ABSTRACT
Background
Phenylalanine and tyrosine (referred to as total aromatic amino acids; TAAs) are essential for protein synthesis, and are precursors for important catecholamines. Current estimated average requirement (EAR) recommendations for TAA during pregnancy are 36 mg·kg−1·d−1, and has not been experimentally determined.
Objectives
The aim was to determine TAA requirements (dietary phenylalanine in the absence of tyrosine) during early and late gestation using the indicator amino acid oxidation (IAAO, with L-[1-13C]leucine) technique.
Methods
Nineteen healthy pregnant women (age 22–38 y) were studied at a range of phenylalanine intakes (5 to 100 mg·kg−1·d−1) in early (13–19 wk) and/or late (33–39 wk) pregnancy for a total of 51 study days. Graded test intakes were provided as 8 hourly isonitrogenous and isocaloric meals. Breath samples were collected for 13C enrichment analysis on an isotope ratio mass spectrometer. A plasma sample was collected and analyzed for phenylalanine and tyrosine concentrations on an amino acid analyzer. The TAA requirement in early and late pregnancy was calculated using 2-phase linear regression crossover analysis that identified breakpoints in 13CO2 production (the requirement) in response to phenylalanine intakes.
Results
TAA requirement during early pregnancy was 44 mg·kg−1·d−1 (95% CI: 28.3, 58.8) and during late pregnancy was 50 mg·kg−1·d−1 (95% CI: 36.1, 63.1). In early and late pregnancy, plasma phenylalanine and tyrosine concentrations rose linearly in response to graded phenylalanine intakes.
Conclusions
Our results suggest that the current EAR of 36 mg·kg−1·d−1 for TAAs is underestimated. When compared with results previously determined in nonpregnant adults, early pregnancy requirements were similar (43 compared with 44 mg·kg−1·d−1, respectively). During late pregnancy, a 14% higher TAA requirement was observed when compared with early pregnancy. The results from this study have potential implications for creating gestation stage-specific TAA recommendations.
Keywords: stable isotopes, phenylalanine, tyrosine, pregnancy, amino acid requirements, indicator amino acid oxidation
Introduction
Pregnancy is associated with changes in dietary energy and nutrient requirements as a result of changes in maternal metabolism and increased tissue accretion (1, 2). This dynamic period in life is accompanied by an increase in blood and extracellular volume, development of the placenta, changes in breast and uterine tissues, and fetal growth. Previously, using the indicator amino acid oxidation (IAAO) and direct amino acid oxidation (DAAO) techniques, we have estimated protein, lysine, and phenylalanine (in the presence of excess tyrosine) requirements during early and late pregnancy (3–5).
Phenylalanine and tyrosine, are required for protein synthesis and are the precursors for neurotransmitters dopamine, norepinephrine, and epinephrine. Phenylalanine is an indispensable amino acid and is converted intracellularily to tyrosine, a conditionally indispensable amino acid, via the enzyme phenylalanine hydroxylase (6). Phenylalanine and tyrosine are referred to as aromatic amino acids (7). Since dietary phenylalanine can provide tyrosine in vivo, phenyalanine requirement estimates derived in the absence of dietary tyrosine is regarded as the total aromatic amino acid (TAA) requirement. We previously estimated the dietary phenylalanine requirement in the presence of excess tyrosine (minimum phenylalanine requirement) (5). However, the TAA requirements have not been experimentally determined in human pregnancies.
Currently, the dietary reference intakes (DRIs) provide an estimated average requirement (EAR) for TAA (phenylalanine + tyrosine) at 36 mg·kg−1·d−1 for pregnant women, compared with an EAR of 27 mg·kg−1·d−1 for nonpregnant adults (7). The techniques used to determine protein and amino acid requirements in humans prior to stable isotope-based methods were considered too invasive for routine application in pregnant women. Therefore, the values for protein and amino acid recommendations that the DRIs provide for pregnant women are factorially calculated and based on total potassium accretion during pregnancy and nitrogen balance studies done in nonpregnant adults (8). These DRI recommendations are static throughout pregnancy and do not account for potential changes in requirements. This is problematic as there are dynamic differences in metabolism and development throughout pregnancy. Our laboratory has determined that there are significant differences in requirements between early and late gestation for protein (25% increase), lysine (37% increase), and minimum phenylalanine (40% increase), indicating that current recommendations should be re-evaluated, and the remaining indispensable amino acid requirements should be experimentally determined in early (13–19 wk) and late (33–39 wk) pregnancy (3–5).
The objective of the current study was to determine the dietary requirement for TAA (phenylalanine in the absence of tyrosine) in early (13–19 wk) and late (33–39 wk) gestation. This was done using the minimally invasive IAAO technique (with L-[1-13C]leucine). We hypothesized that the requirement for TAA in late pregnancy would differ from the requirement in early pregnancy, and that stage-specific requirements would differ from current recommendations.
Methods
Participants
Healthy women who were pregnant with a single child participated in this study at Britsh Columbia Children's Hospital Research Institute within our Clinical Research and Evaluation Unit. All women were aged between 20 and 40 y, with self-reported prepregnancy BMIs between 19 and 28 kg/m2. The participants had no significant nausea or vomiting, gestational diabetes, pre-eclampsia, or other chronic health or pregnancy-induced conditions, and reported all prescription medication and supplement use. All women were taking prenatal vitamins. Written and informed consent was gathered from all participants. An honorarium was provided to participants at the end of each completed study day. This study was approved by British Columbia Children's and Women's Hospital's Research Ethics Board (H17-02924) and was registered at clinicaltrials.gov as NCT03409939. A flow chart with the details of the screening and enrollment process is outlined in Supplemental Figure 1.
Experimental design
The study design was modeled after previous IAAO studies (5, 9). The IAAO technique indirectly measures oxidation of an indicator amino acid (leucine in this study) to determine the dietary requirement of the test amino acids (TAA). The underlying principle of this method is that when an indispensable amino acid intake is deficient for protein synthesis to occur, the remaining amino acids (including the indicator amino acid), will be oxidized since amino acids are not stored in the body for later use (10). With increasing intakes of the test amino acid(s) IAAO will decrease, as amino acids will be incorporated for protein synthesis until the requirement is reached, after which the IAAO will achieve a plateau. The point at which this change occurs represents the breakpoint or mean requirement of the test amino acid(s).
In early (13–19 wk) and late (33–39 wk) gestation, 8 phenylalanine intakes (in the absence of dietary tyrosine; 5, 25, 40, 50, 60, 70, 85, 100 mg·kg−1·d−1) were repeated multiple times by different participants. Since phenylalanine is the precursor to tyrosine, and we aimed to measure the TAA requirement and not the minimum phenylalanine requirement, no tyrosine was provided in the diet.
Each participant completed ≤6 study days within a gestational stage, with ≥5 d between study days. This study is similar to previous pregnancy studies performed by our laboratory determining protein, lysine, and minimum phenylalanine (in the presence of excess tyrosine) requirements (3–5).
Preliminary assessment of participants
The eligibility of each participant was evaluated during a preliminary assessment. Participants were weighed using a digital scale to the nearest 0.1 kg, height was determined to the nearest 0.1 cm using a stadiometer, and body composition was determined using skinfold analysis. Fat mass was assessed using pregnancy-specific equations that account for: sex, age, gestational stage, and 3 skinfold thickness sites (triceps, biceps, and subscapular) measured using Harpenden Skinfold Calipers (Baty International) (5, 11). Fasted (10–12 h) blood glucose was assessed by a finger prick blood glucose monitor (One Touch® Ultra® 2 LifeScan), and a blood glucose cut-off of 6.0 mmol/L was used to screen for gestational diabetes. Resting energy expenditure (REE, kcal/d) was assessed by an open circuit indirect calorimeter with a ventilated hood (Vmax Encore, VIASYS). Glucose and protein in urine were determined by Chemstrip®7 Urinalysis Strips (Roche Diagnostics) to rule out gestational diabetes and risk of pre-eclampsia. With the assistance of food models, detailed 2-d diet records were obtained to create a personalized diet recommendation that prescribed protein intake at 1.5 g·kg−1·d−1. Participants were instructed on how to maintain a 2-d standardized diet prior to each study day, and instructed to take prenatal vitamins to ensure adequacy of micronutrient intake. Analysis of the diet records to measure adherence was carried out using the Food Processor Nutrition Analysis Software.
Study day diets
Participants arrived at the Clinical Research and Evaluation Unit at BC Children's Hospital Research Institute after an overnight fast. Weight, height, urine test strip, and fasted blood glucose were repeated at the beginning of each study day. A randomized phenylalanine intake, by pulling from an envelope, was provided on each study day (5, 25, 40, 50, 60, 70, 85, 100 mg·kg−1·d−1) as 8 hourly meals consisting of flavored liquid formula and protein-free cookies. Each study day protocol is outlined in Supplemental Figure 2. Study test intakes were chosen based on the requirement for TAAs previously determined in adult males, 43 mg·kg−1·d−1, and our earlier reported normal intakes of phenylalanine and tyrosine in pregnant women living in Vancouver (12, 13). Each of these meals provided 1/12th of the participant's daily requirement for energy and nutrients. The short study day, paired with instructions to each participant to consume/resume a normal meal upon finishing the study, were implemented to create a study as minimally invasive and ethical as possible. The range of test intakes that we tested are also within a fairly normal consumption amount, as explained above (12, 13), although we realize restriction of amino acids is not ideal in pregnancy, and the short-term nature of our study (8 h) was to minimize the impact. In addition, we informally collected information about the birth of the infant and outcome of pregnancy, and women have not reported any adverse events. Thus, every attempt was made to ensure the safety of the participants in our studies (3–5). Protein was provided at 1.5 g·kg−1·d−1 and energy was provided at 1.7× the participants' measured REE from the preliminary assessment. The macronutrient distribution on study days was ∼53% carbohydrates, 37% fat, and 10% protein. The formula contained protein-free powder (PFD1: Mead Johnson Nutrition), orange flavored drink powder (Tang and Kool-Aid: Kraft Canada), corn oil (Mazola: ACH Food Companies), and protein as a crystalline L-amino acid mixture (Ajinomoto) modeled after egg-protein composition with the exception of phenylalanine, tyrosine, leucine, serine, and glutamine. Serine and glutamine content were altered depending on the phenylalanine intake to ensure all meals were isonitrogenous. Tyrosine was not provided in the diet. The diets were prepared at BC Children's Hospital Research Institute. On study days, only the experimental diets and water were consumed by participants.
Isotope protocols
Isotope consumption started at meal 5 with priming doses of NaH13CO3 [0.176 mg/kg; 99 atom % excess (APE) Cambridge Isotope Laboratories] and L-[1-13C]leucine (1.727 mg/kg; 99 APE Cambridge Isotope Laboratories). Hourly doses of L-[1-13C]leucine (1.727 mg·kg−1·h−1) was provided in meals 5–8. The mass of nonlabeled L-leucine equivalent to the L-[1-13C]leucine was removed from the diet to provide a constant leucine intake across all 8 meals. Total leucine intake was 65 mg·kg−1·d−1—which is above the DRI's EAR and RDA of 45 and 56 mg·kg−1·d−1, respectively, to ensure leucine intake was sufficient and constant (7). This quantity of the indicator amino acid was chosen to both ensure the dietary requirement was met and to ensure its sensitivity to changes in phenylalanine intake (5, 14).
Sample collection and analysis
Breath samples were collected and analyzed for baseline and isotopic plateau enrichment measurements. Breath bags (single-use collection bags, Easy Sampler System, QuinTron, Terumo Medical) that removed any dead space air were used to collect breath samples in exetainer tubes (Labco). Three baseline samples were collected 45, 30, and 15 min before isotope administration (meal 5). Breath samples were then collected at 150, 180, 195, 210, 225, and 240 min after isotope administration began, during isotopic steady state. Samples were stored at room temperature. The samples were then analyzed for 13C-enrichment in expired breath using a continuous flow isotope ratio mass spectrometer (CF-IRMS IsoPrime100). 13CO2 was quantified in APE over a reference CO2 standard.
One venous blood sample was obtained during each study by a certified phlebotomist in EDTA tubes at the 6th hour of the study day. Samples were taken at this time point to allow for plasma amino acid concentration stabilization. Plasma was isolated via centrifugation (10 min, 2000 × g, 4°C; Sorvall® Biofuge Stratos, Mandel Scientific Co. Ltd) and stored at –80°C until analysis. Ion exchange chromatography with postcolumn ninydrin derivatization was performed using an amino acid analyzer (Hitachi L8900) to determine plasma amino acid concentrations, as previously described (15).
Isotope kinetics
The F13CO2 (rate of 13CO2 released from L-[1-13C]leucine during oxidation) was expressed in μmol 13CO2/kg/h and calculated using the following equation:
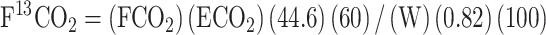 |
(1) |
where FCO2 is the CO2 rate of production (mL/minute), ECO2 is 13CO2 enrichment (APE) in exhaled breath at isotopic steady state, W is the participant's weight (kg), the constants 44.6 μmol/mL and 60 min/h convert F13CO2 to μmol/h, and the factor of 100 converts the APE to a fraction. The 0.82 factors in bicarbonate fixation of 13CO2 in the fed state (16).
Statistical analysis
Subject characteristics and study day assessment results are presented as mean ± SD. The mean requirements for TAA in both early and late pregnancy were estimated from breakpoint analysis of F13CO2 data using a biphase linear regression crossover analysis in SAS (SAS/STAT Ver 9.4), with subject as a random variable, because not all women participated in multiple study days (17–20). This analysis selects the model with the minimum residual SE in a stepwise partitioning of phenylalanine intakes between 2 regression lines. These lines are assessed for a candidate breakpoint with mixed models in order to account for repeated measures within a participant. Using I as the indicator variable, it is equal to 0 for x values left of the breakpoint and 1 for x values to the right of the breakpoint. The model is Y = β0+β1x+β2I+β3Ix, where Y = leucine oxidation or F13CO2, x = phenylalanine intake, β0 = left line intercept, β0+β2 = right line intercept, β1 = left line slope, β1+β3 = right line slope. Therefore, Y = β0+β1x for the left line and Y = (β0+β2) + (β1+β3)x for the right. Equating these, β0+β1x = (β0+β2) + (β1+β3)x and solving for x yields the breakpoint at x = –(β2/β3). We used regression with mixed models by selecting parameter estimates for multiple breakpoint candidates. The model that minimized the Akaike information criteria (AIC), with the highest adjusted R2 and lowest CV was used to select the final breakpoint model.
Using Fiellers Theorem, the 95% CI was determined: 95% CI = breakpoint ± tdf, α/2 × SE, where SE is the SE of the combined regression lines, df is the degrees of freedom associated with the residual mean square of the best fit model, and α is the 95% CI level (3, 4). The statistical difference between the early and late stage breakpoints was assessed using a pooled 2-sample t-test as described previously (5, 21, 22). Linear regression analysis (Graphpad Prism 6, Graphpad Software) was used to analyze the effect of graded phenylalanine intakes on plasma concentrations of phenylalanine and tyrosine. Significance was set at P ≤ 0.05.
Results
Participants
Nineteen women participated in early and/or late gestation (nearly = 10 and nlate = 10), with 1 participant studied at both stages. A total of 51 study days were completed (Table 1 and 2). In early pregnancy, 1 participant completed 6 study days, 1 completed 5 study days, 1 completed 3 study days, 3 completed 2 study days, and 4 completed 1 study day. In late pregnancy, 2 participants completed 5 study days, 3 completed 3 study days, 3 completed 2 study days, and 2 completed 1 study day. No participants reported pregnancies in the 6 mo prior to their current pregnancy, and self-reported prepregnancy BMIs were between 17 and 29 (mean = 24.3 ± 3.5). Women were aged between 22 and 38 y and had appropriate gestational weight gain when compared with current recommendations (23). For the protein standardization diet, our dietary record analysis indicated that participants ate 1.22 ± 0.34 and 1.22 ± 0.49 g·kg−1·d−1 in early and late pregnancy, respectively (Table 2). None of the participants reported use of alcohol, illicit drugs, or cigarettes during their current pregnancy. Two women reported recent use of pyridoxine/doxylamine, 1 reported use of levothyroxine, and 1 reported use of fluoxitine, though no prescription medications were consumed during study days. All women had normal fasted blood glucose concentrations both during preliminary assessments (Table 1) and on study days (Table 2), and no abnormal glucose or protein concentrations in urine were observed.
TABLE 1.
Participant characteristics1
| Characteristic | Early gestation | Late gestation |
|---|---|---|
| Participants, n | (n = 10) | (n = 10) |
| Age, y | 32.3 ± 3.0 | 30.0 ± 5.0 |
| Gestational age, wk | 17.2 ± 2.4 | 34.1 ± 2.5 |
| Prepregnancy BMI,2 kg/m2 | 25.0 ± 3.0 | 23.5 ± 3.8 |
| Fasting blood glucose, mmol/L | 4.8 ± 0.4 | 5.0 ± 0.4 |
| Fat mass,3 % | 30.3 ± 4.6 | 30.2 ± 5.8 |
| Resting energy expenditure,4 kcal/d | 1422 ± 203 | 1387 ± 252 |
Values are mean ± SD.
Based on participant reported prepregnancy weight.
Determined by Skinfold Measurements (Harpenden Skinfold Caliper).
Determined by Open-circuit Indirect-calorimetry (Vmax Encore, VIASYS).
TABLE 2.
Study day assessments of healthy pregnant women during early and late gestation1
| Early gestation | Late gestation | |
|---|---|---|
| Variable | (n2 = 24) | (n2 = 27) |
| Weight, kg | 72.4 ± 10.0 | 71.5 ± 12.7 |
| Fasting blood glucose, mmol/L | 4.9 ± 0.3 | 4.9 ± 0.3 |
| Energy intake, kcal/d | 2417 ± 345 | 2358 ± 430 |
| Phenylalanine intake prior to study day,3 mg · kg−1 · d−1 | 52 ± 15 | 54 ± 23 |
| Tyrosine intake prior to study day,3 mg · kg−1 · d−1 | 43 ± 13 | 43 ± 20 |
| Protein intake prior to study day,3 g · kg−1 · d−1 | 1.22 ± 0.33 | 1.22 ± 0.49 |
Values are mean ± SD.
n refers to number of individual observations from 10 women in each gestation period.
Amount of protein and phenylalanine consumed by participants in the 2 d prior to study day as indicated by dietary records.
Tracer oxidation
In early pregnancy, L-[1-13C]leucine oxidation decreased with increasing phenylalanine intake to a F13CO2 of 2.1 μmol·kg−1·h−1, and then plateaued with phenylalanine intakes above 40 mg·kg−1·d−1 (Figure 1). In late pregnancy, L-[1-13C]leucine oxidation decreased to a F13CO2 of 2.0 μmol·kg−1·h−1, and then plateaued with phenylalanine intakes above 50 mg·kg−1·d−1 (Figure 2).
FIGURE 1.
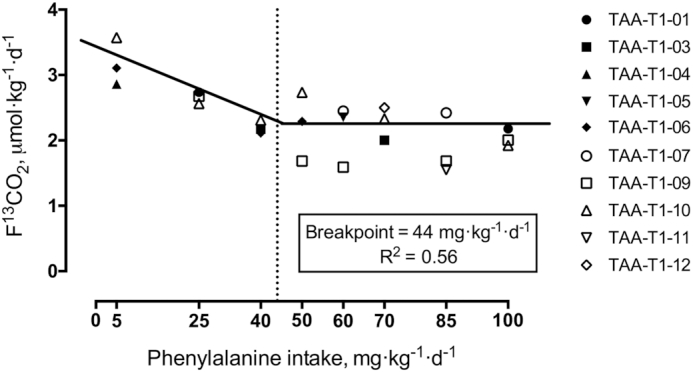
Estimated average requirement of TAA in early gestation using the indicator amino acid oxidation method in healthy pregnant women. Biphase linear regression crossover analysis of L-[1-13C]leucine tracer oxidation (F13CO2, μmol·kg−1·h−1) was used to determine the TAA requirement using the mixed and regression procedure in SAS (SAS/STAT Ver 9.4). TAA requirements were determined to be 44 mg·kg−1·d−1 (R2 = 0.56, 95% CI: 28.3, 58.8 mg·kg−1·d−1; n = 10 women, individual study days = 24). Dashed line indicates the mean requirement. TAA, total aromatic amino acids.
FIGURE 2.
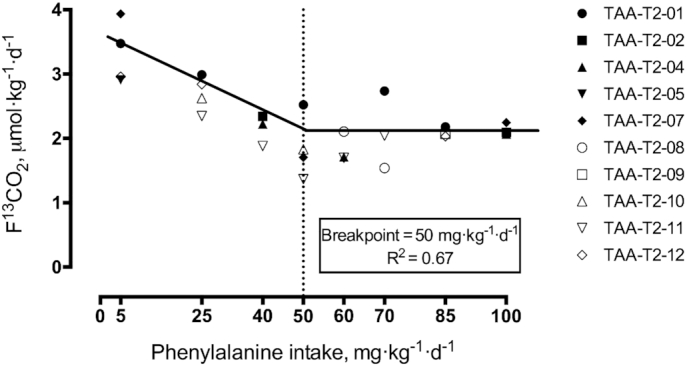
Estimated average requirement of TAA in late gestation using the indicator amino acid oxidation method in healthy pregnant women. Biphase linear regression crossover analysis of L-[1-13C]leucine tracer oxidation (F13CO2, μmol·kg−1·h−1) was used to determine the phenylalanine requirement using the mixed and regression procedure in SAS (SAS/STAT Ver 9.4). Phenylalanine requirements were determined to be 50 mg·kg−1·d−1 (R2 = 0.67, 95% CI: 36.1, 63.1 mg·kg−1·d−1; n = 10 women, individual study days = 27). Dashed line indicates the mean requirement. TAA, total aromatic amino acids.
Biphase linear regression crossover analysis of the early pregnancy data provided a breakpoint (mean requirement) or 43.57 mg·kg−1·d−1 (rounded to 44 for the dietary requirement; R2 = 0.56, 95% CI: 28.3, 58.8 mg·kg−1·d−1). The mean requirement in late pregnancy was determined to be 49.56 mg·kg−1·d−1 (rounded to 50 for the dietary requirement; R2 = 0.67, 95% CI: 36.1, 63.1 mg·kg−1·d−1). Comparison of the early and late stage mean requirements showed a significant difference (P < 0.01).
Plasma amino acids
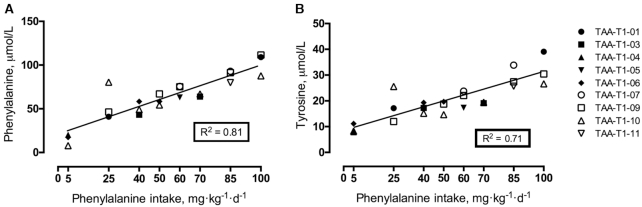
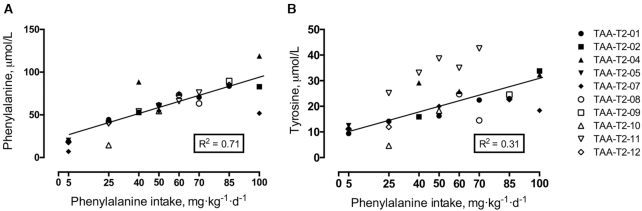
In early pregnancy, plasma phenylalanine concentrations rose linearly (R2 = 0.81, P < 0.01) in response to graded phenylalanine intakes (Figure 3). A similar increasing trend (R2 = 0.71, P < 0.01) was seen in late pregnancy (Figure 4). Plasma concentrations for tyrosine rose linearly (R2 = 0.71, P < 0.01) in early pregnancy (Figure 3) but the late pregnancy rise (R2 = 0.31, P < 0.01) was more variable (Figure 4).
FIGURE 3.
Plasma concentrations of phenylalanine and tyrosine in early pregnancy in response to graded phenylalanine intakes in healthy pregnant women. Linear regression analysis of phenylalanine concentrations (R2 = 0.81, panel A) and tyrosine concentrations (R2 = 0.71, panel B) (n = 10 women, individual study days = 24). TAA, total aromatic amino acids.
FIGURE 4.
Plasma concentrations of phenylalanine and tyrosine in late pregnancy in response to graded phenylalanine intakes in healthy pregnant women. Linear regression analysis of phenylalanine concentrations (R2 = 0.71, panel A) and tyrosine concentrations (R2 = 0.31, panel B) (n = 10 women, individual study days = 27). TAA, total aromatic amino acids.
Discussion
This was the first study, to the best of our knowledge, to experimentally determine TAA (phenylalanine in the absence of dietary tyrosine) requirements in healthy pregnant women. In early pregnancy (13–19 weeks of gestation), the mean requirement was determined to be 44 mg·kg−1·d−1. In late pregnancy (33–39 weeks of gestation), the mean requirement was determined to be 50 mg·kg−1·d−1. Both of these findings are higher than the current DRI and EAR for TAA intake during pregnancy of 36 mg·kg−1·d−1.
In healthy nonpregnant adults using stable isotope-based techniques there are 4 studies that have been conducted previously to determine TAA requirements. The first, published in 1998, employed a 24-h tyrosine balance method as a physiological endpoint for the TAA requirement using 3 phenylalanine intakes (18.5, 35.6, and 96.6 mg·kg−1·d−1) (24). They estimated a tentative requirement of 39 mg·kg−1·d−1, which was higher than the recommendation at the time of 35.6 mg·kg−1·d−1. In 2006, 3 studies were published addressing TAA requirements in healthy adults. The first, using the IAAO technique with L-[1-13C]lysine, used a similar approach as the current study by providing no dietary tyrosine on study days (25). A requirement of 48 mg·kg−1·d−1 was found. Secondly, the IAAO technique was employed while providing L-[1-13C]leucine, to determine the TAA requirement (with an absence of dietary tyrosine) (14). A requirement of 42 mg·kg−1·d−1 was determined. Lastly, Kurpad et al., using a cohort of healthy Indian men and the 24-h indicator amino acid balance method, found a TAA requirement of 38 mg·kg−1·d−1 (26). Following a thorough review of these data, an average requirement was deduced from the available data of 43 mg·kg−1·d−1 for nonpregnant adults (12). Thus, our early pregnancy TAA requirement (44 mg·kg−1·d−1) was similar to requirements previously determined in nonpregnant adults, whereas late pregnancy requirement (50 mg·kg−1·d−1) increased by ∼16%.
In human pregnancies, 3 studies have been done previously in our laboratory to determine protein and amino acids requirements. Using the IAAO technique (with L-[13C]phenylalanine), we determined the mean protein requirements during early (13–19 wk) and late (33–39 wk) pregnancy as 1.22 and 1.52 g·kg−1·d−1, respectively (3). This was the first experimental study in pregnant humans to suggest that the current DRI recommendations (0.88 g·kg−1·d−1) were underestimated, and that the single recommendation throughout the duration of pregnancy was not appropriate (there was a 25% difference in requirement between stages). Next, lysine requirements during early and late pregnancy were determined to be 37 and 50 mg·kg−1·d−1, respectively (4). They were different from the current DRI recommendation of 41 mg·kg−1·d−1, and the late pregnancy requirement was 37% higher than early pregnancy requirement. Most recently, we determined the phenylalanine (in the presence of excess tyrosine at 65 mg·kg−1·d−1) requirements during early and late gestation in healthy pregnant women (5) to be 15 and 21 mg·kg−1·d−1, respectively. The current study suggests that recommendations for TAA at 36 mg·kg−1·d−1 are underestimated (by 22% in early pregnancy and 39% in late pregnancy).
Previously, both the protein and minimum phenylalanine requirements were higher for pregnant women compared with nonpregnant adults (3, 5). This corresponds well to the fact that whole-body protein turnover has been reported to increase in early pregnancy with ∼15% increase in protein synthesis by the end of the first trimester (27–30). Conversely, lysine and TAA (the current study) requirements were similar between early pregnancy and nonpregnant adults. On the one hand, these findings suggest that whereas protein needs increase from early stages of pregnancy, this is not true for all amino acids. It is not entirely clear why phenylalanine requirements increase early in pregnancy, but TAA requirements do not. It is potentially due to phenylalanine requirements increasing in early pregnancy as well as late pregnancy, whereas tyrosine requirements are held more constant throughout the pregnancy. Furthermore as pregnancy progresses, the rate of tissue synthesis and the amino acid composition of newly deposited tissues is variable, potentially accounting for the increase in requirement (as a percent). Both minimum phenylalanine and TAA requirements increased by ∼6 mg·kg−1·d−1 between early and late pregnancy, providing some evidence for this justification.
Since tyrosine is synthesized from phenylalanine in vivo, it has been suggested that dietary tyrosine can spare phenylalanine requirements. The minimum phenylalanine (in the presence of excess tyrosine) requirement in healthy adult males was reported as 9.1 mg·kg−1·d−1 (31). Paired with the data from the TAA requirement study estimates (43 mg·kg−1·d−1), it was reasoned earlier that the TAA requirement that can be met by tyrosine in nonpregnant adults is 78%, and phenylalanine must provide ≥22% of the requirement (12). When comparing our recent pregnancy studies (5) on phenylalanine (15 and 21 mg·kg−1·d−1 in early and late pregnancy, respectively) with the current study, tyrosine spares 66% of the TAA requirement in early pregnancy, and 58% in late pregnancy. This adds to the idea that phenylalanine-specific requirements are increasing more than tyrosine during pregnancy. Additionally, a study by Roberts et al. showed that tyrosine requirement in healthy adults using L-[13C]lysine is 7 mg·kg−1·d−1, and that protein synthesis was optimized when the dietary ratio of phenylalanine:tyrosine was 60:40, which is comparable to human tissue TAA composition (32). Going forward, it would be interesting to investigate the effect of different dietary phenylalanine:tyrosine ratios during human pregnancies.
We are aware of a few limitations with this study, including the small number of subjects, which is similar to our previous pregnancy studies (3–5). Each pregnant woman could not participate in all test intakes due to the dynamic nature of pregnancy. In addition, the determined requirements have a wide and overlapping 95% CI. However, the relatively high R2 values and low AIC and root mean square error indicate robust data, providing the first experimentally determined TAA requirement at different stages of human pregnancy. Lastly, studies like these may use diets that are not like most natural foods. Natural foods do not contain phenylalanine without tyrosine, and do not contain protein that is as highly digestible as crystalline amino acids. However, our results provide a basis for future studies investigating metabolic availability and protein quality for formulas to treat patients with phenylketonuria, who require life-long dietary management involving phenylalanine restriction and tyrosine supplementation, including during pregnancy—maternal phenylketonuria.
In conclusion, TAA requirements (phenylalanine in the absence of dietary tyrosine) determined in healthy pregnant women during early and late gestation were 44 and 50 mg·kg−1·d−1, respectively. The TAA requirements found in early pregnancy are similar to those found previously in adult males (12, 25, 26, 32). Furthermore, the mean late gestation requirement was higher compared with the early gestation requirement by 14%. This has the potential to improve future dietary recommendation guidelines during pregnancy.
Supplementary Material
Acknowledgments
The authors’ contributions were as follows––MAE, KL, ROB, PBP, GCM, and RE: designed the project; MAE: conducted the research; MAE and AJO: analyzed data; MAE, AJO, ROB, PBP, GCM, and RE: wrote the manuscript; RE: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Supported by the Canadian Institute of Health Research (FRN 10321).
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: AIC, Akaike information criterion; APE, atoms % excess; DRI, dietary reference intake; EAR, estimated average requirement; IAAO, indicator amino acid oxidation; REE, resting energy expenditure; TAA, total aromatic amino acid.
Contributor Information
Madeleine A Ennis, British Columbia Children's Hospital Research Institute, British Columbia Children's Hospital, Vancouver, Canada; Department of Pediatrics, University of British Columbia, Vancouver, Canada.
Anna-Joy Ong, British Columbia Children's Hospital Research Institute, British Columbia Children's Hospital, Vancouver, Canada.
Kenneth Lim, Department of Obstetrics and Gynecology, British Columbia Women's Hospital, Vancouver, Canada.
Ronald O Ball, Department of Agricultural, Food and Nutritional Sciences, University of Alberta, Edmonton, Canada.
Paul B Pencharz, Research Institute, The Hospital for Sick Children, Toronto, Canada; Department of Nutritional Sciences, University of Toronto, Toronto, Canada; Department of Pediatrics, University of Toronto, Toronto, Canada.
Glenda Courtney-Martin, Research Institute, The Hospital for Sick Children, Toronto, Canada; Department of Nutritional Sciences, University of Toronto, Toronto, Canada.
Rajavel Elango, British Columbia Children's Hospital Research Institute, British Columbia Children's Hospital, Vancouver, Canada; Department of Pediatrics, University of British Columbia, Vancouver, Canada; School of Population and Public Health, University of British Columbia, Vancouver, Canada.
References
- 1. Kalhan SC. Protein metabolism in pregnancy. Am J Clin Nutr. 2000;71:1249S–55S. [DOI] [PubMed] [Google Scholar]
- 2. King JC. Physiology of pregnancy and nutrient metabolism. Am J Clin Nutr. 2000;71:1218S–25S. [DOI] [PubMed] [Google Scholar]
- 3. Stephens TV, Payne M, Ball RO, Pencharz PB, Elango R. Protein requirements of healthy pregnant women during early and late gestation are higher than current recommendations. J Nutr. 2015;145:73–8. [DOI] [PubMed] [Google Scholar]
- 4. Payne M, Stephens T, Lim K, Ball RO, Pencharz PB, Elango R. Lysine requirements of healthy pregnant women are higher during late stages of gestation compared to early gestation. J Nutr. 2018;148:94–9. [DOI] [PubMed] [Google Scholar]
- 5. Ennis MA, Rasmussen BF, Lim K, Ball RO, Pencharz PB, Courtney-Martin G, Elango R. Dietary phenylalanine requirements during early and late gestation in healthy pregnant women. Am J Clin Nutr. 2020;111:351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flydal MI, Martinez A. Phenylalanine hydroxylase: function, structure, and regulation. IUBMB Life. 2013;65:341–9. [DOI] [PubMed] [Google Scholar]
- 7. Institute of Medicine Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids [Internet]. 2005. [Cited 2020 Sep 18] Available from: https://www.nap.edu/catalog/10490/dietary-reference-intakes-for-energy-carbohydrate-fiber-fat-fatty-acids-cholesterol-protein-and-amino-acids. [DOI] [PubMed] [Google Scholar]
- 8. Elango R, Ball RO. Protein and amino acid requirements during pregnancy. Adv Nutr. 2016;7:839S–44S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elango R, Humayun MA, Ball RO, Pencharz PB. Lysine requirement of healthy school-age children determined by the indicator amino acid oxidation method. Am J Clin Nutr. 2007;86:360–5. [DOI] [PubMed] [Google Scholar]
- 10. Elango R, Ball RO, Pencharz PB. Recent advances in determining protein and amino acid requirements in humans. Br J Nutr. 2012;108(Suppl 2):S22–30. [DOI] [PubMed] [Google Scholar]
- 11. Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. [DOI] [PubMed] [Google Scholar]
- 12. Pencharz PB, Hsu JW-C, Ball RO. Aromatic amino acid requirements in healthy human subjects. J Nutr. 2007;137:1576S–8S.; discussion 1597S–1598S. [DOI] [PubMed] [Google Scholar]
- 13. Stephens TV, Woo H, Innis SM, Elango R. Healthy pregnant women in Canada are consuming more dietary protein at 16- and 36-week gestation than currently recommended by the dietary reference intakes, primarily from dairy food sources. Nutr Res. 2014;34:569–76. [DOI] [PubMed] [Google Scholar]
- 14. Hsu JW-C, Kriengsinyos W, Wykes LJ, Rafii M, Goonewardene LA, Ball RO, Pencharz PB. Leucine is not a good choice as an indicator amino acid for determining amino acid requirements in men. J Nutr. 2006;136:958–64. [DOI] [PubMed] [Google Scholar]
- 15. Rasmussen B, Gilbert E, Turki A, Madden K, Elango R. Determination of the safety of leucine supplementation in healthy elderly men. Amino Acids. 2016;48:1707–16. [DOI] [PubMed] [Google Scholar]
- 16. Hoerr RA, Yu YM, Wagner DA, Burke JF, Young VR. Recovery of 13C in breath from NaH13CO3 infused by gut and vein: effect of feeding. Am J Physiol. 1989;257:E426–38. [DOI] [PubMed] [Google Scholar]
- 17. Wang LA, Goonewardene LA The use of MIXED models in the analysis of animal experiments with repeated measures data. Can J Anim Sci. 2004;84:1–11. [Google Scholar]
- 18. Hayamizu K, Kato M, Hattori S. Determining amino acid requirements from repeated observations on indicator amino acid oxidation method by mixed-effect change-point regression models. J Clin Biochem Nutr. 2011;49:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robbins KR, Saxton AM, Southern LL. Estimation of nutrient requirements using broken-line regression analysis. J Anim Sci. 2006;84:E155–65. [DOI] [PubMed] [Google Scholar]
- 20. Lamberson WR, Firman JD. A comparison of quadratic versus segmented regression procedures for estimating nutrient requirements. Poult Sci. 2002;81:481–4. [DOI] [PubMed] [Google Scholar]
- 21. Kriengsinyos W, Wykes LJ, Goonewardene LA, Ball RO, Pencharz PB. Phase of menstrual cycle affects lysine requirement in healthy women. Am J Physiol Endocrinol Metab. 2004;287:E489–96. [DOI] [PubMed] [Google Scholar]
- 22. Courtney-Martin G, Chapman KP, Moore AM, Kim JH, Ball RO, Pencharz PB. Total sulfur amino acid requirement and metabolism in parenterally fed postsurgical human neonates. Am J Clin Nutr. 2008;88:115–24. [DOI] [PubMed] [Google Scholar]
- 23. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines Weight gain during pregnancy: reexamining the guidelines. [Internet] Rasmussen KM, Yaktine AL, Washington (DC): National Academies Press (US); 2009. [Cited 2017 Aug 8]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK32813/. [PubMed] [Google Scholar]
- 24. Basile-Filho A, Beaumier L, El-Khoury AE, Yu YM, Kenneway M, Gleason RE, Young VR. Twenty-four-hour L-[1-(13)C]tyrosine and L-[3,3-(2)H2]phenylalanine oral tracer studies at generous, intermediate, and low phenylalanine intakes to estimate aromatic amino acid requirements in adults. Am J Clin Nutr. 1998;67:640–59. [DOI] [PubMed] [Google Scholar]
- 25. Hsu JW-C, Goonewardene LA, Rafii M, Ball RO, Pencharz PB. Aromatic amino acid requirements in healthy men measured by indicator amino acid oxidation. Am J Clin Nutr. 2006;83:82–8. [DOI] [PubMed] [Google Scholar]
- 26. Kurpad AV, Regan MM, Raj TDS, Rao VN, Gnanou J, Young VR. The daily phenylalanine requirement of healthy Indian adults. Am J Clin Nutr. 2006;83:1331–6. [DOI] [PubMed] [Google Scholar]
- 27. Kalhan SC, Parimi PS. Transamination of leucine and nitrogen accretion in human pregnancy and the newborn infant. J Nutr. 2006;136:281S–7S. [DOI] [PubMed] [Google Scholar]
- 28. de Benoist B, Jackson AA, Hall JSE, Persaud C. Whole-body protein turnover in Jamaican women during normal pregnancy. Hum Nutr Clin Nutr. 1985;39(3):167–79. [PubMed] [Google Scholar]
- 29. Thompson GN, Halliday D. Protein turnover in pregnancy. Eur J Clin Nutr. 1992;46:411–7. [PubMed] [Google Scholar]
- 30. Duggleby SL, Jackson AA. Higher weight at birth is related to decreased maternal amino acid oxidation during pregnancy. Am J Clin Nutr. 2002;76:852–7. [DOI] [PubMed] [Google Scholar]
- 31. Zello GA, Pencharz PB, Ball RO. Phenylalanine flux, oxidation, and conversion to tyrosine in humans studied with L-[1-13C]phenylalanine. Am J Physiol. 1990;259:E835–43. [DOI] [PubMed] [Google Scholar]
- 32. Roberts SA, Thorpe JM, Ball RO, Pencharz PB. Tyrosine requirement of healthy men receiving a fixed phenylalanine intake determined by using indicator amino acid oxidation. Am J Clin Nutr. 2001;73:276–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.