Abstract
Interleukin (IL)-38 belongs to the IL-1 family and is part of the IL-36 subfamily due to its binding to the IL-36 Receptor (IL-1R6). In the current study, we assessed the anti-inflammatory properties of IL-38 in murine models of arthritis and systemic inflammation. First, the anti-inflammatory properties of the precursor forms of mouse and human IL-38 were compared to forms with truncated N-termini. In mouse bone marrow derived dendritic cells (BMDC), human and mouse IL-38 precursors with a truncation of the two N-terminal amino acids (3–152) suppressed LPS-induced IL-6. Recombinant human IL-38 (3–152) was further investigated for its immunomodulatory potential using four murine models of inflammatory disease: streptococcal cell wall (SCW)-induced arthritis, monosodium urate (MSU) crystal-induced arthritis, MSU crystal-induced peritonitis, and systemic endotoxemia. In each of these models IL-38 significantly reduced inflammation. In SCW and MSU crystal-induced arthritis, joint swelling, inflammatory cell influx, and synovial levels of IL-1β, IL-6, and KC were reduced by 50% or greater. These suppressive properties of IL-38 in SCW-induced arthritis were independent of the anti-inflammatory co-receptor IL-1R8, as IL-38 reduced arthritis equally in IL-1R8 deficient and WT mice. In MSU crystal-induced peritonitis, IL-38 reduced hypothermia also, plasma IL-6 and KC and peritoneal KC levels were reduced by 65–70%. In the LPS endotoxemia model, IL-38 pretreatment reduced systemic IL-6, TNFα and KC. Furthermore, in ex vivo cultured bone marrow, LPS-induced IL-6, TNFα and KC were reduced by 75–90%. Overall, IL-38 exhibits broad anti-inflammatory properties in models of systemic and local inflammation and therefore may be an effective cytokine therapy.
Keywords: Interleukin-38, pathogenesis, IL-1 family, Inflammation
1. Introduction
IL-38 was identified in 2001 as a member of the IL-1 family and assigned the gene symbol IL1F10. IL-38 shares significant homology with IL-1 Receptor antagonist (IL-1Ra) and also IL-36Ra (41% and 43%, respectively) [1, 2]. With the exception of IL-1Ra, all members of the IL-1 family lack a signal peptide and require processing for biological activity [3, 4]. In the case of the IL-38 precursor, the naturally occurring N-termini of processed IL-38 are unknown [5]. Mouse and human IL-38 contain the IL-1 family consensus sequence AXD, where A is an aliphatic amino acid, X is any amino acid and D, aspartic acid. The AXD sequence is found in 10 of the 11 members of the IL-1 family, except IL-1Ra, which has a signal peptide and is readily secreted [3, 4]. Nine amino acids N-terminal from the AXD is a predicted cleavage site for the mature N-terminus, which results in an active cytokine capable of receptor engagement and biological signaling [3, 4]. The nine amino acid interval distance was originally reported for IL-36α, IL-36β, IL-36γ and IL-36Ra in which biological activities increased over 100-fold following removal of only a few N-terminal amino acids [6].
In 2012, our goup made the initial observation that IL-38 binds to the IL-36 Receptor (IL-1R6) and, furthermore, that recombinant human IL-38 reduced IL-17 and IL-22 release by human peripheral blood mononuclear cells (PBMC) stimulated with the yeast Candida albicans [7]. Today, IL-38 is included in the IL-36 subfamily together with IL-36Ra, IL-36α, IL-36β and IL-36γ [8]. The proinflammatory cytokines IL-36α, IL-36β, and IL-36γ bind to IL-1R6 and form induce a signaling complex with the co-receptor IL-1R3. In contrast, IL-38 does not form a complex with the co-receptor IL-1R3. One candidate co-receptor for IL-38 is IL-1R9; however, to date a complex of IL-38 with IL-1R6 and IL-1R9 has not been demonstrated [9, 10]. Han et al. reported that the inhibition of IL-1R9 signaling in activated γδ-T cells depends directly on IL-38, as IL-38 deficiency resulted in amplified IL-1R9 dependent IL-17 production and exacerbated skin inflammation in a mouse model of imiquimod induced psoriasis [10]. Interestingly, in those experiments, the involvement of IL-1R6 in the signaling of IL-38 was not confirmed.
There is growing evidence that IL-38 contributes to the pathogenesis of several inflammatory diseases, including rheumatoid arthritis [11–13], psoriatic arthritis [14, 15], systemic lupus erythematosus [16], asthma [17], and liver injury [18]. In a genome-wide association study with over 80,000 subjects, a SNP in the IL-38 encoding IL1F10 gene was identified as one of 18 genetic markers associated with elevated C-reactive protein levels [19]. This suggests a potential role for IL-38 in regulating inflammatory responses.
In the present study, we investigated whether IL-38 requires N-terminal truncation to enhance its biological activity. Once we established the proper N-terminus for recombinant IL-38 in reducing proinflammatory cytokine release in vitro, we investigated the anti-inflammatory potential of recombinant human IL-38 in vivo in models of joint inflammation, including gouty arthritis, peritonitis, and sepsis.
2. Material and Methods
2.1. Recombinant proteins.
Recombinant mouse and human IL-38 precursor (1–152) and truncated IL-38 (3–152) were expressed in Escherichia coli, refolded and purified to homogeneity (R & D Systems, Minneapolis, MN, USA). The preparations contained less than (0.00015 EU/μg) of endotoxin by the Limulus Amoebocyte Lysate (LAL) assay. The purity of recombinant IL-38 revealed a single band after silver-stained SDS-PAGE under reducing conditions. Anakinra, the recombinant form of the natural IL-1Ra, was obtained from Swedish Orphan Biovitrum (Stockholm, Sweden).
2.2. Mice.
Animal protocols were approved by the University of Colorado Animal Care and Use Committee. Male C57BL/6 mice (10–12 weeks of age, 22–26 g) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). IL-1R8 deficient were generated as reported [21]. Mice received food and water ad libitum and were acclimatized in the animal facility for at least 7 days before use.
2.3. Bone Marrow-Derived Dendritic Cell culture (BMDC).
Mouse bone marrow was flushed from mouse femurs with cold RPMI-1640 culture medium (Corning, Manassas, VA, USA), and passed through a 70 μm cell strainer. The cells were centrifuged at 300 × g for 7 minutes at 4°C and counted with an automated veterinary cell counter (Heska, Loveland, CO, USA). 500,000 cells per well were seeded in flat bottom 96 well plates. Non-adherent cells were removed after 2 hours, and the wells were washed with PBS (Gibco, New York, NY, USA) and adherent cells were differentiated into BMDC with RPMI-1640 containing 10% FBS (Corning), 20 ng/mL mouse GM-CSF, and 20 ng/mL mouse IL-4 (R & D Systems). The differentiation medium was refreshed every other day. After 6 days the cells were treated with RPMI or mouse or human recombinant IL-38 (precursor or 3–152) for one hour before the cells were stimulated with 10 ng/mL lipopolysaccharide (LPS; O55:B5, Sigma-Aldrich, St. Louis, MO, USA). After 24 hours, the supernatants were collected and stored at −20°C until ELISA was performed.
2.4. Streptococcal cell wall arthritis model.
Mice received 1 μg recombinant human IL-38 (3–152) or human serum albumin intraperitoneal (i.p.) 24, 12 and 2 hours before arthritis was induced. 25 μg Streptococcus pyogenenes cell wall (SCW) fragments (Lee Laboratories, Georgia, USA) were instilled into the knee joints of wild type (WT) or IL-1R8 deficient mice in 10 μL of PBS. Mice were sacrificed after 4 hours and the skin of the hind leg was removed. Joint inflammation was visually scored by two blinded investigators using a scale of 0–3 as follows: 0, no swelling; 1, mild; 2, moderate; and 3, severe inflammation [22]. The patella from one of the knee joints lysed in 200 μL 0.5% Triton-X100 in H2O. After three freeze-thawing cycles and centrifugation at 10,000 × g at 4°C for 15 minutes, the supernatants were stored at −20°C until ELISA was performed. The opposing knee joint was fixed in 4% formaldehyde for 7 days before decalcification in 5% formic acid and processing for paraffin embedding. Tissue sections (7 μm) were stained with hematoxylin/eosin. Histopathological changes in the knee joints were scored in the patella/femur region on 5 semi-serial sections. Scoring on a scale from 0–3 was performed by two blinded observers, based on the amount of cells infiltrating the synovial lining and the joint cavity.
2.5. Gouty arthritis model.
Mono sodium urate (MSU) crystals were prepared by diluting 1.0 g of uric acid (Sigma) in 200 mL of sterile water containing 24 g of NaOH. The pH was adjusted to 7.2 with HCl and the preparation was boiled at 100 °C for 6 hours. After cooling to room temperature, the absence of endotoxin was confirmed by LAL assay. The resulting MSU crystals were 5–25 μm in length, resuspended at 30 mg/mL in sterile PBS and stored at 4°C. MSU crystals were combined with RPMI-1640 culture medium containing 20% human serum albumin (Sigma) at a ratio of 1:20 and warmed to 37°C. The mixture was sonicated for 20–25 minutes to aid conjugation and kept at 37°C. To prepare MSU/palmitic acid (C16.0) for injections, a solution of 400 μM C16.0 (Sigma), 20% human serum albumin and 0.4% ethanol in RPMI-1640 culture medium was filter-sterilized (0.2 μm) and mixed in a ratio of 1:1 with 1200 μg/mL MSU crystals. This preparation was stored at 4°C, and before use warmed to room temperature and vortexed. Mice were treated i.p. with 1 μg IL-38 (3–152), or 1 μg human serum albumin, as a vehicle control, at 24, 12 and 2 hours prior to induction of gouty arthritis. Mice were anesthetized and gouty arthritis was induced by i.a. injection of MSU/C16.0 (300 μg/200 μM) in 10 μL into each knee joint. After 4 hours, mice were sacrificed, joint inflammation of both knees was scored as described in the SCW-induced arthritis model. After visual scoring, one knee joint was fixed with in 4% formaldehyde for histology, and scored for cell influx as described above for the SCW-induced arthritis model. From the opposing knee, the patella including the synovial lining was placed in 200 μL of RPMI-1640 containing penicillin-streptomycin in a flat bottomed 96 wells plate. The supernatant was collected after 2 hours of incubation at 37°C, and the synovial lining of the patella was lysed in 200 μL 0.5% Triton-X100, subjected to three freeze-thaw cycles and centrifuged at 4°C for 10,000 × g for 15 minutes. The supernatants were stored at −20°C until ELISA was performed.
2.6. MSU crystal/C16.0-induced peritonitis model.
Mice were pretreated i.p. with human serum albumin as the vehicle or three doses of 1 μg of human IL-38 (3–152) or vehicle at 24, 12 and 2 hours before MSU/C16.0, and a third group was injected with 10 mg/kg Anakinra 1 hour prior to i.p. administration of MSU/C16.0 (1 mg/200 μM) in 200 μL PBS. After 4 hours, the abdominal wall body temperature was measured, mice were anesthetized, exsanguinated from the orbital plexus and sacrificed. The peritoneal cavity was lavaged with 10 mL of ice-cold PBS. The peritoneal fluid and blood were centrifuged at 300 × g for 10 minutes at 4°C, and the cleared peritoneal fluid and plasma were stored at −20°C until ELISA was performed.
2.7. LPS-induced endotoxemia model.
Mice were treated i.p. with saline or human IL-38 (3–152) at 48, 24 and 2 hours before challenge with 5 mg/kg LPS i.p. After 16 hours, mice were anesthetized and plasma was obtained as described for the peritonitis model and the mice were sacrificed. Bone marrow cells from the both femurs were obtained and 100,000 cells per well were seeded in flat bottom 96 well plates in RPMI-1640 medium containing penicillin/streptomycin. After one hour, LPS (100 ng/mL) was added and after 24 hours the supernatants were removed and stored at −20°C until ELISA was performed.
2.8. Cytokine Measurements.
Cytokines were measured using ELISAs for mouse IL-1β, IL-6, TNFα, KC, and IL-10 (DuoSet, R & D Systems) according to the manufacturer’s instructions.
2.9. Analytical size-exclusion
An analytical Superdex-75 (24 mL total volume, GE Healthcare) was used to assess the migratory behavior of IL-38 purified in-house by comparing this behavior to standards (Gel Filtration Calibration Kit LMW, GE Healthcare). Briefly, a chimera of sumo and IL-38 residues 3–152 was synthesized as a gblock (Integrated DNA Technologies, Coralville, Iowa) and cloned into pET21 with an N-terminal 6xHis tag for subsequence expression in BL21/DE3. For a typical 4 L growth, cells were induced at an OD(600) of 0.6 and cells harvested after 5 h at room temperature. Ni affinity (GE Healthcare) was used to purify the chimera, cleaved with the sumo protease purified in-house, applied to a Ni-affinity to remove the His-tagged sumo and protease, and finally concentrated for a preparative Superdex-75. Standards (GE Healthcare) used for analytical size-exclusion included ovalbumin, carbonic anhydrase, and ribonuclease A
2.10. Statistical analysis
The differences between the various conditions were analyzed with the Wilcoxon matched pairs signed rank test or Mann-Whitney U-test as appropriate. Data are presented as mean ±SEM unless otherwise indicated. Data was analyzed using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA). A p-value below 0.05 was considered significant.
3. Results
3.1. N-terminal truncation of IL-38 enhances biological activity.
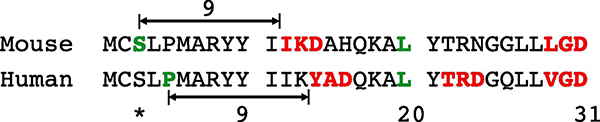
We sought to determine the N-terminus of recombinant IL-38, as IL-38 lacks a signal peptide. Mouse IL-38 contains two AXD sites in the N-terminal domain (Figure 1, shown in red). Therefore, mouse IL-38 with serine-3 (shown in green) as the N-terminus was expressed in E. coli. Similarly, recombinant human IL-38 with serine-3 as the N-terminus to match mouse IL-38 (3–152) was produced, although the predicted N-terminus for human IL-38 is at proline-5.
Figure 1. Sequence alignment of mouse and human IL-38.
The sequence of the first 31 amino acids of mouse and human IL-38 precursors. Amino acids colored in red are AXD consensus sequences within IL-38. The serine-3 colored in green in the mouse IL-38 sequence is the predicted N-terminus based on the consensus sequence IKD. In human IL-38, the predicted N-terminus based on the AXD consensus YAD is proline 5. The asterisk indicates serine-3 as the N-terminus of the recombinant human IL-38 used in these studies.
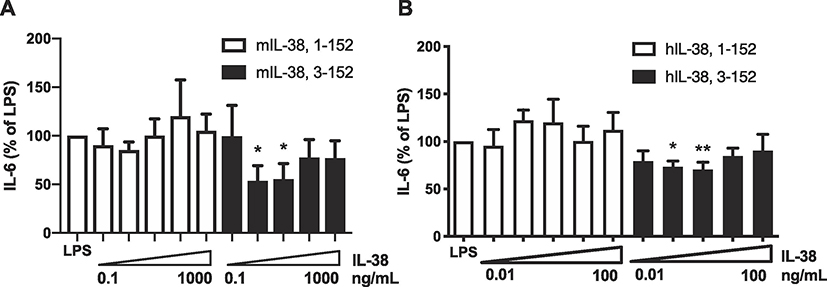
To determine the optimal recombinant IL-38 with optimal biological activities, we compared murine precursor with the cleaved murine IL-38 (3–152). Mouse BMDCs stimulated with LPS released reduced amounts of IL-6 production when incubated with low concentrations of murine 3–152 (p < 0.05), whereas precursor IL-38 was inactive (Figure 2A). Similarly, human truncated IL-38 (3–152) at low concentrations reduced LPS induced IL-6 in BMDC (p < 0.05), whereas precursor human IL-38 did not (Figure 2B). Therefore, we concluded that, in both mouse and human IL-38, truncation of the first two amino acids from precursor IL-38 results in enhanced biological activity. This finding is consistent with removal of one amino acid from the IL-36Ra precursor and an increase in the anti-inflammatory activity of the cytokine [6].
Figure 2. Anti-inflammatory effects of precursor mouse IL-38 compared to N-terminus processed IL-38.
A. Mouse BMDC were incubated with precursor recombinant mouse IL-38 (1–152) or mouse IL-38 (3–152) for one hour followed by stimulation with LPS (10 ng/mL). After 24 hours, the supernatants were assayed for IL-6. The data are shown as mean ±SEM, *p < 0.05, n=6.B. Mouse BMDCs were incubated with precursor or 3–152 recombinant human IL-38 for one hour and then stimulated with LPS (10 ng/mL). After 24 hours, the supernatants were assayed for IL-6. The data are shown as mean ±SEM, *p < 0.05, n=5 per group. In A, the dose-escalation starts a 0.1 ng/mL and increased by a factor of 10 to 1000 ng/mL. In B, the dose-escalation starts a 0.01 ng/mL and increased by a factor of 10 to 100 ng/mL.
3.2. Recombinant human IL-38 reduces inflammation in murine SCW-induced arthritis.
Since truncated IL-38 (3–152) exhibited anti-inflammatory properties in vitro, the anti-inflammatory properties of IL-38 were explored in vivo. Mice were first treated with recombinant human IL-38 (3–152) or vehicle 24, 12 and 2 hours prior to induction of arthritis with SCW fragments. IL-38 pretreatment significantly reduced joint inflammation scores by 60% (Figure 3A) and cell influx score by 40% (Figure 3B) compared to vehicle treated mice (both p < 0.0001).Histological analysis of knee joints from vehicle and IL-38 pretreated mice (Figure 3C and D) reveal reduced histopathology in IL-38 (3–152) treated mice. IL-38 pretreatment reduced levels of IL-1β (p < 0.05) and IL-6 (p < 0.001) in extracts of the synovium by over 50% (Figure 3E–F).
Figure 3. Effect of recombinant IL-38 on SCW fragment-induced arthritis.
Mice were pretreated i.p. with either vehicle of recombinant human IL-38 (3–152) 24, 12 and 2 hours before intraarticular instillation of SCW fragments under the patella into the synovial compartment.A. Macroscopic scoring of exposed inflamed knee joint. Mean ±SEM, ***p < 0.001, n=10 mice.B. cell influx scoring on histological sections from mice shown in A by two blinded investigators. Mean ±SEM, ***p < 0.001.C-D. Representative hematoxylin-eosin stained sections of whole knee joints of vehicle (C) and IL-38 treated mice (D).E-F. Mean ±SEM levels of IL-1β (E) and IL-6 (F) in extracts of the synovial membranes. *p < 0.05,p < 0.001, n=5 per group.
3.3. The anti-inflammatory properties of IL-38 are independent of IL-1R8.
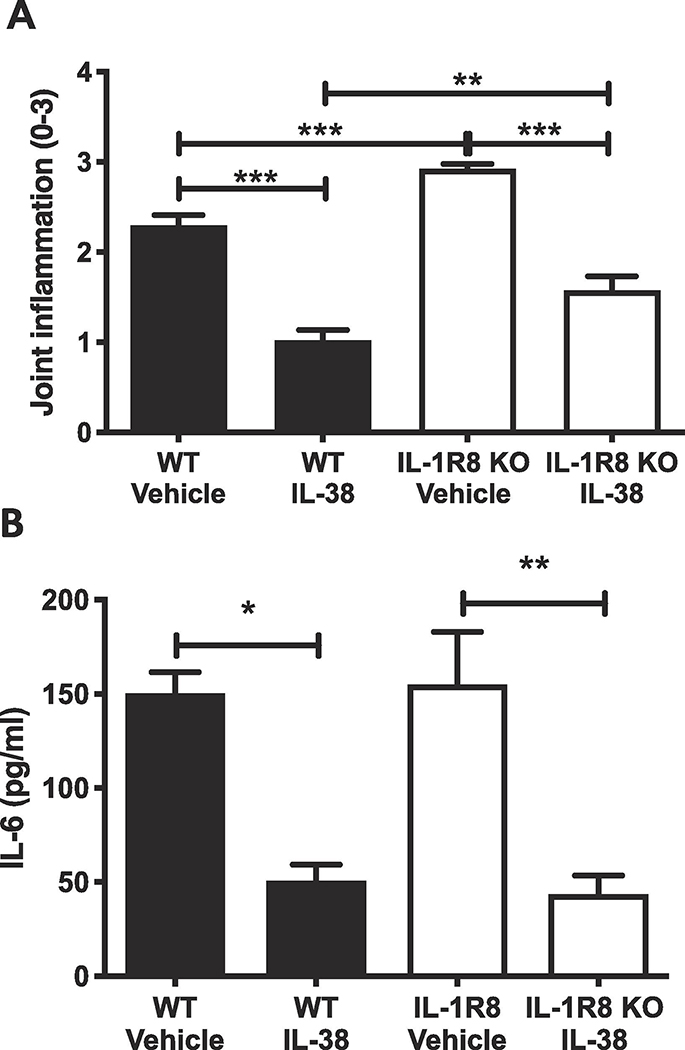
IL-1R8 functions as an anti-inflammatory receptor in the IL-1 family and mice deficient in IL-1R8 exhibit exacerbated inflammatory responses [23–26]. IL-1R8 is required for the anti-inflammatory effects of IL-37 [27–30]. Since IL-38 and IL-37 exhibit similar anti-inflammatory properties, we sought to examine whether IL-1R8 mediates the anti-inflammatory function of IL-38 [7]. To test whether the anti-inflammatory effects of IL-38 are mediated through IL-1R8, SCW fragments were injected the knee joints of WT and IL-1R8 deficient mice to induce arthritis after i.p. treatment with human IL-38 (3–152) or vehicle. Similar to WT mice, IL-1R8 deficient mice pretreated with IL-38 (3–152) showed a 50% reduction in inflammatory score compared to vehicle treated mice (p < 0.0001; Figure 4A). Furthermore, IL-6 levels in the synovial extract of the knee joint were reduced over 70% by human IL-38 (3–152) in both WT and IL-1R8 deficient mice (p < 0.05; Figure 4B). Overall, SCW induced a more severe joint inflammation in IL-1R8 deficient mice compared to WT mice (p < 0.01), which is consistent with previous studies on this receptor [23–26]. These data establish that IL-1R8 is redundant for the anti-inflammatory properties induced by IL-38 and is therefore consistent with the requirement of receptors distinct from that used by IL-37.
Figure 4. Effect of IL-38 on streptococcal cell wall induced arthritis in mice deficient in IL-1R8.
A. Joint inflammation scores of WT mice pretreated with vehicle or with recombinant human IL-38 (3–152) administered intraperitoneally 24, 12 and 2 hours prior to intraarticular instillation of streptococcal cell wall fragments.B. Levels of IL-6 in the synovial extract from mice shown in. Data are presented as mean ±SEM, *p < 0.05, **p < 0.01, ***p < 0.001, n=5 per group.
3.4. The effect of IL-38 on MSU-crystal induced gouty arthritis.
As the incidence of metabolic syndrome increases, so does the number of patients with acute gouty arthritis [31]. Acute attacks of gout are highly responsive to IL-1 blockade with anakinra, the recombinant form of the naturally occurring IL-1Ra [32–35]. Canakinumab, an anti-IL-1β human monoclonal antibody, also effectively reduces the number of gout attacks in gout patients [36–38]. Since IL-38 reduces IL-1β and joint inflammation in SCW-induced arthritis, the potential of recombinant IL-38 to mitigate acute MSU crystal-induced arthritis in mice was investigated.
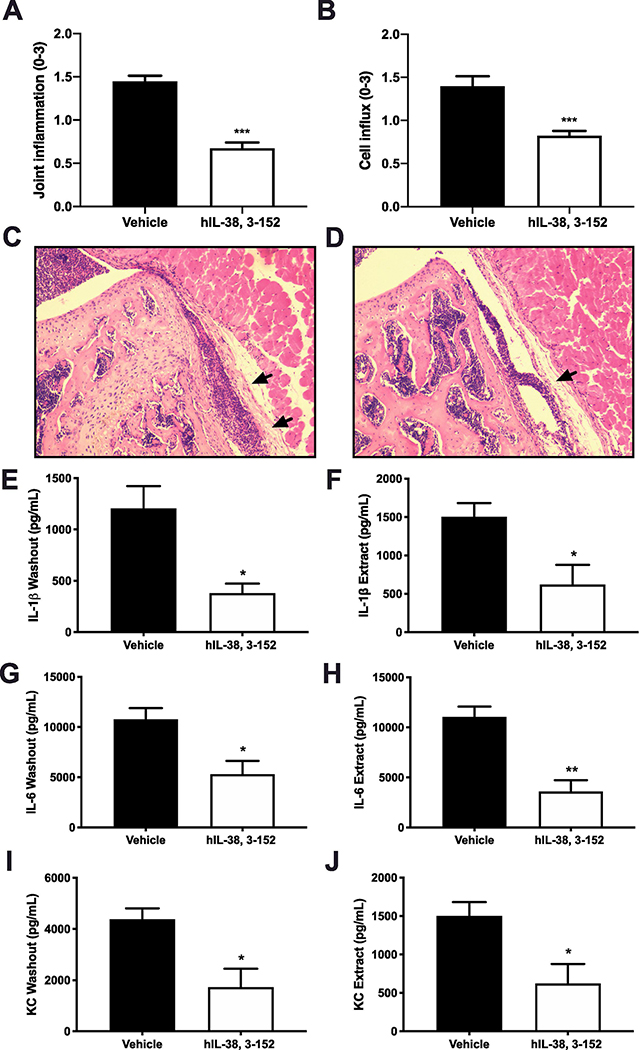
Mice were received 1 μg of recombinant human IL-38 (3–152) intraperitonelly 24, 12 and 2 hours prior to instillation of MSU crystals into a knee joint as described previously [39]. After 4 hours, mice treated with IL-38 showed a 50% reduction in joint inflammation using the visual scoring scale (Figure 5A) and a 40% reduction in cell influx compared to vehicle-treated mice (Figure 5B; p < 0.0001). In addition, mice treated with IL-38 had 60% or greater reduction in IL-1β, IL-6, and KC levels in the washout from cultured synovial membranes and in synovial extracts (p < 0.05; Figure 5E–J).
Figure 5. Effect of recombinant IL-38 on MSU crystal-induced gouty arthritis.
A. Macroscopic joint inflammation scores in mice treated with recombinant IL-38 (3–152) or vehicle prior to intraarticular MSU crystals. The data are presented as mean ±SEM, ***p < 0.001, 5 mice per group, n=10 knee joints.B. Synovial cell influx score of mice shown inA. Mean ±SEM, ***p < 0.001, 5 mice per group, n=5 knee joints.C and D. Representative hematoxylin-eosin stained sections of whole knee joints of vehicle (C) and IL-38 treated (D) mice as shown inA.E, G, I. IL-1β (E), IL-6 (G) and KC (I) levels in the synovial washout.F, H, J. IL-1β (F), IL-6 (H) and KC (J) levels in synovial extracts. The data are presented as mean ±SEM. *p < 0.05, **p < 0.01.
3.5. Effect of IL-38 in inflammatory MSU-induced peritonitis.
To investigate whether the anti-inflammatory properties of IL-38 are similar to those of anakinra, human recombinant IL-38 (3–152) was compared with anakinra in a mouse model of acute peritonitis. Mice were treated with IL-38 or vehicle 24, 12 and 2 hours before the induction of acute peritonitis by MSU crystals. Another group of mice received anakinra (10 mg/kg) 1 hour prior to the instillation of the crystals into the peritoneal cavity. Mice treated with IL-38 or anakinra were protected from hypothermia compared to mice treated with vehicle by approximately 1 °C (p < 0.01; Figure 6A). Hypothermia is indicative of systemic inflammation in mice [27, 40]. The KC levels in the peritoneal fluid was similarly reduced by 70% in mice treated with anakinra or IL-38 compared to vehicle treated mice (p < 0.05; Figure 6B). In addition, anakinra and IL-38 similarly reduced plasma IL-6 and KC levels by 60% or greater (p < 0.05; Figure 6C–D).
Figure 6. Effect of recombinant IL-38 on MSU/C16-induced peritonitis.
Mice received human IL-38 (3–152) or vehicle 48, 24 and 2 hours prior to intraperitoneal instillation of MSU crystals (1 mg/mouse). Another group of mice received anakinra at 10 mg/kg one hour before MSU crystals.A. Abdominal wall temperature 4 hours after MSU crystals, immediately prior to anesthesia and sacrifice.B. Levels of peritoneal fluid KC.C. Plasma IL-6 and D. plasma KC 4 hours after MSU crystals. Data are presented as mean ±SEM, *p < 0.05, **p < 0.01 compared to vehicle, n=5 mice per group. Differences between vehicle and anakinra or vehicle and IL-38 are indicated above the bar.
3.6. Effects of IL-38 on LPS-induced systemic inflammation.
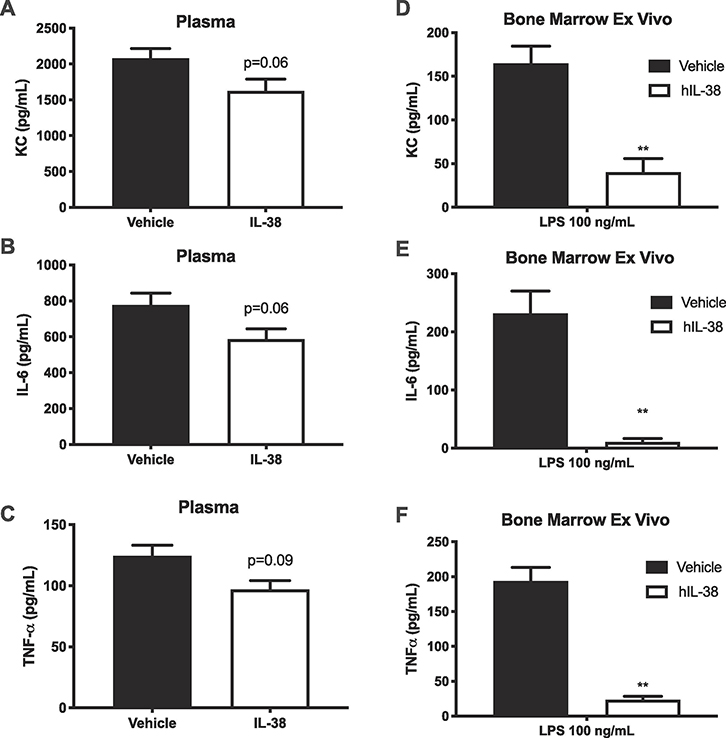
Next the effects of recombinant human IL-38 were investigated in a model of systemic inflammation using LPS-induced endotoxemia. Mice were pre-treated with human recombinant IL-38 (3–152) or vehicle 48, 24 and 2 hours before intraperitoneal administration of LPS (5 mg/kg). Although the circulating levels of KC, IL-6 and TNFα were reduced by 25% (Figure 7A–F), these levels did not reach statistical significance (p = 0.06, p = 0.06 and p = 0.08, respectively). However, ex vivo cultured bone marrow cells from IL-38 treated mice secreted reduced levels of KC, IL-6 and TNFα by 75% after ex vivo culture with LPS for 24 hours (Figure 7D–F; p < 0.01). Taken together, these data show that systemic treatment with IL-38 provides anti-inflammatory properties both locally in isolated joints, as well as systemically.
Figure 7. Effect of recombinant IL-38 on LPS-induced cytokines.
Mice received human IL-38 (3–152) or vehicle 48, 24 and 2 hours prior to intraperitoneal instillation of LPS (5 mg/kg).A-C. Plasma levels of KC, IL-6 and TNFα 16 hours after LPS.D-F. Concentrations of KC, IL-6 and TNFα in supernatants from bone marrow culturedex vivo with 100 ng/mL LPS for 24 hours. Data are shown as mean ±SEM, *p < 0.05, **p < 0.01, n=5 per group.
We investigated whether recombinant IL-38 cytokine is monomeric in solution, similar to other IL-1 family members, or dimeric in solution, similar to IL-37. Using size-exclusion chromotagraphy (Supplement Figure 1), comparative analysis to globular standards clearly reveals that the migratory behavior of IL-38 is that of a monomer in solution and therefore suggests that any pro-inflammatory activity is not due the oligomeric behavior that was previously identified for IL-37 [41].
4. Discussion
N-terminal truncation of precursors of the IL-1 family are required for biological activity. IL-1β, which is first synthesized as an inactive a 31 kDa precursor, is cleaved by caspase-1 to yield the mature 17 kDa biologically active IL-1β. However, similar to other members of the IL-36 subfamily, there is no caspase-1 cleavage site in IL-38 and the IL-38 precursor is likely truncated by limited proteolysis [3]. We hypothesized that the IL-38 precursor requires cleavage by proteases to reach full biological activity [5]. Based on the location of the AXD consensus sequence, murine IL-38 has a cleavage site after the precursor’s first two amino acids, yielding mature 3–152 IL-38. In murine BMDCs stimulated with LPS, we observed that recombinant mouse IL-38 (3–152) inhibited LPS-induced IL-6, whereas untruncated (precursor) IL-38 did not. Human recombinant IL-38 (3–152) similarly suppressed LPS-induced IL-6 in BMDC, but not theprecursor. Besides dendritic cells, IL-38 may have other cellular targets including macrophages [9], γδ-Tcells [10] and regulatory T cells [42]. The relatively small reduction of IL-6 by IL-38 observed in BMDCs may be due to the low expression of IL-1R6 and or IL-1R9 in these cells.
Which and how many N-termini of IL-38 occur naturally remains unknown. As various cells die at sites of inflammation, particularly neutrophils, proteolytic enzymes are released that may process IL-38 precursor extracellularly. Such truncations are likely to yield IL-38 with different N-termini and different degrees of biological activity. Mora et al. first reported that precursor IL-38 is truncated under apoptotic conditions, resulting in increased biological activity, although the N-terminal amino acid was not identified [9]. In that report, the authors describe that recombinant human IL-38 (20–152) reduced IL-1β-induced IL-6 release from macrophages by blocking the JNK/AP1 pathway downstream of IL-1R9 [9]. In contrast, Boutet et al. did not find recombinant IL-38 (20–152) to be anti-inflammatory in macrophages [5]. In the synovium of patients with rheumatoid arthritis, IL-38 expression is elevated compared to healthy subjects [11] and Garraud et al. reported synovial IL-38 with a molecular weight of 14–15 kDa, indicating truncation [5]. In other studies, IL-38 cleavage by caspase-1, chymase, or proteinase 3 was not observed [11]. Harel et al. predicted that IL-38 has cleavage sites for MMP-2, MMP-9 and cathepsin G, and demonstrated in THP-1 cells overexpressing IL-38 that degradation and release of IL-38 can be inhibited by TAPI, an inhibitor of MMPs and TACE [43]. While the naturally occurring as well as most bioactive N-terminally cleaved variant of IL-38 have yet to be determined, the fact that N-terminal truncation is required to increase bioactivity is clear from our observations and and previous reports on IL-38 with amino acids 7–152 [10], 20–152 [9] and 3–152 [44, 45].
Based on the in vitro data obtained with truncated IL-38, we tested recombinant human IL-38 (3–152) in mouse models of inflammatory joint diseases. Our data demonstrate that in arthritis-induced by SCW fragments, systemic treatment with recombinant human IL-38 (3–152) reduced macroscopic joint inflammation, cell influx, and levels of IL-1β and IL-6 in the synovial lining. These data are consistent with a reduction of inflammation reported with IL-38 overexpression [46], and IL-38 deficiency exacerbating inflammation in murine models of arthritis [11].
We next hypothesized that IL-1R8 may be a co-receptor for IL-38 signaling since it exhibits similar anti-inflammatory effects as IL-37, which requires IL-1R8 as a co-receptor to exert its functions [27, 28]. We tested this hypothesis in the SCW-induced arthritis model comparing WT mice with IL-1R8 deficient mice treated with human recombinant IL-38 (3–152) or vehicle. Both WT and IL-1R8 deficient mice showed a marked inflammatory response after intra-articular SCW instillation, with IL-1R8 deficiency giving rise to exacerbated joint inflammation. Pretreatment with recombinant IL-38 reduced inflammation similarly in WT and IL-1R8 deficient mice, indicating that IL-1R8 is redundant for the anti-inflammatory effects induced by IL-38.
Recently, IL-38 was reported to bind IL-1R9, an orphan receptor of the IL-1 family formerly known as IL1RAPL1 and TIGIRR-2 [9]. To date, there is no convincing evidence for an IL-38-IL-1R6-IL-1R9 complex. IL-1R9 is located on the X chromosome, and mutations in IL-1R9 are associated with conditions ranging from X-linked intellectual disability [47] to autism [48]. Of note, the Toll-IL-1R (TIR) domain of IL-1R9 shares 29% homology with IL-1R8-TIR, which is consistent with IL-1R8 not being a co-receptor for IL-38 [49].
We also studied recombinant IL-38 in acute gouty knee arthritis, the prototypical IL-1β mediated model of gouty arthritis. IL-38 treatment reduced macroscopic inflammation, decreased secretion of IL-1β, IL-6, and KC from the synovial membrane and reduced cell-associated IL-1β, IL-6 and KC in the synovial extract. We also studied MSU-crystal induced peritonitis. IL-38 treatment reduced plasma IL-6 and KC, and KC in the peritoneal lavage fluid. These finding were similar to those of anakinra in MSU-crystal induced peritonitis. We conclude that IL-38, similar to anakinra, may have therapeutic ultility in treating inflammation.
Next, we investigated an anti-inflammatory role for IL-38 in murine LPS-induced endotoxemia. Pretreatment with recombinant IL-38 reduced IL-6, TNFα, and KC, in plasma as well as in cultured ex vivo bone marrow cells. In sepsis patients, serum IL-38 concentrations were elevated compared to healthy controls, and higher levels of IL-38 were correlated with reduced leukocyte numbers, and release of IL-6 and TNFα [50]. In addition, an IL-38 neutralizing antibody impaired survival of mice subjected to LPS [50].
The inhibitory activity of IL-38 in vitro is lost at higher concentrations, in contrast to IL-1Ra, a prototypic receptor antagonist that shows greater reduction of inflammation as concentrations are increased [7]. In BMDC, we observed that the optimal concentration of IL-38 for inhibition of LPS-induced IL-6 release is in the low nanogram range of (1–10 ng/mL), whereas this inhibitory property is lost at the higher concentrations of 100 and 1000 ng/mL. There are two explanations for the atypical dose response for IL-38. First, we consider receptor promiscuity for which there is indeed already precedent [7], where higher concentrations of IL-38 may bind to other members of the IL-1 Receptor family beyond IL-1R6, for example IL-1R1 or IL-1R9 [9, 20]. Second, IL-38 could theoretically form homodimers, as spontaneous dimerization of IL-37 occurs [51] and is observed in primary human monocytes [40]. Recent studies reveal that IL-37 dimers prevent IL-37 monomers from suppressing inflammation [41, 52]. However, using analytical size-exclusion chromatraphy here, recombinant IL-38 does not form dimers and thus, we suggest that receptor promiscuity may underly the pro-inflammatory response of IL-38 using elevated concentrations. To unequivocally determine that IL-38 binds various receptors depending on the concentration, additional studies are required.
In summary, we report that systemic treatment with IL-38 dampens the local and systemic inflammatory response. This is in line with recombinant IL-38 alleviating the disease burden in mouse models of systemic lupus erythematosus, psoriasis, allergic asthma and cardiac remodeling after myocardial infarction [15, 44, 53, 54]. Taken together, IL-38 has significant potential to be developed into a therapeutic with the intention of treating immune and inflammatory diseases.
Supplementary Material
Supplemental Figure 1. IL-38 chromatography
Recombinant IL-38 migrates as a monomer on analytical size-exclusion. For comparison, the elution profile of purified IL-38 (red) is shown along with the elution profiles of ovalbumin (43 kDa, dashed black), carbonic anhydrase (29 kDa, black), and ribonucleaseA (13.7 kDa, grey).
Acknowledgements
The authors thank Carlo Marchetti, Tania Azam and Alberto Dinarello for helpful discussions.
Funding
These studies are supported by NIH Grant AI-15614 (to CAD) and The Interleukin Foundation. L.A.B.J. was supported by a Competitiveness Operational Program grant of the Romanian Ministry of European Funds (P_37_762, MySMIS 103587).
Abbreviations:
- BMDC
Bone Marrow Derived Dendritic Cells
- LAL
Limulus Amoebocyte Lysate
- LPS
Lipopolysaccharide
- MSU
Monosodium urate
- PBMC
Peripheral Blood Mononuclear Cells
- SCW
Streptococcus pyogenes cell wall
- WT
Wild Type
Footnotes
Competing Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lin H,Ho AS,Haley-Vicente D,Zhang J,Bernal-Fussell J,Pace AM,Hansen D,Schweighofer K,Mize NK,Ford JE,Cloning and characterization of IL-1HY2, a novel interleukin-1 family member,J Biol Chem 276(23) (2001)20597–602. [DOI] [PubMed] [Google Scholar]
- [2].Bensen JT,Dawson PA,Mychaleckyj JC,Bowden DW,Identification of a novel human cytokine gene in the interleukin gene cluster on chromosome 2q12–14,J Interferon Cytokine Res 21(11) (2001)899–904. [DOI] [PubMed] [Google Scholar]
- [3].Dinarello CA,Overview of the IL-1 family in innate inflammation and acquired immunity,Immunol Rev 281(1) (2018)8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dinarello CA,The IL-1 family of cytokines and receptors in rheumatic diseases,Nat Rev Rheumatol 15(10) (2019)612–32. [DOI] [PubMed] [Google Scholar]
- [5].Garraud T,Harel M,Boutet MA,Le Goff B,Blanchard F,The enigmatic role of IL-38 in inflammatory diseases,Cytokine Growth Factor Rev 39 (2018)26–35. [DOI] [PubMed] [Google Scholar]
- [6].Towne JE,Renshaw BR,Douangpanya J,Lipsky BP,Shen M,Gabel CA,Sims JE,Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36alpha, IL-36beta, and IL-36gamma) or antagonist (IL-36Ra) activity,J Biol Chem 286(49) (2011)42594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].van de Veerdonk FL,Stoeckman AK,Wu G,Boeckermann AN,Azam T,Netea MG,Joosten LA,van der Meer JW,Hao R,Kalabokis V,Dinarello CA,IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist,Proc Natl Acad Sci U S A 109(8) (2012)3001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].van de Veerdonk FL,Netea MG,New Insights in the Immunobiology of IL-1 Family Members,Front Immunol 4 (2013)167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mora J,Schlemmer A,Wittig I,Richter F,Putyrski M,Frank AC,Han Y,Jung M,Ernst A,Weigert A,Brune B,Interleukin-38 is released from apoptotic cells to limit inflammatory macrophage responses,J Mol Cell Biol 8(5) (2016)426–38. [DOI] [PubMed] [Google Scholar]
- [10].Han Y,Mora J,Huard A,da Silva P,Wiechmann S,Putyrski M,Schuster C,Elwakeel E,Lang G,Scholz A,Scholz T,Schmid T,de Bruin N,Billuart P,Sala C,Burkhardt H,Parnham MJ,Ernst A,Brune B,Weigert A,IL-38 Ameliorates Skin Inflammation and Limits IL-17 Production from gammadelta T Cells,Cell Rep 27(3) (2019)835–46. [DOI] [PubMed] [Google Scholar]
- [11].Takenaka SI,Kaieda S,Kawayama T,Matsuoka M,Kaku Y,Kinoshita T,Sakazaki Y,Okamoto M,Tominaga M,Kanesaki K,Chiba A,Miyake S,Ida H,Hoshino T,IL-38: A new factor in rheumatoid arthritis,Biochem Biophys Rep 4 (2015)386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Boutet MA,Bart G,Penhoat M,Amiaud J,Brulin B,Charrier C,Morel F,Lecron JC,Rolli-Derkinderen M,Bourreille A,Vigne S,Gabay C,Palmer G,Le Goff B,Blanchard F,Distinct expression of interleukin (IL)-36alpha, beta and gamma, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and Crohn’s disease,Clin Exp Immunol 184(2) (2016)159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Xu WD,Su LC,He CS,Huang AF,Plasma interleukin-38 in patients with rheumatoid arthritis,Int Immunopharmacol 65 (2018)1–7. [DOI] [PubMed] [Google Scholar]
- [14].Rahman P,Sun S,Peddle L,Snelgrove T,Melay W,Greenwood C,Gladman D,Association between the interleukin-1 family gene cluster and psoriatic arthritis,Arthritis Rheum 54(7) (2006)2321–5. [DOI] [PubMed] [Google Scholar]
- [15].Mercurio L,Morelli M,Scarponi C,Eisenmesser EZ,Doti N,Pagnanelli G,Gubinelli E,Mazzanti C,Cavani A,Ruvo M,Dinarello CA,Albanesi C,Madonna S,IL-38 has an anti-inflammatory action in psoriasis and its expression correlates with disease severity and therapeutic response to anti-IL-17A treatment,Cell Death Dis 9(11) (2018)1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rudloff I,Godsell J,Nold-Petry CA,Harris J,Hoi A,Morand EF,Nold MF,Brief Report: Interleukin-38 exerts antiinflammatory functions and is associated with disease activity in systemic lupus erythematosus,Arthritis Rheumatol 67(12) (2015)3219–25. [DOI] [PubMed] [Google Scholar]
- [17].Chu M,Chu IM,Yung EC,Lam CW,Leung TF,Wong GW,Wong CK,Aberrant expression of novel cytokine IL-38 and regulatory T lymphocytes in childhood asthma,Molecules 21(7) (2016)E933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yuan X,Li Y,Pan X,Peng X,Song G,Jiang W,Gao Q,Li M,IL-38 alleviates concanavalin A-induced liver injury in mice,Int Immunopharmacol 40 (2016)452–57. [DOI] [PubMed] [Google Scholar]
- [19].Dehghan A,Dupuis J,Barbalic M,Bis JC,Eiriksdottir G,Lu C,Pellikka N,Wallaschofski H,Kettunen J,Henneman P,Baumert J,Strachan DP,Fuchsberger C,Vitart V,Wilson JF,Pare G,Naitza S,Rudock ME,Surakka I,de Geus EJ,Alizadeh BZ,Guralnik J,Shuldiner A,Tanaka T,Zee RY,Schnabel RB,Nambi V,Kavousi M,Ripatti S,Nauck M,Smith NL,Smith AV,Sundvall J,Scheet P,Liu Y,Ruokonen A,Rose LM,Larson MG,Hoogeveen RC,Freimer NB,Teumer A,Tracy RP,Launer LJ,Buring JE,Yamamoto JF,Folsom AR,Sijbrands EJ,Pankow J,Elliott P,Keaney JF,Sun W,Sarin AP,Fontes JD,Badola S,Astor BC,Hofman A,Pouta A,Werdan K,Greiser KH,Kuss O,Meyer zu Schwabedissen HE,Thiery J,Jamshidi Y,Nolte IM,Soranzo N,Spector TD,Volzke H,Parker AN,Aspelund T,Bates D,Young L,Tsui K,Siscovick DS,Guo X,Rotter JI,Uda M,Schlessinger D,Rudan I,Hicks AA,Penninx BW,Thorand B,Gieger C,Coresh J,Willemsen G,Harris TB,Uitterlinden AG,Jarvelin MR,Rice K,Radke D,Salomaa V,Willems van Dijk K,Boerwinkle E,Vasan RS,Ferrucci L,Gibson QD,Bandinelli S,Snieder H,Boomsma DI,Xiao X,Campbell H,Hayward C,Pramstaller PP,van Duijn CM,Peltonen L,Psaty BM,Gudnason V,Ridker PM,Homuth G,Koenig W,Ballantyne CM,Witteman JC,Benjamin EJ,Perola M,Chasman DI,Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels,Circulation 123(7) (2011)731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].van de Veerdonk FL,de Graaf DM,Joosten LA,Dinarello CA,Biology of IL-38 and its role in disease,Immunol Rev 281(1) (2018)191–6. [DOI] [PubMed] [Google Scholar]
- [21].Garlanda C,Riva F,Polentarutti N,Buracchi C,Sironi M,De Bortoli M,Muzio M,Bergottini R,Scanziani E,Vecchi A,Hirsch E,Mantovani A,Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family,Proc Natl Acad Sci U S A 101(10) (2004)3522–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Joosten LA,Crisan TO,Azam T,Cleophas MC,Koenders MI,van de Veerdonk FL,Netea MG,Kim S,Dinarello CA,Alpha-1-anti-trypsin-Fc fusion protein ameliorates gouty arthritis by reducing release and extracellular processing of IL-1beta and by the induction of endogenous IL-1Ra,Ann Rheum Dis 75(6) (2016)1219–27. [DOI] [PubMed] [Google Scholar]
- [23].Garlanda C,Riva F,Veliz T,Polentarutti N,Pasqualini F,Radaelli E,Sironi M,Nebuloni M,Zorini EO,Scanziani E,Mantovani A,Increased susceptibility to colitis-associated cancer of mice lacking TIR8, an inhibitory member of the interleukin-1 receptor family,Cancer Res 67(13) (2007)6017–21. [DOI] [PubMed] [Google Scholar]
- [24].Molgora M,Supino D,Mantovani A,Garlanda C,Tuning inflammation and immunity by the negative regulators IL-1R2 and IL-1R8,Immunol Rev 281(1) (2018)233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Garlanda C,Dinarello CA,Mantovani A,The interleukin-1 family: back to the future,Immunity 39(6) (2013)1003–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Garlanda C,Riva F,Bonavita E,Gentile S,Mantovani A,Decoys and Regulatory “Receptors” of the IL-1/Toll-Like Receptor Superfamily,Front Immunol 4 (2013)180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nold-Petry CA,Lo CY,Rudloff I,Elgass KD,Li S,Gantier MP,Lotz-Havla AS,Gersting SW,Cho SX,Lao JC,Ellisdon AM,Rotter B,Azam T,Mangan NE,Rossello FJ,Whisstock JC,Bufler P,Garlanda C,Mantovani A,Dinarello CA,Nold MF,IL-37 requires the receptors IL-18Ralpha and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction,Nat Immunol 16(4) (2015)354–65. [DOI] [PubMed] [Google Scholar]
- [28].Costelloe C,Watson M,Murphy A,McQuillan K,Loscher C,Armstrong ME,Garlanda C,Mantovani A,O’Neill LA,Mills KH,Lynch MA,IL-1F5 mediates anti-inflammatory activity in the brain through induction of IL-4 following interaction with SIGIRR/TIR8,J Neurochem 105(5) (2008)1960–9. [DOI] [PubMed] [Google Scholar]
- [29].Cavalli G,Koenders M,Kalabokis V,Kim J,Tan AC,Garlanda C,Mantovani A,Dagna L,Joosten LA,Dinarello CA,Treating experimental arthritis with the innate immune inhibitor interleukin-37 reduces joint and systemic inflammation,Rheumatology (Oxford) 55(12) (2016)2220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li S,Neff CP,Barber K,Hong J,Luo Y,Azam T,Palmer BE,Fujita M,Garlanda C,Mantovani A,Kim S,Dinarello CA,Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8,Proc Natl Acad Sci U S A 112(8) (2015)2497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Roddy E,Choi HK,Epidemiology of gout,Rheumatic diseases clinics of North America 40(2) (2014)155–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Petite SE,Effectiveness of Anakinra in Acute Gout: A Retrospective Review of Initial and Refractory Therapy,Am J Ther 24(5) (2017)e633–4. [DOI] [PubMed] [Google Scholar]
- [33].Janssen CA,Oude Voshaar MAH,Vonkeman HE,Jansen T,Janssen M,Kok MR,Radovits B,van Durme C,Baan H,van de Laar M,Anakinra for the treatment of acute gout flares: a randomized, double-blind, placebo-controlled, active-comparator, non-inferiority trial,Rheumatology (Oxford) (2019)1344–52. [DOI] [PubMed] [Google Scholar]
- [34].Desmarais J,Chu CQ,Utility of Anakinra in Acute Crystalline Diseases: A Retrospective Study Comparing a University Hospital with a Veterans Affairs Medical Center,J Rheumatol 46(7) (2019)748–50. [DOI] [PubMed] [Google Scholar]
- [35].Vitale A,Cantarini L,Rigante D,Bardelli M,Galeazzi M,Anakinra treatment in patients with gout and type 2 diabetes,Clin Rheumatol 34(5) (2015)981–4. [DOI] [PubMed] [Google Scholar]
- [36].Rossi-Semerano L,Fautrel B,Wendling D,Hachulla E,Galeotti C,Semerano L,Touitou I,Kone-Paut I,Tolerance and efficacy of off-label anti-interleukin-1 treatments in France: a nationwide survey,Orphanet J Rare Dis 10 (2015)19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schlesinger N,Alten RE,Bardin T,Schumacher HR,Bloch M,Gimona A,Krammer G,Murphy V,Richard D,So AK,Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active-controlled, double-blind trials and their initial extensions,Ann Rheum Dis 71(11) (2012)1839–48. [DOI] [PubMed] [Google Scholar]
- [38].So A,De Meulemeester M,Pikhlak A,Yucel AE,Richard D,Murphy V,Arulmani U,Sallstig P,Schlesinger N,Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis: Results of a multicenter, phase II, dose-ranging study,Arthritis Rheum 62(10) (2010)3064–76. [DOI] [PubMed] [Google Scholar]
- [39].Joosten LA,Netea MG,Mylona E,Koenders MI,Malireddi RK,Oosting M,Stienstra R,van de Veerdonk FL,Stalenhoef AF,Giamarellos-Bourboulis EJ,Kanneganti TD,van der Meer JW,Engagement of fatty acids with Toll-like receptor 2 drives interleukin-1beta production via the ASC/caspase 1 pathway in monosodium urate monohydrate crystal-induced gouty arthritis,Arthritis Rheum 62(11) (2010)3237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nold MF,Nold-Petry CA,Zepp JA,Palmer BE,Bufler P,Dinarello CA,IL-37 is a fundamental inhibitor of innate immunity,Nat Immunol 11(11) (2010)1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Eisenmesser EZ,Gottschlich A,Redzic JS,Paukovich N,Nix JC,Azam T,Zhang L,Zhao R,Kieft JS,The E,Meng X,Dinarello CA,Interleukin-37 monomer is the active form for reducing innate immunity,Proc Natl Acad Sci U S A 116(12) (2019)5514–5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ge Y,Huang M,Wu Y,Dong N,Yao Y-M,Interleukin-38 protects against sepsis by augmenting immunosuppressive activity of CD4+CD25+ regulatory T cells,Journal of Cellular and Molecular Medicine 24(2) (2020)2027–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Harel M,Garraud T,Le Goff B,Blanchard F,IL-38 in arthritis maturation and degradation of this new IL-1 family anti-inflammatory cytokine,Ann Rheum Dis 77(Suppl 1) (2018)A38–9. [Google Scholar]
- [44].Sun X,Hou T,Cheung E,Iu TN,Tam VW,Chu IM,Tsang MS,Chan PK,Lam CW,Wong CK,Anti-inflammatory mechanisms of the novel cytokine interleukin-38 in allergic asthma,Cell Mol Immunol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Xie C,Yan W,Quan R,Chen C,Tu L,Hou X,Fu Y,Interleukin-38 is elevated in inflammatory bowel diseases and suppresses intestinal inflammation,Cytokine 127 (2020)154963. [DOI] [PubMed] [Google Scholar]
- [46].Boutet MA,Najm A,Bart G,Brion R,Touchais S,Trichet V,Layrolle P,Gabay C,Palmer G,Blanchard F,Le Goff B,IL-38 overexpression induces anti-inflammatory effects in mice arthritis models and in human macrophages in vitro,Ann Rheum Dis 76(7) (2017)1304–12. [DOI] [PubMed] [Google Scholar]
- [47].Nawara M,Klapecki J,Borg K,Jurek M,Moreno S,Tryfon J,Bal J,Chelly J,Mazurczak T,Novel mutation of IL1RAPL1 gene in a nonspecific X-linked mental retardation (MRX) family,Am J Med Genet A 146a(24) (2008)3167–72. [DOI] [PubMed] [Google Scholar]
- [48].Gao X,Xi G,Niu Y,Zhang S,Fu R,Zheng Z,Zhang K,Lv S,He H,Xue M,Zhang F,A study on the correlation between IL1RAPL1 and human cognitive ability,Neurosci Lett 438(2) (2008)163–7. [DOI] [PubMed] [Google Scholar]
- [49].Khan JA,Brint EK,O’Neill LA,Tong L,Crystal structure of the Toll/interleukin-1 receptor domain of human IL-1RAPL,J Biol Chem 279(30) (2004)31664–70. [DOI] [PubMed] [Google Scholar]
- [50].Xu F,Lin S,Yan X,Wang C,Tu H,Yin Y,Cao J,Interleukin 38 protects against lethal sepsis,J Infect Dis 218(7) (2018)1175–84. [DOI] [PubMed] [Google Scholar]
- [51].Kumar S,Hanning CR,Brigham-Burke MR,Rieman DJ,Lehr R,Khandekar S,Kirkpatrick RB,Scott GF,Lee JC,Lynch FJ,Gao W,Gambotto A,Lotze MT,Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production,Cytokine 18(2) (2002)61–71. [DOI] [PubMed] [Google Scholar]
- [52].Ellisdon AM,Nold-Petry CA,D’Andrea L,Cho SX,Lao JC,Rudloff I,Ngo D,Lo CY,Soares da Costa TP,Perugini MA,Conroy PJ,Whisstock JC,Nold MF,Homodimerization attenuates the anti-inflammatory activity of interleukin-37,Sci Immunol 2(8) (2017). [DOI] [PubMed] [Google Scholar]
- [53].Chu M,Tam LS,Zhu J,Jiao D,Liu H,Cai Z,Dong J,Kai Lam CW,Wong CK,In vivo anti-inflammatory activities of novel cytokine IL-38 in Murphy Roths Large (MRL)/lpr mice,Immunobiology 222(3) (2017)483–93. [DOI] [PubMed] [Google Scholar]
- [54].Wei Y,Lan Y,Zhong Y,Yu K,Xu W,Zhu R,Sun H,Ding Y,Wang Y,Zeng Q,Interleukin-38 alleviates cardiac remodelling after myocardial infarction,J Cell Mol Med (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. IL-38 chromatography
Recombinant IL-38 migrates as a monomer on analytical size-exclusion. For comparison, the elution profile of purified IL-38 (red) is shown along with the elution profiles of ovalbumin (43 kDa, dashed black), carbonic anhydrase (29 kDa, black), and ribonucleaseA (13.7 kDa, grey).