Abstract
Gap junction intercellular communication (GJIC) is necessary for ovarian function, and it is temporospatially regulated during follicular development and ovulation. At outermost layer of the antral follicle, theca cells provide structural, steroidogenic, and vascular support. Inter- and extra-thecal GJIC is required for intrafollicular trafficking of signaling molecules. Because GJIC can be altered by hormones and endocrine disrupting chemicals (EDCs), we tested if any of five common EDCs (bisphenol A (BPA), bisphenol S (BPS), bisphenol F (BPF), perfluorooctanesulfonic acid (PFOS), and triphenyltin chloride (TPT)) can interfere with theca cell GJIC. Since most chemicals are reported to repress GJIC, we hypothesized that all chemicals tested, within environmentally relevant human exposure concentrations, will inhibit theca cell GJICs. To evaluate this hypothesis, we used a scrape loading/dye transfer assay. BPS, but no other chemical tested, enhanced GJIC in a dose- and time-dependent manner in ovine primary theca cells. A signal-protein inhibitor approach was used to explore the GJIC-modulatory pathways involved. Phospholipase C and mitogen-activated protein kinase (MAPK) inhibitors significantly attenuated BPS-induced enhanced GJIC. Human theca cells were used to evaluate translational relevance of these findings. Human primary theca cells had a ~40% increase in GJIC in response to BPS, which was attenuated with a MAPK inhibitor, suggestive of a conserved mechanism. Upregulation of GJIC could result in hyperplasia of the theca cell layer or prevent ovulation by holding the oocyte in meiotic arrest. Further studies are necessary to understand in vitro to in vivo translatability of these findings on follicle development and fertility outcomes.
Keywords: theca cells, bisphenol S, gap junction intercellular communication, connexin, ovary
1. Introduction
Nearly one out of every six couples suffer from infertility; a quarter of which remain etiologically unexplained, and a third are of female origin (Thoma et al., 2013; Practice Committee of the American Society for Reproductive, 2015). The basis for female infertility often relates to ovarian dysfunction and includes diseases that have steroid hormone imbalances such as polycystic ovarian syndrome, premature ovarian insufficiency, and luteal dysfunction (CDC, 2017). There is growing evidence that exposure to environmental contaminants may be a contributing factor to the rising infertility trend over the past four decades as they have been reported to affect hypothalamic-pituitary-gonadal axis regulation alter ovarian function including steroidogenic dysfunction, alteration in follicular development, oocyte maturation, and uterine dysfunction resulting in reduced implantation (Rattan et al., 2017) and, placental dysfunction (Strakovsky and Schantz, 2018; Gingrich et al., 2020).
The ovarian antral follicle is comprised of two distinct cell types that sustain oocyte development and maturation; granulosa and theca. Theca cells are steroidogenic cells at the outer layer of the follicle that provide structural and vascular support, and androgen and progesterone synthesis (Young and McNeilly, 2010). Prior to ovulation, theca cells synthesize testosterone that is transferred extracellularly into neighboring granulosa cells for estrogen synthesis. Intercellular communication within the follicle and transport of testosterone across follicular layers is thus essential for follicular growth and oocyte maturation (Orisaka et al., 2009). Regulated through gonadotropin-mediated events (Sommersberg et al., 2000; Norris et al., 2008; El-Hayek and Clarke, 2015), gap junction intercellular communication (GJIC) within the follicle is also critical for oocyte meiotic arrest and resumption. Granulosa cells allow intercellular transfer of cGMP to the oocyte which indirectly increases the local concentration of cAMP, holding the cell in a state of meiotic arrest via protein kinase A (PKA)-induced cell signaling (Shuhaibar et al., 2015). Reduction in cGMP, driven in part through a mitogen-activated protein kinase (MAPK)-dependent mechanism (Norris et al., 2008), allows phosphodiesterase 3A (PDE3a)-controlled cAMP hydrolysis resulting in meiotic resumption and ovulation (Mehlmann, 2005; Richards and Ascoli, 2018). Theca cells aid in control of the ovulatory process through a steroid feedback loop (Young and McNeilly, 2010) and by stimulation of proliferation and anti-apoptotic responses in granulosa cells (Orisaka et al., 2009). Upon ovulation, granulosa and theca cells undergo luteinization, a cellular differentiation process that includes an estrogen-to-progestogen shift in follicular steroidogenesis. This shift towards progesterone synthesis is essential for fertilization, embryo implantation, and pregnancy maintenance in mammalian species. To note, connexin 37 (Cx37) gap junctions have been implicated in the transfer of important, yet unidentified signals, from the oocyte to the granulosa cells to prevent luteinization prior to ovulation (Winterhager and Kidder, 2015). Theca cells functions are modulated by steroid hormones, growth factors, and bone morphogenetic proteins (BMP) (Young and McNeilly, 2010). Additionally, they are highly sensitive to a variety of stimuli, including nutrition (Williams et al., 2001), stress (Zhu et al., 2016), heat stress (Nteeba et al., 2015), and bacterial insult (Magata et al., 2014). Increasing evidence also suggests that endocrine disrupting chemicals (EDCs) can disrupt ovarian function (Patel et al., 2015; Craig and Ziv-Gal, 2018). However, studies specifically addressing the effects of EDCs on theca cells are scarce, but have identified that dioxins, genistein, tyrphostin and herbimycin can reduce progesterone synthesis (Gregoraszczuk et al., 1999; Grochowalski et al., 2001). In the rat, in utero exposure to di-2-ethylhexyl phthalate, a common plasticizer, reduced the size of the theca cell layer in offspring gestationally exposed (Meltzer et al., 2015). Although theca cells remain fairly understudied as a target of EDC toxicity, theca cells are critical for ovarian function whose alteration can lead to ovarian pathologies, such as polycystic ovarian syndrome, hyperthecosis, primary ovarian insufficiency, and premature ovarian failure, that can result in sub- or infertility (Richards et al., 2018).
Gap junctions are intercellular communication channels that are formed by apposition of connexin proteins organized into hexameric connexons. Once connexons from two adjacent cells dock end-to-end, intercellular channels are formed to allow diffusion of molecules <1 kDa in mass or <1.6 nm in diameter, a process known as gap junction intercellular communication. Assembly and activation of connexon channels can occur through multiple established pathways including protein kinase C (PKC) (Long et al., 2007), PKA (Pidoux et al., 2014), phosphatidyl choline-phospholipase C (PC-PLC) (Machala et al., 2003a; Upham et al., 2008) and MAPK (Warn-Cramer et al., 1996). Connexins are expressed in the ovary of mammalian species (Gershon et al., 2008), including humans (Furger et al., 1996; Wang et al., 2009). There are over twenty connexin genes in vertebrates, which are expressed in a tissue and a cell-specific manner (Sohl and Willecke, 2004b; Srinivas et al., 2018). Connexin 43 (Cx43) is the most highly expressed connexin in the ovary and it is essential for ovarian follicle formation in mice (Juneja et al., 1999; Ackert et al., 2001). However, Cx43 knockout mice are embryonic lethal, making reproduction in the absence of Cx43 difficult to study (Nishii et al., 2014) as no Cx43 ovarian conditional knockout mice have been developed to date. However, when compared to ovaries from Cx43+/+ wildtype mice, cultured ovaries from Cx43−/− knockout mice resulted in folliculogenesis arrest at the primary (Juneja et al., 1999) or secondary follicle stage (Ackert et al., 2001). In the ovary, formation of gap junctions allows the development of a metabolic syncytium between the oocyte and its supporting cells (Kidder and Vanderhyden, 2010), but can also enable theca-to-granulosa paracrine signaling via ATP release (Tong et al., 2007). Connexin expression in the antral follicle is a dynamic process. Specifically, Cx43 has reduced expression during the peri-ovulatory period (Okuma et al., 1996; Granot and Dekel, 2002; Sela-Abramovich et al., 2005; Borowczyk et al., 2006) enabling meiosis resumption (Norris et al., 2008) and the initiation of the ovulatory process (Borowczyk et al., 2006). In mice, theca cell gap junctions are primarily composed of Cx32 and Cx26 (Wright et al., 2001) and have significantly lower levels of GJIC than granulosa cells in the bovine ovary (Johnson et al., 2002). Theca-derived BMP4 and BMP7 contribute to the downregulation of Cx43 and gap junction intercellular communication in granulosa cells (Chang et al., 2013; Chang et al., 2014) through SMAD-dependent signaling (Chang et al., 2013; Rossi et al., 2016) in both mice and humans. Despite this, the role of gap junctions in theca cells is not yet well established.
GJIC plays a central role in coordinating intracellular signal transduction initiated by extracellular signals (endocrine, paracrine, and autocrine); in turn, controlling gene expression of neighboring cells via signaling molecules like cAMP, cGMP, and ATP (Mese et al., 2007; Trosko, 2011; Zong et al., 2016). This process can be modulated by EDCs with steroid hormone binding activity via non-genomic actions, such as cAMP and protein kinase pathway modulation (Vinken et al., 2009). Despite the significant role that intercellular communication plays in the ovarian follicle, to our knowledge the only EDC investigated for ovarian-specific GJIC outcomes is perfluorooctanesulfonic acid (PFOS) (Dominguez et al., 2016), which inhibited granulosa cell to oocyte GJIC (Dominguez et al., 2016). Additionally, bisphenol A (BPA) has been reported to reduce Cx43 and Cx26 mRNA expression in the mouse cumulus-oocyte cell complex (Acuna-Hernandez et al., 2018; Zhang et al., 2019a; Zhang et al., 2019b), and BPA analog bisphenol S (BPS) has been demonstrated to impair oocyte maturation and development (Zalmanova et al., 2017; Desmarchais et al., 2020) revealing the potential of other EDCs to influence GJIC. Taken together, these effects demonstrate the potential for EDCs to alter ovarian GJIC, potentially leading to detrimental reproductive outcomes. Therefore, the purpose of this study was to investigate the effects of known EDCs, specifically BPA, BPS, bisphenol F (BPF), triphenyltin chloride (TPT), and PFOS on GJIC in ovarian theca cells. BPA is a synthetic organic chemical, and the most widely used bisphenol in the manufacturing of epoxy resins and industrial and consumer plastics (Liao et al., 2012; Liao and Kannan, 2013). The recent ban of BPA from certain consumer products (Metz, 2016) has raised concerns about the safe use of other bisphenolic compounds like BPS and BPF. Additionally, we have included two non-bisphenolic EDCs of concern, TPT and PFOS in this study. We hypothesized that all five chemicals would reduce GJIC in theca cells. To test this hypothesis, we used primary theca cells from two monovulatory species: sheep and human. We further investigated the molecular pathways by which these chemicals alter GJIC in ovarian theca cells by using a signal-protein inhibitor approach.
2. Materials and Methods
2.1. Chemicals
All chemicals used in the study are listed in Table 1 and were dissolved in DMSO. All groups were administered the same solvent volume in media (0.1% DMSO). The doses of chemicals used (BPA, BPS and BPF: 1 to 1,000 ng/ml, PFOS: 50 ng/ml, and TPT: 10 ng/ml) were chosen for their environmental exposure relevance as they fall within a magnitude of human exposures determined through biomonitoring studies (ranges of human exposure for BPA: 0.14 – 792 ng/ml in urine, BPS: 0.07 – 211.9 ng/ml in urine, BPF: 0.14 – 298.7 ng/ml in urine, PFOS: 4.6–168 ng/ml in plasma, and TPT: 0.06–204 ng tin/ml in urine) (Heitland and Koster, 2006; CDC, 2016; Fleisch et al., 2017; Worley et al., 2017; Pu et al., 2019).
Table 1.
Chemicals
| Bisphenol A (BPA) | 239658 | 99% | Sigma | 1 – 10,000 ng/ml | 24 h |
| Bisphenol S (BPS) | 146915000 | 99.7% | Acros | 1 – 10,000 ng/ml | 24 h |
| Bisphenol F (BPF) | B47006 | > 98% | Sigma | 1 – 10,000 ng/ml | 24 h |
| Perfluorooctanesulfonic acid (PFOS) | 77282 | 98% | Sigma | 50 ng/ml | 24 h |
| Triphenyltin chloride (TPT) | 45492 | NR | Sigma | 10 ng/ml | 24 h |
| GF109203X (panPKC inhibitor) | NC9686383 | 98% | Enzo | 1 μM | 30 min |
| SB202190 (p38 MAPK inhibitor) | S7067 | > 98% | Sigma | 1 μM | 30 min |
| D609 (PC-PLC inhibitor) | 1437 | > 98% | Tocris | 50 μM | 30 min |
| ET-18-OCH3 (PI-PLC inhibitor) | 3022 | NR | Tocris | 30 μM | 15 min |
| H89 (PKA inhibitor) | B1427 | > 98% | Sigma | 40 μM | 30 min |
| U0126 (MEK1/2 inhibitor) | 1144 | 99% | Tocris | 70 μM | 30 min |
| CW008 (PKA activator) | 5495 | > 98% | Tocris | 0.25 μM | 24 h |
| Phorbol 12-myristate 13-acetate (TPA) | 1201 | > 99% | Tocris | 5 nM | 15 min |
| Lucifer yellow | PK-CA707–80015 | > 99% | Promocell | 0.5 mg/ml | 5 min |
| Rhodamine-dextran | R8881 | NR | Sigma | 0.5 mg/ml | 5 min |
NR: Purity not reported.
2.2. Generation of primary sheep ovarian theca cells, purity, and luteinization
All procedures used in this study were approved by the Institutional Animal Care and Use Committee of Michigan State University and are consistent with the National Research Council’s Guide for the Care and Use of Laboratory Animals and the current Animal Welfare Act. Ovine theca cells were used in this study as they represent an excellent in vitro model to study GJIC in the context of human chemical exposures and ovarian function as sheep and human are both monovulatory, and have similar ovarian sizes, steroid feedback regulatory mechanisms (Nippoldt et al., 1989; Kasa-Vubu et al., 1992), and connexin protein expression to humans (Borowczyk et al., 2006; Winterhager and Kidder, 2015). Isolation of theca cells was performed on eight multiparous Polypay × Dorsett breed sheep at gestational day 120 of pregnancy as previously described (Pu et al., 2019). High circulating progesterone during pregnancy allowed for collection of theca cells from a homogenous subset of follicles not subjected to follicular divergence or dominance. In brief, the theca interna cell layer of antral follicles was isolated by microdissection. Then, theca cells were dispersed using collagenase I (1 mg/ml) supplemented with 10 μg/ml deoxyribonuclease (DNAse I) in Ca2+/Mg2+-free buffer. The cell suspension was filtered, then fractioned on a discontinuous Percoll gradient (44% and 35% Percoll). Plated cells were maintained in basic medium consisting of DMEM/F12 media supplemented with 1% heat inactivated fetal bovine serum (FBS, Cat#: 35– 010-CV, Corning Inc., Corning, NY, USA), 2 mM L-glutamine, 10 mM HEPES, 100 IU/ml penicillin, and 100 μg/ml streptomycin in 5% CO2 at 39°C. Theca cell purity was determined using theca cell markers fibulin 5 (Hatzirodos et al., 2015) and vimentin (Qiu et al., 2018) by immunocytochemistry. Purity of all isolated primary cell cultures was > 95% (Pu et al., 2019). Theca cells were kept frozen at −80 °C until used for GJIC assays. Theca cells luteinization was performed as previously described (Pu et al., 2019). In brief, theca cells were grown in DMEM/F12 medium supplemented with 1% penicillin/streptomycin, 2 mM L-glutamine, 1% heat inactivated FBS, 250 ng/ml ovine luteinizing hormone (LH; U.S. National Hormone and Peptide Program), and 50 ng/ml insulin-like growth factor-1 (IGF-1), and cultured for 72 h.
2.3. Ethics, exclusion criteria, and generation of human ovarian theca cells
Human ovarian tissue was obtained after written informed consent following Institutional Review Board (IRB: 17–1066 M) approval from Sparrow Health System and Michigan State University and is consistent with relevant guidelines and regulations. Ovaries were collected from healthy non-pregnant women (n = 3, aged 31 – 46 years) undergoing surgical uni- or bilateral oophorectomy. Patient parity varied between samples (0–5 births). No patients were taking antiandrogens, insulin sensitizers, or gonadotropins at the time of tissue collection. Exclusion criteria included polycystic ovarian syndrome, ovarian cancer, menopause, drug addiction, HIV, and/or hepatitis B or C diagnoses. Only follicles with a healthy appearance, and ≥ 3 mm in size were chosen to pool for the generation of a theca cell line per individual. All ovaries were processed within 1 h of surgical removal and kept at 4°C. The theca interna cell layer of human antral follicles was isolated as previously described (Pu et al., 2019) and enzyme-digested as described for sheep. Changes to the enzyme digestion for human theca cells included more collagenase I (3 mg/ml) and DNase I (300 IU/ml). Theca cells were kept frozen at −80 °C until used for GJIC assays.
2.4. Cell viability assay
Cell viability was determined using an MTT assay as previously described (Palaniappan et al., 2013). In brief, theca cells (n = 8 primary cultures, with at least 3 replicates per experiment) were seeded into 96-well plates. Cells were treated with increasing concentrations of BPA, BPS, or BPF (0, 1, 10, 20, 50, 100, 200, 500, 1,000, and 10,000 ng/ml) for 72 h, or pathway inhibitors at concentrations and times (15 or 30 min) listed in Table 1. All chemicals tested were dissolved in DMSO to a final concentration of 0.1%. Vehicle group received 0.1% DMSO. Medium was then replaced with MTT working solution (50 μg/ml) and incubated for 4 h. Wells were rinsed, and DMSO added to each. Cell viability was determined by absorbance quantification at 570 nm using a microplate reader (SpectraMax M5e, Molecular Devices, LLC, Sunnyvale, CA, USA). Exposure groups were also visually assessed for cellular morphology differences.
2.5. Experimental design
To test if three bisphenols (BPA, BPS, and BPF), PFOS, and/or TPT alter GJIC, ovine pre-luteinized theca cells were subjected to a GJIC assay as outlined below. Once plated cells reached 90% confluency, media was replaced for exposure media consisting of basic medium supplemented with DMSO (0.1%, vehicle), BPA, BPS, BPF, TPT, or PFOS for 24 h, followed by a 2-h serum starving step, and subsequent gap junction communication assays using a scrape loading dye transfer assay (see below). Bisphenols (BPA, BPS, and BPF) exposure doses (1, 100, and 1,000 ng/ml) were chosen to cover a range of exposures from environmentally relevant to supraphysiological. Given that BPS altered GJIC, BPS was also tested at these additional doses: 10, 200, and 500 ng/ml. We then investigated if the observed GJIC BPS effect occurred in a theca cells independent of their stage (pre-luteinized, luteinizing, and post-luteinized). Luteinizing cells chemical exposure occurred concomitantly with LH during the last 24 h of the 72-h differentiation period, and luteinized cells were exposed after the 72-h luteinizing period in the absence of LH. The effect of BPS (at 200 ng/ml) exposure duration on GJIC was also assessed in pre-luteinized theca cells for 3, 6, 12, and 24 h.
To assess signal transduction mechanisms mediating changes in GJIC, specific gap junction pathway inhibitors (GF109203X, SB202190, D609, ET-18-OCH3, H89, and U0126) and a PKA activator (CW008) were used. Pathways in which these inhibitors function and concentrations and time of exposure at which all test compounds were used as outlined in Table 1. Primary ovine theca cell cultures (n = 8) were used in the aforementioned experiments, with a minimum of 3 replicates per treatment group per experiment. Human theca cell GJIC experiments (n = 3 primary theca cell cultures per treatment group) followed the same method to that described for ovine cells, at a fixed BPS exposure dose (200 ng/ml) and using a MAPK inhibitor (1 μM SB202190) for GJIC pathway analysis.
2.6. Gap junction intercellular communication assay
Theca cells were plated at 50 ×104 cells/dish on a 60 mm-diameter tissue culture dishes (Corning Inc., Corning, NY, USA) and cultured in basic medium supplemented with 10% FBS. After 24 h, media was replaced with normal basic medium (containing only 1% FBS). Confluent cells were passed and seeded at 25 × 104 cells/dish in 35 mm-diameter tissue culture dishes and cultured in basic medium supplemented with 10% FBS. After 24 h, media was replaced with basic medium (1% FBS). Once cells reached 90% confluency, media was replaced for exposure media consisting of basic medium supplemented with DMSO (0.1%, vehicle), BPA, BPS, BPF, TPT, or PFOS for 24 h. To stimulate intercellular communication, cells were serum starved 2-h prior to the GJIC assay (Lin et al., 2003). For the gap junction pathway inhibitor experiments, inhibitors were added in the last 15 to 30 min (Table 1) of the serum starvation step. GJIC was assessed using the scrape loading/dye transfer technique as previously described (Upham, 2011). In brief, cells were washed with Ca2+-Mg2+-PBS and then lucifer yellow and rhodamine-dextran were added to each plate. With gentle pressure, a surgical scalpel blade was rolled through a cell monolayer. Passive diffusion of the lucifer yellow from the loaded cells into the adjacent cells was allowed for 5 min. Due to its high molecular weight and inability to pass through gap junction channels, the rhodamine-dextran was used to visualize the dye loaded cells (not shown). Cells were then washed with Ca2+-Mg2+-PBS, fixed with 4% neutral buffered formalin and stored at 4°C until imaging.
2.7. Image Analyses
To quantify theca cell GJIC, 6 to 10 images per primary cultured cell line per treatment group were captured using an Eclipse TE2000-U inverted microscope (Nikon, Toyko, Japan) with a CoolSNAP-ProCF camera (Media Cybernetics, Rockville, MD, USA). Images were quantified using Fiji image analysis software (Schindelin et al., 2012). The freehand selection tool was used to manually determine the area of co-joined lucifer yellow positive cells, and then averaged to determine the area of dye diffusion. A vehicle group was included in each run, and the average area of dye diffusion was normalized to the vehicle group.
2.8. Statistical analysis
All data are presented as a mean ± SEM. Appropriate transformations were applied, as needed, to account for normality of data. Comparisons among the treatment groups were analyzed by mixed model ANOVA with Tukey posthoc tests using experiment run as a covariate. Differences were considered significant at P < 0.05.
3. Results
3.1. Effects of bisphenols (BPA, BPS, and BPF) on cell viability
Cell viability was unaltered by any bisphenol (range: 1 to 10,000 ng/ml; Fig. 1). Additionally, TPT, PFOS, or inhibitors used at the concentration tested (Table 1) did not affect cell viability (data not shown). Cell morphology was similar across treatment groups and was not affected by exposure to bisphenols, TPT, PFOS, or inhibitors at the concentrations tested.
Figure 1.
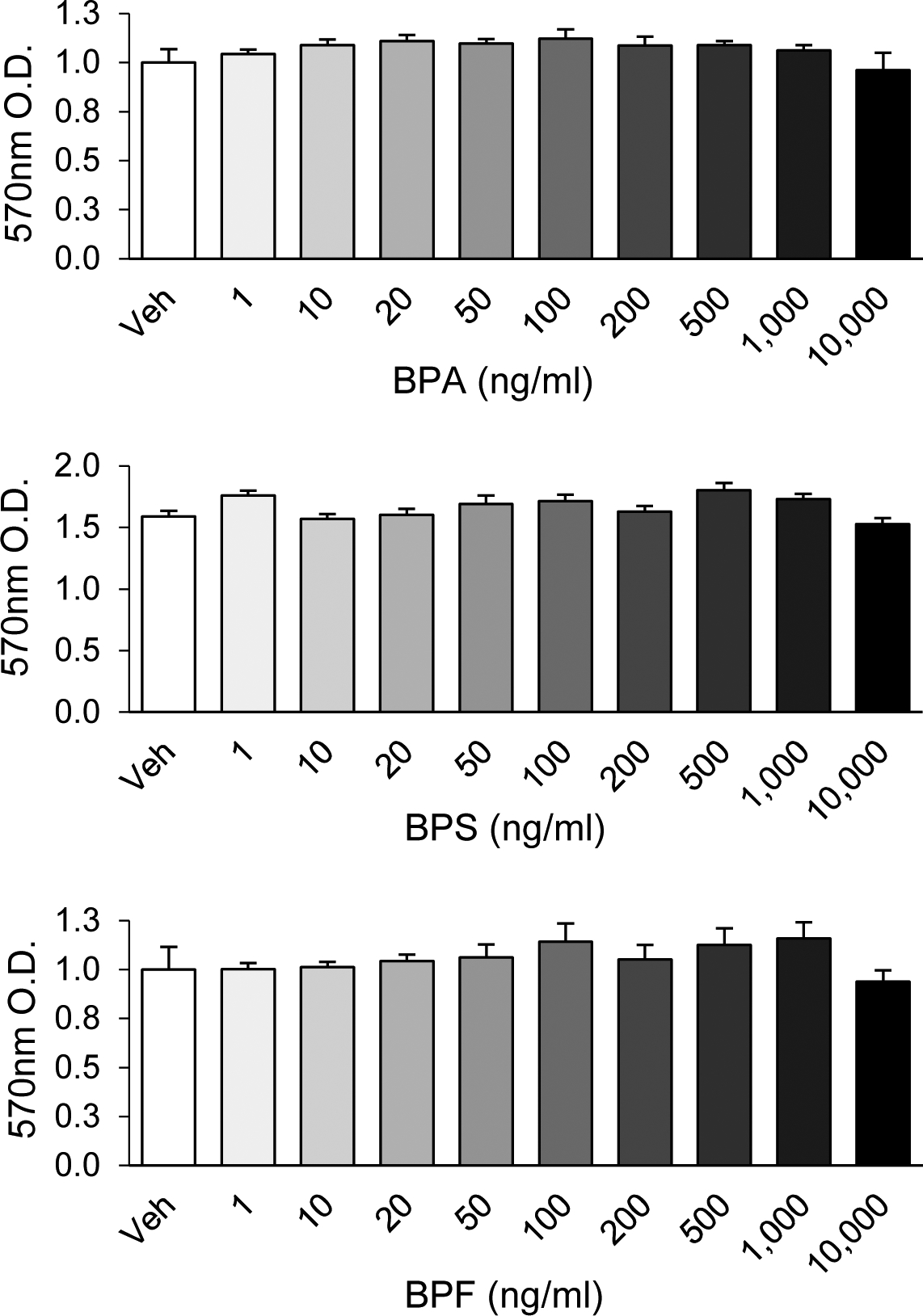
Cytotoxicity assay for bisphenol A (BPA), bisphenol S (BPS), and bisphenol (BPF) in pre-luteinized ovine theca cells after 72 h of exposure (range: 1 to 10,000 ng/ml). Data are expressed as mean ± SEM. N = 3 primary theca cell cultures. No significant differences between exposure concentrations were detected. Veh: DMSO vehicle control. O.D.: optical density.
3.2. BPS enhances gap junction communication in a cell stage- time- and dose-dependent manner
BPA and BPF, at all doses tested (ranging: 1 to 1,000 ng/ml), had no effect on GJIC in pre-luteinized theca cells (Fig. 2, left and center panels) following a 24 h exposure, and thus no further experiments were pursued with these two chemicals. The rhodamine-dextran dye control did not pass beyond the cells cut with the scalpel blade (not shown). PFOS and TPT, additionally, had no effect on GJIC (Fig. 2, right panel). However, pre-luteinized theca cells exposed for 24 h to BPS increased GJIC in a dose-dependent fashion (Fig. 3). GJIC increased starting at an exposure dose as low as 10 ng/ml, peaked at 200 ng/ml, and plateaued at higher doses.
Figure 2.
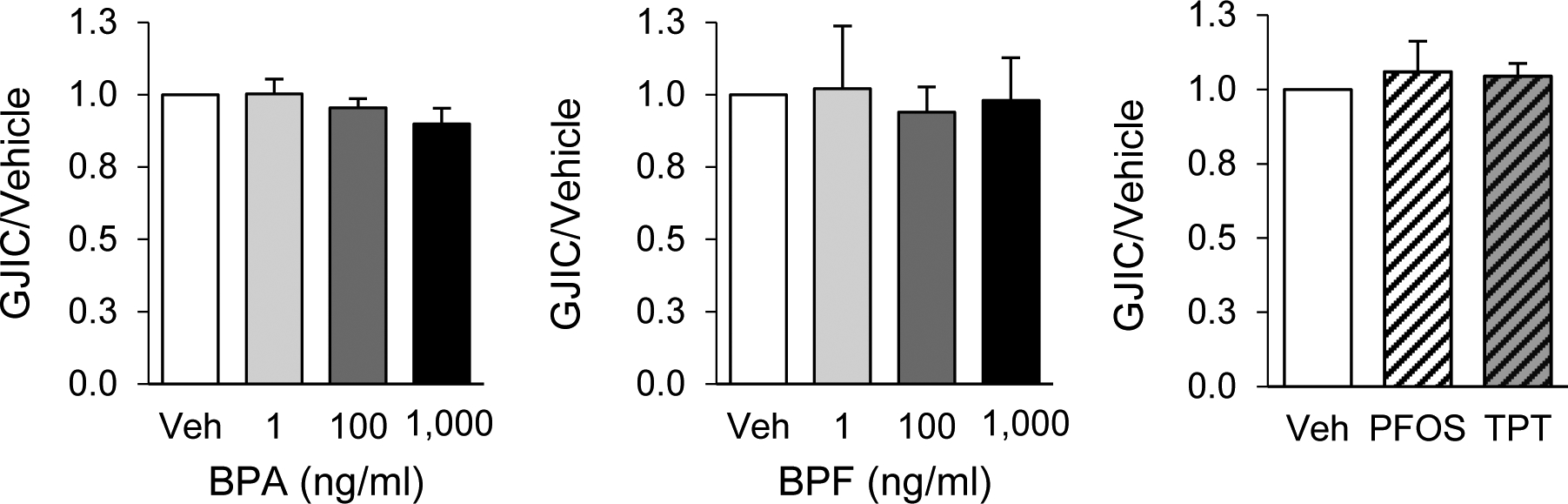
Effects of exposure to common endocrine disrupting chemicals on gap junction intercellular communication (GJIC) in ovine primary theca cells. GJIC was assessed using the scrape loading dye transfer assay in pre-luteinized ovine theca cells exposed to bisphenol A (BPA), bisphenol F (BPF), perfluorooctanesulfonic acid (PFOS; 50 ng/ml), or triphenyltin chloride (TPT; 10 ng/ml). Data are represented as mean ± SEM. The effects were normalized to the vehicle group GJIC. At least 3 different theca cell cultures (each representing a biological replicate) were used for each chemical and dose. Veh: DMSO vehicle control.
Figure 3.
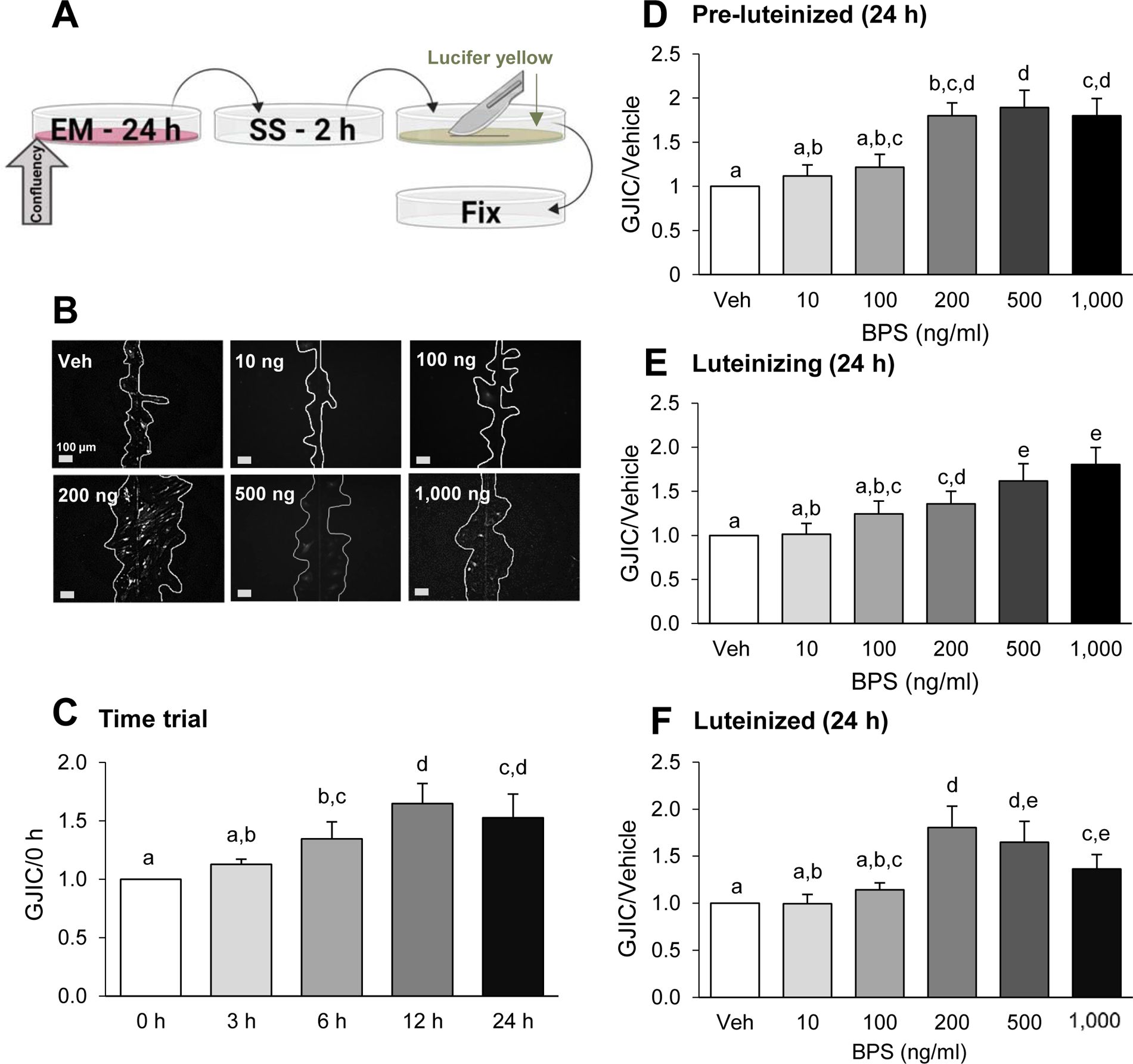
Dose- and time-dependent effects of bisphenol S (BPS) exposure on gap junction intercellular communication (GJIC) in ovine primary theca cells. Scheme of exposure (A): At ~80% confluency, cells are exposed to exposure medium (EM) containing BPS for 24 h, followed by a 2 h serum starvation (SS) period. Cells were then subjected to the scrape loading dye/transfer assay, using lucifer yellow dye (arrow) and a scalpel blade, before fixation (see text for additional details). Representative images from scrape loading dye transfer assay (B) used to assess GJIC in pre-luteinized ovine theca cells. White lines were drawn to facilitate visualization of the lucifer yellow intercellular transfer. BPS effect on GJIC (mean ± SEM) in a time-course in pre-luteinized theca cells (C) and in a dose-response in pre-luteinized (D), luteinizing (E), and luteinized (F) theca cells. The effect of BPS was normalized with the dye transfer in the vehicle control. At least 3 different theca cell cultures (each representing a biological replicate) were used for each time point, BPS dose and cellular stage. Different letters denote statistical differences among treatment groups at P < 0.05. Veh: DMSO vehicle control.
To assess if exposure time affects the BPS-induced increase in GJIC in pre-luteinized theca cells, we used the lowest BPS dose that resulted in the strongest effect (200 ng/ml BPS resulted in ~2-fold increase in GJIC), for exposure times of 3, 6, 12, and 24 h. The increase in GJIC was time-dependent with 12 h and 24 h exposures resulting in the highest GJIC response (Fig. 3C).
To test whether BPS differentially modulates GJIC depending on the luteal stage of the theca cells, we used luteinizing and luteinized theca cells. In luteinizing theca cells, BPS also induced an increase in GJIC. Although less pronounced, BPS’s effect was first observed at 200 ng/ml and displayed a dose-dependent increase up to the maximum dose tested (1,000 ng/ml) (Fig. 3E). In luteinized theca cells, the BPS effect followed a similar trend to the pre-luteinized theca cells but the effect was less robust (Fig. 3F).
3.3. Signal-protein inhibitor/activator evaluation of BPS-enhanced gap junction communication
A signal-protein inhibitor/activator approach was used to evaluate the molecular pathways potentially involved in BPS-induced upregulation of GJIC (Fig. 4A). First, PKA activator CW008 was used alone and in combination with BPS exposure as a positive control to enhance GJIC. CW008 exposure enhanced GJIC in pre-luteinized theca cells both single and in combination with BPS, although the combined effect was comparable to that of BPS alone (Fig. 4B). Additionally, TPA was used as a non-specific negative control due to its ability to inhibit GJIC by internalizing gap junction channels through a protein kinase C/extracellular receptor kinase 1,2 (PKC/ERK1/2)-dependent mechanism (Ruch et al., 2001; Leithe and Rivedal, 2004). The signal transduction pathway inhibitors (D609, ET-18-OCH3, GF109203X, H89, SB202190, and U0126), were used to determine if phosphatidyl choline specific phospholipase C, phosphatidyl inositol specific phospholipase C, protein kinase C, protein kinase A, extracellular receptor kinase- mitogen activated protein kinase were involved in BPS-induced regulation of GJIC, and did not alter the effects of GJIC in the absence of BPS. Co-exposure of BPS and TPA resulted in GJIC like that observed in the vehicle group (Fig. 4C).
Figure 4.
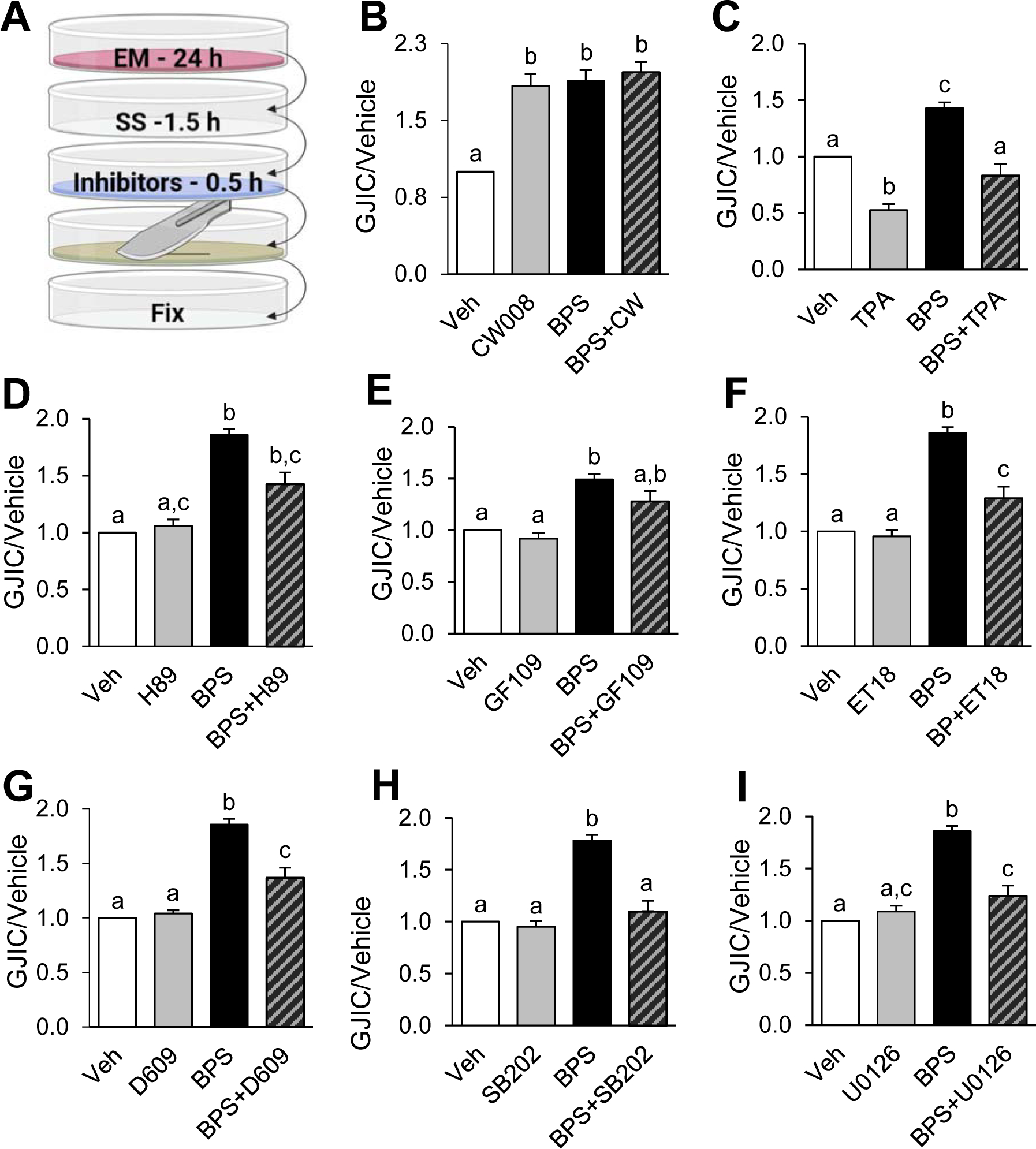
Pathway identification of bisphenol S (BPS)-induced enhanced GJIC in pre-luteinized ovine primary theca cells using chemical modulators. Scheme of exposure to BPS and GJIC inhibitors (A): At ~90% confluency, cells are exposed to exposure medium (EM) containing BPS followed by a 2 h serum starvation (SS) period, where inhibitors were added in the last 15 – 30 min. Cells were then subjected to the scrape loading dye transfer assay, which was run using lucifer yellow (green plate) and a scalpel blade, prior to fixation (see text for additional details). GJIC (mean ± SEM) in pre-luteinized ovine theca cells exposed to BPS with and without: positive control CW008 (PKA activator) (B), negative control phorbol 12-myristate 13-acetate (TPA) (C), or GJIC pathway inhibitors H89 (PKA inhibitor) (D), GF109203X (panPKC inhibitor) (E), ET-18-OCH3 (PI-PLC inhibitor) (F), D609 (PC-PLC inhibitor) (G), SB202190 (SB202, p38 MAPK inhibitor) (H), or U0126 (MEK1/2 inhibitor) (I). See Table 1 for inhibitor exposure times. GJIC is expressed relative to the vehicle group. At least 3 different theca cell cultures (each representing a biological replicate) were used for each inhibitor experiment. Different letters denote statistical differences among treatment groups at P < 0.05. Veh: DMSO vehicle control.
To evaluate the involvement of the PKA pathway, H89 was used as an inhibitor of PKA-induced connexin phosphorylation. H89 partially reduced (by ~23%) the BPS-induced enhanced GJIC (Fig. 4D). PKC pathway activation was tested using GF109203X as a panPKC inhibitor (Fig. 4E), ET-18-OCH3 as a phosphatidylinositol-phospholipase C (PI-PLC)-dependent PKC inhibitor (Fig. 4F), and D609 as a phosphatidylcholine-phospholipase C (PC-PLC)-dependent PKC inhibitor (Fig. 4G). Both PLC-dependent PKC pathway inhibitors were able to significantly attenuate the BPS-induced enhanced GJIC by ~29 and ~31%, respectively. Finally, to understand the role of MAPK in BPS-induced upregulation of GJIC, a p38 MAPK inhibitor (SB202190), and a MEK1/2 inhibitor (U0126) were used. SB202190 was the only inhibitor used to result a full attenuation of the BPS effect (Fig. 4H), while U0126 was only able to partially prevent the BPS-induced enhanced GJIC to that of the vehicle control group by ~33% (Fig. 4I). No pathway inhibitors tested individually reduced GJIC below that of the vehicle control.
3.4. BPS enhances gap junction communication in human theca cells
GJIC in primary isolated human theca cells upon BPS exposure was also tested. At the time and dose optimized in ovine primary theca cell lines (24 h exposure to 200 ng/ml BPS), BPS was able to induce approximately a ~1.5-fold increase in GJIC, which was significantly attenuated with a MAPK inhibitor (Fig. 5).
Figure 5.
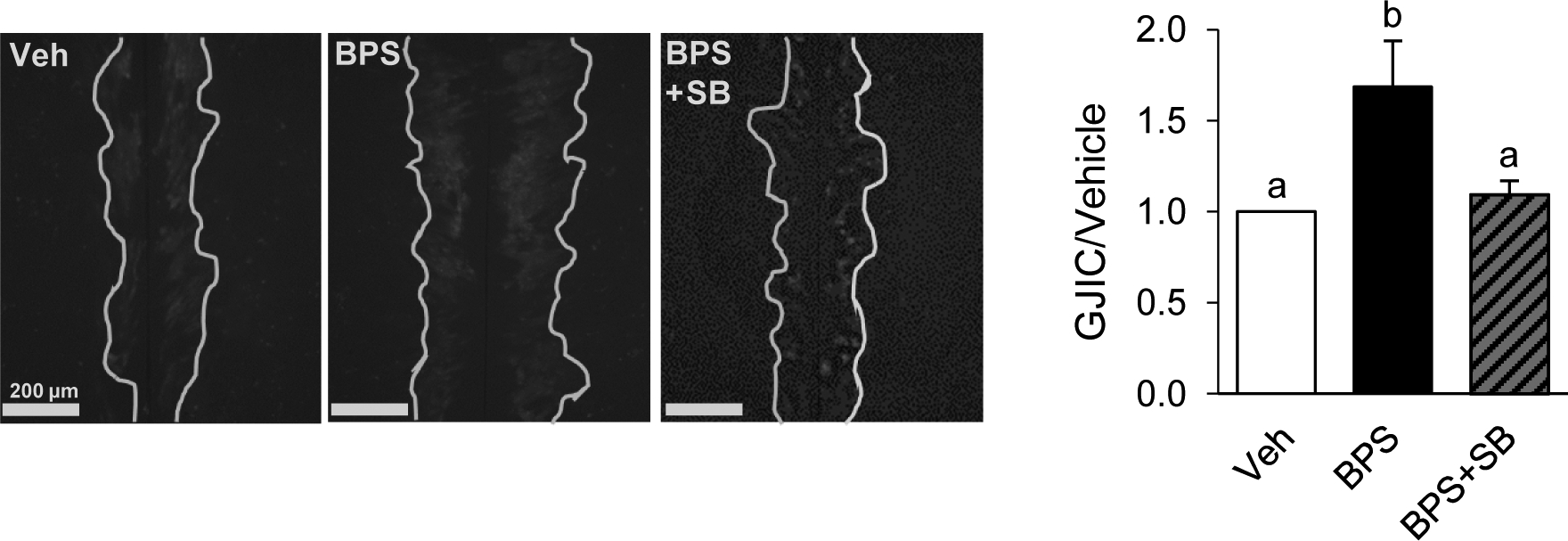
Effect of bisphenol S (BPS) exposure on GJIC in human theca cells. Representative images from scrape loading dye transfer assay used to assess GJIC in pre-luteinized human theca cells. White lines were drawn to facilitate visualization of the lucifer yellow intercellular transfer. GJIC was assessed following exposure to vehicle control (Veh, 0.1% DMSO), BPS, or BPS in combination with MAPK inhibitor SB2020190 (SB, 1 μM). Data are represented as mean ± SEM and were normalized to dye transfer in the vehicle control. Different letters denote statistical differences among treatment groups at P < 0.05. Three different primary human theca cell cultures were used.
4. Discussion
We demonstrated that BPS, but not four other common EDCs (BPA, BPF, TPT, and PFOS), enhanced GJIC in both a dose- and time-dependent manner in ovine primary ovarian theca cells. BPS-induced enhancement of GJIC was also evident in primary human theca cells. The lowest observed adverse effect level (LOAEL) for enhanced GJIC was 10 and 100 ng/ml BPS in pre-luteinized, and luteinizing and luteinized cells, respectively. Importantly, both LOAEL doses fall within environmentally relevant human exposures (Qiu et al., 2018; Zhao et al., 2018). Thus far, chemotherapeutic agents have been shown to enhance GJIC (Vinken et al., 2006; Liu et al., 2013; Xiao et al., 2013; Yang et al., 2014; Sovadinova et al., 2015; Babica et al., 2016a; Wu et al., 2016), while most EDCs (dichlorodiphenyltrichloroethane (Masten et al., 2001), polychlorinated biphenyls (Kang et al., 1996; Machala et al., 2003a), perfluorinated compounds (Upham et al., 1998; Hu et al., 2002; Upham et al., 2009), methoxychlor,vinclozolin (Babica et al., 2016b)), lipid by-products (octadecatetraenoate (Hasler et al., 1991)), cigarette smoke relevant polycyclic aromatic hydrocarbons (Tai et al., 2007; Upham et al., 2008; Osgood et al., 2014; Velmurugan et al., 2015; Osgood et al., 2017; Siegrist et al., 2019), cannabinoids (Δ(9)-tetrahydrocannabinol and cannabinol (Upham et al., 2003)), plant derivatives (licorice root; (Davidson and Baumgarten, 1988; Tanaka et al., 1999)), and pharmaceuticals (phenobarbital; (Klaunig et al., 1990; Ren and Ruch, 1996)) result in a dose- and time-dependent reduction of GJIC. For both PFOS and TPT exposures, only a single environmentally relevant dose was used. As a dose-dependent study was not performed for either of these chemicals, we cannot rule out that, similar to BPS, these chemicals may exert a dose-dependent effect on GJIC.
Epidemiological evidence linking infertility in humans and BPS exposure is currently only available in males (Ghayda et al., 2019), and not females. Other epidemiological associations from BPS exposure are restricted to metabolic outcomes like obesity (Liu et al., 2019) and type 2 diabetes (Ranciere et al., 2019). Our findings and those of others (Zalmanova et al., 2017; Nevoral et al., 2018; Desmarchais et al., 2020) highlights the need for future epidemiological studies to focus on evaluating if exposure to BPS can results in reduced fertility outcomes.
Reduced GJIC, in vivo, is recognized as a hallmark in the early stages of cancer, while late stage or metastatic cancers present with both down- and up-regulated GJIC, particularly during the vascularization of tumors (Aasen et al., 2017), including in the ovary (Hanna et al., 1999). Inhibition of GJIC has been associated with a chemicals’ higher carcinogenic potential (Rosenkranz et al., 2000). On the contrary, substances that enhance GJIC, such as green tea micronutrients genistein and epicatechin (Sai et al., 2000; Ale-Agha et al., 2002) and other plant antioxidants (Nakamura et al., 2005a; Nakamura et al., 2005b; Upham et al., 2007; Sovadinova et al., 2015; Babica et al., 2016a), are hypothesized to have cancer preventive properties, and drugs enhancing GJIC have been viewed as novel chemotherapeutics (Yang et al., 2014; Wu et al., 2016). Despite this, in the context of the ovarian reproductive cycle where GJIC is temporospatially regulated (Kidder and Mhawi, 2002), enhanced GJIC could result in pathological outcomes like hyperplasia of the theca cell layer, a pathology seen in hyperandrogenism (Czyzyk et al., 2017). Importantly, a BPS-induced increase in GJIC, if also occurring intergranulosal or granulosa-to-oocyte, could impair follicle development and oocyte maturation. We are not aware of disease etiologies associated with altered GJIC in the ovary. However, cellular arrest before antral follicle development in Cx43-inhibited human granulosa cells (Winterhager and Kidder, 2015). Importantly, enhanced GJIC has been associated with the development of diabetic cardiomyopathy (Wang et al., 2017) and in the brain is associated with epileptic hypersynchronous neuronal activity (Xie et al., 2015). Upregulation of connexin mRNA expression and protein abundance is implicated in a model for Parkinson’s disease (Xie et al., 2015) and also occurs following prion disease on-set (Lee et al., 2016) highlighting the role of GJIC imbalances during in disease states.
During ovulation, just prior to luteinization, there is a BMP4- and BMP7-mediated gradual loss in GJIC within the preovulatory antral follicle for oocyte maturation to occur (Okuma et al., 1996; Granot and Dekel, 2002; Sela-Abramovich et al., 2005; Borowczyk et al., 2006; Norris et al., 2008; Winterhager and Kidder, 2015). Therefore, BPS-induced enhanced GJIC occurring during the latest stages of preovulatory follicle growth, especially if GJIC is also altered in granulosa cells, could alter transfer of signaling molecules, like BMPs, preventing final oocyte maturation and progression into metaphase II. This may ultimately result in lower oocyte fertilization rates and reduced fertility. BPS also retained the ability to enhance GJIC during and after theca cell luteinization, a time when theca cells shift steroid synthesis from androgens to progesterone.
Estradiol and the xenoestrogen BPA have been previously reported to reduce GJIC via a down regulation in connexin mRNA expression in the ovarian cumulus-oocyte cell complex (Acuna-Hernandez et al., 2018; Zhang et al., 2019a; Zhang et al., 2019b). However, these observations were made on the cumulus-oocyte cell complex in mice, an altricial litter-bearing small mammal with inherently different ovarian physiology than sheep, which is a precocial large mammal who typically bears one or two offspring. Additionally, these observations were made at doses exceeding 2 μM BPA, which exceeds not only BPA doses used in this study, but high-end human exposures (ranges for human exposure to BPA: 0.14 – 792 ng/ml in urine). BPS can also act as a xenoestrogen (Pelch et al., 2019), and the observed BPS-induced enhancement in GJIC could thus potentially be due to upregulation of connexin expression. However, connexin mRNA expression does not necessarily translate to a change in GJIC (Genetos et al., 2012), particularly in the context of EDC exposures (Zhang et al., 2019b), and therefore functional outcomes such as intercellular dye transfer should be assessed when studying changes in GJIC. Since enhanced GJIC occurs following exposure to BPS, the interplay between BPS, connexin expression, and the changing hormonal milieu throughout folliculogenesis and ovulation should be studied further.
To understand the mechanism by which BPS increases GJIC, we co-incubated theca cells with pharmacological inhibitors directed to specific signal-protein targets (Fig. 6). Our results demonstrate that BPS can act through multiple phosphorylation pathways. These pathways occur in parallel or upstream of those commonly implicated in GJIC regulation (Kurtenbach et al., 2014). PKA, PKC, and PLC pathways have redundant roles in the phosphorylation of connexins, or can indirectly regulate other pathways implicated in GJIC (Kanemitsu and Lau, 1993; Cesen-Cummings et al., 1998; Hossain et al., 1998; Ruch et al., 2001; Machala et al., 2003b; Upham et al., 2008). These redundant roles are likely why only a partial, albeit significant, attenuation of BPS-induced enhanced GJIC was achieved by any of the signal-protein inhibitors, both in sheep and human theca cells. To note, these phosphorylation checkpoints are implicated in other cellular processes such as invasion, differentiation, Ca2+ ion signaling and influx, and neuronal responsiveness, cancer metastasis, and the development of diabetes (Sotogaku et al., 2007; Morrison, 2012; Putney and Tomita, 2012; Sassone-Corsi, 2012; Tarafdar and Michie, 2014). Additionally, there are 21 known connexins expressed in humans (Sohl and Willecke, 2004a), at least 6 of which (Cx26, Cx32, Cx37, Cx40, Cx43, and Cx45) are present in the normal human ovary, and at least 11 (Cx26, Cx30, Cx30.3, Cx31, Cx31.1, Cx32, Cx37, Cx40, Cx43, Cx46, and Cx50) have been reported in ovarian cancers (Gershon et al., 2008). Although the function of each connexin is not fully understood, expression of different connexins within the ovary can result in essential pathway redundancy needed to provide compensatory communication mechanisms upon specific pathway malfunction (Sinyuk et al., 2018). This makes the evaluation and identification of specific biochemical pathways altered in BPS-enhanced GJIC challenging.
Figure 6.
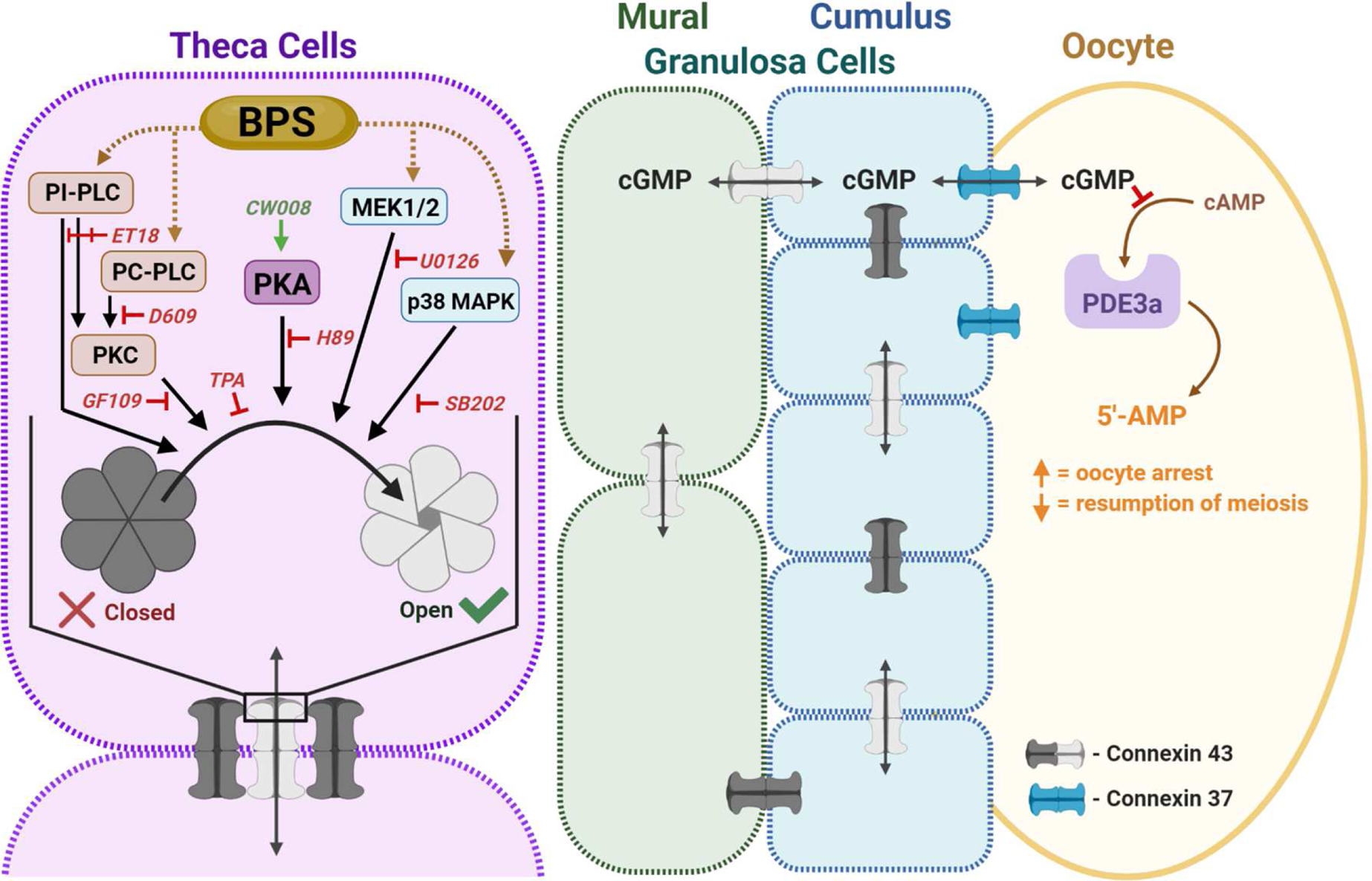
Working model for BPS’ modulation of theca cell GJIC. The antral follicle is formed by theca cells (purple), which provide physical and steroidogenic support to the underlying mural granulosa cells (green), cumulus granulosa cells (blue), and the oocyte (yellow). Connexin 37 and 43 form gap junction channels that connect inter-thecal, inter-granulosal and granulosa- to-oocyte cell communication, while theca-to-granulosa cell communication and hormone transport primarily occurs through paracrine signaling. Alterations in theca cell GJIC could affect the structural integrity and steroidogenic function of the follicle. GJIC in the ovary is temporospatially regulated, in part, by phosphodiesterase 3a (PDE3a), and needed for cellular arrest and meiosis resumption in the oocyte. Connexin phosphorylation signals opening, function, and recycling of gap junction channels. Pathways involved in GJIC regulation include protein kinase C (PKC), protein kinase A (PKA), and mitogen-activated protein kinase (MAPK) signaling. In theca cells, BPS can modulate GJIC through phosphatidylinositol-specific phospholipase C (PI-PLC) independent of PKC activation, as well as through MAPK/ERK kinase (MEK1/2). Like inter-thecal, inter-granulosal and granulosa-to-oocyte cell communication occurs through gap junctions, so we anticipate BPS could affect normal PDE3a-regulation of oocyte arrest and resumption of meiosis through dysregulated GJIC.ET18: ET-18-OCH3, GF109: GF109203X, PC-PLC: phosphatidyl choline-specific phospholipase C, SB202: SB202190, and TPA: phorbol 12-myrisate 13-acetate.
All signal protein inhibitor experiments were conducted in pre-luteinized cells to avoid potential confounding factors that may be introduced during the luteinization protocol, such as active cellular differentiation and the presence of LH in the media. Since the BPS-induced enhanced GJIC was conserved across cell stages, we predict that this effect occurs through the same pathways regardless of the theca cells differentiation stage.
Bisphenols and perfluorinated chemicals, both common classes of EDCs, have recently been reported to reduce GJIC in the ovary both, functionally or through a reduction in connexin expression (Acuna-Hernandez et al., 2018; Dominguez et al., 2019; Lopez-Arellano et al., 2019). To our knowledge, BPS is the first bisphenol congener and EDC reported to enhance GJIC. Importantly, gestational exposure to BPS in sheep reduces the population of progesterone-producing cells in the placenta (Gingrich et al., 2018). This was hypothesized to be due to an imbalance in cellular fusion at the commitment stage, which requires gap junction communication after cell apposition (Gingrich et al., 2018). Additional reproductive outcomes such as delayed onset of puberty (Shi et al., 2019), increased testosterone production (Shi et al., 2019), decreased ovary weight (Nevoral et al., 2018), and decreased number of antral and preantral follicles (Nevoral et al., 2018) are reported outcomes in female mice exposed to BPS in vivo. To our knowledge, neither GJIC or connexin expression was assessed or implicated in any of these BPS-related outcomes.
5. Concluding Remarks
This study provides a novel model to evaluate of the role of GJIC in theca cells. Using this model, we have demonstrated that BPS enhances GJIC in ovine ovarian theca cells. This effect is reproducible in primary human theca cells, highlighting the translational relevance of these findings. The inhibitor approach used demonstrates the promiscuity of BPS in altering multiple signaling pathways regulating GJIC in theca cells (Fig. 6). Importantly, since GJIC alterations can disrupt folliculogenesis, the role of BPS in mediating ovarian-mediated sub- or infertility should be evaluated.
Supplementary Material
Highlights.
Bisphenol S, but not four other endocrine disrupting chemicals, alters GJIC in theca cells.
Bisphenol S enhances GJIC in ovine and human theca cells.
Bisphenol S-enhanced GJIC is partially mediated via the MAPK signaling pathway.
Acknowledgements:
We thank the Michigan State University (MSU) Sheep Teaching and Research Facility for animal procurement and husbandry, and Lisbeth Lockwood (Department of Pediatrics & Human Development, MSU) for technical assistance. Figures 3A, 4A and 6 were developed using biorender.com.
Funding: This work was supported by Michigan State University General Funds, AgBioResearch and the United States Department of Agriculture (USDA) National Institute of Food and Agriculture, and Hatch project. Research reported in this publication was partially supported by the National Institute of Environmental Health Sciences (NIEHS) of the National Institute of Health under Award Number R01ES027863. J.G. was supported in part by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (NICHD) under award number T32HD087166. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aasen T, Mesnil M, Naus CC, Lampe PD, Laird DW, 2017. Gap junctions and cancer: communicating for 50 years. Nat Rev Cancer 17, 74. [DOI] [PubMed] [Google Scholar]
- Ackert CL, Gittens JE, O’Brien MJ, Eppig JJ, Kidder GM, 2001. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol 233, 258–270. [DOI] [PubMed] [Google Scholar]
- Acuna-Hernandez DG, Arreola-Mendoza L, Santacruz-Marquez R, Garcia-Zepeda SP, Parra-Forero LY, Olivares-Reyes JA, Hernandez-Ochoa I, 2018. Bisphenol A alters oocyte maturation by prematurely closing gap junctions in the cumulus cell-oocyte complex. Toxicol Appl Pharmacol 344, 13–22. [DOI] [PubMed] [Google Scholar]
- Ale-Agha N, Stahl W, Sies H, 2002. (−)-Epicatechin effects in rat liver epithelial cells: stimulation of gap junctional communication and counteraction of its loss due to the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Biochem Pharmacol 63, 2145–2149. [DOI] [PubMed] [Google Scholar]
- Babica P, Ctverackova L, Lencesova Z, Trosko JE, Upham BL, 2016a. Chemopreventive Agents Attenuate Rapid Inhibition of Gap Junctional Intercellular Communication Induced by Environmental Toxicants. Nutrition and Cancer-an International Journal 68, 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babica P, Zurabian R, Kumar ER, Chopra R, Mianecki MJ, Park JS, Jasa L, Trosko JE, Upham BL, 2016b. Methoxychlor and Vinclozolin Induce Rapid Changes in Intercellular and Intracellular Signaling in Liver Progenitor Cells. Toxicological Sciences 153, 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowczyk E, Johnson ML, Bilski JJ, Borowicz PP, Redmer DA, Reynolds LP, Grazul-Bilska AT, 2006. Expression of gap junctional connexins 26, 32, and 43 mRNA in ovarian preovulatory follicles and corpora lutea in sheep. Can J Physiol Pharmacol 84, 1011–1020. [DOI] [PubMed] [Google Scholar]
- CDC, 2016. Personal Care and Consumer Product Chemicals and Metabolites (EPHPP_H) in: Survey, N.H.a.N.E. (Ed.). [Google Scholar]
- CDC, 2017. Infertility.
- Cesen-Cummings K, Warner KA, Ruch RJ, 1998. Role of protein kinase C in the deficient gap junctional intercellular communication of K-ras-transformed murine lung epithelial cells. Anticancer Res 18, 4343–4346. [PubMed] [Google Scholar]
- Chang HM, Cheng JC, Leung PC, 2013. Theca-derived BMP4 and BMP7 down-regulate connexin43 expression and decrease gap junction intercellular communication activity in immortalized human granulosa cells. J Clin Endocrinol Metab 98, E437–445. [DOI] [PubMed] [Google Scholar]
- Chang HM, Cheng JC, Taylor E, Leung PC, 2014. Oocyte-derived BMP15 but not GDF9 down-regulates connexin43 expression and decreases gap junction intercellular communication activity in immortalized human granulosa cells. Mol Hum Reprod 20, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig ZR, Ziv-Gal A, 2018. Pretty Good or Pretty Bad? The Ovary and Chemicals in Personal Care Products. Toxicol Sci 162, 349–360. [DOI] [PubMed] [Google Scholar]
- Czyzyk A, Latacz J, Filipowicz D, Podfigurna A, Moszynski R, Jasinski P, Sajdak S, Gaca M, Genazzani AR, Meczekalski B, 2017. Severe hyperandrogenemia in postmenopausal woman as a presentation of ovarian hyperthecosis. Case report and mini review of the literature. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology 33, 836–839. [DOI] [PubMed] [Google Scholar]
- Davidson JS, Baumgarten IM, 1988. Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap-junctional intercellular communication. Structure-activity relationships. J Pharmacol Exp Ther 246, 1104–1107. [PubMed] [Google Scholar]
- Desmarchais A, Teteau O, Papillier P, Jaubert M, Druart X, Binet A, Maillard V, Elis S, 2020. Bisphenol S Impaired In Vitro Ovine Early Developmental Oocyte Competence. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez A, Salazar Z, Arenas E, Betancourt M, Ducolomb Y, Gonzalez-Marquez H, Casas E, Teteltitla M, Bonilla E, 2016. Effect of perfluorooctane sulfonate on viability, maturation and gap junctional intercellular communication of porcine oocytes in vitro. Toxicol In Vitro 35, 93–99. [DOI] [PubMed] [Google Scholar]
- Dominguez A, Salazar Z, Betancourt M, Ducolomb Y, Casas E, Fernandez F, Bahena I, Salomon A, Teteltitla M, Martinez R, Chaparro A, Cuapio P, Salazar-Lopez C, Bonilla E, 2019. Effect of perfluorodecanoic acid on pig oocyte viability, intracellular calcium levels and gap junction intercellular communication during oocyte maturation in vitro. Toxicology in vitro : an international journal published in association with BIBRA 58, 224–229. [DOI] [PubMed] [Google Scholar]
- El-Hayek S, Clarke HJ, 2015. Follicle-Stimulating Hormone Increases Gap Junctional Communication Between Somatic and Germ-Line Follicular Compartments During Murine Oogenesis. Biol Reprod 93, 47. [DOI] [PubMed] [Google Scholar]
- Fleisch AF, Rifas-Shiman SL, Mora AM, Calafat AM, Ye X, Luttmann-Gibson H, Gillman MW, Oken E, Sagiv SK, 2017. Early-Life Exposure to Perfluoroalkyl Substances and Childhood Metabolic Function. Environ Health Perspect 125, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furger C, Cronier L, Poirot C, Pouchelet M, 1996. Human granulosa cells in culture exhibit functional cyclic AMP-regulated gap junctions. Mol Hum Reprod 2, 541–548. [DOI] [PubMed] [Google Scholar]
- Genetos DC, Zhou Z, Li Z, Donahue HJ, 2012. Age-related changes in gap junctional intercellular communication in osteoblastic cells. J Orthop Res 30, 1979–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon E, Plaks V, Dekel N, 2008. Gap junctions in the ovary: expression, localization and function. Mol Cell Endocrinol 282, 18–25. [DOI] [PubMed] [Google Scholar]
- Ghayda RA, Williams PL, Chavarro JE, Ford JB, Souter I, Calafat AM, Hauser R, Minguez-Alarcon L, 2019. Urinary bisphenol S concentrations: Potential predictors of and associations with semen quality parameters among men attending a fertility center. Environ Int 131, 105050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich J, Pu Y, Roberts J, Karthikraj R, Kannan K, Ehrhardt R, Veiga-Lopez A, 2018. Gestational bisphenol S impairs placental endocrine function and the fusogenic trophoblast signaling pathway. Arch Toxicol 92, 1861–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich J, Ticiani E, Veiga-Lopez A, 2020. Placenta Disrupted: Endocrine Disrupting Chemicals and Pregnancy. Trends Endocrinol Metab 31, 508–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot I, Dekel N, 2002. The ovarian gap junction protein connexin43: regulation by gonadotropins. Trends Endocrinol Metab 13, 310–313. [DOI] [PubMed] [Google Scholar]
- Gregoraszczuk E, Slomczynska M, Stoklosowa S, 1999. Effect of genistein, tyrphostin and herbimycin on prolactin-stimulated progesterone production by porcine theca and luteal cells. J Physiol Pharmacol 50, 477–484. [PubMed] [Google Scholar]
- Grochowalski A, Chrzaszcz R, Pieklo R, Gregoraszczuk EL, 2001. Estrogenic and antiestrogenic effect of in vitro treatment of follicular cells with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Chemosphere 43, 823–827. [DOI] [PubMed] [Google Scholar]
- Hanna EA, Umhauer S, Roshong SL, Piechocki MP, Fernstrom MJ, Fanning JD, Ruch RJ, 1999. Gap junctional intercellular communication and connexin43 expression in human ovarian surface epithelial cells and ovarian carcinomas in vivo and in vitro. Carcinogenesis 20, 1369–1373. [DOI] [PubMed] [Google Scholar]
- Hasler CM, Trosko JE, Bennink MR, 1991. Incorporation of n-3 fatty acids into WB-F344 cell phospholipids inhibits gap junctional intercellular communication. Lipids 26, 788–792. [DOI] [PubMed] [Google Scholar]
- Hatzirodos N, Hummitzsch K, Irving-Rodgers HF, Rodgers RJ, 2015. Transcriptome comparisons identify new cell markers for theca interna and granulosa cells from small and large antral ovarian follicles. PLoS One 10, e0119800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitland P, Koster HD, 2006. Biomonitoring of 30 trace elements in urine of children and adults by ICP-MS. Clin Chim Acta 365, 310–318. [DOI] [PubMed] [Google Scholar]
- Hossain MZ, Ao P, Boynton AL, 1998. Platelet-derived growth factor-induced disruption of gap junctional communication and phosphorylation of connexin43 involves protein kinase C and mitogen-activated protein kinase. Journal of cellular physiology 176, 332–341. [DOI] [PubMed] [Google Scholar]
- Hu W, Jones PD, Upham BL, Trosko JE, Lau C, Giesy JP, 2002. Inhibition of gap junctional intercellular communication by perfluorinated compounds in rat liver and dolphin kidney epithelial cell lines in vitro and Sprague-Dawley rats in vivo. Toxicol Sci 68, 429–436. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Redmer DA, Reynolds LP, Bilski JJ, Grazul-Bilska AT, 2002. Gap junctional intercellular communication of bovine granulosa and thecal cells from antral follicles: effects of luteinizing hormone and follicle-stimulating hormone. Endocrine 18, 261–270. [DOI] [PubMed] [Google Scholar]
- Juneja SC, Barr KJ, Enders GC, Kidder GM, 1999. Defects in the germ line and gonads of mice lacking connexin43. Biol Reprod 60, 1263–1270. [DOI] [PubMed] [Google Scholar]
- Kanemitsu MY, Lau AF, 1993. Epidermal growth factor stimulates the disruption of gap junctional communication and connexin43 phosphorylation independent of 12–0-tetradecanoylphorbol 13-acetate-sensitive protein kinase C: the possible involvement of mitogen-activated protein kinase. Mol Biol Cell 4, 837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KS, Wilson MR, Hayashi T, Chang CC, Trosko JE, 1996. Inhibition of gap junctional intercellular communication in normal human breast epithelial cells after treatment with pesticides, PCBs, and PBBs, alone or in mixtures. Environ Health Perspect 104, 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasa-Vubu JZ, Dahl GE, Evans NP, Thrun LA, Moenter SM, Padmanabhan V, Karsch FJ, 1992. Progesterone blocks the estradiol-induced gonadotropin discharge in the ewe by inhibiting the surge of gonadotropin-releasing hormone. Endocrinology 131, 208–212. [DOI] [PubMed] [Google Scholar]
- Kidder GM, Mhawi AA, 2002. Gap junctions and ovarian folliculogenesis. Reproduction 123, 613–620. [DOI] [PubMed] [Google Scholar]
- Kidder GM, Vanderhyden BC, 2010. Bidirectional communication between oocytes and follicle cells: ensuring oocyte developmental competence. Can J Physiol Pharmacol 88, 399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaunig JE, Ruch RJ, Weghorst CM, 1990. Comparative effects of phenobarbital, DDT, and lindane on mouse hepatocyte gap junctional intercellular communication. Toxicol Appl Pharmacol 102, 553–563. [DOI] [PubMed] [Google Scholar]
- Kurtenbach S, Kurtenbach S, Zoidl G, 2014. Gap junction modulation and its implications for heart function. Front Physiol 5, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GH, Jang B, Choi HS, Kim HJ, Park JH, Jeon YC, Carp RI, Kim YS, Choi EK, 2016. Upregulation of Connexin 43 Expression Via C-Jun N-Terminal Kinase Signaling in Prion Disease. J Alzheimers Dis 49, 1005–1019. [DOI] [PubMed] [Google Scholar]
- Leithe E, Rivedal E, 2004. Ubiquitination and down-regulation of gap junction protein connexin-43 in response to 12-O-tetradecanoylphorbol 13-acetate treatment. J Biol Chem 279, 50089–50096. [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K, 2013. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. Journal of agricultural and food chemistry 61, 4655–4662. [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Guo Y, Moon HB, Nakata H, Wu Q, Kannan K, 2012. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ Sci Technol 46, 9138–9145. [DOI] [PubMed] [Google Scholar]
- Lin D, Zhou J, Zelenka PS, Takemoto DJ, 2003. Protein kinase Cgamma regulation of gap junction activity through caveolin-1-containing lipid rafts. Invest Ophthalmol Vis Sci 44, 5259–5268. [DOI] [PubMed] [Google Scholar]
- Liu B, Lehmler HJ, Sun Y, Xu G, Sun Q, Snetselaar LG, Wallace RB, Bao W, 2019. Association of Bisphenol A and Its Substitutes, Bisphenol F and Bisphenol S, with Obesity in United States Children and Adolescents. Diabetes Metab J 43, 59–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Wang J, Zou H, Huang X, 2013. Effect of apigenin on gap junctional intercellular communication in human Tenon’s capsule fibroblasts. Eye Sci 28, 62–67. [PubMed] [Google Scholar]
- Long AC, Colitz CM, Bomser JA, 2007. Regulation of gap junction intercellular communication in primary canine lens epithelial cells: role of protein kinase C. Curr Eye Res 32, 223–231. [DOI] [PubMed] [Google Scholar]
- Lopez-Arellano P, Lopez-Arellano K, Luna J, Flores D, Jimenez-Salazar J, Gavia G, Teteltitla M, Rodriguez JJ, Dominguez A, Casas E, Bahena I, Betancourt M, Gonzalez C, Ducolomb Y, Bonilla E, 2019. Perfluorooctanoic acid disrupts gap junction intercellular communication and induces reactive oxygen species formation and apoptosis in mouse ovaries. Environmental toxicology 34, 92–98. [DOI] [PubMed] [Google Scholar]
- Machala M, Blaha L, Vondracek J, Trosko JE, Scott J, Upham BL, 2003a. Inhibition of gap junctional intercellular communication by noncoplanar polychlorinated biphenyls: Inhibitory potencies and screening for potential mode(s) of action. Toxicol. Sci 76, 102–111. [DOI] [PubMed] [Google Scholar]
- Machala M, Blaha L, Vondracek J, Trosko JE, Scott J, Upham BL, 2003b. Inhibition of gap junctional intercellular communication by noncoplanar polychlorinated biphenyls: inhibitory potencies and screening for potential mode(s) of action. Toxicol Sci 76, 102–111. [DOI] [PubMed] [Google Scholar]
- Magata F, Horiuchi M, Miyamoto A, Shimizu T, 2014. Lipopolysaccharide (LPS) inhibits steroid production in theca cells of bovine follicles in vitro: distinct effect of LPS on theca cell function in pre- and post-selection follicles. J Reprod Dev 60, 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten SJ, Tian M, Upham BL, Trosko JE, 2001. Effect of selected pesticides and their ozonation by-products on gap junctional intercellular communication using rat liver epithelial cell lines. Chemosphere 44, 457–465. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, 2005. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction 130, 791–799. [DOI] [PubMed] [Google Scholar]
- Meltzer D, Martinez-Arguelles DB, Campioli E, Lee S, Papadopoulos V, 2015. In utero exposure to the endocrine disruptor di(2-ethylhexyl) phthalate targets ovarian theca cells and steroidogenesis in the adult female rat. Reproductive toxicology 51, 47–56. [DOI] [PubMed] [Google Scholar]
- Mese G, Richard G, White TW, 2007. Gap junctions: basic structure and function. J Invest Dermatol 127, 2516–2524. [DOI] [PubMed] [Google Scholar]
- Metz CM, 2016. Bisphenol A: Understanding the Controversy. Workplace Health Saf 64, 28–36; quiz 37. [DOI] [PubMed] [Google Scholar]
- Morrison DK, 2012. MAP kinase pathways. Cold Spring Harb Perspect Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Chang CC, Mori T, Sato K, Ohtsuki K, Upham BL, Trosko JE, 2005a. Augmentation of differentiation and gap junction function by kaempferol in partially differentiated colon cancer cells. Carcinogenesis 26, 665–671. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Yoshikawa N, Hiroki I, Sato K, Ohtsuki K, Chang CC, Upham BL, Trosko JE, 2005b. beta-sitosterol from psyllium seed husk (Plantago ovata Forsk) restores gap junctional intercellular communication in Ha-ras transfected rat liver cells. Nutrition and Cancer-an International Journal 51, 218–225. [DOI] [PubMed] [Google Scholar]
- Nevoral J, Kolinko Y, Moravec J, Zalmanova T, Hoskova K, Prokesova S, Klein P, Ghaibour K, Hosek P, Stiavnicka M, Rimnacova H, Tonar Z, Petr J, Kralickova M, 2018. Long-term exposure to very low doses of bisphenol S affects female reproduction. Reproduction 156, 47–57. [DOI] [PubMed] [Google Scholar]
- Nippoldt TB, Reame NE, Kelch RP, Marshall JC, 1989. The roles of estradiol and progesterone in decreasing luteinizing hormone pulse frequency in the luteal phase of the menstrual cycle. The Journal of clinical endocrinology and metabolism 69, 67–76. [DOI] [PubMed] [Google Scholar]
- Nishii K, Shibata Y, Kobayashi Y, 2014. Connexin mutant embryonic stem cells and human diseases. World J Stem Cells 6, 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA, 2008. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development 135, 3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nteeba J, Sanz-Fernandez MV, Rhoads RP, Baumgard LH, Ross JW, Keating AF, 2015. Heat Stress Alters Ovarian Insulin-Mediated Phosphatidylinositol-3 Kinase and Steroidogenic Signaling in Gilt Ovaries. Biol Reprod 92, 148. [DOI] [PubMed] [Google Scholar]
- Okuma A, Kuraoka A, Iida H, Inai T, Wasano K, Shibata Y, 1996. Colocalization of connexin 43 and connexin 45 but absence of connexin 40 in granulosa cell gap junctions of rat ovary. J Reprod Fertil 107, 255–264. [DOI] [PubMed] [Google Scholar]
- Orisaka M, Tajima K, Tsang BK, Kotsuji F, 2009. Oocyte-granulosa-theca cell interactions during preantral follicular development. J Ovarian Res 2, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osgood RS, Upham BL, Bushel PR, Velmurugan K, Xiong KN, Bauer AK, 2017. Secondhand Smoke-Prevalent Polycyclic Aromatic Hydrocarbon Binary Mixture-Induced Specific Mitogenic and Pro-inflammatory Cell Signaling Events in Lung Epithelial Cells. Toxicol. Sci 157, 156–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osgood RS, Upham BL, Hill T III, Helms KL, Velmurugan K, Babica P, Bauer AK, 2014. Polycyclic aromatic hydrocarbon-induced signaling events relevant to inflammation and tumorigenesis in lung cells are dependent on molecular structure. PLoS. One 8, e65150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniappan M, Menon B, Menon KM, 2013. Stimulatory effect of insulin on theca-interstitial cell proliferation and cell cycle regulatory proteins through MTORC1 dependent pathway. Mol Cell Endocrinol 366, 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Zhou C, Rattan S, Flaws JA, 2015. Effects of Endocrine-Disrupting Chemicals on the Ovary. Biol Reprod 93, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelch KE, Li Y, Perera L, Thayer KA, Korach KS, 2019. Characterization of Estrogenic and Androgenic Activities for Bisphenol A-like Chemicals (BPs): In Vitro Estrogen and Androgen Receptors Transcriptional Activation, Gene Regulation, and Binding Profiles. Toxicol Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux G, Gerbaud P, Dompierre J, Lygren B, Solstad T, Evain-Brion D, Tasken K, 2014. A PKA-ezrin-Cx43 signaling complex controls gap junction communication and thereby trophoblast cell fusion. J Cell Sci 127, 4172–4185. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive, M., 2015. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril 103, e44–50. [DOI] [PubMed] [Google Scholar]
- Pu Y, Pearl S, Gingrich J, Jing J, Martin D, Murga-Zamalloa CA, Veiga-Lopez A, 2019. Multispecies study: low-dose tributyltin impairs ovarian theca cell cholesterol homeostasis through the RXR pathway in five mammalian species including humans. Arch Toxicol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW, Tomita T, 2012. Phospholipase C signaling and calcium influx. Adv Biol Regul 52, 152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W, Yang M, Liu S, Lei P, Hu L, Chen B, Wu M, Wang KJ, 2018. Toxic Effects of Bisphenol S Showing Immunomodulation in Fish Macrophages. Environ Sci Technol 52, 831–838. [DOI] [PubMed] [Google Scholar]
- Ranciere F, Botton J, Slama R, Lacroix MZ, Debrauwer L, Charles MA, Roussel R, Balkau B, Magliano DJ, Group DESIRS, 2019. Exposure to Bisphenol A and Bisphenol S and Incident Type 2 Diabetes: A Case-Cohort Study in the French Cohort D.E.S.I.R. Environ Health Perspect 127, 107013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S, Zhou C, Chiang C, Mahalingam S, Brehm E, Flaws JA, 2017. Exposure to endocrine disruptors during adulthood: consequences for female fertility. The Journal of endocrinology 233, R109–R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren P, Ruch RJ, 1996. Inhibition of gap junctional intercellular communication by barbiturates in long-term primary cultured rat hepatocytes is correlated with liver tumour promoting activity. Carcinogenesis 17, 2119–2124. [DOI] [PubMed] [Google Scholar]
- Richards JS, Ascoli M, 2018. Endocrine, Paracrine, and Autocrine Signaling Pathways That Regulate Ovulation. Trends Endocrin Met 29, 313–325. [DOI] [PubMed] [Google Scholar]
- Richards JS, Ren YA, Candelaria N, Adams JE, Rajkovic A, 2018. Ovarian Follicular Theca Cell Recruitment, Differentiation, and Impact on Fertility: 2017 Update. Endocrine reviews 39, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz HS, Pollack N, Cunningham AR, 2000. Exploring the relationship between the inhibition of gap junctional intercellular communication and other biological phenomena. Carcinogenesis 21, 1007–1011. [DOI] [PubMed] [Google Scholar]
- Rossi RO, Costa JJ, Silva AW, Saraiva MV, Van den Hurk R, Silva JR, 2016. The bone morphogenetic protein system and the regulation of ovarian follicle development in mammals. Zygote 24, 1–17. [DOI] [PubMed] [Google Scholar]
- Ruch RJ, Trosko JE, Madhukar BV, 2001. Inhibition of connexin43 gap junctional intercellular communication by TPA requires ERK activation. J Cell Biochem 83, 163–169. [DOI] [PubMed] [Google Scholar]
- Sai K, Kanno J, Hasegawa R, Trosko JE, Inoue T, 2000. Prevention of the down-regulation of gap junctional intercellular communication by green tea in the liver of mice fed pentachlorophenol. Carcinogenesis 21, 1671–1676. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P, 2012. The cyclic AMP pathway. Cold Spring Harb Perspect Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela-Abramovich S, Chorev E, Galiani D, Dekel N, 2005. Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology 146, 1236–1244. [DOI] [PubMed] [Google Scholar]
- Shi M, Sekulovski N, MacLean JA, Whorton A, Hayashi K, 2019. Prenatal Exposure to Bisphenol A Analogues on Female Reproductive Functions in Mice. Toxicol Sci 168, 561–571. [DOI] [PubMed] [Google Scholar]
- Shuhaibar LC, Egbert JR, Norris RP, Lampe PD, Nikolaev VO, Thunemann M, Wen L, Feil R, Jaffe LA, 2015. Intercellular signaling via cyclic GMP diffusion through gap junctions restarts meiosis in mouse ovarian follicles. Proc Natl Acad Sci U S A 112, 5527–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist KJ, Romo D, Upham BL, Armstrong M, Quinn K, Vanderlinden L, Osgood RS, Velmurugan K, Elie M, Manke J, Reinhold D, Reisdorph N, Saba L, Bauer AK, 2019. Early Mechanistic Events Induced by Low Molecular Weight Polycyclic Aromatic Hydrocarbons in Mouse Lung Epithelial Cells: A Role for Eicosanoid Signaling. Toxicol Sci 169, 180–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinyuk M, Mulkearns-Hubert EE, Reizes O, Lathia J, 2018. Cancer Connectors: Connexins, Gap Junctions, and Communication. Front Oncol 8, 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohl G, Willecke K, 2004a. Gap junctions and the connexin protein family. Cardiovasc Res 62, 228–232. [DOI] [PubMed] [Google Scholar]
- Sohl G, Willecke K, 2004b. Gap junctions and the connexin protein family. Cardiovasc. Res 62, 228–232. [DOI] [PubMed] [Google Scholar]
- Sommersberg B, Bulling A, Salzer U, Frohlich U, Garfield RE, Amsterdam A, Mayerhofer A, 2000. Gap junction communication and connexin 43 gene expression in a rat granulosa cell line: Regulation by follicle-stimulating hormone. Biol. Reprod 63, 1661–1668. [DOI] [PubMed] [Google Scholar]
- Sotogaku N, Tully SE, Gama CI, Higashi H, Tanaka M, Hsieh-Wilson LC, Nishi A, 2007. Activation of phospholipase C pathways by a synthetic chondroitin sulfate-E tetrasaccharide promotes neurite outgrowth of dopaminergic neurons. J Neurochem 103, 749–760. [DOI] [PubMed] [Google Scholar]
- Sovadinova I, Babica P, Boeke H, Kumar E, Wilke A, Park J-S, Trosko JE, Upham BL, 2015. Phosphatidylcholine Specific PLC-Induced Dysregulation of Gap Junctions, a Robust Cellular Response to Environmental Toxicants, and Prevention by Resveratrol in a Rat Liver Cell Model. Plos One 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas M, Verselis VK, White TW, 2018. Human diseases associated with connexin mutations. Biochim Biophys Acta Biomembr 1860, 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakovsky RS, Schantz SL, 2018. Impacts of bisphenol A (BPA) and phthalate exposures on epigenetic outcomes in the human placenta. Environ Epigenet 4, dvy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai MH, Upham BL, Olson LK, Tsao MS, Reed DN Jr., Trosko JE, 2007. Cigarette smoke components inhibited intercellular communication and differentiation in human pancreatic ductal epithelial cells. Int J Cancer 120, 1855–1862. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Otsuka A, Tanaka H, Shigenobu K, 1999. Glycyrrhetinic acid-sensitive mechanism does not make a major contribution to non-prostanoid, non-nitric oxide mediated endothelium-dependent relaxation of rat mesenteric artery in response to acetylcholine. Res Commun Mol Pathol Pharmacol 103, 227–239. [PubMed] [Google Scholar]
- Tarafdar A, Michie AM, 2014. Protein kinase C in cellular transformation: a valid target for therapy? Biochem Soc Trans 42, 1556–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM, 2013. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 99, 1324–1331 e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong D, Li TY, Naus KE, Bai D, Kidder GM, 2007. In vivo analysis of undocked connexin43 gap junction hemichannels in ovarian granulosa cells. J Cell Sci 120, 4016–4024. [DOI] [PubMed] [Google Scholar]
- Trosko JE, 2011. The gap junction as a “Biological Rosetta Stone”: implications of evolution, stem cells to homeostatic regulation of health and disease in the Barker hypothesis. J. Cell Commun. Signal 5, 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upham BL, 2011. Role of integrative signaling through gap junctions in toxicology. Curr Protoc Toxicol Chapter 2, Unit2 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upham BL, Blaha L, Babica P, Park JS, Sovadinova I, Pudrith C, Rummel AM, Weis LM, Sai K, Tithof PK, Guzvic M, Vondracek J, Machala M, Trosko JE, 2008. Tumor promoting properties of a cigarette smoke prevalent polycyclic aromatic hydrocarbon as indicated by the inhibition of gap junctional intercellular communication via phosphatidylcholine-specific phospholipase C. Cancer Sci 99, 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upham BL, Deocampo ND, Wurl B, Trosko JE, 1998. Inhibition of gap junctional intercellular communication by perfluorinated fatty acids is dependent on the chain length of the fluorinated tail. Int. J. Cancer 78, 491–495. [DOI] [PubMed] [Google Scholar]
- Upham BL, Guzvic M, Scott J, Carbone JM, Blaha L, Coe C, Li LL, Rummel AM, Trosko JE, 2007. Inhibition of gap junctional intercellular communication and activation of mitogen-activated protein kinase by tumor-promoting organic peroxides and protection by resveratrol. Nutr. Cancer 57, 38–47. [DOI] [PubMed] [Google Scholar]
- Upham BL, Park JS, Babica P, Sovadinova I, Rummel AM, Trosko JE, Hirose A, Hasegawa R, Kanno J, Sai K, 2009. Structure-activity-dependent regulation of cell communication by perfluorinated fatty acids using in vivo and in vitro model systems. Environ. Health Perspect 117, 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upham BL, Rummel AM, Carbone JM, Trosko JE, Ouyang Y, Crawford RB, Kaminski NE, 2003. Cannabinoids inhibit gap junctional intercellular communication and activate ERK in a rat liver epithelial cell line. Int J Cancer 104, 12–18. [DOI] [PubMed] [Google Scholar]
- Velmurugan K, Osgood RS, Xiong KN, Xiong J, Upham BL, Bauer AK, 2015. Secondhand Smoke Prevalent Low Molecular Weight Polycyclic Aromatic Hydrocarbon Effects On Lung Epithelial Cells. American Journal of Respiratory and Critical Care Medicine 191. [Google Scholar]
- Vinken M, Doktorova T, Decrock E, Leybaert L, Vanhaecke T, Rogiers V, 2009. Gap junctional intercellular communication as a target for liver toxicity and carcinogenicity. Crit Rev Biochem Mol Biol 44, 201–222. [DOI] [PubMed] [Google Scholar]
- Vinken M, Henkens T, Vanhaecke T, Papeleu P, Geerts A, Van Rossen E, Chipman JK, Meda P, Rogiers V, 2006. Trichostatin a enhances gap junctional intercellular communication in primary cultures of adult rat hepatocytes. Toxicol Sci 91, 484–492. [DOI] [PubMed] [Google Scholar]
- Wang GY, Bi YG, Liu XD, Han JF, Wei M, Zhang QY, 2017. Upregulation of connexin 43 and apoptosisassociated protein expression by high glucose in H9c2 cells was improved by resveratrol via the autophagy signaling pathway. Mol Med Rep 16, 3262–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Tong D, El-Gehani F, Tekpetey FR, Kidder GM, 2009. Connexin expression and gap junctional coupling in human cumulus cells: contribution to embryo quality. J Cell Mol Med 13, 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warn-Cramer BJ, Lampe PD, Kurata WE, Kanemitsu MY, Loo LW, Eckhart W, Lau AF, 1996. Characterization of the mitogen-activated protein kinase phosphorylation sites on the connexin-43 gap junction protein. J Biol Chem 271, 3779–3786. [DOI] [PubMed] [Google Scholar]
- Williams SA, Blache D, Martin GB, Foot R, Blackberry MA, Scaramuzzi RJ, 2001. Effect of nutritional supplementation on quantities of glucose transporters 1 and 4 in sheep granulosa and theca cells. Reproduction 122, 947–956. [PubMed] [Google Scholar]
- Winterhager E, Kidder GM, 2015. Gap junction connexins in female reproductive organs: implications for women’s reproductive health. Hum Reprod Update 21, 340–352. [DOI] [PubMed] [Google Scholar]
- Worley RR, Moore SM, Tierney BC, Ye X, Calafat AM, Campbell S, Woudneh MB, Fisher J, 2017. Per- and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environ Int 106, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CS, Becker DL, Lin JS, Warner AE, Hardy K, 2001. Stage-specific and differential expression of gap junctions in the mouse ovary: connexin-specific roles in follicular regulation. Reproduction 121, 77–88. [DOI] [PubMed] [Google Scholar]
- Wu JF, Ji J, Dong SY, Li BB, Yu ML, Wu DD, Tao L, Tong XH, 2016. Gefitinib enhances oxaliplatin-induced apoptosis mediated by Src and PKC-modulated gap junction function. Oncol Rep 36, 3251–3258. [DOI] [PubMed] [Google Scholar]
- Xiao J, Zhang G, Qiu P, Liu X, Wu Y, Du B, Li J, Zhou J, Li J, Tan Y, 2013. Tanshinone IIA increases the bystander effect of herpes simplex virus thymidine kinase/ganciclovir gene therapy via enhanced gap junctional intercellular communication. PLoS One 8, e67662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie HY, Cui Y, Deng F, Feng JC, 2015. Connexin: a potential novel target for protecting the central nervous system? Neural Regen Res 10, 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Qin SK, Wu Q, Wang ZS, Zheng RS, Tong XH, Liu H, Tao L, He XD, 2014. Connexin-dependent gap junction enhancement is involved in the synergistic effect of sorafenib and all-trans retinoic acid on HCC growth inhibition. Oncol Rep 31, 540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JM, McNeilly AS, 2010. Theca: the forgotten cell of the ovarian follicle. Reproduction 140, 489–504. [DOI] [PubMed] [Google Scholar]
- Zalmanova T, Hoskova K, Nevoral J, Adamkova K, Kott T, Sulc M, Kotikova Z, Prokesova S, Jilek F, Kralickova M, Petr J, 2017. Bisphenol S negatively affects the meotic maturation of pig oocytes. Sci Rep 7, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wei Q, Gao Z, Ma C, Yang Z, Zhao H, Liu C, Liu J, Zhao X, Ma B, 2019a. G protein-coupled receptor 30 mediates meiosis resumption and gap junction communications downregulation in goat cumulus-oocyte complexes by 17beta-estradiol. J Steroid Biochem Mol Biol 187, 58–67. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wu S, Liu L, Hou X, Jiang J, Wei X, Hao W, 2019b. Effects of bisphenol A on gap junctions in HaCaT cells as mediated by the estrogen receptor pathway. J Appl Toxicol 39, 271–281. [DOI] [PubMed] [Google Scholar]
- Zhao F, Jiang G, Wei P, Wang H, Ru S, 2018. Bisphenol S exposure impairs glucose homeostasis in male zebrafish (Danio rerio). Ecotoxicol Environ Saf 147, 794–802. [DOI] [PubMed] [Google Scholar]
- Zhu HS, Qian Z, Liu HL, Bao ED, 2016. ACTH-induced stress in weaned sows impairs LH receptor expression and steroidogenesis capacity in the ovary. Reprod Biol Endocrinol 14, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong L, Zhu Y, Liang R, Zhao HB, 2016. Gap junction mediated miRNA intercellular transfer and gene regulation: A novel mechanism for intercellular genetic communication. Sci Rep 6, 19884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


