Abstract
Background
Current guidelines recommend viral, autoimmune, coagulation and liver ultrasound testing in intrahepatic cholestasis of pregnancy to exclude alternative diagnoses.
Methods
Electronic health records were searched for investigations and diagnoses in women with raised bile acid concentrations (>10 µmol/L) between January 2016 and December 2017 at two UK maternity units.
Results
Five hundred and thirty-one women had a raised bile acid concentration (median (IQR): 18 (13–32 µmol/L)) at a median gestation of 35.1 (IQR 31.8–37.0) weeks. Out of 531 women, 250 (47.1%) had full virology, autoimmune and ultrasound tests, and 348 (65.5%) had coagulation performed. Positive hepatitis B and C results were previously known. No new Epstein–Barr virus, cytomegalovirus or hepatitis A diagnoses were made. There were 11 positive autoimmune results, but no new diagnoses. No woman had an unexplained prolonged prothrombin time. No ultrasound liver (n = 38) or gallbladder (n = 85) abnormalities were of acute clinical significance.
Conclusion
Intrahepatic cholestasis of pregnancy investigations provided no new diagnoses that influenced clinical management during pregnancy.
Keywords: Cholestasis, pregnancy
Background
Intrahepatic cholestasis of pregnancy (ICP) or obstetric cholestasis is the commonest pregnancy-specific liver disease, affecting approximately 0.7% of pregnancies in multi-ethnic populations in the UK.1 It has a multifactorial aetiology with genetic, hormonal and environmental components,2–4 with higher incidence noted in women of Asian-Pakistani (1.5%) and Asian-Indian (1.2%) origin.5 ICP is diagnosed by the combination of pruritus and elevated bile acids (BAs) in pregnancy, in the absence of another identified cause, with resolution of clinical symptoms and biochemical abnormalities expected within weeks of delivery.
Current guidelines advocate approaching ICP as a diagnosis of exclusion. The Royal College of Obstetricians & Gynaecologists (RCOG) Obstetric Cholestasis Green-top Guideline (last updated in 2011) recommends that ‘other causes of pruritus and abnormal liver function tests should be sought’, suggesting viral testing for Hepatitis A, B, C, Epstein–Barr virus (EBV) and cytomegalovirus (CMV), liver autoimmune testing for chronic active hepatitis and primary biliary cirrhosis, liver ultrasound and that ‘a coagulation screen should be performed’.6 The principal aim of this testing in the antenatal period is to identify alternative causes for the clinical presentation (i.e. pruritus and raised BA concentrations). However, the proportion of women with suspected ICP who have additional investigations and the detection rate for alternative diagnoses or co-morbidities as a result of testing in ICP is uncertain.
Methods
All tests for BA concentrations performed in two London hospital maternity units between 1 January 2016 and 31 December 2017 were reviewed. Women with a peak BA concentration above the upper limit of normal (>10 µmol/L in both units) were identified. Data review was performed to remove records of non-pregnant women and those in whom BA concentration was measured following stillbirth without any clinical symptoms or preceding diagnosis of ICP. Following data cleaning, hospital laboratory databases were reviewed to determine which investigations were performed during pregnancy and the results of these investigations (biochemistry, virology, autoimmune antibodies, coagulation and liver ultrasound) and clinical outcomes were extracted from electronic health record databases. In women with abnormal investigation results and those with severe early-onset disease (defined as a peak BA level of ≥40 µmol/L at <32 weeks’ gestation), all available electronic health records including discharge summaries and clinic letters were reviewed in order to determine whether any new diagnoses were made. Data manipulation and analysis was performed in R version 3.4.3 (The R project for Statistical Computing). ANOVA and Kruskal–Wallis tests for continuous and chi-squared trend test for categorical variables were used to assess for differences between peak BA concentration groups as appropriate.
Results
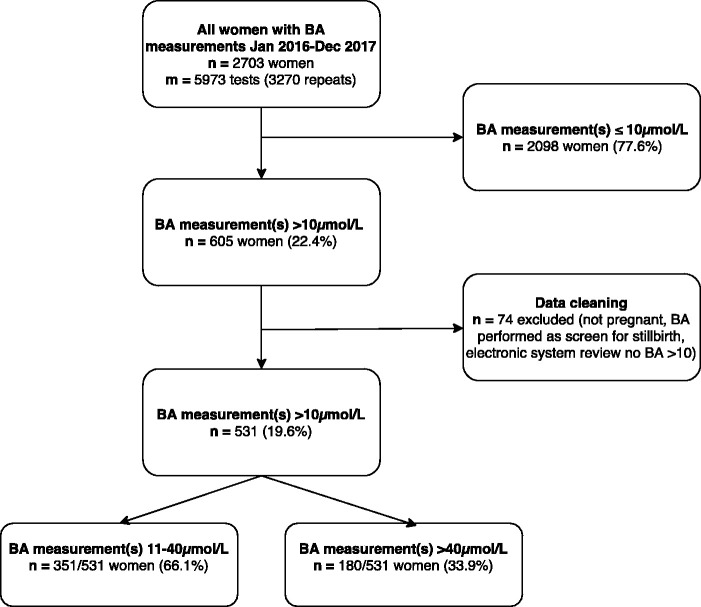
The selection of women with a peak BA concentration greater than 10 µmol/L during pregnancy during the two-year study period is shown in Figure 1. Out of 23,927 deliveries at both hospital sites, 531 (2.2%) women had raised BA concentrations with first raised BA at a median gestation age of 35.1 weeks (interquartile range (IQR) 31.8–37.0). One hundred and eighty-four women (34.7%) had a peak BA concentration greater than or equal to 40 µmol/L during their pregnancy (at a median gestational age of 34.3 (IQR 32.1–36.5) weeks).
Figure 1.
Flow diagram illustrating the selection of women with raised bile acid concentrations in pregnancy. BA: bile acid.
Maternal demographics
The demographics of the women with raised BAs during pregnancy are shown in Table 1. There were 47 (8.9%) twin pregnancies.
Table 1.
Maternal demographics of women with bile acid concentration greater than 10 µmol/L.
| Site 1n = 317 | Site 2n = 214 | All womenn = 531 | |
|---|---|---|---|
| Age (years; median [IQR]) | 34 [30–37] | 31 [27–35] | 33 [29–36] |
| Age category (n (%)) | |||
| <20 | 1 (0.3) | 5 (2.3) | 6 (1.1) |
| 20–29 | 65 (20.5) | 69 (32.2) | 134 (25.2) |
| 30–39 | 184 (58.0) | 123 (57.5) | 307 (57.8) |
| ≥40 | 47 (14.8) | 17 (7.9) | 64 (12.1) |
| Not recorded | 20 (6.3) | 0 (0.0) | 20 (3.7) |
| Ethnic origin (n (%)) | |||
| White | 124 (39.1) | 75 (35.0) | 199 (37.5) |
| Asian | 38 (12.0) | 109 (50.9) | 147 (27.7) |
| Black | 40 (12.6) | 19 (8.9) | 59 (11.0) |
| Other | 7 (2.2) | 6 (2.8) | 13 (2.4) |
| Mixed | 9 (2.8) | 2 (0.9) | 11 (2.1) |
| Not recorded or declined | 99 (31.2) | 3 (1.4) | 102 (19.2) |
| BMI (kg/m2; median [IQR]) | 23.6 [20.8–27.2] | 25.0 [23.0–28.0] | 24.0 [21.9–27.7] |
| BMI category (kg/m2; n (%)) | |||
| <20 | 37 (11.7) | 13 (6.1) | 50 (9.4) |
| 20–24.9 | 131 (41.3) | 84 (39.3) | 215 (40.5) |
| 25–29.9 | 65 (20.5) | 73 (34.1) | 138 (26.0) |
| 30–34.9 | 17 (5.4) | 27 (12.6) | 44 (8.3) |
| ≥35 | 15 (4.7) | 15 (7.0) | 30 (5.6) |
| Not recorded | 52 (16.3) | 2 (0.9) | 54 (10.1) |
| Parity (n (%)) | |||
| 0 | 176 (55.5) | 112 (52.3) | 288 (54.2) |
| 1 | 77 (24.3) | 75 (35.0) | 152 (28.6) |
| 2 | 27 (8.5) | 15 (7.0) | 42 (7.9) |
| >2 | 18 (5.6) | 12 (5.6) | 30 (5.6) |
| Not recorded | 19 (6.0) | 0 (0.0) | 19 (3.6) |
IQR: interquartile range; BMI: body mass index.
Delivery and fetal outcomes
Delivery and fetal outcomes in women are shown in Table 2. Women with a peak BA concentration greater than or equal to 40 µmol/L delivered at earlier gestations. There were high rates of induction of labour (between 38.9% and 54.6%) in all groups, with approximately 50% of women overall being induced and 19% having planned delivery by caesarean section. Overall, 19% of women delivered preterm (prior to 37 weeks’ gestation), of whom 25.7% went into spontaneous preterm labour. Four per cent of women delivered very preterm (at <34 weeks’ gestation), of whom 33.3% had spontaneous onset of preterm labour. There were two stillbirths (0.3% of births) at 31 and 35 weeks’ gestation, both of which occurred in women who had a peak BA concentration greater than or equal to 100 µmol/L during pregnancy, and with additional maternal comorbidities alongside ICP. There was one neonatal death (0.2% of births) of one baby of twins delivered at 32 weeks’ gestation by elective caesarean section for suspected twin to twin transfusion in whom the woman had a peak BA concentration of 42 µmol/L.
Table 2.
Delivery and perinatal outcomes in women with raised bile acid concentrations and grouped by peak bile acid concentration.a
| Peak bile acid concentration (µmol/L) |
|||||
|---|---|---|---|---|---|
| Delivery outcomes | All womenn = 531 | 11–39n = 347 | 40–99n = 130 | 100+ n = 54 | p value* |
| Gestational age at delivery (weeks) | 37.9 [37.1–39.0] | 38.1 [37.3–39.3] | 37.4 [36.5–38.1] | 37.1 [37.0–37.9] | <0.001 |
| Onset | |||||
| Spontaneous labour | 114 (21.5) | 79 (22.8) | 23 (17.7) | 12 (22.2) | 0.332 |
| Induction of labour | 265 (49.9) | 173 (49.9) | 71 (54.6) | 21 (38.9) | 0.540 |
| Elective caesarean section | 103 (19.4) | 69 (19.9) | 26 (20.0) | 8 (14.8) | 0.955 |
| Missing data | 49 (9.2) | 26 (7.5) | 10 (7.7) | 13 (24.1) | |
| Delivery | |||||
| <37 weeks | 101 (19.0) | 52(15.0) | 38 (29.2) | 11 (20.4) | 0.002 |
| Spontaneous onset | 26 (4.9) | 10 (2.9) | 12 (9.2) | 4 (7.4) | |
| Induction of labour | 26 (4.9) | 15 (4.3) | 9 (6.9) | 2 (3.7) | |
| Elective caesarean section | 34 (6.4) | 21 (6.1) | 11 (8.5) | 2 (3.7) | |
| Missing | 15 (2.8) | 6 (1.7) | 6 (4.6) | 3 (5.5) | |
| <34 weeks | 21 (4.0) | 12 (3.5) | 7 (5.4) | 2 (3.7) | 0.631 |
| Spontaneous onset | 7 (1.3) | 3 (0.9) | 3 (2.3) | 1 (1.9) | |
| Induction of labour | 3 (0.6) | 2 (0.6) | 0 (0.0) | 1 (1.9) | |
| Elective caesarean section | 7 (1.3) | 5 (1.4) | 2 (1.5) | 0 (0.0) | |
| Missing | 4 (0.8) | 2 (0.6) | 2 (1.5) | 0 (0.0) | |
| Missing data | 22 (4.1) | 11 (3.2) | 4 (3.1) | 7 (13.0) | |
| Number of babies | |||||
| 1 | 463 (87.2) | 308 (88.8) | 111 (85.4) | 44 (81.5) | 0.431 |
| 2 | 47 (8.9) | 29 (8.4) | 15 (11.5) | 3 (5.6) | |
| Missing data | 21 (4.0) | 10 (2.9) | 4 (3.1) | 7 (13.0) | |
|
Perinatal outcomes (n of babies) |
n = 578 |
n = 376 |
n = 145 |
n = 57 |
|
| Mode of delivery | |||||
| Spontaneous vaginal delivery | 259 (44.8) | 176 (46.8) | 60 (41.4) | 23 (40.4) | 0.454 |
| Emergency caesarean section | 124 (21.5) | 74 (19.7) | 34 (23.4) | 16 (28.1) | 0.163 |
| Elective caesarean section | 104 (18.0) | 71 (18.9) | 26 (17.9) | 7 (12.3) | 0.626 |
| Assisted vaginal delivery | 65 (11.2) | 40 (10.6) | 21 (14.5) | 4 (7.0) | 0.339 |
| Missing data | 26 (4.5) | 15 (4.0) | 4 (2.8) | 7 (12.3) | |
| Birth outcome | |||||
| Live birth | 549 (95.0) | 361 (96.0) | 140 (96.6) | 48 (84.2) | 0.001 |
| Intrauterine death | 2 (0.3) | 0 (0.0) | 0 (0.0) | 2 (3.5) | <0.001 |
| Neonatal death | 1 (0.2) | 0 (0.0) | 1 (0.7) | 0 (0.0) | 0.233 |
| Missing data | 26 (4.5) | 15 (4.0) | 4 (2.8) | 7 (12.3) | |
| Birth weight, g | 3096 (2688–3450) | 3170 (2770–3500) | 2920 (2530–3310) | 3014 (2508–3120) | <0.001 |
| Birth weight <2500 g | 91 (15.7) | 46 (12.2) | 34 (23.4) | 11(19.3) | 0.005 |
| Male | 298 (51.6) | 198 (52.7) | 73 (50.3) | 27 (47.4) | 0.825 |
| Female | 254 (43.9) | 163 (43.4) | 68 (47.9) | 23 (40.4) | |
| Missing data | 26 (4.5) | 15 (4.0) | 4 (2.8) | 7 (12.3) | |
| Neonatal unit admission | 77 (13.3) | 43 (11.4) | 29 (20.0) | 5 (8.8) | 0.030 |
| Missing data | 26 (4.5) | 15 (4.0) | 4 (2.8) | 7 (12.3) | |
aResults displayed as median [interquartile range] or number (%).
*p values denoting results of tests for difference between peak bile acid concentration groups (11–39, 40–99 and ≥100 µmol/L).Significant p-values are highlighted in bold.
Biochemistry testing
Other biochemistry results at the time of first raised BA are summarised in Table 3. Women who had a peak BA concentration greater than or equal to 40 µmol/L during pregnancy presented with higher initial BAs, and with higher alanine transaminase. There were 15 women (2.8%) who had raised bilirubin at time of peak BA, of which 7 (1.3%) cases were elevated sufficiently (>34–42 μmol/L) to cause clinically appreciable jaundice.
Table 3.
First raised bile acid concentration (>10 µmol/L), liver function test results and gestational age at time of first raised bile acid result in all women and grouped by subsequent peak bile acid concentration.
| Peak bile acid concentration (µmol/L) |
|||||
|---|---|---|---|---|---|
| Liver function test results, median [IQR] | All womenn = 531 | 11–39n = 347 | 40–99n = 130 | 100+ n = 54 | p value* |
| First bile acid >10 µmol/L | 18 [13–32] | 15 [12–20] | 41 [25–55] | 59 [25–127] | <0.001 |
| Gestation (weeks) | 35.1 [31.8–37.0] | 35.7 [31.4–37.3] | 34.6 [32.4–36.6] | 33.4 [30.3–36.3] | 0.188 |
| Bilirubin (µmol/L) | 7 [5–10] | 6 [4–9] | 7 [5–11] | 9 [6–15] | <0.001 |
| Alanine transaminase (IU/L) | 40 [20–106] | 31 [17–76] | 72 [36–145] | 78 [40–189] | <0.001 |
| Gamma-glutamyl transferase (IU/L) | 21 [14–35] | 20 [12–31] | 25 [16–44] | 26 [14–38] | 0.021 |
*Comparing biochemistry results between peak bile acid concentration groups 11–39, 40–99 and 100+ µmol/L.Significant p-values are highlighted in bold.
Virology testing
A summary of the virology, autoimmune and ultrasound testing and results is shown in Table 4. Three hundred and eighty (71.6%) women were tested for EBV: of these, 343 (90.3%) women were immune (EBV viral capsid antigen (VCA) IgG positive, IgM negative), 13 (3.4%) were susceptible (EBV VCA IgG and IgM negative), and 24 women (6.3%) were both EBV VCA IgG and IgM positive which can denote recent infection. In these latter cases, further testing for Epstein–Barr nuclear antigen (EBNA) was performed. Twenty-three women tested positive for EBNA denoting past (a minimum of 6–8 weeks between infection and testing) rather than acute infection, and one woman tested EBNA negative; however, she had a low positive EBV VCA IgM result which was reported by the virology team as ‘consistent but not typical of primary EBV infection’, no testing for EBV viral load was performed and no clinical diagnosis was made on review of electronic health records.
Table 4.
Summary of screening investigations (virology, autoimmune and ultrasound) in women with raised bile acid concentration during pregnancy.
| Number (%) of women tested | Number (%) of positive result | Number (%) by peak BA concentration (µmol/L) | Number (%) of known diagnosis | Number (%) of new diagnosis | |||
|---|---|---|---|---|---|---|---|
| Virology | 11–39 | 40–99 | 100+ | ||||
| EBV serology | |||||||
| All women (n = 531) | 380 (71.6) | 1/380 (0.3) | 0/238 (0.0) | 1/99 (1.0) | 0/43 (0.0) | 0/1 (0.0) | 0/188 (0.0) |
| CMV serology | |||||||
| All women (n = 531) | 395 (74.4) | 0/395 (0.0) | – | – | – | – | – |
| Hepatitis C IgG | |||||||
| All women (n = 531) | 421 (79.3) | 3/421 (0.7) | 1/266 (0.4) | 1/109 (0.9) | 1/46 (2.2) | 3/3 (100) | 0/421 (0.0) |
| Hepatitis B sAg | |||||||
| All women (n = 531) | 496 (93.4) | 7/496 (1.4) | 4/327 (1.2) | 2/119 (1.7) | 1/50 (2.0) | 6/7 (85.7)1/7a (14.3) | 0/496 (0.0) |
| Hepatitis A IgM | |||||||
| All women (n = 531) | 387 (72.9) | 0/387 (0.0) | – | – | – | – | – |
| Autoimmune | |||||||
| Smooth muscle Ab | |||||||
| All women (n = 531) | 404 (76.1) | 5/404 (1.2) | 2/259 (0.8) | 3/102 (2.9) | 0/43 (0.0) | 0/5 (0.0) | 0/406 (0.0) |
| Hep2 Antinuclear Ab | |||||||
| Site 1 (n = 317) | 227 (71.6) | 1/227 (0.4) | 1/133 (0.8) | 0/65 (0.0) | 0/29 (0.0) | 1/1b (100) | 0/227 (0.0) |
| Mitochondrial Ab | |||||||
| All women (n = 531) | 403 (75.9) | 5/403 (1.2) | 3/258 (1.1) | 0/102 (0.0) | 2/43 (4.7) | 1/5c (25.0) | 0/405 (0.0) |
| Liver/kidney microsomal Ab | |||||||
| All women (n = 531) | 406 (76.5) | 0/406 (0.0) | – | – | – | – | – |
| Imaging | |||||||
| Liver ultrasound | |||||||
| All women (n = 531) | 326 (61.4) | 38/326 (11.7) | 24/199 (12.1) | 7/86 (8.1) | 7/41 (17.1) | – | 0/327 (0.0) |
| Gallbladder ultrasound | |||||||
| All women (n = 531) | 323 (60.8) | 85/323 (26.3) | 45/197 (22.8) | 26/86 (30.2) | 14/40 (35.0) | – | 0/324 (0.0) |
EBV: Epstein–Barr virus; CMV: cytomegalovirus; sAg: surface antigen; Ab: antibody.
aNot booked at GSTT, single attendance.
bKnown Ro and anti-PL-12 positive anti-synthetase syndrome positive interstitial lung disease prior to pregnancy.
cKnown rheumatoid arthritis prior to pregnancy.
Of the 395 (74.4%) women tested for CMV, 170 (43.0%) were immune (CMV IgG positive, CMV IgM negative/not tested in two cases), 181 (45.8%) tested negative for CMV IgM (IgG testing was not performed), 37 (9.4%) were susceptible to CMV infection (CMV IgG and IgM negative), and seven women (1.7%) tested positive for CMV IgG and IgM which can denote recent infection. In these seven women, three were tested for CMV DNA and were all negative, two had repeat CMV serology with no interval change (thus excluding acute infection) and in two cases booking serology was tested and CMV IgG was positive suggesting the women were immune and the CMV IgM was a false positive in pregnancy.
Seven (1.4%) of the 496 (93.4%) women tested for Hepatitis B infection had Hepatitis B surface antigen detected, of whom six had known diagnoses, including two in whom the diagnosis had been made at booking in the same pregnancy. The status of one woman who tested positive for Hepatitis B infection could not be ascertained as she had a single attendance at one hospital site whilst visiting London and had booked her pregnancy elsewhere.
Of the 421 (79.3%) women tested for Hepatitis C infection, three (0.7%) were positive, in all of whom the diagnosis had been made prior to the pregnancy. Of the 387 (72.9%) women tested for Hepatitis A infection, there were no positive IgM results.
Autoimmune testing
Autoantibody testing for smooth muscle antibodies
Four hundred and four (76.1%) women were tested for smooth muscle antibodies of whom five (1.2%) were positive. None of the women with positive smooth muscle antibodies tested positive for any of the viral infections, anti-mitochondrial antibodies, or liver/kidney microsomal antibodies. ICP only was the recorded diagnosis in all cases on review of the electronic health records and clinic letters and none were referred to gastroenterology or hepatology services. Further details are given in Supplementary Text 1.
Autoantibody testing for antinuclear antibodies
Hep-2 antinuclear antibody (ANA) testing was performed as part of the ICP investigations at one hospital site only. Of the 227 women tested for Hep-2 ANA, 31 (13.7%) had antibodies detected, with only two at dilutions of 1:640 or 1:1280. Most women had known pre-existing diagnoses, including autoimmune diseases. On review of electronic health records and clinic letters, no new diagnoses other than ICP were made as a result of testing. Further details are given in Supplementary Text 2.
Autoantibody testing for anti-mitochondrial antibodies and liver kidney microsomal antibodies
Four hundred and three (75.9%) women were tested for anti-mitochondrial antibodies, of whom five (1.2%) were positive. Three women were referred to gastroenterology/hepatology services and discharged after further investigations. In the remaining cases, no referrals to specialist services were made and no new clinical diagnoses other than ICP were evident on electronic health records and clinic letters. Further details are given in Supplementary Text 3. Of the 406 women tested for liver kidney microsomal antibody there were no positive results.
Ultrasound testing for comorbidities and additional diagnoses
In total 327 (61.6%) women had a liver and gallbladder ultrasound as part of ICP investigations. There were high rates of co-incidental findings on imaging, shown in Supplementary Table 1. Eighty-five women (26.2%) had gallbladder abnormalities detected including 60 women (18.5%) with gallstones and/or biliary sludge, 12 (3.7%) with a polyp, 10 (3.1%) with previous cholecystectomy, and three with other minor abnormalities (debris noted in gallbladder, gallbladder wall thickened, mild gallbladder oedema).
Coagulation testing
Three hundred and forty-eight women (65.5%) had coagulation tests performed. Three had a prolongation of prothrombin time; one also had severe pre-eclampsia and HELLP (haemolysis, elevated liver enzymes, low platelets) syndrome necessitating delivery at 30 weeks’ gestation, and two had acute fatty liver of pregnancy.
Diagnoses in women presenting with early onset rise in BA concentrations
There were 23 women (4.3%) who had a peak BA concentration greater than or equal to 40 µmol/L at less than 32 weeks’ gestation, 10 (43.5%) of whom had a peak BA concentration greater than or equal to 100 µmol/L during pregnancy. In 23 cases, 12 (52.2%) ICP was the only liver diagnosis during pregnancy with no additional comorbidities of note. In the remaining cases, pre-existing comorbidities, alternative explanations for the abnormal liver function tests, and/or additional pregnancy complications were present. Full details are given in Supplementary Text 4 and Supplementary Table 2.
Characteristics of testing
In a total of 531 women, 250 (47.1%) with raised BAs during pregnancy had complete virology, autoimmune and ultrasound tests. Only 24 (4.5%) women had no additional tests. There were no significant differences in the proportion of women with peak BA concentration of 11–39, 40–99 or greater than or equal to 100 µmol/L or more that had full virology testing (p = 0.244, 62.8%, 70.0%, 70.4% tested, respectively), or autoimmune testing (p = 0.514, 74.4%, 78.5%, 79.6% tested, respectively). However, women with higher peak BAs were more likely to have a liver ultrasound (p = 0.015, 57.3%, 66.2%, 75.9%, respectively).
Cost of additional investigations in ICP
The cost for a full set of ICP blood investigations (virology and autoimmune) for a single woman, estimated utilising Viapath Laboratory Services costs, was £130.67; the cost of an abdominal ultrasound within the NHS is estimated to be £150; coagulation screen costs £3.50. Therefore, we estimate around £35,000 per year was spent investigating women across the two hospitals. If all women had received all tests as recommended by the RCOG Green-top guidelines this would equate to £75,000 per year across the two sites.
Discussion
Principal findings
In our study cohort of pregnant women with raised serum BA concentrations greater than 10 µmol/L, there were no new diagnoses that resulted in ongoing specialist management detected from additional investigations routinely performed in clinical practice. Approximately one in five women who had BAs tested in pregnancy had elevated serum BA concentrations greater than 10 µmol/L. Of the women with raised BA concentrations, approximately half of women had a full set of virology, autoimmune and ultrasound testing investigations as currently recommended by national guidelines. Our data show that amongst women tested, over 95% were already immune to EBV. The cost of performing additional routine testing for ICP is substantial, for minimal, if any, new clinical information. There were no antenatal characteristics relating to the women, or the initial BA concentration, that identified a sub-group of women who might benefit from additional testing.
Strengths and weaknesses
The strength of our study is that it is a two-centre study in a large city with a multi-ethnic population addressing a real-world, clinical conundrum. However, as we selected women with raised BAs in pregnancy rather than a clinically defined population of women with suspected ICP, it is possible that our study included women with alternative diagnoses, increasing our denominator and so diluting our reported rates of investigation. BAs can be raised in several hepatobiliary conditions including hepatitis, obstructive jaundice and cirrhosis7 as well as in pre-eclampsia, but BAs are not usually tested in these conditions during or outside of pregnancy. In addition, the electronic health record review was limited to the hospital at which the initial BA test was performed and where the care during pregnancy and the postpartum period was provided, so it is possible that some women with hepatic diseases, e.g. autoimmune hepatitis, were not ascertained, if electronic records did not adequately capture other specialty involvement subsequent to the pregnancy. However, as pregnancy outcome data were available for all but 25 (4.3%) women, it appears that the majority of women received antenatal care and delivered within the two hospital Trusts.
Comparison with other studies
In a UK series of 70 patients with ICP, ultrasound scans revealed gallstones in 10% of women, and two (2.9%) cases of Hepatitis C were detected as a result of virology testing.8 In a French series reporting clinical characteristics of 50 women with ICP, virology testing did not reveal any concomitant liver disease and ultrasound examination was normal in all women.9 In a published Scandinavian cohort of 91 women negative virology testing and normal liver ultrasound defined the ICP cohort and the number of women excluded on the basis of positive virology results were not reported.10 One case in our study had genetic variation in ABCB4 and ABCB11, consistent with the findings of a UK study in which pathogenic mutations in these biliary transporters were identified in approximately 20% cases.11 None of these studies specifically investigated the clinical utility of additional investigations in ICP. Recommendations in the RCOG guidelines for management of ICP were based on expert opinion alone.
Implications for clinicians and policymakers
Our findings suggest that the currently recommended routine additional investigations have no diagnostic yield and that a more targeted testing approach should be advocated. This is especially pertinent in an era in which healthcare efficiency is increasingly relevant, and the Academy of Medical Royal Colleges is encouraging avoidance of unnecessary testing and procedures through the Choosing Wisely programme (see: http://www.choosingwisely.co.uk/about-choosing-wisely-uk/). This extends to autoantibody testing, where a focus on not testing persons with a low pre-test probability of disease has been proposed.12 Reducing unselected testing would save money and allow resources to be focussed on women with atypical disease, clinical uncertainty, women with additional comorbidities or if postpartum resolution does not occur.
Conclusion
Our data suggest little diagnostic value of routine additional testing investigations in ICP in women presenting with typical features. A more targeted and individualised approach could be used, based on factors such as atypical clinical symptoms, presence of comorbidities, severity of ICP, gestational age at presentation or failure of liver function tests to normalise postpartum.
Supplemental Material
Supplemental Material for Detection of additional abnormalities or co-morbidities in women with suspected intrahepatic cholestasis of pregnancy by Frances Conti-Ramsden, Michael McEwan, Rachel Hill, Julie Wade, Georgina Abraham, Olivia Buckeldee, Catherine Williamson, Caroline L Knight, Joanna Girling and Lucy C Chappell in Obstetric Medicine: The Medicine of Pregnancy
Footnotes
Author's note: Joanna Girling and Lucy C Chappell are both the senior authors of this manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: There was no specific funding required for this project. FCR is an NIHR Academic Clinical Fellow; LCC is supported by a National Institute for Health Research Professorship, RP-2014-05-019.
Ethical approval: The study was registered as an audit at both local hospital sites and approved by the Trust Audit Committee. Audit Numbers: GSTT1000 (Site 1), WCSLA 043 (Site 2).
Informed consent: Informed consent was not sought for the present study because it was a retrospective audit.
Guarantor: LCC is the guarantor of this article.
Contributorship: LCC and JG designed the study. FCR, MM, RH, CLK, GA, OB and JW performed data collection. FCR and RH performed data analysis. FCR, CW, LCC and JG interpreted the results. The first manuscript draft was written by FCR and re-drafted and approved by all other authors.
References
- 1.Kenyon AP, Tribe RM, Nelson-Piercy C, et al. Pruritus in pregnancy: a study of anatomical distribution and prevalence in relation to the development of obstetric cholestasis. Obstet Med 2010; 3(1): 25–29. [DOI] [PMC free article] [PubMed]
- 2.Ovadia C, Williamson C. Intrahepatic cholestasis of pregnancy: recent advances. Clin Dermatol 2016; 34: 327–334. [DOI] [PubMed] [Google Scholar]
- 3.Dixon PH, Williamson C. The pathophysiology of intrahepatic cholestasis of pregnancy. Clin Res Hepatol Gastroenterol 2016; 40: 141–153. [DOI] [PubMed] [Google Scholar]
- 4.Marschall H-U. Management of intrahepatic cholestasis of pregnancy. Expert Rev Gastroenterol Hepatol 2015; 9: 1273–1279. [DOI] [PubMed] [Google Scholar]
- 5.Abedin P, Weaver JB, Egginton E. Intrahepatic cholestasis of pregnancy: prevalence and ethnic distribution. Ethn Health 1999; 4: 35–37. [DOI] [PubMed] [Google Scholar]
- 6.Kenyon AP, Girling J. Obstetric cholestasis. RCOG Green-top Guideline No. 43, 2011, pp.1–14.
- 7.Neale G, Lewis B, Weaver V, et al. Serum bile acids in liver disease. Gut 1971; 12: 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenyon AP, Piercy CN, Girling J, et al. Obstetric cholestasis, outcome with active management: a series of 70 cases. BJOG 2002; 109: 282–288. [DOI] [PubMed] [Google Scholar]
- 9.Bacq Y, Sapey T, Bréchot MC, et al. Intrahepatic cholestasis of pregnancy – A French prospective study. Hepatology 1997; 26: 358–364. [DOI] [PubMed] [Google Scholar]
- 10.Heinonen S, Kirkinen P. Pregnancy outcome with intrahepatic cholestasis. Obstet Gynecol 1999; 94: 189–193. [DOI] [PubMed] [Google Scholar]
- 11.Dixon PH, Sambrotta M, Chambers J, et al. An expanded role for heterozygous intrahepatic cholestasis of pregnancy. Sci Rep 2017; 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abeles AM, Abeles M. The clinical utility of a positive antinuclear antibody test result. Am J Med 2013; 126: 342–348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Detection of additional abnormalities or co-morbidities in women with suspected intrahepatic cholestasis of pregnancy by Frances Conti-Ramsden, Michael McEwan, Rachel Hill, Julie Wade, Georgina Abraham, Olivia Buckeldee, Catherine Williamson, Caroline L Knight, Joanna Girling and Lucy C Chappell in Obstetric Medicine: The Medicine of Pregnancy