Capsule summary
Psoriasis-associated suppression of the skin-specific chemokine/receptor CCL27/CCR10 axis leads to enhanced pathogenic IL-17A/IL-22-producing skin T cell activation and inflammation.
Keywords: psoriasis, immune dysregulation, CCL27, CCR10, IL-17A, IL-22, γδT cells, αβT cells, innate lymphoid cells (ILCs), imiquimod, microneedles
To the editor
Skin inflammatory symptoms of psoriasis are predominantly caused by pathogenic cytokines such as IL-17A/IL-22 overproduced by dysregulated T cells. Targeting pathogenic cytokines is effective in treatment of the symptoms but does not treat the T cell dysregulation. Molecular mechanisms responsible for the T cell dysregulation are currently poorly understood. We report here that psoriasis-associated suppression of CCL27/CCR10-derived immune regulatory signals contributes to enhanced IL-17A/IL-22-producing skin T cell over-activation and inflammation.
CCL27 is a skin-specific chemokine expressed by skin epithelial cells, and its receptor, CCR10, is expressed by skin lymphocytes1. The CCL27/CCR10 axis has been long suggested to promote skin inflammatory diseases including psoriasis by facilitating skin pathogenic T cell migration2. However, clinical studies found that CCL27 is severely suppressed in lesional skin of psoriatic patients3, 4, inconsistent with a role of the CCL27/CCR10 axis in promoting psoriasis. Using mouse models, we recently discovered that CCR10 is important for homeostatic establishment of resident lymphocytes in healthy skin but dispensable for T cell migration to inflamed skin5, 6, 7, supporting an opposite hypothesis that loss of CCL27/CCR10-derived regulatory signals might actually lead to enhanced skin T cell over-activation in psoriasis.
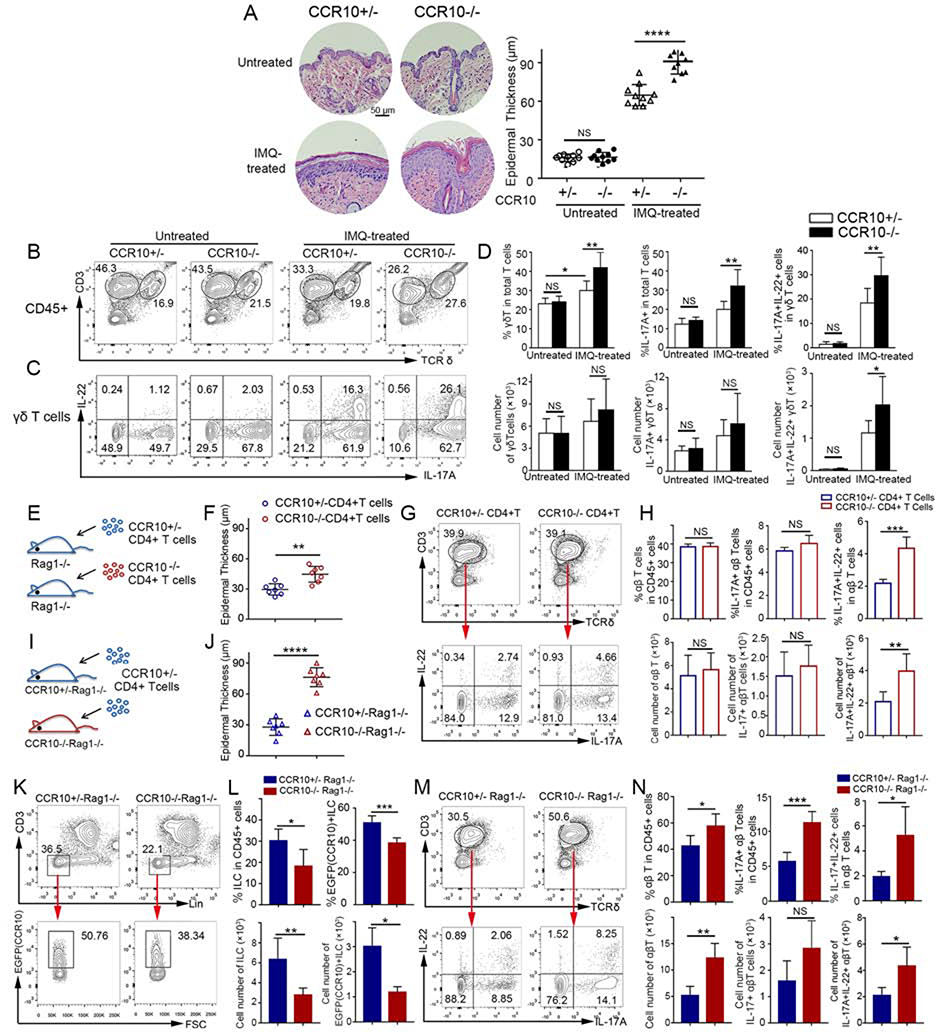
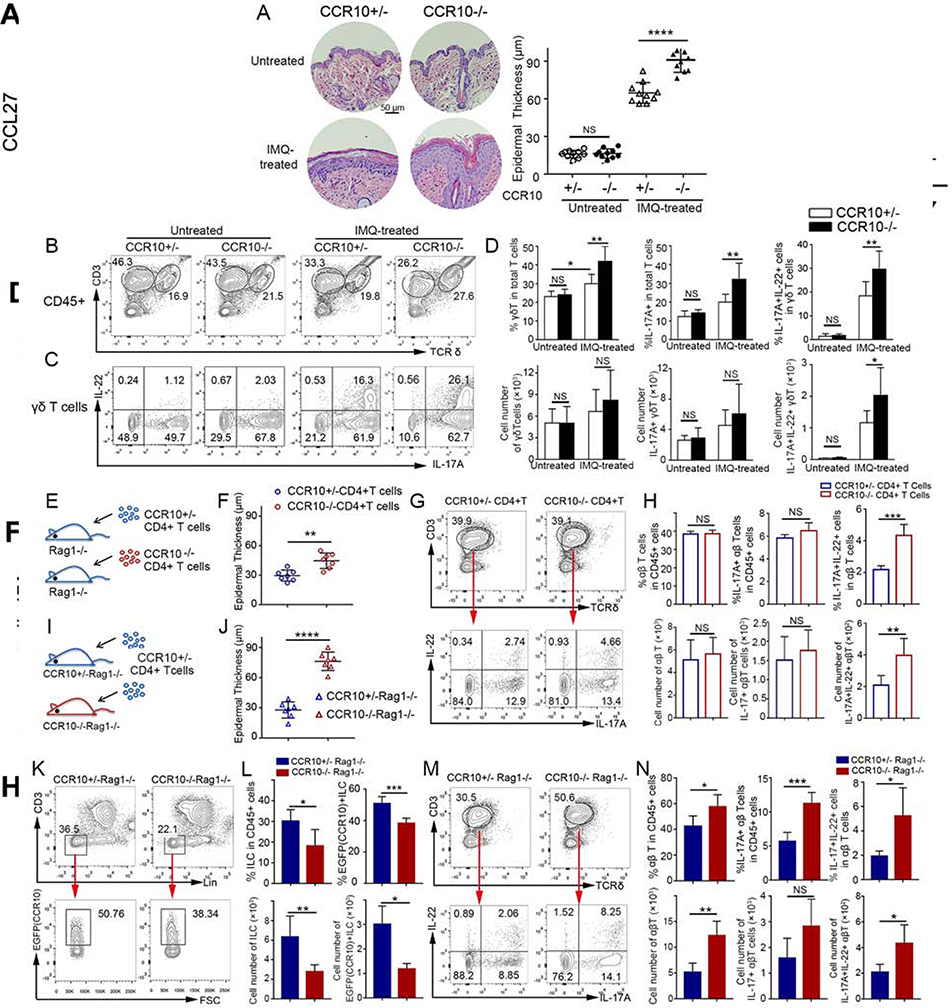
We tested this hypothesis first in CCR10-knockout (KO)/EGFP-knockin (KI) mice using an imiquimod (IMQ)-induced model of psoriasis, in which topical IMQ application induces skin inflammation mainly through activation of IL-17A/IL-22-producing γδT (γδT17) cells8. In CCR10-KO/EGFP-KI mice, the CCR10 coding sequence was replaced with a coding sequence for EGFP that could be used to report the CCR10 expression5. IMQ-treated homozygous CCR10-KO/EGFP-KI (CCR10EGFP/EGFP or CCR10−/− for simplicity) mice had more severe skin inflammation than IMQ-treated heterozygous (CCR10+/EGFP or CCR10+/−) littermates while untreated CCR10+/− or CCR10−/− mice had no inflammation (Fig. 1A, Fig. E1A). Associated with this, IMQ-treated CCR10−/− mice had higher expansion of skin IL-17A+, particularly IL-17A+IL-22+, γδT cells than IMQ-treated CCR10+/− littermates (Fig. 1B-D). Untreated CCR10−/− and CCR10+/− mice had similar skin IL-17A+ γδT cells, few of which expressed IL-22 (Fig. 1B-D). Skin γδT cells of IMQ-treated CCR10−/− and CCR10+/− mice had lower EGFP(CCR10) than their untreated controls (Fig. E1B), suggesting downregulation of CCR10 in activated γδT cells. These results indicate that CCR10-derived signals suppress the IMQ-induced activation of IL-17A/IL-22-producing skin γδT cells and inflammation.
Figure 1.
Enhanced IL-17A/IL-22-producing skin T cell activation and inflammation in IMQ-treated CCR10-knockout mice. (A,B,C,D) H&E stained sections and average epidermal thickness (A), flow cytometric (FC) analysis of γδT cells (B) and their IL-17A/IL-22 production (C), and average percentages and numbers of IL-17A/IL-22+ γδT cells (D) of untreated and 5 day-IMQ-treated skin of CCR10−/− and CCR10+/− mice. (E) Scheme of reconstitution of Rag1−/− mice with CCR10−/− or CCR10+/− CD4+ αβT cells. (F,G,H) Average epidermal thickness (F), FC analysis of donor αβT cells and their IL-17A/IL-22 production (G), and average percentages and numbers of IL-17A/IL-22+ αβT cells (H) of the IMQ-treated skin of Rag1−/− mice reconstituted with CCR10−/− or CCR10+/− CD4+ αβT cells. (I) Scheme of reconstitution of CCR10+/−Rag1−/− or CCR10−/−Rag1−/− mice with same CCR10+/− CD4+ αβT cells. (J,K,L,M,N) Average epidermal thickness (J), FC analysis (K) and percentages and numbers (L) of EGFP(CCR10)+ host ILCs, and FC analysis (M) and percentages and numbers (N) of IL-17A/IL-22+ donor αβT cells of the IMQ-treated skin of CCR10+/−Rag1−/− and CCR10−/−Rag1−/− mice reconstituted with CCR10+/− CD4+ αβT cells. One dot represents one sample (A,F,J). N=5–9 (D) and 5 each (H,L,N). NS: not significant, *P<0.05, **P<0.01, ***P<0.001, ***P<0.0001.
Besides γδT17 cells, IL-17-producing CD4+ αβT (Th17) cells and innate lymphoid cells (ILCs) are also involved in psoriasis. IMQ-treated CCR10−/− mice had higher expansion of IL-17A+IL-22+ CD4+ skin αβT cells than IMQ-treated CCR10+/− mice (Fig. E2A-C), suggesting that CCR10 also restricts Th17 cell activation. Associated with this, there were more significantly reduced percentages of EGFP(CCR10)+ αβT cells in IMQ-treated skin of CCR10−/− than CCR10+/− mice since activated IL-17A+IL-22+ skin αβT cells had downregulated CCR10(EGFP) compared to IL-17A-IL-22− or IL-17A+IL-22− skin αβT cells (Fig. E3A-B). On the other hand, IMQ-treated CCR10−/− and CCR10+/− mice had similar percentages of IL-17A+ skin ILCs, which did not express IL-22 (Fig. E4A-B). However, IMQ-treated CCR10−/− mice had lower percentages of EGFP(CCR10)+ skin ILCs than IMQ-treated CCR10+/− mice (Fig. E4C). Since CCR10+ ILCs are involved in homeostatic regulation of skin αβT cells7, these results suggest that CCR10 might support maintenance of regulatory ILCs to restrict the skin T cell activation and inflammation.
To demonstrate further that CCR10 was important in restricting activation of Th17 cells to suppress skin inflammation, we applied IMQ to the skin of Rag1−/− mice that were reconstituted with CCR10−/− or CCR10+/− CD4+ αβT cells (Fig. 1E). In this setting, only αβT cells of the two donor origins had different CCR10-knockout statuses. Indeed, IMQ-treated Rag1−/− mice reconstituted with CCR10−/− αβT cells had more severe skin inflammation than IMQ-treated Rag1−/− mice reconstituted with CCR10+/− αβT cells (Fig. 1F, Fig. E5A). Associated with this, there was higher expansion of IL-17A+IL-22+ skin αβT (CD3+TCRδ-) cells of CCR10−/− than CCR10+/− donor origin (Fig. 1G-H). IL-17A+IL-22+ skin αβT cells expressed lower EGFP(CCR10) than IL-17A-IL-22− or IL-17A+IL-22− counterparts (Fig. E5B), again indicating downregulation of CCR10 in activated Th17 cells.
To test further whether CCR10 supported regulatory ILCs to restrict skin Th17 cell activation and inflammation, we applied IMQ to the skin of CCR10+/−Rag1−/− or CCR10−/−Rag1−/− mice that were reconstituted with same CCR10+/− CD4+ αβT cells (Fig. 1I). In this setting, ILCs of CCR10+/−Rag1−/− and CCR10−/−Rag1−/− recipients had different CCR10-knockout statuses whereas donor αβT cells had no CCR10 defect. Compared to IMQ-treated CCR10+/−Rag1−/− recipients, IMQ-treated CCR10−/−Rag1−/− recipients had significantly worse skin inflammation (Fig. 1J, Fig. E6). IMQ-treated CCR10−/−Rag1−/− recipients had fewer total and EGFP(CCR10)+ skin ILCs than IMQ-treated CCR10+/−Rag1−/− recipients (Fig. 1K-L), confirming that CCR10 is important for maintenance of skin ILCs with regulatory function on helper T cells. Supporting this notion, donor αβT (CD3+TCRδ-) cells in the IMQ-treated skin of CCR10−/−Rag1−/− recipients expressed IL-17A and IL-22 more than those of CCR10+/−Rag1−/− recipients did (Fig. 1M-N). Together, these results demonstrate that CCR10-dervied signals restrain over-activation of IL-17A/IL-22+ γδT and αβT cells directly and indirectly (through promoting regulatory ILCs) to suppress skin inflammation.
We then tested whether delivery of exogenous CCL27 into the skin could reduce IMQ-induced inflammation in wild-type mice to determine biological importance of psoriasis-associated suppression of CCL27. Like human psoriatic skin3, 4, IMQ-applied inflamed mouse skin had downregulated CCL27 expression (Fig. 2A), making it an appropriate model to test the effect of exogenous CCL27 delivery.
Fig. 2.
Microneedle-mediated trans-epidermal delivery of CCL27 reduces IMQ-induced skin inflammation. (A) Real-time RT-PCR analysis of the CCL27 expression in untreated and 5-day IMQ-treated mouse skin. Normalized to GAPDH. (B) Image of a whole area of skin injected with arrays of microneedles carrying DyLight 650-labeled protein (Red). (C) H&E-stained sections and average epidermal thickness of the skin treated with CCL27- or control (Ctrl) microneedles. Mice were applied with IMQ daily, injected with microneedles on days 0/3/5, analyzed on day 6. (D,E) FC analysis of TCRδ+CD3+ γδT and TCRδ-CD3+ (αβ)T cells (D), and percentages and numbers of γδT cells (E) in the CCL27- and control microneedle-treated skin. (F,G,H) FC analysis (F), and percentages and numbers of IL-17A+ γδT cells (G) and IL-17A+ αβT cells (H) in the CCL27- and control microneedle-treated skin. In the panels D-H, mice were applied with IMQ daily, treated with microneedles on days 3/5, analyzed on day 6. One dot represents one sample (A,C,E,G,H). *P<0.05, **P<0.01.
Microneedle patches made with the biocompatible polymers polyvinylpyrrolidone and polyvinyl alcohol were used to increase efficacy of the CCL27 delivery9. After insertion into the skin, tips of microneedles dissolve, allowing for delivery of cargoes evenly across an area of the skin (Fig. 2B, Fig. E7A-B).
Compared to untreated or empty microneedle-treated skin, CCL27-loaded microneedle-treated skin had reduced inflammation (Fig. 2C, Fig. E7C). Correlating with this, CCL27 treatment significantly reduced IMQ-induced expansion of skin γδT cells (Fig. 2D-E, Fig. E7D). Furthermore, CCL27-treated skin contained significantly lower percentages of IL-17A+ γδT cells and IL-17A+ αβT cells than control-treated skin (Fig. 2F,G,H).
In sum, we discovered that impairment of the CCL27/CCR10-mediated immune regulation contributes to increased skin IL-17-producing T cell activation and inflammation in mouse models of psoriasis, suggesting that its restoration might be a new therapeutic strategy against the disease. Clinical relevance and molecular mechanisms of the CCL27/CCR10-mediated inhibition of psoriasis are worth further investigation (“Discussion” in Online Repository).
Supplementary Material
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers AR064831 and R01AR070887 (to N.X) and R01AR073364 (to Y.W.). The content is solely the responsibility of the authors and does not necessarily represent official views of the funding agencies. We thank the staff of Penn State Microscopy and Cytometry Facility for excellent technical support.
Supported by NIH grants AR064831 and R01AR070887 (to N.X) and R01AR073364 (to Y.W.).
Footnotes
All authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Xiong N, Fu Y, Hu S, Xia M, Yang J. CCR10 and its ligands in regulation of epithelial immunity and diseases. Protein Cell. 2012;3(8):571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Homey B, Alenius H, Muller A, Soto H, Bowman EP, Yuan W, et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8(2):157–65. [DOI] [PubMed] [Google Scholar]
- 3.Gudjonsson JE, Ding J, Johnston A, Tejasvi T, Guzman AM, Nair RP, et al. Assessment of the psoriatic transcriptome in a large sample: additional regulated genes and comparisons with in vitro models. J Invest Dermatol. 2010;130(7):1829–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riis JL, Johansen C, Vestergaard C, Bech R, Kragballe K, Iversen L. Kinetics and differential expression of the skin-related chemokines CCL27 and CCL17 in psoriasis, atopic dermatitis and allergic contact dermatitis. Exp Dermatol. 2011;20(10):789–94. [DOI] [PubMed] [Google Scholar]
- 5.Jin Y, Xia M, Sun A, Saylor CM, Xiong N. CCR10 is important for the development of skin-specific gammadeltaT cells by regulating their migration and location. J Immunol. 2010;185(10):5723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia M, Hu S, Fu Y, Jin W, Yi Q, Matsui Y, et al. CCR10 regulates balanced maintenance and function of resident regulatory and effector T cells to promote immune homeostasis in the skin. J Allergy Clin Immunol. 2014;134(3):634–44 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J, Hu S, Zhao L, Kaplan DH, Perdew GH, Xiong N. Selective programming of CCR10(+) innate lymphoid cells in skin-draining lymph nodes for cutaneous homeostatic regulation. Nat Immunol. 2016;17(1):48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182(9):5836–45. [DOI] [PubMed] [Google Scholar]
- 9.Coyne J, Davis B, Kauffman D, Zhao N, Wang Y. Polymer Microneedle Mediated Local Aptamer Delivery for Blocking the Function of Vascular Endothelial Growth Factor. ACS Biomater Sci Eng. 2017;3(12):3395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.