ABSTRACT
The collective findings from human microbiome research, randomized controlled trials on specific microbes (i.e., probiotics), and associative studies of fermented dairy consumption provide evidence for the beneficial effects of the regular consumption of safe live microbes. To test the hypothesis that the inclusion of safe, live microbes in the diet supports and improves health, we propose assessment of the types and evidentiary quality of the data available on microbe intake, including the assembly and evaluation of evidence available from dietary databases. Such an analysis would help to identify gaps in the evidence needed to test this hypothesis, which can then be used to formulate and direct initiatives focused on prospective and randomized controlled trials on live microbe consumption. Outcomes will establish whether or not the evidence exists, or can be generated, to support the establishment of dietary recommendations for live microbes.
Keywords: fermented food, probiotics, live dietary microbes, dietary guidelines, bioactive, gut microbiome, NHANES, International Scientific Association for Probiotics and Prebiotics
Introduction
For most of human evolution, diets were based on raw and unprocessed foods. The microorganisms associated with unprocessed foods were largely adventitious and originated from plant, animal, and environmental sources. With the transition from hunter–gatherer to more settled communities, ∼10,000–20,000 y ago, fermented foods containing large and diverse populations of microbes emerged as staples in numerous societies (1). Diets underwent further changes in the 19th and 20th centuries because of the development of modern food processing and preservation methods. Diets in high-income countries with large urban populations now consist of many highly processed foods. Further, water sources are treated to minimize microbial contamination. As a result, people live in increasingly hygienic environments with fewer and less diverse microbial exposures (2).
Despite the obvious public health benefits that more hygienic foods and environments provide, there may also be unforeseen negative health consequences as a result of these reductions in microbial exposures. Some research suggests that diet contributes to the rise over the past century of many contemporary chronic immune, metabolic, and other “lifestyle” diseases (3, 4). These diseases include asthma, eczema, lupus, type 1 diabetes, celiac disease, food allergies, multiple sclerosis, Crohn disease, and rheumatoid arthritis (5). The potential for a microbial role in the emergence of those diseases is addressed in “the old friends hypothesis” (6), which suggests that exposure to nonharmful or commensal microbes in foods is an important, beneficial source of microbial stimuli for the immune system. Live safe microbes obtained from daily intake in the diet may “engage” with the mucosal surfaces of the digestive tract, fine-tuning the immune system, bolstering gut function, and reinforcing the ability of the human symbiont to mitigate susceptibility to the development of chronic diseases (Figure 1). Immune regulatory activities of live microorganisms may also contribute to health by dampening an overactive inflammatory response produced by Western diets low in live and safe microbes (7).
FIGURE 1.
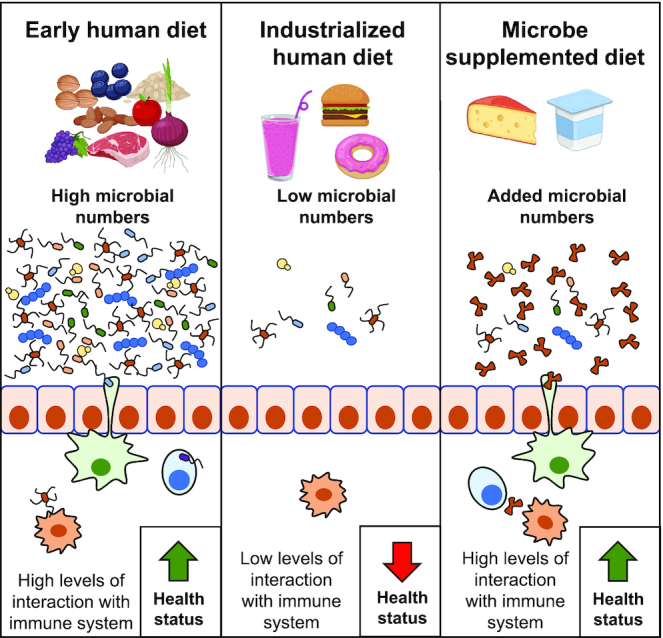
Higher levels of live safe microbes in the diet could engage with the immune system and prevent, limit, or ameliorate diseases connected to immune dysfunction.
Results of numerous human studies on probiotics are consistent with the premise that the ingestion of certain commensal microbes can benefit human health (8). Probiotics are defined as live microbes that, when administered in adequate amounts, confer a health benefit on the host (9). Although probiotic strain differences may account for heterogeneity in some health outcomes, accumulated evidence from human studies and meta-analyses point to common health benefits derived from consuming sufficient doses of safe strains of a range of species (9, 10). Indeed, the number of strains capable of conferring health benefits may greatly exceed the number subjected to rigorous testing, as practical considerations limit the range of strains subjected to clinical evaluation. Because some probiotics are highly related to the microorganisms used to make fermented foods, it is reasonable to hypothesize that health benefits may also accrue from consumption of fermented foods when the viability of those microbes is retained until the time of consumption. Indeed, fermented foods have been associated with a wide range of health benefits (11–14). We therefore hypothesize that the regular consumption of safe, live microbes, although not essential, may confer health-promoting properties to mitigate or reduce risk of disease. We acknowledge that although dead (inactivated) microbes and microbial metabolites may also confer impacts on human health, they are outside the scope of this paper. The lack of data and the inability to quantify dead (inactivated) microbes significantly hinders the inclusion of this group at this time. Health benefits conferred by individual microbial metabolites, such as acetic acid, are addressed separately (15).
This paper builds on discussions held at the 2019 International Scientific Association for Probiotics and Prebiotics (ISAPP) Annual Meeting in Antwerp, Belgium, and on previous efforts (16–20). ISAPP is a nonprofit association dedicated to advancing the science of probiotics and prebiotics. At the 2019 ISAPP meeting, authors CH and BH organized a half-day workshop comprising invited experts and industry representatives to discuss how the hypothesis that live microbes could provide a health benefit could be rigorously tested, perhaps leading to a recognized dietary recommendation for consumption of live microorganisms.
Human holobiont–diet connection
Humans evolved in a microbial world. Microbes have been the primary colonizers of our planet for billions of years, and all multicellular organisms have had to find ways to cope with this abundance and diversity of microscopic life. Throughout the course of human evolution, microbes colonized all bodily surfaces, including mucosal surfaces associated with alimentary, respiratory, and vaginal tracts. Regular contact with microbes in the air, on surfaces, and in foods and beverages also meant that our ancestors needed to evolve effective microbial containment and management strategies. Humans and animals developed physicochemical barriers, including a largely impenetrable skin and intestinal mucosa, an acidic stomach, and adaptive and cell-mediated immune systems. It is notable that the mucosa-associated lymphoid tissue of the gut contains more plasma B cells (the white blood cells responsible for making most antibodies) than the lymphoid nodes, spleen, and bone marrow combined (21). Rather than only eliminating or excluding microorganisms, these strategies may have evolved to select for and maintain specific microorganisms, and as such, humans and their associated microbiota should be regarded as holobionts (22). The human digestive tract alone houses trillions of resident microbes that perform vital roles in immune, neurological, and metabolic functions.
Although numerous factors can affect gut microbiome composition and function, including microbiota-depleting medications (23), diet is a particularly influential driver of gut microbiome composition (24). Although some diets, such as those high in fats and sugars, may be detrimental to normal gut microbiome function, other diets support the growth of saccharolytic members of the gut microbiome that are critical to producing intestinal short-chain fatty acids essential for gut health (25). The gut microbiota can be influenced through diets high in fibers (12) and inclusion of prebiotics (26), probiotics (27), and consumption of fermented foods (28). The interplay among diet, the gut microbiome, and human health is at the center of our interest in understanding the beneficial role that consumption of safe, live dietary microbes may have in health.
The types and numbers of microbes in food
Microbes in food include bacteria, yeasts, and molds, and the amounts and types vary depending on the food, the food source, and the extent of processing the food has undergone. The goal of most food-preservation strategies is to inhibit growth or inactivate microbes, either collectively or by targeting specific groups such as pathogens or spoilage microorganisms. Microbial loads in dry, frozen, or thermally processed foods are low (29, 30). Further, processing steps combined with hygienic practices that minimize microbial exposure have led to relatively low numbers of microbes in both raw and minimally processed foods (31).
Surveys of the microbial content of fresh fruits and vegetables show that the quantities of microbes on those foods range widely, but are often <106 CFU/g (32). Although lactic acid bacteria (33) and fungi (34) are found on fresh produce, members of the Proteobacteria (including Pantoea, Pseudomonas, and Xanthomonas) frequently dominate (35–37). As fresh produce is now recognized as a frequent source of foodborne disease outbreaks, pre- and postharvest approaches to reduce microbial loads are being continuously updated in order to reduce the risk of foodborne pathogen contamination (38, 39). Such efforts, although necessary to prevent foodborne disease, also reduce the numbers of nonpathogenic microbes. Therefore, fresh produce, which is often washed and packaged, contributes relatively few live microbes to the daily diet of consumers.
In contrast, data from published studies on the amounts of live microorganisms in fermented foods show that those foods could serve as major sources of ingested microbes (40). Populations that widely consume fermented foods (e.g., yogurt) may ingest 108–1011 CFU/d according to estimates made in multiple reports (27, 40). However, those numbers were not based on empirical data and therefore may overestimate actual consumption. Lang et al. (41) attempted to estimate the number of ingested microbes based on consumption of actual meals. These authors developed 3 representative daily meal plans and enumerated the microbes remaining after meal preparation. The results showed that 2 of these meal plans provided <107 CFU/d. The third meal plan delivered >109 CFU/d, driven mostly by the yogurt and cultured milk contained in the diet.
Microbes in our diet: are they good for us?
The link between consumption of live microbes and health comes from intervention and associative studies on fermented foods and also from randomized controlled trials (RCTs) on specific live microbes, known as probiotics. Studies have shown that fermented foods, and dairy foods in particular, are linked to a reduced risk of cardiovascular disease (42, 43), reduced risk of weight gain (44), reduced risk of type 2 diabetes (45), healthier metabolic profiles (blood lipids, blood glucose, blood pressure, and insulin resistance) (46), and altered immune responses (47, 48) [see also reviews (11–13)]. One systematic review, which included RCTs, cohort studies, case-control studies, and cross-sectional studies, concluded that consumption of yogurt and fermented milk was associated with improvements in gastrointestinal and cardiovascular health, cancer risk, weight management, diabetes and metabolic health, and bone density (18). Furthermore, many RCTs with a range of therapeutic and preventive endpoints have been conducted on probiotic-containing yogurt or fermented milks and on probiotic supplement products (49). Many positive outcomes have been reported for interventions with live microorganisms, including treatment of colic (50), functional gut symptoms (51, 52), and acute pediatric diarrhea (53) and for reducing the risk of antibiotic-associated diarrhea (54, 55), necrotizing enterocolitis (56), Clostridioides difficile–associated diarrhea (57), lactose intolerance symptoms (58), and acute upper respiratory tract infections (59), among many others. For the purposes of dietary recommendations, studies on generally healthy populations are of primary relevance, but taken together, these studies provide a strong rationale for the value to human health of consuming live microbes. However, no studies have aimed to assess the specific contribution of safe, live microbes (numbers and types of living microbes) in either fermented foods or in diets as a whole on health outcomes. Therefore, utilizing these data to evaluate our hypothesis that live dietary microbes contribute to health remains a challenge.
Testing the hypothesis that live dietary microbes contribute to health
When considering options for developing a dietary recommendation for consuming live microbes, we recognize that the non-nutritive, non-essential nature of live dietary microbes and the limited available evidence makes establishing an RDA unfeasible. Adequate intake (AI) guidance is another approach that may offer more opportunities because, unlike RDAs, AIs are not based on estimated average requirement values. Evidence supporting an AI must ultimately be able to inform an estimate of the average daily amount of the nutrient that should be consumed by members of a healthy population. However, this level of intake must also be tied to minimizing the likelihood of nutrient inadequacy, disease, or compromised functional status. Because negative consequences of a lack of live microbe intake are not currently known, an AI may not be feasible. A framework for incorporating bioactive food components into dietary recommendations has been proposed (60). The evidence requirements for this approach may align better with our knowledge level for a range of live microbe intakes with demonstrated efficacy for identified health outcomes and may reflect a more likely attainable goal (61, 62).
Can we use existing dietary databases to test the benefits of live microbes?
We propose that evaluating available evidence from dietary databases is the first step to determining if there are quantifiable health benefits from consuming living microbes. This can be done by utilizing relevant data from existing observational studies, such as the NHANES, NutriNet-Santé, Korea National Health and Nutrition Examination Survey, and the European Prospective Investigation into Cancer and Nutrition. To date, several studies used NHANES to address research questions about yogurt and/or probiotic consumption that could help inform dietary recommendations for living microbes (Table 1). However, studies more specifically focused on general live microbe consumption are needed.
TABLE 1.
Examples of peer-reviewed studies that used NHANES to study research questions relating to consumption of live microbe–containing food and health outcomes
| Study reference no. | Study design | Population | Intervention/comparator | Outcome | AOR (95% CI) | AHR (95% CI) |
|---|---|---|---|---|---|---|
| (63) | Secondary analysis of NHANES 2003–2006 survey data | Adult, civilian, noninstitutionalized, residential USA | ≥3 vs. <2 servings/wk yoghurt or probiotic supplement | Prevalent proteinuric kidney disease | 0.76 (0.61, 0.94) | — |
| (64) | Secondary analysis of NHANES 1999–2014 | Adult, civilian, noninstitutionalized, residential USA | Yogurt and/or probiotic supplementation vs. neither | Obesity | 0.83 (0.76, 0.92) | — |
| (65) | Secondary analysis of NHANES 1999–2010 | Adult (≥20 y old), civilian, noninstitutionalized, residential USA | Top vs. bottom 4th of yogurt consumption | Mortality | — | 0.89 (0.83, 0.94)* |
*When additional covariates for body mass index, hypertension, and diabetes were included, the beneficial effect of yogurt was slightly attenuated to AHR=0.93 (0.85, 1.01)
In order for this approach to result in high-quality and actionable data, appropriate research questions aimed to identify useful relations between live microbe consumption and health or disease endpoints should be formulated. Such an effort will need to do the following: 1) identify foods that contain living microbes, 2) provide estimates of the numbers of living microbes in foods, 3) propose specific biomarkers or disease endpoint(s) that are affected by live microbe consumption, and 4) assess the hypothesized exposure/outcome through rigorous hypothesis testing and effect size estimation.
Limitations to using existing databases
For an existing cohort study to be useful for determining the relation between a disease outcome and dietary exposure, a food frequency assessment, or other dietary assessment, must capture the food or nutrient exposure of interest. Therefore, the extent to which existing dietary questionnaires provide direct or indirect data on the intake of live microbes must be determined. Large data collection surveys, such as the NHANES (66) and the National Institutes of Health Automated Self-Administered 24-Hour Dietary Recall (67), should specifically be reviewed for data on foods that may have large microbial loads. Delineating factors such as cooked compared with raw, fermented compared with nonfermented, and canned compared with fresh could be important. Combined with knowledge of the microbial load of such foods, estimates of microbe consumption could be calculated. However, it is not clear if existing databases provide the level of granularity in food description that would be required. These questionnaires may capture intakes of yogurt, kombucha, or other fermented products. However, because intakes of these products are typically low in existing large prospective cohorts in the United States and Canada, it may be difficult to quantify any relation between intake of fermented foods and health or disease endpoints.
Research questions posed in evidence-informed health guideline–development formats can be specified by population, interventions/exposures, comparators, and outcomes of interest (PICO) elements (68, 69). However, specifications for PICO elements can be very complex. The scope of study could include populations of varying compositions with respect to health status (70), multiple formulations of the intervention, numerous outcomes, and even multiple indicators of the same outcome. Simple and transparent specification of these elements for individual studies will be required. For example, a National Academy of Medicine expert panel charged with establishing principles for evaluating evidence for dietary reference intakes based on chronic disease recommended using a single outcome indicator on the hypothesized causal pathway between the nutrient or other food substance and the chronic disease of interest (71). Additionally, to avoid “fishing expeditions” and spurious conclusions, the research questions (including specific outcomes) that will be examined should be stated from the start (72), ideally in a study registry, in a published study protocol, or by an alternative means that would permit readers to verify that the question was posed before the analysis was conducted.
Observational food intake studies are also prone to internal validity threats, including selection, information, and confounding biases. For example, the NHANES consists of cross-sectional measurement of participants and thus lacks follow-up measurements. As a consequence, such studies cannot fully establish temporal, let alone causal, relations among factors being assessed. This limits the value of their findings for informing public health recommendations or can lead to opaque recommendations (73) or misinterpretations (74). Thus, it is important to develop and apply analytical methods for large cohort databases that can result in robust and transparent inferences while acknowledging that the strength of causal inferences from observational studies may be limited by confounding and other factors that cannot be fully controlled.
New prospective and randomized controlled studies
We recognize that existing evidence may not be sufficient to inform the determination of dietary recommendations for live microbes. Reduced levels of live microorganisms in foods is associated with other long-standing changes to dietary patterns (such as the intake of highly processed foods) that risk negative health effects, constituting a potential confounder. Therefore, new studies may need to be developed using study designs such as secondary analyses of national health surveys as well as new prospective cohort studies that include the collection of relevant biological samples, which might contribute to mechanistic understanding.
More RCTs on fermented foods containing live microbes, including probiotics, are also needed. Such studies are challenging in part due to the limitations of research on food intakes (75). One approach can include setting the background diet of subjects to provide control over potential dietary confounders. This method entails providing people with all their meals or keeping track of what foods at what quantity are eaten over the study duration. Another challenge inherent to the design of RCTs on fermented foods is the choice of proper controls, including maintenance of equivalent nutrient content and blinding of sensorially and texturally complex fermented foods. Another difficulty encountered in conducting RCTs on fermented food is that the microbiological composition of the food may not be accurately described, which is the case for food resulting from uncontrolled fermentation processes. RCTs on consumption of live probiotics contained in dietary supplements are also relevant and avoid many of the complications that the study of fermented foods entails. Such studies are typically easier to placebo control than other food studies. To our knowledge, no studies have directly compared effects of probiotic supplements and fermented foods. Furthermore, we recognize that differentiating among effects of live compared with dead microbes or microbial metabolites will be a challenge for testing our hypothesis. Initiating and advancing these efforts will also be complemented by in vitro and animal model studies that help to focus the key considerations needed for dietary studies in humans.
Conclusions
Even though recommendations for the consumption of live, safe microbes to health are largely absent from dietary guidelines (76, 77), we believe that available evidence supports the hypothesis that inclusion of safe live microbes in the diet may support and improve health. We are not suggesting that consumption of live microbes will address a deficiency condition but that, analogous to dietary fiber consumption, they may improve some parameter of health. To evaluate this hypothesis, existing data should be evaluated in order to articulate missing information. To address this proposition, we suggest the following practical steps:
Analyze existing databases. Unrestricted grants could be solicited from interested, nonprofit organizations such as ISAPP, or from industry. An initial seed grant might be sufficient to develop indications of what relevant data are available and what data gaps exist. Appropriate research questions relating consumption of live microbe-containing food and health outcomes will need to be developed. Several challenges will need to be addressed for such efforts, including the absence of information on live microbe composition of foods in dietary databases and managing confounding factors present in microbe-containing foods that might also impact health, such as dead microbes, microbial metabolites, and food components.
Coordinate with key health organizations. Outreach to the NIH, the USDA, the National Academy of Sciences, or other national or global organizations should be undertaken. This effort could include meetings organized with such agencies along with nutrition, microbiology, food science, and clinical experts able to inform the dietary recommendation process and determine the evidence required to set a dietary recommendation for live microbes.
Develop needed data on dietary live microbes. Once existing databases are probed, gaps in knowledge required to address the hypothesis must be compiled. It is anticipated that a large prospective study, ideally with collection of relevant biological samples, may be needed to enable robust conclusions about the consumption of live microbe-containing food and health outcomes.
Perform research as an iterative process. This process will be needed to adjust and refine the scope to specify which taxa (e.g., only those generally recognized as safe species), food formats, requirements for cell viability, necessity for certain microbial metabolites or compounds (e.g., DNA, peptidoglycan, and other macromolecules), and different impacts of dietary microbes depending on stage in life (e.g., infancy compared with adulthood).
All steps should be coordinated with groups having similar goals. Interested stakeholders should be consulted to develop appropriate research paths and funding sources to make needed progress that will lead to evidence-based conclusions on the role of live dietary microbes in human health.
Acknowledgments
We are grateful for the exchange of ideas that occurred among the industry and academic scientists who participated in the 2019 ISAPP meeting discussion group on the topic of “RDA for live microbes.” These experts, in addition to the authors, were Sylvie Binda, Tom Boileau, Amrish Chawla, Claire Demaël, Arno Greyling, Irene Lenoir-Wijnkoop, Greg Leyer, Sonja Nodland, Noelle Patno, Bruno Pot, Seppo Salminen, Jan Willem Sanders, Wilbert Sybesma, Jessica ter Haar, and Gabriel Vinderola. ISAPP is an organization a nonprofit organization governed by a volunteer board of directors of academic scientists. ISAPP is funded by member companies, but ISAPP's activities and outputs are not governed, stipulated, or controlled by industry members. ISAPP's mission is to provide objective, science-based information on probiotics, prebiotics, fermented foods, and related substances.
The authors’ responsibilities were as follows—MLM, CH, RH, JS, DJT, DM, and MES: wrote the paper; MES: compiled submitted contributions; MLM, CH, RH, DJT, and DM: extensively edited submitted contributions; and all authors: read and approved the final manuscript.
Notes
MLM and CH share first authorship.
Author disclosures: MLM has been compensated for consulting, speaking fees, or service on advisory boards for the Kerry Health and the Nutrition Institute and the Icelandic Milk & Skyr Corporation; CH is a scientific advisor and shareholder for Artugen Therapeutics and has research grants funded by Kerry Foods, ADARE Pharmaceutical, and Janssen; RH received grants or honoraria from Mead Johnson Nutrition, Pharmavite, Danone, Beachbody, and PepsiCo and is a co-owner of Synbiotic Health; DJT has been compensated for consulting from Synbiotic Health; DJM has been compensated for consulting for Dupont; MES declares compensation for consulting, speaking fees, or service on advisory boards from Associated British Foods, California Dairy Research Foundation, Cargill, Church & Dwight, Danone North America, Danone Research, fairlife, Georgetown, GlaxoSmithKline, Kerry, Mead Johnson, Pepsico, Probi, Trouw, Visalia Dairy Company, Winclove, and Yakult; and receiving travel expense reimbursement from ILSI NA, the Nebraska Food for Health Center, and the United States Pharmacopeia; and all authors had their travel expenses paid by the International Scientific Association for Probiotics and Prebiotics (ISAPP) for these authors to assemble at the 2019 ISAPP meeting.
Abbreviations used: AI, adequate intake; ISAPP, International Scientific Association for Probiotics and Prebiotics; PICO, population, interventions/exposures, comparators, and outcomes of interest; RCT, randomized controlled trial.
Contributor Information
Maria L Marco, Department of Food Science & Technology, University of California, Davis, CA, USA.
Colin Hill, APC Microbiome Ireland and School of Microbiology, University College Cork, Cork, Ireland.
Robert Hutkins, Department of Food Science and Technology, University of Nebraska, Lincoln, NE, USA.
Joanne Slavin, Department of Food Science and Nutrition, University of Minnesota, St. Paul, MN, USA.
Daniel J Tancredi, Department of Pediatrics and Center for Healthcare Policy and Research, University of California Davis School of Medicine, Sacramento, CA, USA.
Daniel Merenstein, Department of Family Medicine, Georgetown University, Washington DC, USA.
Mary Ellen Sanders, International Scientific Association for Probiotics and Prebiotics, Centennial, CO, USA.
References
- 1. Tamang JP, Cotter PD, Endo A, Han NS, Kort R, Liu SQ, Mayo B, Westerik N, Hutkins R. Fermented foods in a global age: East meets West. Comp Rev Food Sci Food Safety. 2020;19:184–217. [DOI] [PubMed] [Google Scholar]
- 2. Rodriguez-Monforte M, Sanchez E, Barrio F, Costa B, Flores-Mateo G. Metabolic syndrome and dietary patterns: a systematic review and meta-analysis of observational studies. Eur J Nutr. 2017;56:925–47. [DOI] [PubMed] [Google Scholar]
- 3. Christ A, Lauterbach M, Latz E. Western diet and the immune system: an inflammatory connection. Immunity. 2019;51:794–811. [DOI] [PubMed] [Google Scholar]
- 4. Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “Western-lifestyle” inflammatory diseases. Immunity. 2014;40:833–42. [DOI] [PubMed] [Google Scholar]
- 5. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375:2369–79. [DOI] [PubMed] [Google Scholar]
- 6. Rook GA. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: darwinian medicine and the “hygiene” or “old friends” hypothesis. Clin Exp Immunol. 2010;160:70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Margina D, Ungurianu A, Purdel C, Tsoukalas D, Sarandi E, Thanasoula M, Tekos F, Mesnage R, Kouretas D, Tsatsakis A. Chronic inflammation in the context of everyday life: dietary changes as mitigating factors. IJERPH. 2020;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanders ME, Merenstein D, Merrifield CA, Hutkins R. Probiotics for human use. Nutr Bull. 2018;43. [Google Scholar]
- 9. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S et al. . Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14. [DOI] [PubMed] [Google Scholar]
- 10. Sanders ME, Benson A, Lebeer S, Merenstein DJ, Klaenhammer TR. Shared mechanisms among probiotic taxa: implications for general probiotic claims. Curr Opin Biotechnol. 2018;49:207–16. [DOI] [PubMed] [Google Scholar]
- 11. Marco ML, Heeney D, Binda S, Cifelli CJ, Cotter PD, Foligne B, Ganzle M, Kort R, Pasin G, Pihlanto A et al. . Health benefits of fermented foods: microbiota and beyond. Curr Opin Biotechnol. 2017;44:94–102. [DOI] [PubMed] [Google Scholar]
- 12. Dimidi E, Cox SR, Rossi M, Whelan K. Fermented foods: definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gille D, Schmid A, Walther B, Vergeres G. Fermented food and non-communicable chronic diseases: a review. Nutrients. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanlier N, Gokcen BB, Sezgin AC. Health benefits of fermented foods. Crit Rev Food Sci Nutr. 2019;59:506–27. [DOI] [PubMed] [Google Scholar]
- 15. Samad A, Azlan A, Ismail A. Therapeutic effects of vinegar: a review. Curr Opin Food Sci. 2016;8:56–61. [Google Scholar]
- 16. Hill C, Sanders ME. Rethinking “probiotics.”. Gut Microbes. 2013;4:269–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hill C. RDA for microbes—are you getting your daily dose?. Biochemist. 2018;40. [Google Scholar]
- 18. Savaiano DA, Hutkins R. Yogurt, cultured fermented milk and health: a systematic review, Nutr Rev.. 2020, May 23; nuaa013, doi: 10.1093/nutrit/nuaa01;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bell V, Ferrao J, Fernandes T. Nutritional guidelines and fermented food frameworks. Foods. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chilton SN, Burton JP, Reid G. Inclusion of fermented foods in food guides around the world. Nutrients. 2015;7:390–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spencer J, Sollid LM. The human intestinal B-cell response. Mucosal Immunol. 2016;9:1113–24. [DOI] [PubMed] [Google Scholar]
- 22. van de Guchte M, Blottiere HM, Dore J. Humans as holobionts: implications for prevention and therapy. Microbiome. 2018;6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vich Vila A, Collij V, Sanna S, Sinha T, Imhann F, Bourgonje AR, Mujagic Z, Jonkers D, Masclee AAM, Fu J et al. . Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun. 2020;11:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N et al. . Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–5. [DOI] [PubMed] [Google Scholar]
- 25. McBurney MI, Davis C, Fraser CM, Schneeman BO, Huttenhower C, Verbeke K, Walter J, Latulippe ME. Establishing what constitutes a healthy human gut microbiome: state of the science, regulatory considerations, and future directions. J Nutr. 2019;149:1882–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Derrien M, van Hylckama Vlieg JE. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015;23:354–66. [DOI] [PubMed] [Google Scholar]
- 28. Taylor BC, Lejzerowicz F, Poirel M, Shaffer JP, Jiang L, Aksenov A, Litwin N, Humphrey G, Martino C, Miller-Montgomery S et al. . Consumption of fermented foods is associated with systematic differences in the gut microbiome and metabolome. mSystems. 2020;5:e00901–19.. doi: 10.1128/mSystems.00901-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Institute of Food Technologists Evaluation and definition of potentially hazardous foods: a report of the Institute of Food Technologists for the Food and Drug Administration of the United States Department of Health and Human Services. 2001[cited2020. Jul 3] [Internet]; Available from: https://www.fda.gov/files/food/published/Evaluation-and-Definition-of-Potentially-Hazardous-Foods.pdf [Google Scholar]
- 30. Feroz F, Shimizu H, Nishioka T, Mori M, Sakagami Y. Bacterial and fungal counts of dried and semi-dried foods collected from Dhaka, Bangladesh, and their reduction methods. Biocontrol Sci. 2016;21:243–51. [DOI] [PubMed] [Google Scholar]
- 31. Sonnenburg ED, Sonnenburg JL. The ancestral and industrialized gut microbiota and implications for human health. Nat Rev Microbiol. 2019;17:383–90. [DOI] [PubMed] [Google Scholar]
- 32. Becker B, Stoll D, Schulz P, Kulling S, Huch M. Microbial contamination of organically and conventionally produced fresh vegetable salads and herbs from retail markets in Southwest Germany. Foodborne Pathogens and Disease. 2019;16:269–75. [DOI] [PubMed] [Google Scholar]
- 33. Zwielehner J, Handschur M, Michaelsen A, Irez S, Demel M, Denner EB, Haslberger AG. DGGE and real-time PCR analysis of lactic acid bacteria in bacterial communities of the phyllosphere of lettuce. Mol Nutr Food Res. 2008;52:614–23. [DOI] [PubMed] [Google Scholar]
- 34. Tournas VH, Katsoudas E. Mould and yeast flora in fresh berries, grapes and citrus fruits. Int J Food Microbiol. 2005;105:11–7. [DOI] [PubMed] [Google Scholar]
- 35. Jackson CR, Randolph KC, Osborn SL, Tyler HL. Culture dependent and independent analysis of bacterial communities associated with commercial salad leaf vegetables. BMC Microbiol. 2013;13:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dees MW, Lysoe E, Nordskog B, Brurberg MB. Bacterial communities associated with surfaces of leafy greens: shift in composition and decrease in richness over time. Appl Environ Microbiol. 2015;81:1530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu AO, Leveau JHJ, Marco ML. Abundance, diversity and plant-specific adaptations of plant-associated lactic acid bacteria. Environmental Microbiology Reports. 2020;12:16–29. [DOI] [PubMed] [Google Scholar]
- 38. Lynch MF, Tauxe RV, Hedberg CW. The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol Infect. 2009;137:307–15. [DOI] [PubMed] [Google Scholar]
- 39. Gao C, Montoya L, Xu L, Madera M, Hollingsworth J, Purdom E, Singan V, Vogel J, Hutmacher RB, Dahlberg JA et al. . Fungal community assembly in drought-stressed sorghum shows stochasticity, selection, and universal ecological dynamics. Nat Commun. 2020;11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rezac S, Kok CR, Heermann M, Hutkins R. Fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lang JM, Eisen JA, Zivkovic AM. The microbes we eat: abundance and taxonomy of microbes consumed in a day's worth of meals for three diet types. PeerJ. 2014;2:e659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buendia JR, Li Y, Hu FB, Cabral HJ, Bradlee ML, Quatromoni PA, Singer MR, Curhan GC, Moore LL. Regular yogurt intake and risk of cardiovascular disease among hypertensive adults. Am J Hypertens. 2018;31:557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang K, Chen X, Zhang L, Deng Z. Fermented dairy foods intake and risk of cardiovascular diseases: a meta-analysis of cohort studies. Crit Rev Food Sci Nutr. 2020;60:1189–94. [DOI] [PubMed] [Google Scholar]
- 44. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen M, Sun Q, Giovannucci E, Mozaffarian D, Manson JE, Willett WC, Hu FB. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med. 2014;12::215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang H, Livingston KA, Fox CS, Meigs JB, Jacques PF. Yogurt consumption is associated with better diet quality and metabolic profile in American men and women. Nutr Res. 2013;33:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Alm JS, Swartz J, Lilja G, Scheynius A, Pershagen G. Atopy in children of families with an anthroposophic lifestyle. Lancet. 1999;353:1485–8. [DOI] [PubMed] [Google Scholar]
- 48. Ulven SM, Holven KB, Gil A, Rangel-Huerta OD. Milk and dairy product consumption and inflammatory biomarkers: an updated systematic review of randomized clinical trials. Adv Nutr. 2019;10:S239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Merenstein DJ, Sanders ME, Tancredi DJ. Probiotics as a Tx resource in primary care. J Fam Pract. 2020;69: E1–E10. [PubMed] [Google Scholar]
- 50. Sung V, D'Amico F, Cabana MD, Chau K, Koren G, Savino F, Szajewska H, Deshpande G, Dupont C, Indrio F et al. . Lactobacillus reuteri to Treat Infant Colic: A Meta-analysis. Pediatrics. 2018 Jan;141. [DOI] [PubMed] [Google Scholar]
- 51. Eskesen D, Jespersen L, Michelsen B, Whorwell PJ, Muller-Lissner S, Morberg CM. Effect of the probiotic strain Bifidobacterium animalis subsp. lactis, BB-12(R), on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: a randomised, double-blind, placebo-controlled, parallel-group trial. Br J Nutr. 2015;114:1638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang Y, Li L, Guo C, Mu D, Feng B, Zuo X, Li Y. Effects of probiotic type, dose and treatment duration on irritable bowel syndrome diagnosed by Rome III criteria: a meta-analysis. BMC Gastroenterol. 2016;16:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Szajewska H, Kolodziej M, Gieruszczak-Bialek D, Skorka A, Ruszczynski M, Shamir R. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children - a 2019 update. Aliment Pharmacol Ther. 2019;49:1376–84. [DOI] [PubMed] [Google Scholar]
- 54. Guo Q, Goldenberg JZ, Humphrey C, El Dib R, Johnston BC. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019;4:CD004827, doi: 10.1002/14651858.CD004827.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Szajewska H, Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793–801. [DOI] [PubMed] [Google Scholar]
- 56. AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2014, 9: 584–671.. doi: 10.1002/ebch.1976. [DOI] [PubMed] [Google Scholar]
- 57. Goldenberg JZ, Yap C, Lytvyn L, Lo CK, Beardsley J, Mertz D, Johnston BC. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12: CD006095. doi: 10.1002/14651858.CD006095.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. EFSA Panel on Dietetic Products NaA Scientific Opinion on the substantiation of health claims related to live yoghurt cultures and improved lactose digestion (ID 1143, 2976) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010;8:1763 10.2903/j.efsa.2010.1763. [DOI] [Google Scholar]
- 59. Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015:Feb 3;(2):CD006895, doi: 10.1002/14651858.CD006895.pub3. [DOI] [PubMed] [Google Scholar]
- 60. Ellwood K, Balentine DA, Dwyer JT, Erdman JW Jr., Gaine PC, Kwik-Uribe CL. Considerations on an approach for establishing a framework for bioactive food components. Adv Nutr. 2014;5:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gaine PC, Balentine DA, Erdman JW Jr., Dwyer JT, Ellwood KC, Hu FB, Russell RM. Are dietary bioactives ready for recommended intakes?. Adv Nutr. 2013;4:539–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lupton JR, Atkinson SA, Chang N, Fraga CG, Levy J, Messina M, Richardson DP, van Ommen B, Yang Y, Griffiths JC et al. . Exploring the benefits and challenges of establishing a DRI-like process for bioactives. Eur J Nutr. 2014;53(Suppl 1):1–9.. doi: 10.1007/s00394-014-0666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yacoub R, Kaji D, Patel SN, Simoes PK, Busayavalasa D, Nadkarni GN, He JC, Coca SG, Uribarri J. Association between probiotic and yogurt consumption and kidney disease: insights from NHANES. Nutr J. 2016; 15: 10. doi: 10.1186/s12937-016-0127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lau E, Neves JS, Ferreira-Magalhaes M, Carvalho D, Freitas P. Probiotic ingestion, obesity, and metabolic-related disorders: results from NHANES, 1999–2014. Nutrients. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mazidi M, Mikhailidis DR, Sattar N, Howard G, Graham I, Banach M, Meta-Anal LBP. Consumption of dairy product and its association with total and cause specific mortality—a population-based cohort study and meta-analysis. Clin Nutr. 2019;38:2833–45. [DOI] [PubMed] [Google Scholar]
- 66. Centers for Disease Control and Prevention National Health and Nutrition Examination Survey 2020[cited2020. Sep 16]; Available from: https://www.cdc.gov/nchs/nhanes/: [Google Scholar]
- 67. National Institutes of Health NCI Automated Self-Administered 24-Hour (ASA24®) Dietary Assessment Tool. 2020[cited 2020 Sep 16] [Internet]; Available from: https://epi.grants.cancer.gov/asa24/#:∼:text=The%20Automated%20Self%2DAdministered%2024,also%20known%20as%20food%20diaries. [Google Scholar]
- 68. Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123:A12–3. [PubMed] [Google Scholar]
- 69. Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, Alderson P, Glasziou P, Falck-Ytter Y, Schunemann HJ. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64:395–400. [DOI] [PubMed] [Google Scholar]
- 70. Pray LA, Yaktine AL, National Academies of Sciences Engineering and Medicine (US). Food and Nutrition Board. Global harmonization of methodological approaches to nutrient intake recommendations: proceedings of a workshop Washington (DC): The National Academies Press; 2018. [Google Scholar]
- 71. Kumanyika SK, Oria M. National Academies of Sciences Engineering and Medicine (US). Committee on the Development of Guiding Principles for the Inclusion of Chronic Disease Endpoints in Future Dietary Reference Intakes. National Academies of Sciences Engineering and Medicine (US). Food and Nutrition Board, National Academies of Sciences Engineering and Medicine (US). Health and Medicine Division. Guiding principles for developing dietary reference intakes based on chronic disease. Developing dietary reference intakes based on chronic disease, Washington (DC): The National Academies Press; 2017. [PubMed] [Google Scholar]
- 72. NIH US National Library of Medicine. clinicaltrials.gov. Considerations for observational studies and expanded access records. 2019; [Internet]. Available from: https://clinicaltrials.gov/ct2/manage-recs/how-register#considerations [Google Scholar]
- 73. Schwedhelm C, Schwingshackl L, Agogo GO, Sonestedt E, Boeing H, Knuppel S. Associations of food groups and cardiometabolic and inflammatory biomarkers: does the meal matter?. Br J Nutr. 2019 Sep 28;122:707–16. [DOI] [PubMed] [Google Scholar]
- 74. Ioannidis JPA. The challenge of reforming nutritional epidemiologic research. JAMA. 2018;320:969–70. [DOI] [PubMed] [Google Scholar]
- 75. Rubin R. Backlash over meat dietary recommendations raises questions about corporate ties to nutrition scientists. JAMA. 2020, 323, 401–4., doi: 10.1001/jama.2019.21441. [DOI] [PubMed] [Google Scholar]
- 76. Ebner S, Smug LN, Kneifel W, Salminen SJ, Sanders ME. Probiotics in dietary guidelines and clinical recommendations outside the European Union. WJG. 2014;20:16095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Smug LN, Salminen S, Sanders ME, Ebner S. Yoghurt and probiotic bacteria in dietary guidelines of the member states of the European Union. Beneficial Microbes. 2014;5:61–6. [DOI] [PubMed] [Google Scholar]


