ABSTRACT
Background
The integration of time with dietary patterns throughout a day, or temporal dietary patterns (TDPs), have been linked with dietary quality but relations to health are unknown.
Objective
The association between TDPs and selected health status indicators and obesity, type 2 diabetes (T2D), and metabolic syndrome (MetS) was determined.
Methods
The first-day 24-h dietary recall from 1627 nonpregnant US adult participants aged 20–65 y from the NHANES 2003–2006 was used to determine timing, amount of energy intake, and sequence of eating occasions (EOs). Modified dynamic time warping (MDTW) and kernel k-means algorithm clustered participants into 4 groups representing distinct TDPs. Multivariate regression models determined associations between TDPs and health status, controlling for potential confounders, and adjusting for the survey design and multiple comparisons (P <0.05/6).
Results
A cluster representing a TDP with evenly spaced, energy balanced EOs reaching ≤1200 kcal between 06:00 to 10:00, 12:00 to 15:00, and 18:00 to 22:00, had statistically significant and clinically meaningful lower mean BMI (P <0.0001), waist circumference (WC) (P <0.0001), and 75% lower odds of obesity compared with 3 other clusters representing patterns with much higher peaks of energy: 1000–2400 kcal between 15:00 and 18:00 (OR: 5.3; 95% CI: 2.8, 10.1), 800–2400 kcal between 11:00 and 15:00 (OR: 4.4; 95% CI: 2.5, 7.9), and 1000–2600 kcal between 18:00 and 23:00 (OR: 6.7; 95% CI: 3.9, 11.6).
Conclusions
Individuals with a TDP characterized by evenly spaced, energy balanced EOs had significantly lower mean BMI, WC, and odds of obesity compared with the other patterns with higher energy intake peaks at different times throughout the day, providing evidence that incorporating time with other aspects of a dietary pattern may be important to health status.
Keywords: temporal, dietary patterns, body mass index, waist circumference, adults
Introduction
The prevalence of obesity has increased globally and represents a major public health concern. Obesity is both an outcome and a contributor to chronic disease development including type 2 diabetes (T2D) and metabolic syndrome (MetS) (1, 2). Behavioral habits like dietary intake are underlying modifiable risk factors for obesity and chronic disease (3, 4). Traditional investigation of the diet-health relation has focused on singular behaviors (e.g., breakfast skipping) (5–7) or aspects of dietary intake (e.g., individual nutrients) (8–10) in relation to health outcomes; however, numerous aspects of behavior and dietary components could interact to influence health (3). Dietary patterns refer to a way of conceptualizing multiple dietary exposures including the quantities, proportions, frequencies, and combinations of different foods and beverages in diets, as a multifaceted construct (3, 11). This multidimensional approach allows for a more inclusive examination of the diet-health relation that might reveal stronger associations between indicators of health and the role of diet compared with single nutrients or food group approaches (11, 12).
Temporality, or timing, of eating and the influence on health is a recent area of interest (13–17). Most of the accumulated evidence has evaluated timing of dietary intake in a classification-based way, e.g., characterizing participants as early energy consumers or later energy consumers based on the timing of the majority of their energy intake throughout the day followed by regression to determine links with health (18–21). Similar classification-based designation of breakfast skippers compared with those who eat breakfast suggests that breakfast skipping is associated with higher BMI and impaired glucose metabolism manifesting as higher fasting plasma glucose and hemoglobin A1c concentrations in adults (5, 6). Moreover, studies that examined the association of late-night eating with health reported a higher risk of obesity, MetS, and inflammation in late-night eaters compared with early eaters (18, 22–24). Collectively, these studies demonstrate that time of eating could be associated with health. However, the studies are limited by the focus on eating occasions (EOs) at a single time span or part of the day with disregard to EOs at other times of the day. Yet, the amount of energy or nutrient consumed at a certain time may affect the amount consumed at following EOs or be related to total energy intake throughout the day (25). Thus, understanding whether and how patterns of intake over a day, including the timing, amount of energy, and sequence of EOs, are linked with health status will advance knowledge of the importance of these multiple factors to health. Additionally, insight into whether and to what extent the integration of these factors determines health status may advance opportunities for early detection of behavioral patterns that predispose to obesity and chronic disease.
Data-driven methods including cluster and factor analyses and investigator-driven methods including index-based analysis (26, 27) have previously been used to determine dietary patterns and evaluate their association with health. However, few studies focused on dietary patterns have included multiple aspects of dietary intake over time. Eicher-Miller et al. uniquely created temporal dietary patterns (TDPs) by integrating time, amount of energy, and sequence of EOs through a 24-h day using a novel distance measure based on the dynamic time warping (DTW) technique combined with cluster analysis (28). This work showed that a TDP characterized by moderate and proportionally equivalent energy consumed at evenly spaced EOs throughout a day was associated with higher dietary quality among US adults aged 20–65 y compared with other TDPs (28). Considering the elevated disease risk associated with poor dietary quality, this finding supports hypotheses that TDPs may also be linked with health; however, this relation has not been examined. Thus, the aim of this study was to investigate whether TDPs determined using DTW and a kernel k-means clustering approach are associated with selected health status indicators and obesity, T2D, and MetS in adult men and women in the USA. The hypothesis, building on evidence by Eicher-Miller et al. (28), is that a TDP characterized by moderate and proportionally equivalent energy consumed during evenly spaced EOs will emerge and associate with improved health status compared with other TDPs not exhibiting these characteristics.
Methods
Participants and data collection
NHANES is a cross-sectional survey of the noninstitutionalized, civilian, US population that uses a complex, stratified, multistage probability cluster sampling method (29). The National Center for Health Statistics (NCHS), a program of the US CDC, administers NHANES. The NCHS Research Ethics Review Board granted approval and documented consent was obtained from all participants (30). The NHANES survey protocol includes an in-person household interview followed by a health examination in a mobile examination center. During the in-person household interview, sociodemographic data including age, sex, race/ethnicity, and poverty to income ratio (PIR) were collected using an in-depth questionnaire (29). The health examination included the collection of a 24-h dietary recall, anthropometric measurements, and laboratory tests.
Analytic sample
Four years of NHANES data, 2003–2006, were combined for this analysis. The analytic sample included nonpregnant US adults aged 20–65 y with reliable 24-h recall dietary data, and complete anthropometric and health status indicator data (n = 1627) (Supplemental Figure 1). Pregnant women, children, adolescents, and adults older than retirement age were excluded because their daily patterns may include variations characteristic to the life stages they represent.
Anthropometric assessment and laboratory tests
Selected health status indicators were chosen for their previous links with dietary components (31–35). Details of NHANES methods have been widely reported but are summarized briefly here. Weight was measured to the nearest 0.1 kg using a digital scale (36). Height and waist circumference (WC) were measured with a stadiometer and tape measure, respectively, to the nearest 0.1 cm (36). BMI was calculated as weight in kilograms divided by height in meters squared (37).
A phlebotomist obtained blood samples from participants according to a standardized protocol (38, 39). Fasting plasma glucose and triglycerides were assessed after participants fasted for 8 to 24 h. Fasting plasma glucose was measured using a hexokinase method with a Roche/Hitachi 911 (cycle 03–04) or a Roche Cobas Mira (cycle 2005–2006) (40, 41). Triglycerides were measured enzymatically (42, 43). Hemoglobin A1c, total cholesterol, and HDL cholesterol were based on samples taken regardless of fasting state. Hemoglobin A1c was measured with HPLC using Primus CLC 330 and Primus CLC 385 (Primus Corporation) in the 2003–2004 cycle and using Tosoh A1c 2.2 Plus Glycohemoglobin Analyzer (Tosoh Medics, Inc.) in the 2005–2006 cycle (44, 45). Total cholesterol was measured enzymatically. An instrument change occurred in NHANES 2005–2006 for total cholesterol, but the method and laboratory location were the same as in the 2003–2004 survey (46, 47). HDL cholesterol was analyzed using a direct immunoassay method from 2003–2006 (47, 48). There was a change in equipment to measure HDL cholesterol from 2005–2006, however, the laboratory method and location were the same as in 2003–2004 (47, 48). Blood pressure was measured using a mercury sphygmomanometer, with systolic and diastolic blood pressures determined based on ≤4 measures (49); if >1 measurement was obtained, the first was not considered, and the remaining measurements were averaged; otherwise, the first measurement was used.
Dietary data assessment
The first reliable 24-h dietary recall collected using the USDA's Automated Multiple-Pass Method (50) was used to determine energy intake, time of intake, and sequence of EOs throughout a day (51). A reliable dietary recall indicates that a participant has a food record that specifies each individual food consumed, the quantity in grams and nutrient amounts per food component (51). The USDA Food and Nutrient Database for Dietary Studies (FNDDS) for 2003–2004 data (USDA FNDDS, version 2.0) and 2005–2006 data (USDA FNDDS, version 3.0) were used to convert reported dietary intake information into gram amounts and to determine their energy values (kcal).
Assessment of energy misreporting
Energy misreporting was examined as research shows that misreporting could bias the relation between TDPs and adiposity (18, 52). Energy misreporting was assessed as the ratio of reported total energy intake to estimated energy requirement (EER) (53). EER was calculated using the Dietary Reference Intake equations for adults based on sex, weight, height, and physical activity (PA) level (54). Using accelerometry data from 1 valid weekday revealed that participants in this sample spent most of their time (minutes/day) in sedentary behavior. Calculation of PA level was attempted using methods by Gerrior et al. (55), however, the method resulted in very high estimates of activity levels and thus tended to overestimate energy expenditure. Therefore, a low active PA level (≥1.4 to <1.6) was used which conforms with national data showing that most adults spend their time in sedentary behavior or light activity (56, 57).
TDPs
A detailed description of the methodology used to determine the TDPs has been previously described (58) with 1 minor change in this study in which patterns were developed based on absolute amount of energy intake rather than fractional energy intake data computed over a 24-h period. Briefly, 1 24-h dietary recall was used to develop time series of length 24, with each entry representing absolute amount of energy during an hourly time interval. The absolute energy and hourly time stamps of nonzero EOs were extracted from the time series to form the compact representation as defined in (58, 59). Based on our previous work on pattern dietary intake, several distance measures were investigated including the constrained DTW with Sakoe-Chiba band (CDTW) and the modified dynamic time warping (MDTW) (60). Both CDTW and MDTW belong to the elastic distance family and find the optimal matching path among EOs in 2 time series (60). The matching is “optimal” in the sense that the summed difference between matched EOs is minimized. The Sakoe-Chiba band in CDTW and the weight parameter β in MDTW are controlling parameters to avoid pathological matchings (e.g., matching morning to evening EOs). Although the Sakoe-Chiba band rigorously limits the maximum time difference between matched EOs, the weight parameter β controls the matching through a time difference penalty term: larger β indicates more penalty on matching EOs that are different in time. Bands ranging between 60 and 720 min (60-min increments) and β ranging from 0 to 10 (2 increments) were explored in this project, and parameter values outside of these ranges were omitted as they did not bring significant changes in the clustering results. Further, the distance measures were coupled with the kernel k-means algorithm (61) to partition the time series into several clusters such that EOs are more similar within the same cluster and more dissimilar among different clusters. Cluster number k = 4 was selected to divide the population into clusters representing similar TDPs to maintain consistency with the previous development of temporal patterning (28, 58, 60). MDTW β=10 was selected out of each distance measure pairing of CDTW bandwidth = 420 and MDTW β=10 with k-means clustering, based on inferential analyses with health status indicators prioritized as: 1) most significant differences between the 6 pairwise comparisons, 2) highest model R2 values, and 3) largest difference between highest and lowest mean of health status indicators.
Statistical analysis
The Rao Scott F adjusted chi-square statistic determined significant differences among clusters by characteristics: survey year (2003–2004 and 2005–2006), sex (male or female), race/ethnicity (Mexican American and other Hispanic, Non-Hispanic white, Non-Hispanic black, and other-race including multirace), age groups (20–34, 35–49, and 50–65 y), PIR, and BMI classified as underweight (<18.5 kg/m2), normal weight (18.5–24.9), overweight (25.0–29.9), and obese (≥30.0) (37). PIR, calculated as reported household income divided by the federal poverty guideline for household income, was divided into 6 categories: 0–0.99, 1–1.99, 2–2.99, 3–3.99, 4–4.99, and ≥5. Ratios <1 indicate a PIR below the officially defined poverty threshold (62).
Disease categories included obesity, T2D, and MetS. The T2D definition was based on elevated fasting plasma glucose (≥126 mg/dL), hemoglobin A1c (≥6.5%), or self-report of: “yes” in response to the question “have you ever been told by a doctor you have diabetes?”, or to the use of glucose-lowering medications (63). The National Cholesterol Education Program Adult Treatment Panel III definition of MetS was applied to classify this condition based on the presence of 3 or more of the following risk factors: 1) WC (>102 cm for males, >88 cm for females); 2) triglycerides (>150 mg/dL) or taking antihyperlipidemic medications; 3) HDL cholesterol (<40 mg/dL in males, <50 mg/dL in females); 4) hypertension (>130/>85 mmHg) or taking antihypertensive medications; and 5) impaired fasting glucose (>110 mg/dL) or taking glucose-lowering medications (64).
ANOVA determined differences in mean of health status indicators by TDPs. ANOVA model assumptions were met for all models except for hemoglobin A1c, fasting plasma glucose, and systolic blood pressure, where an additional nonparametric Kruskal–Wallis test was used; the nonsignificant P value results aligned with those of ANOVA which are consistently featured. Multiple linear regression models determined associations between 4 TDPs and health status indicators. For risk of obesity, T2D, and MetS, multivariate logistic regression was used to estimate ORs comparing the 4 TDPs. For both linear and logistic models, potential confounders included survey year, sex, age group, race/ethnicity, PIR, energy misreporting [energy intake (EI):EER] and BMI (except for models with BMI, WC, and obesity as the outcome). The EI:EER ratio was used as a continuous covariate in the analyses based on methods by Murakami and Livingstone as this technique has been shown to result in similar findings when compared with excluding implausible reporters while avoiding selection bias (53, 65). Appropriate survey weights were constructed for the 2003–2006 survey years as directed by the NCHS (66). Sampling weights were rescaled so that the sum of the weights matched the survey population at the midpoint of the 4 y covering 2003–2006. Adjustment for the complex survey design including clustering and stratification was completed following NCHS guidelines (67). Comparisons between groups were considered statistically significant when P <0.05/6 (Tukey–Kramer type adjustment for multiple comparisons). Analyses were completed using SAS Survey procedures and inferential analysis version 9.4.
Visualization
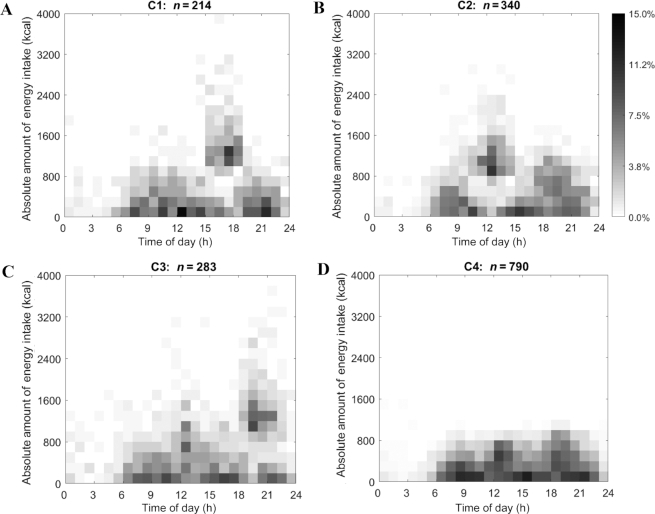
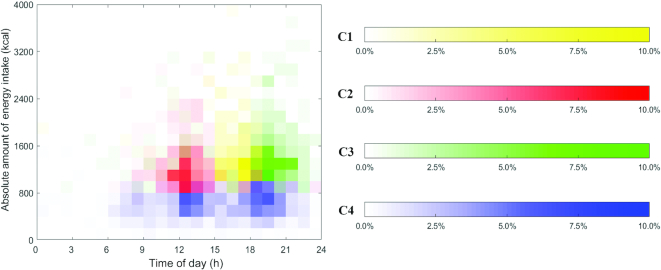
The visualization (Figure 1) illustrates the distribution of nonzero EOs in each cluster using heat maps. Each EO in the heat map is marked by its time stamp (x-axis) and absolute amount of energy intake (y-axis). Time ranged from 00:00 to 24:00 the next day with absolute energy intake ranging from 0 kcal to 4000 kcal at a particular time. The proportion of individuals reporting EOs (certain absolute energy intake and time stamp) is indicated through shading and ranged from 0.0% to 15.0% of each cluster. Darker shading signifies that a greater proportion of that particular cluster reported that specific energy intake at that specific time. Figure 1 exhibits 4 distinct TDPs of energy intake. Figure 2 adds color to differentiate the 4 clusters.
FIGURE 1.
Heat maps for MDTW clusters (A–D) which depict the absolute amount of energy intake ranging from 0 kcal to 4000 kcal (y-axis) for US adults aged 20–65 y as drawn from NHANES 2003–2006 over a 24-h day from time 00:00 to time 24:00 the next day (x-axis). The proportion of the sample is indicated by the inverse gray-scale legend with 0.0% of the cluster participants to 15.0% of the cluster participants. MDTW, modified dynamic time warping.
FIGURE 2.
Heat maps for MDTW clusters (C1–C4) which depict the distribution of the largest EO within each cluster for US adults aged 20–65 y as drawn from NHANES 2003–2006. The absolute amount of energy intake for each participant in the cluster ranged from 0 kcal to 4000 kcal (y-axis) over a 24-h d from 00:00 to 24:00 the next day (x-axis). The proportion of the sample is indicated by the inverse color-scale legend with 0.0% of the cluster participants to 10.0% of the cluster participants. EO, eating occasion; MDTW, modified dynamic time warping.
Results
Characteristics of participants in the 4 clusters representing TDPs are shown in Table 1. The number of participants was similar between Clusters 1, 2, and 3, though Cluster 4, characterized by evenly spaced energy balanced EOs, included the highest number of participants, ∼2 times the total number in the other clusters. Significant differences were present among clusters by sex (P <0.0001), age (P = 0.001), and BMI (P = 0.03), but not by survey year, race/ethnicity, or PIR. Compared with the other 3 clusters, Cluster 4 had a proportionally greater representation of females compared with males (64.7% compared with 35.3%). Cluster 4 included a higher proportion of ages 50–65 y (44.6%) compared with the other age groups, specifically 20–34 y (23.5%) and 35–49 y (31.9%). In respect to BMI, normal weight was more heavily represented in Cluster 4 (30.8%) compared with the other clusters (22.9–28.3%), whereas the obese category was prominent in Clusters 2 and 3 (38.5% and 39.2%, respectively) compared with Clusters 1 and 4 (34.6% and 33.2%, respectively).
TABLE 1.
Characteristics of clusters representing temporal dietary patterns of US adults aged 20–65 y as drawn from the NHANES 2003–2006 (n = 1627)1
| Characteristic | Total (n) | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | P value2 |
|---|---|---|---|---|---|---|
| Total | 1627 | 214 (13.2) | 340 (20.9) | 283 (17.4) | 790 (48.5) | |
| Survey year | 0.66 | |||||
| 2003–2004 | 804 | 116 (54.2) | 170 (50.0) | 134 (47.3) | 384 (48.6) | |
| 2005–2006 | 823 | 98 (45.8) | 170 (50.0) | 149 (52.7) | 406 (51.4) | |
| Sex | <0.0001 | |||||
| Male | 839 | 134 (62.6) | 216 (63.5) | 210 (74.2) | 279 (35.3) | |
| Female | 788 | 80 (37.4) | 124 (36.5) | 73 (25.8) | 511 (64.7) | |
| Race/ethnicity | 0.35 | |||||
| Mexican American | 350 | 56 (26.2) | 84 (24.7) | 47 (16.6) | 163 (20.6) | |
| Other Hispanic | 46 | 4 (1.9) | 10 (2.9) | 3 (1.1) | 29 (3.7) | |
| Non-Hispanic white | 826 | 112 (52.3) | 168 (49.4) | 157 (55.5) | 389 (49.2) | |
| Non-Hispanic black | 331 | 33 (15.4) | 61 (17.9) | 66 (23.3) | 171 (21.6) | |
| Other | 74 | 9 (4.2) | 17 (5.0) | 10 (3.5) | 38 (4.8) | |
| Age group, y | 0.001 | |||||
| 20–34 | 458 | 72 (33.6) | 99 (29.1) | 101 (35.7) | 186 (23.5) | |
| 35–49 | 548 | 67 (31.3) | 130 (38.2) | 99 (35.0) | 252 (31.9) | |
| 50–65 | 621 | 75 (35.0) | 111 (32.6) | 83 (29.3) | 352 (44.6) | |
| PIR | 0.98 | |||||
| 0–0.99 | 219 | 28 (13.1) | 43 (12.6) | 40 (14.1) | 108 (13.7) | |
| 1.00–1.99 | 352 | 51 (23.8) | 76 (22.4) | 60 (21.2) | 165 (20.9) | |
| 2.00–2.99 | 221 | 27 (12.6) | 41 (12.1) | 36 (12.7) | 117 (14.8) | |
| 3.00–3.99 | 254 | 34 (15.9) | 59 (17.4) | 44 (15.5) | 117 (14.8) | |
| 4.00–4.99 | 152 | 21 (9.8) | 32 (9.4) | 23 (8.1) | 76 (9.6) | |
| ≥5.00 | 370 | 46 (21.5) | 80 (23.5) | 69 (1.6) | 175 (22.2) | |
| BMI3 | 0.03 | |||||
| Underweight | 20 | 3 (1.4) | 3 (0.9) | 4 (1.4) | 10 (1.3) | |
| Normal weight | 466 | 49 (22.9) | 94 (27.6) | 80 (28.3) | 243 (30.8) | |
| Overweight | 562 | 88 (41.1) | 112 (32.9) | 88 (31.1) | 274 (34.7) | |
| Obese | 579 | 74 (34.6) | 131 (38.5) | 111 (39.2) | 263 (33.2) |
n(%); total numbers do not always add up to sample size due to missing values.
Rao Scott F adjusted χ2P value is a goodness-of-fit, 1-sided test; statistical significance is indicated when P <0.05.
BMI categories were defined per the WHO (37).
PIR, poverty to income ratio.
Characteristics of TDPs
Compared with the other 3 clusters, the absolute amount of energy intake in Cluster 4 was moderate, reaching ≤1200 kcal for each of the 3 main EOs throughout the day from 06:00 to 23:00 with a greater proportion (∼10%) of the cluster engaging in EOs from 06:00 to 10:00, 12:00 to 15:00, and 18:00 to 22:00 (Figure 1 and Table 2). In contrast, the other 3 clusters revealed patterns with 1 distinct peak in absolute amount of energy intake. For instance, participants in Cluster 1 consumed a lower amount of energy (reaching ≤1200 kcal) at earlier hours of the day between 07:00 and 13:00, compared to a peak in intake between 15:00 and 18:00 with a higher proportion of the cluster (∼12%) consuming between 1000 and 2400 kcal. Energy intake tended to be lower towards later hours of the day and reached up to 1000 kcal between 19:00 and 23:00. Participants in Cluster 2 had a lower energy intake between 06:00 and 10:00, reaching ≤1000 kcal, compared with a peak reaching ≤2400 kcal from 11:00 to 15:00 (a higher proportion of the cluster, ∼10%, consumed energy ranging between 800 and 1600 kcal), followed by intake reaching ≤1400 kcal between 17:00 and 22:00. Finally, Cluster 3 exhibited a spread-out pattern in regards to amount of energy consumed with energy intake reaching ≤1400 kcal between 07:00 and 13:00 and a much higher energy intake ranging between 1000 and 2600 kcal towards later hours of the day between 18:00 and 23:00 (a higher proportion of the cluster, ∼8–10%, consumed energy between 1000 and 1600 kcal). Figure 2 represents the distribution of the largest EO for each cluster and confirms patterns observed in Figure 1 in which Clusters 1, 2, and 3 exhibited distinct peaks in energy intake at different times of the day, whereas Cluster 4 displayed energy balanced EOs with no distinct peaks.
TABLE 2.
Qualitative description of clusters representing temporal dietary patterns of US adults aged 20–65 y as drawn from the NHANES 2003–2006 (n = 1627)
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | |
|---|---|---|---|---|
| n (%) | 214 (13.2) | 340 (20.9) | 283 (17.4) | 790 (48.6) |
| Overall temporal pattern | Lower energy intake during earlier (07:00–13:00) and later (19:00–23:00) hours of the day with absolute energy reaching ≤ ∼1200 and 1000 kcal, respectively | Lower energy intake during later (17:00–22:00) hours of the day with absolute energy reaching ≤1400 kcal | Lower energy intake during earlier hours (07:00–13:00) with absolute energy reaching ≤1400 kcal | Moderate and consistent energy intake throughout the day |
| Time of peak in energy intake | 15:00–18:00 | 11:00–15:00 | 18:00–23:00 | No peaks |
| Range of absolute energy at peak energy intake occasion (kcal) | 1000–2400 | 800–2400 | 1000–2600 | Does not exceed 1200 |
Association of TDPs with adiposity and chronic disease
Significant differences in mean BMI were present between Clusters 3 and 4 in the unadjusted model (P <0.05; Supplemental Table 1). Significant differences in mean WC and odds of obesity relative to normal weight status were present between Clusters 1 and 4, 2 and 4, and 3 and 4 in the unadjusted model (P <0.01 and P <0.05, respectively; Supplemental Tables 2and3). Analysis to examine the dependence of BMI, WC, and odds of obesity relative to normal weight status on cluster in the adjusted models indicated significant differences between Clusters 1 and 4, 2 and 4, and 3 and 4 (all P <0.0001), whereas there were no significant differences in all other cluster comparisons (Tables 3–5). The differences in mean BMI, mean WC, and odds of obesity were greatest between Clusters 3 and 4 (BMI: β=4.8 ± 0.4, WC: β=12.7 ± 1.2 cm, obesity OR: 6.7; 95% CI: 3.9, 11.6), similar to the results of the unadjusted model (BMI: β=1.1 ± 0.3, WC: β=5.9 ± 0.9 cm, obesity OR: 1.7; 95% CI: 1.2, 2.4; Supplemental Tables 1–3).
TABLE 3.
Mean BMI (kg/m2) and covariate-adjusted regression model results for clusters representing temporal dietary patterns of US adults aged 20–65 y as drawn from the NHANES 2003–20061
| Adjusted models2 | n (%) | BMI3 (kg/m2) | β4 ± SEE (95% CI) compared to Cluster 2 | β4 ± SEE (95% CI) compared to Cluster 3 | β4 ± SEE (95% CI) compared to Cluster 4 |
|---|---|---|---|---|---|
| Cluster 1 | 214 (13.2) | 29.1 ± 6.3 | 0.2 ± 0.5 (–1.3, 1.6) | –0.9 ± 0.6 (–2.4, 0.7) | 4.0 ± 0.6 (2.3, 5.6*) |
| Cluster 2 | 340 (20.9) | 29.1 ± 6.3 | –1.0 ± 0.5 (–2.4, 0.4) | 3.8 ± 0.6 (2.2, 5.3*) | |
| Cluster 3 | 283 (17.4) | 29.2 ± 6.4 | 4.8 ± 0.4 (3.7, 6.0*) | ||
| Cluster 4 | 790 (48.6) | 28.4 ± 6.2 |
Differences in mean BMI were present among Clusters 3 and 4 at P <0.05 in the unadjusted model (Supplemental Table 1).
Models were adjusted for survey year, sex, age, race/ethnicity, poverty to income ratio, and energy misreporting (EI:EER).
Values are mean ± SD.
ß represents the difference between mean BMI of cluster and reference cluster. Differences in mean BMI are different than those between raw means because they represent differences in least square means.
Significance level: *P <0.0001
EER, estimated energy requirement; EI, energy intake; SEE, standard error of the estimate.
TABLE 5.
OR of obesity relative to normal weight status and covariate-adjusted regression model results for clusters representing temporal dietary patterns of US adults aged 20–65 y as drawn from the NHANES 2003–20061
| Adjusted models2 | n (%) | OR3,4 (95% CI) compared to Cluster 2 | OR3,4 (95% CI) compared to Cluster 3 | OR3,4 (95% CI) compared to Cluster 4 |
|---|---|---|---|---|
| Cluster 1 | 214 (13.2) | 1.2 (0.7, 2.2) | 0.8 (0.4, 1.5) | 5.3 (2.8, 10.1*) |
| Cluster 2 | 340 (20.9) | 0.7 (0.4, 1.2) | 4.4 (2.5, 7.9*) | |
| Cluster 3 | 283 (17.4) | 6.7 (3.9, 11.6*) | ||
| Cluster 4 | 790 (48.6) |
Differences among clusters in OR of obesity in the unadjusted model were similar to those in the adjusted model at P <0.05 (Supplemental Table 3).
Models were adjusted for survey year, sex, age, race/ethnicity, poverty to income ratio, and energy misreporting (EI:EER).
OR represents OR of obesity of cluster and reference cluster.
Obesity defined as BMI ≥30 kg/m2 (37).
Significance level: *P <0.0001.
EER, estimated energy requirement; EI, energy intake.
TABLE 4.
Mean WC (cm) and covariate-adjusted regression model results for clusters representing temporal dietary patterns of US adults aged 20–65 y as drawn from the NHANES 2003–20061
| Adjusted models2 | n (%) | WC (cm)3 | β4 ± SEE (95% CI) compared to Cluster 2 | β4 ± SEE (95% CI) compared to Cluster 3 | β4 ± SEE (95% CI) compared to Cluster 4 |
|---|---|---|---|---|---|
| Cluster 1 | 214 (13.2) | 99.4 ± 15.4 | 0.4 ± 1.5 (–3.5, 4.4) | –2.5 ± 1.4 (–6.4, 1.4) | 10.2 ± 1.5 (6.2, 14.3*) |
| Cluster 2 | 340 (20.9) | 99.5 ± 15.1 | –2.9 ± 1.4 (–6.6, 0.9) | 9.8 ± 1.4 (6.1, 13.5*) | |
| Cluster 3 | 283 (17.4) | 100.2 ± 15.9 | 12.7 ± 1.2 (9.5, 15.8*) | ||
| Cluster 4 | 790 (48.6) | 96.1 ± 15.1 |
Differences among clusters in mean WC in the unadjusted model were similar to those in the adjusted model at P <0.01 (Supplemental Table 2).
Models were adjusted for survey year, sex, age, race/ethnicity, poverty to income ratio, and energy misreporting (EI:EER).
Values are mean ± SD.
ß represents the difference between mean WC of cluster and reference cluster. Differences in mean WC are different than those between raw means because they represent differences in least square means.
Significance level: *P <0.0001
EER, estimated energy requirement; EI, energy intake; SEE, standard error of the estimate; WC, waist circumference.
Regarding the other health status indicators, T2D, and MetS investigated, there were 3 significant differences in mean HDL cholesterol between Clusters 1 and 3, 2 and 4, and 3 and 4 (P <0.05) in the unadjusted model (Supplemental Table 4), however, one significant difference was observed in the adjusted model between Clusters 1 and 2 (Supplemental Table 5). There was also 1 significant difference in mean total cholesterol between Clusters 1 and 3 (P <0.05) in the unadjusted model (Supplemental Table 6), but this difference was not observed in the adjusted model (Supplemental Table 7). Moreover, there were no significant differences amongst clusters in any of the other examined health status indicators, T2D, and MetS in both unadjusted and adjusted models (Supplemental Tables 8-21).
Discussion
TDPs generated from 1 24-h recall are associated with BMI, WC, and obesity but not with any of the other health status indicators or diseases investigated. To our knowledge, this is the first study to assess the association of TDPs based on timing, amount, and sequence of EOs throughout a 24-h period with health in an adult US population, while adjusting for potential confounders including energy misreporting. The mean differences in BMI and WC associated with TDPs were both statistically significant and clinically meaningful, implicating a potential relevance to disease management and clinical application (68–70). Thus, observed mean differences in these health status indicators may suggest that TDPs could be an important health exposure that requires further exploration. Reverse causation in the observed associations cannot be ruled out using the cross-sectional study design, nevertheless, the aim of this study was not to establish causation but to investigate whether developed TDPs using a novel methodology meaningfully link to health regardless of the direction of this association. A few studies have assessed the temporal patterning of energy intake in adults throughout the day (28, 71). Using the latent class analysis approach, Leech et al. found a “conventional” pattern defined by evenly spaced meals and snacks consumed at conventional times in Australia, similar to Cluster 4 found in this study, to be associated with lower odds of overweight or obesity and central overweight or obesity in women compared with another pattern characterized by a higher eating frequency (71). Moreover, findings from the current study support previous work which revealed that a TDP characterized by 3 evenly spaced, energy balanced EOs throughout the day was linked with improved dietary quality (28, 60).
The findings of a significantly lower mean BMI and WC and odds of obesity relative to normal weight status in Cluster 4 compared with all other clusters indicates that a pattern with evenly spaced energy balanced EOs consumed throughout a day may be more advantageous to health compared to patterns with 1 distinct peak in absolute amount of energy intake. Regular intervals of energy intake throughout the day have a positive impact on risk factors for diabetes mellitus and heart disease (15). In fact, irregular patterns of total energy intake, i.e., with intake limited to 1 portion of the day or continuously throughout the day, seem to be less advantageous for the maintenance of body weight and optimum cardiometabolic health compared with a more intentional eating strategy which entails eating at planned intervals to distribute total energy intake during the day (15). Other than having regular EOs of moderate energy, intermittent fasting, which involves cycling between periods of fasting (or reduced energy intake) and eating over a given period, has gained considerable attention with reported benefits including weight loss and improved metabolic markers (72, 73). Time-restricted feeding, a modified intermittent fasting protocol, allows ad libitum food intake within specific time frames (from 3–4 h to 10–12 h) with an extended fasting period (12–21 h) per day (74). In the current study, none of the patterns coincided with these criteria as all 4 TDPs revealed EOs starting from 6:00 to 23:00 (17 h of feeding) with no indication of a prolonged fasting period. Nevertheless, it is important to note that cluster descriptions characterize the group and do not represent individuals.
Furthermore, compared with the evenly spaced energy balanced pattern of Cluster 4, both Clusters 2 and 3 had a significantly greater mean BMI, mean WC, and odds of obesity relative to normal weight status. The observed differences between these means was smaller between Clusters 4 and 2, exhibiting a pattern of higher energy intake occurring at earlier hours of the day (11:00 to 15:00), than between Clusters 4 and 3, exhibiting a pattern of higher energy intake occurring towards the later hours of the day (18:00 to 23:00). Models controlled for total energy intake; thus, findings may indicate that observed differences may be explained by temporal differences in these patterns. Evidence from epidemiologic studies suggests a positive association between evening meal consumption and obesity. For example, in a study of 1245 middle-aged adults, consuming greater energy at dinner (≥48%) compared with <33% or 33–48%, was associated with a 2.33-fold greater odds of developing obesity (75). Findings from this analysis also revealed a greater magnitude of difference in mean BMI and WC, and odds of obesity in a pattern with later meal intake; yet, instead of assessing the timing of a single meal or energy intake across stratified time spans, this study examined TDPs based on a novel data-driven approach which integrates several aspects of dietary patterns.
The finding of no significant differences in examined health status indicators among Clusters 1, 2, and 3 was unexpected. These clusters were similar in terms of number of EOs, however, they differed in the timing of the highest energy intake occasion: Cluster 1 (15:00 to 18:00), Cluster 2 (11:00 to 15:00), and Cluster 3 (18:00 to 23:00). Notably, the association between evening meal intake and measures of adiposity remains inconclusive (76); specifically, some observational studies showed a positive association between evening meal intake and weight, BMI, and/or odds of overweight (18, 77, 78), whereas others found no association (79–81), which may help explain the findings of this study.
Interestingly, TDPs were associated with long-term markers of health including BMI and WC, whereas no significant associations were found between patterns and other examined health indicators including serum biomarkers, especially fasting plasma glucose and triglycerides, which may more closely reflect dietary intake reported in the 24-h recalls. Leech et al. reported a “later lunch” temporal eating pattern characterized by a later lunch EO (between 13:00 to 14:00) to be associated with higher systolic and diastolic blood pressures compared with a “conventional” pattern (lunch at 12:00) in women (82); however, no such associations were found between TDPs and blood pressure in the current study. These results may be an artifact of laboratory procedures or may be explained by large intra- and interindividual variability in serum biomarkers and blood pressure compared with BMI and WC. Otherwise, findings may indicate that TDPs more strongly associate with long-term health status indicators; however, more research is needed to further elucidate these findings.
Sociodemographic characteristics such as those included in this study (Table 1) have been shown to be associated with diet-related differences in health. Limited studies have examined how energy distribution throughout the day may differ between population groups and results suggest potential differences by sociodemographic factors (83). For instance, females have been reported to be generally more regulated in their eating patterns compared with males (84). Striegel-Moore et al. found that males are more likely than females to engage in night eating (85), which is consistent with the higher proportion of males in Cluster 3 with the latest meal intake occasion (18:00 to 23:00) compared with Cluster 4 characterized by evenly spaced energy balanced EOs. Moreover, Cluster 4 also included a higher observed proportion of the age group 50–65 y compared with all other clusters; a regular meal pattern has been more commonly observed in older adults compared with young adults, where the latter group has been described as having a more “de-synchronized” eating pattern (83, 84).
Daily dietary patterns that may be associated with behavioral factors that were outside of the scope of this study include exercise and sleep timing. As such, insight into how these behavioral components interact within a day, and overall, as part of a lifestyle pattern may unfold stronger associations with health compared to when they are considered separately. Such data has become more available through the use of technology-assisted assessment tools that target dietary and activity patterns and could be potentially integrated to determine whether or how timing of these behaviors interact in relation with health. Moreover, the use of traditional nutrition epidemiology analysis along with data-driven methods to integrate time into these behavioral patterns holds promise to explore how these temporal patterns link to health and with further development, this evidence may provide insight to inform population-level dietary and PA guideline recommendations.
The strengths of the current analyses include the use of a data-driven approach that integrates amount, time of eating, and sequence of EOs throughout the day for the development of TDPs. Additionally, this approach avoids between-subject variation that participants may have in regard to EO definitions. Limitations of this study include the cross-sectional nature, which provides a snapshot of the participants’ dietary intake and cannot demonstrate causation. Also, the sample size is small and represents ∼8% of the original sample of participants included in survey years 2003–2006; therefore, study results should be interpreted with caution. Notably, sample size attrition is mostly attributable to the selected age range 20–65 y and the inclusion of health status indicators examined in a fasting subsample of participants (both criteria resulted in loss of ∼84% of the original sample). Moreover, patterns were developed based on 1 24-h dietary recall; however, the inclusion of a second recall would have further limited our sample size; also, since information regarding the distribution of timing of dietary patterns over multiple days is unknown, exploration of the time, amount, and sequence of dietary intake over multiple days represents a research gap for future study. Furthermore, in this sample, ∼60% of recalls were collected on a weekday and since different TDPs may emerge on weekends, future studies should consider this investigation.
This article demonstrates that TDPs are associated with differences in BMI, WC, and obesity. Individuals with a TDP characterized by evenly spaced, energy balanced EOs exhibited significantly lower mean BMI, mean WC, and odds of obesity relative to normal weight status compared with the other 3 patterns characterized by distinct peaks in energy intake at different times throughout the day. The incorporation of time to the concept of dietary patterns including amount and sequence of EOs may be important to determine links with health and could provide insight into the detection of behavioral patterns that predispose to obesity and chronic disease.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—HAE-M, ED, SG, AB, EAR, EH, MA, JG, and LL: designed the research; MA, JG, and LL: analyzed data; MA: wrote the original manuscript. HAE-M, EAR, EH, SG, AB, ED, and JG: contributed to the revision of the manuscript; and all authors: read and approved the final manuscript.
Notes
This research was supported by the National Cancer Institute of the NIH under award number R21CA224764 and Purdue University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Prior to issuing a press release concerning the outcomes of this research, please notify the NIH awarding Institute/Center in advance to allow for coordination.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figure 1 and Supplemental Tables 1–21 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: CDTW, constrained with Sakoe-Chiba band dynamic time warping; DTW, dynamic time warping; EER, estimated energy requirement; EI, energy intake; EO, eating occasion; FNDDS, Food and Nutrient Database for Dietary Studies; MDTW, modified dynamic time warping; MetS, metabolic syndrome; NCHS, National Center for Health Statistics; PA, physical activity; PIR, poverty to income ratio; TDP, temporal dietary pattern; T2D, type 2 diabetes; WC, waist circumference.
Contributor Information
Marah M Aqeel, Department of Nutrition Science, Purdue University, West Lafayette, IN, USA.
Jiaqi Guo, School of Electrical and Computer Engineering, Purdue University, West Lafayette, IN, USA.
Luotao Lin, Department of Nutrition Science, Purdue University, West Lafayette, IN, USA.
Saul B Gelfand, School of Electrical and Computer Engineering, Purdue University, West Lafayette, IN, USA.
Edward J Delp, School of Electrical and Computer Engineering, Purdue University, West Lafayette, IN, USA.
Anindya Bhadra, Department of Statistics, Purdue University, West Lafayette, IN, USA.
Elizabeth A Richards, School of Nursing, Purdue University, West Lafayette, IN, USA.
Erin Hennessy, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Heather A Eicher-Miller, Department of Nutrition Science, Purdue University, West Lafayette, IN, USA.
References
- 1. Nguyen NT, Nguyen X-MT, Lane J, Wang P. Relationship between obesity and diabetes in a US adult population: findings from the National Health and Nutrition Examination Survey, 1999–2006. Obes Surg. 2011;21:351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Després J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. [DOI] [PubMed] [Google Scholar]
- 3. Dietary Guidelines Advisory Committee Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Washington (DC): USDA, Agricultural Research Service; 2015. [Google Scholar]
- 4. Klein S, Sheard NF, Pi-Sunyer X, Daly A, Wylie-Rosett J, Kulkarni K, Clark NG. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. Diabetes Care. 2004;27:2067–73. [DOI] [PubMed] [Google Scholar]
- 5. Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL. The relationship between breakfast skipping, chronotype, and glycemic control in type 2 diabetes. Chronobiol Int. 2014;31:64–71. [DOI] [PubMed] [Google Scholar]
- 6. Kollannoor-Samuel G, Chhabra J, Fernandez ML, Vega-López S, Pérez SS, Damio G, Calle MC, D'Agostino D, Pérez-Escamilla R. Determinants of fasting plasma glucose and glycosylated hemoglobin among low income Latinos with poorly controlled type 2 diabetes. J Immigr Minor Health. 2011;13:809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deshmukh-Taskar P, Nicklas TA, Radcliffe JD, O'Neil CE, Liu Y. The relationship of breakfast skipping and type of breakfast consumed with overweight/obesity, abdominal obesity, other cardiometabolic risk factors and the metabolic syndrome in young adults. The National Health and Nutrition Examination Survey (NHANES): 1999–2006. Public Health Nutr. 2013;16:2073–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Dam RM, Seidell JC. Carbohydrate intake and obesity. Eur J Clin Nutr. 2007;61:S75–99. [DOI] [PubMed] [Google Scholar]
- 9. Viuda-Martos M, López-Marcos MC, Fernández-López J, Sendra E, López-Vargas JH, Pérez-Álvarez JA. Role of fiber in cardiovascular diseases: a review. Compr Rev Food Sci Food Saf. 2010;9:240–58. [Google Scholar]
- 10. Muldowney S, Kiely M. Vitamin D and cardiometabolic health: a review of the evidence. Nutr Res Rev. 2011;24:1–20. [DOI] [PubMed] [Google Scholar]
- 11. Reedy J, Krebs-Smith SM, Hammond RA, Hennessy E. Advancing the science of dietary patterns research to leverage a complex systems approach. J Acad Nutr Diet. 2017;117:1019–22. [DOI] [PubMed] [Google Scholar]
- 12. Jacques PF, Tucker KL. Are dietary patterns useful for understanding the role of diet in chronic disease?. Am J Clin Nutr. 2001;73:1–2. [DOI] [PubMed] [Google Scholar]
- 13. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Almoosawi S, Vingeliene S, Karagounis LG, Pot GK. Chrono-nutrition: a review of current evidence from observational studies on global trends in time-of-day of energy intake and its association with obesity. Proc Nutr Soc. 2016;75:487–500. [DOI] [PubMed] [Google Scholar]
- 15. St-Onge M-P, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, Varady K. Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation. 2017;135:e96–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garaulet M, Gómez-Abellán P. Timing of food intake and obesity: a novel association. Physiol Behav. 2014;134:44–50. [DOI] [PubMed] [Google Scholar]
- 17. Beccuti G, Monagheddu C, Evangelista A, Ciccone G, Broglio F, Soldati L, Bo S. Timing of food intake: sounding the alarm about metabolic impairments? A systematic review. Pharmacol Res. 2017;125:132–41. [DOI] [PubMed] [Google Scholar]
- 18. Wang JB, Patterson RE, Ang A, Emond JA, Shetty N, Arab L. Timing of energy intake during the day is associated with the risk of obesity in adults. J Hum Nutr Diet. 2014;27:255–62. [DOI] [PubMed] [Google Scholar]
- 19. Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women: effect of high-calorie breakfast vs. dinner. Obesity. 2013;21:2504–12. [DOI] [PubMed] [Google Scholar]
- 20. Raynor HA, Li F, Cardoso C. Daily pattern of energy distribution and weight loss. Physiol Behav. 2018;192:167–72. [DOI] [PubMed] [Google Scholar]
- 21. Hermengildo Y, López-García E, García-Esquinas E, Pérez-Tasigchana RF, Rodríguez-Artalejo F, Guallar-Castillón P. Distribution of energy intake throughout the day and weight gain: a population-based cohort study in Spain. Br J Nutr. 2016;115:2003–10. [DOI] [PubMed] [Google Scholar]
- 22. Berg C, Lappas G, Wolk A, Strandhagen E, Torén K, Rosengren A, Thelle D, Lissner L. Eating patterns and portion size associated with obesity in a Swedish population. Appetite. 2009;52:21–6. [DOI] [PubMed] [Google Scholar]
- 23. Kutsuma A, Nakajima K, Suwa K. Potential association between breakfast skipping and concomitant late-night-dinner eating with metabolic syndrome and proteinuria in the Japanese population. Scientifica. 2014;2014:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marinac CR, Sears DD, Natarajan L, Gallo LC, Breen CI, Patterson RE. Frequency and circadian timing of eating may influence biomarkers of inflammation and insulin resistance associated with breast cancer risk. PLoS One. 2015;10:e0136240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Castro JM. The time of day and the proportions of macronutrients eaten are related to total daily food intake. Br J Nutr. 2007;98:1077–83. [DOI] [PubMed] [Google Scholar]
- 26. Reedy J, Wirfalt E, Flood A, Mitrou PN, Krebs-Smith SM, Kipnis V, Midthune D, Leitzmann M, Hollenbeck A, Schatzkin A et al. . Comparing 3 dietary pattern methods – cluster analysis, factor analysis, and index analysis – with colorectal cancer risk: The NIH-AARP Diet and Health Study. Am J Epidemiol. 2010;171:479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reedy J, Mitrou PN, Krebs-Smith SM, Wirfält E, Flood A, Kipnis V, Leitzmann M, Mouw T, Hollenbeck A, Schatzkin A et al. . Index-based dietary patterns and risk of colorectal cancer. Am J Epidemiol. 2008;168:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eicher-Miller HA, Khanna N, Boushey CJ, Gelfand SB, Delp EJ. Temporal dietary patterns derived among the adult participants of the National Health and Nutrition Examination Survey 1999–2004 are associated with diet quality. J Acad Nutr Diet. 2016;116:283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Centers for Disease Control and Prevention , National Health and Nutrition Examination Survey. About the National Health and Nutrition Examination Survey. [Internet]. [Accessed 15 Dec, 2019]. Available from: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. [Google Scholar]
- 30. National Center for Health Statistics, National Center for Health Statistics Research Ethics Review Board (ERB) Approval. [Internet]. [Accessed 25 Dec, 2019]. Available from: http://www.cdc.gov/nchs/nhanes/irba98.htm. [Google Scholar]
- 31. Parks EJ. Effect of dietary carbohydrate on triglyceride metabolism in humans. J Nutr. 2001;131:2772S–4S. [DOI] [PubMed] [Google Scholar]
- 32. Fock KM, Khoo J. Diet and exercise in management of obesity and overweight: diet and exercise for weight management. J Gastroenterol Hepatol. 2013;28:59–63. [DOI] [PubMed] [Google Scholar]
- 33. Rossi M, Negri E, Bosetti C, Dal Maso L, Talamini R, Giacosa A, Montella M, Franceschi S, La Vecchia C. Mediterranean diet in relation to body mass index and waist-to-hip ratio. Public Health Nutr. 2008;11:214–17. [DOI] [PubMed] [Google Scholar]
- 34. De Paula TP, Steemburgo T, de Almeida JC, Dall'Alba V, Gross JL, de Azevedo MJ. The role of Dietary Approaches to Stop Hypertension (DASH) diet food groups in blood pressure in type 2 diabetes. Br J Nutr. 2012;108:155–62. [DOI] [PubMed] [Google Scholar]
- 35. Zhou X, Xue H, Duan R, Liu Y, Zhang L, Harvey L, Cheng G. The cross-sectional association of energy intake and dietary energy density with body composition of children in Southwest China. Nutrients. 2015;7:5396–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McDowell MA, Fryar CD, Ogden CL, Flegal KM. Anthropometric Reference Data for Children and Adults: United States, 2003–2006. Hyattsville (MD): NCHS, National Health Statistics Reports; 2008. [PubMed] [Google Scholar]
- 37. World Health Organization, Body Mass Index-f [Internet]. [Accessed 10 Feb, 2020]. Available from: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi. [Google Scholar]
- 38. Centers for Disease Control and Prevention, National Center for Health Statistics NHANES 2003–2004 Procedures Manual. [Internet]. [Accessed 21 Dec, 2019]. Available from: https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Manuals.aspx?BeginYear=2003. [PubMed] [Google Scholar]
- 39. Centers for Disease Control and Prevention, National Center for Health Statistics NHANES 2005–2006 Procedures Manual. [Internet]. [Accessed 21 Dec, 2019]. Available from: https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Manuals.aspx?BeginYear=2005. [PubMed] [Google Scholar]
- 40. Centers for Disease Control and Prevention, National Center for Health Statistics NHANES 2003–2004 Data Documentation Laboratory Assessment: Plasma Fasting Glucose, Serum C-Peptide & Insulin (L10AM_C). [Internet]. [Accessed 28 Dec, 2019]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/L10AM_C.htm. [Google Scholar]
- 41. Centers of Disease Control and Prevention, National Center for Health Statistics NHANES 2005–2006 Data Documentation Laboratory Assessment: Plasma Fasting Glucose & Insulin (GLU_D). [Internet]. [Accessed 28 Dec, 2019]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/GLU_D.htm. [Google Scholar]
- 42. Centers for Disease Control and Prevention, National Center for Health Statistics NHANES 2003–2004 Data Documentation Laboratory Assessment: Cholesterol-LDL & Triglycerides (L13AM_C). [Internet]. [Accessed 28 Dec, 2019]. Available from:https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/L13AM_C.htm. [Google Scholar]
- 43. Centers for Disease Control and Prevention, National Center for Health Statistics NHANES 2005–2006 Data Documentation Laboratory Assessment: Cholesterol - LDL, Triglyceride & Apoliprotein (TRIGLY_D). [Internet]. [Accessed 28 Dec, 2019]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/TRIGLY_D.htm. [Google Scholar]
- 44. Centers for Disease Control and Prevention, National Center for Health Statistics NHANES 2003–2004 Data Documentation Laboratory Assessment: Glycohemoglobin (L10_C). [Internet]. [Accessed 29 Dec, 2019]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/L10_C.htm. [Google Scholar]
- 45. Centers for Disease Control and Prevention, National Center for Health Statistics NHANES 2005–2006 Data Documentation Laboratory Assessment: Glycohemoglobin (GHB_D). [Internet]. [Accessed 29 Dec, 2019]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/GHB_D.htm. [Google Scholar]
- 46. Centers for Disease Control and Prevention, National Center for Health Statistics NHANES 2005–2006 Data Documentation Laboratory Assessment: Total Cholesterol (TCHOL_D). [Internet]. [Accessed 21 Dec, 2019]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/TCHOL_D.htm. [Google Scholar]
- 47. Centers for Disease Control and Prevention, National Center for Health Statistics NHANES 2003–2004 Data Documentation Laboratory Assessment: Cholesterol - Total & HDL (l13_c). [Internet]. [Accessed 28 Dec, 2019]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/L13_C.htm. [Google Scholar]
- 48. Centers for Disease Control and Prevention, National Center for Health Statistics NHANES 2005–2006 Data Documentation Laboratory Assessment: HDL-cholesterol (HDL_C). [Internet]. [Accessed 25 Dec, 2019]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/HDL_D.htm. [Google Scholar]
- 49. Mellen PB, Gao SK, Vitolins MZ, Goff DC. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch Intern Med. 2008;168:308–14. [DOI] [PubMed] [Google Scholar]
- 50. Agricultural Research Service, U.S. Department of Agriculture USDA Automated Multiple-Pass Method. [Internet]. [Accessed 13 Jan, 2020]. Available from:http://www.ars.usda.gov/Services/docs.htm?docid=7710up. [Google Scholar]
- 51. Centers for Disease Control and Prevention, National Center for Health Statistics NHANES Dietary Data. [Internet]. [Accessed 20 Dec, 2019]. Available from: https://wwwn.cdc.gov/nchs/nhanes/Search/DataPage.aspx?Component=Dietary. [Google Scholar]
- 52. Leech RM, Worsley A, Timperio A, McNaughton SA. The role of energy intake and energy misreporting in the associations between eating patterns and adiposity. Eur J Clin Nutr. 2018;72:142–7. [DOI] [PubMed] [Google Scholar]
- 53. Murakami K, Livingstone MBE. Associations between meal and snack frequency and overweight and abdominal obesity in US children and adolescents from National Health and Nutrition Examination Survey (NHANES) 2003–2012. Br J Nutr. 2016;115:1819–29. [DOI] [PubMed] [Google Scholar]
- 54. Institute of Medicine Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. [Internet] Washington (DC): The National Academies Press; 2005. [Accessed 12 Feb, 2020]. Available from: 10.17226/10490. [DOI] [Google Scholar]
- 55. Gerrior S, Juan W, Basiotis P. An easy approach to calculating estimated energy requirements. Prev Chronic Dis. 2006;3:A129. [PMC free article] [PubMed] [Google Scholar]
- 56. Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, Mcdowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–8. [DOI] [PubMed] [Google Scholar]
- 57. Evenson KR, Wen F, Metzger JS, Herring AH. Physical activity and sedentary behavior patterns using accelerometry from a national sample of United States adults. Int J Behav Nutr Phys Act. 2015;12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Khanna N, Eicher-Miller HA, Boushey CJ, Gelfand SB, Delp EJ. Temporal Dietary Patterns Using Kernel k-Means Clustering. In: 2011 IEEE International Symposium on Multimedia. Dana Point (CA); 2011. p. 375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Khanna N, Eicher-Miller HA, Verma HK, Boushey CJ, Gelfand SB, Delp EJ. Modified dynamic time warping (MDTW) for estimating temporal dietary patterns. : 2017 IEEE Global Conference on Signal and Information Processing (GlobalSIP), Montreal (QC); 2017. p. 948–52. [Google Scholar]
- 60. Eicher-Miller HA, Gelfand S, Hwang Y, Delp E, Bhadra A, Guo J. Distance metrics optimized for clustering temporal dietary patterning among U.S. adults. Appetite. 2020;144:104451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dhillon IS, Guan Y, Kulis B. Kernel k-means, spectral clustering and normalized cuts. In: Proceedings of the tenth ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. Seattle (WA); 2004. p. 551–6. [Google Scholar]
- 62. United States Census Bureau How the Census Bureau Measures Poverty, Poverty Thresholds. [Internet]. [Accessed 22 Mar, 2020]. Available from: https://www.census.gov/topics/income-poverty/poverty/guidance/poverty-measures.html. [Google Scholar]
- 63. American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. [DOI] [PubMed] [Google Scholar]
- 64. Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–14. [DOI] [PubMed] [Google Scholar]
- 65. Jessri M, Lou WY, L'Abbé MR. Evaluation of different methods to handle misreporting in obesity research: evidence from the Canadian national nutrition survey. Br J Nutr. 2016;115:147–59. [DOI] [PubMed] [Google Scholar]
- 66. Centers for Disease Control and Prevention, National Center for Health Statistics Specifying Weighting Parameters. [Internet]. [Accessed 26 Dec, 2019]. Available from: https://www.cdc.gov/nchs/tutorials/nhanes/surveydesign/weighting/intro.htm. [Google Scholar]
- 67. Centers for Disease Control and Prevention, National Center for Health Statistics Survey Design Factors. [Internet]. [Accessed 15 Apr, 2020]. Available from: https://www.cdc.gov/nchs/tutorials/nhanes/surveydesign/sampledesign/intro.htm. [Google Scholar]
- 68. Bodegard J, Sundström J, Svennblad B, Östgren CJ, Nilsson PM, Johansson G. Changes in body mass index following newly diagnosed type 2 diabetes and risk of cardiovascular mortality: a cohort study of 8486 primary-care patients. Diabetes Metab. 2013;39:306–13. [DOI] [PubMed] [Google Scholar]
- 69. Mulligan AA, Lentjes MAH, Luben RN, Wareham NJ, Khaw K-T. Changes in waist circumference and risk of all-cause and CVD mortality: results from the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) cohort study. BMC Cardiovasc Disord. 2019;19:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cerhan JR, Moore SC, Jacobs EJ, Kitahara CM, Rosenberg PS, Adami H-O, Ebbert JO, English DR, Gapstur SM, Giles GG et al. . A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89:335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Leech RM, Timperio A, Livingstone KM, Worsley A, McNaughton SA. Temporal eating patterns: associations with nutrient intakes, diet quality, and measures of adiposity. Am J Clin Nutr. 2017;106:1121–30. [DOI] [PubMed] [Google Scholar]
- 72. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Patterson RE, Sears DD. Metabolic effects of intermittent fasting. Annu Rev Nutr. 2017;37:371–93. [DOI] [PubMed] [Google Scholar]
- 74. Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, Palma A, Gentil P, Neri M, Paoli A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bo S, Musso G, Beccuti G, Fadda M, Fedele D, Gambino R, Gentile L, Durazzo M, Ghigo E, Cassader M. Consuming more of daily caloric intake at dinner predisposes to obesity. A 6-year population-based prospective cohort study. PLoS One. 2014;9:e108467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fong M, Caterson ID, Madigan CD. Are large dinners associated with excess weight, and does eating a smaller dinner achieve greater weight loss? A systematic review and meta-analysis. Br J Nutr. 2017;118:616–28. [DOI] [PubMed] [Google Scholar]
- 77. Morse SA, Ciechanowski PS, Katon WJ, Hirsch IB. Isn't this just bedtime snacking? The potential adverse effects of night-eating symptoms on treatment adherence and outcomes in patients with diabetes. Diabetes Care. 2006;29:1800–4. [DOI] [PubMed] [Google Scholar]
- 78. Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity. 2011;19:1374–81. [DOI] [PubMed] [Google Scholar]
- 79. Almoosawi S, Prynne CJ, Hardy R, Stephen AM. Time-of-day and nutrient composition of eating occasions: prospective association with the metabolic syndrome in the 1946 British birth cohort. Int J Obes. 2013;37:725–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Aljuraiban GS, Chan Q, Oude Griep LM, Brown IJ, Daviglus ML, Stamler J, Van Horn L, Elliott P, Frost GS, INTERMAP Research Group . The impact of eating frequency and time of intake on nutrient quality and body mass index: the INTERMAP study, a population-based study. J Acad Nutr Diet. 2015;115:528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kant AK, Graubard BI. Secular trends in patterns of self-reported food consumption of adult Americans: NHANES 1971–1975 to NHANES 1999–2002. Am J Clin Nut. 2006;84:1215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Leech RM, Timperio A, Worsley A, McNaughton SA. Eating patterns of Australian adults: associations with blood pressure and hypertension prevalence. Eur J Nutr. 2019;58:1899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wittig F, Hummel E, Wenzler G, Heuer T. Energy and macronutrient intake over the course of the day of German adults: A DEDIPAC-study. Appetite. 2017;114:125–36. [DOI] [PubMed] [Google Scholar]
- 84. Lund TB, Gronow J. Destructuration or continuity? The daily rhythm of eating in Denmark, Finland, Norway and Sweden in 1997 and 2012. Appetite. 2014;82:143–53. [DOI] [PubMed] [Google Scholar]
- 85. Striegel-Moore RH, Franko DL, Thompson D, Affenito S, Kraemer HC. Night eating: prevalence and demographic correlates. Obesity. 2006;14:139–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.