Abstract
This study investigated the role of the PI3K/Akt pathway in cadmium (Cd) induced malignant transformation of normal prostate epithelial (PWR1E and RWPE1) cells. Both PWR1E and RWPE1 cells were exposed to 10μM Cd for one year and designated as Cd-PWR1E and Cd-RWPE1. Cd-RWPE1 cells robustly formed tumors in athymic nude mice. Functionally, Cd-exposure induced tumorigenic attributes indicated by increased wound healing, migration and invasion capabilities in both cell lines. RT2-array analysis revealed many oncogenes including P110α, Akt, mTOR, NFKB1 and RAF were induced whereas tumor suppressor (TS) genes were attenuated in Cd-RWPE1. This was validated by individual quantitative-real-time-PCR at transcriptional and by immunoblot at translational levels. These results were consistent in Cd-PWR1E vs parental PWR1E cells. Gene Set Enrichment Analysis revealed that five prostate cancer (PCa) related pathways were enriched in Cd-exposed cells compared to their normal controls. These pathways include the KEGG- Pathways in cancer, Prostate Cancer Pathway, ERBB, Apoptosis and MAPK pathways. We selected up- and down-regulated genes randomly from the PI3K/Akt pathway array and profiled these in the TCGA/GDC prostate-adenocarcinoma (PRAD) patient cohort. An upregulation of oncogenes and downregulation of TS genes was observed in PCa compared to their normal controls. Taken together, our study reveals that the PI3K/Akt signaling is one of the main molecular pathways involved in Cd-driven transformation of normal prostate epithelial cells to malignant form. Understanding the molecular mechanisms involved in the Cd-driven malignant transformation of normal prostate cells will provide a significant insight to develop better therapeutic strategies for Cd-induced prostate cancer.
Keywords: Cadmium, Prostate cancer, PI3K/Akt pathway
Introduction
Cadmium (Cd) is a toxic heavy metal and a major chemical pollutant in the industrial and agricultural environment. Technological progress and industrialization in most economically developed countries resulted in an excessive use of Cd that enhanced its dissemination in the environment (UNEP, 2010; WHO, 2010). Cd is thus recognized as an occupational health hazard and a threat to animal health (Julin et al., 2012). Atmospheric cadmium deposition or contamination in the soil due to increased use of Cd containing fertilizer is a threat to public health. Food is thus a prime reason of Cd exposure in majority parts of the world and the extent of exposure depends mainly on dietary intake (Mezynska and Brzoska, 2018). The International Agency for Research on Cancer (IARC) classified Cd as a human carcinogenic agent (IARC, 1993). Previous studies reveal that exposure of Cd is associated with adverse implications on bones, kidneys, liver and triggers the induction of cancer (Liu et al., 2009).
Like other metals, Cd exposure enhances oxidative stress or production of reactive oxygen species (ROS) which is often related to Cd toxicity and carcinogenesis. As described by Liu et al (Liu et al., 2009), hydrogen peroxide, superoxide anion, and hydroxyl radicals, generated due to Cd overload, are often associated with the activation of redox sensitive transcription factors (e.g., NF-κB, AP-1 and Nrf2) and a variation of ROS mediated gene expression leading to tissue damage and tumorigenesis (Waalkes, 2003). Furthermore, studies have proven that this metal has a broad spectrum of genetic and epigenetic effects that affect all phases of carcinogenesis (Waisberg et al., 2003; Bertin and Averbeck, 2006; Costa et al., 2017; Salemi et al., 2017). Cd modifies the expression of several genes related to carcinogenesis, such as c-myc, c-jun, and c-fos; stress response genes such as glutathione; metallothionein and related genes; transcription and transduction factors (Waisberg et al., 2003).
Several studies have demonstrated the association of Cd exposure and prostate cancer risk (Mezynska and Brzoska, 2018; Rapisarda et al., 2018). Human prostate cell in vitro studies have also shown the role of Cd in malignancies (Waalkes, 2003). A recent meta-analysis evaluated the association between prostate cancer risk and Cd exposure (Ju-Kun et al., 2016). Results suggest that exposure to higher Cd concentrations represents a risk factor for prostate cancer. These observations, together with in vivo data suggests a carcinogenic role for Cd in inducing prostate cancer. However, the underlying molecular mechanisms of Cd induced prostate cancer are largely unexplored (Waalkes and Rehm, 1994; Pal et al., 2017). Prostate cancer is among the top ten diagnosed cancers and is second leading cause of cancer deaths in 2020 in the United States. As per the recent estimates, 191,930 men will be diagnosed with prostate cancer and 33,330 deaths will occur in 2020 (Siegel et al., 2020). Prostate cancer cells undergo a plethora of molecular changes that lead to the uncontrolled growth properties. Activation of PI3K/Akt pathway is one of the major molecular alterations that drive the most aggressive forms of prostate cancer (Mulholland et al., 2012; Nacerddine et al., 2012). This pathway coordinates complex multifunctional molecular and functional events that mediate tumor progression and metastasis (Majumder and Sellers, 2005; Kalaany and Sabatini, 2009). Thus, active PI3K/AKT pathway is critical to prostate cancer progression and metastasis. PI3K/AKT/mTOR pathway has been reported to be activated by various chemical toxicants to induce malignant cell transformation (Jing et al., 2012; Li and Wang, 2014; Roy et al., 2014). . In this study, we investigated the role of PI3K/Akt pathway in Cd induced malignant transformation of normal prostate epithelial cells.
Materials and Methods
Human prostate epithelial cell lines and their culture
Human normal prostate epithelial cell lines RWPE1 and PWR1E were purchased from American Type Culture Collection (ATCC) (Manassas, VA). DNA short-tandem repeat analyses by ATCC were employed for their authentication. Cells were maintained at 37°C in an incubator and grown in keratinocyte serum free growth medium containing 5 ng/mL human recombinant epidermal growth factor, 0.05 mg/mL bovine pituitary extract (Gibco, Carlsbad, CA) and 1x penicillin/streptomycin.
Chemicals, assays and reagents used in the study
Cadmium chloride (CdCl2) was purchased from Sigma (St. Louis, MO). cDNA synthesis kit and TaqMan primer assays and kits were purchased from Biorad (Hercules, CA) and ThermoFisher Scientific (South San Francisco, CA), respectively. RT2 profiler reagents and array plate for PI3K/Akt pathway (Cat. # PAHS-058Z) were purchased from Qiagen (Redwood city, CA).
In vivo studies
We performed a pilot experiment with four mice to determine the cancerous transformation of normal RWPE1 cells after Cd-exposure. RWPE1 or Cd-RWPE1 cells (1x107) were subcutaneously implanted in nude mice in both right and left flanks (Balb c nu/nu; Harlan Laboratories Inc., Indianapolis, IN) (Figure 1A). Thus, we had four replicates for each cell line. Tumors were measured weekly and volume calculated as per the formula (Length x width (2))/2. All animal care and monitoring were in accordance with the UCSF, VA Medical Center and National Institutes of Health guidelines for the care and use of laboratory animals.
Figure 1.
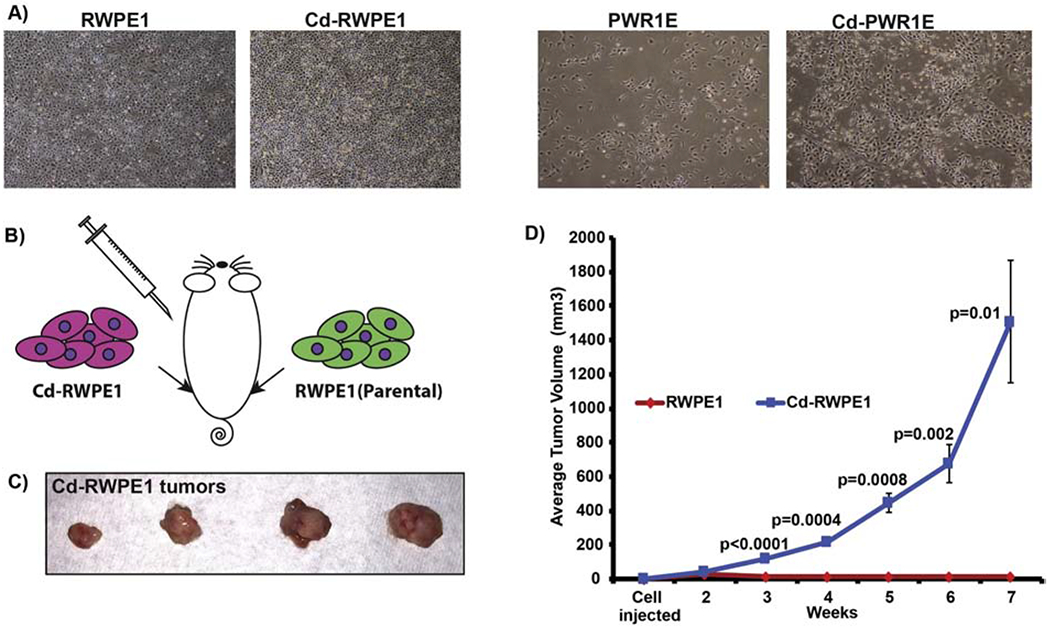
Cell phenotype and tumor formation in nude mice. Parental (RWPE1/PWR1E) and cadmium-exposed (Cd-RWPE1/Cd-PWR1E) cells under the reflected light microscope (A). Sketch showing injection scheme (B). Tumor formation by Cd-RWPE1 cells (C-D). Batch matched parental RWPE1 cells did not result in any tumors (D). Tumor volume over the course of experiment (D). There were four replicates for each cell line. Error bars ±SE.
Extraction of RNA
Extraction of RNA was performed as described previously (Dasgupta et al., 2020). Briefly, RNA from cells was extracted using combination of TRIzol reagent (Thermo Fisher Scientific) and RNeasy columns (Qiagen). RNase-Free DNase kit (Qiagen) was employed for DNase digestion. NanoDrop ND-1000 (NanoDrop Technologies, Wilmingon, DE) was used to measure RNA concentration and quality.
PI3K/Akt pathway profiler array analysis
RT2 PCR profiler array PAHS-058Z (Qiagen) was used for determining expression of PI3K/Akt pathway members. Samples from three biological replicates from the parental normal cells (PWR1E and RWPE1) and Cd exposed cells (Cd-PWR1E and Cd-RWPE1) were used for RNA extraction. Pooled RNA was used for cDNA synthesis and RT2 array analysis. Relative quantification changes in expression of genes between parental and Cd-exposed groups were determined by real time PCR and data analyzed by using Gene Globe Data Analysis Software (Qiagen). The RT2 profiler array has 84 PI3K/Akt pathway genes, five endogenous control genes, positive and negative PCR controls.
Validation of array results by qRT-PCR
cDNA was synthesized from RNA (1 ug) employing iScript Reverse Transcription Supermix (Biorad). TaqMan assays were performed with inventoried gene assays using a 7500 Fast Real Time PCR System ((Thermo Fisher Scientific). GAPDH served as an endogenous control. The change in relative expression of genes was calculated by using the formula: Fold change in gene expression, 2−ΔΔCt = 2−wherein ΔCt = Ct (detected genes) − Ct (GAPDH) and Ct represents threshold cycle number.
Wound healing assay
To determine whether Cd exposure induces increased wound healing capability in normal prostate epithelial cells, a scratch was made on cell monolayer. Percent wound closure was analyzed by comparing the width of the wound at 24 vs 0 hour of the same cell line. The data represents an average of three biological replicates.
Migration and invasion assays
For migration and invasion, cytoselect assay kits (Cell Biolabs, Inc) were employed following manufacturer’s instructions. Briefly, 10x104 cells in 300 μl of serum-free media were added to the top chamber, and the lower chamber contained 400 μl of keratinocyte growth medium having 5 ng/mL EGF and 0.05 mg/mL bovine pituitary extract (Gibco/Invitrogen, Carlsbad, CA). Cells that migrated or invaded on the lower side of the chamber after 16hrs of incubation at 37°C in a 5% CO2 incubator were stained either with crystal violet (Cd-RWPE1 and parental RWPE1) or with the stain provided in the kit (Cd-PWR1E and parental PWR1E) and air dried. Migrated/invaded cells were photographed using a light microscope. Extraction of the stain was performed with the extraction solution in the kit and absorbance was read on SpectraMax plate reader (Molecular Devices, LLC., San Jose, CA). Percent migration or invasion was analyzed based on the absorbance values. The data is represented by average percent migrated or invaded cells.
Western blot analysis
Protein was extracted from cultured cells employing radioimmunoprecipitation assay (RIPA) buffer (ThermoFisher Scientific) following the manufacturer’s instructions, 1x protease and phosphatase inhibitor cocktail (ThermoFisher Scientific) was added to the RIPA buffer. Protein from three biological replicates were pooled together and resolved on 10 or 15% sodium dodecyl sulfate (SDS) polyacrylamide gel. Membranes were blocked with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) and probed with specific antibodies for PI3KCA (4249; Cell Signaling Technology), p-c-RAF1 (Ser338) (9427; Cell Signaling Technology), CCND1 (2922, Cell Signaling Technology), pAkt (Ser473) (4060; Cell Signaling Technology) and GAPDH (sc-32233; Santa Cruz Biotechnology).
Human gene expression in TCGA data
TCGA Wanderer (http://maplab.imppc.org/wanderer/) is an interactive viewer to explore gene expression data in human cancers and it was used to obtain gene expression data in the prostate adenocarcinoma (PRAD) cohort (PCa n=374; normal n==52).
Characterization of enriched pathways by gene set enrichment analysis (GSEA)
To analyze the enriched pathways in Cd-exposed cells vs. normal parental control cells, profiler array expression data were subjected to Broad Institute, “Gene Set Enrichment Analysis (GSEA) version 3.0”. Parental cells served as controls for computing enrichment score (ES) and normalized enrichment scores (NES).
Statistical data analyses
Gene Globe software (Qiagen, Redwood city, CA) was employed to determine the expression of the 84 PI3K/Akt pathway gene in Cd-exposed Cd-RWPE1/Cd-PWR1E vs. normal parental RWPE1/PWR1E samples. The 35 CT value was set as cut-off. Significant fold upregulation cut off was considered 2 fold or higher and significant downregulation cut-off was considered as <0.5. Fold-Change (2^ (- Delta Delta CT) is the normalized gene expression (2^(- Delta CT)) in the Cd-exposed (Cd-RWPE1/CdPWR1E) samples divided by the normalized gene expression (2^ (- Delta CT)) in the parental normal (RWPE1/PWR1E) samples. Other statistical data analyses were done using GraphPad Prism 5 and/or MedCalc version 10.3.2. Quantified data is based on the average of at least triplicate samples or as indicated. Error bars in the figures represent standard deviation or standard error of the mean and a p-value <0.05 was considered statistically significant.
Results
Cadmium induces aggressive oncogenic characteristics in normal prostate epithelial cells
We used normal human prostate epithelial cell lines RWPE1 and PWR1E for this study. RWPE1 (ATCC CRL-11609) are epithelial cells derived from the peripheral zone of a histologically normal adult human prostate. These epithelial cells were transfected with a single copy of the human papilloma virus 18 (HPV-18) to establish the RWPE1 cell line (Bello et al., 1997). PWR1E (ATCC CRL-11611) was developed from human prostatic epithelial cells, derived from a normal prostate with mild hyperplasia and immortalized with an adenovirus 12-SV40 hybrid virus (Ad12-SV40). Both cell lines organize into acini and secrete PSA into the lumen when exposed to androgen (Bello-DeOcampo et al., 2001; Webber et al., 2001). The most remarkable characteristics include growth stimulation, increased androgen receptor expression and PSA induction in response to androgens, which evidently denotes their prostatic epithelial origin. We followed the previous report for levels of Cd exposure (Achanzar et al., 2001). RWPE1 and PWR1E cells were subjected to 10μM Cd-exposure for a year. This chronic Cd-exposure changed these normal cells into oncogenic cells and were designated as Cd-RWPE1 and Cd-PWR1E. Unlike parental RWPE1 and PWR1E cells, Cd-RWPE1 and Cd-PWR1E cells showed a multiple layer growth and were more adhesive. However, no dramatic changes were observed in both cell lines post Cd-exposure (Figure 1A). 10μM Cd dose was selected as it lies within the estimated Cd range of 11-28μM (assuming that 1g of wet tissue equals 1 ml) in the prostates of men that have no known occupational Cd-exposure (Elinder, 1985; Achanzar et al., 2001). We performed a pilot experiment with four nude mice (Balb c nu/nu) to get a definitive confirmation of cadmium induced malignant transformation of RWPE1 cells in vivo. We injected 1x107 Cd-RWPE1 and parental RWPE1 cells subcutaneously in mice (Figure 1B). In all we had four replicates for each cell line. Cd-RWPE1 cells formed tumors in all four mice with an average tumor volume of 1509 mm3 at week seven (Figure 1C–D). Palpable tumors were formed at 3 weeks after Cd-RWPE1 cell implantation. At termination of the experiment significant tumor burden was observed in Cd-RWPE1 inoculated mice (Figure 1D). Tumors were not observed in mice that received batch matched normal parental RWPE1 cells (Figure 1D).
Cadmium exposure modulates PI3K/Akt pathway genes
To decipher the effect of Cd on the PI3K/Akt signaling, we used a PI3K/Akt pathway array plate. This array plate contains 84 PI3K/Akt pathway genes (Supplemental Table 1) and 5 housekeeping control genes. In addition, the array plate has an assay to determine contamination by genomic DNA. Additional 3 assays are provided each for showing positive and negative PCR reaction controls. The 35 Ct value was set as cut-off for the analysis. Scatter plot analysis showed that many genes in the PI3K/Akt signaling are differentially expressed in Cd-RWPE1 cells vs. normal parental RWPE1 cells (Figure 2A). Fold change in expression was normalized to the expression in housekeeping genes and this relative fold change indicated a wide upregulation of most of the PI3K/AKT pathway genes in both Cd-RWPE1 and Cd-PWR1E compared to normal parental cells (Supplemental Table 1). In Cd-RWPE1 cells, 14 genes were significantly upregulated and 7 genes were significantly downregulated compared to normal RWPE1 (Table 1). Whereas in Cd-PWR1E vs. PWR1E cells, 23 genes were upregulated and 2 genes were significantly downregulated (Table 1). The cut-off for significance for upregulated fold change was 2 and for the downregulated was 0.5 fold. The upregulation in gene expression ranged from 2 to 7 fold and downregulation ranged from 0.08 to 0.47 fold in Cd-RWPE1 vs RWPE1 (Table 1). Whereas in Cd-PWR1E vs PWR1E cells, the upregulation was from 2 to 17. Heat map of Cd-RWPE1 vs RWPE1 indicates the overall fold-change in gene expression involved in the PI3K/Akt pathway (Figure 2B). Supplemental Table 1 has details for all PI3K/Akt pathway genes and their relative fold regulation including array location for both cell lines.
Figure 2.
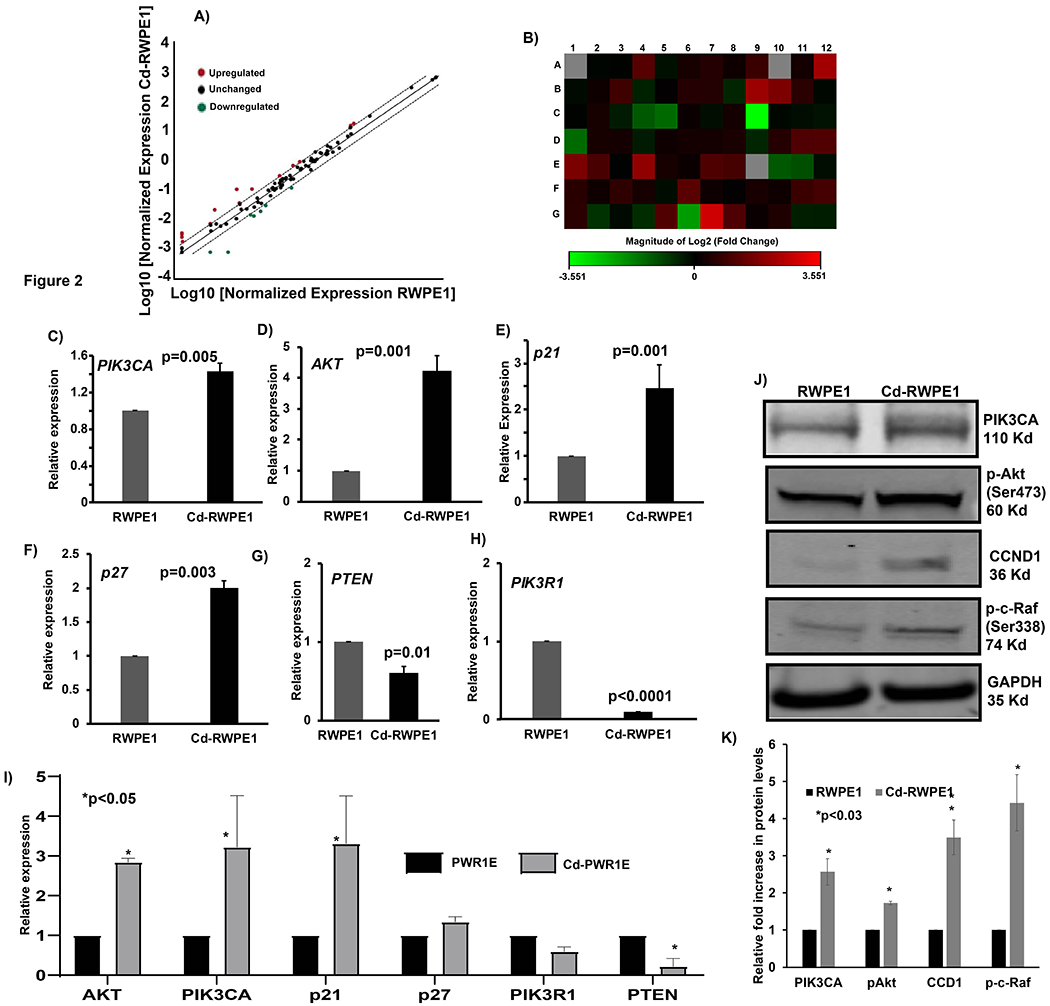
Differential expression by RT2 profiler array, real-time PCR and immunoblot analyses of the PI3K/Akt signaling pathway. Scatter plot representing the array results. Upregulated genes are indicated by red points and downregulated genes are in green (A). Heat Map shows the fold changes in expression of 84 PI3K/Akt pathway genes in Cd-RWPE1 vs. RWPE1 cells (B). The complete gene names, location on array and fold changes are indicated in Supplemental Table 1. TaqMan gene expression (C-I) and immunoblot (J) analyses of selected PI3K/Akt pathway genes. Graphical representation of the relative fold upregulation in protein levels based on the average intensity of the target proteins in all replicates and normalized to their endogenous controls (K). Error bars ±SD. Experiments were performed three times and each time there were three replicates in TaqMan assays. For RT2 profiler array RNA from three biological replicates was pooled together for the cDNA synthesis for all four cell lines.
Table 1.
Significantly up- (>2 fold) and down-regulated (<0.5) genes in Cd exposed (Cd-RWPE1; Cd-PWR1E) compared to parental normal (RWPE1; PWR1E) cells.
| Upregulated genes: Cd-PWR1E vs PWR1E | Upregulated gene: Cd-RWPE1 vs RWPE1 | |||||
|---|---|---|---|---|---|---|
| Position | Symbol | Fold Change | Position | Symbol | Fold Change | |
| A01 | ADAR | 4.19 | A04 | AKT3 | 2.27 | |
| A05 | APC | 2.48 | A12 | CDKN1B | 4.76 | |
| A06 | BAD | 3.17 | B09 | ELK1 | 4.54 | |
| A08 | CASP9 | 5.24 | B10 | FASLG | 3.29 | |
| A11 | CDC42 | 2.87 | D11 | MYD88 | 2.09 | |
| B02 | CSNK2A1 | 2.29 | D12 | NFKB1 | 2.19 | |
| B05 | EIF4B | 2.55 | E01 | NFKBIA | 3.26 | |
| B08 | EIF4G1 | 2.2 | E04 | PDGFRA | 4.10 | |
| B11 | FKBP1A | 2.36 | E07 | PDPK1 | 2.12 | |
| B12 | FOS | 13.78 | F06 | RAC1 | 2.34 | |
| C05 | GRB2 | 3.08 | G05 | TCL1A | 2.22 | |
| C06 | GSK3B | 2.23 | G07 | TLR4 | 7.30 | |
| C08 | HSPB1 | 8.78 | G08 | TOLLIP | 2.06 | |
| D02 | ITGB1 | 5.13 | H02 | B2M | 2.31 | |
| D06 | MAPK14 | 6.41 | Downregulated gene: Cd-RWPE1 vs RWPE1 | |||
| D07 | MAPK3 | 4.6 | C04 | GRB10 | 0.43 | |
| D11 | MYD88 | 16.82 | C05 | GRB2 | 0.36 | |
| F03 | PTEN | 3.45 | C09 | IGF1 | 0.09 | |
| F06 | RAC1 | 2.6 | D01 | IRS1 | 0.36 | |
| F07 | RAF1 | 2.69 | E10 | PIK3R1 | 0.43 | |
| F09 | RBL2 | 3.25 | E11 | PIK3R2 | 0.47 | |
| F10 | RHEB | 2.09 | G06 | TIRAP | 0.22 | |
| F11 | RHOA | 3.54 | ||||
| Downregulated gene: Cd-PWRE1 vs PWRE1 | ||||||
| E05 | PDK1 | 0.1 | ||||
| F02 | PRKCZ | 0.5 | ||||
Quantitative real-time PCR (qRT-PCR) validation of array results
We selected genes randomly from the array results to confirm the Cd-induced modulation in gene expression by qRT-PCR analysis. We used TaqMan gene expression assay system for qRT-PCR. Expression of genes such as PIK3CA (p=0.005; Figure 2C), Akt3 (p=0.001; Figure 2D), p21 (p=0.001; Figure 2E), p27 (p=0.003; Figure 2F), PTEN (p=0.01; Figure 2G) and PIK3R1 (p<0.0001; Figure 2H) were differentially expressed in Cd-RWPE1 vs. normal RWPE1 cells. These results were consistent in Cd-PWR1E compared to normal parental PWR1E (Figure 2I) cells. For qRT-PCR analysis, GAPDH was utilized as the endogenous control.
Cadmium induced translational modulation of PI3K/Akt pathway genes
We performed immunoblot assays to examine the effect of Cd on the translation of PI3K/Akt pathway genes. A significant increase in the protein levels of PI3KCA, p-c-RAF(Ser338), pAkt(Ser473) and CCND1 was observed in Cd-RWPE1 in comparison to the parental RWPE1 cells (Figure 2J). These results along with the qRT-PCR data indicate that Cd modulates the PI3K/Akt pathway at both transcriptional and translational levels.
Cadmium exposure induces tumorigenic characteristics in normal prostate epithelial cells
The PI3K/Akt pathway drives aggressive forms of prostate cancer and metastasis. We sought to investigate the effect of Cd on the tumorigenic functional attributes such as wound healing capability, migration and invasion of both Cd-RWPE1 and Cd-PWR1E vs. normal parental controls.
Cd-exposure induces increased wound healing capability in normal cells
Cd exposed cells were more proficient than parental normal cells in closing an artificial wound created over a confluent cell monolayer (Figure 3A–B, G–H). Cd-RWPE1 cells showed 66% wound closure at 24 vs 0 hours in these cells. Similarly, parental RWPE1 cells showed 29% wound closure at same time points (Figure 3A–B). Cd-PWR1E showed ~60% wound closure at 24 vs 0 hours, whereas parental PWR1E showed ~30% wound closure at the same time points (Figure 3G–H). These results reveal that Cd exposure increased the wound healing ability of normal prostate epithelial cells which is an attribute of malignant transformation.
Figure 3. Cadmium exposure induces aggressive tumorigenic characteristics in normal prostate epithelial cells.
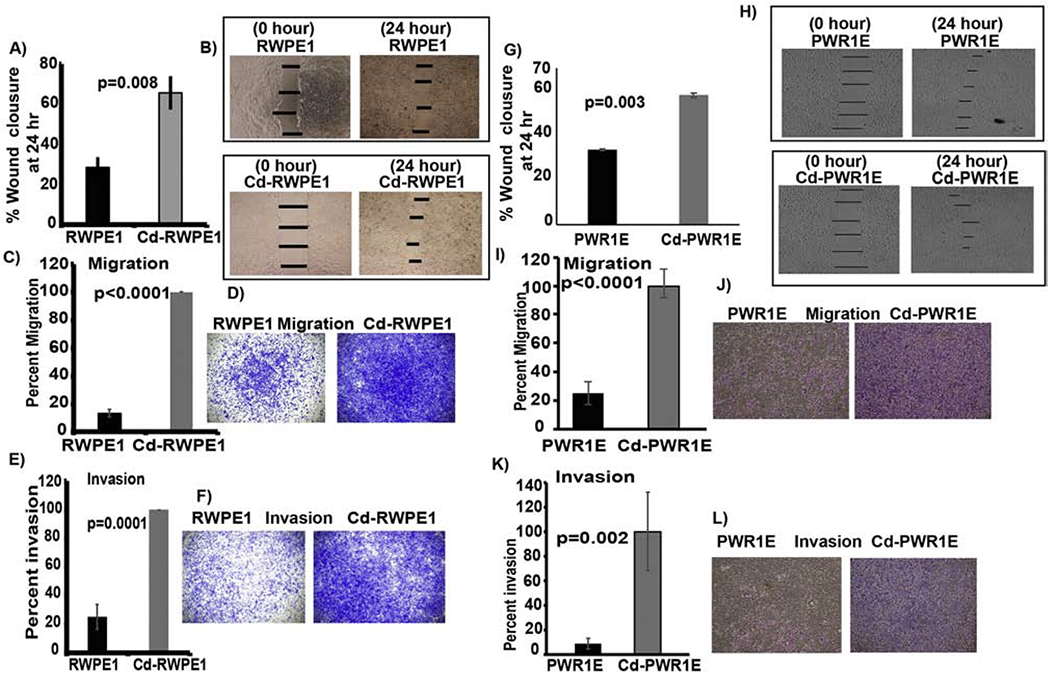
Wound healing assay. Percent artificial wound closure after 24 hours (A; G). Representative pictures of the artificial wound at 0 and 24 hours of cell culture (B; H). Error bar ±SD. Chemotactic cell migration and invasion assay. Average percent migration (C; I) and invasion (E; K) in Cd-exposed (Cd-RWPE1 and Cd-PWR1E) cells compared to their normal parental (RWPE1 and PWR1E) controls. Data are represented as the mean ±SD from at least three replicates. Representative pictures of migration (D; J) and invasion (F; L) in both cell lines.
Cd-exposure induces increased cell migration and invasion in normal cells
To discern whether Cd-mediated modulation of the PI3K/Akt pathway has aggressive phenotypic implications, we performed in vitro chemotactic trans-well invasion and migration assays. A robust increase in migration of Cd-RWPE1 (67%, p<0.0001; Figure 3C–D) and Cd-PWR1E (75%, p<0.0001; Figure 3I–J) cells was observed compared to their parental controls. Similarly, a significant increase in invasion was observed in Cd-RWPE1 (62%, p<0.0001; Figure 3E–F) and Cd-PWR1E (90%, p=0.002; Figure 3K–L) compared to parental controls. These results confirm that aggressive tumorigenic characteristics are induced in normal prostate epithelial cells upon Cd-exposure. These phenotypic experiments reveal that increased wound healing, migration and invasion are core aspects of cadmium-induced aggressive phenotypic transformation of normal prostate epithelial cells. The molecular mechanism underlying these Cd-induced functional effects is thus partly via the modulation of PI3K/Akt pathway.
Enrichment of prostate cancer related pathways in Cd-exposed normal cells analyzed by gene set enrichment analysis (GSEA)
For GSEA analysis, we utilized curated gene sets (C2) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway mapping. All the modulated genes in Cd-exposed (Cd-RWPE1; Cd-PWR1E) cells vs. normal parental (RWPE1; PWR1E) cells were analyzed in comparison to the KEGG pathway database to investigate the pathways that are likely to be correlated with our RT2-array results. We observed 5 overlapping enriched pathways in Cd-treated cells (Cd-RWPE1) which were negatively correlated with parental RWPE1 (Figure 4A–E). The overlapping pathways include KEGG Apoptosis (ES=0.56, NES=1), KEGG ERBB (ES=0.25, NES=1), KEGG MAPK (ES=0.48, NES=1), KEGG Pathways in cancer (ES=0.33, NES=1) and KEGG Prostate Cancer pathways (ES=0.35, NES=1) (Figure 4A–E). A list of genes differentially expressed in these pathways are shown in Figure 5. Interestingly, all these pathways are implicated in prostate cancer progression and metastasis. Similar results were obtained in Cd-PWR1E vs PWR1E (Supplemental Figure 1, 2). These results further confirm that the PI3K/Akt pathway is one of the main and important pathways involved in Cd-driven cancerous transformation of normal prostate cells.
Figure 4. Gene set enrichment analysis (GSEA) for the characterization of gene expression in Cd-exposed compared to normal controls.
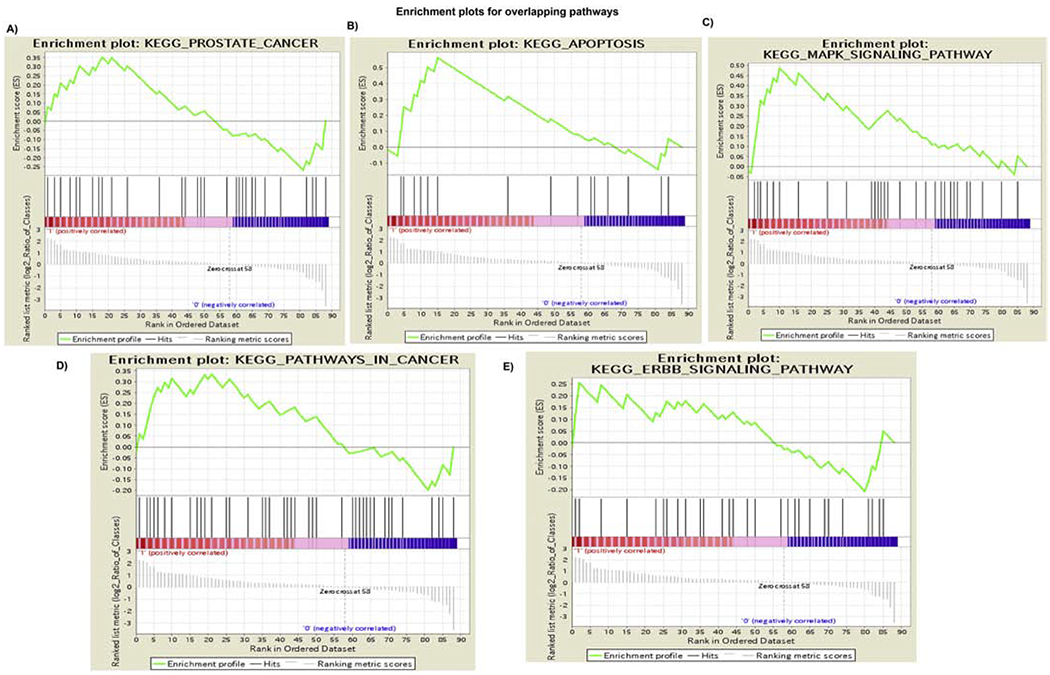
Cd-RWPE1 is denoted as “1” and parental normal RWPE1 as “0” in the plots. Five overlapping pathways are enriched in Cd-RWPE1 vs. Parental RWPE1 as control. These pathways are KEGG_Prostate cancer Pathway (A). KEGG_Apoptosis (B). KEGG_MAPK (C). KEGG_Pathways in cancer (D). KEGG_ERBB (E) pathways. Same pathways were enriched in Cd-PWR1E vs. normal parental PWR1E cells (Supplemental Figures 1 and 2).
Figure 5. Up- and down-regulated genes that are correlated with the pathway enrichment plots (A-E) in Cd-RWPE1 compared to the parental RWPE1.
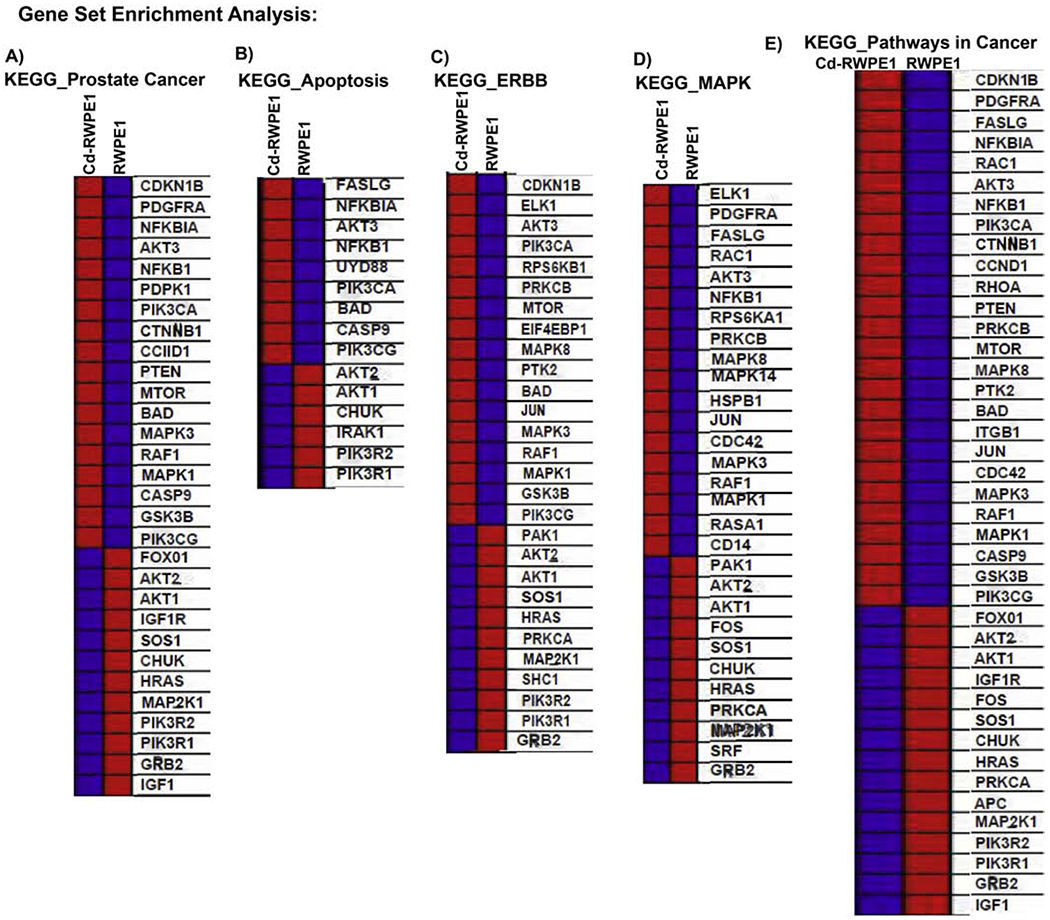
Overexpressed genes are in red and underexpressed genes are in blue. These lists correspond to each gradient red and blue bars located at the middle of Enrichment plots in the Figure 4A–E and represent the genes that are assigned in these plots.
Genes modulated by Cd exposure are implicated in prostate cancer
We randomly selected genes that were modulated by Cd-exposure and determined their expression in TCGA/GDC prostate adenocarcinoma (PRAD) cohort (PCa n= 374; normal n=52). The expression profiles of PTEN and PIK3R1 were significantly downregulated whereas Akt1, Akt2 and MTOR (Figure 6) were overexpressed in cancer compared to normal prostate samples. The overexpressed genes are oncogenic whereas under-expressed genes are tumor suppressor components in the PI3K/Akt pathway and are implicated in prostate carcinogenesis.
Figure 6:
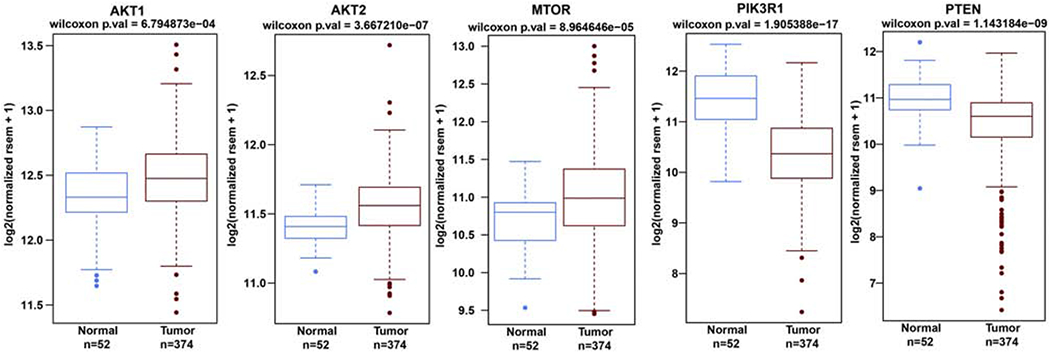
Genes modulated by Cd-exposure are differentially expressed in the TCGA data base. Box plot analysis of the PI3K/Akt pathway genes in the TCGA prostate adenocarcinoma (PRAD) cohort (cancer=374; normal=52). * p≤0.05.
Discussion
Cadmium is reported to be a type I carcinogen by the International Agency for Cancer Research (IARC, 1993; Mulware, 2013). Multiple risk factors are responsible for development of prostate cancer that include dietary factors, androgen, obesity and exposure to cadmium (Allott et al., 2013). Epidemiologic studies investigating the association between Cd-exposure and prostate cancer susceptibility have yielded inconsistent findings. Reports based on literature meta-analysis suggest Cd exposure is associated with prostate cancer development (Ju-Kun et al., 2016; Wei et al., 2017; Mezynska and Brzoska, 2018).Whereas, some studies have reported insignificant or no association between Cd exposure and prostate cancer risk (Thun et al., 1985; Chen et al., 2009; Sawada et al., 2012; Eriksen et al., 2015; Chen et al., 2016a). The identification of environmental factors responsible for the development of aggressive prostate cancer phenotype is a promising area of study. In this study we aimed to investigate the effect of Cd-exposure in inducing wound healing, migration and invasion in normal prostate epithelial cells (RWPE1 and PWR1E). Increased wound healing, migration and invasion are the hallmarks of metastatic prostate cancer. We subjected these normal prostate cells to 10uM Cd in culture for a year guided by a previous publication (Achanzar et al., 2001). Initially, we confirmed the malignant transformation of cadmium exposed RWPE1 cells (Cd-RWPE1) in a pilot experiment by inoculating the Cd-RWPE1 cells into nude mice. We observed robust tumor formation with Cd-RWPE1 in nude mice. Our results are consistent with a previous study that reported inoculation of nude mice with the cadmium-transformed human prostate epithelial cells resulted in formation of highly invasive and metastatic epithelial tumors (Achanzar et al., 2001). Comparable results were observed in rats, where chronic cadmium exposure induced proliferative lesions and prostatic tumors (Waalkes et al., 1988; Waalkes et al., 1999a; Waalkes et al., 1999b).
The PI3K/Akt pathway is constitutively active in prostate cancer and drives cancer progression to advanced metastatic disease (Gao et al., 2003; Majumder and Sellers, 2005; Shukla et al., 2007; Sun et al., 2007; Wang et al., 2007; Taylor et al., 2010; Tanaka et al., 2011; Baiz et al., 2012; Harashima et al., 2012; Imamura et al., 2012; Lin et al., 2012; Mulholland et al., 2012; Nacerddine et al., 2012). This pathway coordinates complex multifunctional events that govern tumor-associated processes such as cell survival, cell cycle, growth epithelial-mesenchymal transition (EMT), migration and angiogenesis (Chan et al., 1999; Datta et al., 1999; Testa and Bellacosa, 2001; Brazil et al., 2002; Majumder and Sellers, 2005; Kalaany and Sabatini, 2009). Our results show that Cd exposure induced oncogenes while suppressing the tumor suppressor genes in the PI3K/Akt pathway. Cd exposure modulated the PI3K/Akt pathway genes at both the transcriptional and translational level. A previous study reported the Cd-exposure of human metastatic prostate cancer cell line (1-LN) increased its proliferation via induction of signaling pathways such as protein kinase C, farnesyl transferase, MEK1/2, ERK1/2, p38MAPK and PI3-kinase (PI3K) (Misra et al., 2003). We observed that Cd triggered induction of the PI3K/Akt pathway induced aggressive malignant phenotype in normal prostate epithelial cells. This was indicated by enhanced wound healing capability, migration and invasion. Unregulated cell migration and invasion are common features of cancer cells that favor metastatic dissemination. In addition, the frequency of genomic aberrations in the PI3K/Akt pathway correlates with prostate cancer progression, rising from 50% in primary to 100% in metastatic tumors (Taylor et al., 2010). From these results we infer that Cd exposure induces an invasive and metastatic phenotype in normal prostate epithelial cells partly via induction of the PI3K/Akt pathway, though further studies are needed to examine this phenomenon in vivo.
We applied Gene Set Enrichment Analysis (GSEA) using our PCR array expression data for the PI3K/Akt pathway. GSEA is a powerful computational method that connects gene expression with biological processes present across phenotypes (Subramanian et al., 2005). Comparative transcriptomics for all the modulated genes was performed in both Cd-RWPE1/Cd-PWR1E arrays for enrichment of pathways keeping parental RWPE1/PWR1E cells as control. RT2 array data of the PI3K/Akt pathway overlapped with five other pathways indicated by enrichment of the KEGG-prostate cancer pathway, KEGG-ERBB, KEGG-Apoptosis, KEGG-MAPK and KEGG-Pathways in cancer in Cd-RWPE1/CD-PWR1E vs parental normal controls. Interestingly all these pathways are implicated in prostate carcinogenesis and regulation of these pathways was reported to be effective in preventing the progression and metastasis of prostate cancer (Fu et al., 2003; Maroni et al., 2004; Mulholland et al., 2012). Our results are consistent with a previous systems biology approach study that showed the enrichment of the PI3K/Akt pathway along with other pathways involved in the carcinogenic activity of cadmium (Chen et al., 2016b). Further, we randomly selected PI3K/Akt pathway genes and profiled these in publicly available TCGA/GDC matched prostate adenocarcinoma(PRAD) data set (cancer=374; normal=52). An upregulation of the oncogenic components and downregulation of the PI3k/Akt pathway tumor suppressor components in prostate cancer compared to normal prostate samples was observed. It has been reported that protooncogene activation and tumor suppressor gene inactivation are responsible for Cd-induced carcinogenesis (Hartwig, 2010; Asara et al., 2013). This data further supports our finding that the PI3K/Akt pathway is the major molecular signaling underlying the Cd-induced cancerous transformation of normal prostate cells. However, other pathways may also be involved and need to be further investigated.
Taken together, our study demonstrates that enhanced PI3K/Akt signaling is one of the main molecular mechanisms underlying Cd-induced prostate cancer. Functionally, Cd-induced PI3K/Akt pathway lead to aggressive tumorigenic characteristics in normal prostate cells. Further, we show that five overlapping signaling pathways may mediate the carcinogenic effects following Cd exposure. Identification of key molecular pathways and their functional analyses will provide critical information regarding their contribution to Cd induced malignant transformation. In addition, this study highlights the PI3K/Akt pathway as a promising therapeutic target to regulate invasive and metastatic prostate cancer.
Supplementary Material
Supplemental Figure 1. Gene set enrichment analysis (GSEA) in Cd-PWR1E vs parental PWR1E. Overlapping pathways enriched in Cd-PWR1E vs. PWR1E as control. Similar to Cd-RWPE1 vs RWPE1, same pathways such as KEGG_Prostate cancer Pathway, KEGG_Apoptosis, KEGG_MAPK, KEGG_Pathways in cancer and KEGG_ERBB pathways were enriched in Cd-PWR1E vs parental PWR1E cells.
Supplemental Figure 2. Modulated expression of genes that are correlated with the pathway enrichment plots in Cd-RWPE1 vs parental RWPE1. Overexpression is shown in red and lower expression if indicated by blue. These lists correspond to the gradient red and blue bars that are located at the middle of Enrichment plots in Supplemental Figure 1 and represent the genes that are included in the plots.
Supplemental Table 1: List of genes in the PI3K/Akt pathway array and their differential expression represented as the relative fold change in Cd exposed (Cd-RWPE1; Cd-PWR1E) compared to parental normal (RWPE1; PWR1E) cells.
Normal prostate epithelial RWPE1 and PWR1E cells were exposed to cadmium (Cd).
Cadmium exposed RWPE1 (Cd-RWPE1) cells formed tumors in nude mice.
Molecular mechanism involved the PI3K/Akt pathway genes.
Activation of these genes was observed at transcription and translation levels.
Gene Set Enrichment Analysis showed enrichment of prostate cancer related pathways.
Acknowledgements:
We thank Dr. Roger Erickson for his support and assistance with the preparation of the manuscript.
Funding
This study was supported by the Department of Veterans Affairs VA Merit Review 101 BX001123, VA Senior Research Career Scientist Award (Rajvir Dahiya, IK6-BX004473), and the National Institutes of Health / National Cancer Institute RO1CA199694, RO1CA196848.
Abbreviations:
- PCa
Prostate cancer
- Cd
Cadmium
- TS
Tumor suppressor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no potential conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Achanzar WE, Diwan BA, Liu J, Quader ST, Webber MM, Waalkes MP, 2001. Cadmium-induced malignant transformation of human prostate epithelial cells. Cancer Res 61, 455–458. [PubMed] [Google Scholar]
- Allott EH, Masko EM, Freedland SJ, 2013. Obesity and prostate cancer: weighing the evidence. Eur Urol 63, 800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asara Y, Marchal JA, Carrasco E, Boulaiz H, Solinas G, Bandiera P, Garcia MA, Farace C, Montella A, Madeddu R, 2013. Cadmium modifies the cell cycle and apoptotic profiles of human breast cancer cells treated with 5-fluorouracil. Int J Mol Sci 14, 16600–16616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiz D, Pinder TA, Hassan S, Karpova Y, Salsbury F, Welker ME, Kulik G, 2012. Synthesis and Characterization of a Novel Prostate Cancer-Targeted Phosphatidylinositol-3-kinase Inhibitor Prodrug. J Med Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-DeOcampo D, Kleinman HK, Deocampo ND, Webber MM, 2001. Laminin-1 and alpha6beta1 integrin regulate acinar morphogenesis of normal and malignant human prostate epithelial cells. Prostate 46, 142–153. [DOI] [PubMed] [Google Scholar]
- Bello D, Webber MM, Kleinman HK, Wartinger DD, Rhim JS, 1997. Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis 18, 1215–1223. [DOI] [PubMed] [Google Scholar]
- Bertin G, Averbeck D, 2006. Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 88, 1549–1559. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Park J, Hemmings BA, 2002. PKB binding proteins. Getting in on the Akt. Cell 111, 293–303. [DOI] [PubMed] [Google Scholar]
- Chan TO, Rittenhouse SE, Tsichlis PN, 1999. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem 68, 965–1014. [DOI] [PubMed] [Google Scholar]
- Chen C, Xun P, Nishijo M, Carter S, He K, 2016a. Cadmium exposure and risk of prostate cancer: a meta-analysis of cohort and case-control studies among the general and occupational populations. Sci Rep 6, 25814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Duan X, Li M, Huang C, Li J, Chu R, Ying H, Song H, Jia X, Ba Q, Wang H, 2016b. Systematic network assessment of the carcinogenic activities of cadmium. Toxicol Appl Pharmacol 310, 150–158. [DOI] [PubMed] [Google Scholar]
- Chen YC, Pu YS, Wu HC, Wu TT, Lai MK, Yang CY, Sung FC, 2009. Cadmium burden and the risk and phenotype of prostate cancer. BMC Cancer 9, 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa C, Miozzi E, Teodoro M, Briguglio G, Rapisarda V, Fenga C, 2017. New insights on ‘old’ toxicants in occupational toxicology (Review). Mol Med Rep 15, 3317–3322. [DOI] [PubMed] [Google Scholar]
- Dasgupta P, Kulkarni P, Bhat NS, Majid S, Shiina M, Shahryari V, Yamamura S, Tanaka Y, Gupta RK, Dahiya R, Hashimoto Y, 2020. Activation of the Erk/MAPK signaling pathway is a driver for cadmium induced prostate cancer. Toxicol Appl Pharmacol 401, 115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME, 1999. Cellular survival: a play in three Akts. Genes Dev 13, 2905–2927. [DOI] [PubMed] [Google Scholar]
- Elinder CG, 1985. Normal values for cadmium in human tissue, blood and urine in different countries . In Friberg L, Elinder CG, Kjellström T, Nordberg GF, (Ed.), Cadmium and health: A toxicological and epidemiological appraisal. CRC Press, Boca Raton, FL, pp. 81–102. [Google Scholar]
- Eriksen KT, HaIkjaer J, Meliker JR, McElroy JA, Sorensen M, Tjonneland A, Raaschou-Nielsen O, 2015. Dietary cadmium intake and risk of prostate cancer: a Danish prospective cohort study. BMC Cancer 15, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Smith PC, Zhang L, Rubin MA, Dunn RL, Yao Z, Keller ET, 2003. Effects of raf kinase inhibitor protein expression on suppression of prostate cancer metastasis. J Natl Cancer Inst 95, 878–889. [DOI] [PubMed] [Google Scholar]
- Gao N, Zhang Z, Jiang BH, Shi X, 2003. Role of PI3K/AKT/mTOR signaling in the cell cycle progression of human prostate cancer. Biochem Biophys Res Commun 310, 1124–1132. [DOI] [PubMed] [Google Scholar]
- Harashima N, Inao T, Imamura R, Okano S, Suda T, Flarada M, 2012. Roles of the PI3K/Akt pathway and autophagy in TLR3 signaling-induced apoptosis and growth arrest of human prostate cancer cells. Cancer Immunol Immunother 61, 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig A, 2010. Mechanisms in cadmium-induced carcinogenicity: recent insights. Biometals 23, 951–960. [DOI] [PubMed] [Google Scholar]
- IARC, 1993. IARC monographs on the evaluation of carcinogenic risks to humans., Lyon, pp. 1972–1993. [Google Scholar]
- Imamura Y, Sakamoto S, Endo T, Utsumi T, Fuse M, Suyama T, Kawamura K, Imamoto T, Yano K, Uzawa K, Nihei N, Suzuki H, Mizokami A, Ueda T, Seki N, Tanzawa H, Ichikawa T, 2012. FOXA1 Promotes Tumor Progression in Prostate Cancer via the Insulin-Like Growth Factor Binding Protein 3 Pathway. PLoS One 7, e42456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y, Liu LZ, Jiang Y, Zhu Y, Guo NL, Barnett J, Rojanasakul Y, Agani F, Jiang BH, 2012. Cadmium increases HIF-1 and VEGF expression through ROS, ERK, and AKT signaling pathways and induces malignant transformation of human bronchial epithelial cells. Toxicol Sci 125, 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju-Kun S, Yuan DB, Rao HF, Chen TF, Luan BS, Xu XM, Jiang FN, Zhong WD, Zhu JG, 2016. Association Between Cd Exposure and Risk of Prostate Cancer: A PRISMA-Compliant Systematic Review and Meta-Analysis. Medicine (Baltimore) 95, e2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julin B, Wolk A, Bergkvist L, Bottai M, Akesson A, 2012. Dietary cadmium exposure and risk of postmenopausal breast cancer: a population-based prospective cohort study. Cancer Res 72, 1459–1466. [DOI] [PubMed] [Google Scholar]
- Kalaany NY, Sabatini DM, 2009. Tumours with PI3K activation are resistant to dietary restriction. Nature 458, 725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Wang G, 2014. Computer-aided targeting of the PI3K/Akt/mTOR pathway: toxicity reduction and therapeutic opportunities. IntJ Mol Sci 15, 18856–18891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Sun X, Feng T, Zou H, Jiang Y, Liu Z, Zhao D, Yu X, 2012. ADAM17 regulates prostate cancer cell proliferation through mediating cell cycle progression by EGFR/PI3K/AKT pathway. Mol Cell Biochem 359, 235–243. [DOI] [PubMed] [Google Scholar]
- Liu J, Qu W, Kadiiska MB, 2009. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol 238, 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder PK, Sellers WR, 2005. Akt-regulated pathways in prostate cancer. Oncogene 24, 7465–7474. [DOI] [PubMed] [Google Scholar]
- Maroni PD, Koul S, Meacham RB, Koul HK, 2004. Mitogen Activated Protein kinase signal transduction pathways in the prostate. Cell Commun Signal 2, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezynska M, Brzoska MM, 2018. Environmental exposure to cadmium-a risk for health of the general population in industrialized countries and preventive strategies. Environ Sci Pollut Res Int 25, 3211–3232. [DOI] [PubMed] [Google Scholar]
- Misra UK, Gawdi G, Pizzo SV, 2003. Induction of mitogenic signalling in the 1LN prostate cell line on exposure to submicromolar concentrations of cadmium+. Cell Signal 15, 1059–1070. [DOI] [PubMed] [Google Scholar]
- Mulholland DJ, Kobayashi N, Ruscetti M, Zhi A, Tran LM, Huang J, Gleave M, Wu H, 2012. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res 72, 1878–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulware SJ, 2013. Trace elements and carcinogenicity: a subject in review. 3 Biotech 3, 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacerddine K, Beaudry JB, Ginjala V, Westerman B, Mattiroli F, Song JY, van der Poel H, Ponz OB, Pritchard C, Cornelissen-Steijger P, Zevenhoven J, Tanger E, Sixma TK, Ganesan S, van Lohuizen M, 2012. Akt-mediated phosphorylation of Bmi1 modulates its oncogenic potential, E3 ligase activity, and DNA damage repair activity in mouse prostate cancer. J Clin Invest 122, 1920–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal D, Suman S, Kolluru V, Sears S, Das TP, Alatassi H, Ankem MK, Freedman JH, Damodaran C, 2017. Inhibition of autophagy prevents cadmium-induced prostate carcinogenesis. Br J Cancer 117, 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapisarda V, Miozzi E, Loreto C, Matera S, Fenga C, Avola R, Ledda C, 2018. Cadmium exposure and prostate cancer: insights, mechanisms and perspectives. Front Biosci (Landmark Ed) 23, 1687–1700. [DOI] [PubMed] [Google Scholar]
- Roy R, Singh SK, Chauhan LK, Das M, Tripathi A, Dwivedi PD, 2014. Zinc oxide nanoparticles induce apoptosis by enhancement of autophagy via PI3K/Akt/mTOR inhibition. Toxicol Lett 227, 29–40. [DOI] [PubMed] [Google Scholar]
- Salemi R, Marconi A, Di Salvatore V, Franco S, Rapisarda V, Libra M, 2017. Epigenetic alterations and occupational exposure to benzene, fibers, and heavy metals associated with tumor development (Review). Mol Med Rep 15, 3366–3371. [DOI] [PubMed] [Google Scholar]
- Sawada N, Iwasaki M, Inoue M, Takachi R, Sasazuki S, Yamaji T, Shimazu T, Endo Y, Tsugane S, 2012. Long-term dietary cadmium intake and cancer incidence. Epidemiology 23, 368–376. [DOI] [PubMed] [Google Scholar]
- Shukla S, Maclennan GT, Hartman DJ, Fu P, Resnick MI, Gupta S, 2007. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int J Cancer 121, 1424–1432. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A, 2020. Cancer statistics, 2020. CA Cancer J Clin 70, 7–30. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP, 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102, 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LC, Luo J, Mackey LV, Fuselier JA, Coy DH, 2007. A conjugate of camptothecin and a somatostatin analog against prostate cancer cell invasion via a possible signaling pathway involving PI3K/Akt, alphaVbeta3/alphaVbeta5 and MMP-2/-9. Cancer Lett 246, 157–166. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Yoshida M, Tanimura H, Fujii T, Sakata K, Tachibana Y, Ohwada J, Ebiike H, Kuramoto S, Morita K, Yoshimura Y, Yamazaki T, Ishii N, Kondoh O, Aoki Y, 2011. The selective class I PI3K inhibitor CH5132799 targets human cancers harboring oncogenic PIK3CA mutations. Clin Cancer Res 17, 3272–3281. [DOI] [PubMed] [Google Scholar]
- Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL, 2010. Integrative genomic profiling of human prostate cancer. Cancer Cell 18, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa JR, Bellacosa A, 2001. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci U S A 98, 10983–10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thun MJ, Schnorr TM, Smith AB, Halperin WE, Lemen RA, 1985. Mortality among a cohort of U.S. cadmium production workers--an update. J Natl Cancer Inst 74, 325–333. [PubMed] [Google Scholar]
- UNEP, 2010. Interim review of scientific information on cadmium. United Nations Environment Programe (UNEP), pp. [Google Scholar]
- Waalkes MP, 2003. Cadmium carcinogenesis. Mutat Res 533, 107–120. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Anver M, Diwan BA, 1999a. Carcinogenic effects of cadmium in the noble (NBL/Cr) rat: induction of pituitary, testicular, and injection site tumors and intraepithelial proliferative lesions of the dorsolateral prostate. Toxicol Sci 52, 154–161. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Anver MR, Diwan BA, 1999b. Chronic toxic and carcinogenic effects of oral cadmium in the Noble (NBL/Cr) rat: induction of neoplastic and proliferative lesions of the adrenal, kidney, prostate, and testes. J Toxicol Environ Health A 58, 199–214. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Rehm S, 1994. Chronic toxic and carcinogenic effects of cadmium chloride in male DBA/2NCr and NFS/NCr mice: strain-dependent association with tumors of the hematopoietic system, injection site, liver, and lung. Fundam Appl Toxicol 23, 21–31. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Rehm S, Riggs CW, Bare RM, Devor DE, Poirier LA, Wenk ML, Henneman JR, Balaschak MS, 1988. Cadmium carcinogenesis in male Wistar [Crl:(WI)BR] rats: dose-response analysis of tumor induction in the prostate and testes and at the injection site. Cancer Res 48, 4656–4663. [PubMed] [Google Scholar]
- Waisberg M, Joseph P, Hale B, Beyersmann D, 2003. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 192, 95–117. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kreisberg JI, Ghosh PM, 2007. Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer. Curr Cancer Drug Targets 7, 591–604. [DOI] [PubMed] [Google Scholar]
- Webber MM, Quader ST, Kleinman HK, Bello-DeOcampo D, Storto PD, Bice G, DeMendonca-Calaca W, Williams DE, 2001. Human cell lines as an in vitro/in vivo model for prostate carcinogenesis and progression. Prostate 47, 1–13. [DOI] [PubMed] [Google Scholar]
- Wei T, Jia J, Wada Y, Kapron CM, Liu J, 2017. Dose dependent effects of cadmium on tumor angiogenesis. Oncotarget 8, 44944–44959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2010. Preventing disease through health environments Exposure to cadmium: a major public health concern. . World Health Orgnaization (WHO), Geneva, Switzerland, pp. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Gene set enrichment analysis (GSEA) in Cd-PWR1E vs parental PWR1E. Overlapping pathways enriched in Cd-PWR1E vs. PWR1E as control. Similar to Cd-RWPE1 vs RWPE1, same pathways such as KEGG_Prostate cancer Pathway, KEGG_Apoptosis, KEGG_MAPK, KEGG_Pathways in cancer and KEGG_ERBB pathways were enriched in Cd-PWR1E vs parental PWR1E cells.
Supplemental Figure 2. Modulated expression of genes that are correlated with the pathway enrichment plots in Cd-RWPE1 vs parental RWPE1. Overexpression is shown in red and lower expression if indicated by blue. These lists correspond to the gradient red and blue bars that are located at the middle of Enrichment plots in Supplemental Figure 1 and represent the genes that are included in the plots.
Supplemental Table 1: List of genes in the PI3K/Akt pathway array and their differential expression represented as the relative fold change in Cd exposed (Cd-RWPE1; Cd-PWR1E) compared to parental normal (RWPE1; PWR1E) cells.


