005). The CB1 receptor is also expressed extensively outside the CNS, especially in the gut, liver, reproductive system, skeletal muscles, cardiovascular system, and epidermis (for review, (Zou and Kumar, 2018)).
The CB2 receptor, which shares 44% sequencing homology with the CB1 receptor protein, is predominantly expressed peripherally (e.g., spleen, liver Kupffer cells, adipose, bone, reproductive and cardiovascular systems, GI tract as well as circulating immune cells) (for reviews, (Bie et al., 2018; Howlett et al., 2002)). The CB2 receptor originally appeared to have little CNS expression under normal physiological conditions, with early evidence suggesting it was restricted to the brainstem, hippocampal CA2/3 pyramidal neurons, and highly-inducible reactive microglia (following inflammation or injury) (Bie et al., 2018; Stempel et al., 2016; Van Sickle et al., 2005). Though initial studies were unable to detect the CB2 receptor in healthy brains (Carlisle et al., 2002; Chakrabarti et al., 1995; Derocq et al., 1995; Galiegue et al., 1995; Griffin et al., 1999; Schatz et al., 1997; Sugiura et al., 2000), there is evidence now for CB2 receptor immunoreactivity in the spinal cord as well as various rodent brain regions (e.g., cerebellum, hippocampus, striatum, cerebral cortex) (Gong et al., 2006; Onaivi et al., 2006; Xi et al., 2011).
Endogenous cannabinoid agonists are rapidly degraded (Fu et al., 2011), in part, by the actions of two hydrolase enzymes. Monoacylglycerol lipase (MAGL) is the key enzyme in the hydrolysis and degradation of 2-AG, while fatty acid amide hydrolase (FAAH) mediates anandamide degradation. Inhibition of MAGL and FAAH may increase endocannabinoid levels, an attractive drug development target that is discussed below in Section 4.5.
Knowledge of endocannabinoid receptor systems is expanding via the discovery of endocannabinoid interactions with non-CB GPCRs, deorphanized GPCRs such as GPR55 (GPRs), transient receptor potential (TRP) channels, and peroxisome proliferator-activated nuclear receptors (PPARs) (Pertwee et al., 2010). Continued investigation of these novel mechanisms will expand our understanding of the endocannabinoid system.
2.2. UNDERSTANDING THE FIRST GENERATION OF MEDICALLY-APPROVED SCBS: CANNABINOID ISOLATION AND SYNTHESIS
Phytocannabinoids occur naturally in the cannabis plant and interact with the endocannabinoid system. While there are over 100 known phytocannabinoids (ElSohly et al., 2016), research has predominantly focused on THC and cannabidiol (CBD), the two most abundant phytocannabinoids in cannabis. The structures and stereochemistry of CBD and THC were described in 1963 and 1964, respectively, when they were first isolated from the cannabis plant by Raphael Mechoulam. Just one year later, in 1965, Mechoulam synthetized (±)-Δ9-THC and (±)-CBD (and later, each of their enantiomers) in the laboratory (Mechoulam and Burstein, 1973; Mechoulam and Hanus, 2000). Since then, the pharmacokinetics and mechanisms of action of THC and CBD were reviewed in detail (Agurell et al., 1986; Ashton, 2001; Grotenhermen, 2003; Huestis and Smith, 2018; Maykut, 1985). In this review, studies of both synthetic and plant-derived THC and CBD are discussed because both were employed in clinical studies and some studies do not specify the source of the drug used, but we distinguish these when possible. To reiterate Bonn-Miller et al. (2019), “chemistry is an exact science with respect to chemical composition and structure,” therefore there is little reason to believe that plant-derived and synthetic forms of either THC or CBD will perform differently in clinical studies.
3. MEDICALLY-APPROVED SYNTHETIC CANNABINOIDS IN HUMANS: THC AND THC-LIKE DRUGS
3.1. Experimental Investigation, FDA Approval, and Therapeutic Uses of THC and THC-Like Drugs
In this section, we review the first generation of medically-approved SCBs in humans: synthetic THC (dronabinol) and the THC-analog nabilone. THC binds to the CB1 (Ki: 5.05 – 53.3 nM) and CB2 (Ki: 3.13 – 75.3 nM) receptors as evidenced by displacement binding studies utilizing human cannabinoid receptors (for reviews, (Pertwee, 1997, 2008)). Similarly, the THC-analog nabilone showed a similar profile at the CB1 receptor in vitro (Ki = 22.3 ± 6.6 nM). Nabilone also exhibited greater efficacy than THC, that is, nabilone produced greater cAMP inhibition and [35S]GTPγS binding than THC in rodent brain homogenates (Matsuda et al., 1990).
In the 1980’s, the United States Food and Drug Administration (FDA) approved dronabinol and nabilone for anorexia associated with weight loss in patients with autoimmune deficiency syndrome (AIDS) and for chemotherapy-induced nausea or vomiting. These approvals were based on extensive RCTs assessing safety, tolerability, and efficacy (Borgelt et al., 2013). Pain relief is one of the most physiologically and clinically substantiated uses of THC (National Academies of Sciences and Medicine, 2017; Whiting et al., 2015). To date, over 40 RCTs explored plant-derived and synthetic THC or nabilone for various types of chronic pain with encouraging findings, specifically for neuropathies. However, many of these trials were of short duration, efficacy was assessed as an adjunct to other analgesics, and findings were not consistent across chronic pain conditions (Cooper and Abrams, 2019; National Academies of Sciences and Medicine, 2017). Several studies are underway to further understand the clinical utility of THC or nabilone for pain, including assessing the use of dronabinol for neuropathic back pain (NCT02460692) and nabilone for acute pain in inflammatory bowel disease (NCT03422861).
Recent and ongoing clinical trials suggest that THC or nabilone have therapeutic potential for numerous additional health problems. For instance, dronabinol and nabilone were explored for the treatment of Cannabis Use Disorder (CUD) with positive findings (Budney et al., 2007; Haney et al., 2004; Herrmann et al., 2016; Lintzeris et al., 2019; Trigo et al., 2018; Vandrey et al., 2013). THC or nabilone for the treatment of post-traumatic stress disorder (PTSD) is another area of interest with promising findings. In a small pilot study, nabilone demonstrated efficacy in the treatment of sleep-related symptoms of PTSD in military personnel (Jetly et al., 2015); the potential therapeutic effects of dronabinol for PTSD are also under evaluation (i.e., NCT03008005). For aging-related disorders, nabilone demonstrated efficacy for levodopa-induced dyskinesia in Parkinson’s disease (Sieradzan et al., 2001) and dronabinol decreased anorexia, disturbed behavior (Volicer et al., 1997), and improved nighttime agitation (Walther et al., 2006) in Alzheimer’s disease; ongoing clinical trials are assessing dronabinol (NCT02792257) and nabilone (NCT02351882) in the treatment of Alzheimer’s-associated agitation. In summary, investigations with synthetic THC and THC-like compounds for a wide range of conditions provide positive signals. However, aside from the approved uses of these drugs (i.e., nausea associated with chemotherapy and weight loss in AIDS), chronic pain, and CUD, no more than a few studies per indication rigorously evaluated the therapeutic potential of these drugs.
The future of THC-like SCB development is vast, but one promising direction is to administer THC in combination with CBD, especially for the treatment of pain and spasticity. This combination is advantageous, in part, due to the unique mechanisms of action of THC and CBD. THC targets CB1/2 receptors such as those expressed in the peripheral, spinal, and supraspinal pain systems (Überall, 2020). Meanwhile, a recent preclinical study demonstrated that 7 days of CBD treatment normalized impaired 5-HT neurotransmission, reduced mechanical allodynia, and decreased anxiety-like behavior in a model of neuropathic pain (De Gregorio et al., 2019). In this light, considering that anxiety and stress are hypothesized drivers of chronic pain, a second advantage of the THC:CBD combination is that CBD may also improve anxiety-like behavior associated with neuropathic pain, in part, by modulating the potential anxiogenic effects of THC (Überall, 2020).
Currently, plant-derived THC in the form of an oromucosal spray (nabiximols or Sativex), which delivers 2.7 mg THC in combination with 2.5 mg CBD per actuation, is approved in the UK, Germany, and Switzerland for multiple sclerosis (MS) related spasticity and in Canada for pain associated with MS and cancer (Etges et al., 2016); it is not approved in the United States for any indication at this time. Recent studies suggest that nabiximols may also reduce the severity of cannabis withdrawal in CUD patients (Allsop et al., 2014). Similar clinical investigations in cannabis users employing synthetic, pharmaceutical grade THC and/or CBD (for example, from STI Pharmaceuticals) are published (Hindocha et al., 2015; Solowij et al., 2019)
3.2. Toxicity, Safety, and Abuse Liability of THC and THC-like SCBs
Dronabinol use in humans is associated with numerous, potential adverse reactions. First, neuropsychiatric adverse events were observed in some clinical trials, and dronabinol was reported to exacerbate depression, mania, and schizophrenia and to impair cognitive function (Marinol (dronabinol) full prescribing information). For example, in antiemetic clinical trials, significant CNS symptoms followed oral dronabinol doses of 0.4 mg/kg (28 mg per 70 kg patient). Dronabinol use is also associated with hemodynamic instability (assessed as hypo- or hypertension, fainting, or tachycardia) and is not recommended for patients with cardiovascular disorders or those using concomitant medications with similar cardiac effects (e.g., amphetamines). Dronabinol patients also reported seizures, and dronabinol use is discouraged in patients using anti-epileptic medications. Other common adverse reactions reported in clinical trials (incidence >1%) include nausea, vomiting, and euphoria, discussed below.
Relevant to dronabinol misuse, a dose-related “high” (easy laughing, elation, and heightened awareness) was reported by patients receiving dronabinol in both the antiemetic (24%) and the lower dose appetite stimulant clinical trials (8%) (Marinol (dronabinol) full prescribing information). A human laboratory study revealed that dronabinol produced positive subjective effects in cannabis users (Hart et al., 2005) and dependence (Haney et al., 1999); however, evidence of dronabinol misuse or diversion in community settings is scarce (Calhoun et al., 1998). Thus, dronabinol was progressively rescheduled from Schedule I to Schedule III under the Drug Enforcement Administration Controlled Substances Act. Nonetheless, the FDA discourages dronabinol prescribing to patients with a history of substance misuse and encourages regular assessment abuse potential in dronabinol patients.
Adverse events associated with nabilone use are similar to dronabinol, likely due to their shared mechanisms of action. Nabilone use is associated with tachycardia and hypotension and may exacerbate symptoms of mania, depression, or schizophrenia. The most common adverse reactions to nabilone in placebo-controlled studies included vertigo (52%), drowsiness (52%), and dry mouth (36%). In active-controlled studies (using prochlorperazine), nabilone elicited drowsiness (66%), vertigo (59%), and euphoria (38%) (Cesamet (nabilone) full prescribing information). To this end, nabilone is currently a Schedule II drug in the U.S. and is indicated by the FDA as having potential for misuse, but it appears to have a low incidence of misuse and diversion (for review, (Ware and St Arnaud-Trempe, 2010)).
Nabiximols were generally well-tolerated as indicated by data from 941 patients in an observational registry following approval of nabiximols in Europe. Here, 32% percent of patients stopped treatment, with ~25% of those citing adverse events. The most common treatment-related AEs included dizziness (2.3%) and fatigue (1.7%).
4. SYNTHETIC CANNABINOIDS NOT APPROVED FOR MEDICAL USE IN HUMANS: THERAPEUTIC POTENTIAL AND ADVERSE EFFECTS
4.1. High-Affinity CB1/2 Receptor Agonists
In this section, we discuss the development of high-affinity, high-efficacy CB1/2 receptor agonists that elicit cannabimimetic effects similar to THC. The structural classes of high-affinity, high-efficacy CB1/2 receptor agonists are incredibly diverse, with hundreds of compounds characterized. These include (example compounds in parentheses): adamantoylindoles (AB-001, AKB48), aminoalkylindoles (WIN 55,212-2), benzoylindoles (AM694, RCS-4), cyclohexylphenols (CP 47,497, CP 55,940), dibenzopyrans (HU-210), indazole carboxamides (ADB-PINACA), naphthoylindoles (JWH-018), naphthylmethylindoles (JWH-175), naphthylmethylindenes (JWH-176), napthoylpyrroles (JWH-307), phenylacetylindoles (JWH-250), quinolinyl ester indoles (PB-22), and tetramethylcyclopropylindoles (UR-144, XLR-11). These compounds were originally developed for research purposes as investigational tools to probe the function of the endogenous cannabinoid system; however, progress in this area was impeded because most ligands exhibit poor selectivity between the CB1 and CB2 receptors (Castaneto et al., 2014). Generally, though, many of these compounds exhibit (1) greater affinity for CB1 and CB2 receptors and/or (2) full agonist activity at the CB1/2 receptors relative to the partial agonist actions of THC (for example, JWH-018, JWH-073, AM-1248, CP-55-940, WIN-55,512-2, and HU-210). Thorough reviews of these pharmacological profiles are published elsewhere (Castaneto et al., 2014; Fantegrossi et al., 2014).
4.1.1. Therapeutic Potential
High-affinity CB1/2 receptor agonists are typically associated with use in pre-clinical research to understand the function of the endocannainoid system or with the illicit SCB products being misused for their intoxicating effects. However, it is also possible that these compounds could be developed as therapeutic medications. The approval of dronaboinol and nabilone demonstrate that CB1/2 agonism has medicinal value. Positive preclinical data from animal studies modeling neuropathic, inflammatory, and cancer pain indicate that these ligands could treat different types of pain that are difficult to treat in clinical practice (for review, (Tamba et al., 2020)). Moreover, in an animal model of vomiting (emesis), the order of potency for reducing emesis frequency and the percentage of animals vomiting was CP55,940 > WIN55,212-2 > Δ9-THC (Darmani, 2001). Thus, high-affinity, high-efficacy cannabinoid medications (e.g., full agonists) may confer better therapeutic effects than dronabinol or nabilone.
However, few controlled clinical studies have examined high-affinity CB1/2 receptor agonists in humans. Clinical testing of the CB1/2 receptor full agonist Org 28611 (SCH-900,111) was conducted as a possible treatment for postoperative pain following dental impaction surgery (NCT00782951); this trial was terminated at phase 2 because the drug did not demonstrate superiority to existing analgesics. At a laboratory in the Netherlands, six participants inhaled vapor from the high-affinity CB1/2 receptor full agonist JWH-018 (2 or 3 mg) (Theunissen et al., 2018; Toennes et al., 2017). Both doses of JWH-018 were well tolerated with no serious side effects reported, but no therapeutic endpoints were evaluated in this study.
In sum, a case could be made to pursue high-affinity, high-efficacy CB1/2 agonists as therapeutics, but, given the documented cases of acute and, in some cases, fatal adverse events associated with use of these compounds outside of medicine, care would need to be exercised with respect to dose and formulation. Specifically, efficacy at low doses would need to be established and an oral route of delivery may reduce the likelihood of misuse or overdose. Restriction of use to hospital settings or other controlled environments may also help reduce the risk of adverse consequences related to therapeutic use of this class of compounds.
4.1.2. Toxicity, Safety, and Abuse Liability
A wide range of adverse effects are associated with the use of high-affinity CB1/2 receptor agonists obtained from the illicit drug market. Reports related to illicit SCB toxicity from emergency rooms, poison control centers, police reports, driving under the influence of drug cases, and psychiatric clinics were published. Acute adverse effects associated with high-affinity, high-efficacy CB1/2 receptor agonists include toxic gastrointestinal, renal, cardiovascular, pulmonary, and CNS effects (Logan et al., 2017). Common overt adverse effects reported were cognitive impairment, agitation, nausea, seizures, and psychosis. The CB1/2 receptor agonist XLR-11 was specifically linked to acute kidney injury (Buser et al., 2014), agitated delirium associated with ADB-PINACA, and severe illness and death following MAB-CHMINACA (ADB-CHMINACA) exposure (Trecki et al., 2015). In July 2016 in New York, 33 people exposed to AMB-FUBINACA became “zombie-like,” dropping onto the sidewalks in a small area of Brooklyn (Adams et al., 2017). Two cases of SCB use resulting in Hallucinogen Persisting Perception Disorder were also reported (Lerner et al., 2014). SCB overdose reports include a number of fatalities in which the cause of death was attributed to high-affinity, high-efficacy CB1/2 receptor agonist exposure, which highlights a key difference between, for example, the toxicity of CB1/2 receptor full agonists versus a partial agonist such as THC. In post-mortem cases, the exact mechanism of toxicity for SCBs is unclear.
Substance use disorder, marked by severe withdrawal upon cessation of use, may also occur with frequent use of high-affinity, high-efficacy CB1/2 receptor agonists (Cooper, 2016). The types of withdrawal symptoms reported by these CB1/2 receptor agonists users are similar to those associated with cannabis/THC withdrawal, including headache, sweating, anxiety, depression, agitation/aggression, difficulty concentrating, restlessness, sleep disturbance, and nausea (Vandrey et al., 2012; Zimmermann et al., 2009).
4.2. Novel CB1/2 Receptor Agonist Development: Selective CB2 Receptor Agonists and Peripherally-Restricted CB1 Receptor Agonists
In light of the toxicity, abuse liability, and challenging regulatory status of CNS-active, high-affinity, high-efficacy CB1/2 receptor agonists, novel strategies were developed to stimulate the CB1 and CB2 receptors with limited potential for adverse events. We discuss two of these pharmacological mechanisms below: selective CB2 receptor agonists and peripherally-restricted CB1 receptor agonists. Selective CB2 receptor agonists, such as ajulemic acid and GW842166, were probed for potential anti-inflammatory effects. Ajulemic acid (JBT-101) exhibited roughly 12-fold greater affinity for the CB2 receptor (Ki = 51 nM) over the CB1 receptor (Ki = 628 nM) assessed via radioligand binding (performed in HEK-293T transfected with human CB1 and CB2 receptors) using the tritiated CB1 receptor antagonist SR141716 (rimonabant) (Tepper et al., 2014). GW842166 exhibited moderate binding affinity for CB2 receptors (Ki = 63 nM) and no appreciable CB1 receptor agonist activity in human CB1 and CB2 recombinant receptor assays (Giblin et al., 2007).
Peripherally-restricted CB1 receptor agonists like AZD1940 are compelling as they lack the negative CNS effects (e.g. intoxication, impairment of cognitive functioning) of centrally acting CB1/2 receptor agonists. The peripheral restriction of AZD1940 was demonstrated using positron emission tomography measurements in cynomolgus monkey after intravenous injection of [11C] AZD1940 (Schou et al., 2013). The following sections explore the therapeutic potential and adverse events associated with ajulemic acid, GW842166, and AZD1940.
4.2.1. Therapeutic Effects
Ajulemic acid showed promise in preclinical models of inflammation, fibrosis, and pain (Adams et al., 2017; Dyson et al., 2005; Johnson et al., 2007; Stebulis et al., 2008; Zurier et al., 2009). Clinically, ajulemic acid was effective for chronic neuropathic pain potentially by activation of the PPAR-γ receptor (Karst et al., 2003). It is now being investigated for dermatomyositis (NCT03813160; NCT02466243), lupus (NCT03093402), and diffuse cutaneous systemic sclerosis (NCT03398837; NCT02465437). GW842166, another CB2 receptor agonist with low affinity for CB1 receptors, was explored for the treatment of acute inflammatory pain. However, an earlier study failed to demonstrate effectiveness for acute pain associated with molar extraction (Ostenfeld et al., 2011). Another study assessed the drug’s effectiveness for pain control in patients with osteoarthritis; results of the trial are unknown (NCT00479427).
The peripherally restricted CB1 and CB2 receptor agonist AZD1940 was developed to target pain without psychoactive effects. The compound failed to elicit analgesic effects in an experimental model of pain (Kalliomaki et al., 2013) or after molar extraction (Kalliomaki et al., 2013). There are no ongoing trials of AZD1940.
4.2.2. Toxicity, Safety, and Abuse Liability
Ajulemic acid and GW842166 were safe and well tolerated. The most prominent ajulemic acid adverse effects compared to placebo were dry mouth and tiredness. GW842166 administration was associated with headache (observed in all treatment groups) followed by nausea, pyrexia, pharyngolaryngeal pain and syncope. Mild AZD1940 CNS effects were observed for ‘high’ and ‘sedated’. Dose-dependent mild-to-moderate CNS-related and gastrointestinal adverse events were reported following treatment with AZD1940.
4.3. CB1 Receptor Antagonists/Inverse Agonists
The mechanisms discussed above involved stimulation of the CB1 and/or CB2 receptors using partial or full agonists, and some of these ligands show therapeutic potential with limited adverse events. An additional strategy is to block function of the CB1 receptor using CB1 receptor antagonists/inverse agonists. Several synthetic, selective CB1 receptor antagonists/inverse agonists were evaluated clinically, especially for the treatment of obesity and smoking cessation. Clinical evaluation of these compounds for obesity were informed by their anti-obesity effects in rodents, for example, transient reductions in food intake, body weight, adiposity, and corrections in insulin resistance (Ravinet Trillou et al., 2003). Similarly, clinical development of CB1 receptor antagonist/inverse agonists for smoking cessation were based, in part, on animal studies demonstrating reductions in nicotine self-administration (Cohen et al., 2002) and nicotine-induced conditioned place preference (Le Foll and Goldberg, 2005).
Rimonabant was first described as a potent and selective CB1 receptor antagonist with 100-fold selectivity over the CB2 receptor in studies employing CHO cells transfected with isolated CB receptors from the rat frontal cortex (Rinaldi-Carmona et al., 1994). In cloned human CB1 and CB2 receptors, rimonabant exhibited high CB1 receptor selectivity (Ki = 25 nM) over the CB2 receptor (Ki > 1000 nM) (Lange et al., 2005). Rimonabant was later identified as a CB1 receptor inverse agonist in functional assays using human recombinant CB1 and CB2 receptors (MacLennan et al., 1998). Surinabant (SR147778) was identified as a potent and selective CB1 receptor inverse agonist with nanomolar affinity (Ki = 3.5 nM) for the human recombinant CB1 receptor and low affinity (Ki = 400 nM) for the human recombinant CB2 receptor (Rinaldi-Carmona et al., 2004). Taranabant (MK-0364) was identified as a potent and selective inverse agonist (Ki = 0.13 nM) at the human recombinant CB1 receptor with approximately 1000-fold selectively over the CB2 receptor (Fong et al., 2007) Finally, tetrahydrocannabivarin (THCV, GWP42004) is a CB1 receptor antagonist (Ki = 46.6–75.4 nM for the mouse CB1 receptor) and partial CB2 receptor agonist (Ki = 62.8 at the human recombinant CB2 receptor) (Pertwee, 2007; Thomas et al., 2005). Although plant-derived THCV is under clinical investigation, it is discussed here since two synthetic analogs showed similar pharmacological profiles in vitro and in vivo (Pertwee et al., 2007).
4.3.1. Therapeutic Potential
SR141716 (later named Rimonabant, Acomplia, Zimulti), the first CB1 receptor inverse agonist (MacLennan et al., 1998), was developed as a medication to reduce obesity and normalize metabolic syndrome (Huestis et al., 2001). The drug was approved in Europe and Brazil to treat obesity in 2006 and 2007, respectively. In the US, rimonabant underwent clinical trial testing for both obesity and smoking cessation and showed promise in clinical studies for both indications (Boesten et al., 2012; Pi-Sunyer et al., 2006; Robinson et al., 2018). The drug was also assessed as a possible pharmacotherapeutic strategy for CUD and decreased subjective ratings of cannabis intoxication (Huestis et al., 2007; Huestis et al., 2001). However, rimonabant was withdrawn from use in Europe and Brazil, and from the application for FDA approval in 2008, due to increased suicidal ideation and depression (see 4.3.2. Toxicity, Safety, and Abuse Liability). After approval was withdrawn, some clinical studies of rimonabant continued (Boggs et al., 2012), but it is not currently marketed for clinical use. Rather, it is largely used as a research tool to block the effects of CB1 agonists in preclinical studies.
Taranabant, another CB1 receptor inverse agonist (Armstrong et al., 2007), was tested for smoking cessation (Morrison et al., 2010), weight loss (Aronne et al., 2010; Kipnes et al., 2010; Proietto et al., 2010; Wadden et al., 2010), and lipid and glycemic endpoints (Aronne et al., 2010; Kipnes et al., 2010; Proietto et al., 2010). Taranabant failed to assist in smoking cessation, but did show significant effectiveness for weight loss and improvement in glycemic parameters.
Surinabant was investigated as a smoking cessation agent and a potential pharmacotherapy for CUD. Similar to rimonabant, surinabant significantly decreased cannabis intoxication and cardiovascular effects (Klumpers et al., 2013). However, the drug failed to improve rates of smoking cessation compared to placebo, although it was successful in decreasing post-smoking cessation weight gain (Tonstad and Aubin, 2012).
THCV is of interest for the treatment of obesity-associated glucose intolerance and as a strategy to decrease the negative subjective and cognitive effects of cannabis. Although THCV did not affect high-density lipoprotein (HDL) concentrations in patients with type 2 diabetes mellitus, it significantly decreased fasting glucose and improved pancreatic beta cell function, suggesting its therapeutic potential to improve glycemic control in type 2 diabetes (Jadoon et al., 2016). In line with other CB1 receptor antagonists, THCV successfully decreased subjective effects of THC and some cognitive endpoints (Englund et al., 2016).
4.3.2. Toxicity, Safety, and Abuse Liability
Though clinical studies with CB1 receptor inverse agonists showed considerable therapeutic promise, adverse effects led to the discontinuation of their development as pharmacotherapies. In clinical trials of rimonabant, approximately 83% of patients reported at least one adverse event across treatment groups, and more patients receiving rimonabant withdrew from the study due to psychiatric, nervous system, and gastrointestinal tract adverse events (Pi-Sunyer et al., 2006). Compared to patients receiving placebo, upper respiratory tract infection, nasopharyngitis, nausea, influenza, diarrhea, arthralgia, anxiety, insomnia, viral gastroenteritis, dizziness, depressed mood, and fatigue were reported in ≥5% of patients receiving 20 mg of rimonabant. While rimonabant was generally well tolerated, with nausea the most common drug-related adverse event, it was withdrawn from all commercial markets in 2008 due to increases in depression and suicidal ideation observed during pharmacovigilance monitoring. Psychiatric adverse effects associated with taranabant, another CB1 receptor inverse agonist, led to discontinuation of its development for weight loss (Koch, 2010).
Surinabant was generally safe and well-tolerated with minimal adverse effects. Adverse effects were mild to moderate and transitory, with 56% percent of the treated group reporting adverse events (headache 28%, somnolence 17%, and nausea 17%) compared to 42% of the placebo group (Klumpers et al., 2013). In a second study, the most common adverse events associated with surinabant were headache, nausea, insomnia, anxiety, nasopharyngitis, diarrhea, and excess sweating (Tonstad and Aubin, 2012). THCV was also well tolerated with a similar percentage of adverse effects reported by the THCV (91.7%), and placebo (92.9%) groups; decreased appetite was the most common adverse effect (Jadoon et al., 2016). No CB1 inverse agonist or antagonist exhibited significant abuse liability or potential for misuse in clinical testing completed to date.
4.4. Allosteric Modulation of the CB1 Receptor
In this review, we discussed strategies to target the CB1 and CB2 receptor orthosteric binding sites, or the sites to which endogenous agonists bind. Additionally, much attention was drawn to the development of CB1 receptor allosteric modulators. GPCR allosteric modulators often lack intrinsic efficacy but influence the binding or efficacy of orthosteric ligands, which can modulate downstream second messenger signaling (May et al., 2007). In this way, allosteric modulators may not produce receptor overactivation or downregulation that can occur in response to repeated orthosteric ligand administration, which may reduce adverse events (Wootten et al., 2013). Thus, CB1 receptor allosteric modulators provide a novel pharmacologic mechanism to mitigate the rate or severity of adverse effects commonly observed with exogenous orthosteric ligand administration.
Positive allosteric modulators (PAMs) increase receptor function through an increase in agonist affinity or efficacy (Gentry et al., 2015). Several CB1 receptor PAMs are characterized preclinically, including RTI-371, ZCZ-011, GAT211 (and its enantiomers), and lipoxin A4 (LXA4), an endogenous, fatty-acid metabolite of arachidonic acid (for review, (Nguyen et al., 2017)). Below, we describe clinical studies using the CB1 receptor PAM LXA4.
Negative allosteric modulators (NAMs) decrease receptor function through a decrease in agonist affinity or efficacy (Gentry et al., 2015). Several CB1 receptor NAMs are described, including ORG27569, PSNCBAM-1, peptide endocannabinoids (“pepcans”), CBD, and pregnenolone (for review, (Nguyen et al., 2017)). Given the diverse receptor mechanism for CBD, it is described later in the text (Section 4.6.)
4.4.1. Therapeutic Potential
Positive allosteric modulator LXA4:
LXA4 expression was detected in mouse brain, and does not compete for the CB1 receptor orthosteric binding site (Pamplona et al., 2012). Rather, LXA4 enhances affinity of the endocannabinoid anandamide at the CB1 receptor both in vitro and in vivo, suggesting positive allosteric modulation of the CB1 receptor (Pamplona et al., 2012). Clinically, the safety and efficacy of topically-administered LXA4 was evaluated for the treatment of infantile eczema in a double-blind, placebo-controlled study. 60 patients ages 1-12 were randomized to receive placebo or a LXA4-containing cream for ten days. Relative to placebo, the LXA4 cream relieved eczema severity, induced recovery, and improved patient quality of life (Wu et al., 2013). Another clinical trial to evaluate a LXA4 analog for safety and preliminary efficacy to treat gingivitis is registered, but its status is unknown (NCT02342691). However, in these studies, LXA4 was interrogated for its anti-inflammatory properties and not CB1 receptor PAM activity per se; clinical studies evaluating LXA4 through a CB1 receptor PAM mechanism of action were not initiated, to our knowledge.
Negative allosteric modulator pregnenolone:
Stimulation of CB1 receptors by THC increases the synthesis of pregnenolone, a steroid hormone precursor produced by the adrenal gland. Pregnenolone then provides negative feedback and reduces THC effects by allosterically decreasing CB1 receptor response (Vallee et al., 2014). Pregnenolone demonstrates promise in the treatment of schizophrenia/schizoaffective disorder, bipolar disorder, and substance use disorders. When administered with certain antipsychotic medications, pregnenolone reduced negative symptoms (Ritsner et al., 2014), visual attention deficits (Kreinin et al., 2017) and general functioning (Kardashev et al., 2018). In bipolar disorder, pregnenolone significantly increased depression remission rates (Brown et al., 2014). Several ongoing studies are exploring whether pregnenolone and derivatives can treat substance use disorders, including CUD (NCT03717272; NCT02439814), tobacco withdrawal (NCT00900900), mood disorders associated with concurrent alcohol use disorder (NCT02582905), and symptoms of bipolar disorder (NCT01409096).
4.4.2. Toxicity, Safety, and Abuse Liability
The CB1 receptor PAM LXA4 was well tolerated, and no clinical adverse events were reported. All safety parameters were within normal limits: blood counts, urine/feces examinations, electrocardiogram, and liver and kidney function tests (Wu et al., 2013). The CB1 receptor NAM pregnenolone is well tolerated, with no new treatment-related adverse events associated with its administration. No clinically significant changes in vital signs, electrocardiograms, or clinical laboratory variables were noted with treatment (Kardashev et al., 2018).
4.5. Non-CB Receptor Mechanisms: Inhibiting Endocannabinoid-Degrading Enzymes FAAH and MAGL
Another novel pharmacological mechanism for SCB development is to target hydrolase enzymes that regulate endocannabinoid metabolism; increasing the concentration of endocannabinoids could circumvent the adverse events associated with exogenous CB1/2 receptor ligands (Mallet et al., 2016). For example, the membrane enzyme FAAH hydrolyzes anandamide (Watkins and Kim, 2014), while FAAH-knockout mice exhibit enhanced levels of anandamide, as well as CB1 receptor-mediated hypoalgesia in thermal nociceptive tests (Cravatt et al., 2001; Lichtman et al., 2004). SCBs that inhibit FAAH or MAGL are in development as potential therapeutics for a variety of health conditions.
4.5.1. Therapeutic Potential
FAAH is a metabolic enzyme that breaks down the endogenous cannabinoid anandamide (Cravatt et al., 1996). Reversible and irreversible FAAH inhibitors may provide novel strategies to treat pain, inflammation, mood, anxiety, and sleep disorders. One of these FAAH inhibitors, BIA 10-2474, was under development to treat neuropathic pain; however, it failed during a phase I clinical human trial due to serious adverse events including mortality (see Section 4.5.2.). Phase 2 studies of another FAAH inhibitor, JNJ-42165279, were temporarily suspended in light of findings with BIA 10-2474. However, JNJ-42165278 exhibited fewer adverse effects (Zannikos et al., 2014), and is currently under investigation to treat social anxiety disorder (NCT02432703), autism spectrum disorder (NCT03664232), and anxious distress in major depressive disorders (NCT02498392). Recent studies suggest that one particular FAAH inhibitor (PF-04457845) may be useful for treating CUD (D’Souza et al., 2019). This compound reduced cannabis withdrawal symptoms, lowered self-reported cannabis use, and lowered urinary THCCOOH concentrations in men with CUD. PF-04457845 is currently being assessed for CUD in a multi-site trial (NCT03386487) and is also being tested for Tourette syndrome (NCT02134080). ASP3652 is a FAAH inhibitor proposed to reduce the excitability of urinary tract afferents, including nociceptors, thus having the potential to treat chronic prostatitis/chronic pelvic pain syndrome. However, an adaptive, double-blind, placebo-controlled trial failed to find the drug effective (Wagenlehner et al., 2017). URB597 (KD-4103), a potent selective FAAH inhibitor that was phase 1-ready (but not yet evaluated) to target pain, anxiety, and depression (Piomelli et al., 2006), is currently registered for the study of its effects on schizophrenia-associated symptoms (NCT00916201).
Another strategy to target the endocannabinoid system is by preventing degradation of 2-AG, an endocannabinoid that binds to both CB1 and CB2 receptors and is hydrolyzed by MAGL (Dinh et al., 2002; Makara et al., 2005). Early studies showed that inhibiting MAGL may be helpful in treating Tourette syndrome (ABX-1431) (Jiang and van der Stelt, 2018) and follow up studies are underway (NCT03625453). Several ongoing trials also assess the effects of this compound on pain (NCT03138421; NCT02929264; NCT03447756).
4.5.2. Toxicity, Safety, and Abuse Liability
There were serious adverse effects in six participants in the first phase I clinical trial of a FAAH inhibitor, BIA 10-2474, including one death and two volunteers with serious neurological damage at repeated daily doses of 50 mg. Single escalating doses up to 100 mg, and repeated doses below 20 mg produced no serious adverse effects. After careful investigation it was concluded that BIA 10-2474 was not selective to the inhibition of FAAH, and reacted with multiple lipases, which disrupted human cortical neurons (van Esbroeck et al., 2017).
The available data from completed clinical trials indicate that more selective FAAH and MAGL inhibitors are well tolerated (D’Souza et al., 2019; Mayo et al., 2020). A phase I study of the FAAH inhibitor JNJ-42165279 had few adverse effects of mild intensity, including a minor transient increase in liver transaminases at the highest doses in a few cases (Zannikos et al., 2014). ASP3652, a FAAH inhibitor investigated for the treatment of men with chronic prostatitis/chronic pelvic pain syndrome, was generally safe and well-tolerated (Wagenlehner et al., 2017). Abuse liability of these inhibitors are not yet assessed. Interestingly, no FAAH inhibitor produced adverse effects that are typically associated with CB1 agonism, such as impairment in cognition, motor coordination, or psychosis.
4.6. Diverse Mechanisms of Action: A Discussion of Cannabidiol (CBD)
CBD is discussed independently due to its diverse mechanisms of action, which are reviewed extensively elsewhere (Campos et al., 2012; Devinsky et al., 2014). Briefly, CBD exhibits low affinity for the CB1 (Ki = 27542 nM) and CB2 (Ki = 2399 - 4200 nM) receptors, evidenced by displacement studies using the tritiated CB1/2 receptor agonist CP55940 and/or with membranes from cultured cells transfected with human cannabinoid receptors (for reviews, (Pertwee, 1997, 2008)). Rather, the mechanisms for many of CBD’s behavioral effects likely include 5-HT1A receptor agonism (Russo et al., 2005). In rodents, the acute anxiolytic and antidepressant-like effects may be attributable to 5-HT1A receptor neurotransmission in key brain areas related to emotional or defensive responses, e.g., medial prefrontal cortex, dorsal periaqueductal grey, and/or bed nucleus of the stria terminalis (for review, (Campos et al., 2012)). Additionally, activation of transient receptor potential vanilloid-1 (TRPV1) channels is implicated in the acute antipsychotic effects elicited by CBD (Bisogno et al., 2001). A recent review of in vivo pharmacological data suggests that the mechanism of action of CBD underlying the reduction of seizures may include TPRV1 agonism and GPR55 receptor antagonism (Gray and Whalley, 2020; Ryberg et al., 2007). Other potential mechanisms mediating CBD behavioral effects require continued investigation but may include CB1 receptor antagonism or NAM activity (Thomas et al., 2007), CB2 receptor inverse agonism (Thomas et al., 2007), FAAH/anandamide transporter inhibition (Bisogno et al., 2001), PPARɤ receptor agonism (O’Sullivan et al., 2009), allosteric modulation of mu or delta opioid receptors (Kathmann et al., 2006), or a host of other mechanisms that were concisely reviewed (Campos et al., 2012; Devinsky et al., 2014). Continued studies are needed to definitively link these CBD mechanisms of action to each potential therapeutic indication and adverse event profile that we discuss below.
To this end, we acknowledge that plant-derived CBD, for example, the FDA-approved CBD therapeutic Epidiolex®, is used in many of these studies. However, several studies did not state whether plant-derived or synthetic CBD was used. Considering that synthetic CBD is used in both preclinical and clinical studies, the studies reviewed below utilized either plant-derived or synthetic CBD.
4.6.1. Therapeutic Potential
CBD demonstrated positive effects in preclinical models of numerous diseases and is widely used for therapeutic purposes and general health in jurisdictions where medicinal cannabis use and/or hemp products are legal. In 2018, a plant-derived CBD oral formulation (98% pure, Epidiolex®) was approved by the FDA for the treatment of Lennox-Gastaut and Dravet syndromes in patients two years and older (Devinsky et al., 2017; Devinsky et al., 2018), and was recently approved to treat tuberous sclerosis. This marks the first FDA approval of a cannabis-based drug, the first approval of CBD for any indication, and the first FDA approval of a cannabinoid in over 3 decades (Rubin, 2018). Studies expanding on these findings are ongoing, including the potential of combined THC and CBD to treat adults with treatment-resistant epilepsy (NCT03808935). Note, however, that many controlled studies of CBD utilize synthetic CBD rather than CBD extracted from cannabis.
CBD had antipsychotic effects in patients with schizophrenia, perhaps through slowed anandamide degradation (Leweke et al., 2012). Recent clinical trials also demonstrate that CBD reduces positive symptoms of schizophrenia (McGuire et al., 2018), but does not improve cognitive deficits (Boggs et al., 2018). There are several ongoing clinical trials that build on these findings to assess CBD effects on schizophrenia (e.g., NCT02926859).
CBD is also of interest in the treatment of substance use disorders broadly, after initial positive signals were detected in studies related to opioid and tobacco use disorders. CBD reduced cue-induced craving and anxiety in abstinent heroin users (Hurd et al., 2019). Additionally, following a preliminary study showing that CBD reduced cigarette consumption (Morgan et al., 2013), CBD was shown to reduce the salience and pleasantness of cigarette cues in dependent smokers (Hindocha et al., 2018). Additional studies to explore CBD as a potential pharmacotherapy for substance use disorders are underway.
Several investigations reported positive effects of acute CBD administration on decreasing anxiety-related endpoints (Bergamaschi et al., 2011; Crippa et al., 2011; Fusar-Poli et al., 2009; Linares et al., 2019). One recent placebo-controlled RCT showed that CBD significantly reduced anxiety in adolescents with social anxiety disorder after 4 weeks of treatment (Masataka, 2019). CBD also was explored for movement disorders including Parkinson’s (Chagas et al., 2014; Lotan et al., 2014; Sieradzan et al., 2001) and Huntington’s disease (Consroe et al., 1991) with mixed findings. Despite the prevalence of CBD use for pain (Corroon and Phillips, 2018), only two studies assessed its impact when administered alone for chronic pain (Notcutt et al., 2004; Wade et al., 2003), also with mixed findings. With several placebo-controlled clinical trials registered assessing CBD for anxiety (NCT03549819, NCT04086342), motor symptoms associated with Parkinson’s disease (NCT03582137), and chronic pain (NCT03984565, NCT03215940), a substantial increase in data related to the potential efficacy of CBD for these disorders and symptoms will soon emerge. Given CBD’s complex pharmacology, research isolating the exact mechanisms of its therapeutic effects could help with the development of more targeted novel medications.
4.6.2. Toxicity, Safety, and Abuse Liability
CBD is used for a variety of health conditions in the absence of sufficient evidence of its effectiveness, and without regard for potential adverse effects and toxicity. The complexity of CBD pharmacology offers tremendous therapeutic potential, but also the potential for adverse events and drug-drug interactions (for reviews, (Huestis et al., 2019; Sholler et al., 2020; White, 2019)). While generally safe and well-tolerated, the high doses of CBD studied for epilepsy and psychiatric disorders may elicit CBD-induced drug-drug interactions, hepatic abnormalities, diarrhea, fatigue, vomiting, and somnolence. In the Epidiolex® FDA approval notification, CBD-associated adverse events were similar to other anti-epileptics and included suicidal thoughts, suicide attempts, agitation, depression, aggression, and panic attacks. In a 2016 open-label clinical trial of 214 patients with treatment-resistant epilepsy, 79% of patients reported adverse events, 25% somnolence, 11% convulsions, and more than 5% reported fatigue, lethargy, convulsions, status epilepticus, changes in concentrations of concomitant antiepileptic drugs, gait disturbance, and sedation (Devinsky et al., 2016). It is important to note that the participants in these trials had serious illnesses and were taking a number of concomitant medications. Oral CBD does not produce intoxicating or misuse-related effects (Babalonis et al., 2017; Haney et al., 2016), and withdrawal symptoms do not occur after abrupt cessation of chronic use of high dose (1500 mg/day) oral CBD (Taylor et al., 2020).
5. PROMISING DIRECTIONS FOR CONTINUED SYNTHETIC CANNABINOID RESEARCH
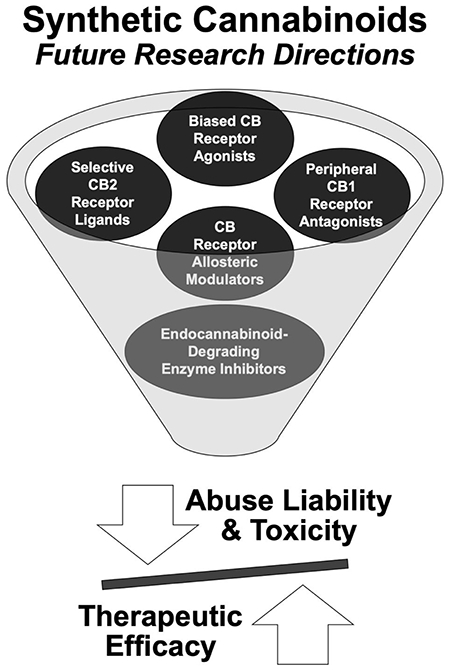
In this review, we highlight directions for SCB research that hold promise in the development of efficacious therapeutics with decreased susceptibility for misuse and CUD. First, continued development of CB1 receptor allosteric modulators (Section 4.4) should be prioritized and may mitigate the rate or severity of adverse effects commonly observed with exogenous orthosteric CB1 receptor ligands. For example, we highlight ongoing studies evaluating the CB1 receptor negative allosteric modulator pregnenolone to treat CUD, tobacco withdrawal, and mood disorders. CB1 receptor negative allosteric modulation could circumvent the adverse events associated with CB1 receptor antagonists/inverse agonists (e.g., psychiatric, nervous system, and gastrointestinal tract adverse events). Second, endocannabinoid-degrading enzyme inhibitors (Section 4.5) such as reversible and irreversible FAAH inhibitors show promise to treat pain, inflammation, mood, anxiety, sleep disorders, and CUD. As continued research unfolds, focus should be placed on developing more selective FAAH and MAGL inhibitors to improve tolerability (D’Souza et al., 2019; Mayo et al., 2020) and to evaluate abuse liability of these inhibitors, which are not yet assessed. Third, selective CB2 receptor agonists (section 4.2) draw great interest as these ligands do not appear to exhibit abuse liability or toxicity similar to selective CB1 receptor agonists (Pertwee, 2009). As CB2 receptor agonist development continues, an important goal is to design ligands that are selective over the homologous CB1 receptor in order to overcome the adverse events, abuse liability, and toxicity associated with this receptor target.
In the sections below, we present additional directions for SCB research – not previously detailed in this review – that may produce efficacious therapeutics with limited toxicity and abuse liability.
5.1. Peripheral CB1 Receptor Antagonists/Inverse Agonists
As discussed previously, the CB1 receptor inverse agonist rimonabant showed promise as a novel therapeutic to treat obesity, but was removed from approved markets due to adverse psychiatric events including increased rates of depression and suicidal ideation (Christensen et al., 2007; Le Foll et al., 2009; Rucker et al., 2007). In response to the withdrawal of rimonabant from the international market, attention shifted to the development of CB1 receptor antagonists/inverse agonists that are restricted to the periphery, considering that a peripherally-restricted ligand might avoid the serious psychiatric events associated with central CB1 receptor inhibition. The CNS drug development field converged upon novel strategies to modify the physicochemical properties of a drug to alter blood brain barrier permeability (for review, (Hitchcock and Pennington, 2006)), leading to the development of several peripherally-restricted CB1 receptor antagonists/inverse agonists. To date, peripherally-restricted CB1 receptor antagonists/inverse agonists show promise in preclinical studies as potential therapeutics to treat obesity (Le Foil et al., 2009). The peripherally-restricted, neutral CB1 receptor antagonist LH-21, which exhibits poor CNS penetration, decreased food intake upon acute administration in Zucker rats (Pavon et al., 2008). Additionally, the peripherally-restricted CB1 receptor inverse agonist JD5037 was demonstrated as equally efficacious to its brain-penetrant parent compound to reduce appetite, body weight, and insulin resistance in diet-induced obese mice independent of occupying central CB1 receptors (Tam et al., 2012). Further, the peripherally-restricted CB1 receptor antagonist/CB2 receptor agonist URB447 decreased food intake and weight gain in mice (LoVerme et al., 2009). While blood brain barrier-impermeable CB1 receptor antagonists/inverse agonists may deter adverse psychiatric events, further research is needed to determine if these same ligands would be useful to treat CNS disorders, an area that may be expanded by using validated rodent models.
5.2. Biased Cannabinoid Receptor Agonists
An alternative strategy to diminish toxicity and adverse events associated with high-affinity, high-efficacy CB1/2 receptor agonists is to “bias” their intracellular signaling; that is, to potentiate intracellular signaling pathways that may underlie their therapeutic efficacy and attenuate pathways that may drive adverse events. Biased agonism is the concept that different agonists can exhibit functional selectivity in addition to binding selectivity. Biased agonists act on the same GPCR in the same tissue, but they are thought to stimulate unique effector systems, possibly due to these agonists stabilizing different receptor conformations (Ibsen et al., 2017; Kenakin, 2011). Although it was demonstrated that the CB1 and CB2 receptors couple to various effector systems including the Gαi/o protein but also Gαs, Gαq/11, and the β-arrestin proteins (Pinto et al., 1994), the development of functionally-selective, biased SCB agonists is in its infancy (for reviews, see (Laprairie et al., 2017; Wouters et al., 2019)). However, the therapeutic potential for biased agonists can be gleaned from the study of other GPCRs. For example, Allen and colleagues discovered several β-arrestin2-biased agonists at the dopamine D2 receptor including UNC9975, which displayed potent antipsychotic-like activity without inducing motoric side effects in inbred C57BL/6 mice in vivo (Allen et al., 2011). Moreover, Brust and colleagues developed a G protein-biased kappa opioid receptor agonist that exhibited antinociceptive and antipruritic efficacy in mice, but did not induce sedation or reductions in dopamine release in mice in vivo (Brust et al., 2016). Ligand bias for at least six SCB CB1 agonists was characterized (functional selectivity shown in parentheses), including JWH-018 (G proteins = β-arrestin in Chinese hamster ovary cells), HU-210 (Gαo > Gαi = Gαs > Gαq in Sf9 cells), and CP55,940 (Gαs > β-arrestin1 > Gαi > Gαq > Gβγ in STHdhQ7/Q7 cells) (Ford et al., 2017; Khajehali et al., 2015; Laprairie et al., 2016; Laprairie et al., 2014). Biased SCB agonist development, particularly those that target the CB1 receptor, could generate compounds that exhibit improved targeting with reduced side effect profiles (Wouters et al., 2019).
5.3. Cannabinoid Biosynthesis from Non-Cannabis Sources
Cannabinoid research was historically limited by conflicting federal and state cannabis laws (for a detailed discussion, see Section 5.4.). Even with a federal license permitting research on Schedule I substances, the structural complexity and low abundance of many cannabinoids in the cannabis plant limits bulk chemical synthesis of these compounds (Luo et al., 2019). To navigate these limitations associated with cannabis plant material, several laboratories successfully biosynthesized major cannabinoids from non-cannabis sources, for example, through the generation of cannabinoid-producing yeast. Several laboratories successfully engineered biosynthetic pathways for the synthesis of cannabinoids in the yeast species Saccharomyces cerevisiae (Luo et al., 2019; Zirpel et al., 2017; Zirpel et al., 2015), as well as Pichia pastoris (Zirpel et al., 2015). Synthesizing cannabinoids independent of cannabis plant material may alleviate regulatory burdens and also permit the scaling up of phytocannabinoids otherwise difficult to obtain for future controlled laboratory research.
5.4. Regulatory Considerations for SCB Research
Research into the endocannabinoid system was historically limited by the legal scheduling of cannabinoids. Although medicinal use of cannabis is now legal in many places, cannabis and dozens of SCBs remain controlled substances in most countries. Investigators conducting research on these substance must obtain special research exceptions, which can be time consuming, costly, and significantly impede research progress (Mead, 2017).
In this review, we reinforce that some SCBs (especially high-affinity, high-efficacy CB1/2 receptor agonists) are susceptible to misuse, and a range of adverse events. However, there is significant variability in the extent to which SCBs possess abuse liability or acute toxicity. Indeed, this review and others (Loewinger et al., 2013) highlight that greater attention should be given to SCB behavioral pharmacology, neurochemistry, and toxicology when designing and implementing SCB-focused policies, considering that some SCB classes may yield high therapeutic value and low illicit abuse liability (for example, selective CB2 receptor agonists, biased CB receptor agonists). Blanket policies that place all SCB chemicals (as well as cannabis) into the most restrictive control categories impede research into cannabinoid mechanisms of action, therapeutic development, and ultimately, clinical use. Overall, limiting SCB regulatory control to compounds that are identified in the illicit market as being substances of abuse is important so that SCB therapeutic development can continue without major restrictions on research implementation.
6. CONCLUSIONS: A VISION FOR FUTURE SCB RESEARCH
Findings from extensive preclinical and clinical research demonstrate the therapeutic potential of SCB compounds, including the two marketed drugs dronabinol (synthetic THC) and nabilone (synthetic THC analog). Simultaneously, synthesis of highly-potent SCB compounds gave rise to a novel class of abused drugs, creating a significant public health burden, but also leading to scheduling of many SCB compounds, which impeded research progress in this area. In response to these challenges, we propose several directions for continued SCB research that may yield effective therapeutics with decreased abuse liability: CB1 receptor allosteric modulators, endocannabinoid-degrading enzyme inhibitors, peripheral CB1 receptor antagonists/inverse agonists, selective CB2 receptor ligands, biased cannabinoid receptor agonists, cannabinoid biosynthesis from non-cannabis sources. Together, these strategies could lead to the discovery of new, therapeutically useful SCBs and reduce their negative public health impact.
HIGHLIGHTS:
Synthetic cannabinoids (SCBs) are both approved medications and misused illicit drugs.
SCBs exhibit a diverse pharmacology, which is reviewed in detail.
Randomized clinical trials (RCT) demonstrate therapeutic potential for several SCBs.
SCB abuse liability and toxicity vary considerably based on pharmacology.
SCB targets for continued drug development are proposed.
ACKNOWLEDGEMENTS:
RV has been paid as a consultant or scientific advisory board member for Zynerba Pharmaceuticals, Canopy Health Innovations Inc., FSD Pharma, and Present Life Corporation outside the submitted work. MAH and ZDC have no competing interests in relation to the work described. MAH served as a consultant for Canopy Health Innovations Inc., and PinneyAssociates. In the past year, ZDC served as a consultant to Beckley Canopy Therapeutics and served on the scientific advisory board of FSD Pharma. All other authors report no conflicts of interest that are relevant to the analyses in this manuscript. This work was supported by the National Institute on Drug Abuse (NIDA) Training Grant T32DA07209, NIDA grants R01-DA043075 (Vandrey) and R01-DA047296 (Cooper), and NCCIH grant R01-1AT010762 (Cooper).
ABBREVIATIONS:
- 2-AG
2-arachidonoylglycerol
- 5-HT
5-hydroxytryptamine; serotonin
- AIDS
Acquired immunodeficiency syndrome
- Anandamide
N-arachidonoylethanolamine
- CB1R
Cannabinoid type 1 receptor
- CB2R
Cannabinoid type 2 receptor
- cAMP
Cyclic adenosine monophosphate
- CBD
Cannabidiol
- CNS
Central nervous system
- CUD
Cannabis Use Disorder
- CYP
Cytochrome P450
- DAGL
Diacylglycerol lipases
- FAAH
Fatty acid amide hydrolase
- FDA
Food and Drug Administration
- GPCR
G protein-coupled receptor
- GPR
Orphan GPCR
- HDL
High-density lipoproteins
- HIV
Human immunodeficiency virus
- LXA4
Lipoxin A4
- MAGL
Monoacylglycerol lipase
- MS
Multiple sclerosis
- NAM
Negative allosteric modulator
- NAPE-PLD
N-acyl-phosphatidyl-ethanolamine-selective phospholipase D
- NPS
Novel psychoactive substances
- PAM
Positive allosteric modulator
- PPAR
Peroxisome proliferator-activated nuclear receptors
- PTSD
Post-traumatic stress disorder
- RCT
Randomized controlled trials
- SCB
Synthetic cannabinoid
- THC
Δ9-tetrahydrocannabinol
- THCV
Tetrahydrocannabivarin
- TRP
Transient receptor potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams AJ, Banister SD, Irizarry L, Trecki J, Schwartz M, and Gerona R (2017). “Zombie” Outbreak Caused by the Synthetic Cannabinoid AMB-FUBINACA in New York. N Engl J Med 376, 235–242. [DOI] [PubMed] [Google Scholar]
- Agurell S, Halldin M, Lindgren JE, Ohlsson A, Widman M, Gillespie H, and Hollister L (1986). Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev 38, 21–43. [PubMed] [Google Scholar]
- Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M, Peterson S, Yadav PN, Huang XP, Feng B, et al. (2011). Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci U S A 108, 18488–18493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, Rivas GR, Holland RM, Muhleisen P, Norberg MM, et al. (2014). Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry 71, 281–291. [DOI] [PubMed] [Google Scholar]
- Armstrong HE, Galka A, Lin LS, Lanza TJ Jr., Jewell JP, Shah SK, Guthikonda R, Truong Q, Chang LL, Quaker G, et al. (2007). Substituted acyclic sulfonamides as human cannabinoid-1 receptor inverse agonists. Bioorg Med Chem Lett 17, 2184–2187. [DOI] [PubMed] [Google Scholar]
- Aronne LJ, Tonstad S, Moreno M, Gantz I, Erondu N, Suryawanshi S, Molony C, Sieberts S, Nayee J, Meehan AG, et al. (2010). A clinical trial assessing the safety and efficacy of taranabant, a CB1R inverse agonist, in obese and overweight patients: a high-dose study. Int J Obes (Lond) 34, 919–935. [DOI] [PubMed] [Google Scholar]
- Ashton CH (2001). Pharmacology and effects of cannabis: a brief review. Br J Psychiatry 178, 101–106. [DOI] [PubMed] [Google Scholar]
- Babalonis S, Haney M, Malcolm RJ, Lofwall MR, Votaw VR, Sparenborg S, and Walsh SL (2017). Oral cannabidiol does not produce a signal for abuse liability in frequent marijuana smokers. Drug Alcohol Depend 172, 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Solis E Jr., Watterson LR, Marusich JA, Fantegrossi WE, and Wiley JL (2014). Baths salts, spice, and related designer drugs: the science behind the headlines. J Neurosci 34, 15150–15158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi MM, Queiroz RH, Chagas MH, de Oliveira DC, De Martinis BS, Kapczinski F, Quevedo J, Roesler R, Schroder N, Nardi AE, et al. (2011). Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology 36, 1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bie B, Wu J, Foss JF, and Naguib M (2018). An overview of the cannabinoid type 2 receptor system and its therapeutic potential. Curr Opin Anaesthesiol 31, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R, and Di Marzo V (2001). Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 134, 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesten JE, Kaper J, Stoffers HE, Kroon AA, and van Schayck OC (2012). Rimonabant improves obesity but not the overall cardiovascular risk and quality of life; results from CARDIO-REDUSE (CArdiometabolic Risk reDuctIOn by Rimonabant: the Effectiveness in Daily practice and its USE). Fam Pract 29, 521–527. [DOI] [PubMed] [Google Scholar]
- Boggs DL, Kelly DL, McMahon RP, Gold JM, Gorelick DA, Linthicum J, Conley RR, Liu F, Waltz J, Huestis MA, et al. (2012). Rimonabant for neurocognition in schizophrenia: a 16-week double blind randomized placebo controlled trial. Schizophr Res 134, 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs DL, Surti T, Gupta A, Gupta S, Niciu M, Pittman B, Schnakenberg Martin AM, Thurnauer H, Davies A, D’Souza DC, et al. (2018). The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl) 235, 1923–1932. [DOI] [PubMed] [Google Scholar]
- Bonn-Miller MO, Babson KA, and Vandrey R (2014). Using cannabis to help you sleep: heightened frequency of medical cannabis use among those with PTSD. Drug Alcohol Depend 136, 162–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgelt LM, Franson KL, Nussbaum AM, and Wang GS (2013). The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy 33, 195–209. [DOI] [PubMed] [Google Scholar]
- Brown ES, Park J, Marx CE, Hynan LS, Gardner C, Davila D, Nakamura A, Sunderajan P, Lo A, and Holmes T (2014). A randomized, double-blind, placebo-controlled trial of pregnenolone for bipolar depression. Neuropsychopharmacology 39, 2867–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, Zhou L, Stahl EL, Cameron MD, Scarry SM, et al. (2016). Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci Signal 9, ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Moore BA, and Bahrenburg B (2007). Oral delta9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend 86, 22–29. [DOI] [PubMed] [Google Scholar]
- Buser GL, Gerona RR, Horowitz BZ, Vian KP, Troxell ML, Hendrickson RG, Houghton DC, Rozansky D, Su SW, and Leman RF (2014). Acute kidney injury associated with smoking synthetic cannabinoid. Clin Toxicol (Phila) 52, 664–673. [DOI] [PubMed] [Google Scholar]
- Calhoun SR, Galloway GP, and Smith DE (1998). Abuse potential of dronabinol (Marinol). J Psychoactive Drugs 30, 187–196. [DOI] [PubMed] [Google Scholar]
- Campos AC, Moreira FA, Gomes FV, Del Bel EA, and Guimaraes FS (2012). Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc Lond B Biol Sci 367, 3364–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle SJ, Marciano-Cabral F, Staab A, Ludwick C, and Cabral GA (2002). Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int Immunopharmacol 2, 69–82. [DOI] [PubMed] [Google Scholar]
- Castaneto MS, Gorelick DA, Desrosiers NA, Hartman RL, Pirard S, and Huestis MA (2014). Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend 144, 12–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneto MS, Wohlfarth A, Desrosiers NA, Hartman RL, Gorelick DA, and Huestis MA (2015). Synthetic cannabinoids pharmacokinetics and detection methods in biological matrices. Drug Metab Rev 47, 124–174. [DOI] [PubMed] [Google Scholar]
- Chagas MH, Zuardi AW, Tumas V, Pena-Pereira MA, Sobreira ET, Bergamaschi MM, dos Santos AC, Teixeira AL, Hallak JE, and Crippa JA (2014). Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J Psychopharmacol 28, 1088–1098. [DOI] [PubMed] [Google Scholar]
- Chakrabarti A, Onaivi ES, and Chaudhuri G (1995). Cloning and sequencing of a cDNA encoding the mouse brain-type cannabinoid receptor protein. DNA Seq 5, 385–388. [DOI] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, and Astrup A (2007). Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 370, 1706–1713. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Voltz C, Steinberg R, and Soubrie P (2002). SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol 13, 451–463. [DOI] [PubMed] [Google Scholar]
- Consroe P, Laguna J, Allender J, Snider S, Stern L, Sandyk R, Kennedy K, and Schram K (1991). Controlled clinical trial of cannabidiol in Huntington’s disease. Pharmacol Biochem Behav 40, 701–708. [DOI] [PubMed] [Google Scholar]
- Cooper ZD (2016). Adverse Effects of Synthetic Cannabinoids: Management of Acute Toxicity and Withdrawal. Curr Psychiatry Rep 18, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, and Abrams DI (2019). Considering abuse liability and neurocognitive effects of cannabis and cannabis-derived products when assessing analgesic efficacy: a comprehensive review of randomized-controlled studies. Am J Drug Alcohol Abuse 45, 580–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corroon J, and Phillips JA (2018). A Cross-Sectional Study of Cannabidiol Users. Cannabis Cannabinoid Res 3, 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, and Lichtman AH (2001). Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A 98, 9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, and Gilula NB (1996). Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384, 83–87. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FL, Martin-Santos R, Simoes MV, Bhattacharyya S, Fusar-Poli P, Atakan Z, et al. (2011). Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol 25, 121–130. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Cortes-Briones J, Creatura G, Bluez G, Thurnauer H, Deaso E, Bielen K, Surti T, Radhakrishnan R, Gupta A, et al. (2019). Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: a double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry 6, 35–45. [DOI] [PubMed] [Google Scholar]
- Darmani NA (2001). Delta(9)-tetrahydrocannabinol and synthetic cannabinoids prevent emesis produced by the cannabinoid CB(1) receptor antagonist/inverse agonist SR 141716A. Neuropsychopharmacology 24, 198–203. [DOI] [PubMed] [Google Scholar]
- Davidson C, Opacka-Juffry J, Arevalo-Martin A, Garcia-Ovejero D, Molina-Holgado E, and Molina-Holgado F (2017). Spicing Up Pharmacology: A Review of Synthetic Cannabinoids From Structure to Adverse Events. Adv Pharmacol 80, 135–168. [DOI] [PubMed] [Google Scholar]
- De Gregorio D, McLaughlin RJ, Posa L, Ochoa-Sanchez R, Enns J, Lopez-Canul M, Aboud M, Maione S, Comai S, and Gobbi G (2019). Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain 160, 136–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derocq JM, Segui M, Marchand J, Le Fur G, and Casellas P (1995). Cannabinoids enhance human B-cell growth at low nanomolar concentrations. FEBS Lett 369, 177–182. [DOI] [PubMed] [Google Scholar]
- Devane WA, Breuer A, Sheskin T, Jarbe TU, Eisen MS, and Mechoulam R (1992). A novel probe for the cannabinoid receptor. J Med Chem 35, 2065–2069. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, Katz R, Di Marzo V, Jutras-Aswad D, Notcutt WG, et al. (2014). Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 55, 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, Scheffer IE, Thiele EA, and Wright S (2017). Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N Engl J Med 376, 2011–2020. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, Miller I, Flamini R, Wilfong A, Filloux F, et al. (2016). Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol 15, 270–278. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, Greenwood SM, Roberts C, Checketts D, VanLandingham KE, et al. (2018). Effect of Cannabidiol on Drop Seizures in the Lennox-Gastaut Syndrome. N Engl J Med 378, 1888–1897. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, and Piscitelli F (2015). The Endocannabinoid System and its Modulation by Phytocannabinoids. Neurotherapeutics 12, 692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, and Piomelli D (2002). Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A 99, 10819–10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration (2011). Schedules of controlled substances: temporary placement of five synthetic cannabinoids into schedule I. Federal Register 76, 11075–11078. [PubMed] [Google Scholar]
- Dyson A, Peacock M, Chen A, Courade JP, Yaqoob M, Groarke A, Brain C, Loong Y, and Fox A (2005). Antihyperalgesic properties of the cannabinoid CT-3 in chronic neuropathic and inflammatory pain states in the rat. Pain 116, 129–137. [DOI] [PubMed] [Google Scholar]
- ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, and Church JC (2016). Changes in Cannabis Potency Over the Last 2 Decades (1995–2014): Analysis of Current Data in the United States. Biol Psychiatry 79, 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund A, Atakan Z, Kralj A, Tunstall N, Murray R, and Morrison P (2016). The effect of five day dosing with THCV on THC-induced cognitive, psychological and physiological effects in healthy male human volunteers: A placebo-controlled, double-blind, crossover pilot trial. J Psychopharmacol 30, 140–151. [DOI] [PubMed] [Google Scholar]
- Etges T, Karolia K, Grint T, Taylor A, Lauder H, Daka B, and Wright S (2016). An observational postmarketing safety registry of patients in the UK, Germany, and Switzerland who have been prescribed Sativex((R)) (THC:CBD, nabiximols) oromucosal spray. Ther Clin RiskManag 12, 1667–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Moran JH, Radominska-Pandya A, and Prather PL (2014). Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to A(9)-THC: mechanism underlying greater toxicity? Life Sci 97, 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong TM, Guan XM, Marsh DJ, Shen CP, Stribling DS, Rosko KM, Lao J, Yu H, Feng Y, Xiao JC, et al. (2007). Antiobesity efficacy of a novel cannabinoid-1 receptor inverse agonist, N-[(1S,2S)-3-(4-chlorophenyl)-2-(3-cyanophenyl)-1-methylpropyl]-2-methyl-2-[[5-(trifluoromethyl)pyridin-2-yl]oxy]propanamide (MK-0364), in rodents. J Pharmacol Exp Ther 321, 1013–1022. [DOI] [PubMed] [Google Scholar]
- Ford BM, Franks LN, Tai S, Fantegrossi WE, Stahl EL, Berquist MD, Cabanlong CV, Wilson CD, Penthala NR, Crooks PA, et al. (2017). Characterization of structurally novel G protein biased CB1 agonists: Implications for drug development. Pharmacol Res 125, 161–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraguas-Sanchez AI, Fernandez-Carballido A, and Torres-Suarez AI (2016). Phyto-, endo- and synthetic cannabinoids: promising chemotherapeutic agents in the treatment of breast and prostate carcinomas. Expert Opin Investig Drugs 25, 1311–1323. [DOI] [PubMed] [Google Scholar]
- Fu J, Bottegoni G, Sasso O, Bertorelli R, Rocchia W, Masetti M, Guijarro A, Lodola A, Armirotti A, Garau G, et al. (2011). A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat Neurosci 15, 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, Seal M, Surguladze SA, O’Carrol C, Atakan Z, et al. (2009). Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry 66, 95–105. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, and Casellas P (1995). Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem 232, 54–61. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, and Mechoulam R (1964). Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. Journal of the American Chemical Society 86, 1646–1647. [Google Scholar]
- Gentry PR, Sexton PM, and Christopoulos A (2015). Novel Allosteric Modulators of G Protein-coupled Receptors. J Biol Chem 290, 19478–19488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblin GM, O’Shaughnessy CT, Naylor A, Mitchell WL, Eatherton AJ, Slingsby BP, Rawlings DA, Goldsmith P, Brown AJ, Haslam CP, et al. (2007). Discovery of 2-[(2,4-dichlorophenyl)amino]-N-[(tetrahydro- 2H-pyran-4-yl)methyl]-4-(trifluoromethyl)- 5-pyrimidinecarboxamide, a selective CB2 receptor agonist for the treatment of inflammatory pain. J Med Chem 50, 2597–2600. [DOI] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, and Uhl GR (2006). Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res 1071, 10–23. [DOI] [PubMed] [Google Scholar]
- Gray RA, and Whalley BJ (2020). The proposed mechanisms of action of CBD in epilepsy. Epileptic Disorders 22, S10–S15. [DOI] [PubMed] [Google Scholar]
- Griffin G, Wray EJ, Tao Q, McAllister SD, Rorrer WK, Aung MM, Martin BR, and Abood ME (1999). Evaluation of the cannabinoid CB2 receptor-selective antagonist, SR144528: further evidence for cannabinoid CB2 receptor absence in the rat central nervous system. Eur J Pharmacol 377, 117–125. [DOI] [PubMed] [Google Scholar]
- Grotenhermen F (2003). Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 42, 327–360. [DOI] [PubMed] [Google Scholar]
- Gummin DD, Mowry JB, Spyker DA, Brooks DE, Fraser MO, and Banner W (2017). 2016 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th Annual Report. Clin Toxicol (Phila) 55, 1072–1252. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, and Foltin RW (2004). Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology 29, 158–170. [DOI] [PubMed] [Google Scholar]
- Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, Gray KM, McRae-Clark A, Lofwall MR, Sparenborg S, et al. (2016). Oral Cannabidiol does not Alter the Subjective, Reinforcing or Cardiovascular Effects of Smoked Cannabis. Neuropsychopharmacology 41, 1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Comer SD, and Foltin RW (2005). Reinforcing effects of oral Delta9-THC in male marijuana smokers in a laboratory choice procedure. Psychopharmacology (Berl) 181, 237–243. [DOI] [PubMed] [Google Scholar]
- Herrmann ES, Cooper ZD, Bedi G, Ramesh D, Reed SC, Comer SD, Foltin RW, and Haney M (2016). Effects of zolpidem alone and in combination with nabilone on cannabis withdrawal and a laboratory model of relapse in cannabis users. Psychopharmacology (Berl) 233, 2469–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindocha C, Freeman TP, Grabski M, Stroud JB, Crudgington H, Davies AC, Das RK, Lawn W, Morgan CJA, and Curran HV (2018). Cannabidiol reverses attentional bias to cigarette cues in a human experimental model of tobacco withdrawal. Addiction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindocha C, Freeman TP, Schafer G, Gardener C, Das RK, Morgan CJ, and Curran HV (2015). Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: a randomised, double-blind, placebo-controlled study in cannabis users. Eur Neuropsychopharmacol 25, 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock SA, and Pennington LD (2006). Structure-brain exposure relationships. J Med Chem 49, 7559–7583. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, et al. (2002). International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54, 161–202. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Boyd SJ, Heishman SJ, Preston KL, Bonnet D, Le Fur G, and Gorelick DA (2007). Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology (Berl) 194, 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, and Frank RA (2001). Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry 58, 322–328. [DOI] [PubMed] [Google Scholar]
- Huestis MA, and Smith ML (2018). Cannabinoid Markers in Biological Fluids and Tissues: Revealing Intake. Trends Mol Med 24, 156–172. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Solimini R, Pichini S, Pacifici R, Carlier J, and Busardo FP (2019). Cannabidiol Adverse Effects and Toxicity. Curr Neuropharmacol 17, 974–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, Spriggs S, Alishayev J, Winkel G, Gurgov K, Kudrich C, Oprescu AM, and Salsitz E (2019). Cannabidiol for the Reduction of Cue-Induced Craving and Anxiety in Drug-Abstinent Individuals With Heroin Use Disorder: A Double-Blind Randomized Placebo-Controlled Trial. Am J Psychiatry 176, 911–922. [DOI] [PubMed] [Google Scholar]
- Ibsen MS, Connor M, and Glass M (2017). Cannabinoid CB1 and CB2 Receptor Signaling and Bias. Cannabis Cannabinoid Res 2, 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadoon KA, Ratcliffe SH, Barrett DA, Thomas EL, Stott C, Bell JD, O’Sullivan SE, and Tan GD (2016). Efficacy and Safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and Lipid Parameters in Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Pilot Study. Diabetes Care 39, 1777–1786. [DOI] [PubMed] [Google Scholar]
- Jetly R, Heber A, Fraser G, and Boisvert D (2015). The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology 51, 585–588. [DOI] [PubMed] [Google Scholar]
- Jiang M, and van der Stelt M (2018). Activity-Based Protein Profiling Delivers Selective Drug Candidate ABX-1431, a Monoacylglycerol Lipase Inhibitor, To Control Lipid Metabolism in Neurological Disorders. J Med Chem 61, 9059–9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DR, Stebulis JA, Rossetti RG, Burstein SH, and Zurier RB (2007). Suppression of fibroblast metalloproteinases by ajulemic acid, a nonpsychoactive cannabinoid acid. J Cell Biochem 100, 184–190. [DOI] [PubMed] [Google Scholar]
- Kalliomaki J, Annas P, Huizar K, Clarke C, Zettergren A, Karlsten R, and Segerdahl M (2013). Evaluation of the analgesic efficacy and psychoactive effects of AZD1940, a novel peripherally acting cannabinoid agonist, in human capsaicin-induced pain and hyperalgesia. Clin Exp Pharmacol Physiol 40, 212–218. [DOI] [PubMed] [Google Scholar]
- Kardashev A, Ratner Y, and Ritsner MS (2018). Add-On Pregnenolone with L-Theanine to Antipsychotic Therapy Relieves Negative and Anxiety Symptoms of Schizophrenia: An 8-Week, Randomized, Double-Blind, Placebo-Controlled Trial. Clin Schizophr Relat Psychoses 12, 31–41. [PubMed] [Google Scholar]
- Karst M, Salim K, Burstein S, Conrad I, Hoy L, and Schneider U (2003). Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: a randomized controlled trial. Jama 290, 1757–1762. [DOI] [PubMed] [Google Scholar]
- Kathmann M, Flau K, Redmer A, Trankle C, and Schlicker E (2006). Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch Pharmacol 372, 354–361. [DOI] [PubMed] [Google Scholar]
- Kenakin T (2011). Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther 336, 296–302. [DOI] [PubMed] [Google Scholar]
- Khajehali E, Malone DT, Glass M, Sexton PM, Christopoulos A, and Leach K (2015). Biased Agonism and Biased Allosteric Modulation at the CB1 Cannabinoid Receptor. Mol Pharmacol 88, 368–379. [DOI] [PubMed] [Google Scholar]
- Kipnes MS, Hollander P, Fujioka K, Gantz I, Seck T, Erondu N, Shentu Y, Lu K, Suryawanshi S, Chou M, et al. (2010). A one-year study to assess the safety and efficacy of the CB1R inverse agonist taranabant in overweight and obese patients with type 2 diabetes. Diabetes Obes Metab 12, 517–531. [DOI] [PubMed] [Google Scholar]
- Klumpers LE, Roy C, Ferron G, Turpault S, Poitiers F, Pinquier JL, van Hasselt JG, Zuurman L, Erwich FA, and van Gerven JM (2013). Surinabant, a selective cannabinoid receptor type 1 antagonist, inhibits Delta9-tetrahydrocannabinol-induced central nervous system and heart rate effects in humans. Br J Clin Pharmacol 76, 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch L (2010). Obesity: Taranabant no longer developed as an antiobesity agent. Nat Rev Endocrinol 6, 300. [DOI] [PubMed] [Google Scholar]
- Kreinin A, Bawakny N, and Ritsner MS (2017). Adjunctive Pregnenolone Ameliorates the Cognitive Deficits in Recent-Onset Schizophrenia: An 8-Week, Randomized, Double-Blind, Placebo-Controlled Trial. Clin Schizophr Relat Psychoses 10, 201–210. [PubMed] [Google Scholar]
- Lange JH, van Stuivenberg HH, Coolen HK, Adolfs TJ, McCreary AC, Keizer HG, Wals HC, Veerman W, Borst AJ, de Looff W, et al. (2005). Bioisosteric replacements of the pyrazole moiety of rimonabant: synthesis, biological properties, and molecular modeling investigations of thiazoles, triazoles, and imidazoles as potent and selective CB1 cannabinoid receptor antagonists. J Med Chem 48, 1823–1838. [DOI] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, and Denovan-Wright EM (2017). Cannabinoid receptor ligand bias: implications in the central nervous system. Curr Opin Pharmacol 32, 32–43. [DOI] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Kelly ME, and Denovan-Wright EM (2016). Biased Type 1 Cannabinoid Receptor Signaling Influences Neuronal Viability in a Cell Culture Model of Huntington Disease. Mol Pharmacol 89, 364–375. [DOI] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Kelly ME, Dupre DJ, and Denovan-Wright EM (2014). Type 1 cannabinoid receptor ligands display functional selectivity in a cell culture model of striatal medium spiny projection neurons. J Biol Chem 289, 24845–24862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, and Goldberg SR (2005). Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl) 178, 481–492. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Gorelick DA, and Goldberg SR (2009). The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology (Berl) 205, 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner AG, Goodman C, Bor O, and Lev-Ran S (2014). Synthetic Cannabis Substances (SPS) Use and Hallucinogen Persisting Perception Disorder (HPPD): Two Case Reports. Isr J Psychiatry Relat Sci 51, 277–280. [PubMed] [Google Scholar]
- Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, Klosterkotter J, Hellmich M, and Koethe D (2012). Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2, e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, and Cravatt BF (2004). Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain 109, 319–327. [DOI] [PubMed] [Google Scholar]
- Linares IM, Zuardi AW, Pereira LC, Queiroz RH, Mechoulam R, Guimaraes FS, and Crippa JA (2019). Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Braz J Psychiatry 41, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintzeris N, Bhardwaj A, Mills L, Dunlop A, Copeland J, McGregor I, Bruno R, Gugusheff J, Phung N, Montebello M, et al. (2019). Nabiximols for the Treatment of Cannabis Dependence: A Randomized Clinical Trial. JAMA Intern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewinger GC, Oleson EB, and Cheer JF (2013). Using dopamine research to generate rational cannabinoid drug policy. Drug Test Anal 5, 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan BK, Mohr ALA, Friscia M, Krotulski AJ, Papsun DM, Kacinko SL, Ropero-Miller JD, and Huestis MA (2017). Reports of Adverse Events Associated with Use of Novel Psychoactive Substances, 2013-2016: A Review. J Anal Toxicol 41, 573–610. [DOI] [PubMed] [Google Scholar]
- Lotan I, Treves TA, Roditi Y, and Djaldetti R (2014). Cannabis (medical marijuana) treatment for motor and non-motor symptoms of Parkinson disease: an open-label observational study. Clin Neuropharmacol 37, 41–44. [DOI] [PubMed] [Google Scholar]
- LoVerme J, Duranti A, Tontini A, Spadoni G, Mor M, Rivara S, Stella N, Xu C, Tarzia G, and Piomelli D (2009). Synthesis and characterization of a peripherally restricted CB1 cannabinoid antagonist, URB447, that reduces feeding and body-weight gain in mice. Bioorg Med Chem Lett 19, 639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HC, and Mackie K (2016). An Introduction to the Endogenous Cannabinoid System. Biol Psychiatry 79, 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Reiter MA, d’Espaux L, Wong J, Denby CM, Lechner A, Zhang Y, Grzybowski AT, Harth S, Lin W, et al. (2019). Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 567, 123–126. [DOI] [PubMed] [Google Scholar]
- Mackie K (2005). Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol, 299–325. [DOI] [PubMed] [Google Scholar]
- MacLennan SJ, Reynen PH, Kwan J, and Bonhaus DW (1998). Evidence for inverse agonism of SR141716A at human recombinant cannabinoid CB1 and CB2 receptors. Br J Pharmacol 124, 619–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makara JK, Mor M, Fegley D, Szabo SI, Kathuria S, Astarita G, Duranti A, Tontini A, Tarzia G, Rivara S, et al. (2005). Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus. Nat Neurosci 8, 1139–1141. [DOI] [PubMed] [Google Scholar]
- Mallet C, Dubray C, and Duale C (2016). FAAH inhibitors in the limelight, but regrettably. Int J Clin Pharmacol Ther 54, 498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masataka N (2019). Anxiolytic Effects of Repeated Cannabidiol Treatment in Teenagers With Social Anxiety Disorders. Front Psychol 10, 2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, and Bonner TI (1990). Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564. [DOI] [PubMed] [Google Scholar]
- May LT, Leach K, Sexton PM, and Christopoulos A (2007). Allosteric modulation of G protein-coupled receptors. Annu Rev Pharmacol Toxicol 47, 1–51. [DOI] [PubMed] [Google Scholar]
- Maykut MO (1985). Health consequences of acute and chronic marihuana use. Prog Neuropsychopharmacol Biol Psychiatry 9, 209–238. [DOI] [PubMed] [Google Scholar]
- Mayo LM, Asratian A, Linde J, Morena M, Haataja R, Hammar V, Augier G, Hill MN, and Heilig M (2020). Elevated Anandamide, Enhanced Recall of Fear Extinction, and Attenuated Stress Responses Following Inhibition of Fatty Acid Amide Hydrolase: A Randomized, Controlled Experimental Medicine Trial. Biol Psychiatry 87, 538–547. [DOI] [PubMed] [Google Scholar]
- McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, Taylor A, and Wright S (2018). Cannabidiol (CBD) as an Adjunctive Therapy in Schizophrenia: A Multicenter Randomized Controlled Trial. Am J Psychiatry 175, 225–231. [DOI] [PubMed] [Google Scholar]
- Mead A (2017). The legal status of cannabis (marijuana) and cannabidiol (CBD) under U.S. law. Epilepsy Behav 70, 288–291. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, and Burstein SH (1973). Marijuana: Chemistry, Pharmacology, Metabolism and Clinical Effects. Contributors-Burstein SH [And Others] (Academic Press; ). [Google Scholar]
- Mechoulam R, and Hanuš L.r. (2000). A historical overview of chemical research on cannabinoids. Chemistry and physics of lipids 108, 1–13. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, and Parker LA (2013). The endocannabinoid system and the brain. Annu Rev Psychol 64, 21–47. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Das RK, Joye A, Curran HV, and Kamboj SK (2013). Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict Behav 38, 2433–2436. [DOI] [PubMed] [Google Scholar]
- Morrison MF, Ceesay P, Gantz I, Kaufman KD, and Lines CR (2010). Randomized, controlled, double-blind trial of taranabant for smoking cessation. Psychopharmacology (Berl) 209, 245–253. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, E., and Medicine (2017). The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research (National Academies Press; ). [PubMed] [Google Scholar]
- Nguyen T, Li JX, Thomas BF, Wiley JL, Kenakin TP, and Zhang Y (2017). Allosteric Modulation: An Alternate Approach Targeting the Cannabinoid CB1 Receptor. Med Res Rev 37, 441–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notcutt W, Price M, Miller R, Newport S, Phillips C, Simmons S, and Sansom C (2004). Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 ‘N of 1’ studies. Anaesthesia 59, 440–452. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SE, Sun Y, Bennett AJ, Randall MD, and Kendall DA (2009). Time-dependent vascular actions of cannabidiol in the rat aorta. Eur J Pharmacol 612, 61–68. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, et al. (2006). Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci 1074, 514–536. [DOI] [PubMed] [Google Scholar]
- Ostenfeld T, Price J, Albanese M, Bullman J, Guillard F, Meyer I, Leeson R, Costantin C, Ziviani L, Nocini PF, et al. (2011). A randomized, controlled study to investigate the analgesic efficacy of single doses of the cannabinoid receptor-2 agonist GW842166, ibuprofen or placebo in patients with acute pain following third molar tooth extraction. Clin J Pain 27, 668–676. [DOI] [PubMed] [Google Scholar]
- Pamplona FA, Ferreira J, Menezes de Lima O Jr., Duarte FS, Bento AF, Forner S, Villarinho JG, Bellocchio L, Wotjak CT, Lerner R, et al. (2012). Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc Natl Acad Sci U S A 109, 21134–21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavon FJ, Serrano A, Perez-Valero V, Jagerovic N, Hernandez-Folgado L, Bermudez-Silva FJ, Macias M, Goya P, and de Fonseca FR (2008). Central versus peripheral antagonism of cannabinoid CB1 receptor in obesity: effects of LH-21, a peripherally acting neutral cannabinoid receptor antagonist, in Zucker rats. J Neuroendocrinol 20 Suppl 1, 116–123. [DOI] [PubMed] [Google Scholar]
- Pertwee RG (1997). Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther 74, 129–180. [DOI] [PubMed] [Google Scholar]
- Pertwee RG (2007). Cannabinoids and multiple sclerosis. Mol Neurobiol 36, 45–59. [DOI] [PubMed] [Google Scholar]
- Pertwee RG (2008). The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol 153, 199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG (2009). Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol 156, 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, et al. (2010). International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2). Pharmacol Rev 62, 588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Thomas A, Stevenson LA, Ross RA, Varvel SA, Lichtman AH, Martin BR, and Razdan RK (2007). The psychoactive plant cannabinoid, Delta9-tetrahydrocannabinol, is antagonized by Delta8- and Delta9-tetrahydrocannabivarin in mice in vivo. Br J Pharmacol 150, 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, and Rosenstock J (2006). Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. Jama 295, 761–775. [DOI] [PubMed] [Google Scholar]
- Pinto JC, Potie F, Rice KC, Boring D, Johnson MR, Evans DM, Wilken GH, Cantrell CH, and Howlett AC (1994). Cannabinoid receptor binding and agonist activity of amides and esters of arachidonic acid. Mol Pharmacol 46, 516–522. [PubMed] [Google Scholar]
- Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, Dasse O, Monaghan EP, Parrott JA, and Putman D (2006). Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597). CNS Drug Rev 12, 21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietto J, Rissanen A, Harp JB, Erondu N, Yu Q, Suryawanshi S, Jones ME, Johnson-Levonas AO, Heymsfield SB, Kaufman KD, et al. (2010). A clinical trial assessing the safety and efficacy of the CB1R inverse agonist taranabant in obese and overweight patients: low-dose study. Int J Obes (Lond) 34, 1243–1254. [DOI] [PubMed] [Google Scholar]
- Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, and Soubrie P (2003). Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 284, R345–353. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Congy C, Martinez S, Oustric D, Perio A, Poncelet M, Maruani J, Arnone M, Finance O, et al. (2004). SR147778 [5-(4-bromophenyl)-1-(2,4-dichlorophenyl)-4-ethyl-N-(1-piperidinyl)-1H-pyrazole-3 -carboxamide], a new potent and selective antagonist of the CB1 cannabinoid receptor: biochemical and pharmacological characterization. J Pharmacol Exp Ther 310, 905–914. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D, et al. (1994). SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350, 240–244. [DOI] [PubMed] [Google Scholar]
- Ritsner MS, Bawakny H, and Kreinin A (2014). Pregnenolone treatment reduces severity of negative symptoms in recent-onset schizophrenia: an 8-week, double-blind, randomized add-on two-center trial. Psychiatry Clin Neurosci 68, 432–440. [DOI] [PubMed] [Google Scholar]
- Robinson JD, Cinciripini PM, Karam-Hage M, Aubin HJ, Dale LC, Niaura R, and Anthenelli RM (2018). Pooled analysis of three randomized, double-blind, placebo controlled trials with rimonabant for smoking cessation. Addict Biol 23, 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgman C, Kinzie E, and Leimbach E (2011). Bad Mojo: use of the new marijuana substitute leads to more and more ED visits for acute psychosis. Am J Emerg Med 29, 232. [DOI] [PubMed] [Google Scholar]
- Rubin R (2018). The Path to the First FDA-Approved Cannabis-Derived Treatment and What Comes Next. Jama 320, 1227–1229. [DOI] [PubMed] [Google Scholar]
- Rucker D, Padwal R, Li SK, Curioni C, and Lau DC (2007). Long term pharmacotherapy for obesity and overweight: updated meta-analysis. Bmj 335, 1194–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, and Parker KK (2005). Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res 30, 1037–1043. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, and Greasley PJ (2007). The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 152, 1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz AR, Lee M, Condie RB, Pulaski JT, and Kaminski NE (1997). Cannabinoid receptors CB1 and CB2: a characterization of expression and adenylate cyclase modulation within the immune system. Toxicol Appl Pharmacol 142, 278–287. [DOI] [PubMed] [Google Scholar]
- Schou M, Varnäs K, Jucaite A, Gulyás B, Halldin C, and Farde L (2013). Radiolabeling of the cannabinoid receptor agonist AZD1940 with carbon-11 and PET microdosing in non-human primate. Nucl Med Biol 40, 410–414. [DOI] [PubMed] [Google Scholar]
- Sholler DJ, Schoene L, and Spindle TR (2020). Therapeutic Efficacy of Cannabidiol (CBD): a Review of the Evidence From Clinical Trials and Human Laboratory Studies. Current Addiction Reports, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieradzan KA, Fox SH, Hill M, Dick JP, Crossman AR, and Brotchie JM (2001). Cannabinoids reduce levodopa-induced dyskinesia in Parkinson’s disease: a pilot study. Neurology 57, 2108–2111. [DOI] [PubMed] [Google Scholar]
- Solowij N, Broyd S, Greenwood LM, van Hell H, Martelozzo D, Rueb K, Todd J, Liu Z, Galettis P, Martin J, et al. (2019). A randomised controlled trial of vaporised Delta(9)-tetrahydrocannabinol and cannabidiol alone and in combination in frequent and infrequent cannabis users: acute intoxication effects. Eur Arch Psychiatry Clin Neurosci 269, 17–35. [DOI] [PubMed] [Google Scholar]
- Stebulis JA, Johnson DR, Rossetti RG, Burstein SH, and Zurier RB (2008). Ajulemic acid, a synthetic cannabinoid acid, induces an antiinflammatory profile of eicosanoids in human synovial cells. Life Sci 83, 666–670. [DOI] [PubMed] [Google Scholar]
- Stempel AV, Stumpf A, Zhang HY, Özdoğan T, Pannasch U, Theis AK, Otte DM, Wojtalla A, Rácz I, Ponomarenko A, et al. (2016). Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron 90, 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, Suhara Y, Takayama H, and Waku K (2000). Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J Biol Chem 275, 605–612. [DOI] [PubMed] [Google Scholar]
- Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T, Szanda G, Mukhopadhyay B, Chedester L, Liow JS, et al. (2012). Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab 16, 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamba BI, Stanciu GD, Urîtu CM, Rezus E, Stefanescu R, Mihai CT, Luca A, Rusu-Zota G, Leon-Constantin MM, Cojocaru E, et al. (2020). Challenges and Opportunities in Preclinical Research of Synthetic Cannabinoids for Pain Therapy. Medicina (Kaunas) 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Crockett J, Tayo B, Checketts D, and Sommerville K (2020). Abrupt withdrawal of cannabidiol (CBD): A randomized trial. Epilepsy Behav 104, 106938. [DOI] [PubMed] [Google Scholar]
- Tepper MA, Zurier RB, and Burstein SH (2014). Ultrapure ajulemic acid has improved CB2 selectivity with reduced CB1 activity. Bioorg Med Chem 22, 3245–3251. [DOI] [PubMed] [Google Scholar]
- Theunissen EL, Hutten N, Mason NL, Toennes SW, Kuypers KPC, de Sousa Fernandes Perna EB, and Ramaekers JG (2018). Neurocognition and subjective experience following acute doses of the synthetic cannabinoid JWH-018: a phase 1, placebo-controlled, pilot study. Br J Pharmacol 175, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, and Pertwee RG (2007). Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol 150, 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Stevenson LA, Wease KN, Price MR, Baillie G, Ross RA, and Pertwee RG (2005). Evidence that the plant cannabinoid Delta9-tetrahydrocannabivarin is a cannabinoid CB1 and CB2 receptor antagonist. Br J Pharmacol 146, 917–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toennes SW, Geraths A, Pogoda W, Paulke A, Wunder C, Theunissen EL, and Ramaekers JG (2017). Pharmacokinetic properties of the synthetic cannabinoid JWH-018 and of its metabolites in serum after inhalation. J Pharm Biomed Anal 140, 215–222. [DOI] [PubMed] [Google Scholar]
- Tonstad S, and Aubin HJ (2012). Efficacy of a dose range of surinabant, a cannabinoid receptor blocker, for smoking cessation: a randomized controlled clinical trial. J Psychopharmacol 26, 1003–1009. [DOI] [PubMed] [Google Scholar]
- Trecki J, Gerona RR, and Schwartz MD (2015). Synthetic Cannabinoid-Related Illnesses and Deaths. N Engl J Med 373, 103–107. [DOI] [PubMed] [Google Scholar]
- Trigo JM, Soliman A, Quilty LC, Fischer B, Rehm J, Selby P, Barnes AJ, Huestis MA, George TP, Streiner DL, et al. (2018). Nabiximols combined with motivational enhancement/cognitive behavioral therapy for the treatment of cannabis dependence: A pilot randomized clinical trial. PLoS One 13, e0190768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Überall MA (2020). A Review of Scientific Evidence for THC:CBD Oromucosal Spray (Nabiximols) in the Management of Chronic Pain. J Pain Res 13, 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee M, Vitiello S, Bellocchio L, Hebert-Chatelain E, Monlezun S, Martin-Garcia E, Kasanetz F, Baillie GL, Panin F, Cathala A, et al. (2014). Pregnenolone can protect the brain from cannabis intoxication. Science 343, 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Esbroeck ACM, Janssen APA, Cognetta AB 3rd, Ogasawara D, Shpak G, van der Kroeg M, Kantae V, Baggelaar MP, de Vrij FMS, Deng H, et al. (2017). Activity-based protein profiling reveals off-target proteins of the FAAH inhibitor BIA 10-2474. Science 356, 1084–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, et al. (2005). Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310, 329–332. [DOI] [PubMed] [Google Scholar]
- Vandrey R, Dunn KE, Fry JA, and Girling ER (2012). A survey study to characterize use of Spice products (synthetic cannabinoids). Drug Alcohol Depend 120, 238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Stitzer ML, Mintzer MZ, Huestis MA, Murray JA, and Lee D (2013). The dose effects of short-term dronabinol (oral THC) maintenance in daily cannabis users. Drug Alcohol Depend 128, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volicer L, Stelly M, Morris J, McLaughlin J, and Volicer BJ (1997). Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int J Geriatr Psychiatry 12, 913–919. [PubMed] [Google Scholar]
- Wadden TA, Fujioka K, Toubro S, Gantz I, Erondu NE, Chen M, Suryawanshi S, Carofano W, Johnson-Levonas AO, Shapiro DR, et al. (2010). A randomized trial of lifestyle modification and taranabant for maintaining weight loss achieved with a low-calorie diet. Obesity (Silver Spring) 18, 2301–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade DT, Robson P, House H, Makela P, and Aram J (2003). A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil 17, 21–29. [DOI] [PubMed] [Google Scholar]
- Wagenlehner FME, van Till JWO, Houbiers JGA, Martina RV, Cerneus DP, Melis J, Majek A, Vjaters E, Urban M, Ramonas H, et al. (2017). Fatty Acid Amide Hydrolase Inhibitor Treatment in Men With Chronic Prostatitis/Chronic Pelvic Pain Syndrome: An Adaptive Double-blind, Randomized Controlled Trial. Urology 103, 191–197. [DOI] [PubMed] [Google Scholar]
- Walther S, Mahlberg R, Eichmann U, and Kunz D (2006). Delta-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology (Berl) 185, 524–528. [DOI] [PubMed] [Google Scholar]
- Ware MA, and St Arnaud-Trempe E (2010). The abuse potential of the synthetic cannabinoid nabilone. Addiction 105, 494–503. [DOI] [PubMed] [Google Scholar]
- Watkins BA, and Kim J (2014). The endocannabinoid system: directing eating behavior and macronutrient metabolism. Front Psychol 5, 1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CM (2019). A Review of Human Studies Assessing Cannabidiol’s (CBD) Therapeutic Actions and Potential. J Clin Pharmacol 59, 923–934. [DOI] [PubMed] [Google Scholar]
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, et al. (2015). Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. Jama 313, 2456–2473. [DOI] [PubMed] [Google Scholar]
- Wootten D, Christopoulos A, and Sexton PM (2013). Emerging paradigms in GPCR allostery: implications for drug discovery. Nat Rev Drug Discov 12, 630–644. [DOI] [PubMed] [Google Scholar]
- Wouters E, Walraed J, Banister SD, and Stove CP (2019). Insights into biased signaling at cannabinoid receptors: synthetic cannabinoid receptor agonists. Biochem Pharmacol 169, 113623. [DOI] [PubMed] [Google Scholar]
- Wu SH, Chen XQ, Liu B, Wu HJ, and Dong L (2013). Efficacy and safety of 15(R/S)-methyl-lipoxin A(4) in topical treatment of infantile eczema. Br J Dermatol 168, 172–178. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, and Gardner EL (2011). Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nat Neurosci 14, 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannikos P, Wennerholm M, Van der Ark P, Vaccaro N, Schmidt M, and Rassnick S (2014). The pharmacokinetics (PK), pharmacodynamics (PD), safety, and tolerability of JNJ-42165279, a potent and selective inhibitor of fatty acid amide hydrolase (FAAH) in healthy subjects. In Neuropsychopharmacology, pp. S291–S472. [Google Scholar]
- Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, and Schulz K (2009). Withdrawal phenomena and dependence syndrome after the consumption of “spice gold”. Dtsch Arztebl Int 106, 464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirpel B, Degenhardt F, Martin C, Kayser O, and Stehle F (2017). Engineering yeasts as platform organisms for cannabinoid biosynthesis. J Biotechnol 259, 204–212. [DOI] [PubMed] [Google Scholar]
- Zirpel B, Stehle F, and Kayser O (2015). Production of Delta9-tetrahydrocannabinolic acid from cannabigerolic acid by whole cells of Pichia (Komagataella) pastoris expressing Delta9-tetrahydrocannabinolic acid synthase from Cannabis sativa L. Biotechnol Lett 37, 1869–1875. [DOI] [PubMed] [Google Scholar]
- Zou S, and Kumar U (2018). Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW (2006). History of cannabis as a medicine: a review. Braz J Psychiatry 28, 153–157. [DOI] [PubMed] [Google Scholar]
- Zurier RB, Sun YP, George KL, Stebulis JA, Rossetti RG, Skulas A, Judge E, and Serhan CN (2009). Ajulemic acid, a synthetic cannabinoid, increases formation of the endogenous proresolving and anti-inflammatory eicosanoid, lipoxin A4. Faseb j 23, 1503–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]


