Figure 6.
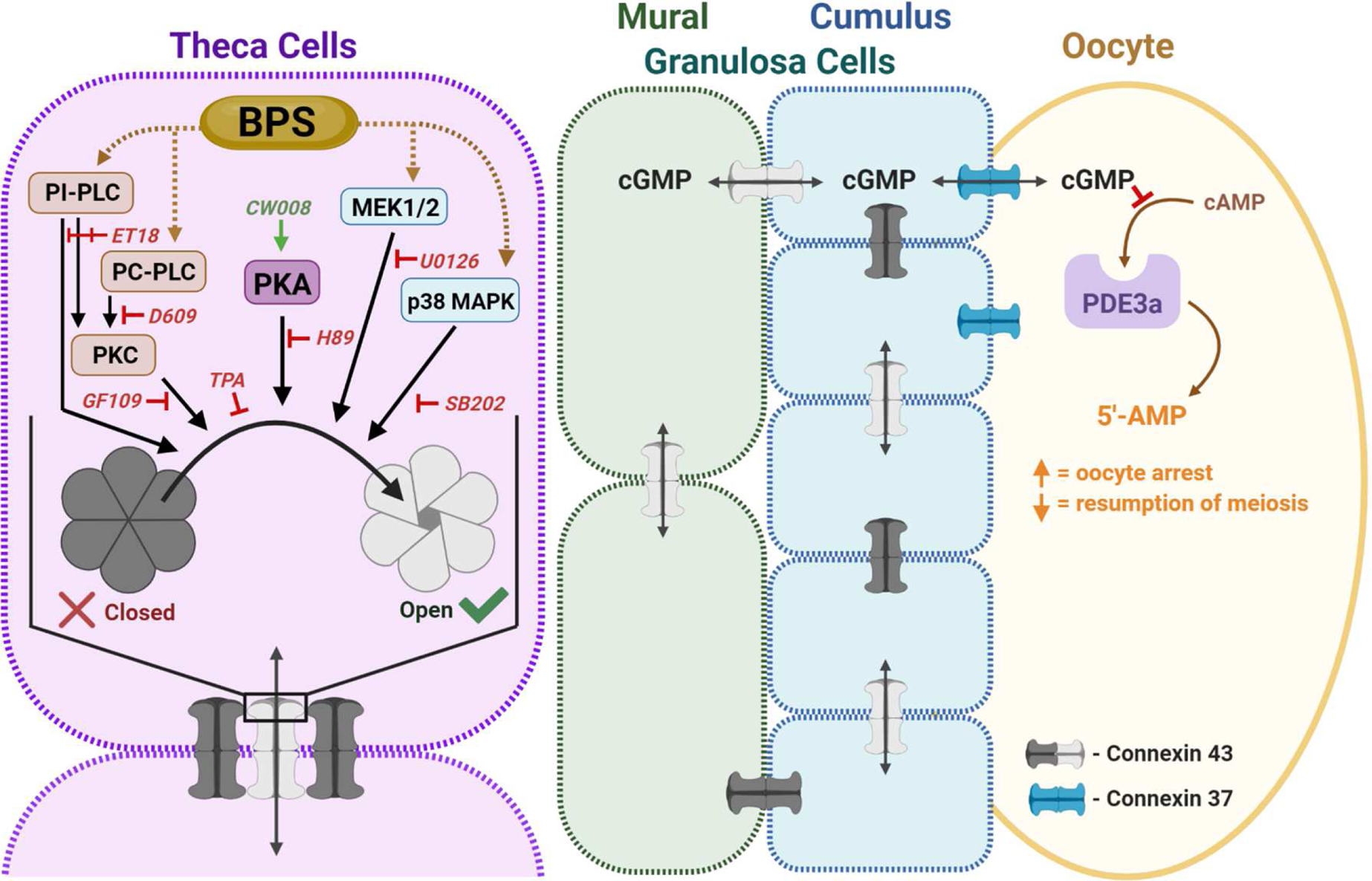
Working model for BPS’ modulation of theca cell GJIC. The antral follicle is formed by theca cells (purple), which provide physical and steroidogenic support to the underlying mural granulosa cells (green), cumulus granulosa cells (blue), and the oocyte (yellow). Connexin 37 and 43 form gap junction channels that connect inter-thecal, inter-granulosal and granulosa- to-oocyte cell communication, while theca-to-granulosa cell communication and hormone transport primarily occurs through paracrine signaling. Alterations in theca cell GJIC could affect the structural integrity and steroidogenic function of the follicle. GJIC in the ovary is temporospatially regulated, in part, by phosphodiesterase 3a (PDE3a), and needed for cellular arrest and meiosis resumption in the oocyte. Connexin phosphorylation signals opening, function, and recycling of gap junction channels. Pathways involved in GJIC regulation include protein kinase C (PKC), protein kinase A (PKA), and mitogen-activated protein kinase (MAPK) signaling. In theca cells, BPS can modulate GJIC through phosphatidylinositol-specific phospholipase C (PI-PLC) independent of PKC activation, as well as through MAPK/ERK kinase (MEK1/2). Like inter-thecal, inter-granulosal and granulosa-to-oocyte cell communication occurs through gap junctions, so we anticipate BPS could affect normal PDE3a-regulation of oocyte arrest and resumption of meiosis through dysregulated GJIC.ET18: ET-18-OCH3, GF109: GF109203X, PC-PLC: phosphatidyl choline-specific phospholipase C, SB202: SB202190, and TPA: phorbol 12-myrisate 13-acetate.
