Abstract
Background
There remains no gold standard management for deep shoulder periprosthetic joint infection (PJI). This case series aims to present our experience of two-stage revision arthroplasty, including eradication of infection and reoperation rates.
Methods
We retrospectively reviewed patients undergoing revision arthroplasty for shoulder PJI between 2006 and 2015. Cases were confirmed using Musculoskeletal Infection Society (MSIS) and American Academy of Orthopaedic Surgeons (AAOS) guidelines. TSA removal, debridement and irrigation preceded antibiotic-loaded cement spacer insertion and a minimum of six weeks intravenous antibiotics. Reimplantation was performed as a second stage following a negative aspirate.
Results
Twenty-eight patients underwent a first stage procedure (mean age 69 years; 16 male, 12 female). Propionibacterium acnes, Methicillin-sensitive Staphylococcus aureus, Coagulase-negative Staphylococcus and Staphylococcus epidermidis were the commonest microorganisms cultured. Five cases had mixed growths and six cases provided no growth. Three patients did not proceed to a second stage. Twenty-five patients underwent reimplantation (mean interval 6.7 months), with 80% remaining infection-free (mean follow-up 38.3 months).
Discussion
Managing complex and late presentation shoulder PJI with two-stage revision is associated with high rates of infection eradication (80%). In the absence of a management consensus, our experience supports two-stage revision arthroplasty for eradicating infection in this complex patient group.
Keywords: total shoulder arthroplasty, total shoulder replacement, periprosthetic joint infection, two-stage revision arthroplasty, revision shoulder arthroplasty, reimplantation, infection eradication
Introduction
Total shoulder arthroplasty (TSA) is an effective treatment for rheumatoid arthritis, primary osteoarthritis, acute fracture and arthritis secondary to trauma. TSA failure can result from infection, instability, component loosening, malposition or wear and periprosthetic fracture.1 Deep periprosthetic joint infection (PJI) is the most common complication in the first two years following TSA and is associated with potentially devastating outcomes.2,3 The incidence of PJI ranges from 0% to 3.9% after primary anatomic TSA4–6 and approximately 5% for primary reverse polarity TSA.7 Treatment options include long-term suppressive antibiotic therapy, debridement with antibiotics and implant retention (DAIR), excision arthroplasty, exchange arthroplasty by single- or two-stage revision, arthrodesis and amputation. Implant retention, either following debridement or with suppressive antibiotics alone, is associated with high failure rates,8 however limited success is seen in early infections.9–11 Resection arthroplasty combined with antibiotic-impregnated spacers often provide comfort at rest, however poor functional outcomes are expected and is therefore reserved for refractory, low-demand or elderly patients.12–14 Excision arthroplasty often results in poor functional outcomes,8,14,15 whilst arthrodesis has high failure rates.16 Revision arthroplasty may restore function, but can be technically challenging due to bone loss and soft tissue scarring. The majority of published PJI studies relate to the hip and knee arthroplasty, with two-stage revision considered the ‘gold standard’ treatment.17,18 Although many centres also regard two-stage revision to be the treatment of choice for shoulder PJI,19,20 there is no consensus regarding the relative indications for single- or two-stage revision.21
Diagnosing shoulder PJI requires a high index of suspicion due to indolent infection proving common.1,22 In contrast to the lower limb, PJI of the shoulder frequently presents with non-specific pain and stiffness, with classic features of hyperpyrexia (fever, chills, sweats, rigors) or local inflammation (erythema, induration, calor, sinus formation) often remaining absent due to a high incidence of low virulence organisms, including Propionibacterium acnes and Staphylococcus epidermidis.23–25 Previously considered non-pathogenic, P. acnes is frequently cited as the most common causative organism in shoulder PJI24,26 and occurs in 0.9–1.9% of all TSA; however, this is likely an underestimate as diagnosis is unreliable.27 Polymicrobial infection is not uncommon.28
The aim of this study is to contribute to the published literature pertaining to shoulder PJI management by presenting our experience of two-stage revision arthroplasty, with respect to the presenting features of the infected joint, microbiological profile, eradication of infection and reoperation rates.
Materials and methods
Patients
We reviewed the medical records and imaging of all patients undergoing revision arthroplasty for infected TSA at our institution between 2006 and 2015. Patients undergoing revision for apparently aseptic reasons (fracture, instability, aseptic loosening) who then had positive results for intra-operative samples were excluded, since they did not have the same antibiotic regime and thorough debridement as those known to be infected pre-operatively. Twenty-eight patients underwent a first stage revision procedure with removal of all implants. We recorded the indication for primary surgery, the time from primary surgery to first stage revision, whether the patient was diabetic or receiving treatment with steroids, whether the implants showed evidence of radiological loosening, the organisms isolated from intra-operative specimens and whether the patient remained free from infection at the time of most recent follow-up. Ethical approval was provided by our institution's research and development committee.
Pre-operative
Diagnosing shoulder PJI can be difficult, especially when compared to the lower limb, and no single set of criteria are accepted as the gold standard.29 We confirmed shoulder PJI using the guidelines of the Musculoskeletal Infection Society29 (MSIS) (see Table 1), which complement the earlier recommendations of the American Academy of Orthopaedic Surgeons (AAOS).30
Table 1.
Criteria for diagnosing periprosthetic joint infection.31
| A definite diagnosis of PJI can be made when one of the following three conditions are met: | |
| 1. | There is a sinus tract communicating with the prosthesis; or |
| 2. | A pathogen is isolated by culture from at least two separate tissue or fluid samples obtained from the affected prosthetic joint; or |
| 3. | Four of the following six criteria exist: |
| a. Elevated serum erythrocyte sedimentation rate (ESR) or serum C-reactive protein (CRP) concentration b. Elevated synovial white blood cell (WBC) count c. Elevated synovial neutrophil percentage (PMN%) d. Presence of purulence in the affected joint e. Isolation of a microorganism in one culture of periprosthetic tissue or fluid, or f. Greater than five neutrophils per high-power field in five high-power fields observed from histologic analysis of periprosthetic tissue at ×400 magnification. Infection may be present if fewer than four of these criteria are met. | |
All patients had anteroposterior and axillary radiographs to assess for any evidence of loosening. Whenever possible rheumatoid medications – including steroids, disease modifying anti-rheumatic drugs and biologic agents – were discontinued in the peri-operative period. Diabetic control was optimised if necessary. Antibiotics were stopped at least two weeks pre-operatively if patients were taking them.
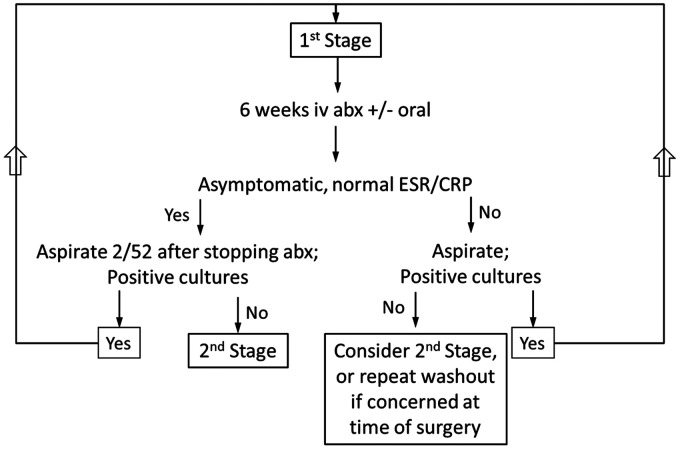
Our management protocol is detailed herein, and a summary is presented in Figure 1.
Figure 1.
Summary of our management protocol.
First stage
All surgery was carried out via a deltopectoral approach with the patient in the beach chair position. A thorough debridement was carried out, including removal of all infected soft tissue and bone, and excision of the sinus tract(s) if present. Multiple samples of fluid, soft tissue and bone were sent for microscopy and culture before prophylactic antibiotics were administered. Tissue samples were collected by a non-standardised method at the start of the review period. However, since the latter stage of the review we have sampled using an infection tray with five instrument sets. Throughout the review period we utilised five separate microbiology pots and a single histology pot. Once samples had been taken empirical antibiotic treatment was started with intravenous tazocin, teicoplanin and amikacin. In the case of penicillin allergy tazocin was withheld. All prosthetic components and cement were removed using osteotomes, curettes, bone nibblers and a Midas Rex Legend High-Speed Burr (Medtronic, Minneapolis, MN, USA) and Ultradrive-3 Ultrasonic Revision System (Biomet, Warsaw, IN, USA) if required. Copious irrigation with 0.9% saline was performed. A spacer moulded from antibiotic-laden bone cement (ALBC) pre-loaded with gentamycin and clindamycin (Heraeus Medical, Hanau, Germany) was inserted. All wounds were closed over a suction drain (removed after 24 h).|
Post-operative antimicrobial treatment was continued with tazocin 4.5 g TDS IV, teicoplanin 10 mg/kg OD IV and amikacin 15 mg/kg OD IV. Amikacin was discontinued after two doses, and treatment was adjusted after microbiology results and sensitivities were available. If there were no positive samples then treatment was continued with teicoplanin alone to cover the Gram-positive bacteria that are the most frequent infecting organisms. Antibiotics were administered on an out-patient basis using a peripherally inserted central catheter (PICC).
Patients were reviewed in the surgical clinic at two weeks and six weeks post-operatively, with monitoring of inflammatory markers (WCC, ESR and CRP). In addition, patients were reviewed by the Out Patient Antimicrobial Therapy (OPAT) team, consisting of consultant microbiologists, clinical nurse specialists and pharmacists, with weekly telephone consultations in addition to clinical review. If there were no clinical signs of infection, and inflammatory markers had returned to normal, the antibiotics were discontinued after six weeks. Two weeks following the cessation of antibiotics all patients had a fluoroscopically-guided aspiration of the affected joint under aseptic conditions in the operating theatre, with samples sent for microbiological analysis. The decision to proceed with reimplantation was dependant on the following criteria being met: (1) no clinical signs of infection, (2) antibiotic treatment having been completed and (3) negative aspirate result and normal inflammatory markers following cessation of antibiotics for at least two weeks.21
If at the six week post-operative review there were clinical signs of infection, or inflammatory markers had not normalised, patients underwent an aspiration as described above. If the aspirate was positive, then patients underwent a repeat first stage procedure (debridement and lavage). If the aspirate was negative then a second stage procedure was planned in line with the above criteria, but with a low threshold for making an intra-operative decision to repeat the first stage (rather than reimplant new prosthetic components) if there was concern regarding the possibility of continuing infection.
Second stage
Second stage surgery was carried out via the same deltopectoral approach, with the patient again in the beach chair position. Further fluid, soft tissue and bone samples were sent for microbiological analysis. If purulent fluid was identified during the second stage an intra-operative decision to repeat the first stage was made (see Figure 1). In the absence of purulent fluid, the antibiotic spacers were removed and the humerus and glenoid were prepared for reimplantation. The choice of implant was dependent on the residual bone stock and the integrity of the rotator cuff tendons. All implants were inserted using ALBC pre-loaded gentamycin and clindamycin. We chose not to use bespoke antibiotic regimens given the then unproven therapeutic effect for definitive component reimplantation after periprosthetic joint infection combined with the potential risk of diminishing cementation properties. The wound was closed, using an absorbable subcuticular suture, over a suction drain (removed at 24 h post-op), and the arm was rested in a sling. Gentle passive range of motion exercises were commenced on the first post-operative day. After six weeks the sling was removed and unrestricted range of motion exercises were initiated, progressing to strengthening exercises at three months post-operatively.
Results
Twenty-eight patients underwent a first stage procedure, with a mean age of 69 years (range 37–82 years). Sixteen patients were male and 12 were female. The indication for the primary arthroplasty were acute fracture (11 patients), rotator cuff arthropathy (8), osteoarthritis (8) and osteomyelitis following septic native joint (1). The mean time from index arthroplasty to first stage revision was 56.1 months (range 2–206 months). The delays were in part due to cases being referred for a tertiary opinion from secondary care providers. Intra-operative samples from the first stage identified the following organisms (from patients with single or mixed growths): Methicillin-sensitive Staphylococcus aureus (MSSA) in four patients, P. acnes in five, Coagulase-negative Staphylococcus (CNS) in four, S. epidermidis in four, Enterococcus species in two and the remaining seen in one patient each: Corynebacterium striatum, Escherichia coli, Group B Streptococcus (GBS), Methicillin-resistant S. aureus (MRSA) and Proteus species. Five patients had mixed growths (including CNS/GBS, MSSA/Corynebacterium, P. acnes/Proteus species, E. coli/MSSA, MSSA, Proteus species); and no growths were seen in six patients.
Although it was intended for all 28 cases to be managed with a second stage, three patients had not proceeded to a second stage by the time of this paper. One case refused surgery, and two cases underwent further surgery in the form of a definitive single-stage revision (with reimplantation). The decision to proceed with a single stage revision followed careful consideration of their medical comorbidities (including high body mass index), normal serial WCC and inflammatory markers and an intra-operative assessment demonstrating no overt signs of infection. Intraoperative samples from the first of these definitive single stage revisions isolated S. capitis (in one of the five samples) which was considered a contaminant; and at 25 months follow-up the patient remains asymptomatic for infection with normal WCC and inflammatory markers. Intraoperative samples from the second definitive single stage revision isolated P. acnes in four of the five samples, and it was decided to treat with 12 weeks of intravenous clindamycin after consideration of the local and systemic risks of repeat revision surgery. Serum WCC and inflammatory markers normalised in the first five weeks of antibiotic therapy and have remained unremarkable until recent review at 14 months post-revision surgery. The patient does not demonstrate any clinical or radiographic signs of infection; however, definitive confirmation through aspiration has not been performed. It is recognised that a successful first stage revision (and the decision to proceed with a second stage) can be challenging in some cases, especially those with complex medical comorbidities.
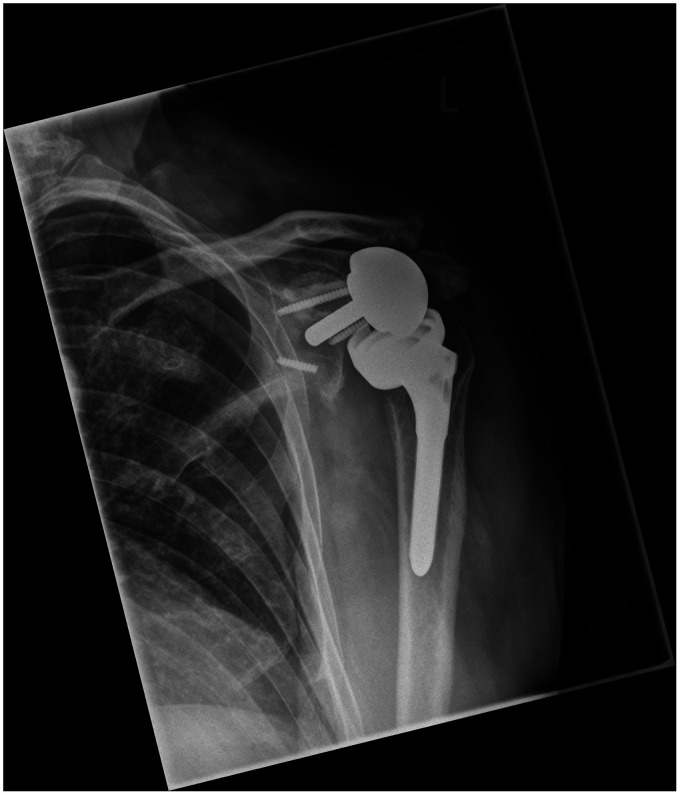
Twenty-five patients underwent reimplantation of a TSA after a mean interval of 6.7 months (range 2–21 months) after the first stage (see Table 2). The 25 second stage patients included 14 males and 11 females (mean age 70.2 years; range 37–82 years). Fifteen of these 25 cases (60%) had radiographic evidence of implant loosening (see Figure 2). The indication for the primary arthroplasty was acute fracture (11 patients), rotator cuff arthropathy (6), osteoarthritis (7) and osteomyelitis (1). First stage microbiological results and patient comorbidities can be viewed in Table 2. In six patients prolonged culture identified no growths; however, pre-operative clinical and serum assessment and intraoperative review were considered suggestive of PJI, in line with MSIS criteria, and the known difficulties in culturing low virulence organisms (such as P. acnes) and the resultant high risk of false negative sampling.
Table 2.
Summary of two-stage revision cases.
| Case | Sex | Age (y) | Indication for primary TSA | X-ray loosening | Year of revision | Delay between first and second stages (m) | Intra- operative culture result | Comorbidities | Out- patient follow-up (m) | Reinfection |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 78 | OM | Y | 2006 | 8 | CNS | – | D | Y |
| 2 | F | 69 | F | N | 2007 | 6 | NG | – | 62 | N |
| 3 | F | 65 | F | Y | 2007 | 5 | CNS/GBS | RA | D | N |
| 4 | M | 67 | F | Y | 2007 | 5 | NG | – | 73 | N |
| 5 | F | 75 | OA | Y | 2008 | 4 | E | H, O | 9 | N |
| 6 | F | 81 | OA | Y | 2009 | 16 | CNS | RA | 88 | N |
| 7 | M | 56 | F | Y | 2010 | 7 | MSSA | – | 69 | Y |
| 8 | F | 79 | OA | Y | 2010 | 5 | NG | – | 51 | N |
| 9 | M | 82 | F | N | 2011 | 14 | MRSA | PD | 62 | Y |
| 10 | F | 72 | F | Y | 2012 | 5 | NG | DM, H | 37 | N |
| 11 | F | 78 | F | N | 2012 | 5 | PA | DM | 48 | N |
| 12 | M | 70 | RCA | Y | 2013 | 6 | NG | – | 18 | N |
| 13 | F | 70 | RCA | N | 2013 | 21 | NG | A | 25 | N |
| 14 | F | 64 | F | N | 2013 | 2 | SE | – | 40 | N |
| 15 | M | 71 | RCA | Y | 2013 | 6 | PA | – | 42 | N |
| 16 | M | 70 | OA | Y | 2013 | 4 | SE | – | 39 | N |
| 17 | M | 37 | F | N | 2013 | 7 | C/MSSA | O | 37 | Y |
| 18 | M | 65 | F | N | 2014 | 2 | MSSA | – | 35 | Y |
| 19 | F | 77 | RCA | N | 2014 | 6 | SE | – | 29 | N |
| 20 | M | 69 | OA | N | 2014 | 6 | CNS | – | 20 | N |
| 21 | M | 79 | F | Y | 2014 | 6 | SE | L | 26 | N |
| 22 | M | 71 | RCA | Y | 2014 | 4 | PA | – | 19 | N |
| 23 | F | 67 | OA | N | 2014 | 7 | E | – | 23 | N |
| 24 | M | 77 | OA | Y | 2014 | 8 | P/PA | – | 18 | N |
| 25 | M | 67 | RCA | Y | 2015 | 2 | PA | Dep | 11 | N |
OM: osteomyelitis; F: fracture; OA: osteoarthritis; RCA: rotator cuff arthropathy; Y: yes; N: no; D: deceased; NG: no growth; CNS: coagulase-negative Staphylococcus; GBS: Group B Streptococcus; E: Enterococcus spp.; MRSA: Methicillin-resistant Staphylococcus aureus; PA: Propionibacterium acnes; SE: Staphylococcus epidermidis; C: Corynebacteria; MSSA: Methicillin-sensitive Staphylococcus aureus; P: Proteus ssp; RA: rheumatoid arthritis; PD: Parkinson's disease; DM: diabetes mellitus; H: essential hypertension; A: asthma; O: obesity; L: lymphoma; Dep: depression.
Figure 2.
Left shoulder anteroposterior (AP) plain radiograph demonstrating a severely loosened reverse-type TSA.
All 25 patients that proceeded to a second stage had a negative microbiology outcome from the guided-aspirate after a minimum of two weeks post-cessation of antibiotic therapy. Patients proceeding to a second stage were followed up clinically (with serial plain radiographs) for a minimum of nine months (mean 38.3, range 9–88 months), which excludes two cases that died of other causes. Of the patients treated with two-stage revision arthroplasty, 20 of 25 remained free from infection (80%).
Discussion
The literature reports recurrent infection rates of between 0% and 37% following two-stage revision for shoulder PJI.32,33 Our study is one of only two to review exclusively the efficacy of two-stage revision arthroplasty in the management of shoulder PJI. We demonstrated 80% eradication using this treatment modality, which compares to only 63% eradication by Strickland et al. (see Table 3).33 It has been demonstrated that lower limb PJI occurring within four weeks of surgery, termed ‘early’ using a temporal classification,34 can often be successfully managed with implant retention, lavage, debridement and antibiotics, with late PJI requiring implant replacement.35,36 All cases in the current series presented for a tertiary opinion ‘late’ in the clinical course and were therefore not suitable for debridement and implant retention. The vast majority of published studies pertain to hip and knee PJI. A systematic review of lower limb single-stage and two-stage revision arthroplasty concluded that both approaches afforded similar reinfection rates; however, the authors cautioned that the quality of the studies was generally poor and that randomised trials were required to compare more accurately revision arthroplasty outcomes.37
Table 3.
Summary of shoulder PJI studies that include revision arthroplasty.
| Study | Patients (TSAs) | Management (cases per treatment) | Three most commonly isolated organisms in order of incidence | Mean follow-up in months (range) | Eradication rate (%) |
|---|---|---|---|---|---|
| Coste et al.13 | 42 (49) | Multiple - Antibiotics (5) - Resection (10) - Debridement (8) - Single-stage (3) - Two-stage (10) - Spacer/other (3) | Staphylococcus epidermidis Propionibacterium acnes Staphylococcus aureus | 32 (12–96) | 71 |
| Ince et al.35 | 16 (16) | Single-stage | CNS Propionibacterium sp. Streptococcus | 70 (13–160) | 100 |
| Strickland et al.38 | 17 (19) | Two-stage | Propionibacterium sp. CNS Staphylococcus aureus | 35 (24–80) | 63 |
| Beekman et al.6 | 11 (11) | Single-stage | Propionibacterium acnes CNS Escherichia coli | 24 (12–36) | 90.9 |
| Romanò et al.39 | 44 (44) | Multiple - Two-stage (17) - Spacer (15) - Resection (6) - Debridement (5)a | CNS Staphylococcus aureus Propionibacterium acnes | 41 (21–98) | 95.5 |
| Klatte et al.40 | 35 (35)b | Single-stage | Staphylococcus epidermidis Propionibacterium acnes Staphylococcus capitis | 56 (13–162) | 94 |
| Jacquot et al.37 | 32 (32) | Multiple - Two-stage (14) - Debridement (13) - Resection (6) - Single-stage (5) | CNS Propionibacterium acnes Otherc | 37 (12–137) | 81 |
| This study | 28 (28) | Two-stage | Propionibacterium acnes MSSA CNS/Staphylococcus epidermidis | 38.3 (9–88) | 80d |
CNS: coagulase negative staphylococcus; MSSA: methicillin sensitive Staphylococcus aureus.
aThree debridement cases received exchange of mobile parts.
bIncludes nine cases previously reported by Ince et al. (see main text).
cOther: remaining cultures had equal incidence.
d80% indicates the eradication rate for the 25 cases managed with second-stage revision with TSA reimplantation.
A review of the literature regarding treatment of shoulder PJI highlights the paucity of high-level studies, with retrospective series forming the overwhelming majority of the evidence.9,16,21,32 The multi-centre retrospective series published by Romanò et al.32 and Jacquot et al.15 compared multiple treatment options (including single- and two-stage revision and debridement alone); however, the studies made different conclusions, with the former demonstrating comparable eradication rates between two-stage revision, permanent antibiotic-loaded spacer and resection arthroplasty, and the latter reporting superior outcomes for single- and two-stage revision arthroplasties. Jacquot et al. described improved function and infection eradication rates (81% success at 37 months) associated with single-stage revision, however small study numbers prevented them from recommending a single-stage. Studies comparing multiple treatment groups are limited by selection bias, with patients managed on a case-by-case basis in the absence of standardised management algorithms. The most complex cases are more likely to be managed with two-stage revision.
Much of the literature reviewing a single treatment option relates to single-stage revision. Ince et al. retrospectively reviewed 16 cases and reported complete eradication with a mean follow-up of 5.8 years.41 Like the current study, the group utilised pre-operative aspiration, antibiotic-impregnated bone cement and antibiotic duration depended on clinical findings and a fall in CRP. Klatte et al., working in the same centre, reviewed 35 patients in 2013 (including nine cases previously reported in 2005) and presented a 6% reinfection rate (4.7 years mean follow-up).42 The latter study did not recommend single-stage revision above other treatment options but did highlight a shortened in-patient stay when compared to two-stages. P. acnes was shown to be a significant PJI burden, with Klatte et al. and the current study both demonstrating this skin commensal to be the second most common organism. A smaller, single-stage series reported 91% eradication in 10 cases (two years mean follow-up), and they cited the advantage of avoiding glenoid wear from the spacer when utilising a single-stage.43
Our series demonstrated five cases of P. acnes infection. This Gram-positive, anaerobic bacillus has low virulence characteristics and commonly colonises the sebaceous glands of the skin,3 especially the chest, back and axillae.44,45 The deltopectoral approach is a risk factor for deep wound inoculation25; however, this is the established approach for arthroplasty. Both male gender and humeral component loosening are recognised as significant pre-operative predictors of positive P. acnes cultures.46–48 Although too few in number to make definitive conclusions, four of the five cases of P. acnes in the current study were male and all these male cases demonstrated evidence of pre-operative radiographic humeral component loosening (the single female case did not).
A high index of suspicion is required when diagnosing shoulder PJI due to indolent infection being commonplace.1,22 We highlight that patients presenting with any combination of non-specific pain, stiffness or instability complicating a TSA should focus ones attention to the possibility of shoulder PJI, even in the absence of classic signs and symptoms of infection.22 Although serum inflammatory markers and white blood cell (WBC) count are essential during the initial work-up, they have low sensitivity in the shoulder.49–52 For example, serum white WBC has a sensitivity of only 7%.51 Although the current study monitored WCC, CRP and ESR we did not find absolute values to be any more predictive of infection eradication beyond a positive or negative result in general. The 2nd International Consensus Meeting on Prosthetic Joint Infection in 2018 concluded that WCC, CRP and ESR have poor sensitivity in the diagnosis of shoulder PJI.21 Plain radiographs are also essential but are neither sensitive nor specific for indolent shoulder PJI.1 Radiographic humeral component loosening and osteolysis in painful but otherwise aseptic TSAs are associated with a threefold and tenfold increase in P. acnes culture rates, respectively.48 In the current study, only 18 of the 28 cases exhibited radiological evidence of loosening, which supports radiological assessment in combination with clinical review and blood markers. Magnetic resonance imaging (MRI) is of limited use due to local metal artefact; however, we occasionally utilise computed tomography (CT) to assess implant loosening, evaluate periprosthetic bone stock and/or permit bespoke computer-aided design and manufacture (CAD CAM) prostheses. We rarely use Technetium Tc 99m bone scanning as it cannot differentiate between infection and aseptic failure, and Indium In 111-labelled WBC scanning accuracy is poor in PJI.33 Pre-operative synovial fluid culture from joint aspiration remains an important investigation for shoulder PJI, however high false positive rates are common1,21,33,41; and clinically relevant infection can be difficult to distinguish from contamination, especially for P. acnes.51,53,54 A positive aspirate culture in broth alone in the absence of additional evidence of infection is likely to be contamination and repeat aspiration should be considered.1,31 There is increasing consensus regarding the pre-operative work-up of suspected shoulder PJI.1,25 Although guidelines of the MSIS and AAOS have been widely accepted for lower limb PJI, some have proposed the need for shoulder-specific criteria given the added complexity of indolent infection.54
We support synovial fluid aspirates and periprosthetic tissue (or fluid) specimens being incubated for an extended period due to the high prevalence of low virulence organisms. Intraoperative inspection may help predict the presence of P. acnes, especially when pre-operative aspirates prove inconclusive, with membrane formation and the presence of cloudy fluid being predictors, albeit subjective, for P. acnes infection.21,48 Intraoperative tissue culture following an antibiotic-free period remains of paramount importance.55 MSIS guidance recommends obtaining ‘at least two’ intra-operative samples (tissue or fluid) for microbiological analysis,56 whilst the AAOS recommends ‘multiple’ samples.30 Four to five samples are generally considered optimal, with five recommended by the 2nd International Consensus Meeting on Prosthetic Joint Infection.21,57–60 Although we performed two-stage revision with the expectation that all TSAs were infected, we aim to harvest odd sample numbers to avoid inconclusive results. As previously discussed, samples were collected by a non-standardised method at the start of the review period. However, since the latter stage of the review we sampled using an infection tray with five instrument sets in keeping with the work of Atkins et al.57 Despite the retrieval process having changed during the study, we utilised separate microbiology pots and a single histology pot for all cases. In addition to the change in our sampling protocol, the current study is further limited by a small sample size, the retrospective study design and the absence of a control group for comparison. However, when compared to the other published studies (all retrospective series) we present the largest number of two-stage revision cases (see Table 3).
Two-stage revision did not successfully eradicate infection in 5 of the 25 cases in our study. Upon review of these cases, pre-operative work-up would not have predicted these failures and, unfortunately, we are unable to identify common patient risk factors or suggest alternative treatment strategies for this group. The decision to proceed to a second stage was dependent upon satisfactory clinical assessment, a review of WCC and inflammatory markers and a negative inter-stage aspirate result. Although infection following the second stage could be related to new microbial contamination at the second stage, false negative aspirates cannot be excluded. Despite the potential for uneven distribution of organisms making detection more difficult,25 there is limited evidence to recommend pre-reimplantation open or arthroscopic tissue biopsy in these patients.21 In the absence of a gold standard diagnostic test we support the use of our tripartite strategy, including (1) no clinical signs of infection, (2) antibiotic treatment having been completed and (3) negative aspirate result and normal inflammatory markers following cessation of antibiotics for at least two weeks.
In the future, polymerase chain reaction (PCR) techniques could be utilised in cases with a high clinical suspicion but associated with negative cultures61; however, it's efficacy in shoulder PJI remains unproven.21 Our institution routinely incubates microbiology samples for at least 14 days, which is supported by much of the literature.62,63 Incubation periods of up to 21 days has been recommended60,64; however, periods beyond 14 days are associated with increased false positive rates.39,65,66 Although frozen section histological analysis of periprosthetic tissue is supported by both the MSIS and AAOS,29,30 low sensitivity has been demonstrated for P. acnes.54 Future management decisions may be more accurately guided by antimicrobial peptides (e.g. alpha-defensin), pro-inflammatory cytokines (e.g. IL-1 beta, IL-6), interferon-gamma, tumour necrosis factor-alpha (TNF-alpha) and vascular endothelial growth factor (VEGF), which have demonstrated encouraging results in the hip and knee.38,67–70 Synovial fluid alpha-defensin and IL-6 have shown good diagnostic accuracy for shoulder PJI, including for low virulence organisms such as P. acnes67,71; however, testing for leucocyte esterase has proven unreliable in shoulder PJI.72
Conclusion
Shoulder PJI is a potentially devastating complication, and antibiotic suppression alone or long-term suppressive antibiotic therapy combined with debridement, antibiotics and implant retention (DAIR) have unacceptably high failure rates, with many patients requiring future excision arthroplasty. Attempts to salvage the implants should be restricted to patients presenting with infection in the immediate post-operative period. The lack of a comparison group prevents us from recommending two-stage revision above other treatment choices. Randomised controlled trials are required to more accurately compare reinfection rates and functional outcomes for patients with shoulder PJI. The current study demonstrates high eradication rates (80%) in complex ‘late’ shoulder PJI cases managed at a national tertiary referral department by two-stage revision. A multi-disciplinary approach to management is essential for optimising patient outcomes. In the absence of a management consensus for shoulder PJI, our experience supports two-stage revision arthroplasty as an effective treatment strategy for eradicating infection in this complex patient group.
Acknowledgement
This article was presented as a free paper podium presentation at the British Elbow and Shoulder Society (BESS) annual meeting 2015, Sheffield.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Review and Patient Consent: Ethical approval sought but not required (Royal National Orthopaedic Hospital NHS Research & Development Committee). This study was a retrospective review of imaging and case notes, and as such individual patients' consent was deemed to be unnecessary and was not obtained, in line with local R&D policy.
References
- 1.Updegrove GF, Armstrong AD, Kim HM. Preoperative and intraoperative infection workup in apparently aseptic revision shoulder arthroplasty. J Shoulder Elbow Surg 2015; 24: 491–500. [DOI] [PubMed] [Google Scholar]
- 2.Dines JS, Fealy S, Strauss EJ, et al. Outcomes analysis of revision total shoulder replacement. J Bone Joint Surg Am 2006; 88: 1494–1500. [DOI] [PubMed] [Google Scholar]
- 3.Portillo ME, Salvadó M, Alier A, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Rel Res 2013; 471: 3672–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohsali KI, Wirth MA, Rockwood CA. Complications of total shoulder arthroplasty. J Bone Joint Surg Am 2006; 88: 2279–2292. [DOI] [PubMed] [Google Scholar]
- 5.Braman JP, Sprague M, Bishop J, et al. The outcome of resection shoulder arthroplasty for recalcitrant shoulder infections. J Shoulder Elbow Surg 2006; 15: 549–553. [DOI] [PubMed] [Google Scholar]
- 6.Jerosch J, Schneppenheim M. Management of infected shoulder replacement. Arch Orthop Trauma Surg 2003; 123: 209–214. [DOI] [PubMed] [Google Scholar]
- 7.Farshad M, Gerber C. Reverse total shoulder arthroplasty – from the most to the least common complication. Int Orthop 2010; 34: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coste JS, Reig S, Trojani C, et al. The management of infection in arthroplasty of the shoulder. Bone Joint J 2004; 86: 65–69. [PubMed] [Google Scholar]
- 9.Boileau P, Melis B, Duperron D, et al. Revision surgery of reverse shoulder arthroplasty. J Shoulder Elbow Surg 2013; 22: 1359–1370. [DOI] [PubMed] [Google Scholar]
- 10.Gardner J, Gioe TJ, Tatman P. Can this prosthesis be saved? Implant salvage attempts in infected primary TKA. Clin Orthop Rel Res 2011; 469: 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lora-Tamayo J, Murillo O, Iribarren JA, et al. A large multicenter study of methicillin–susceptible and methicillin–resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis 2013; 56: 182–194. [DOI] [PubMed] [Google Scholar]
- 12.Rispoli DM, Sperling JW, Athwal GS, et al. Pain relief and functional results after resection arthroplasty of the shoulder. Bone Joint J 2007; 89: 1184–1187. [DOI] [PubMed] [Google Scholar]
- 13.Themistocleous G, Zalavras C, Stine I, et al. Prolonged implantation of an antibiotic cement spacer for management of shoulder sepsis in compromised patients. J Shoulder Elbow Surg 2007; 16: 701–705. [DOI] [PubMed] [Google Scholar]
- 14.Weber P, Utzschneider S, Sadoghi P, et al. Management of the infected shoulder prosthesis: a retrospective analysis and review of the literature. Int Orthop 2011; 35: 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacquot A, Sirveaux F, Roche O, et al. Surgical management of the infected reversed shoulder arthroplasty: a French multicenter study of reoperation in 32 patients. J Shoulder Elbow Surg 2015; 24: 1713–1722. [DOI] [PubMed] [Google Scholar]
- 16.Clare DJ, Wirth MA, Groh GI, et al. Shoulder arthrodesis. J Bone Joint Surg Am 2001; 83: 593–600. [DOI] [PubMed] [Google Scholar]
- 17.Haleem AA, Berry DJ, Hanssen AD. The Chitranjan Ranawat Award: mid-term to long-term followup of two-stage reimplantation for infected total knee arthroplasty. Clin Orthop Rel Res 2004; 428: 35–39. [DOI] [PubMed] [Google Scholar]
- 18.Leone JM, Hanssen AD. Management of infection at the site of a total knee arthroplasty. J Bone Joint Surg Am 2005; 87: 2335–2348. [DOI] [PubMed] [Google Scholar]
- 19.Mileti J, Sperling JW, Cofield RH. Reimplantation of a shoulder arthroplasty after a previous infected arthroplasty. J Shoulder Elbow Surg 2004; 13: 528–531. [DOI] [PubMed] [Google Scholar]
- 20.Zhang AL, Feeley BT, Schwartz BS, et al. Management of deep postoperative shoulder infections: is there a role for open biopsy during staged treatment? J Shoulder Elbow Surg 2015; 24: e15–20. [DOI] [PubMed] [Google Scholar]
- 21.The second international consensus meeting on prosthetic joint infection (part III, shoulder), https://icmphilly.com/document/icm-2018-shoulder-document/ (accessed 10 December 2018).
- 22.Foruria AM, Fox TJ, Sperling JW, et al. Clinical meaning of unexpected positive cultures (UPC) in revision shoulder arthroplasty. J Shoulder Elbow Surg 2013; 22: 620–627. [DOI] [PubMed] [Google Scholar]
- 23.Achermann Y, Vogt M, Leunig M, et al. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J Clin Microbiol 2010; 48: 1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly JD, II, Hobgood ER. Positive culture rate in revision shoulder arthroplasty. Clin Orthop Relat Res 2009; 467: 2343–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shields MV, Abdullah L, Namdari S. The challenge of Propionibacterium acnes and revision shoulder arthroplasty: a review of current diagnostic options. J Shoulder Elbow Surg 2016; 25: 1034–1040. [DOI] [PubMed] [Google Scholar]
- 26.Kim SJ, Kim JH. Unexpected positive cultures including isolation of Propionibacterium acnes in revision shoulder arthroplasty. Chin Med J 2014; 127: 3975–3979. [PubMed] [Google Scholar]
- 27.Achermann Y, Goldstein EJ, Coenye T, et al. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev 2014; 27: 419–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saltzman MD, Marecek GS, Edwards SL, et al. Infection after shoulder surgery. J Am Acad Orthop Surg 2011; 19: 208–218. [DOI] [PubMed] [Google Scholar]
- 29.Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Rel Res 2011; 469: 2992–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Della Valle C, Parvizi J, Bauer TW, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg 2010; 18: 760–770. [DOI] [PubMed] [Google Scholar]
- 31.Barrack RL, Jennings RW, Wolfe MW, et al. The Coventry Award. The value of preoperative aspiration before total knee revision. Clin Orthop Relat Res 1997; 345: 8–16. . [PubMed] [Google Scholar]
- 32.Romanò CL, Borens O, Monti L, et al. What treatment for periprosthetic shoulder infection? Results from a multicentre retrospective series. Int Orthop 2012; 36: 1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strickland JP, Sperling JW, Cofield RH. The results of two-stage re-implantation for infected shoulder replacement. Bone Joint J 2008; 90: 460–465. [DOI] [PubMed] [Google Scholar]
- 34.McPherson EJ, Tontz W, Jr, Patzakis M, et al. Outcome of infected total knee utilizing a staging system for prosthetic joint infection. Am J Orthop 1999; 28: 161–165. [PubMed] [Google Scholar]
- 35.Cui Q, Mihalko WM, Shields JS, et al. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am 2007; 89: 871–882. [DOI] [PubMed] [Google Scholar]
- 36.Hanssen AD, Osmon DR. Evaluation of a staging system for infected hip arthroplasty. Clin Orthop Rel Res 2002; 403: 16–22. [DOI] [PubMed] [Google Scholar]
- 37.Leonard HA, Liddle AD, Burke Ó, et al. Single-or two-stage revision for infected total hip arthroplasty? A systematic review of the literature. Clin Orthop Rel Res 2014; 472: 1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacovides CL, Parvizi J, Adeli B, et al. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty 2011; 26: 99–103. [DOI] [PubMed] [Google Scholar]
- 39.Fink B, Makowiak C, Fuerst M, et al. The value of synovial biopsy, joint aspiration and C-reactive protein in the diagnosis of late peri-prosthetic infection of total knee replacements. Bone Joint J 2008; 90: 874–878. [DOI] [PubMed] [Google Scholar]
- 40.Athwal GS, Sperling JW, Rispoli DM, et al. Deep infection after rotator cuff repair. J Shoulder Elbow Surg 2007; 16: 306–311. [DOI] [PubMed] [Google Scholar]
- 41.Ince A, Seemann K, Frommelt L, et al. One-stage exchange shoulder arthroplasty for peri-prosthetic infection. Bone Joint J 2005; 87: 814–818. [DOI] [PubMed] [Google Scholar]
- 42.Klatte TO, Junghans K, Al-Khateeb H, et al. Single-stage revision for peri-prosthetic shoulder infection. Bone Joint J 2013; 95: 391–395. [DOI] [PubMed] [Google Scholar]
- 43.Beekman PD, Katusic D, Berghs BM, et al. One-stage revision for patients with a chronically infected reverse total shoulder replacement. Bone Joint J 2010; 92: 817–822. [DOI] [PubMed] [Google Scholar]
- 44.Grice EA, Kong HH, Conlan S, et al. Topographical and temporal diversity of the human skin microbiome. Science 2009; 324: 1190–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel A, Calfee RP, Plante M, et al. Propionibacterium acnes colonization of the human shoulder. J Shoulder Elbow Surg 2009; 18: 897–902. [DOI] [PubMed] [Google Scholar]
- 46.Hudek R, Sommer F, Kerwat M, et al. Propionibacterium acnes in shoulder surgery: true infection, contamination, or commensal of the deep tissue? J Shoulder Elbow Surg 2014; 23: 1763–1771. [DOI] [PubMed] [Google Scholar]
- 47.Koh CK, Marsh JP, Drinković D, et al. Propionibacterium acnes in primary shoulder arthroplasty: rates of colonization, patient risk factors, and efficacy of perioperative prophylaxis. J Shoulder Elbow Surg 2016; 25: 846–852. [DOI] [PubMed] [Google Scholar]
- 48.Pottinger P, Butler-Wu S, Neradilek MB, et al. Prognostic factors for bacterial cultures positive for Propionibacterium acnes and other organisms in a large series of revision shoulder arthroplasties performed for stiffness, pain, or loosening. J Bone Joint Surg Am 2012; 94: 2075–2083. [DOI] [PubMed] [Google Scholar]
- 49.Berbari E, Mabry T, Tsaras G, et al. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am 2010; 92: 2102–2109. [DOI] [PubMed] [Google Scholar]
- 50.Piper KE, Fernandez-Sampedro M, Steckelberg KE, et al. C-reactive protein, erythrocyte sedimentation rate and orthopedic implant infection. PLoS One 2010; 5: e9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Topolski MS, Chin PY, Sperling JW, et al. Revision shoulder arthroplasty with positive intraoperative cultures: the value of preoperative studies and intraoperative histology. J Shoulder Elbow Surg 2006; 15: 402–406. [DOI] [PubMed] [Google Scholar]
- 52.Villacis D, Merriman JA, Yalamanchili R, et al. Serum interleukin-6 as a marker of periprosthetic shoulder infection. J Bone Joint Surg Am 2014; 96: 41–45. [DOI] [PubMed] [Google Scholar]
- 53.Dodson CC, Craig EV, Cordasco FA, et al. Propionibacterium acnes infection after shoulder arthroplasty: a diagnostic challenge. J Shoulder Elbow Surg 2010; 19: 303–307. [DOI] [PubMed] [Google Scholar]
- 54.Grosso MJ, Frangiamore SJ, Ricchetti ET, et al. Sensitivity of frozen section histology for identifying Propionibacterium acnes infections in revision shoulder arthroplasty. J Bone Joint Surg Am 2014; 96: 442–447. [DOI] [PubMed] [Google Scholar]
- 55.Malekzadeh D, Osmon DR, Lahr BD, et al. Prior use of antimicrobial therapy is a risk factor for culture-negative prosthetic joint infection. Clin Orthop Rel Res 2010; 468: 2039–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parvizi J, Jacovides C, Zmistowski B, et al. Definition of periprosthetic joint infection: is there a consensus? Clin Orthop Rel Res 2011; 469: 3022–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atkins BL, Athanasou N, Deeks JJ, et al. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. J Clin Microbiol 1998; 36: 2932–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parvizi J, Azzam K, Ghanem E, et al. Periprosthetic infection due to resistant staphylococci: serious problems on the horizon. Clin Orthop Rel Res 2009; 467: 1732–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parvizi J, Jacovides C, Antoci V, Ghanem E. Diagnosis of periprosthetic joint infection: the utility of a simple yet unappreciated enzyme. J Bone Joint Surg Am 2011; 93: 2242–2248. [DOI] [PubMed] [Google Scholar]
- 60.Warme WJ, Hsu JE. Definition of a ‘true’ periprosthetic shoulder infection still eludes us. J Bone Joint Surg Am 2015; 97: e56. [DOI] [PubMed] [Google Scholar]
- 61.Zmistowski B, Della Valle C, Bauer TW, et al. Diagnosis of periprosthetic joint infection. J Orthop Res 2014; 32: S98–S107. [DOI] [PubMed] [Google Scholar]
- 62.Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol 2011; 49: 2490–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frangiamore SJ, Saleh A, Grosso MJ, et al. Early versus late culture growth of Propionibacterium acnes in revision shoulder arthroplasty. J Bone Joint Surg Am 2015; 97: 1149–1158. [DOI] [PubMed] [Google Scholar]
- 64.Matsen FA, Butler-Wu S, Carofino BC, et al. Origin of propionibacterium in surgical wounds and evidence-based approach for culturing propionibacterium from surgical sites. J Bone Joint Surg Am 2013; 95: e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schäfer P, Fink B, Sandow D, et al. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis 2008; 47: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 66.Shannon SK, Mandrekar J, Gustafson DR, et al. Anaerobic thioglycolate broth culture for recovery of Propionibacterium acnes from shoulder tissue and fluid specimens. J Clin Microbiol 2013; 51: 731–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frangiamore SJ, Saleh A, Grosso MJ, et al. α-Defensin as a predictor of periprosthetic shouler infection. J Shoulder Elbow Surg 2015; 24: 1021–1027. [DOI] [PubMed] [Google Scholar]
- 68.Gollwitzer H, Dombrowski Y, Prodinger PM, et al. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg Am 2013; 95: 644–651. [DOI] [PubMed] [Google Scholar]
- 69.Guenther D, Kokenge T, Jacobs O, et al. Excluding infections in arthroplasty using leucocyte esterase test. Int Orthop 2014; 38: 2385–2390. [DOI] [PubMed] [Google Scholar]
- 70.Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One 2014; 9: e89045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frangiamore SJ, Saleh A, Kovac MF, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg 2015; 97: 63–70. [DOI] [PubMed] [Google Scholar]
- 72.Nelson GN, Paxton ES, Narzikul A, et al. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg 2015; 24: 1421–1426. [DOI] [PubMed] [Google Scholar]