Abstract
Background.
Programs such as the Pediatric Access Line in Washington state have shown decreases in antipsychotic medication use by youth with non-psychotic disorders. Program outcomes have been studied with observational designs. This manuscript describes the protocol for Targeted and Safer Use of Antipsychotics in Youth (SUAY), a randomized controlled trial of psychiatrist review of prescriptions and facilitated access to psychosocial care. The aim of the intervention is to reduce the number of person-days of antipsychotic use among participants.
Methods.
Recruitment occurs at 4 health systems. Targeted enrollment is 800 youth aged 3–17 years. Clinicians are block randomized to intervention versus usual care prior to the study. Youth are nested within the arm of the prescribing clinician. Clinicians in the intervention group receive an EHR-based best practice alert with options to expedite access to psychosocial care and all medication orders are reviewed by a child and adolescent psychiatrist with feedback provided to the prescriber. The primary outcome is person-days of antipsychotic medication use in the 6 months following the initial order. All randomized individuals contribute data regardless of their level of participation (including declining all services).
Discussion.
The trial has been approved by the institutional review boards at each of the 4 sites. The intervention has 4 novel design features including automated recruitment using a best practice alert, psychiatrist medication order review and consultation, telephone navigation to psychosocial care, and telemental health visits. Recruitment began in March of 2018 and will be completed in June 2020. Follow-up will be completed December 31, 2020.
Trial registration:
Keywords: antipsychotic, children, adolescents, psychiatry consults
INTRODUCTION
Beginning in the mid 1990’s, rates of prescribing antipsychotic medications to children and adolescents grew tremendously [1–6] and remain high in both Medicaid and commercially insured populations [6–10]. There is concern about over-prescribing due to potentially severe cardio-metabolic side effects[11, 12]. There are a number of existing algorithms/guidelines for the use of antipsychotic medications including the Texas Medication Algorithm Project (TMAP)[13], Treatment Recommendations for the use of Antipsychotics for Aggressive Youth (TRAAY),[14] and the Practice Parameter for the Use of Atypical Antipsychotic Medications in Children and Adolescents[15]. A recent publication by the Substance Abuse and Mental Health Services Administration also provided guidance on strategies to promote best practice in antipsychotic prescribing[16]. However, all of these guidelines are silent on the clinical resources needed to implement the recommendations. Access to psychosocial interventions as an alternative to antipsychotics is limited by geography, the size of the clinician workforce[17–20], and in some cases prohibitive costs. Access to child and adolescent psychiatrists is also severely limited[17]. Systems of care are needed that enable guideline-concordant care; not just guidelines and prescribing algorithms.
A “second opinion” approach is being used in several states to encourage guideline-concordant care where orders for psychotropic medications exceed recommended doses, involve more than one medication in the same class, or are written for pre-school-age children. Orders meeting these criteria are subject to review by a child and adolescent psychiatrist in order to obtain prior authorization for the order. This workflow is limited in effectiveness because the clinical decision making has already occurred. Though short-term, “emergency” prescription fills may be allowed, the process can also result in significant delay in youth/families obtaining appropriate medications. Nevertheless, programs such as the Pediatric Access Line in Washington state have shown dramatic decreases in antipsychotic medication use by youth with non-psychotic disorders[21].
Based on these results and trends in prescribing, the Mental Health Research Network and Nationwide Children’s Hospital are conducting a pragmatic trial designed to test a 4 component intervention: (1) an EHR-based best practice alert regarding the appropriate first-line use of antipsychotic medications, (2) offers of psychiatry consultations to all prescribing clinicians, (3) assistance to the family with navigating the mental health system to access appropriate, possibly alternative services, and (4) expedited access to psychosocial treatments using telemental health and in-person visits. Our rationale is that an approach with both a prescribing algorithm and augmented clinical workflow is: (a) flexible and patient/family-centered across healthcare settings and technology platforms; (b) collegial, with child and adolescent psychiatrist support for clinical decision making; (c) supportive, with case management (“navigation”) to maximize use of effective, first-line alternatives to antipsychotic medications; and (d) accessible, by expanding access to psychosocial interventions for youth and parents/guardians using telemental health. We will examine whether this program reduces the total person-time using antipsychotic medications in the first six months from treatment initiation compared to a usual care control group.
METHODS
Overview
We began by engaging child and adolescent healthcare providers in primary care and psychiatry for input on how to optimize the design of the proposed intervention[22]. Following principles of human centered design[23], we interviewed providers to understand their antipsychotic prescribing practices and intervention workflow preferences. These results informed adaptations to components of the intervention to ensure both feasibility and potential for effectiveness.
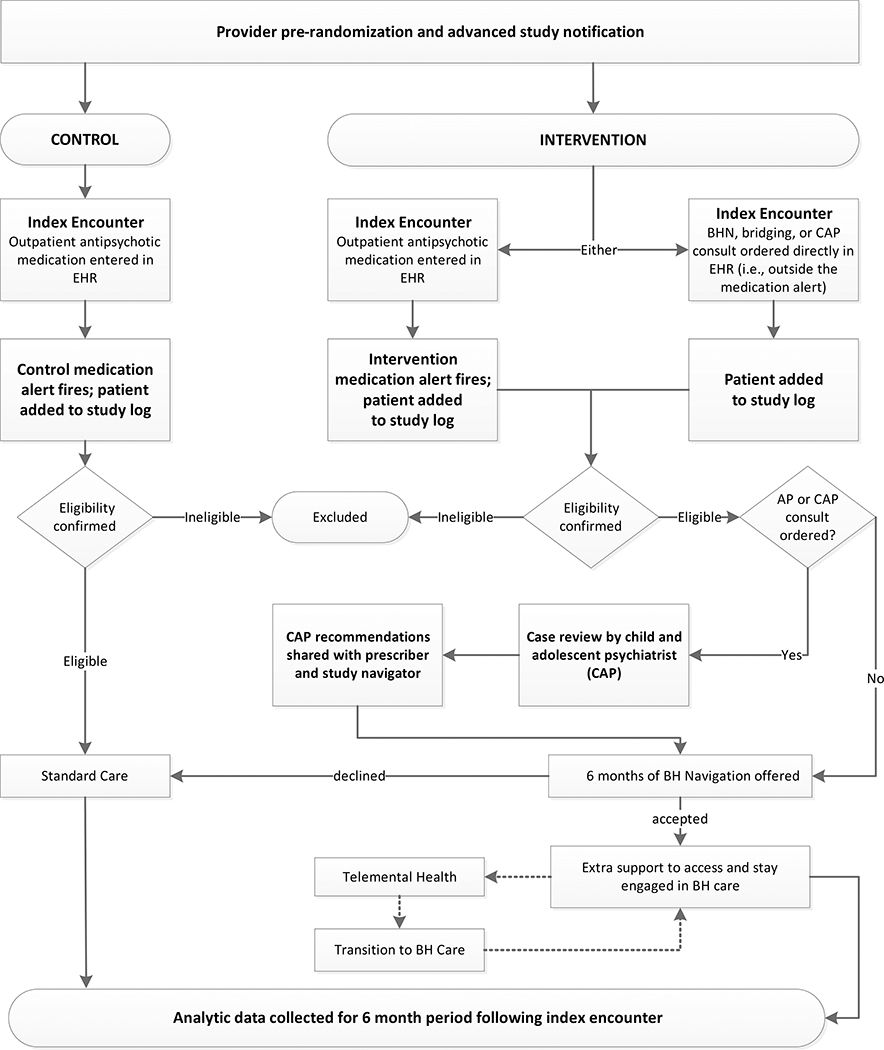
Figure 1 provides an overview of the study design. At participating health systems, one of two best practice alerts (BPAs) appear in the electronic health record (EHR) EpicCare when a randomized prescriber enters an outpatient antipsychotic (AP) order for patients meeting study eligibility criteria per a programmed algorithm. Both of these best practice alerts reference Choosing Wisely® guidelines for prescribing antipsychotics to youth who do not suffer from a psychotic disorder. Informed by provider input[22], the intervention arm BPA also offers the prescriber the following options: (a) remove the medication order, (b) request a provider-to-provider consult with a child and adolescent psychiatrist (CAP) to discuss the case, (3) order behavioral health (BH) navigation and/or telemental health therapy sessions for the patient. All patients identified automatically continue in usual care. Those for whom an intervention arm BPA fired in the encounter are offered extra support engaging (or maintaining engagement) in psychotherapy within the health system or with a contracted provider in the health system’s network.
Figure 1:
Overall Study Design
When warranted, intervention condition services may also include an offer of telemental health (TMH) delivered by a study therapist (clinic-to-home) or in-person therapy sessions (up to 9 sessions of either). The intervention services offered to adolescents and families are meant to supplement usual care, not to replace it. Participants in both arms are free to receive any other services normally available, including pharmacotherapy, individual or group psychotherapy, school-based counseling, or inpatient treatment. BH navigation services are provided primarily by telephone and any study-based therapy by HIPAA compliant clinic-to-home telemental health conferencing or onsite. The percentage of children/youth taking an antipsychotic at the end of the study period, and the number of person-days of antipsychotic use among participants during the study period will be assessed based on medication orders placed within the health system EHR (i.e., provider behavior). Primary evaluation will compare these outcomes by treatment assignment over the 6 months following the index encounter, regardless of level of participation in the intervention program.
Study settings
Study recruitment is conducted within 4 health systems: Kaiser Permanente (KP) Washington, KP Colorado, KP Northwest, and Nationwide Children’s Hospital of Ohio. These health systems provide general medical care as well as mental health specialty care to their respective patient populations. Patients are representative of each health system’s geographic service area in terms of race/ethnicity and age of the pediatric populations served.
Eligibility
Youth included in the trial are:
Receiving a new order for antipsychotic medication at an outpatient encounter.
Aged ≥ 3 and < 18 years on the encounter date when the mediation was ordered.
English-speaking per health system records.
Kaiser Permanente enrollees seen in the integrated group practice; Nationwide Children’s Hospital patients.
Youth are excluded when:
There is a recorded diagnosis of a psychotic disorder, mania, autism spectrum disorder, or intellectual disability in the health system EHR.
An outpatient order for an antipsychotic medication (except prochlorperazine) exists in the health system’s EHR record within the prior 180 days (i.e., to exclude youth with medication refills and switching between antipsychotic medications).
The antipsychotic medication order occurs in an inpatient setting or emergency department setting.
They are KP Colorado patients enrolled in a Medicaid plan in which mental health services are carved out, resulting in inadequate ascertainment of subsequent MH treatments and services.
Randomization and subject accrual
Randomization is at the level of provider; all patients prescribed antipsychotic medication by a given provider are nested within the same study condition as that provider. All providers authorized to order medications within the EHR were randomized at the KP sites and stratified according to the likelihood they would prescribe an antipsychotic based on the prior 2 years of prescription orders. All psychiatrists were assigned to the “likely to prescribe” category. At Nationwide Children’s Hospital, only authorized prescribers in the Psychiatry and Developmental and Behavioral Pediatrics departments were randomized. Randomization was not stratified at Nationwide because both departments were considered ‘likely to prescribe.’
Patients meeting study criteria accrue to the study using “best practice alerts” (BPAs). One of two BPAs is triggered when an order for an antipsychotic is entered in the EHR at an outpatient encounter. Patients inherit the study arm assignment of the prescribing provider and are automatically logged into a registry in the EHR for secondary evaluation of eligibility via targeted chart review by study staff. Intervention arm providers may also accrue study patients by directly ordering study intervention service(s) for a patient without triggering the intervention BPA (i.e., order a consult with the study psychiatrist, navigation for BH services, including “bridging” sessions with a study therapist). These orders can be placed by intervention arm providers regardless of whether the order for the antipsychotic is completed.
Invitation and consent for intervention arm providers and patients
Randomized providers receive a memo from health system leaders to inform providers that the quality improvement research project is being carried out. After receiving the CAP’s suggestions, intervention arm providers are sent messages in the EHR by a study navigator at least 3 days prior to reaching out to the patient/family. Providers may decline the study invitation on behalf of their patient. A positive response, or no provider response within 3 business days results in the navigator reaching out to the patient/family by phone to offer study services. Navigators provide a brief description of the program and elements of informed consent (e.g., study purpose, procedures, participant rights, etc.). Up to 3 cycles of outreach attempts are made to offer intervention arm services. Cycles are 2 weeks apart. For each cycle of outreach, the navigator makes up to 3 attempts to reach the subject within a 1-week period, with up to two messages left. Each participant can decline participation at the time of the initial offer or any time thereafter.
The age of consent for mental health care varies across the states participating in the study (Washington, Oregon, Ohio, Colorado). Youth protected by mental health care privacy laws had the right to consent and not involve a parent, consent to involve a parent, or decline.
Control arm providers and patients
At the time of randomization, control arm providers receive a general memo about a quality improvement research study that will be conducted in the health system. The control arm study BPA message includes a simple reference to Choosing Wisely® safety guidelines for prescribing antipsychotics to youth without psychotic disorders. Usual care arm patients/participants are never contacted by study staff. Providers are not notified regarding participant enrollment.
Child and adolescent psychiatrist review intervention
Rationale
The provider intervention is inspired by the principle of collegial review from the Washington State Pediatric Access Line service[21, 24] and informed by interviews with providers[22]. All antipsychotic medication orders meeting criteria are reviewed by a study CAP via chart review. The study psychiatrist may suggest changes in the type(s) of medication prescribed, changes in dose, and changes or initiation of psychotherapeutic interventions. The intervention is designed to encourage the use of first-line treatments and reduce the duration of antipsychotic medication use.
Antipsychotic prescribing guideline
Prior to the study, we convened a national consensus panel to develop a guideline for prescribing antipsychotic medications to youth based on the target symptoms or complaints reported by youth and families. The study psychiatrists use this guideline to make treatment suggestions to prescribing clinicians.
Study CAPs were internal to the health system at 3 sites: Nationwide Children’s Hospital, KP Colorado and KP Northwest. At these sites, delivery of the case review to the prescriber was supported by existing functions in the health system EHRs including e-consults, telephone notes, and ‘assist’ notes routed to the prescriber – depending on the usual work flow in each system. KP Washington utilized a CAP at Seattle Children’s Hospital. This CAP was external to the health system and granted read-only access to the KP Washington EHR. Referrals and reviews were faxed between KP Washington and Seattle Children’s. At all sites, the study CAP reached out by phone to an intervention arm prescriber who actively ordered a CAP consult or if the CAP needed additional information about the case to complete a review.
Training and supervision
Training of study CAPS across sites was conducted via site visits and teleconference, led by clinical investigators from the KP Washington site and Dr. Robert Hilt of Seattle Children’s Hospital. Initial training covered the overall study protocol, clinical guidance for the safer prescribing of antipsychotic medications for youth (SUAY) developed by a national consensus panel in phase 1 of the contract, informatics tools and CAP review template forms, and best practices for providing unsolicited advice to prescribers, including fellow psychiatrists.
Ongoing telephone ‘supervision’ for all CAPs was led by the KP Washington principal investigator and Dr. Bob Hilt of Seattle Children’s Hospital. Meetings are scheduled monthly throughout the enrollment period for case discussion, collegial support, and procedural Q&A. In keeping with the pragmatic trial practices and principles[25], no detailed monitoring of intervention fidelity (e.g., review of content of CAP recommendations to the SUAY clinical guidance document) is conducted.
Engagement with health system stakeholders
Meetings with clinical leaders at sites were held during the development phases of the contract (including adolescent medicine/primary care, mental health specialty care, and clinical informatics) to develop consensus regarding content and workflow of the provider intervention, including:
Language for initial outreach messages to all randomized providers;
Language for the intervention best practice alert;
Processes for study CAPs to communicate with prescribers inside the health system.
Family/Youth intervention
Rationale
Parents, guardians and adolescents often have difficulty navigating systems of behavioral health care[26–30]. Therapists and psychiatrists often belong to different health systems or have independent clinics, reducing the opportunity for team care and continuity for these complex patients. Across Kaiser Permanente regions, psychotherapy is often provided by clinicians external to the integrated delivery system. Similarly, youth who receive care at hospitals such as NCH often receive parts of their mental health care in the hospital and part in the community. Furthermore, it is frequently difficult for patients and families to identify therapists in the community who provide a clinically appropriate therapeutic modality of therapy with demonstrated effectiveness for this patient population, and who are accepting patients.
Finally, it is often the case that youth and families are not motivated to participate in psychotherapy. Youth often decline to attend therapy sessions and it is often difficult to coordinate work and school schedules with multiple therapy sessions over time.
BH Navigator role
The navigator role is two-fold. First, navigators reach out to youth and families to assess barriers to mental health counseling, problem-solve identified barriers, and encourage and motivate engagement in care if they are not currently attending psychotherapy sessions. Second, navigators help youth and families find clinicians who offer particular modalities of evidence based mental health counseling suitable/relevant to the presenting issues, and who are accepting new patients.
TMH therapist role
There is typically a delay in identifying therapists with particular skills in working with these (often complex) patients, and who are accepting patients. The role of the study therapist is to provide short-term (up to 9 one-hour sessions) psychotherapy to youth in an effort to engage them quickly. The study therapist, in cooperation with the navigator, also works to transition youth to longer-term care with non-study clinicians as soon as possible.
Navigators and therapists are employees of the health systems at all 4 sites. Delivery of the BH navigation intervention to youth/families is supported by the following functions in the health system EHRs: Secure messaging for navigators’ communication with primary care and mental health specialty providers; population-management and reporting tools for case management and follow-up.
Training and supervision
BH navigators are credentialed mental health professionals. Training of navigators was conducted via videoconference and teleconference, led by Deborah King, LICSW of the KP Washington site. Initial training included:
1 hour of general training to project aims and overall protocol;
2 hours of training on initial outreach and interventional offering; and
4 hours of specific training regarding intervention delivery, motivational interviewing, informatics tools, and study procedures.
Ongoing telephone consultation and oversight for all study navigators was led by Ms. King from the KP Washington site. Navigator meetings were initially held weekly and scheduled bi-weekly thereafter. Consistent with the practices and principles of pragmatic trials[25], no detailed monitoring of intervention fidelity (e.g., review of case notes or contact logs) was conducted.
Engagement with health system stakeholders
Meetings with clinical leaders at all sites were held during the development phases of the contract (including adolescent medicine/primary care, mental health specialty care, and clinical informatics) to develop consensus regarding content and workflow of the provider and teen/family intervention components, including:
Processes for study navigators to communicate with study CAPs within and outside of the health system;
Processes for study navigators and therapists to communicate with providers inside the health system;
Processes for coordinating navigation services and study therapy with other care and support patients may be receiving from the health system (e.g., following an inpatient stay).
Outcomes definitions
Our primary outcomes are percent of patients taking antipsychotic medication at 6 months post enrollment and total person-months of antipsychotic use by youth, based on medication order data. As secondary outcomes, we will also compare the percent of patients taking antipsychotic medication at 6 months post enrollment and total person-months of antipsychotic use by youth, based on pharmacy prescription dispensing data.
Statistical Analysis plan
All primary analyses will follow an intent-to-treat approach, including all individuals assigned to an intervention arm regardless of the amount of intervention received.
Primary Analysis 1
We will estimate the odds ratio (OR) of antipsychotic use observed at 6 months between intervention and control. For this analysis, we will use a logistic regression model where the outcome is a binary indicator of antipsychotic use observed at 6 months. Binary treatment arm assignment and psychiatric hospitalization in the prior year will be the only covariates included in the regression model. The estimated coefficient associated with treatment is interpreted as the log-OR of observing antipsychotic use at six months for an intervention patient vs. a control patient. Patient is the unit of analysis, GEE will be used to account for correlation within provider, and robust standard errors (with working independence covariance matrix assumed) will be calculated to construct a 95% CI around the estimated OR. Our null hypothesis is that the OR is equal to 1, corresponding to no statistically significant difference between antipsychotic use observed at 6 months. We will reject the null hypothesis if the lower bound of the 95% CI is above 1 (increased use in treatment arm) or the upper bound is below 1 (decreased use in treatment arm).
Exploratory subgroup analysis will be the Heterogeneity of Treatment Effects (HTE), conducted using adjustment variables in the model. To perform HTE analyses, we will use the same analysis procedure for the primary outcome but now including adjustment variables indicated above separately, with an interaction term between the treatment and the covariate. HTE analyses are prespecified for: provider type (MH vs. non-MH), , gender, age, race/ethnicity (composite variable), new health plan enrollee, and insurance (Medicaid vs. non-Medicaid).
Primary analysis 2
Our primary analysis examines antipsychotic medication use, as measured by days’ supply ordered, in the 6 months following patient enrollment.
We will estimate the relative risk (RR) of antipsychotic use between intervention and control during the 6 months post-enrollment. For this analysis, we will use Poisson regression where the outcome is the total days of antipsychotic supply (maximum of 181) for each enrolled patient and the offset is the number of days of follow-up. For the primary analysis, treatment arm and psychiatric hospitalization in the prior year will be the only covariates included in the regression model (additional covariate adjustment will be done as a sensitivity analysis), and the estimated coefficient for treatment arm is interpreted as the log-RR of antipsychotic use for an intervention patient vs. a control patient. Patient will be the unit of analysis for this regression model, and the regression model will be fit using generalized estimating equations (GEE) to account for correlation within provider. We will estimate standard errors using a robust (sandwich) covariance estimation (assuming a working independence covariance structure) and construct a 95% confidence interval (CI) around the estimated RR. Our null hypothesis is that the RR is equal to 1, corresponding to no statistically significant difference between antipsychotic order rates in treatment and control patients. If the upper bound of the 95% CI is below 1, we will reject the null hypothesis and conclude that the RR of antipsychotic use is lower for intervention vs. control patients in the 6 months following enrollment (and vice-versa for a 95% CI above 1).
Secondary Analyses
We have several secondary analyses planned:
Compare Emergency Department and Urgent Care visit frequency, both for psychiatric crises and for other reasons between the study groups;
Compare the percentage of youth with baseline and follow-up critical laboratory assessments (BMI measurement and metabolic panel laboratory results);
Compare the percentage of study clinicians with at least one change in antipsychotic regimen (discontinuation, treatment adjustment, and therapy referral);
Compare the percentage of patients attending two or more system-provided psychotherapy appointments in the 6 months following study enrollment
Compare the percent of eligible patients with any antipsychotic order after the provider’s first exposure to the BPA.
Sample size
We plan to enroll 800 eligible patients in the trial (400 per arm), with up to 360 eligible patients in each arm (720 total) remaining enrolled at 6 months of follow-up (i.e., 10% lost to follow-up). Assuming a P<0.05 alpha level and based on an anticipated 95% of patients in the control arm using antipsychotics at 6 months, this sample will ensure over 95% power to detect a 6% reduction in the percent of participants with any order for antipsychotic medications at 6 months. Furthermore, we expect to have 80% power to detect a 13% reduction in subgroups with exposures occurring with 25% prevalence, and 80% power to detect a 17% reduction in subgroups with exposures occurring with 18% prevalence (e.g., racial/ethnic minorities). A minimal clinically important difference of 10% was prespecified to allow for evaluation of clinical significance as well as statistical significance as well as statistical significance. We have over 90% power to detect a 10% reduction in antipsychotic use at 6 months for patients in the intervention arm given a rate of 85%, 90%, or 95% use in the control arm.
Enrollment progress
The trial is funded by National Institute of Mental Health through a contract administered by the National Institute on Drug Abuse. A pilot study was completed at 2 of the 4 trial sites (KP Washington and Nationwide Children’s Hospital) to demonstrate feasibility and acceptability of the intervention to patients and providers. The trial was implemented at KP Washington in March 2018, expanding to 3 sites by August 2018. A fourth site (KP Northwest) initiated the protocol in May 2019. The study had enrolled 728 eligible subjects as of June 1, 2020 with targeted enrollment of 800 participants.
Ethical and regulatory approval
The study protocol and procedures were reviewed and approved by Institutional Review Boards at all four participating health systems.
Waiver of informed consent
Waivers of consent are granted at each site for both providers and patients. Patients accrue to the study arm assigned to the treating provider without first consenting the youth participants or parents/guardians. This modified Zelen design is necessary because we intend to test whether the offer of intervention services changes the duration of use of APs and uptake of navigation services and psychotherapy. Including only those who consent to participation would result in a highly biased group of study participants who are willing to change their use of services. Study staff have no contact with control arm participants. Outcomes will be measured solely through the use of administrative data as we have done in a suicide prevention trial[31].
While it is not practicable to obtain teen/parental consent prior to enrollment and randomization due to the study design, it is practicable to provide appropriate information to youth/families at the time intervention services are offered. As described above, navigators discuss elements of informed consent to intervention participants. This includes a short description of the study purpose, procedures, potential risks, and the right to decline the intervention offered. Participants who decline the offer to participate are still included in the outcomes analysis (using administrative data).
Defining minimal risk
Regulations concerning the protections of human research subjects allow for waivers of consent/assent for research involving no more than minimal risk. Our proposal to waive the requirement for informed consent was viewed differently by IRBs across the four health systems. Two IRBs expedited the protocol, two others brought it for full Board review. Extensive conversations and multiple IRB submissions were required at the KP Washington site to come to agreement on the following:
SUAY was a minimal risk intervention in a high-risk population;
Those in usual care receive the same care they would have had the study not occurred. No services or medications are restricted or withheld from control arm providers or patients;
The autonomy of the prescribing clinician is in no way diminished. They are free to follow or ignore the CAP advise based on their first-hand knowledge of the clinical scenario and their best clinical judgement. In addition, no services or medications are restricted or withheld from intervention arm providers or patients;
Participants are free to receive any other services that are normally available. Participants are assigned to the offer of intervention, with no obligation to participate. Invitational phone scripts clearly state the offerings are part of a research study, make no promise of benefit, and advise that participation is voluntary.
At all participating health systems, notices regarding privacy practices advise patients specifically about the use of health records for research purposes. Members who have previously requested exclusion from research contact are excluded from the study best practice alerts.
Monitoring for adverse events
The traditional approach[32] for monitoring of adverse events in a clinical trial (i.e., immediate reporting and review to assess “relatedness” to study participation) was not appropriate for the study. First, external claims for inpatient hospitalizations as well as any death data would not be available for 3 to 6 months or more following an event. Second, the study has no contact with control arm patients. We can only use events that generate administrative claims to compare rates of suicide attempt, emergency department, and urgent care use. Only by comparing these rates between the usual care control and the intervention arm can any possible increased risk be observed.
Interim analysis of benefit or harm
Interim analyses to evaluate the benefit of the provider or family/youth intervention are not planned. Early detection of a benefit of either of these interventions is unlikely, and early termination of intervention delivery would return all subjects to care-as-usual and therefore not offer any added protection for ongoing or potential participants.
Interim analysis to test for evidence of significant harm (increased risk of death, increased use of inpatient or urgent/emergent health care services) in the intervention group compared to the usual care group is planned. Clear evidence that the intervention resulted in increased risk of such harm would warrant suspending the delivery of the intervention and assignment of additional patients to that arm. Interim analyses comparing risks of these adverse events between study arms are conducted three times per year. Interim harm reports are delivered to the National Institute of Mental Health Data and Safety Monitoring Board by the study statistician. The rest of the investigative team is blinded to these reports.
Data and resource sharing
The following deliveries will be made to the National Institutes of Health at the end of the contractual period: (1) de-identified version of the analytic dataset for the pragmatic clinical trial; (2) a statistical report addressing the research questions of the study, and (3) a cost-benefit analysis of the intervention. Additional study resources (e.g., invention materials, clinical algorithm, specifications, computer code, etc.) will be shared at or before the publication of study results.
DISCUSSION
This trial promoting the safer and targeted use of antipsychotic medications in youth has important health system implications. By 2014, 31 states had active programs to manage antipsychotic prescribing to youth insured by Medicaid[16]. This trial will answer whether automatic psychiatrist review of antipsychotic medication orders meeting criteria, combined with navigator outreach and navigated access to psychosocial interventions reduces the use of these medications by youth who do not have a diagnosis for a psychotic disorder, mania, irritability associated with autism, or intellectual disability. The trial has several novel design elements that might inform the conduct of future trials and implementation of study results.
Study setting
We deliberately chose trial performance sites with different characteristics that will increase the generalizability of study findings. The three Kaiser Permanente sites limit recruitment to patients seen in the integrated group practice where coordination between pediatricians and child and adolescent psychiatrists is easier; there is a shared medical record between pediatricians, psychiatrists, and therapists; and the health system (by virtue of being both an insurer and a care provider) can better align payment and delivery of care. On the other hand, the departments of Developmental Pediatrics and Psychiatry at Nationwide Children’s Hospital see a higher volume of patients and more acutely ill patients, treat a greater proportion of youth insured by Medicaid, and have more external clinicians with whom coordination is necessary (but who do not share the same EHR).
Staffing
Our trial is different from a purely pragmatic design insofar as the navigators at 3 sites are clinically trained staff employed by the embedded research institutes. At NCH, the navigators are clinical staff who already perform other care coordination with youth/families and providers within and outside the hospital setting. Thus, we will learn something about the design of staffing for navigator roles and the degree to which they need to be closely tied to a patient’s care team.
Regarding psychiatrist staffing, Kaiser Permanente Washington employed a child and adolescent psychiatrist outside the Kaiser system to perform reviews of medication orders and charts. The remaining sites employed psychiatrists within the delivery system that are familiar to other clinicians. This affords an opportunity to learn about how collegial review offered from someone “outside” will be received by prescribers of antipsychotics. It will also allow us to learn about how reviews are received when performed by colleagues in the same department – sometimes when the reviewer is more junior than the person whose medication order is reviewed.
Pre-randomization and subject accrual
We obtained lists of all providers in each system with prescribing authority (limited to two departments at NCH) and randomized them to the intervention and control arms. This allowed programming in the EHR that would trigger the control arm or intervention arm best practice alert and assign the patient to the respective arm of the provider. This approach has pros and cons. One benefit is that patients are automatically entered into a database of eligible patients. This is efficient from a staffing and cost perspective because it is not necessary to advertise or approach patients in waiting rooms. One limitation is that a best practice alert is triggered and the patient identified as potentially eligible before the encounter is closed and any new, potentially exclusionary diagnoses are entered as part of the qualifying visit or medication order. Thus, exclusion diagnoses entered for the first time at a visit where a new antipsychotic medication is ordered cannot be used to exclude patients in advance programmatically. A secondary eligibility step, via brief chart review, is necessary to confirm eligibility.
Another interesting component of patient recruitment is that all patients and families are contacted by telephone to offer navigation to psychosocial services. At the Kaiser Permanente sites, the age of consent for mental health is 12 years (Washington) 14 years (Oregon) and 15 years (Colorado). The age of consent for mental health treatment in Ohio is 15; however, At NCH, all parents/guardians are made aware of the youth’s mental health service use. Reaching out to offer study services by telephone to youth older than the age of consent means that we must obtain each adolescent’s permission to disclose information to parents/guardians before we can tell parents/guardians the purpose of the phone call. Parents/guardians are understandably suspicious when the navigator informs them that we must obtain their child’s permission to talk to them. Most parents/guardians have allowed the study navigator to speak to their adolescent children.
Nature of Clinical Review
As highlighted in the recent SAMHSA guidance[16] on promoting best practices in antipsychotic prescribing, relatively little is known about the effectiveness of mandatory review programs. In this trial, all antipsychotic medication orders meeting inclusion criteria are reviewed by the study child and adolescent psychiatrist – including a brief chart review of the clinical circumstances. A review form is completed and sent to the prescribing provider within no more than10 business days of the encounter where the order was submitted. Our preliminary observations suggest that some providers (particularly psychiatrists) feel this is unnecessary oversight. There was also concern about legal liability if the recommendations of the review were not followed. In other cases, providers are highly appreciative for the second opinion and schedule follow-up telephone calls. We have also observed across-the-board increases in the amount of documentation concerning the clinical decision-making around prescribing the antipsychotic medication after exposure to the intervention.
CONCLUSION
This trial will advance our understanding of the effectiveness of prescription review programs that include facilitation of access to first-line mental health treatments. The approach and novel design elements will inform the development of systems that enable guideline-concordant care and thereby improve outcomes with lower risk of serious medication side effects.
TRIAL STATUS
Enrollment is ongoing and is expected to be complete in June 2020. Follow-up will end December 31, 2020.
Acknowledgments
Funding acknowledgement: This project is supported by the National Institute of Mental Health (NIMH) of the National Institutes of Health (NIH) under contract number HHSN271201600002C, A Targeted Approach to a Safer Use of Antipsychotics in Youth (total award $9,658,552; no project costs were financed by nongovernmental sources). This information or content and conclusions are those of the author and should not be construed as the official position or policy of, nor should any endorsements be inferred by NIMH or NIH.
Footnotes
Financial Disclosures:
In the last two years, BV was consultant for Medice and Lundeck Pharmaceuticals, and for the law firms Goodwin & Procter and Haynes & Boone. He holds no stocks of pharmaceutical companies.
Trial registration number and trial register: Clinicaltrials.gov NCT03448575
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Olfson M, Marcus SC, Weissman MM, Jensen PS, National trends in the use of psychotropic medications by children, J Am Acad Child Adolesc Psychiatry 41(5) (2002) 514–21. [DOI] [PubMed] [Google Scholar]
- [2].Zito JM, Safer DJ, dosReis S, Gardner JF, Boles M, Lynch F, Trends in the prescribing of psychotropic medications to preschoolers, JAMA 283(8) (2000) 1025–30. [DOI] [PubMed] [Google Scholar]
- [3].Zito JM, Safer DJ, DosReis S, Gardner JF, Magder L, Soeken K, Boles M, Lynch F, Riddle MA, Psychotropic practice patterns for youth: a 10-year perspective, Arch Pediatr Adolesc Med 157(1) (2003) 17–25. [DOI] [PubMed] [Google Scholar]
- [4].Zito JM, Safer DJ, Sai D, Gardner JF, Thomas D, Coombes P, Dubowski M, Mendez-Lewis M, Psychotropic Medication Patterns Among Youth in Foster Care, Pediatrics 121(1) (2008) e157–e163. [DOI] [PubMed] [Google Scholar]
- [5].Crystal S, Olfson M, Huang C, Pincus H, Gerhard T, Broadened Use Of Atypical Antipsychotics: Safety, Effectiveness, And Policy Challenges, Health Affairs 28(5) (2009) w770–w781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Olfson M, Crystal S, Huang C, Gerhard T, Trends in antipsychotic drug use by very young, privately insured children, J Am Acad Child Adolesc Psychiatry 49(1) (2010) 13–23. [DOI] [PubMed] [Google Scholar]
- [7].Penfold RB, Hunkeler EM, Madden JM, Cummings JR, Owen-Smith AA, Rossom RC, Lu C, Lynch FL, Waitzfelder BE, Coleman KA, Ahmedani BK, Beck AL, Zeber JE, Simon GE, Use of antipsychotic medications in pediatric populations: what do the data say?, Curr Psychiatry Rep In Press (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Olfson M, Blanco C, Liu SM, Wang S, Correll CU, National Trends in the Office-Based Treatment of Children, Adolescents, and Adults With Antipsychotics, Arch Gen Psychiatry (2012) 1–10. [DOI] [PubMed] [Google Scholar]
- [9].Merikangas KR, He JP, Rapoport J, Vitiello B, Olfson M, Medication use in US youth with mental disorders, JAMA Pediatr 167(2) (2013) 141–8. [DOI] [PubMed] [Google Scholar]
- [10].Crystal S, Mackie T, Fenton MC, Amin S, Neese-Todd S, Olfson M, Bilder S, Rapid Growth Of Antipsychotic Prescriptions For Children Who Are Publicly Insured Has Ceased, But Concerns Remain, Health Aff (Millwood) 35(6) (2016) 974–82. [DOI] [PubMed] [Google Scholar]
- [11].Brunette MF, Cotes RO, de Nesnera A, McHugo G, Dzebisashvili N, Xie H, Bartels SJ, Use of Academic Detailing With Audit and Feedback to Improve Antipsychotic Pharmacotherapy, Psychiatr Serv 69(9) (2018) 1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nicol GE, Yingling MD, Flavin KS, Schweiger JA, Patterson BW, Schechtman KB, Newcomer JW, Metabolic Effects of Antipsychotics on Adiposity and Insulin Sensitivity in Youths: A Randomized Clinical Trial, JAMA Psychiatry 75(8) (2018) 788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pliszka SR, Crismon ML, Hughes CW, Corners CK, Emslie GJ, Jensen PS, McCracken JT, Swanson JM, Lopez M, Texas D Consensus Conference Panel on Pharmacotherapy of Childhood Attention Deficit Hyperactivity, The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder, J Am Acad Child Adolesc Psychiatry 45(6) (2006) 642–57. [DOI] [PubMed] [Google Scholar]
- [14].Pappadopulos E, Macintyre Ii JC, Crismon ML, Findling RL, Malone RP, Derivan A, Schooler N, Sikich L, Greenhill L, Schur SB, Felton CJ, Kranzler H, Rube DM, Sverd J, Finnerty M, Ketner S, Siennick SE, Jensen PS, Treatment recommendations for the use of antipsychotics for aggressive youth (TRAAY). Part II, J Am Acad Child Adolesc Psychiatry 42(2) (2003) 145–61. [DOI] [PubMed] [Google Scholar]
- [15].Findling RL, Drury S, Jensen PS, Rapoport J, Practice Parameter for the Use of Atypical Antipsychotic Medications in Children and Adolescents, (2011) 27.
- [16].Substance Abuse and Mental Health Services Administration, Guidance on Strategies to Promote Best Practice in Antipsychotic Prescribing for Children and Adolescents, Office of Chief Medical Officer. Substance Abuse and Mental Health Services Administration, Rockville, MD, 2019. [Google Scholar]
- [17].Cama S, Malowney M, Smith AJB, Spottswood M, Cheng E, Ostrowsky L, Rengifo J, Boyd JW, Availability of Outpatient Mental Health Care by Pediatricians and Child Psychiatrists in Five U.S. Cities, Int J Health Serv 47(4) (2017) 621–635. [DOI] [PubMed] [Google Scholar]
- [18].Straus JH, Sarvet B, Behavioral health care for children: the massachusetts child psychiatry access project, Health Aff (Millwood) 33(12) (2014) 2153–61. [DOI] [PubMed] [Google Scholar]
- [19].Harrison J, Wasserman K, Steinberg J, Platt R, Coble K, Bower K, The Five S’s: A Communication Tool for Child Psychiatric Access Projects, Curr Probl Pediatr Adolesc Health Care 46(12) (2016) 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Finnerty M, Neese-Todd S, Pritam R, Leckman-Westin E, Bilder S, Byron SC, Hudson Scholle S, Crystal S, Olfson M, Access to Psychosocial Services Prior to Starting Antipsychotic Treatment Among Medicaid-Insured Youth, J Am Acad Child Adolesc Psychiatry 55(1) (2016) 69–76 e3. [DOI] [PubMed] [Google Scholar]
- [21].Barclay RP, Penfold RB, Sullivan D, Boydston L, Wignall J, Hilt RJ, Decrease in Statewide Antipsychotic Prescribing after Implementation of Child and Adolescent Psychiatry Consultation Services, Health Serv Res 52(2) (2017) 561–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hartzler AL, Ralston JD, A.H. T, Kelleher KJ, Penfold RB, Designing Safer Use of Antipsychotics Among Youths: A Human-Centered Approach to an Algorithm-Based Solution, Psychiatr Serv 70(10) (2019) 944–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Johnson CM, Johnson TR, Zhang J, A user-centered framework for redesigning health care interfaces, J Biomed Inform 38(1) (2005) 75–87. [DOI] [PubMed] [Google Scholar]
- [24].Hilt RJ, Romaire MA, McDonell MG, Sears JM, Krupski A, Thompson JN, Myers J, Trupin EW, The Partnership Access Line: evaluating a child psychiatry consult program in Washington State, JAMA Pediatr 167(2) (2013) 162–8. [DOI] [PubMed] [Google Scholar]
- [25].Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, Tunis S, Bergel E, Harvey I, Magid DJ, Chalkidou K, A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers, J Clin Epidemiol 62(5) (2009) 464–75. [DOI] [PubMed] [Google Scholar]
- [26].Markoulakis R, Turner M, Wicik K, Weingust S, Dobbin K, Levitt A, Exploring Peer Support Needs of Caregivers for Youth with Mental Illness or Addictions Concerns in Family Navigation Services, Community Ment Health J 54(5) (2018) 555–561. [DOI] [PubMed] [Google Scholar]
- [27].Markoulakis R, Chan S, Levitt A, Identifying the key features and outcomes of family navigation services for mental health and/or addictions concerns: a Delphi study, BMC Health Serv Res 19(1) (2019) 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Godoy L, Hodgkinson S, Robertson HA, Sham E, Druskin L, Wambach CG, Beers LS, Long M, Increasing Mental Health Engagement From Primary Care: The Potential Role of Family Navigation, Pediatrics 143(4) (2019). [DOI] [PubMed] [Google Scholar]
- [29].Yang Y, Dillon EC, Li M, Li J, Erlich KJ, Heneghan AM, Becker DF, Primary care provider utilization and satisfaction with a health system navigation program for adolescents with behavioral health needs, Transl Behav Med 9(3) (2019) 549–559. [DOI] [PubMed] [Google Scholar]
- [30].Sanci L, Kauer S, Thuraisingam S, Davidson S, Duncan AM, Chondros P, Mihalopoulos C, Buhagiar K, Effectiveness of a Mental Health Service Navigation Website (Link) for Young Adults: Randomized Controlled Trial, JMIR Ment Health 6(10) (2019) e13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Simon GE, Beck A, Rossom R, Richards J, Kirlin B, King D, Shulman L, Ludman EJ, Penfold R, Shortreed SM, Whiteside U, Population-based outreach versus care as usual to prevent suicide attempt: study protocol for a randomized controlled trial, Trials 17(1) (2016) 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].NIH Collaboratory Grand Rounds Presentation: Data and safety monitoring in pragmatic trials. . https://dcricollab.dcri.duke.edu/sites/NIHKR/KR/GR%20Slides%2002-13-15.pdf. (Accessed June 17, 2019.