Abstract
Tissue resident memory T cells (TRM) in the lungs are pivotal for protection against repeated infection with respiratory viruses. However, the gradual loss of these cells over time and the associated decline in clinical protection represent a serious limit in the development of efficient T-cell based vaccines against respiratory pathogens. Here, using an adenovirus expressing influenza nucleoprotein (AdNP) we show that CD8 TRM in the lungs can be maintained for at least one year post-vaccination. Our results reveal that lung TRM continued to proliferate in-situ 8 months after AdNP vaccination. Importantly, this required airway vaccination and antigen persistence in the lung, as non-respiratory routes of vaccination failed to support long-term lung TRM maintenance. Additionally, parabiosis experiments show that in AdNP vaccinated mice, the lung TRM pool is also sustained by continual replenishment from circulating memory CD8 T cells that differentiate into lung TRM, a phenomenon not observed in influenza infected parabiont partners. Concluding, these results demonstrates key requirements for long-lived cellular immunity to influenza virus, knowledge that could be utilized in future vaccine design.
Keywords: Tissue Resident T cells, CD8 T cells, Adenovirus, Vaccines, Antigen persistence
Introduction
CD8 tissue resident memory T cells (TRM) in the lungs represent a subset of memory T cells essential for optimal control of respiratory virus infections 1, 2. Since TRM were identified a decade ago, they have been found in various tissues including gut, skin, lung, reproductive tract, liver and brain3–7. TRM are cells that reside in non-lymphoid tissues and function as a first line of defense against secondary infections. In addition to their unique anatomic location, TRM have transcriptional profiles distinct from both central and effector memory T cells (TCM and TEM, respectively) 8–10. In peripheral tissues CD8 TRM cells are primarily identified by their expression of the tissue retention markers CD69 and CD103, combined with the exclusion of cells that stain with an intravascular label 11. However, not all TRM are created equal. In the skin, TRM remain in the tissue long after infection and antigen clearance 12. Conversely, lung TRM are lost a few months after an acute infection, leading to the loss of protection from secondary challenge1, 13. The reason for this rapid decay is still under investigation, but a harsh environment in the lungs and airways is likely to contribute. Recently published evidence show that the airway environment causes transcriptional and epigenetic changes in the memory T cells resulting from amino acid stravation and leading to increased apoptosis14. Previous reports have suggested residual antigen is important for continued development of virus-specific CD8 T cells after viral clearance following influenza infection 15. However, the potential role of residual antigen for the differentiation and maintenance of lung TRM has not been investigated.
The discovery of the protective capacity of CD8 lung TRM is of the utmost interest to the global vaccine community and, in particular, influenza vaccine research. However, the gradual waning of protection over time represents a serious limitation to the practical application of this finding. Therefore, a full understanding of the requirements for long-term lung TRM maintenance would allow informed vaccine design to induce long-standing protective cellular immunity. Several studies have shown that adeno-based (Ad) vectors were effective at inducing CD8 T cell mediated protection against influenza 16–19. In addition to this, Ad vectors have been investigated as vaccines for both cancer and infections such as yellow fever, malaria and Listeria monocytogenes 20–23. There are many advantages to the use of Ad vectors; they are easy to produce in high titers, have the potential to express large inserts and the vector itself functions as an efficient adjuvant 24, 25. The possibility to enhance the immune response induced by Ad vectors further has been under investigation. One approach involves encoding of signals within the Ad vector to provide additional help to the CD8+ T cell response 26, 27. Previous work also investigated how the route of vaccination impacts the duration of protection against influenza virus. Mice vaccinated both intranasally (i.n.) and subcutaneously (s.c.) with an Ad vector expressing influenza nucleoprotein (AdNP) were protected from challenge longer than mice vaccinated by either the i.n. or s.c. route alone (Uddback et al., 2016). However, these studies did not address the mechanism(s) of increased duration of protection, nor did they address the impact of AdNP vaccination on the development and longevity of CD8+ TRM in the lung and airways.
In the present study, we highlight the connection between persistent antigen expression following AdNP vaccination and maintenance of lung CD8 TRM. We find, in contrast to the rapid loss of lung TRM following influenza infection, mice primed with AdNP maintain a substantially larger population of lung TRM for up to one year post vaccination (p.v.). Using Nur77GFP reporter mice, we show lung TRM of AdNP immunized mice continue to interact with cognate antigen for at least three months p.v.. The expanded lung TRM population in AdNP immunized mice is maintained in the lungs and airways by both in situ proliferation and continual replenishment of the lung TRM pool from the circulation. Finally, we show that i.n. administration of AdNP is critical for the increased longevity of lung and airway TRM. These results highlight the potential benefits of Ad vector vaccination, and underscore the importance of prolonged antigen expression in the lung for extended T-cell mediated protection against respiratory infections.
Results
AdNP induced antigen specific TRM are maintained long-term in lung and BAL
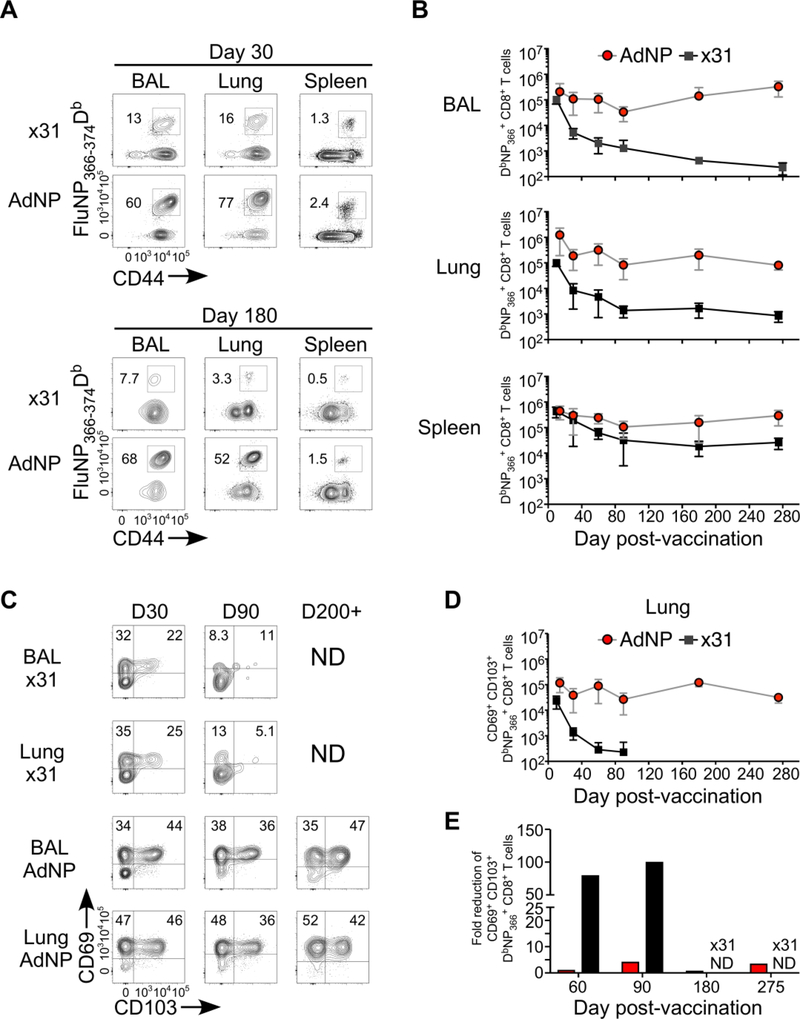
To investigate the mechanism(s) underlying the increased duration of protection in AdNP immunized mice, we first examined the longevity of the CD8 T cells induced by AdNP and compared it to that generated by Influenza A/HK×31 (×31). After priming with either AdNP or ×31, spleen, bronchoalveolar lavage (BAL), and lungs were isolated at various time points and numbers of DbNP366 tetramer positive cells were determined by flow cytometry. For analysis of lung TRM cells, intravital labeling was performed prior to exsanguation, labelling all cells within the circulation, ensuring that we could discriminate lung TRM cells from cells in the lung vasculature28. Already at day 30 p.v. (Fig 1A), there is a substantially larger population of DbNP366+ T cells in the lungs and airways of AdNP immunized mice. At day 180 (Fig 1A), the proportion of DbNP366+ cells in lungs and airways of AdNP primed mice remain at the same level, or higher, whereas in ×31 infected mice, the proportion of DbNP366+ cells declines substantially. Importantly, comparing the number of DbNP366+ cells between AdNP and ×31 primed mice, we see that, despite that both generate high numbers in the acute phase (day 10–14), the numbers diverge as early as day 30 in lungs and airways (Fig 1B). Where numbers of DbNP366+ cells in ×31 infected mice rapidly decline, numbers are maintained in AdNP vaccinated mice up to at least day 275 p.v.. Additionally, a greater proportion of DbNP366+ cells express both CD69 and CD103 in the AdNP mice as early as day 30 p.v. (Fig 1C). Even more striking, by day 90 (Fig 1C) the proportion of CD69+CD103+ cells is maintained at around thirty percent in AdNP mice that declines to about five percent ×31 infected mice. Examining AdNP mice at day 210, there is a further enrichment of CD69+CD103+ cells within the DbNP366+ population (Fig 1C). A similar trend is observed when analyzing the absolute number (Fig 1D), with the number of cells in the lungs and airways of AdNP mice remaining stable up to day 210 p.v.. In contrast, numbers of CD69+CD103+ cells in the ×31 primed mice is reduced drastically after the acute phase (Fig 1D and 1E) and this population is almost completely lost by day 90. In addition to these data, a substantial number of DbNP366+ cells were still found in the lung and airways 580 days p.v., further illustating the long lived maintence of the population in AdNP vaccinated mice (Fig S1). The protective capacity of the AdNP induced CD8 T cell response long after immunization have been previously demonstrated18. Furthermore, through knock out and depletion experiments, we previously showed that the NP specific CD8 T cells are responsible for the protective immunity established by AdNP vaccination. Importantly, we confirmed that the cellular immunity induced by AdNP vaccination was protective more than 255 days p.v. (Fig S2). Taken together, these data indicate that the long-lasting immunity induced by AdNP is due to increased duration of CD8 TRM in the lung and airways.
Figure 1.
AdNP induced antigen specific TRM are maintained long-term in lung and BAL. C57BL/6 mice were immunized with AdNP sub-cutaneous (s.c.) in the footpad and i.n. or with HKx31 (x31) Influenza i.n. Lung, BAL and Spleen were isolated for DbNP366tetramer analysis. (A) Representative plots. (B) Kinetics of absolute number of DbNP366+ CD8 T cells. (C) Representative plots of residency markers CD69 and CD103 and (D) absolute numbers of CD69+CD103+DbNP366+ CD8 T cells. (E) Fold reduction of CD69+CD103+ T cells. (B+D) Dots and bars represents mean and SD. (E) Dots represent fold reduction for each time point. All time points are representative of three indivudal experiments with 5 mice in each.
Antigen persists in the lungs and airways after AdNP vaccination
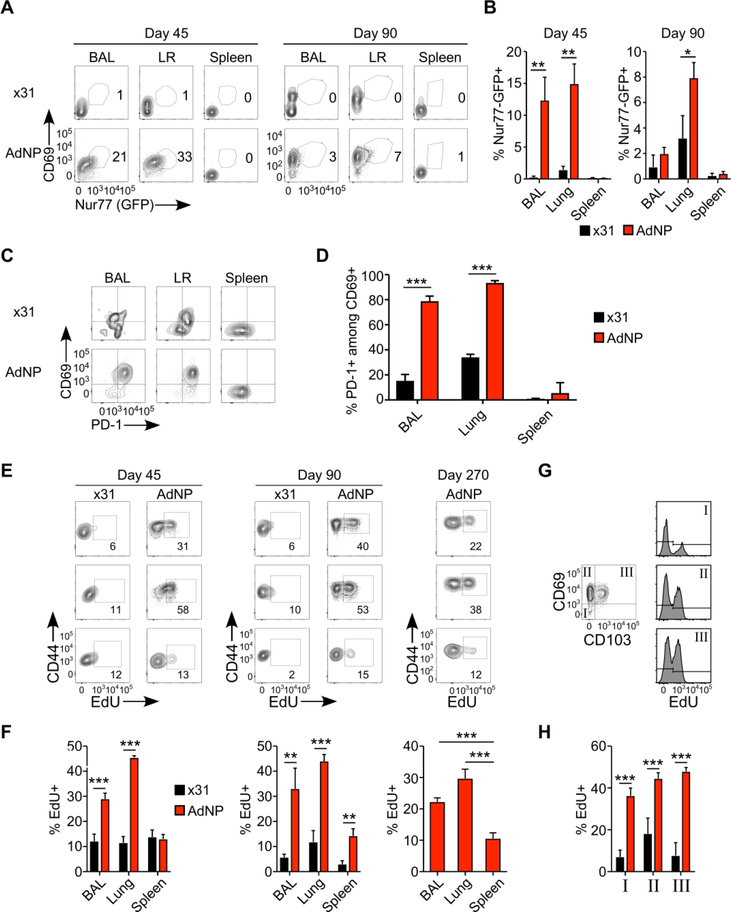
Several studies have investigated the effect of persistent antigen on the CD8 T cell population following vaccination with adenoviral vectors 29, 30. It is well established that low level persistent antigen can stimulate T cells without causing exhaustion. This phenomenon, known as memory inflation has been studied in both murine Cytomegalovirus and Ad infections 29, 31–33. Moreover, previous work has shown that antigen encounter in the lung is required for establishment of CD8 TRM34–36. Importantly, genomic material from adenoviruses and adeno-vectors persists in the tissue after immunization, in some cases up to a year post-injection 30, 37. In the present study, we confirmed persistence of the NP antigen by immunofluorescence microscopy in lungs 110 days after AdNP vaccination (Fig S4). However, the effect of this low level antigen persistence on lung TRM has never been studied. To investigate this, we utilized a Nur77GFP reporter mouse to visualize antigen-dependent CD8 T cell stimulation in vivo. The Nur77GFP signal is transient and quickly lost after withdrawal of antigen stimulation, making the readout of GFP in Nur77GFP mice a useful tool to investigate if lung TRM have recently encountered their cognate antigen. Nur77GFP mice were primed with ×31 or AdNP and 45 or 90 days later BAL, lungs and spleens were isolated and Nur77 expression analyzed. As illustrated, there is a significantly higher proportion of Nur77+CD69+ cells among the DbNP366+ CD8 T cells in the lungs and airways of AdNP mice at day 45 (Fig 2A and B), compared to ×31 mice. The frequency of Nur77+CD69+ decreases in both groups at day 90, however, we still find significantly more Nur77+CD69+ T cells in the lung TRM of AdNP vaccinated mice There is no difference in the frequency of Nur77+ cells between ×31 and AdNP mice when expression was analyzed based on TRM expression of CD69 and CD103 (data not shown).
Figure 2.
Antigen persists in the lungs and airways after AdNP vaccination (A-B) C57BL/6xNur77GFP mice were immunized with AdNP s.c.+i.n. or with x31 i.n. 45 or 90 days p.v., spleen, lung and BAL were isolated for analysis of Nur77 expression. (A) Representative plots. (B) Percentages of Nur77+ cells within the DbNP366+ T cells. (C-D) C57BL/6 were immunized with AdNP i.n.+s.c. and 60 days later cell were isolated from BAL, lung and spleen and PD-1 and CD69 expression was analysed in the DbNP366 tetramer+ T cells. (C) Representative plots (D) percentage PD-1 and CD69 expression within the DbNP366 tetramer positive population. (E-H) For proliferation studies, EdU incorporation was analysed in vaccinated C57BL/6 mice. 45, 90 and 270 days p.v., spleen, lung and BAL were isolated for analysis of EdU incorporation and CD44 in DbNP366+T cells. (E) Representative plots (F) Percentage of EdU+ out of DbNP366+ T cells (G) EdU incorporation in different subpopulations expressing CD69 and CD103 (H) Percentage of EdU in different CD69 and CD103 subpopulations. *: p<0.05, **:p<0.01, The figures are representative of three indivudal experiments with 5 mice in each.
Chronic PD-1 expression is believed to reflect ongoing antigen stimulation in TRM populations 1, 38; therefore, we examined PD-1 expression on CD8 lung TRM as an additional indicator of persistent antigen stimulation (Fig 2C and D). The majority of the DbNP366+ T cells in the BAL and lung of AdNP primed mice expressed PD-1 at day 60 and the proportion of CD69+PD-1+ cells was significantly higher in lungs and BAL of AdNP vaccinated mice compared with cells from ×31 primed mice. PD-1 expression in AdNP mice was maintained until the last time point studied (day 275, data not shown). Since prolonged PD-1 expression can indicate a state of T-cell exhaustion 39, it is important to stress that CD8 TRM in AdNP vaccinated mice still show protection from PR8 challenge up to 255 days p.v. (Fig S2). To directly address possible concerns regarding the functional relevance of PD-1+ expression on the airway T cells, the cellular response and its protective capacities after AdNP immunization were evaluated in PD-1−/− mice (Fig S3). We found no significant difference in the numbers of DbNP366+ TRM, nor was there a significant difference in viral titers in the lungs 5 days after PR8 challenge compared to wild-type (WT) mice. We also examined TIM-3 expression, as TIM-3 has also been implicated in an exhausted T cell phenotype 40. DbNP366+ cells in AdNP immunized mice had little to no expression of TIM-3 (data not shown).
Next, we hypothesized that the persistent antigen in AdNP immunized mice results in continual in situ proliferation and thereby maintains the TRM population. To evaluate proliferation, we administered EdU in the drinking water for a period of 7 days in both AdNP and ×31 primed mice. Both at day 45 and day 90, mice immunized with AdNP have a significantly higher proportion of EdU+ cells in the BAL and lungs compared to ×31 infected mice (Fig 2E and F). Incredibly, we found EdU incorporation in DbNP366+ T cells of AdNP immunized mice as far out as 270 days p.v., with significantly greater incorporation in the BAL and lungs (Fig 2E and F). Due to the low number of DbNP366+ T cells in ×31 infected mice after day 90, we did not investigate EdU incorporation in these mice at later time points. Moreover, we observed that all lung TRM subsets based on expression of CD69 and CD103 expression had a significantly higher proportion of EdU+ NP-specific cells in AdNP immunized mice than observed in ×31 infected mice (Fig 2G and H). Taken together with the previous results, these data indicate the persistence of local antigen in the lung is driving activation and continued proliferation of lung TRM following AdNP vaccination.
Persistent antigen in AdNP immunized mice pull circulating cells into the TRM pool
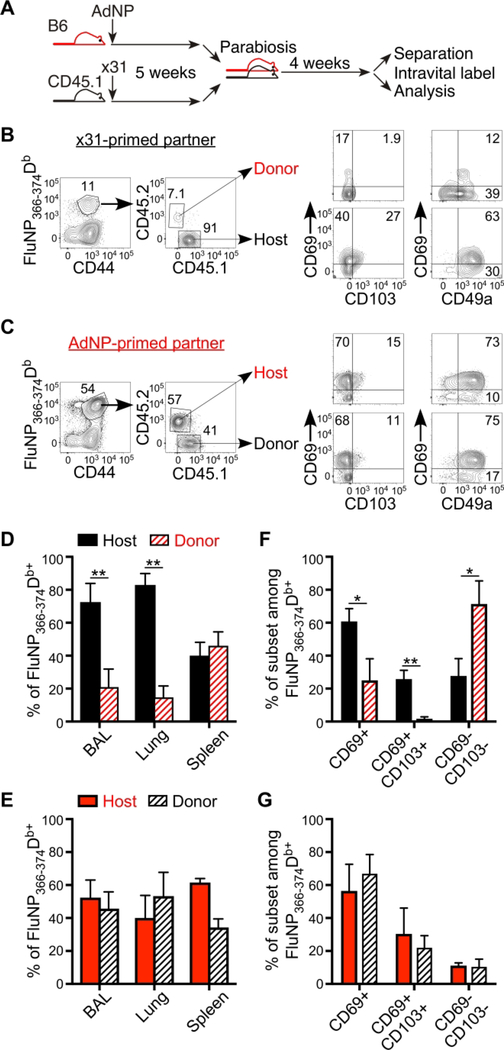
It is well established that antigen is required for establishment and maintenance of lung TRM following influenza infection 15, 34, 35. However, we previously showed that i.n. vaccination with AdNP alone was not enough to induce long-lasting protection (Uddback et al 2016) and hypothesized that the circulating population of NP-specific T cells induced by s.c. vaccination provides a pool of cells for continual recruitment and establishment of “new” TRM following AdNP vaccination. To directly address this hypothesis, we utilized a parabiosis approach. Briefly, we immunized CD45 congenic mice with either AdNP or influenza ×31 and 35 days later parabiotic surgery was performed. Twenty-eight days later, parabiont partners were separated and spleen, lungs and BAL were analyzed (Fig 3A). Our data confirms a previous report showing that very few partner cells become resident in the BAL and lung of ×31 infected mice 35. Importantly, the proportion of donor cells found in the spleen is about 50%, indicating that equilibrium of recirculating memory cells was achieved (Fig 3B and 3D). These data are in stark contrast to parabiont partners immunized with AdNP, where AdNP had induced substantial recruitment of partner cells into all organ sites analyzed. Notably, in both BAL and lungs, about 50% of DbNP366+ cells were from the ×31-infected partner (Fig 3C and 3E). In addition, partner cells in the lungs of AdNP vaccinated mice show similar expression of the TRM markers CD69, CD103, and CD49a compared to host cells, indicating that the recruited T cells differentiate into TRM within the lungs. (Fig 3C, right plots and 3G). In contrast, a very low proportion (about 1%) of the cells that had migrated from the partner into the lung and airways of the ×31 infected mice expressed these tissue retention markers, indicating these are likely effector memory T cells transiting through the tissue (Fig 3B right plots and 3F). These results provide compelling evidence that AdNP vaccination results in persistent antigen expression in the lungs of vaccinated mice, allowing circulating CD8 T cells to be continuously recruited into TRM pool.
Figure 3.
Persistent antigen in AdNP immunized mice pull circulating cells into the TRM pool (A) AdNP and x31 primed mice were joined in a parabiosis surgery at early memory (5 weeks) and DbNP366+ T cells were analysed after full equilibrium was reached 4 weeks after joining. (B) Representative plots of CD69, CD103 and CD49a expression in DbNP366+ T cells in x31-primed partner (C) corresponding plots as in (B) for AdNP primed partner. (D+F) Percentage of host and donor cells in x31 primed partner (E+G) Percentage of host and donor in AdNP primed *: p<0.05, **:p<0.01, ***:p<0.001. The figure is representative of two individual experiments with 3 parabiosis pair in each.
AdNP intranasal inoculation is indispensable for sustaining the lung TRM pool
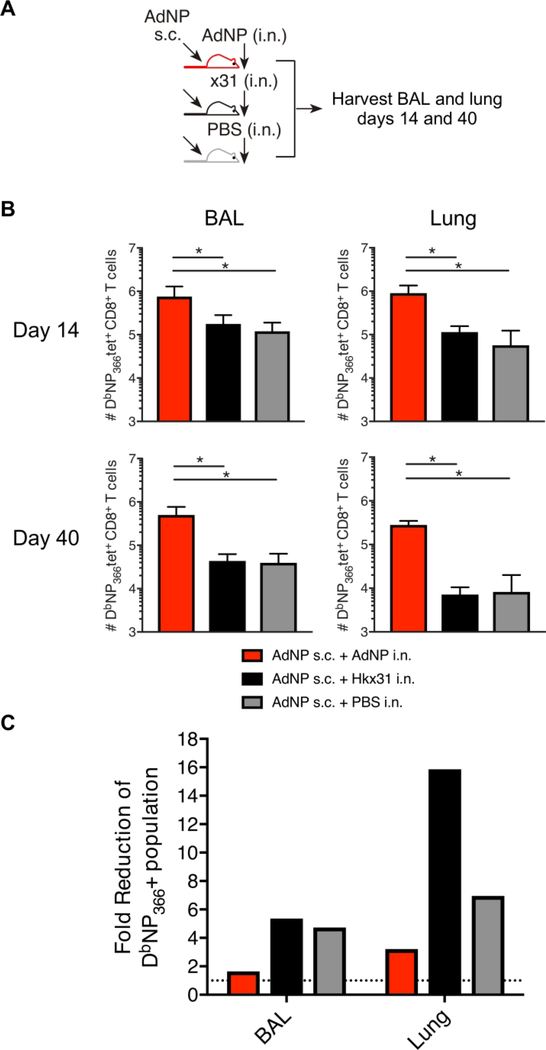
In order to further support our hypothesis of persistent local antigen expression as critical for maintenance of the lung TRM population, we compared the NP-specific T-cell response in mice immunized both s.c. and i.n. with AdNP to mice vaccinated s.c. with AdNP and infected i.n. with ×31 or mock-infected i.n. with PBS (Fig 4A). By day 14 (Fig 4B), mice immunized i.n. with AdNP had significantly more cells in the airway and lungs compared to mice inoculated i.n. with ×31. At day 40 post priming, there were still significantly more DbNP366+ T cells in in the airways and lungs of mice immunized with AdNP i.n. Importantly, regardless of s.c. administration of AdNP, the fold reduction of DbNP366+ T cells in the lungs and airways between day 14 and 40 was greater in mice that received i.n. ×31 or PBS (Fig 4C). This emphasizes the necessity of local persistent antigen for the maintenance of a long-lived airway and lung TRM population following AdNP vaccination.
Figure 4.
AdNP intranasal inoculation is indispensable for sustaining the lung TRM pool (A) C57BL/6 mice were immunized with AdNP s.c.+i.n. or, AdNP s.c.+x31 i.n or, AdNP s.c.+PBS i.n. 14 and 40 days p.v. spleen, BAL and lungs were isolated and analysed for DbNP366+ tetramer cells. (B) Absolut numbers of DbNP366+ CD8 T cells in lung and BAL. (C) Fold reduction of DbNP366+ CD8 T cells in lung and BAL between day 14 and day 40. Dotted line represent 1= no fold reduction. *: p<0.05. The figure is representative of two indivudal experiments per time point with 5 mice in each.
Discussion
Influenza virus infections represent a global health burden and currently available vaccines are inefficient. First, they are directed at a moving target, the main surface molecule, hemagglutinin, (HA), which is subject to substantial genetic variation as a result of both genetic drift as well as genetic shift. This creates a need for repeated vaccinations to sustain at least some protection. Second, the vaccine-induced immune response to HA consists largely of circulating IgG, whereas local immunity in the airways is limited. One way to remedy both of these deficiencies is to replace the current vaccine approach with one that induces a potent local T-cell response in the respiratory tract. Unlike antibodies, T cells also target the internal viral antigens, which are not subject to the same kind of selection as the surface molecules and therefore much more conserved between strains of influenza. However, a primary challenge in generating effective T-cell based vaccines against respiratory pathogens, is the rapid decline of the lung TRM population. However, our recently published results have indicated that combined local and systemic immunization in mice using AdNP induces a long-lasting protective CD8 population18. Until now the underlying reasons for this sustained response has not been investigated. In this study, we provide evidence indicating that TRM in AdNP vaccinated mice continue to encounter antigen in the lungs up to at least 3 months p.v., undergo in situ proliferation, and are continuously recruited into the lung from the circulating memory T cell pool.
First, our data provided evidence for long-standing proliferation of NP specific cells in the lungs of Ad immunized mice in contrast to flu infected mice. Second, we observed ongoing recruitment of circulating memory cells as a consequence of prolonged antigen expression. Previous research has suggested that the TRM population of influenza primed mice is maintained through a dynamic process consisting of a high apoptotic rate concurrent with replenishment from circulating memory cells, and that this recruitment is independent of local antigen41. Based on our parabiosis experiments, we found little to no evidence of recruitment of circulating cells into the lung TRM population in influenza primed mice. Moreover, a circulating memory T cell population generated by s.c. AdNP immunization of i.n. influenza primed mice, did not suffice to maintain the lung TRM population. Together, our data shows the importance of prolonged local antigen expression for continuous recruitment of circulating T cells into the the lung TRM pool.
These data further emphasize that TRM across tissues cannot be treated as one homogenous population, and that the requirements for establishment and maintenance is as diverse as the anatomic locations in which they reside. For example, antigen is not required for the establishment of skin TRM and persistent antigen has not been shown to be necessary for their long-term maintenance12. Thus, it is highly plausible that long-term maintenance of a stable TRM population in any given tissue is dictated by how effectively local proliferation and ongoing recruitment from other memory cell subsets balances the local apoptotic rate.
Most importantly, all of these parameters are influenced by the microenvironment in the tissues of relevance. It should be acknowledged that other components of the immune system, such as APCs and even NP-specific antibodies, may also play a role in the maintenance of the CD8 T cell population 42. We have previously shown that CD8 T cells are the primary mechanism of protection after AdNP vaccination, but to what extent NP-specific antibodies are affected by the persiting antigen, and their contribution to protective immunity, remains to be investigated18. In the lungs, the TRM are subject to a relatively harsh environment, causing a high rate of apoptosis14. While we cannot significantly change the environment of the lung, we have shown it is possible to expand and maintain the TRM population by prolonged antigen stimulation. As stated previously, this strategy will open new doors for development of vaccines against flu and other viral respiratory diseases. In this context it should be kept in mind that the dual vaccination approach applied for Ad immunized mice allows for antigen persistence in both the lungs and the periphery and both of these sources are likely contributing factors in the sustained response. It has been clearly documented that repeated antigen exposures gradually improve the quality of the primed cells, local cells become less prone to apoptosis, and circulating precursors develop a higher propensity to home to the lungs and differentiate into TRM 43, 44. The prolonged presence of antigen in the periphery is likely to have the same effect, in addition to increasing the number of activated circulating precursors. Consistent with this possibility, we see limited contraction following the initial T-cell burst in s.c. Ad immunized mice, and phenotypic analysis have revealed evidence of ongoing activation e.g. the prolonged expression of CD43, on remaining antigen specific CD8 T cells 45, 46. The degree to which these factors, local antigen versus prolonged circulation of relevant precursors, contribute to the long-term immunity observed is not clear, but our previous results clearly show that combined s.c. and i.n. vaccination is superior to i.n. vaccination alone18. Notably, due to the delicate nature of the lung, any vaccination strategy which results in persistent antigen must be carefully assessed for the induction of localized persistent inflammation and immunopathology that could be detrimental to the host. In conclusion, the results presented in this report show us that we can achieve a stable T cell response in the lung with AdNP vaccination. Importantly, we also show that this long-lived T cell response is due to the presence of persistent local antigen. Not only is antigen required for TRM formation as previously documented34, but unlike the situation in most other organ sites, persistent antigen is essential for the continual replenishment and long-term maintenance of TRM population in the lungs.
Methods
Experimental models
6–8 weeks old C57BL/6 mice from Taconic Biosciences were used in this study. All mice were rested upon arrival for at least 1 week. PepBoy/J, Nur77GFP (Nur77-GFPCre B6–820), and PD-1−/− (B6.Cg-Pdcd1tm1.1Shr/J) mice were purchase from Jackson Laboratory. All experimental procedures were approved by the national animal ethics committee (The Animal Experiments Inspectorate or IACUC) of the University of Copenhagen, Emory University, and Kindai University and were conducted in accordance with national guidelines; the mice were housed in an AAALAC accredited facility in accordance with good animal practice as defined by FELASA.
Virus and vaccines
All mice receiving intranasal (i.n.) inoculation were first anaesthetized by intraperitoneal (i.p.) injection with avertin (2,2,2 tribromoethanol in 2-methyl-2-butanol, 250 mg/kg). Influenza infection with A/Hong Kong/×31 (×31) was used at a dosage of 30,000 EID50 in 30 ul HBSS and administrated i.n. after avertin anesthesia. For Influenza challenge, A/Puerto Rico/8/34 (PR8) was used at a dose of 3LD50 in 30ul i.n. The production of the replication deficient adenovirus type 5 expressing influenza PR8 nucleoprotein (AdNP) used in this study has previously been described 18. Mice were immunized with 2×107 plaque forming units (PFU) in 30 μl of PBS i.n. and 30 ul s.c. in the right foot pad after anesthesia with avertin.
Preparation of single-cell suspensions
To isolate resident lymphocytes in lung, mice were intravenously injected with 1.5ug anti-CD3e [145–2C11] fluorophore conjugated antibody in 200 ul PBS in the tail vein 11, 28. 5 minutes post injection mice were anesthetized using avertin and exsanguated. This was followed by harvest of BAL and other tissues 47. After isolation lungs were digested with 5g/L Collagenase D (Roche) and 2×106 Units/L DNAse (Sigma) for 30 min 37 C. Samples were enriched by centrifugation in a 40%/80% Percoll gradient to isolate lymphocytes. Spleen and MLN were mechanically dissociated and passed through a 70 μm nylon filter prior to staining.
Antibodies for flow cytometry
Cells were first blocked for unspecific binding with αCD16/32 followed by staining with NP366–374/Db tetramer conjugated to allophycocyanin (APC) or Brilliant Violet 421 (BV421). Tetramer labelled cells was incubated with fluorophore conjugated antibodies CD8α (clone 53–6.7), CD8β (clone H35 17.2) CD44 (clone IM7), CD45.1 (clone 30-F11), CD45.2 (clone 104) CD69 (clone H1.2F3), CD103 (clone 2E7), PD-1 and live/dead stain Zombie NIR. EdU staining was performed using Click It Plus Alexa Flour 647 Assay kit (Invitrogen) according to manufacturer’s instructions. Samples were analyzed on a Fortessa LSR II (BD Biosciences). Data analysis was conducted using FlowJo v10 software (TreeStar). Gates for CD69, CD103 and PD-1 were set using fluorescence minus one samples. All antibodies were purchased from Biolegend. Relevant tetramers were kindly provided by Søren Buus, Department of Immunology and Microbiology and the NIH tetramer core facility.
Parabiosis
For parabiosis surgery, mice were anesthetized, and a clipper was used to remove flank hair. This was followed by a longitudinal skin incision from the knee to the elbow on a single lateral side along with a 1-cm lateral peritoneal incision. Suturing each reciprocal peritoneal opening joined the two mice together. To hold the mice in the upright position, two mattress stiches were made on the lateral edges of the skin section. Also, the dorsal and ventral sides of the skin section of each mouse were further joined with wound clips. Equilibrium was confirmed 10 days after surgery with a blood sample.
MDCK plaque assay
Lungs were homogenized in 1%BSA in PBS 9X the lung weight to obtain a 10% w/v suspension. The mixture was homogenized using sterilized sand, mortar and pestle. This was followed by centrifugation at 600 g, 15 min, 4°C. The supernatant was transferred to a new tube and kept on ice until use. 4.5 ×104 MDCK cells were seeded in 100 ul medium in 96-well plates and the following day, lung supernatant was added in 10-fold dilutions in media containing DMEM 1965 medium with 2 mM L-glutamin, 200 IU/ml penicillin, 50 μg/ml streptomycin, 0.2% BSA, 1% sodium-pyruvate and 5 units/ml TPCK Trypsin for 2 hours. Virus was then removed and samples were incubated for 48 hours, 37°C, 5% CO2, with an 1:1 mixture of medium containing 2× minimum essential medium (MEM) eagle supplemented with 0.4% BSA, 10% NaHCO3, 2% Streptomycin, 2% penicillin and 5 units/ml TPCK trypsin and 1.8% methyl cellulose. After incubation overlay was removed and cells were fixed with 4% formaldehyde in PBS for 30 minutes at room temperature (RT) and permeabilized using warm 0.5% Triton-X in Hanks balanced salt solution for 10 minutes at RT. After permeabilization cells were incubated with primary α-influenza nucleocapsid A mAb (Nordic Biosite) diluted 1:1500 in 10% FBS in PBS at 37°C, 5% CO2. Following the primary antibody, cells were incubated with secondary goat α-mouse HRP conjugated mAb (Dako) diluted 1:500 in 10% FBS in PBS at 37C. After the secondary antibody, substrate was added containing 3 mg/ml 3-amino-9-ethylcarbazole and 0.07% H2O2 and 5 mM citrate phosphate buffer pH5 and incubated at RT for 30 min. After this plaque forming units were counted and calculated per g lung according to the following formula:
Immunofluorescence Microscopy
For OCT imaging, mice were infected with the specified agent and harvested at indicated days. The mice were euthanized by 2,2,2-tribromoethanol overdose and the ribcage was dissected off. An incision was made in the trachea and the airways were inflated with 0.75 mL OCT via an 18-gauge iv cathedar. The trachea was tied using 5–0 silk suture on a reverse cutting needle and the heart, lungs and thymus were removed en bloc and flash frozen in OCT. Sections were cut at 7 um and transferred to slides for staining. Slides were fixed in 75 % acetone / 25 % ethanol solution for 10 minutes and blocked with 10 % donkey serum, 10 % mouse serum, 10 % rat serum, 1 ug/mL anti CD16/32 clone 2.4G2, and 5 % FCS in PBS for 30 minutes on ice. Slides were stained with primary antibodies for 30 minutes on ice and secondary reagents for 15 minutes on ice. Coverslips were placed with ProFade Gold mounting media and imaged using a Zeiss AxioObserver microscope using Zen 2 software. Antibodies used include anti-EpCAM-A647 (clone G8.8, Biolegend), anti-Collagen-IV goat pAb (part # AB769, EMD Millipore), Donkey anti-goat-A405 (part # 705–475-147, Jackson Immunoresearch), anti-influenza-nucleoprotein-FITC (clone 431, abcam), anti-FITC-A488 (part # A-11090, Invitrogen), andanti-CD11c-A594 (clone N418, Biolegend).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7. ANOVA was used for statistical testing for all experiments and where pair-wise comparison was made Mann-Whitney rank test was used. *:p<0.05, **p<0.01, ***p<0.001.
Supplementary Material
Acknowledgements
This project was supported by the Danish Research Council and ‘Fonden til Lægevidenskabens Fremme’ for grant support. IEMU is the recipient of a PhD scholarship from the Faculty of Health and Medical Sciences, University of Copenhagen. We would also like to acknowledge the support by NIH grants HL122559, HL138508, and Centers of Excellence in Influenza Research and Surveillance contract HHSN272201400004C (to J.E.K.), and Grant-in-Aid for Young Scientists (A) 24689043, Grant-in-Aid for Scientific Research (C) 16K08850 from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and grants from Takeda Science Foundation, Daiichi-Sankyo Foundation of Life Science, Uehara Memorial Foundation, and Kanae Foundation for Promotion of Medical Science (to S.T.). S.L.H. was supported by NIH grant F31 HL136101. We recognize contributions from the Children’s Healthcare of Atlanta and Emory University Pediatric Flow Cytometry Core for cell sorting and the NIH Tetramer Core Facility (contract HHSN272201300006C).
Footnotes
No conflict of interest exists
References
- 1.Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol 2014; 95(2): 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinnear E, Lambert L, McDonald JU, Cheeseman HM, Caproni LJ, Tregoning JS. Airway T cells protect against RSV infection in the absence of antibody. Mucosal Immunol 2018; 11(1): 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 2009; 10(5): 524–530. [DOI] [PubMed] [Google Scholar]
- 4.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates nonmigratory memory CD8+ T(RM) cells providing global skin immunity. Nature 2012; 483(7388): 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med 2010; 207(3): 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol 2013; 14(5): 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stelma F, de Niet A, Sinnige MJ, van Dort KA, van Gisbergen K, Verheij J et al. Human intrahepatic CD69 + CD8+ T cells have a tissue resident memory T cell phenotype with reduced cytolytic capacity. Sci Rep 2017; 7(1): 6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM et al. T-box Transcription Factors Combine with the Cytokines TGF-beta and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity 2015; 43(6): 1101–1111. [DOI] [PubMed] [Google Scholar]
- 9.Wakim LM, Woodward-Davis A, Liu R, Hu Y, Villadangos J, Smyth G et al. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol 2012; 189(7): 3462–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar BV, Ma W, Miron M, Granot T, Guyer RS, Carpenter DJ et al. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep 2017; 20(12): 2921–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc 2014; 9(1): 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A 2012; 109(18): 7037–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilchuk P, Hill TM, Guy C, McMaster SR, Boyd KL, Rabacal WA et al. A Distinct Lung-Interstitium-Resident Memory CD8(+) T Cell Subset Confers Enhanced Protection to Lower Respiratory Tract Infection. Cell Rep 2016; 16(7): 1800–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayward SL, Scharer CD, Cartwright EK, Takamura S, Li ZT, Boss JM et al. Environmental cues regulate epigenetic reprogramming of airway-resident memory CD8(+) T cells. Nat Immunol 2020; 21(3): 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zammit DJ, Turner DL, Klonowski KD, Lefrancois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity 2006; 24(4): 439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coughlan L, Sridhar S, Payne R, Edmans M, Milicic A, Venkatraman N et al. Heterologous Two-Dose Vaccination with Simian Adenovirus and Poxvirus Vectors Elicits Long-Lasting Cellular Immunity to Influenza Virus A in Healthy Adults. EBioMedicine 2018; 29: 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powell TJ, Peng Y, Berthoud TK, Blais ME, Lillie PJ, Hill AV et al. Examination of influenza specific T cell responses after influenza virus challenge in individuals vaccinated with MVA-NP+M1 vaccine. PLoS One 2013; 8(5): e62778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uddback IE, Pedersen LM, Pedersen SR, Steffensen MA, Holst PJ, Thomsen AR et al. Combined local and systemic immunization is essential for durable T-cell mediated heterosubtypic immunity against influenza A virus. Sci Rep 2016; 6: 20137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitelli A, Quirion MR, Lo CY, Misplon JA, Grabowska AK, Pierantoni A et al. Vaccination to conserved influenza antigens in mice using a novel Simian adenovirus vector, PanAd3, derived from the bonobo Pan paniscus. PLoS One 2013; 8(3): e55435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassi MR, Larsen MA, Kongsgaard M, Rasmussen M, Buus S, Stryhn A et al. Vaccination with Replication Deficient Adenovectors Encoding YF-17D Antigens Induces Long-Lasting Protection from Severe Yellow Fever Virus Infection in Mice. PLoS Negl Trop Dis 2016; 10(2): e0004464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen S, Steffensen MA, Jensen BA, Schluter D, Christensen JP, Thomsen AR. Adenovirus-based vaccine against Listeria monocytogenes: extending the concept of invariant chain linkage. J Immunol 2013; 191(8): 4152–4164. [DOI] [PubMed] [Google Scholar]
- 22.Lasaro MO, Ertl HC. New insights on adenovirus as vaccine vectors. Mol Ther 2009; 17(8): 1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheehy SH, Duncan CJ, Elias SC, Biswas S, Collins KA, O’Hara GA et al. Phase Ia clinical evaluation of the safety and immunogenicity of the Plasmodium falciparum blood-stage antigen AMA1 in ChAd63 and MVA vaccine vectors. PLoS One 2012; 7(2): e31208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bett AJ, Prevec L, Graham FL. Packaging capacity and stability of human adenovirus type 5 vectors. J Virol 1993; 67(10): 5911–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamen A, Henry O. Development and optimization of an adenovirus production process. J Gene Med 2004; 6 Suppl 1: S184–192. [DOI] [PubMed] [Google Scholar]
- 26.Zhou AC, Wagar LE, Wortzman ME, Watts TH. Intrinsic 4–1BB signals are indispensable for the establishment of an influenza-specific tissue-resident memory CD8 T-cell population in the lung. Mucosal Immunol 2017; 10(5): 1294–1309. [DOI] [PubMed] [Google Scholar]
- 27.Jensen BA, Steffensen MA, Nielsen KN, Christensen JP, Thomsen AR, Holst PJ. Co-expression of tumor antigen and interleukin-2 from an adenoviral vector augments the efficiency of therapeutic tumor vaccination. Mol Ther 2014; 22(12): 2107–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson KG, Sung H, Skon CN, Lefrancois L, Deisinger A, Vezys V et al. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol 2012; 189(6): 2702–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolinger B, Sims S, O’Hara G, de Lara C, Tchilian E, Firner S et al. A new model for CD8+ T cell memory inflation based upon a recombinant adenoviral vector. J Immunol 2013; 190(8): 4162–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tatsis N, Fitzgerald JC, Reyes-Sandoval A, Harris-McCoy KC, Hensley SE, Zhou D et al. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood 2007; 110(6): 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH et al. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol 2003; 170(4): 2022–2029. [DOI] [PubMed] [Google Scholar]
- 32.Holtappels R, Pahl-Seibert MF, Thomas D, Reddehase MJ. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62L(lo) memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J Virol 2000; 74(24): 11495–11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holst PJ, Orskov C, Thomsen AR, Christensen JP. Quality of the transgene-specific CD8+ T cell response induced by adenoviral vector immunization is critically influenced by virus dose and route of vaccination. J Immunol 2010; 184(8): 4431–4439. [DOI] [PubMed] [Google Scholar]
- 34.McMaster SR, Wein AN, Dunbar PR, Hayward SL, Cartwright EK, Denning TL et al. Pulmonary antigen encounter regulates the establishment of tissue-resident CD8 memory T cells in the lung airways and parenchyma. Mucosal Immunol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takamura S, Yagi H, Hakata Y, Motozono C, McMaster SR, Masumoto T et al. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J Exp Med 2016; 213(13): 3057–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pizzolla A, Nguyen THO, Smith JM, Brooks AG, Kedzieska K, Heath WR et al. Resident memory CD8(+) T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci Immunol 2017; 2(12). [DOI] [PubMed] [Google Scholar]
- 37.Finn JD, Bassett J, Millar JB, Grinshtein N, Yang TC, Parsons R et al. Persistence of transgene expression influences CD8+ T-cell expansion and maintenance following immunization with recombinant adenovirus. J Virol 2009; 83(23): 12027–12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakai S, Kauffman KD, Schenkel JM, McBerry CC, Mayer-Barber KD, Masopust D et al. Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J Immunol 2014; 192(7): 2965–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006; 443(7109): 350–354. [DOI] [PubMed] [Google Scholar]
- 40.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009; 10(1): 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slutter B, Van Braeckel-Budimir N, Abboud G, Varga SM, Salek-Ardakani S, Harty JT. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci Immunol 2017; 2(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leon B, Ballesteros-Tato A, Randall TD, Lund FE. Prolonged antigen presentation by immune complex-binding dendritic cells programs the proliferative capacity of memory CD8 T cells. J Exp Med 2014; 211(8): 1637–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Braeckel-Budimir N, Varga SM, Badovinac VP, Harty JT. Repeated Antigen Exposure Extends the Durability of Influenza-Specific Lung-Resident Memory CD8(+) T Cells and Heterosubtypic Immunity. Cell Rep 2018; 24(13): 3374–3382 e3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tatsis N, Lin SW, Harris-McCoy K, Garber DA, Feinberg MB, Ertl HC. Multiple immunizations with adenovirus and MVA vectors improve CD8+ T cell functionality and mucosal homing. Virology 2007; 367(1): 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steffensen MA, Holst PJ, Steengaard SS, Jensen BA, Bartholdy C, Stryhn A et al. Qualitative and quantitative analysis of adenovirus type 5 vector-induced memory CD8 T cells: not as bad as their reputation. J Virol 2013; 87(11): 6283–6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jahn ML, Steffensen MA, Christensen JP, Thomsen AR. Analysis of adenovirus-induced immunity to infection with Listeria monocytogenes: Fading protection coincides with declining CD8 T cell numbers and phenotypic changes. Vaccine 2018; 36(20): 2825–2832. [DOI] [PubMed] [Google Scholar]
- 47.McMaster SR, Wilson JJ, Wang H, Kohlmeier JE. Airway-Resident Memory CD8 T Cells Provide Antigen-Specific Protection against Respiratory Virus Challenge through Rapid IFN-gamma Production. J Immunol 2015; 195(1): 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.