Abstract
Fish oil (FO) and olive oil (OO) supplementations attenuate the cardiovascular responses to inhaled concentrated ambient particles in human volunteers. This study was designed to examine the cardiovascular effects of ozone (O3) exposure and the efficacy of FO and OO-enriched diets in attenuating the cardiovascular effects from O3 exposure in rats. Rats were fed either a normal diet (ND), a diet enriched with 6% FO or OO starting at 4 weeks of age. Eight weeks following the start of these diet, animals were exposed to filtered air (FA) or 0.8 ppm O3, 4 hr/day for 2 consecutive days. Immediately after exposure, cardiac function was measured as the indices of left-ventricular developed pressure (LVDP) and contractility (dP/dtmax and dP/dtmin) before ischemia. In addition, selective microRNAs (miRNAs) of inflammation, endothelial function, and cardiac function were assessed in cardiac tissues to examine the molecular alterations of diets and O3 exposure. Pre-ischemic LVDP and dP/dtmax were lower after O3 exposure in rats fed ND but not FO and OO. Cardiac miRNAs expressions were altered by both diet and O3 exposure. Specifically, O3-induced up-regulation of miR-150-5p and miR-208a-5p were attenuated by FO and/or OO. miR-21 was up-regulated by both FO and OO after O3 exposure. This study demonstrated that O3-induced cardiovascular responses appear to be blunted by FO and OO diets. O3-induced alterations in miRNAs linked to inflammation, cardiac function, and endothelial dysfunction support these pathways are involved, and dietary supplementation with FO or OO may alleviate these adverse cardiovascular effects in rats.
Keywords: ozone, fish oil diet, olive oil diet, cardiac function, cardiac contractility, microRNA
INTRODUCTION
Air pollution is among the leading risk factors for mortality in the world. Ozone (O3), one of 6 criteria air pollutants, has been studied extensively and well-established for causing respiratory mortality and morbidity, decrements in lung function, increases in lung inflammation, and respiratory symptoms (Kim et al., 2011; Schelegle et al., 2009). Recent epidemiological studies reported that O3 also causes cardiovascular mortality and morbidity. An epidemiological study demonstrated that a 10 ppb increase in the previous week’s O3 was associated with a 0.64% increase in cardiovascular and respiratory mortality (Bell et al., 2004). Controlled exposure studies showed that O3 increased vascular and systemic inflammation and caused cardiac autonomic dysfunction (Arjomandi et al., 2015; Devlin et al., 2012).
Intervention has been sought to reduce the adverse health effects of air pollutants at individual levels. Among those interventions, dietary supplementation with omega-3 fatty acids (Lin et al., 2019; Romieu et al., 2005; Tong, 2016; Tong et al., 2015; Tong et al., 2012) and vitamins (Zhong et al., 2017) have reduced particulate matter (PM)-induced cardiac autonomic dysfunction, inflammation, endothelial dysfunction and dyslipidemia. The present study was aimed to explore whether unsaturated fatty acid supplements could modulate the cardiopulmonary effects from O3 exposure in an animal model. We have previously demonstrated that fish oil protected the vascular system against acute O3-induced contractile response but increased pulmonary injury/inflammation in rats at baseline (Snow et al., 2018). The present study focused on whether FO and OO could attenuate acute O3 exposure-induced cardiac effects.
Recent studies have revealed important roles for microRNAs (miRNAs) in the cardiovascular system (Latronico et al., 2007) and environmental exposure (Vrijens et al., 2015). miRNAs represent a class of small noncoding RNAs that negatively regulate target gene expression at the post-transcriptional level by inhibiting the translation of protein from mRNA or by promoting the degradation of mRNA (Lee et al., 2003). They are expressed in a tissue-specific manner, including heart tissue and vasculature, where differential expression patterns have been linked with inflammation (Recchiuti et al., 2011; Urbich et al., 2008; Yang et al., 2018) and oxidative stress (Li et al., 2012; Pereira et al., 2015). miRNAs also participate in cardiac events such as myocardial growth and necrosis (Wang et al., 2015), coronary artery disease (Karakas et al., 2017), myocardial infarction (Dong et al., 2009; Yang et al., 2018; Zampetaki et al., 2012), and endothelial proliferation (Schober et al., 2014). This study assessed the association of cardiac miRNAs with O3 exposure and whether dietary supplementation altered this association in rat hearts.
MATERIALS AND METHODS
Animals
Three-week-old male Wistar Kyoto (WKY) rats were purchased from Charles River Laboratories (Raleigh, NC). Animals were housed two per cage at 23 ± 1°C and kept on a 12h light/dark cycle. All animal received Purina 5001 rodent diet until the start of dietary regimen (Ralston Purina Laboratories, St. Louis, MO). Diet and water were provided ad libitum. All experimental protocols were approved by the U.S. EPA’s Institutional Animal Care and Use Committee (IACUC).
Diets
Animals were fed either a standard laboratory diet (Purina 5001, Ralston Purina Laboratories, St. Louis, MO) or a diet enriched with 6% by weight fish oil (Teklad Custom Research Diets #TD.140729, Harlan Laboratories, Inc., Indianapolis, IN) or olive oil (Teklad Custom Research Diets #TD.140727) (Table 1) starting at 4 weeks of age for 8 weeks until the end of experiment as has been reported in our previous study (Snow et al., 2018). Animals were given water and food ad libitum. Diets were refrigerated and fresh food was replaced every 2–3 days throughout the course of this study to minimize oxidation.
Table 1.
Compositions of fish oil and olive oil diet.
| Fatty Acids (g/kg) | Normal Diet | Fish Oil Diet (TD 140729) | Olive Oil Diet (TD 140727) |
|---|---|---|---|
| Total Fat | 57 | 72 | 72 |
| Saturated Fat | 15.6 | 18.3 | 10.5 |
| Monounsaturated Fat | 16.0 | 15.8 | 46.1 |
| Polyunsaturated Fat | 10 | 25.9 | 12.1 |
| Saturated Fat (% of fat) | unknown | 30.5 | 15.3 |
| Monounsaturated Fat (% of fat) | unknown | 26.3 | 67.1 |
| Polyunsaturated Fat (% of fat) | unknown | 43.2 | 17.6 |
| 14:0 Myristic acid | unknown | 5.4 | 0 |
| 16:0 Palmitic acid | unknown | 10.7 | 8.3 |
| 16:1 Palmitoleic acid | unknown | 7.2 | 0.6 |
| 18:0 Stearic acid | unknown | 2.2 | 2.2 |
| 18:1 Oleic acid | unknown | 8.6 | 45.5 |
| 18:2 Linoleic acid (LA) | 12.2 | 6.5 | 10.7 |
| 18:3 Alpha-linolenic acid (ALA) | 1.0 | 1.7 | 1.4 |
| 18:4 Stearidonic acid (SDA) | unknown | 1.8 | 0 |
| 20:4 Eicosatetraenoic acid (ETA) | unknown | 0.9 | 0 |
| 20:5 Eicosapentaenoic acid (EPA) | 1.9* | 8.1 | 0 |
| 22:5 Docosapentaenoic acid (DPA) | unknown | 0.6 | 0 |
| 22:6 Docosahexaenoic acid (DHA) | unknown | 6.3 | 0 |
| n-6: n-3 ratio | 3.5 | 0.4 | 7.6 |
Unknown, values for individual fatty acids are not routinely measured for this diet.
This concentration represents total omega-3 fatty acids content (https://www.labsupplytx.com/wp-content/uploads/2012/10/5001.pdf).
Ozone Exposures
Ozone was generated in the animal exposure chambers as previously described (Snow et al., 2018). After the animals were on the respective diets for 8 weeks, they were exposed to either filtered air (FA) or 0.8 ppm O3 for 4h/day for 2 consecutive days. The average (mean ± SEM) air temperature (75.01 ± 0.21°F), relative humidity (49.84 ± 0.30), and O3 concentration (0.8045 ± 0.0027 ppm) were monitored continuously. Ozone at 0.8 ppm concentration in rats produces demonstrable pulmonary and systemic changes that allows for assessing alleviating effect of diet (Snow et al., 2018). This concentration of O3 is many folds higher than what could be encountered environmentally. However, it is comparable to humans inhaling 0.2 ppm O3 while undergoing intermittent moderate exercise based on the evidence that resting rats retain 1/4th the O3 lung dose of humans exposed with intermittent exercise (Hatch et al., 2013). Two consecutive days of exposure protocol was chosen to allow for maximum lung injury and systemic response that is not influenced by adaptation that is apparent on day 3 of O3 exposure (Miller et al., 2016).
Cardiac Function
Two cohorts of animals were used in this study with identical dietary and exposure conditions. Cohort 1 was used to assess the cardiovascular responses (n=8/group); Cohort 2 was used for tissues collection (n=8/group) (Snow et al., 2018). As described previously (Tong et al., 2009), within 2–4 h after second day O3 exposure, rats from cohort 1 were anesthetized with an intraperitoneal injection of sodium pentobarbital (80 mg/kg body weight). After intravenous heparin (100 units) injection the hearts were rapidly excised and placed in ice-cold Krebs-Henseleit buffer. The aortas were cannulated and perfused retrograde at constant pressure of 100 cmH2O. The non-recirculating perfusate was a Krebs-Henseleit buffer containing (in mmol/L) 120 NaCl, 5.9 KCl, 1.2 MgSO4, 1.75 CaCl2, 25 NaHCO3, and 11 glucose. The buffer was aerated with 95% O2 and 5% CO2, and maintained at pH 7.4 and 37°C.
For assessment of contractile function, a latex balloon on the tip of a polyethylene catheter was inserted through the left atrium into the left ventricle. The catheter was connected to a pressure transducer (Argon Medical Devices, Athens, TX) at the same height as the heart. The left ventricular balloon was inflated to 0–5 cmH2O. A PowerLab system was used to collect and process the heart rate, left ventricular developed pressure (LVDP=LV peak minus end-diastolic pressure), and contractility (dP/dt) data (AD Instruments, Milford, MA). All hearts were perfused for 20 min when the baseline measurements were taken prior to initiating 20 min of global no-flow ischemia followed by 2 hr reperfusion. Onset of ischemic contracture was detected when the left ventricular pressure began to increase during ischemia. Recovery of LVDP, expressed as % of initial pre-ischemic LVDP was measured at 60 min of reperfusion after 20 min of ischemia.
Cardiac necrosis was evaluated as described previously (Tong et al., 2010; Tong et al., 2009), at the end of 2 hr of reperfusion. Hearts were perfused with 30 ml of 1% solution of 2,3,5-triphenyltetrazolium chloride (TTC) dissolved in Krebs-Henseleit buffer, then incubated in 1% TTC at 37°C for 10 min, and then fixed in formalin. The area of necrosis was measured by taking cross-sectional slices through the ventricles, which were then photographed using a digital camera mounted on a stereomicroscope. The resulting images were quantified by measuring the areas of stained (viable tissue) versus unstained tissue (infarct) with the use of Adobe Photoshop. Infarct size was expressed as a percentage of the total ventricular section and averaged from four images.
Cardiac Gene Expression
A portion of left ventricular tissues isolated consistently across all animals from cohort 2 was homogenized with RNA lysis buffer for RNA extraction following the manufacturer’s protocol (Quick-RNA Miniprep Kit, Zymo Research). RNA concentrations were measured using a Qubit 4.0 fluorometer with the Qubit RNA BR Assay kit (Thermo Fisher). The cDNA product was amplified in a StepOne Real-Time PCT system (Applied Biosystems) using SYBR® Green Supermix (BioRad) and validated primers (Supplemental Table) specific to each gene target of tumor necrosis factor alpha (TNFα), heme oxygenase-1 (HO1), or superoxide dismutase 2 (SOD2). Expression of hypoxanthine phosphoribosyltransferase-1 (HPRT1) gene was utilized as a standard reference gene and all data are presented as normalized fold change expression compared to FA controls in normal diet hearts using the ΔΔCt method (Livak and Schmittgen, 2001).
Cardiac miRNAs Measurement
miRNA was isolated from left ventricular tissues consistently across all animals from cohort 2. Briefly, ventricles were grinded with motor and pestle and lysed with Trizol reagent. PureLink miRNA isolation kit (Invitrogen) was used to isolate miRNA following the manufacture instruction. To quantify and characterize the integrity of the isolated miRNA, spectrophotometric evaluation was performed using Nanodrop (Thermo Scientific). All the miRNA whose A260 (absorbance at 260 nm) value was more than 0.15 after eluting off the PureLink column was used for further experiments. The ratio of the readings at 260 nm and 280 nm (A260/A280) was also measured in order to check the purity of the isolated RNA and the sample with ratio of more than 0.9 was used for experiments. For further and more accurate purity and integrity estimation of the isolated miRNA, Bioanalyzer 2100 (Agilent Technologies) was used.
Custom TaqMan advanced miRNA micro fluidic array cards were used to measure miRNA expression. Forty-five miRNA candidates related to air pollutants, diet, inflammation, and oxidative stress, endothelial and cardiovascular function were selected for the array card (Table 2). miRNA expression levels were performed on TaqMan array cards (Thermo Fisher) according to manufacturer’s recommended protocol. Briefly, 3 ng of total RNA was reverse transcribed to cDNA using the TaqMan Advanced miRNA cDNA synthesis kit (Thermo Fisher). For TaqMan array qPCR reaction, the pre-amplified product was mixed with TaqMan Universal PCR Master Mix and loaded into the array cards (Thermo Fisher) according to manufacturer’s recommended protocol. Real time PCR was performed on 7900HT Fast Real-Time PCR system (Thermo Fisher). Raw Ct values were normalized by a pre-selected reference miRNA (miRNA-16). The resulting ΔCt values were then normalized for FA/normal diet group. All reactions were performed in triplicate.
Table 2.
List of microRNAs to the custom array card.
| Target | Assay ID | Target | Assay ID |
|---|---|---|---|
| rno-miR-1 | rno480888_mir | rno-miR-133 | rno481491_mir |
| rno-miR-9 | rno481285_mir | rno-miR-135b-3p | rno478710_mir |
| rno-miR-10 | rno480895_mir | rno-miR-142-5p | rno481324_mir |
| rno-miR-17-1-3p | rno480957_mir | rno-miR-143-5p | rno480936_mir |
| rno-miR-19-3p | rno479228_mir | rno-miR-145 | rno480938_mir |
| rno-miR-21 | rno481342_mir | rno-miR-146a-5p | rno481451_mir |
| rno-miR-22-5p | rno477987_mir | rno-miR-146b-5p | rno480941_mir |
| rno-miR-23a | rno481350_mir | rno-miR-150-5p | rno480947_mir |
| rno-miR-25 | rno481352_mir | rno-miR-155-5p | rno480953_mir |
| rno-miR-26a-5p | rno481013_mir | rno-miR-181a | rno481485_mir |
| rno-miR-27b-5p | rno481016_mir | rno-miR-199a-5p | rno480984_mir |
| rno-miR-28-5p | rno481018_mir | rno-miR-208a-5p | rno478762_mir |
| rno-miR-29c-5p | rno481034_mir | rno-miR-214-5p | rno481471_mir |
| rno-miR-30a-5p | rno479448_mir | rno-miR-221-5p | rno481349_mir |
| rno-miR-92a-3p | rno481275_mir | rno-miR-222-5p | rno481006_mir |
| rno-miR-99b-5p | rno481284_mir | rno-miR-224-5p | rno481010_mir |
| rno-miR-106b-5p | rno478412_mir | rno-miR-328 | rno481059_mir |
| rno-miR-107-3p | rno478254_mir | rno-miR-350 | rno481086_mir |
| rno-miR-122-5p | rno480899_mir | rno-miR-378a-5p | rno481145_mir |
| rno-miR-125 | rno480906_mir | rno-miR-486 | rno481183_mir |
| rno-miR-126-3p | rno481318_mir | rno-miR-582-5p | rno481409_mir |
| rno-miR-128-3p | rno480912_mir | rno-miR-872-5p | rno481423_mir |
| rno-miR-132 | rno480919_mir |
Prediction and Functional Analysis of miRNA Targets
Network analysis on predicted gene (mRNA) targets of miRNAs was carried out using Ingenuity Pathway Analysis (IPA®, QIAGEN Redwood City, www.qiagen.com/ingenuity). Briefly, miRNA target gene prediction was based on “Moderate” and “High” predictions and “Experimentally Observed” associations captured within the primary IPA database, which is populated with data derived from miRecords, TarBase, TargetScan, as well as Ingenuity Expert Findings. Canonical and “tox” pathway-level analyses were carried out using this predicted mRNA gene target list. Significance of canonical pathway enrichment was determined by Fischer’s exact test (p<0.05).
Statistical Analysis
Data are expressed as means ± SEM. For statistical analysis of cardiac function, gene expression, and miRNAs data, SigmaPlot 14.0 software was used. A Two-Way ANOVA was performed using diet (ND, FO, or OO) and exposure (FA or O3) as independent factors followed by Holm-Sidak’s post-hoc test for all pairwise multiple comparison procedures. The statistical significance levels were set at p<0.05.
RESULTS
FO and OO diets had no effects on baseline heart rate, LVDP, dP/dt after FA exposure compared to the normal diet (ND). When compared to FA, baseline heart rate before ischemia in the ND and FO groups increased by O3 exposure but did not reach statistical significance; however, heart rate was increased after O3 exposure (265±8 bpm) in the OO group compared to FA exposure (232±12 bpm; p=0.05) (Figure 1A). No marked differences in baseline coronary artery flow rate were observed among the groups. Compared to FA (219.0±20.6 cm H2O), baseline LVDP was markedly lower in hearts from O3 exposure (169.9±6.3 cm H2O; p=0.03) in the ND group but not in the FO and OO groups (Figure 1B). Baseline LVDP after O3 exposure was not significantly different among groups (Figure 1B). Exposure to O3 decreased baseline left ventricular contractility. Compared to FA exposure (4982±396 cm H2O/s), baseline rate of contraction (dP/dtmax) was significantly lower after O3 exposure (4052±153 cm H2O/s; p=0.04) in the ND group but not in the FO and OO groups (Figure 2A). There were no significant differences of dP/dtmax among the groups exposed to FA. The baseline rate of relaxation (dP/dtmin) was not significantly altered after O3 exposure (−3367±184 cm H2O/s) compared to FA (−3853±619 cm H2O/s) in the ND group (Figure 2B). FO and OO diets did not significantly affect baseline dP/dtmin.
Figure 1.
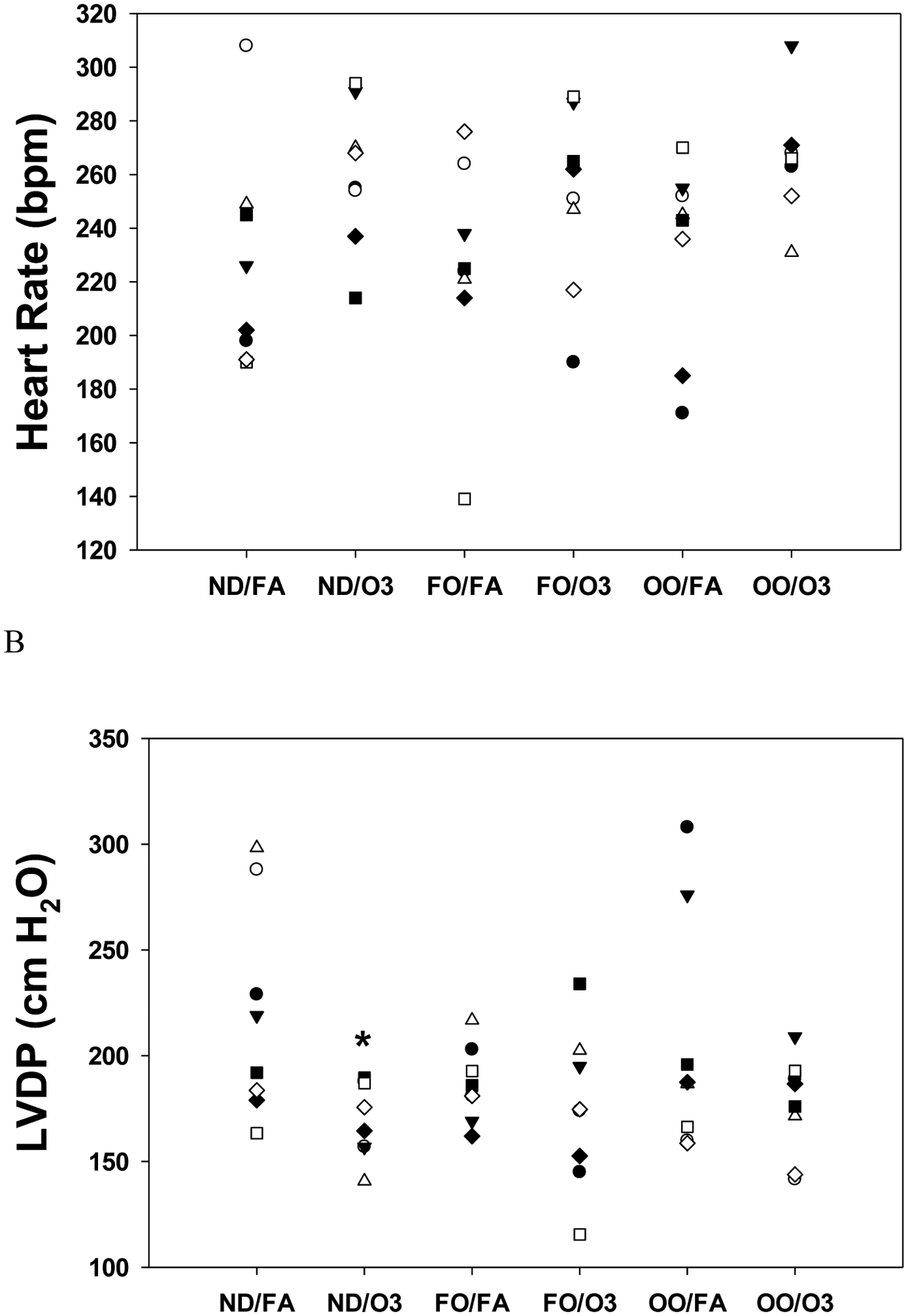
Heart rate and cardiac function in isolated perfused hearts before ischemia after rats were fed with 8 weeks of fish oil (FO), olive oil (OO), or normal diet (ND). Heart rate (A) and left ventricular developed pressure (LVDP) (B) at baseline prior to ischemia in rat hearts isolated 2–4 hr after inhalation exposure to filtered air (FA) or two days of intermittent O3 for 4 hr/day as described in METHODS. n = 8 in each group. *p < 0.05; significantly different than FA group within same diet.
Figure 2.
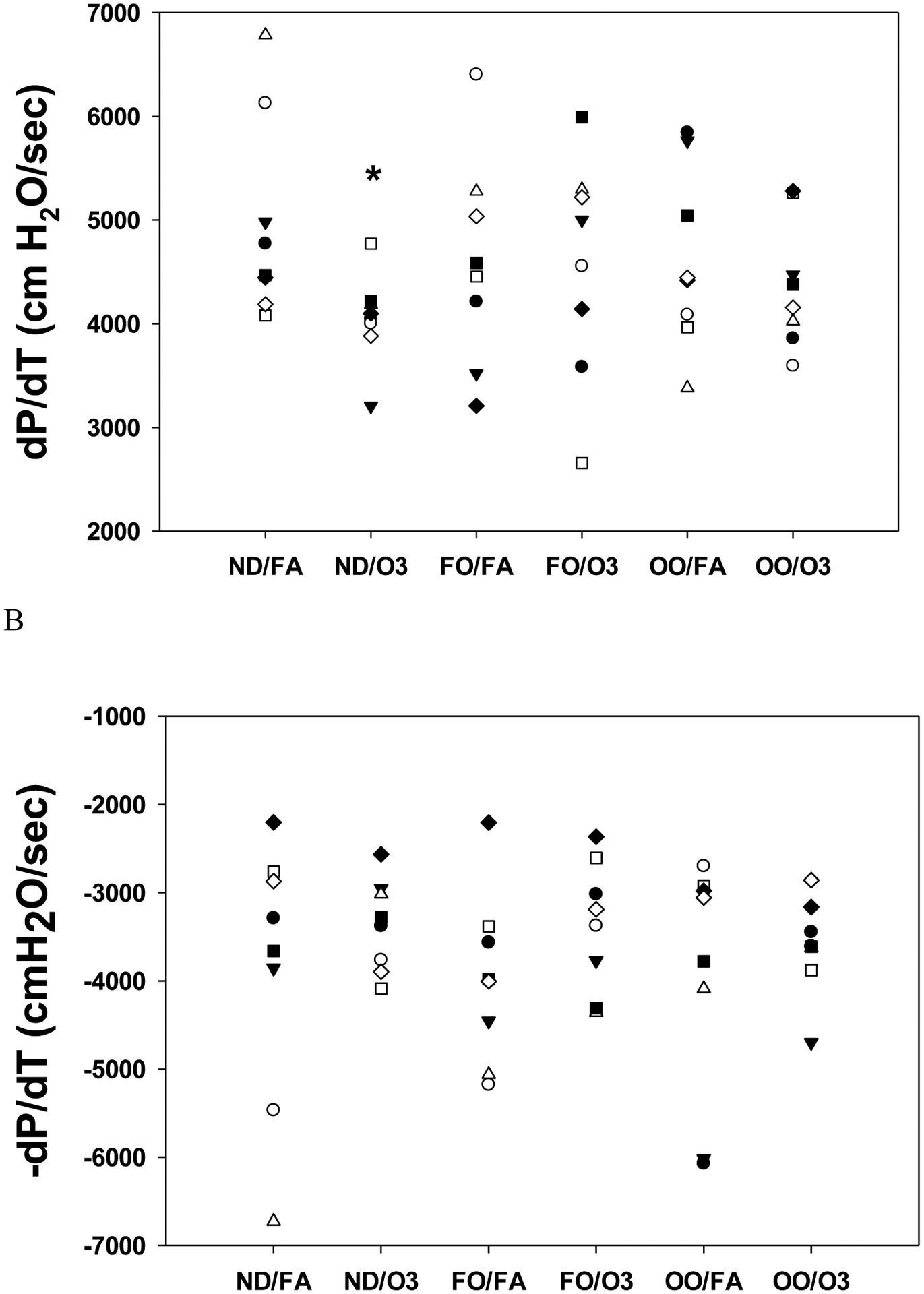
Cardiac contractility in isolated perfused hearts before ischemia after rats were fed with 8 weeks of fish oil (FO), olive oil (OO), or normal diet (ND). The cardiac contractility assessed by maximum (A) and minimum (B) dP/dt at baseline prior to ischemia in rat hearts isolated 2–4 hr after inhalation exposure to filtered air (FA) or two days of intermittent O3 for 4 hr/day as described in METHODS. n = 8 in each group. *p < 0.05; significantly different than FA group within same diet.
Compared to the ND group after FA exposure (18.1±0.6 min), time to ischemic contracture was markedly advanced in the OO group after FA exposure (15.6±0.7 min; p=0.02) during 20 min of ischemia. In addition, O3 exposure delayed the onset to ischemic contracture (17.6±0.5 min; p=0.05) compared to FA in the OO group. Post-ischemic recovery of LVDP (% LVDP) at 60 min after reperfusion appeared better after O3 exposure in the ND group but did not reach statistical significance compared to FA (Figure 3A). There was no significant difference of %LVDP in FO and OO groups compared to the ND group (Figure 3A). Infarct size measured at 2 h after reperfusion was not different among the groups (Figure 3B).
Figure 3.
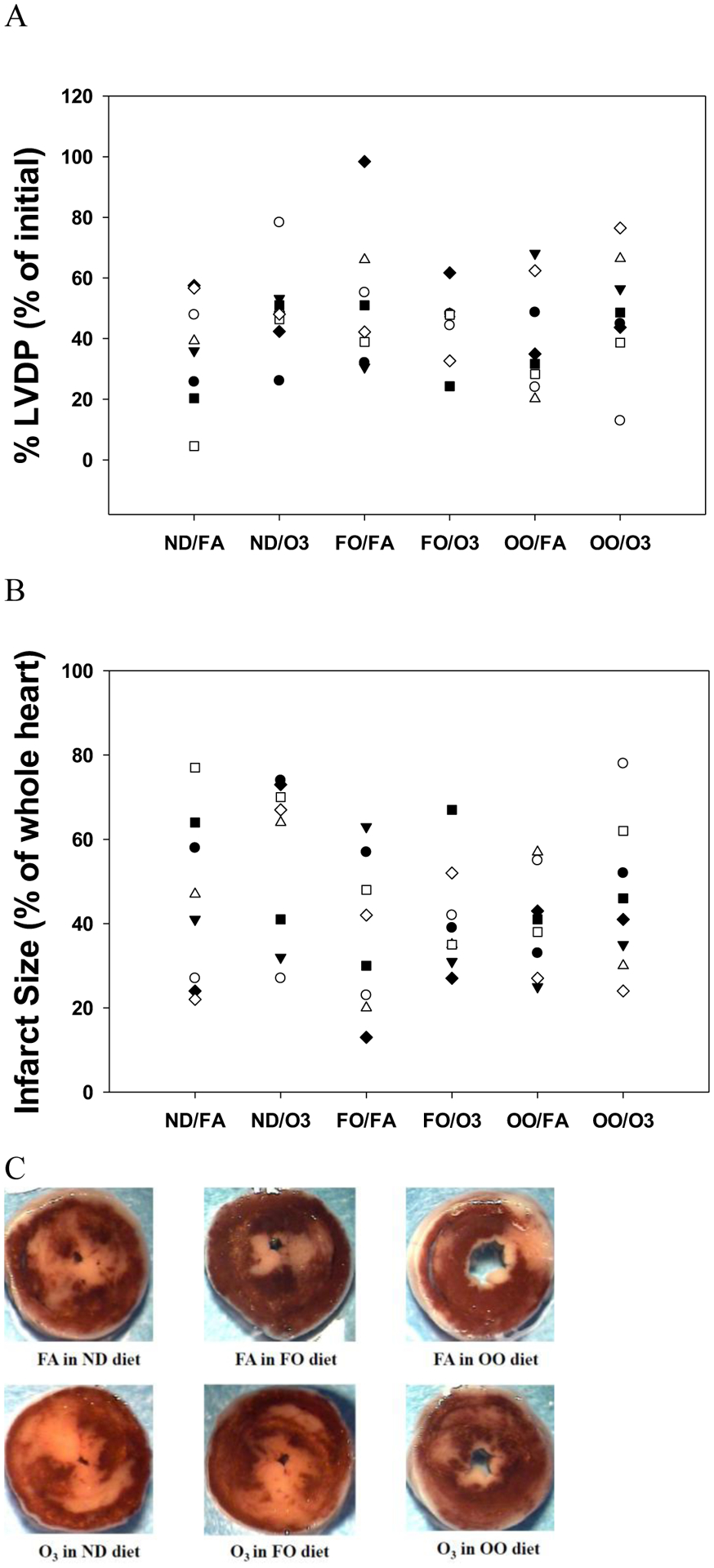
Recovery of post-ischemic cardiac function and necrosis in rat hearts after rats were fed with 8 weeks of fish oil (FO), olive oil (OO), or normal diet (ND). Recovery of left ventricular developed pressure (LVDP) (A), expressed as a percentage of the initial baseline pre-ischemic LVDP was measured after 60 min of reperfusion and infarct size (B), expressed as a percentage of the whole heart was measured after 2 hr of reperfusion in rat hearts isolated 2–4 hr after inhalation exposure to filtered air (FA) or two days of intermittent O3 for 4 hr/day. (C) representative images of rat heart sections stained with 1% solution of 2,3,5-triphenyltetrazolium chloride (TTC). Red-stained areas indicate viable tissue and white areas indicate infracted tissue. n = 8 in each group.
To investigate if acute exposure to O3 induce oxidative stress and inflammation in cardiac tissue and whether the FO or OO diet will modify the effects, mRNA expression levels of genes were measured. Compared with FA, O3 exposure did not significantly change the expression of HO1, SOD2 and TNFα genes regardless of diet groups (Figure 4).
Figure 4.
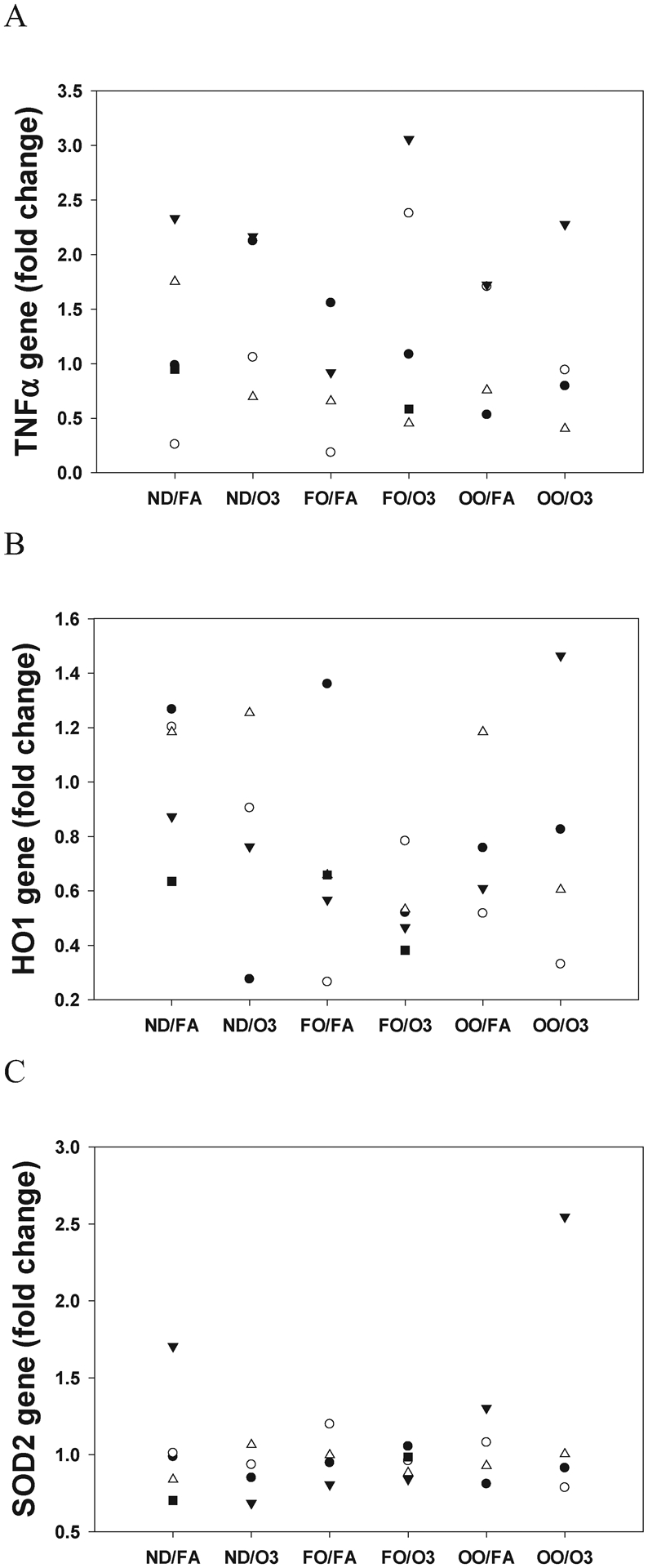
Oxidative stress and inflammatory genes expression in rat heart tissue. Hearts were collected at necropsy following a 2-day exposure to filtered air (FA) or O3 in animals fed with 8 weeks of fish oil (FO), olive oil (OO), or normal diet (ND). Cardiac RNA was extracted and measured for mRNA expression of tumor necrosis factor alpha (TNFα), heme oxygenase-1 (HO1), and superoxide dismutase 2 (SOD2) by qPCR. Fold change was calculated over FA/ND group. *P < 0.05; statistically significant difference compared with FA hearts. n=4–5 in each group.
Twenty-four of 45 miRNAs selected on the array cards were detected. Among these cardiac miRNAs, 10 miRNAs were altered by either diet supplements or O3 exposure (Table 3). miR-150-5p was slightly up-regulated by O3 exposure in the ND group (p=0.099 vs. FA), where both FO (p<0.001 vs. ND) and OO (p=0.009 vs. ND) attenuated the O3 exposure induced up-regulation of miR-150-5p. There was an interaction between exposure and diet (p=0.05; F=3.42) for miR-150-5p. miR-208a-5p expression was increased by O3 exposure in the ND (p=0.027 vs. FA) but was reduced by FO diet (p=0.01 vs. ND). There was a significant interaction between exposure and diet (p=0.043; F=3.656) for miR-208a-5p. miR-107-3p expression was decreased by O3 exposure in the ND (p=0.022 vs. FA). Both FO (p=0.053 vs. ND) and OO (p=0.06 vs. ND) slightly reduced miR-107-3p expression after FA exposure. There was a significant interaction between exposure and diet (p=0.016; F=5.062). miR-145 expression was also down-regulated by O3 exposure (p=0.036 vs. FA) and there was a significant interaction between exposure and diet (p=0.03; F=4.127). However, level of miR-29c-5p was up-regulated by O3 in the OO group (p=0.038 vs. FA). There was a significant interaction between exposure and diet for miR-29c-5p (p=0.041; F=3.72).
Table 3.
microRNA expression to exposure and dietary supplementation.
| miRNA | Normal Diet | Fish Oil Diet | Olive Oil Diet | |||
|---|---|---|---|---|---|---|
| FA | O3 | FA | O3 | FA | O3 | |
| miR-107-3p | 1.0±0.194 | 0.411±0.137* | 0.425±0.112 | 0.196±0.112 | 0.468±0.123 | 0.789±0.137 |
| miR-126-3p | 1.0±0.328 | 0.903±0.232 | 1.409±0.189 | 1.468±0.189 | 0.911±0.207 | 1.193±0.232 |
| miR-145 | 1.0±0.209 | 0.382±0.181* | 0.646±0.148 | 0.455±0.148 | 0.396±0.162 | 0.815±0.181 |
| miR-150-5p | 1.0±0.168 | 1.41±0.168 | 0.859±0.15 | 0.48±0.137# | 0.563±0.15 | 0.657±0.68# |
| miR-181a | 1.0±0.258 | 0.73±0.182 | 0.959±0.149 | 0.52±0.149 | 0.381±0.163 | 0.618±0.182 |
| miR-208a-5p | 1.0±0.537 | 2.682±0.465* | 1.276±0.38 | 0.71±0.38# | 0.902±0.416 | 1.888±0.465 |
| miR-21 | 1.0±0.38 | 0.829±0.269 | 1.497±0.22 | 1.815±0.22# | 0.859±0.241 | 1.75±0.269*# |
| miR-29c-5p | 1.0±0.353 | 0.759±0.25 | 0.579±0.204 | 0.285±0.204 | 0.634±0.223 | 1.377±0.25* |
| miR-486 | 1.0±0.273 | 0.806±0.193 | 0.999±0.157 | 0.797±0.157 | 0.558±0.172 | 1.236±0.193* |
| miR-872-5p | 1.0±0.236 | 0.5±0.167 | 0.796±0.136 | 0.804±0.136 | 0.524±0.149 | 0.771±0.167 |
Data are means ± SE. FA, filter air;
p<0.05, significantly different than FA group within same diet;
p<0.05, significantly different than normal diet group for the same exposure; n=5–6 in each group.
Expression of miR-21 was slightly decreased after O3 exposure in the ND hearts but significantly up-regulated by O3 (p=0.022 vs. FA) in the OO hearts. miR-21 was also significantly up-regulated by OO diet (p=0.049; OO vs. ND) and FO diet (p=0.029; FO vs. ND) after O3 exposure but not after FA exposure. There was a significant interaction between exposure and diet (p=0.043; F=3.66) for miR-21. miR-486 was up-regulated by O3 exposure (p=0.016 vs. FA) in the OO group but not in the ND and FO groups. There was a significant interaction between exposure and diet (p=0.039; F=3.805).
IPA provides an independent method of assessing the role of predicted miRNA gene targets. In this regard, functional gene network analysis of the predicted gene targets of the 4 miRNAs (miR-107-3p, miR-145, miR-150-5p, and miR-208a-5p) altered by O3 exposure in the ND group was performed. These gene target predictions were based on moderate to stringent criteria (using TargetScan, TarBase, miRecords, IPA Expert Findings databases curated in IPA), resulting in a gene target list of 2497 unique genes (Supplemental File 1). Canonical and toxicological analyses of heart-relevant pathways revealed “Cardiovascular Disease” and “Cardiovascular System Development and Function” as the top enriched disease-related pathways. Canonical pathway analysis indicated involvement of Fcγ receptor and Rac signaling, sphingomyelin metabolism, and cardiogenesis pathways. Cardiotoxicity pathway enrichment included cardiac enlargement, arrythmia, arteriopathy, and fibrosis.
O3 exposure altered the expression of a few miRNAs in FO group (miR-150-5p, miR-208a-5p, and miR-21) that was significantly different from the ND group. IPA indicated 975 matched gene targets for these miRNAs (Supplemental File 2). While “Cardiovascular Disease” was enriched using this target gene list in the top disease-related pathways, more general indicators of cellular dysfunction were enriched, including “DNA Replication, Recombination, and Repair”, “Molecular Transport”, and “Cellular Compromise”. The canonical pathways associated targets were p38 MAPK signaling, citrulline biosynthesis, and cell cycle. Top cardiotoxicity pathways included cardiac dysfunction, cardiac damage, cardiac degeneration, and increased levels of ALT and LDH. Top toxicological function analysis was characterized by increases in depolarization of mitochondria and mitochondrial membrane and decreases in transmembrane potential of mitochondria and mitochondrial membrane.
Finally, we examined the canonical pathway enrichment of the predicted target genes of O3-responsive miRNAs in the OO group. Similarly, since this group only included 3 miRNAs (miR-21, miR-29c-5p, and miR-486) in the OO group altered by O3 exposure, we used a more relaxed gene target prediction, resulting in 650 potential target genes (Supplemental File 3). Again, disease pathway enrichment indicated “Cardiovascular System Development and Function” as a top 3 hit, and the top cardiotoxicity lists included cardiac enlargement, cardiac necrosis and death, cardiac damage, cardiac stenosis, and cardiac regeneration. The top toxicity targets were increases damage of mitochondria, decreases depolarization of mitochondria and mitochondrial membrane, and decreases transmembrane potential of mitochondria and mitochondrial membrane. The top canonical pathways included Tec kinase signaling TRK signaling and ILK signaling.
DISCUSSION
Emerging evidence suggests that O3 contributes to cardiovascular disease and death (Di et al., 2017; Environmental Protection Agency, 2020). In the present study, acute two-day intermittent O3 exposure was associated with decreased cardiac function and altered miRNAs expression related to cardiac and endothelial function in cardiac tissues. In addition, supplementation with fish oil or olive oil modified the O3 exposure-induced cardiovascular responses.
A new study reported that a decade of ambient O3 exposure was associated with arterial injury by increasing rate of carotid wall intima-media thickness progression and risk of new plaque formation in a healthy elderly cohort (Wang et al., 2019). Intima-media thickness of artery is noninvasive marker for subclinical vascular disease and associated with cardiovascular diseases and events (Cheng et al., 2002; Stein et al., 2008). Risk factors for intima-media thickness include endothelial dysfunction, increased endothelial cell adhesiveness and permeability, increases in procoagulant, vasoconstrictive, and inflammatory molecules, increases in cytokines and chemokines, increased oxidative stress, and proliferation and migration of smooth muscle cells (Stein et al., 2008). An animal study showed that inhaled O3 increased arterial dysfunction, oxidative stress, mitochondrial DNA damage, and atherogenesis (Chuang et al., 2009). We have also shown that acute O3 exposure induced vasoconstriction of aorta from a separate cohort of animals (Snow et al., 2018), supporting O3-induced endothelial cell injury and vascular effect. Because miRNAs are upstream regulators of gene expression and are involved in various physiological and pathological processes, and many miRNAs have functional roles in the vasculature involved in the regulation of vessel growth, remodeling, and inflammation control (Jae and Dimmeler, 2020), we examined the expression of miRNAs implicated in these processes. We showed that expression of endothelial miRNAs, miR-150-5p, miR-107-3p, miR-145, miR-181a, and miR-872-5p was altered by O3 exposure. miR-150-5p regulates endothelial cell migration and its expression is up-regulated by oxidative stress (Zhang et al., 2010). miR-181a is known to play a role in endothelial cell fate decision (Kazenwadel et al., 2010). Therefore, alteration of these miRNAs by O3 exposure suggests that O3-induced vascular effects may be mediated through these endothelial miRNAs. Furthermore, FO completely abolished the O3-induced vasoconstriction (Snow et al., 2018) and FO and OO significantly attenuated the up-regulation of miR-150-5p by O3, suggesting that endothelial miRNAs may mediate the protection of dietary supplements on O3-induced endothelial dysfunction.
We have previously demonstrated that acute O3 exposure (4 hrs of 0.245 ppm O3) decreased cardiac contractility and heart rate in mice (McIntosh-Kastrinsky et al., 2013). In agreement, the present study showed that O3 exposure (4 hrs of 0.8 ppm of O3 for 2 days) resulted in more cardiac effects of decreased LVDP and dP/dt in the ND group, suggesting that acute O3 exposure suppressed cardiac function and cardiac contractility. These findings are supported by more human and animal studies. An epidemiological study reported that the risk of out-of-hospital cardiac arrest was increased following short-term exposure to ambient O3 by analyzing record of the Swedish Register for Cardiopulmonary Resuscitation and they further discovered that the elevated cardiac arrest risk was not associated with previous hospitalizations for cardiovascular diseases of acute myocardial infarction, heart failure, arrhythmias, diabetes, hypertension, and stroke (Raza et al., 2019). In animal studies, Tankersley et al. (Tankersley et al., 2013) showed that exposure to 0.5 ppm of O3 for 3 consecutive days reduced LVDP, stroke volume and cardiac output in mice. Perepu et al. (Perepu et al., 2012) demonstrated that long-term O3 exposure (8 h/day of 0.8 ppm for 28 or 56 days) altered cardiac function by significantly reduction of LVDP, dP/dtmax, dP/dtmin, and elevation of LVEDP, as well as increased inflammatory markers TNF-α and lipid peroxidation in rats. Their findings suggested that myocardial dysfunction subsequent to long-term O3 exposure in rats may be associated with a decrease in antioxidant reserve and an increase in production of inflammatory mediators (Perepu et al., 2012). However, the present study with acute O3 exposure did not result in significant changes of expression of selected inflammatory and oxidative stress genes in cardiac tissues.
Inflammation and oxidative stress pathways involved in cardiovascular effects of air pollution exposure are supported by both human and animal studies (Brook et al., 2010; Miller, 2020). To reduce the health risks from air pollution exposure at individual levels, efforts to intervene the adverse health effects by reducing inflammation and oxidative stress could be achieved through dietary and pharmacological measures (Tong, 2016). Omega-3 fatty acids (Lin et al., 2019; Tong et al., 2012) and olive oil (Lim et al., 2019; Tong et al., 2015) have been shown to offer protection against particulate matter-induced cardiovascular effects in humans. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), two marine source of omega-3 fatty acids, offer cardioprotection through anti-inflammation and antioxidant properties (Mason et al., 2020). A highly purified EPA significantly reduced the risk of cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization in at-risk patients (Bhatt et al., 2019). Two most recent prospective 24-year cohorts report that higher OO intake (>7g/d) was associated with a lower risk of coronary heart disease and total cardiovascular disease in US adults that was associated with lower levels of circulating inflammatory biomarkers (Guasch-Ferre et al., 2020). The present study examined whether supplementation with FO and OO that have anti-inflammatory and antioxidant properties could mitigate the cardiovascular effects from O3 exposure in animals. The findings on LVDP and dP/dt suggest that the adverse cardiac effects were attenuated by FO and OO, which is consistent with finding that FO protects the O3-induced vascular effect (Snow et al., 2018). In addition to anti-inflammation and antioxidant effects, polyunsaturated fatty acids including FO act on voltage-gated ion channels, such as voltage-gated sodium, potassium, calcium, and proton channels, as well as calcium-activated potassium and transient receptor potential channels resulting in physiological and pharmacological effects (Demaison et al., 2000; Elinder and Liin, 2017; Leifert et al., 2001; Leifert et al., 2000). Therefore, a trend of lower baseline pre-ischemic LVDP and contractility in the FO hearts could be due to modulation of these voltage-gated ion channels. Future research warrants investigation on the modulation of cardiac ion channels by air pollutants.
Emerging evidence suggest that miRNAs could be potential indicators of air pollution exposure (Chen et al., 2020; Vrijens et al., 2015). Therefore, we further examined expression of cardiac miRNAs related to cardiac function regulation and found that expression of miR-21, miR-145, miR-208a-5p, miR-29c-5p, miR-486 was altered by O3 exposure. Li et al. (Li et al., 2012) reported that miR-145 protected cardiomyocytes against oxidative stress-induced apoptosis through targeting the mitochondria pathway. Consistently, we observed that miR-145 expression was significantly downregulated by O3 exposure in the ND hearts and slightly up-regulated by OO. Qiao et al. (Qiao et al., 2019) demonstrated that miR-21 inhibits cardiomyocyte apoptosis by targeting programmed cell death 4 (PDCD4), promotes angiogenesis by activating PTEN/Akt signaling. miR-21 also prevents excessive inflammation and cardiac dysfunction after myocardial infarction in an animal model (Yang et al., 2018). Furthermore, Chen et al. (Chen et al., 2018) showed that expression of miR-21 which targeted IL-1 and ICAM-1 was negatively associated with PM2.5 levels in healthy human subjects. Furthermore, it is proposed that the anti-inflammatory property of essential fatty acids is through the specialized proresolving lipid mediators including resolvin, which synthesized from EPA, DHA, and marine oils, are important class of mediators for acute inflammation resolution (Serhan and Levy, 2018). A study examining the role of miRNAs in resolution of acute inflammation demonstrated that resolvin D1 up-regulated miR-21 and down-regulated miR-208a-5p in a disease model of peritonitis (Recchiuti et al., 2011). miR-208a-5p is a regulator of cardiac hypertrophy and conduction (Callis et al., 2009). In the present study, we showed that FO significantly decreased the O3 up-regulated expression of miR-208a and both FO and OO diet significantly upregulated miR-21 expression after O3 exposure.
Autonomic nervous system is one of the pathways mediates the cardiovascular effects of air pollutant exposure. Increases in heart rates indicates an altered autonomic control of the heart in association with air pollution (Peters et al., 1999). Ambient O3 was associated with increases in heart rates (lag01) in healthy young volunteers (Song et al., 2020) and in coronary disease patients (Zhang et al., 2018). However, in the ex vivo model where autonomic nervous system control on the heart is not involved, the trend of increases in heart rates after O3 exposure might be due to cardiac compensation to reduced LVDP and contractility induced by O3 exposure in the present study.
Interestingly, we observed a significant early onset of ischemic contracture in the OO hearts after FA exposure and O3 exposure significantly delayed the onset to ischemic contracture in the OO hearts. Ischemic contracture occurs in the heart after a period of global ischemia and contracture is initiated when the cellular ATP content decreases (Steenbergen et al., 1990). The time of onset of contracture can be delayed by reduced myocardial energy demand, increase myocardial energy supply or reduce cellular calcium influx. The time of onset of contracture may be advanced by reduced the energy supply (Hearse et al., 1977). The onset of ischemic contracture is also regulated by glucose flux rate (Owen et al., 1990) and glycogen content (Cross et al., 1996). We hypothesized that diets and O3 may influence energy metabolism in myocardium resulting in altered ischemic contracture. To support this hypothesis, we also observed that FA-exposed animals receiving OO enriched diets tended to develop glucose intolerance which was exacerbated by O3 (Snow et al. 2020 TAAP, manuscript being revised). In addition, acute O3 exposure increased extracellular ATP in human bronchial epithelial cells, protecting against O3 toxicity (Ahmad et al., 2005).
In conclusion, we show that exposure to intermittent two days of O3 resulted in lower cardiac function and contractility which were attenuated by supplementation with FO and OO. Expression of miRNAs related to endothelial and cardiac function and inflammation was altered by O3 exposure that was modulated by either FO and/or OO diets. These findings suggest that dietary supplementation may modulate the O3-induced cardiovascular effects through miRNAs.
Supplementary Material
Acknowledgements:
The authors thank Laura Taylor for the technical support. We acknowledge the help of Dr. Mark Higuchi and Mr. Allen Ledbetter (retired) of the US EPA for ozone inhalation exposures.
Funding: This work was supported by the US EPA Intramural Research Program.
List of abbreviations:
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FA
filtered air
- FO
fish oil
- LVDP
left-ventricular developed pressure
- ND
normal diet
- O3
ozone
- OO
olive oil
- PM
particulate matter
Footnotes
Publisher's Disclaimer: Disclaimer: The research described in this article has been reviewed by the Center for Public Health and Environmental Assessment, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does the mention of trade names of commercial products constitute endorsement or recommendation for use.
Availability of data and materials: The datasets used during the current study are available from the corresponding author on reasonable request.
Competing interests: The authors declare that they have no competing interests.
References
- Ahmad S, Ahmad A, McConville G, Schneider BK, Allen CB, Manzer R, Mason RJ and White CW (2005) Lung epithelial cells release ATP during ozone exposure: signaling for cell survival. Free Radic Biol Med 39:213–226. [DOI] [PubMed] [Google Scholar]
- Arjomandi M, Wong H, Donde A, Frelinger J, Dalton S, Ching W, Power K and Balmes JR (2015) Exposure to medium and high ambient levels of ozone causes adverse systemic inflammatory and cardiac autonomic effects. Am J Physiol Heart Circ Physiol 308:H1499–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, McDermott A, Zeger SL, Samet JM and Dominici F (2004) Ozone and short-term mortality in 95 US urban communities, 1987–2000. JAMA 292:2372–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr., Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM and Investigators R-I (2019) Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med 380:11–22. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr., Whitsel L, Kaufman JD, American Heart Association Council on E, Prevention CotKiCD, Council on Nutrition PA and Metabolism (2010) Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121:2331–2378. [DOI] [PubMed] [Google Scholar]
- Callis TE, Pandya K, Seok HY, Tang RH, Tatsuguchi M, Huang ZP, Chen JF, Deng Z, Gunn B, Shumate J, Willis MS, Selzman CH and Wang DZ (2009) MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest 119:2772–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Xu Y, Rappold A, Diaz-Sanchez D and Tong H (2020) Effects of ambient ozone exposure on circulating extracellular vehicle microRNA levels in coronary artery disease patients. J Toxicol Environ Health A 83:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Li H, Cai J, Wang C, Lin Z, Liu C, Niu Y, Zhao Z, Li W and Kan H (2018) Fine Particulate Air Pollution and the Expression of microRNAs and Circulating Cytokines Relevant to Inflammation, Coagulation, and Vasoconstriction. Environ Health Perspect 126:017007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KS, Mikhailidis DP, Hamilton G and Seifalian AM (2002) A review of the carotid and femoral intima-media thickness as an indicator of the presence of peripheral vascular disease and cardiovascular risk factors. Cardiovasc Res 54:528–538. [DOI] [PubMed] [Google Scholar]
- Chuang GC, Yang Z, Westbrook DG, Pompilius M, Ballinger CA, White CR, Krzywanski DM, Postlethwait EM and Ballinger SW (2009) Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. Am J Physiol Lung Cell Mol Physiol 297:L209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross HR, Opie LH, Radda GK and Clarke K (1996) Is a high glycogen content beneficial or detrimental to the ischemic rat heart? A controversy resolved. Circ Res 78:482–491. [DOI] [PubMed] [Google Scholar]
- Demaison L, Blet J, Sergiel JP, Gregoire S and Argaud D (2000) Effect of dietary polyunsaturated fatty acids on contractile function of hearts isolated from sedentary and trained rats. Reprod Nutr Dev 40:113–125. [DOI] [PubMed] [Google Scholar]
- Devlin RB, Duncan KE, Jardim M, Schmitt MT, Rappold AG and Diaz-Sanchez D (2012) Controlled exposure of healthy young volunteers to ozone causes cardiovascular effects. Circulation 126:104–111. [DOI] [PubMed] [Google Scholar]
- Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, Dominici F and Schwartz JD (2017) Air Pollution and Mortality in the Medicare Population. N Engl J Med 376:2513–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES and Zhang C (2009) MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem 284:29514–29525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinder F and Liin SI (2017) Actions and Mechanisms of Polyunsaturated Fatty Acids on Voltage-Gated Ion Channels. Front Physiol 8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency U (2020) Integrated Science Assessment (ISA) of Ozone and Related Photochemical Oxidants (Final Report), pp EPA/600/R-620/012, U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- Guasch-Ferre M, Liu G, Li Y, Sampson L, Manson JE, Salas-Salvado J, Martinez-Gonzalez MA, Stampfer MJ, Willett WC, Sun Q and Hu FB (2020) Olive Oil Consumption and Cardiovascular Risk in U.S. Adults. J Am Coll Cardiol 75:1729–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch GE, McKee J, Brown J, McDonnell W, Seal E, Soukup J, Slade R, Crissman K and Devlin R (2013) Biomarkers of Dose and Effect of Inhaled Ozone in Resting versus Exercising Human Subjects: Comparison with Resting Rats. Biomark Insights 8:53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearse DJ, Garlick PB and Humphrey SM (1977) Ischemic contracture of the myocardium: mechanisms and prevention. Am J Cardiol 39:986–993. [DOI] [PubMed] [Google Scholar]
- Jae N and Dimmeler S (2020) Noncoding RNAs in Vascular Diseases. Circ Res 126:1127–1145. [DOI] [PubMed] [Google Scholar]
- Karakas M, Schulte C, Appelbaum S, Ojeda F, Lackner KJ, Munzel T, Schnabel RB, Blankenberg S and Zeller T (2017) Circulating microRNAs strongly predict cardiovascular death in patients with coronary artery disease-results from the large AtheroGene study. Eur Heart J 38:516–523. [DOI] [PubMed] [Google Scholar]
- Kazenwadel J, Michael MZ and Harvey NL (2010) Prox1 expression is negatively regulated by miR-181 in endothelial cells. Blood 116:2395–2401. [DOI] [PubMed] [Google Scholar]
- Kim CS, Alexis NE, Rappold AG, Kehrl H, Hazucha MJ, Lay JC, Schmitt MT, Case M, Devlin RB, Peden DB and Diaz-Sanchez D (2011) Lung function and inflammatory responses in healthy young adults exposed to 0.06 ppm ozone for 6.6 hours. Am J Respir Crit Care Med 183:1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latronico MV, Catalucci D and Condorelli G (2007) Emerging role of microRNAs in cardiovascular biology. Circ Res 101:1225–1236. [DOI] [PubMed] [Google Scholar]
- Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S and Kim VN (2003) The nuclear RNase III Drosha initiates microRNA processing. Nature 425:415–419. [DOI] [PubMed] [Google Scholar]
- Leifert WR, Dorian CL, Jahangiri A and McMurchie EJ (2001) Dietary fish oil prevents asynchronous contractility and alters Ca(2+) handling in adult rat cardiomyocytes. J Nutr Biochem 12:365–376. [DOI] [PubMed] [Google Scholar]
- Leifert WR, Jahangiri A, Saint DA and McMurchie EJ (2000) Effects of dietary n-3 fatty acids on contractility, Na+ and K+ currents in a rat cardiomyocyte model of arrhythmia. J Nutr Biochem 11:382–392. [DOI] [PubMed] [Google Scholar]
- Li R, Yan G, Li Q, Sun H, Hu Y, Sun J and Xu B (2012) MicroRNA-145 protects cardiomyocytes against hydrogen peroxide (H(2)O(2))-induced apoptosis through targeting the mitochondria apoptotic pathway. PLoS One 7:e44907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CC, Hayes RB, Ahn J, Shao Y, Silverman DT, Jones RR and Thurston GD (2019) Mediterranean Diet and the Association Between Air Pollution and Cardiovascular Disease Mortality Risk. Circulation 139:1766–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Chen R, Jiang Y, Xia Y, Niu Y, Wang C, Liu C, Chen C, Ge Y, Wang W, Yin G, Cai J, Clement V, Xu X, Chen B, Chen H and Kan H (2019) Cardiovascular Benefits of Fish-Oil Supplementation Against Fine Particulate Air Pollution in China. J Am Coll Cardiol 73:2076–2085. [DOI] [PubMed] [Google Scholar]
- Livak KJ and Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Mason RP, Libby P and Bhatt DL (2020) Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arterioscler Thromb Vasc Biol 40:1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh-Kastrinsky R, Diaz-Sanchez D, Sexton KG, Jania CM, Zavala J, Tilley SL, Jaspers I, Gilmour MI, Devlin RB, Cascio WE and Tong H (2013) Photochemically altered air pollution mixtures and contractile parameters in isolated murine hearts before and after ischemia. Environ Health Perspect 121:1344–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, Snow SJ, Henriquez A, Schladweiler MC, Ledbetter AD, Richards JE, Andrews DL and Kodavanti UP (2016) Systemic metabolic derangement, pulmonary effects, and insulin insufficiency following subchronic ozone exposure in rats. Toxicol Appl Pharmacol 306:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR (2020) Oxidative stress and the cardiovascular effects of air pollution. Free Radic Biol Med 151:69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen P, Dennis S and Opie LH (1990) Glucose flux rate regulates onset of ischemic contracture in globally underperfused rat hearts. Circ Res 66:344–354. [DOI] [PubMed] [Google Scholar]
- Pereira BL, Arruda FC, Reis PP, Felix TF, Santos PP, Rafacho BP, Goncalves AF, Claro RT, Azevedo PS, Polegato BF, Okoshi K, Fernandes AA, Paiva SA, Zornoff LA and Minicucci MF (2015) Tomato (Lycopersicon esculentum) Supplementation Induces Changes in Cardiac miRNA Expression, Reduces Oxidative Stress and Left Ventricular Mass, and Improves Diastolic Function. Nutrients 7:9640–9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perepu RS, Dostal DE, Garcia C, Kennedy RH and Sethi R (2012) Cardiac dysfunction subsequent to chronic ozone exposure in rats. Mol Cell Biochem 360:339–345. [DOI] [PubMed] [Google Scholar]
- Peters A, Perz S, Doring A, Stieber J, Koenig W and Wichmann HE (1999) Increases in heart rate during an air pollution episode. Am J Epidemiol 150:1094–1098. [DOI] [PubMed] [Google Scholar]
- Qiao L, Hu S, Liu S, Zhang H, Ma H, Huang K, Li Z, Su T, Vandergriff A, Tang J, Allen T, Dinh PU, Cores J, Yin Q, Li Y and Cheng K (2019) microRNA-21–5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J Clin Invest 129:2237–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A, Dahlquist M, Jonsson M, Hollenberg J, Svensson L, Lind T and Ljungman PLS (2019) Ozone and cardiac arrest: The role of previous hospitalizations. Environ Pollut 245:1–8. [DOI] [PubMed] [Google Scholar]
- Recchiuti A, Krishnamoorthy S, Fredman G, Chiang N and Serhan CN (2011) MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J 25:544–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu I, Tellez-Rojo MM, Lazo M, Manzano-Patino A, Cortez-Lugo M, Julien P, Belanger MC, Hernandez-Avila M and Holguin F (2005) Omega-3 fatty acid prevents heart rate variability reductions associated with particulate matter. Am J Respir Crit Care Med 172:1534–1540. [DOI] [PubMed] [Google Scholar]
- Schelegle ES, Morales CA, Walby WF, Marion S and Allen RP (2009) 6.6-hour inhalation of ozone concentrations from 60 to 87 parts per billion in healthy humans. Am J Respir Crit Care Med 180:265–272. [DOI] [PubMed] [Google Scholar]
- Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H, Hristov M, Wang S, Kiessling F, Olson EN and Weber C (2014) MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med 20:368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN and Levy BD (2018) Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest 128:2657–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow SJ, Cheng WY, Henriquez A, Hodge M, Bass V, Nelson GM, Carswell G, Richards JE, Schladweiler MC, Ledbetter AD, Chorley B, Gowdy KM, Tong H and Kodavanti UP (2018) Ozone-Induced Vascular Contractility and Pulmonary Injury Are Differentially Impacted by Diets Enriched With Coconut Oil, Fish Oil, and Olive Oil. Toxicol Sci 163:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Zhu J, Tian G, Li H, Li H, An Z, Jiang J, Fan W, Wang G, Zhang Y and Wu W (2020) Short time exposure to ambient ozone and associated cardiovascular effects: A panel study of healthy young adults. Environ Int 137:105579. [DOI] [PubMed] [Google Scholar]
- Steenbergen C, Murphy E, Watts JA and London RE (1990) Correlation between cytosolic free calcium, contracture, ATP, and irreversible ischemic injury in perfused rat heart. Circ Res 66:135–146. [DOI] [PubMed] [Google Scholar]
- Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS and American Society of Echocardiography Carotid Intima-Media Thickness Task F (2008) Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 21:93–111; quiz 189–190. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Georgakopoulos D, Tang WY, Abston E, Bierman A and Sborz N (2013) Effects of ozone and particulate matter on cardiac mechanics: role of the atrial natriuretic peptide gene. Toxicol Sci 131:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H (2016) Dietary and pharmacological intervention to mitigate the cardiopulmonary effects of air pollution toxicity. Biochim Biophys Acta 1860:2891–2898. [DOI] [PubMed] [Google Scholar]
- Tong H, Cheng WY, Samet JM, Gilmour MI and Devlin RB (2010) Differential cardiopulmonary effects of size-fractionated ambient particulate matter in mice. Cardiovascular toxicology 10:259–267. [DOI] [PubMed] [Google Scholar]
- Tong H, McGee JK, Saxena RK, Kodavanti UP, Devlin RB and Gilmour MI (2009) Influence of acid functionalization on the cardiopulmonary toxicity of carbon nanotubes and carbon black particles in mice. Toxicology and applied pharmacology 239:224–232. [DOI] [PubMed] [Google Scholar]
- Tong H, Rappold AG, Caughey M, Hinderliter AL, Bassett M, Montilla T, Case MW, Berntsen J, Bromberg PA, Cascio WE, Diaz-Sanchez D, Devlin RB and Samet JM (2015) Dietary Supplementation with Olive Oil or Fish Oil and Vascular Effects of Concentrated Ambient Particulate Matter Exposure in Human Volunteers. Environ Health Perspect 123:1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Rappold AG, Diaz-Sanchez D, Steck SE, Berntsen J, Cascio WE, Devlin RB and Samet JM (2012) Omega-3 fatty acid supplementation appears to attenuate particulate air pollution-induced cardiac effects and lipid changes in healthy middle-aged adults. Environ Health Perspect 120:952–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbich C, Kuehbacher A and Dimmeler S (2008) Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res 79:581–588. [DOI] [PubMed] [Google Scholar]
- Vrijens K, Bollati V and Nawrot TS (2015) MicroRNAs as potential signatures of environmental exposure or effect: a systematic review. Environ Health Perspect 123:399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JX, Zhang XJ, Li Q, Wang K, Wang Y, Jiao JQ, Feng C, Teng S, Zhou LY, Gong Y, Zhou ZX, Liu J, Wang JL and Li PF (2015) MicroRNA-103/107 Regulate Programmed Necrosis and Myocardial Ischemia/Reperfusion Injury Through Targeting FADD. Circ Res 117:352–363. [DOI] [PubMed] [Google Scholar]
- Wang M, Sampson PD, Sheppard LE, Stein JH, Vedal S and Kaufman JD (2019) Long-Term Exposure to Ambient Ozone and Progression of Subclinical Arterial Disease: The Multi-Ethnic Study of Atherosclerosis and Air Pollution. Environ Health Perspect 127:57001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang B, Zhou Q, Wang Y, Liu X, Liu Z and Zhan Z (2018) MicroRNA-21 prevents excessive inflammation and cardiac dysfunction after myocardial infarction through targeting KBTBD7. Cell Death Dis 9:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampetaki A, Willeit P, Tilling L, Drozdov I, Prokopi M, Renard JM, Mayr A, Weger S, Schett G, Shah A, Boulanger CM, Willeit J, Chowienczyk PJ, Kiechl S and Mayr M (2012) Prospective study on circulating MicroRNAs and risk of myocardial infarction. J Am Coll Cardiol 60:290–299. [DOI] [PubMed] [Google Scholar]
- Zhang S, Breitner S, Cascio WE, Devlin RB, Neas LM, Diaz-Sanchez D, Kraus WE, Schwartz J, Hauser ER, Peters A and Schneider A (2018) Short-term effects of fine particulate matter and ozone on the cardiac conduction system in patients undergoing cardiac catheterization. Part Fibre Toxicol 15:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Liu DQ, Chen X, Li J, Li LM, Bian Z, Sun F, Lu JW, Yin YA, Cai X, Sun Q, Wang KH, Ba Y, Wang QA, Wang DJ, Yang JW, Liu PS, Xu T, Yan QA, Zhang JF, Zen K and Zhang CY (2010) Secreted Monocytic miR-150 Enhances Targeted Endothelial Cell Migration. Mol Cell 39:133–144. [DOI] [PubMed] [Google Scholar]
- Zhong J, Trevisi L, Urch B, Lin X, Speck M, Coull BA, Liss G, Thompson A, Wu S, Wilson A, Koutrakis P, Silverman F, Gold DR and Baccarelli AA (2017) B-vitamin Supplementation Mitigates Effects of Fine Particles on Cardiac Autonomic Dysfunction and Inflammation: A Pilot Human Intervention Trial. Sci Rep 7:45322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


