Abstract
PTSD in adolescents is common and debilitating. In contrast to adult PTSD, relatively little is known about the neurobiology of adolescent PTSD, nor how current treatments may alter adolescent neurodevelopment to allow recovery from PTSD. Improving our understanding of biological mechanisms of adolescent PTSD, taken in the context of neurodevelopment, is crucial for developing novel and personalized treatment approaches. In this review, we highlight prevailing constructs of PTSD and current findings on these domains in adolescent PTSD. Notably, little data exist in adolescent PTSD for prominent adult PTSD constructs, including threat learning and attentional threat bias. Most work to date has examined general threat processing, emotion regulation, and their neural substrates. These studies suggest that adolescent PTSD, while phenomenologically similar to adult PTSD, shows unique neurodevelopmental substrates which may impair recovery, but could also be targeted in the context of adolescent neuroplasticity to improve outcomes. Both cross-sectional and longitudinal data suggest abnormal frontolimbic development compared to typically developing youth, a pattern which may differ from resilient youth. Whether current treatments such as trauma-focused psychotherapy engage these targets and restore healthy neurodevelopment remains an open question. We end our review by highlighting emerging areas and knowledge gaps that could be addressed to better characterize the biology underlying adolescent PTSD. Emerging studies in computational modeling of decision making, caregiver-related transmission of traumatic stress, and other areas may offer new targets which could harness adolescent neurobehavioral plasticity to improve resilience and recovery for some of our most vulnerable youth.
Keywords: trauma, PTSD, neurodevelopment, adolescence, neuroimaging, resilience
Introduction
Approximately two-thirds of youth are exposed to trauma by late adolescence, and many develop PTSD as a result (1). By age 18, 8% of traumatized youth have met criteria for a diagnosis of PTSD, with numbers rising up to 40% in cases of sexual abuse and assault (1). In addition to the psychological suffering imposed, PTSD is associated with lower academic achievement and high rates of comorbidity including anxiety and depressive disorders (2). Strikingly, PTSD carries the highest risk of all mental illnesses for first suicide attempt in adolescents and young adults (3). Childhood trauma and PTSD also impart tremendous societal cost in terms of health care utilization and financial outlay, costing the United States an estimated $2 trillion annually (4,5). Current treatments for adolescent PTSD, which rely primarily on trauma-focused cognitive therapy, achieve only small to moderate effect sizes (6,7), leaving many youth unrecovered even if they are able to access skilled therapists. Remarkably, there are currently no evidence-based pharmacological options for treating adolescent PTSD. While the aforementioned therapies target presumed domains of dysfunction in adolescent PTSD, advancing our neurobiological understanding of the illness will be critical for tailoring current treatments and developing novel interventions for affected adolescents.
In this review, we aim to summarize our current understanding of the neurodevelopmental substrates of adolescent PTSD. However, PTSD is not a biological construct in and of itself. Thus, dysfunction is best understood across a constellation of cognitive, emotional, and biological systems. Accordingly, we focus the review on neurodevelopment in systems most commonly implicated in PTSD (e.g., emotion regulation). We also discuss emerging areas of study, including large-scale neural network approaches, computational modeling of decision making and caregiver transmission of traumatic stress. Finally, we consider how findings in these domains may inform the prevention and treatment of adolescent PTSD. We also refer readers to Supplement, which contains expanded discussion of prominent constructs and emerging areas implicated in adolescent PTSD.
Developmental considerations: Adolescence as a period of biological change and reorganization
Adolescence is characterized by dramatic changes in physiological and neuroendocrine systems, along with reorganization of neural systems subserving executive function, socioemotional processing, and emotion regulation. A more complete discussion of normative physiological change can be found elsewhere in this special issue (8). Pubertal onset is known to be associated with altered sensitivity of subcortical regions, notably the amygdala and striatum, to emotional stimuli albeit with mixed findings (9,10). This, coupled with the more prolonged development of frontoparietal regions involved in cognitive control and emotion regulation, is thought to underlie, in part, the rapid increase in affective disorders in adolescence (11). PTSD is no exception, in that it shows a marked increase in prevalence through adolescence (12). However, this has been difficult to separate from the concomitant increase in trauma exposure in adolescence (1,13), which may itself be driven in part by social reorienting to peers and increased social risk taking [see this issue (8,14)]. Sex hormones also influence the development of circuitry underlying cognitive-emotional interactions, though in nuanced ways (15,16), and which may contribute to the characteristic greater PTSD prevalence (2–3 fold) in adolescent females compared to males. As we explore neurodevelopmental substrates implicated in adolescent PTSD, we attempt to incorporate both age and pubertal influences on these systems in ways that may heighten PTSD risk, contribute to sex differences, and impact potential treatment response for adolescent PTSD.
Neural correlates of PTSD in adolescents
In this section, we briefly summarize neuroimaging findings in adolescent PTSD with an emphasis on neurodevelopment in emotion processing circuitry. However, an important question remaining in the field is whether these alterations represent transdiagnostic risk mechanisms for trauma-induced psychopathology, or whether certain neural substrates are specific to adolescent PTSD. We refer readers to recent reviews on this topic (17–19). While we focus here mainly on univariate investigations of brain function, recent studies have begun characterizing network-level alterations in pediatric PTSD (see Supplement).
The prefrontal cortex, amygdala, and hippocampus are implicated in numerous cognitive processes, but in the context of trauma and PTSD have largely been investigated with respect to constructs of threat learning and extinction, threat reactivity, and emotion regulation (see Supplement). As noted above, relative delays in prefrontal versus subcortical maturation (20,21) are thought to underlie, in part, the tendency for increased emotional reactivity in adolescence. Structural studies show relatively early maturation of amygdala and hippocampus which nonetheless continue into adolescence (21,22). However, prefrontal cortical regions show structural maturation well into adolescence and early adulthood, characterized by cortical thinning (20). Accordingly, functional imaging studies show that amygdala reactivity to negative faces and images decreases with age in adolescence (23–27), and is accompanied by greater structural and functional connectivity with the medial prefrontal cortex (mPFC) in particular (23,24,27). These normative patterns likely underlie, in part, decreased reactivity to negative content and enhanced emotion-regulation capacity through adolescence (17). Notably, these neurodevelopmental patterns are embedded in the larger process of adolescent brain remodeling characterized by changes in association, limbic, and subcortical circuits subserving higher order processes (e.g., executive function, social cognition, and mentalizing) (28,29).
Structural brain studies most consistently show reduced gray matter volume in the ventromedial (vm)PFC, and either reduced or age-related decline in hippocampal volume in adolescent PTSD (30,31). In contrast, traumatized adolescents without PTSD show increased hippocampal volume and maintained vmPFC volume (32). In a longitudinal extension of our prior work, we examined structural brain development over one year in adolescents with PTSD compared to typically developing (TD) adolescents. Here, we found stable reductions in vmPFC and ventrolateral (vl)PFC volume in adolescent PTSD (33). The vmPFC has diverse functions, and in the context of PTSD, is notable for its role in stimulus valuation and inhibition of threat responses and amygdala reactivity (34). The vlPFC has also been heavily implicated in emotion regulation through its role in attentional control, response inhibition, and emotion perception (35–37), and has direct anatomical connections to the amygdala (38). Notably, reduced volume in both vmPFC and vlPFC were associated with greater symptom severity in adolescents with PTSD. Finally, we found evidence of abnormal development in the dorsolateral (dl)PFC, characterized by a normative decrease in volume in TD youth, but absence of decline in adolescents with PTSD. Given the role of the dlPFC in emotion regulation and particularly reappraisal (37,39), the lack of dlPFC volume change could represent delayed development contributing to the persistence of PTSD. Alternatively, absent dlPFC volume change could serve to maintain plasticity in this key regulatory region while illness is ongoing. Further studies will be needed to better understand the functional and behavioral correlates of abnormal gray matter structure and development in adolescent PTSD, and whether such patterns differ by sex and pubertal stage.
In functional brain studies in adolescent trauma and PTSD, most work has focused on general threat processing and reactivity, with some additional study under resting state conditions. As noted in prior reviews (17,40,41), childhood trauma and adversity are broadly associated with increased amygdala reactivity to negative stimuli and decreased resting functional coupling between the amygdala, hippocampus and vmPFC irrespective of PTSD status (42,43). Such changes may serve an adaptive response allowing, for example, enhanced threat detection and learning (17). Conversely, maintained or enhanced coupling between the amygdala and dorsal/lateral prefrontal regions involved in emotional appraisal and regulation may be an important factor for resilience in trauma-exposed adolescents (44,45). Supporting this notion, childhood trauma is associated with increased prefrontal recruitment and amygdala-prefrontal coupling during emotion regulation in healthy adolescents or when adjusting for affective symptoms (44,46–49). In contrast, adolescents with PTSD show reduced prefrontal engagement, as well as reduced coupling between regulatory prefrontal regions (vlPFC, dorsomedial [dm]PFC) and amygdala (17).
Importantly, adolescence may be a key period determining risk and resilience trajectories of prefrontal-amygdala and -hippocampal development. In our prior work, we identified age-related abnormalities in prefrontal-amygdala function in adolescent PTSD. Specifically, while TD adolescents exhibit decreased amygdala reactivity and increased amygdala-vmPFC coupling to emotional stimuli with age, adolescents with PTSD show the reverse pattern (17), exhibiting a neural profile similar to adult PTSD by late adolescence. Our recent longitudinal extension further supports the notion of abnormal prefrontal-amygdala and -hippocampal development in adolescent PTSD. Specifically, while TD adolescents show increased connectivity between vmPFC-amygdala, vlPFC-amygdala, and dlPFC-hippocampus over one year, adolescents with PTSD show the reverse pattern and independent of pubertal stage (33).
Declining dlPFC-hippocampus connectivity was further associated with greater symptom severity, suggesting that ongoing disruptions in prefrontal-amygdala and -hippocampal development may contribute to persistence or worsening of PTSD in adolescence. We next discuss how these circuits may be involved in treatment and remission in adolescent PTSD.
Longitudinal imaging studies examining treatment and remission of adolescent PTSD
To date, few studies have examined the neural correlates of treatment intervention or remission in adolescent PTSD. In a preliminary study of TF-CBT in adolescent girls with PTSD (N=23), we found that more differential activation of the amygdala to threat vs. neutral faces pre-treatment predicted greater symptom reduction over a 12-week course of TF-CBT (50). Additionally, this study showed that pre- to post-treatment change in suppression during reappraisal of negative images was associated with symptom reduction (51). While this study lacked a comparison group, these initial findings suggest that enhanced neural differentiation of threat-neutral content prior to treatment may allow for greater use of TF-CBT skills including trauma narrative exposure, while functioning in this network itself may be a potential target of TF-CBT.
A more recent study examined the impact of TF-CBT on neural function in adolescents with PTSD relative to TD adolescents (N=40) at baseline and post-treatment (5 months). Using a face processing task, only the posterior cingulate/precuneus showed a group by time effect using whole-brain analysis. Here, independent of face emotion, youth with PTSD showed reduced activation over the treatment course, which further correlated with symptom reduction (52). In ROI-based analyses, youth with PTSD also showed reductions over time in hippocampus, amygdala, midcingulate cortex (MCC), dmPFC, and vlPFC activation to neutral faces relative to TD adolescents. Of these, change in hippocampus and MCC activation were associated with total PTSD symptom reduction. While this study lacked a PTSD treatment comparison group, these findings suggest that symptom improvement in adolescents with PTSD may be mediated in part by reduced engagement of prefrontal-amygdala and -hippocampal circuitry to neutral faces, potentially enhancing threat discrimination.
Finally, we recently reported structural brain correlates of persistence and remission of adolescent PTSD in a naturalistic longitudinal study (53). Here, we examined cortical morphometry and subcortical volume change in adolescents with persistent or remitted PTSD at one year relative to TD adolescents (N=55). Adolescents with persistent PTSD showed contraction of vlPFC surface area compared to both remitters and TD. In contrast, PTSD remission was associated with expansion of frontal pole surface area and vmPFC thickness over time. Across clinical groups, vmPFC thickness was inversely associated with symptom severity. While limited in sample size, these findings suggest that structural plasticity, particularly in prefrontal regions involved in emotion regulation, may be responsible for PTSD recovery in adolescents. The frontal pole and vmPFC are notable given findings in a controlled study in adult PTSD demonstrating that prolonged exposure enhanced frontopolar activation and connectivity with the vmPFC during cognitive reappraisal, which were further associated with symptom improvement (54). However, to more precisely define treatment and recovery substrates in adolescent PTSD, future neuroimaging clinical trials are warranted incorporating an expanded sample size, multimodal imaging, and a treatment control arm to determine treatment-specific biomarkers.
Trauma sensitive periods, stress acceleration, and implications for adolescent PTSD
At present, little is known about developmental trauma sensitive periods in risk for adolescent PTSD, nor how childhood trauma may alter the pace of brain maturation to confer such risk. This area is also confounded with increasing trauma load as youth age, leaving an open question of whether to interpret any differences as trauma sensitive periods or cumulative stress effects. However, neuroimaging studies of trauma exposure and adolescent PTSD offer potential clues to differential brain maturation with trauma and PTSD. In our prior cross-sectional work, youth with PTSD paradoxically show lower amygdala reactivity, greater dmPFC activation, and greater amygdala-vmPFC connectivity at younger ages (<15 years), a pattern which appears to reverse by late adolescence and independent of age at index trauma, PTSD duration, or pubertal stage (26,55). A retrospective study in adults also found that preadolescent abuse was associated with blunting of amygdala reactivity, while adolescent abuse was associated with increased amygdala reactivity (56). Additionally, studies of youth exposed to environmental adversity suggest accelerated functional brain maturation including prefrontal-amygdala connectivity (57,58). These findings suggest a potential stress acceleration in the development of circuitry supporting socioemotional processing and regulation (18). We have speculated that such compensatory neurodevelopment may be degraded by adolescence in vulnerable youth with additional trauma exposure (17). While further study is clearly needed to map neurodevelopmental responses to trauma and risk for adolescent PTSD, these findings raise the intriguing possibility of unique biotypes of adolescent PTSD dependent on trauma characteristics (19) including age of trauma exposure, cumulative stress effects, type of trauma exposure, as well as early stress acceleration responses which may alter the development of socioemotional regulatory systems.
A biopsychosocial model of adolescent PTSD: Emerging directions
The current review is by no means exhaustive, but hopefully highlights three main observations. First, there is a general lack of research specifically conducted in adolescent PTSD and an overreliance on models and findings derived from the adult PTSD literature (see Supplement). Second, most biological studies of adolescent PTSD have not been placed in the normative context of adolescent neurodevelopment, which is particularly important given the enhanced neuroplasticity characteristic of this period. Third, PTSD likely impacts multiple functional domains, yet most studies to date have focused on general threat processing and emotion regulation (see Supplement for additional constructs). The specific functional domains affected are likely to be different for each individual, underscoring the fact that PTSD itself is not a unitary or biological construct.
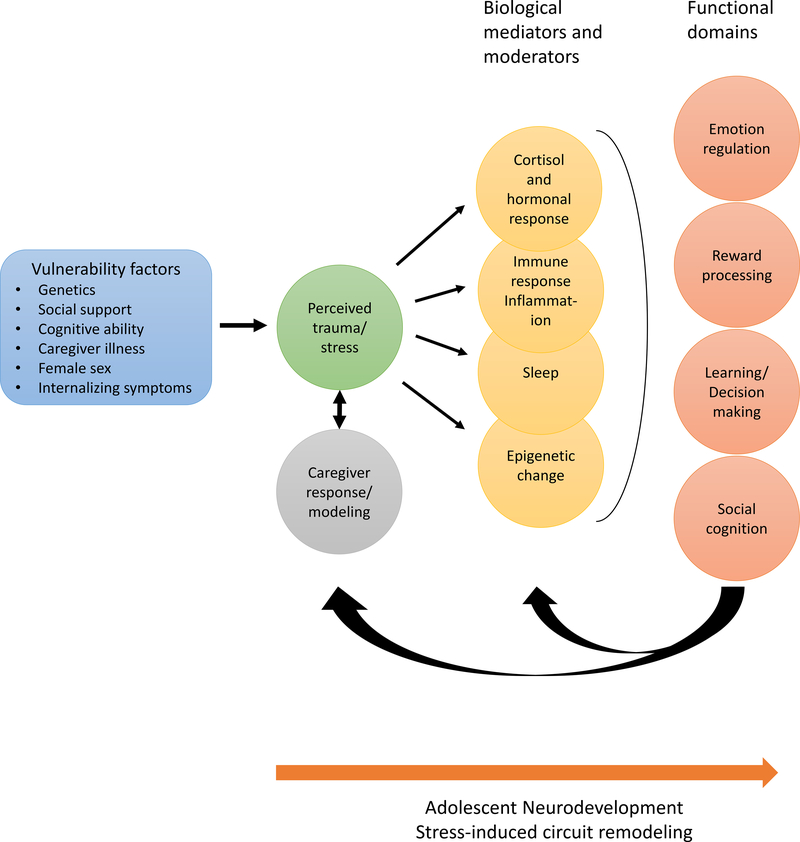
In Figure 1, we propose a working model of adolescent PTSD that incorporates multiple socioenvironmental influences, mediating biological systems, and functional domains relevant for understanding and treating adolescent PTSD, though it is by no means an exhaustive model. Consistent with dimensional approaches to psychopathology, we purposefully leave out a final pathway towards a diagnostic construct. Instead, we suggest that the impact of trauma and PTSD on these functional domains could be more informative for elucidating the neurobiology of adolescent PTSD and generating precision medicine approaches. In this model, known vulnerability factors such as genetic variation, low social support, female sex, or pre-existing internalizing symptoms interact (along with the type and severity of trauma) to influence an adolescent’s perception of a traumatic event (59,60). Further, interactive influences come from responses and modeling of caregivers (and peers) which may ameliorate or worsen an adolescent’s perception of trauma. Perceived trauma may then activate multiple biological systems involved in the stress response including release of cortisol and proinflammatory cytokines (61), altered sleep function (62), and coincident epigenetic changes (63) that may further the stress response. Activation of these biological pathways, in turn, can negatively impact neural function and development of systems underlying emotion regulation (17), reward processing (64), learning and decision making (14,64), and social cognition (65). Functional compromise in these domains, such as heightened threat reactivity, reduced emotion regulation and reward learning, poor extinction learning, and misinterpretation of social information may subsequently exacerbate perceived trauma/stress and negatively impact the caregiver relationship or caregiver modeling in a vicious cycle. By the same token, maintained functioning in these domains and caregiver/peer influence could serve to ameliorate the adolescent’s trauma perception and hasten recovery in biological systems. We again refer readers to Supplement for expanded discussion of biological systems and functional domains implicated in the above model. While most of these sociobiological systems and functional domains have received limited or no study in adolescent PTSD, below we highlight emerging research which could offer fruitful targets for intervention and improving outcomes for adolescent PTSD.
Figure 1 :
Psychosocial and biological model linking early life trauma exposure to multiple systems and functional domains likely contributing to risk or resilience for adolescent PTSD. Pre-trauma factors such as genetic loading and social support (far left) moderate the impact of trauma (i.e. perceived trauma) on youth. In the peritraumatic period, caregiver (as well as peer) responses and emotional modeling further influence adolescent perception of the trauma and sense of safety in a reciprocal manner. The cumulative or ongoing perceived threat may then impact multiple biological systems in youth, which in turn impact neurodevelopmental processes involving multiple functional domains (far right). In an iterative feedback loop, changes in functional domains may ameliorate or exacerbate biological stress response systems such as sleep biology or inflammation, as well as caregiver and adolescent perceived threat and emotion regulation. This feedback loop may again ameliorate or exacerbate changes in biological stress response systems. With exception of emotion regulation, most of the biological systems and functional domains listed have received little study in adolescent PTSD.
Learning and decision making
Adolescence is characterized by changes in learning and decision making that may have important implications for understanding adolescent PTSD [this issue (14,66)], such as increased risky decision-making during social or emotional contexts (67,68). The burgeoning field of computational psychiatry may shed light on important mechanisms of learning and decision-making in early life trauma and adolescent PTSD. In contrast to standard laboratory tasks that present stimuli to which participants can only react and not interact (e.g., emotional face viewing), modeling 1) how individuals make decisions about when, whether, and how to pursue reward and avoid threat, and 2) how individuals learn from experience to update expectations about reward and threat, may provide a more realistic laboratory paradigm for understanding the development and treatment of PTSD. Mathematical modeling of decision-making during these tasks formalizes cognitive hypotheses about behavior that can be explicitly quantified and tested against alternative models. As a recent example, we demonstrated that increased trauma load in adolescent girls was associated with impaired reward learning performance, greater variability in action selection, and decreased encoding of negative reward prediction errors in the salience network during decision-making (69,70). In adult PTSD, recent computational modeling work (71,72) suggests that increased PTSD severity is related to heightened encoding of associability [a dynamic learning rate reflecting increased attentional salience in response to volatility in the learning context (73)] in the anterior insula, ventral striatum, and amygdala. It is not currently clear how this modeling work interacts with normative neurodevelopment occurring during adolescence and if similar results would be expected in adolescent PTSD. An additional domain that will be important to characterize is how adolescents with PTSD make decisions about reward in the context of threat [approach-avoidance conflict learning (74)]. Specifically, the mechanisms by which adolescents prioritize sacrificing reward (e.g., peer interaction) for the sake of avoiding potential threat/trauma reminders presumably involves a complex interaction between higher-order goals and beliefs, valuations of reward, and expectations for threat. Relatedly, how adolescents incorporate signals from caregivers (or peers, see also below) in their decision-making will be important for fully characterizing the mechanisms underlying development and maintenance of adolescent PTSD.
Additionally, whereas most prior work has utilized relatively simplistic models of learning (e.g., Rescorla-Wagner models), emerging work in computational neuroscience emphasizes model-based decision-making strategies (75–78), in which individuals form abstract cognitive maps of a learning environment to inform prospective decision-making. Particularly relevant for treatment of PTSD with exposure therapy, classic model-free conceptualizations (e.g., Rescorla-Wagner models) do not explain return of fear following extinction (75,79–81). However, model-based theories readily explain this phenomenon and thereby implicate higher-order learning mechanisms (e.g., frontoparietal network) in threat extinction and exposure therapy. Incorporating model-based decision-making approaches into future research in adolescent PTSD may provide better characterization of nuanced biases in decision-making with potential for rapid clinical translation in this population.
Caregiver transmission of traumatic stress
Caregiver transmission of traumatic stress to the child involves both behaviorally modeled and genetic influences. Regarding modeling, caregivers are a vital source of information on numerous domains including social cognition, emotion regulation, and threat-safety discrimination. Thus, caregiver function and modeling are likely to have a major impact on PTSD risk in adolescents. For example, parental anxiety has direct environmental transmission to their adolescent offspring (82), which can be mitigated by parent coaching (83). Parental anxious rearing also mediates the effects of stressful life events on youth anxiety through early adolescence (84). More specific to PTSD, parental PTSD is associated with child distress and behavior problems and altered HPA axis functioning, particularly when both parent and child have been exposed to interpersonal violence (85,86). Furthermore, maternal emotion dysregulation increases risk for child PTSD symptoms (87), while lower levels of parent distress and PTSD following a child’s trauma predict more favorable outcomes for the child (88). Finally, in TF-CBT, which incorporates both caregiver and child, improvements in parent distress and symptomatology mediate broad improvements in internalizing and externalizing symptoms in youth with PTSD (89,90). While an early area of research, these studies suggest that caregiver modeling is likely to have a significant impact on shaping adolescent development of processes including emotion regulation and threat-safety discrimination to influence both risk for and progression of PTSD in adolescents. However, to date studies have focused primarily on correlation of caregiver-child subjective reports using cross-sectional designs. While admittedly a complex area of study, our understanding of caregiver modeling and PTSD risk in adolescence could be enhanced through longitudinal designs incorporating bidirectional influence of caregiver and child function (following prior literature examining caregiver-child interactions), and utilizing more objective measures of these functional domains to assess caregiver influence on disease course in adolescent PTSD. For example, studies could test how well psychophysiological measures of caregiver emotion regulation or threat-safety discrimination “transfer” to their adolescent, and whether these processes are maladaptive in the context of caregiver and/or child psychopathology. Finally, genetic transmission of traumatic stress has gained increasing recognition, with studies demonstrating epigenetic alterations particularly in child cortisol and glucocorticoid pathways beginning prenatally (91). Thus, future studies would ideally capture both parental modeling as well as genetic pathways to more fully understand intergenerational transmission of traumatic stress and risk for PTSD in adolescence.
Implications for intervention
Altogether, the studies and models posited above point to an urgent need to better understand the underlying neurobiology of adolescent PTSD and translate such knowledge into neuroscience-guided treatments. With extant studies, there are already a number of salient points regarding treatment implications for adolescent PTSD. First, neurobiological studies indicate that adolescent PTSD is not simply a recapitulation of adult PTSD – there are underlying neurodevelopmental processes such as reduced threat extinction, altered risk tolerance, and decision making that need to be considered in tailoring treatment for adolescents with PTSD. Treatments may therefore need to be properly tailored for adolescents by, for example, increasing or augmenting the number of exposure sessions in psychotherapy, increasing focus on emotion regulation capacity (e.g. CBT, mindfulness), and more explicitly incorporating peer support in treatment to enhance perceived social support. Second, PTSD itself is not a biological or unitary construct. Interventions therefore need to consider actual domains of dysfunction rather than trying to target a syndrome. Ideally, we would clinically profile an adolescent in every system/domain shown in Figure 1 and target those areas accordingly. This could mean, for example, specifically targeting the inflammatory response, augmenting sleep, and improving decision making abilities for a given youth. Third, neuroimaging studies have identified key circuits, notably the dlPFC, that could be targeted in neuromodulation trials to determine whether augmenting this circuit can improve clinical outcomes. Fourth, studies to date suggest that while adolescents with PTSD show a pernicious neurodevelopmental trajectory, they also remain in a window of increased neuroplasticity. Future trials would be warranted to examine the developmental period of intervention (e.g. early vs. late adolescence vs. adult) to determine unique developmental influences and guide treatment tailoring and policy. Fifth, and of no surprise to clinicians treating adolescents, the socioenvironmental context needs to be more fully considered in adolescent PTSD. Caregivers are key in this equation even for adolescents. Studies suggest that parenting behaviors are an important predictor of adolescent PTSD following trauma (92), while caregiver depression moderates psychotherapy outcomes for both adolescent depression and PTSD (93,94). Social support also appears to reduce the effect that adversity has on neural indices of threat processing and emotion regulation in youth (95), suggesting that interventions targeting the family system could have both behavioral and neurodevelopment benefits for adolescents suffering from PTSD.
Conclusion
In summary, adolescent PTSD remains a complicated disorder affecting many adolescents and portends poor outcomes well into adulthood. Mitigating the effects of trauma and PTSD in adolescents will require further investment in longitudinal, neurobiological research assessing multiple functional domains. Given the inherent difficulties in recruiting and studying trauma-exposed youth, our field would benefit from a consortium-based approach to expand sample sizes needed to more fully address trauma heterogeneity, neurodevelopmental patterns of risk and resilience, and sensitive periods for both trauma impact and intervention. Such an effort is currently underway (41) and, coupled with individual research programs, promises to accelerate discovery and treatment for some of our most vulnerable youth.
Supplementary Material
Acknowledgements and financial disclosures
Dr. Herringa’s research is supported by the National Institute of Mental Health R01MH117141 and R01MH115910. Prior funding for work described here also comes from the NIMH (K08MH100267), the American Academy of Child & Adolescent Psychiatry, the Brain and Behavior Research Foundation, and the University of Wisconsin Institute for Clinical and Translational Research (NIH/NCATS UL1TR000427). Dr. Cisler’s research is supported by NIMH grants MH119132 and MH108753.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McLaughlin KA, Koenen KC, Hill ED, Petukhova M, Sampson NA, Zaslavsky AM, Kessler RC (2013): Trauma exposure and posttraumatic stress disorder in a national sample of adolescents. J Am Acad Child Adolesc Psychiatry 52: 815–830.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warshaw MG, Fierman E, Pratt L, Hunt M, Yonkers KA, Massion AO, Keller MB (1993): Quality of life and dissociation in anxiety disorder patients with histories of trauma or PTSD. Am J Psychiatry 150: 1512–1516. [DOI] [PubMed] [Google Scholar]
- 3.Miche M, Hofer PD, Voss C, Meyer AH, Gloster AT, Beesdo-Baum K, Lieb R (2018): Mental disorders and the risk for the subsequent first suicide attempt: results of a community study on adolescents and young adults. Eur Child Adolesc Psychiatry 27: 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson C, Florence C, Klevens J (2018): The economic burden of child maltreatment in the United States, 2015. Child Abuse Negl 86: 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merrick MT, Ford DC, Ports KA, Guinn AS, Chen J, Klevens J, et al. (2019): Vital Signs: Estimated Proportion of Adult Health Problems Attributable to Adverse Childhood Experiences and Implications for Prevention - 25 States, 2015–2017. MMWR Morb Mortal Wkly Rep 68: 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutermann J, Schwartzkopff L, Steil R (2017): Meta-analysis of the Long-Term Treatment Effects of Psychological Interventions in Youth with PTSD Symptoms. Clin Child Fam Psychol Rev 20: 422–434. [DOI] [PubMed] [Google Scholar]
- 7.Morina N, Koerssen R, Pollet TV (2016): Interventions for children and adolescents with posttraumatic stress disorder: A meta-analysis of comparative outcome studies. Clin Psychol Rev 47: 41–54. [DOI] [PubMed] [Google Scholar]
- 8.Pfeifer JH (n.d.): Social development, brain development, and psychopathology in adolescence. Biological Psychiatry. [Google Scholar]
- 9.Vijayakumar N, Op de Macks Z, Shirtcliff EA, Pfeifer JH (2018): Puberty and the human brain: Insights into adolescent development. Neurosci Biobehav Rev 92: 417–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai J, Scherf KS (2019): Puberty and functional brain development in humans: Convergence in findings? Developmental Cognitive Neuroscience 39: 100690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ladouceur CD (2012): Neural systems supporting cognitive-affective interactions in adolescence: the role of puberty and implications for affective disorders. Front Integr Neurosci 6: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merikangas KR, He J-P, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. (2010): Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 49: 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall AD (2016): Developmental Timing of Trauma Exposure Relative to Puberty and the Nature of Psychopathology Among Adolescent Girls. J Am Acad Child Adolesc Psychiatry 55: 25–32.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somerville LH (n.d.): Risk-taking and the adolescent brain. Biological Psychiatry. [Google Scholar]
- 15.Sisk CL, Zehr JL (2005): Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol 26: 163–174. [DOI] [PubMed] [Google Scholar]
- 16.Laube C, van den Bos W, Fandakova Y (2020): The relationship between pubertal hormones and brain plasticity: Implications for cognitive training in adolescence. Dev Cogn Neurosci 42: 100753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herringa RJ (2017): Trauma, PTSD, and the Developing Brain. Curr Psychiatry Rep 19: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaughlin KA, Colich NL, Rodman AM, Weissman DG (2020): Mechanisms linking childhood trauma exposure and psychopathology: a transdiagnostic model of risk and resilience. BMC Medicine 18: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohodes EM, Kitt ER, Baskin-Sommers A, Gee DG (2020): Influences of early-life stress on frontolimbic circuitry: Harnessing a dimensional approach to elucidate the effects of heterogeneity in stress exposure. Developmental Psychobiology n/a. 10.1002/dev.21969 [DOI] [PubMed] [Google Scholar]
- 20.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. (2004): Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101: 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, Nishijo H (2012): Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS ONE 7: e46970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. (1996): Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex 6: 551–560. [DOI] [PubMed] [Google Scholar]
- 23.Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. (2013): A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci 33: 4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vink M, Derks JM, Hoogendam JM, Hillegers M, Kahn RS (2014): Functional differences in emotion processing during adolescence and early adulthood. Neuroimage 91: 70–76. [DOI] [PubMed] [Google Scholar]
- 25.Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin R, et al. (2017): The transition from childhood to adolescence is marked by a general decrease in amygdala reactivity and an affect-specific ventral-to-dorsal shift in medial prefrontal recruitment. Dev Cogn Neurosci 25: 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keding TJ, Herringa RJ (2016): Paradoxical Prefrontal-Amygdala Recruitment to Angry and Happy Expressions in Pediatric Posttraumatic Stress Disorder. Neuropsychopharmacology 41: 2903–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swartz JR, Carrasco M, Wiggins JL, Thomason ME, Monk CS (2014): Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: a multi-modal imaging approach. Neuroimage 86: 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasa F, Romero-Garcia R, Kitzbichler MG, Seidlitz J, Whitaker KJ, Vaghi MM, et al. (2020): Conservative and disruptive modes of adolescent change in human brain functional connectivity. Proc Natl Acad Sci USA. 10.1073/pnas.1906144117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Constantinidis C, Luna B (2019): Neural Substrates of Inhibitory Control Maturation in Adolescence. Trends in Neurosciences 42: 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kribakaran S, Danese A, Bromis K, Kempton MJ, Gee DG (2020): Meta-analysis of Structural Magnetic Resonance Imaging Studies in Pediatric Posttraumatic Stress Disorder and Comparison With Related Conditions. BPS: CNNI 5: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keding TJ, Herringa RJ (2015): Abnormal structure of fear circuitry in pediatric post-traumatic stress disorder. Neuropsychopharmacology 40: 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morey RA, Haswell CC, Hooper SR, De Bellis MD (2016): Amygdala, Hippocampus, and Ventral Medial Prefrontal Cortex Volumes Differ in Maltreated Youth with and without Chronic Posttraumatic Stress Disorder. Neuropsychopharmacology 41: 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heyn SA, Keding TJ, Ross MC, Cisler JM, Mumford JA, Herringa RJ (2019): Abnormal Prefrontal Development in Pediatric Posttraumatic Stress Disorder: A Longitudinal Structural and Functional Magnetic Resonance Imaging Study. BPS: CNNI 4: 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiser J, Koenigs M (2018): The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biological Psychiatry 83: 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiba Y, Santangelo AM, Roberts AC (2016): Beyond the Medial Regions of Prefrontal Cortex in the Regulation of Fear and Anxiety. Front Syst Neurosci 10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chick CF, Rolle C, Trivedi HM, Monuszko K, Etkin A (2020): Transcranial magnetic stimulation demonstrates a role for the ventrolateral prefrontal cortex in emotion perception. Psychiatry Res 284:112515. [DOI] [PubMed] [Google Scholar]
- 37.Morawetz C, Bode S, Derntl B, Heekeren HR (2017): The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: A meta-analysis of fMRI studies. Neurosci Biobehav Rev 72: 111–128. [DOI] [PubMed] [Google Scholar]
- 38.Ghashghaei HT, Hilgetag CC, Barbas H (2007): Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage 34: 905–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. (2014): Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cereb Cortex 24: 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tottenham N, Galvan A (2016): Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci Biobehav Rev 70: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLaughlin KA, Weissman D, Bitran D (2019): Childhood Adversity and Neural Development: A Systematic Review. Annual Review of Developmental Psychology 1: 277–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, Essex MJ (2013): Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci USA 110: 19119–19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birn RM, Patriat R, Phillips ML, Germain A, Herringa RJ (2014): Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depress Anxiety 31: 880–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herringa RJ, Burghy CA, Stodola DE, Fox ME, Davidson RJ, Essex MJ (2016): Enhanced prefrontal-amygdala connectivity following childhood adversity as a protective mechanism against internalizing in adolescence. Biol Psychiatry Cogn Neurosci Neuroimaging 1: 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodman AM, Jenness JL, Weissman DG, Pine DS, McLaughlin KA (2019): Neurobiological Markers of Resilience to Depression Following Childhood Maltreatment: The Role of Neural Circuits Supporting the Cognitive Control of Emotion. Biol Psychiatry 86: 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marusak HA, Martin KR, Etkin A, Thomason ME (2015): Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology 40: 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLaughlin KA, Peverill M, Gold AL, Alves S, Sheridan MA (2015): Child Maltreatment and Neural Systems Underlying Emotion Regulation. J Am Acad Child Adolesc Psychiatry 54: 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elsey J, Coates A, Lacadie CM, McCrory EJ, Sinha R, Mayes LC, Potenza MN (2015): Childhood trauma and neural responses to personalized stress, favorite-food and neutral-relaxing cues in adolescents. Neuropsychopharmacology 40: 1580–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fonzo GA, Huemer J, Etkin A (2016): History of childhood maltreatment augments dorsolateral prefrontal processing of emotional valence in PTSD. Journal of Psychiatric Research 74: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cisler JM, Sigel BA, Kramer TL, Smitherman S, Vanderzee K, Pemberton J, Kilts CD (2015): Amygdala response predicts trajectory of symptom reduction during Trauma-Focused Cognitive-Behavioral Therapy among adolescent girls with PTSD. J Psychiatr Res 71: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cisler JM, Sigel BA, Steele JS, Smitherman S, Vanderzee K, Pemberton J, et al. (2016): Changes in functional connectivity of the amygdala during cognitive reappraisal predict symptom reduction during trauma-focused cognitive-behavioral therapy among adolescent girls with post-traumatic stress disorder. Psychol Med 1–11. [DOI] [PubMed] [Google Scholar]
- 52.Garrett A, Cohen JA, Zack S, Carrion V, Jo B, Blader J, et al. (2019): Longitudinal changes in brain function associated with symptom improvement in youth with PTSD. J Psychiatr Res 114: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heyn SA, Herringa RJ (2019): Longitudinal cortical markers of persistence and remission of pediatric PTSD. Neuroimage Clin 24: 102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fonzo GA, Goodkind MS, Oathes DJ, Zaiko YV, Harvey M, Peng KK, et al. (2017): Selective Effects of Psychotherapy on Frontopolar Cortical Function in PTSD. Am J Psychiatry 174: 1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolf RC, Herringa RJ (2016): Prefrontal-Amygdala Dysregulation to Threat in Pediatric Posttraumatic Stress Disorder. Neuropsychopharmacology 41: 822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu J, Lowen SB, Anderson CM, Ohashi K, Khan A, Teicher MH (2019): Association of Prepubertal and Postpubertal Exposure to Childhood Maltreatment With Adult Amygdala Function. JAMA Psychiatry 76: 843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. (2013): Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA 110: 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gur RE, Moore TM, Rosen AFG, Barzilay R, Roalf DR, Calkins ME, et al. (2019): Burden of Environmental Adversity Associated With Psychopathology, Maturation, and Brain Behavior Parameters in Youths. JAMA Psychiatry 76: 966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brewin CR (2014): Episodic memory, perceptual memory, and their interaction: foundations for a theory of posttraumatic stress disorder. Psychol Bull 140: 69–97. [DOI] [PubMed] [Google Scholar]
- 60.Lancaster CL, Cobb AR, Lee H-J, Telch MJ (2016): The role of perceived threat in the emergence of PTSD and depression symptoms during warzone deployment. Psychol Trauma 8: 528–534. [DOI] [PubMed] [Google Scholar]
- 61.Nusslock R, Miller GE (2016): Early-Life Adversity and Physical and Emotional Health Across the Lifespan: A Neuroimmune Network Hypothesis. Biol Psychiatry 80: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colvonen PJ, Straus LD, Acheson D, Gehrman P (2019): A Review of the Relationship Between Emotional Learning and Memory, Sleep, and PTSD. Curr Psychiatry Rep 21: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daskalakis NP, Rijal CM, King C, Huckins LM, Ressler KJ (2018): Recent Genetics and Epigenetics Approaches to PTSD. Curr Psychiatry Rep 20: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stringaris A (n.d.): Reward processing and adolescent depression. Biological Psychiatry. [Google Scholar]
- 65.Stevens JS, Jovanovic T (2019): Role of social cognition in post-traumatic stress disorder: A review and meta-analysis. Genes Brain Behav 18: e12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blakemore S-J (n.d.): Cognition and adolescent brain development. Biological Psychiatry. [Google Scholar]
- 67.Blakemore S-J, Robbins TW (2012): Decision-making in the adolescent brain. Nat Neurosci 15: 1184–1191. [DOI] [PubMed] [Google Scholar]
- 68.Foulkes L, Blakemore S-J (2018): Studying individual differences in human adolescent brain development. Nat Neurosci 21: 315–323. [DOI] [PubMed] [Google Scholar]
- 69.Cisler JM, Esbensen K, Sellnow K, Ross M, Weaver S, Sartin-Tarm A, et al. (2019): Differential Roles of the Salience Network During Prediction Error Encoding and Facial Emotion Processing Among Female Adolescent Assault Victims. BPS: CNNI 4: 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lenow J, Cisler J, Bush K (2018): Altered Trust Learning Mechanisms Among Female Adolescent Victims of Interpersonal Violence. J Interpers Violence 33: 159–179. [DOI] [PubMed] [Google Scholar]
- 71.Brown VM, Zhu L, Wang JM, Frueh BC, King-Casas B, Chiu PH (2018): Associability-modulated loss learning is increased in posttraumatic stress disorder. Elife 7 10.7554/eLife.30150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Homan P, Levy I, Feltham E, Gordon C, Hu J, Li J, et al. (2019): Neural computations of threat in the aftermath of combat trauma. Nat Neurosci. 10.1038/s41593-018-0315-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Le Pelley ME (2004): The role of associative history in models of associative learning: A selective review and a hybrid model. Quarterly Journal of Experimental Psychology Section B 57: 193–243. [DOI] [PubMed] [Google Scholar]
- 74.Kirlic N, Young J, Aupperle RL (2017): Animal to human translational paradigms relevant for approach avoidance conflict decision making. Behaviour research and therapy 96: 14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cochran AL, Cisler JM (2019): A flexible and generalizable model of online latent-state learning. PLoS computational biology 15: e1007331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ (2011): Model-based influences on humans’ choices and striatal prediction errors. Neuron 69: 1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doll BB, Simon DA, Daw ND (2012): The ubiquity of model-based reinforcement learning. Current opinion in neurobiology 22: 1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Glascher J, Daw N, Dayan P, O’Doherty JP (2010): States versus rewards: dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron 66: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Redish AD, Jensen S, Johnson A, Kurth-Nelson Z (2007): Reconciling reinforcement learning models with behavioral extinction and renewal: implications for addiction, relapse, and problem gambling. Psychological review 114: 784. [DOI] [PubMed] [Google Scholar]
- 80.Gershman SJ, Blei DM, Niv Y (2010): Context, learning, and extinction. Psychol Rev 117: 197–209. [DOI] [PubMed] [Google Scholar]
- 81.Gershman SJ, Niv Y (2012): Exploring a latent cause theory of classical conditioning. Learn Behav 40: 255–268. [DOI] [PubMed] [Google Scholar]
- 82.Eley TC, McAdams TA, Rijsdijk FV, Lichtenstein P, Narusyte J, Reiss D, et al. (2015): The Intergenerational Transmission of Anxiety: A Children-of-Twins Study. Am J Psychiatry 172: 630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ginsburg GS, Drake KL, Tein J-Y, Teetsel R, Riddle MA (2015): Preventing Onset of Anxiety Disorders in Offspring of Anxious Parents: A Randomized Controlled Trial of a Family-Based Intervention. Am J Psychiatry 172: 1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Platt R, Williams SR, Ginsburg GS (2016): Stressful Life Events and Child Anxiety: Examining Parent and Child Mediators. Child Psychiatry Hum Dev 47: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lambert JE, Holzer J, Hasbun A (2014): Association between parents’ PTSD severity and children’s psychological distress: a meta-analysis. J Trauma Stress 27: 9–17. [DOI] [PubMed] [Google Scholar]
- 86.Leen-Feldner EW, Feldner MT, Knapp A, Bunaciu L, Blumenthal H, Amstadter AB (2013): Offspring psychological and biological correlates of parental posttraumatic stress: review of the literature and research agenda. Clin Psychol Rev 33: 1106–1133. [DOI] [PubMed] [Google Scholar]
- 87.Powers A, Stevens JS, O’Banion D, Stenson AF, Kaslow N, Jovanovic T, Bradley B (2020): Intergenerational transmission of risk for PTSD symptoms in African American children: the roles of maternal and child emotion dysregulation. Psychological trauma: theory, research, practice, and policy ePub: ePub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pine DS, Cohen JA (2002): Trauma in children and adolescents: risk and treatment of psychiatric sequelae. BiolPsychiatry 51: 519–531. [DOI] [PubMed] [Google Scholar]
- 89.Cohen JA, Mannarino AP (2015): Trauma-focused Cognitive Behavior Therapy for Traumatized Children and Families. Child Adolesc Psychiatr Clin N Am 24: 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yasinski C, Hayes AM, Ready CB, Cummings JA, Berman IS, McCauley T, et al. (2016): In-Session Caregiver Behavior Predicts Symptom Change in Youth Receiving Trauma-Focused Cognitive Behavioral Therapy (TF-CBT). J Consult Clin Psychol. 10.1037/ccp0000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bhattacharya S, Fontaine A, MacCallum PE, Drover J, Blundell J (2019): Stress Across Generations: DNA Methylation as a Potential Mechanism Underlying Intergenerational Effects of Stress in Both Post-traumatic Stress Disorder and Pre-clinical Predator Stress Rodent Models. Front Behav Neurosci 13: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Valentino K, Berkowitz S, Stover CS (2010): Parenting behaviors and posttraumatic symptoms in relation to children’s symptomatology following a traumatic event. J Trauma Stress 23: 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martin CG, Everett Y, Skowron EA, Zalewski M (2019): The Role of Caregiver Psychopathology in the Treatment of Childhood Trauma with Trauma-Focused Cognitive Behavioral Therapy: A Systematic Review. Clin Child Fam Psychol Rev 22: 273–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brent DA, Brunwasser SM, Hollon SD, Weersing VR, Clarke GN, Dickerson JF, et al. (2015): Effect of a Cognitive-Behavioral Prevention Program on Depression 6 Years After Implementation Among At-Risk Adolescents: A Randomized Clinical Trial. JAMA Psychiatry 72: 1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wymbs NF, Orr C, Albaugh MD, Althoff RR, O’Loughlin K, Holbrook H, et al. (2020): Social supports moderate the effects of child adversity on neural correlates of threat processing. Child Abuse & Neglect 102: 104413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.