Abstract
Recent study suggests that protofibril-formation of amyloidogenic proteins (APs) might be involved in evolvability, an epigenetic inheritance of multiple stresses, in various biological systems. In cancer, evolvability of multiple APs, such as p53, γ-synuclein and the members of the calcitonin family of peptides, might be involved in various features, including increased cell proliferation, metastasis and medical treatment resistance. In this context, the objective of this paper is to explore the potential therapeutic benefits of reduced APs evolvability against cancer. Notably, the same APs are involved in the pathogenesis of neurodegenerative disease and cancer. Given the unsatisfactory outcomes of recent clinical trial of Aβ immunotherapy in Alzheimer's disease, it is possible that suppressing the aggregation of individual APs might also be not effective in cancer. As such, we highlight the adiponectin (APN) paradox that might be positioned upstream of AP aggregation in both neurodegenerative disease and cancer, as a common therapeutic target in both disease types. Provided that the APN paradox due to APN resistance under the diabetic conditions might promote AP aggregation, suppressing the APN paradox combined with antidiabetic treatments might be effective for the therapy of both neurodegenerative disease and cancer.
Keywords: Amyloidogenic proteins (APs), Evolvability, Neurodegenerative disease, Adiponectin (APN) paradox, APN resistance, Type 2 diabetes mellitus
Introduction
Emerging evidence suggests that amyloid might play an important role in epigenetics. The concept of amyloidogenic evolvability was primarily described in yeast prions where phenotypic diversity in yeast due to amyloid-regulated genetic variation may be a powerful survival strategy against multiple environmental stressors, which is transmissible to offspring during cell division [1]. Considering that both yeast and human brain that face multiple environmental challenges, coping with stress is a critical requirement in both circumstances. Based on this, we described that evolvability of amyloidogenic proteins (APs) relevant to various neurodegenerative diseases, such as amyloid-β (Aβ) in Alzheimer's disease (AD) and α-synuclein (αS) in Parkinson's disease (PD), might be important in the human brain [2]. Although evolvability may be beneficial in reproduction, neurodegenerative disease might be manifest through anatagonistic pleiotropy mechanism during aging [3].
Given that both neurodegenerative conditions and cancer are exposed to stressful conditions associated with AP misfolding, the evolvability of APs, such as p53 and γ-synuclein (γS), might play a critical role in various aspects of cancer pathogenesis, including cell proliferation, resistance, and metastasis [4]. Then, it is natural to predict that APs evolvability could be a therapeutic target in cancer. In this context, the main objective of the present paper is to discuss whether targeting AP evolvability can have potential therapeutic benefit against aging-associated cancers that are associated with expressions of multiple APs. As such, common APs might be involved in both neurodegenerative disease and cancer, assuming that similar pathogenic mechanisms are shared between these disorders. Since APs evolvability may be increased by the adiponectin (APN) paradox, a key phenomenon in aging-associated chronic diseases, we suggest that the APN paradox could be a potential target for the therapy of aging-associated cancers.
Amyloid evolvability in cancer
Many cancers, especially in the advanced stages, are characterized by aberrant cell growth and metastasis, and frequently acquire a resistance against a number of anti-neoplastic treatments, such as chemotherapy, irradiation, and hormonal therapy. Although the precise mechanism underlying such transformative tumor characteristics remains elusive, it is possible that adaptation to multiple stressors associated with such therapies might be relevant.
Amyloidogenic evolvability could underlie the mechanism of adaptation to multiple stressors. Among a number of APs, p53 and γS, the 2 major cancer-relevant APs, might be involved this phenomenon [4]. p53 is a master regulatory protein that is ubiquitously expressed in the nucleus and regulates various cellular processes, including apoptosis, DNA repair and cell cycle control, in response to genotoxic stress [4]. In the majority of cancers, mutant p53 not only loses tumor suppression, but often gains additional oncogenic functions that endow cells with growth and survival advantages [4]. On the other hand, γS is a cytoplasmic protein of unknown functions that belongs to the αS family of peptides, which was first identified as a breast cancer-specific gene [5]. The expression of γS has been found in a variety of cancers, including pancreatic, liver, prostate, ovarian, bladder, lung, and cervical cancers [5].
Notably, both p53 and γS have the propensity to form amyloid-like fibrils [4,5]. Supposing that p53 and γS are primarily involved in evolvability against nuclear and cytoplasmic stressors, respectively (Figure 1), it is reasonable to speculate that there might be other APs associated with evolvability against extracellular stressors. In this regard, systemic amyloidosis is associated with many extracellular APs, including immunoglobulin light chains and serum amyloid A proteins, transthyretin, gelsolin, cystatin C, apolipoprotein A1, and lysozyme [6]. It is also noteworthy that plasma Aβ levels in patients are increased in some types of cancer [7].
Figure 1.
Schematic of amyloidogenic evolvability of various APs relevant to cancer. Diagram illustrating amyloidogenic evolvability in cancer. Cancers are confronted with diverse stresses. For instance, it is known that radiation therapy, chemotherapy and various causes of DNA damages may lead to DNA replication stress in the nucleus (Stress N). Furthermore, cytoplasmic stresses may include mitochondrial oxidative stress, proteotoxic stress, metabolic stress, ER stress (Stressors C), whereas extracellular stresses such as hypoxia, inflammation, hyperthermia, mechanical stresses, pH change (Stress E) may occur due to alteration of microenvironment [46], [47], [48]. In response to multiple stressors, protofibrillar oligomerization of APs, including p53, γS, tau, AM, and CT, may be induced, which might confer resistance against each stressor in parental tumor cells. Subsequently, the stress information may be delivered from primary tumors to progeny tumors and to metastatic tumors by transmission of APs through a prion-like propagation, namely amyloidogenic evolvability. AD, Alzheimer's disease; AM, adrenomedullin; AP, amyloidogenic protein.
Also, the calcitonin (CT) family of peptides may be important because the members of this family are aberrantly expressed in various types of cancers (Figure 1). For instance, expression of CT has been well characterized in medullary thyroid cancer [8]. Furthermore, a role for CT family peptides and receptors in prostate cancer and bone metastasis has been well established [9]. Moreover, some members of the CT family, such as adrenomedullin (AM) and amylin, have been implicated in the pathogenesis of pancreatic cancer [10]. Consistent with this, AM was shown to stimulate pancreatic cell proliferation and invasion in an autocrine manner via the AM receptor in cell cultures [11]. Furthermore, it is noteworthy that pancreatic adenocarcinoma has a high incidence of diabetes, profound insulin resistance, and high circulating amylin concentrations [12], suggesting that increased amylin might be secreted in cancer. Collectively, these results are in line with the role of the CT family of peptides in evolvability against diverse stressors in the extracellular apace (Figure 1). Since amyloid-fibril structures of the CT family of peptides have been mainly investigated for amylin and CT [13,14], further investigations might be required.
Notably, the aggregation propensities of amyloidogenic proteins (APs) were shown using the hemagglutinin (HA) epitope-tagged Sup35NM domain (NM-HA) expressed in neuroblastoma cells [15], which might be comparable to the AP transmission to progeny cells in cancer, but not applicable to neurodegenerative diseases since post-mitotic neurons that no longer divide. It is important to clarify whether this is a passive phenomenon or an active mechanism might be involved.
Involvement of common APs in neurodegenerative disease and cancer evolvability
Of significant importance, APs relevant to cancer are also involved in the pathogenesis of neurodegeneration. In support of this, p53, a central molecular actor in cancer, has been well characterized in various neurodegenerative diseases, including AD, PD and Huntington's disease (HD) [16,17]. In AD, levels of p53 were enhanced in the AD brain (Figure 2A, a), which may maintain tau hyperphosphorylation. Furthermore, it was shown that p53 promotes neuronal death or survival via transcription-dependent mechanisms. Moreover, soluble Aβ oligomers increase p53 amount and activity promoting downstream p53 effects. The interactions of p53 with tau and Aβ may represent potential p53-based therapeutics for AD [18]. In PD, it was shown that dopaminergic neuron‐specific deletion of p53 gene is neuroprotective in an experimental PD mouse model [19]. Finally, consistent with the role of p53 in the pathogenesis of HD, p53 mediated cellular dysfunction and behavioral abnormalities in both in vitro and in vivo models of HD [20].
Figure 2.
Histological analyses of the increased expression of APs both in cancer and in neurodegenerative disease. A, a. Immunohistochemistry shows that p53 immunoreactivity in prostatic adenocarcinoma (left upper) is much stronger than that in normal control (left down). Scale bar = 100 µm. Immunoflorescence study of p53 is increased in AD brain (right upper) compared to in healthy brain (right down). Scale bar = 50 µm. b. Immunohistochemistry shows that γS immunoreactivity is strongly observed in human prostatic adenocarcinoma (left upper), but is hardly detectable in normal control (left down). Scale bar = 100 µm. On the other hand, γS immunoreactivity is increased in DLB (right upper) compared to in healthy brain (right down). Scale bar = 20 µm. c. Increased tau immunoreactivity is strongly observed in human breast cancer (left upper), but is faint in normal control (left down). Scale bar = 100 µm. On the other hand, tau immunoreactivity in AD (right upper) is increased creased compared to healthy controls (right down). Scale bar = 25 µm. d. Immunohistochemistry shows that AM immunoreactivity is strongly observed in human pancreatic adenocarcinoma (left upper), but is hardly detectable in chronic pancreatitis (left down). Scale bar = 100 µm. On the other hand, AM immunoreactivity is increased in AD compared to in healthy brain. Scale bar = 25 µm. Reprinted and modified from Kaczorowski et al. [49] (a), Farmer et al. [50] (a), Chen et al. [51] (b), Galvin et al. [52] (b), Matrone et al. [53] (c), Waragai et al. [54] (c), Ramachandran et al.[11] (d), and Ferrero et al. [55] (d) with permission. B, Protein aggregates accumulate in human metastatic melanoma. a. Immunofluorescence images with Proteostat (red) and DAPI (blue) staining on human normal skin (upper, left), samples of primitive melanomas (upper, right), melanoma metastases in brain (down, left) and melanoma metastases in lung (down, right). Scale bar = 30 µm. b. Quantitation of Proteostat-positive dots in primitive vs metastatic melanoma tissues: 6 tissues from metastatic lesions and 6 from primitive melanoma lesions were analyzed. For each tissue, 2 sections were quantified. T-test analysis was applied. T-test analysis, **P < 0.01 (N = 6, data are mean ± SEM). Modified from Matafora et al. [30] with permission. AD, Alzheimer's disease; AM, adrenomedullin; AP, amyloidogenic protein.
On the other hand, γS, primarily identified as a breast cancer-specific gene product, may be similarly relevant. Indeed, it was subsequently shown that γS expression is elevated in the advanced stages of many types of cancers, including breast, ovarian, lung, liver, esophagus, colon, and prostate (Figure 2A, b) [5]. γS has also been implicated in the axonal pathology of α-synucleinopathies, such as dementia with Lewy bodies (DLB) (Figure 2b), neurodegeneration with brain iron accumulation, type 1, and other neurodegenerative conditions including retinal degeneration and amyotrophic lateral sclerosis [5]. Notably, γS related proteins, αS and β-synuclein, neurodegeneration stimulator, and inhibitor respectively, are also involved in the pathogenesis of cancer. Indeed, expression of αS was observed in a variety of brain tumors showing neuronal differentiation [21]. Furthermore, expression of α-, β-, and γS was observed in glial tumors and medulloblastomas [22], and αS was also expressed in malignant melanoma [23].
In addition to γS, tau, the major AP expressed in AD (Figure 2A, c) and other tauopathies, might be involved in evolvability against the cytoplasmic stressors (Figure 1). Indeed, tau is expressed in many cancers, such as pancreatic, colon, lung, breast, and prostate cancers (Figure 2A, c) [24,25]. Of an interest, tau mutations might be related to genome instability and loss of chromosome integrity [26]. Regarding cancer-related secreted APs, increased expression of AM was increased not only in tissues of pancreatic cancer, but also in AD brain [27] (Figure 2A, d). Furthermore, amylin was shown to interact and co-deposit with Aβ and tau protein in AD brain, thereby contributing to diabetes-associated dementia [28]. Moreover, protofibrillar CT oligomers induced both impaired LTP and NMDA-mediated neurotoxicity, suggesting that that CT might contribute to neurodegeneration [29]. Taken together, it is likely that virtually all APs are commonly involved in both neurodegenerative disease and cancer pathogenesis, indicating that a shared mechanism of evolvability might be operant in 2 distinctly different types of disorders.
Although APs are characterized by formation of amyloid fibrils in the pathogenesis of neurodegenerative disease, it is unclear whether increased expression of APs is associated with aggregation or fibrillar formation in cancer (Figure 2A). Providing that AP-protofibrils with intrinsically disordered protein structural diversities might be beneficial for cancer evolvability, the fibrillar formation of APs are also involved in cancer. Consisted with our hypothetic view, accumulation of amyloid aggregates was shown in metastatic melanoma [30]. Thus, it is speculated that expression of APs is associate with fibrillar formation in other cancers.
As far as we know, there are presently little papers referring to the functional connection between APs aggregation and either cancer or neurodegenerative diseases. In this regard, however, one possibility is that structural alteration of APs at monomer level might be relevant to the stress resistance. In support of this notion, it was shown that expression of γS monomer assesses by western blot analysis was correlated with resistance against irradiation in cultured cells [31]. Further investigations are definitely warranted.
Targeting amyloid evolvability in cancer therapy
Presuming that amyloidogenic evolvability might be critical in various aspects of cancer development, this mechanism might represent an important therapeutic target. For this, a dose reduction of APs either at the mRNA level by antisense oligonucleotide technology (ASO) [32] or at the protein level by immunotherapy might reduce formation of AP protofibrils, resulting in reduced amyloidogenic evolvability, and leading to delayed cancer progression. Based on a view that the functions of p53 in the cancer pathogenesis might be altered due to the protein misfolding, Silva et al. proposed that inhibiting p53 aggregation through various approaches, including the use of small-molecule and peptide stabilizers of mutant p53, zinc administration, gene therapy, alkylating and DNA intercalators, and blockage of p53-MDM2 interaction, might be therapeutic for cancers [33,34]. This is consistent with our view suggesting that decreasing evolvability of p53 against nuclear stresses might enhance the effects of cancer therapies and mitigate cancer recurrence.
However, as described above, different APs might be simultaneously involved in evolvability against diverse stressors in multiple cellular locations, including the nucleus, cytoplasm, and the extracellular space. Therefore, we presume that simultaneous interference with the aggregation of several APs might be necessary for proper therapeutic efficacy. One may even argue that suppressing the aggregation of APs might be necessary, but not sufficient, for the effective treatment of cancer. Indeed, the situation might be comparable to that of neurodegenerative disease, in which recent difficulties of AD clinical trials using Aβ immunotherapy suggests that inhibition of aggregation of Aβ may be not be sufficient for the therapy of AD [35].
Role of the APN paradox in the pathogenesis of the aging-associated cancer
Provided this rationale, we specifically highlight a possible role for the APN paradox in promoting aggregation of APs (Figure 3). APN is a multifunctional adipokine that suppresses inflammation and sensitizes insulin receptor signaling [36]. Although APN is protective in many experimental systems, APN is detrimental in aging-associated circulatory diseases, including chronic heart failure and chronic kidney disease, the so-called APN paradox [36]. Currently, the mechanism of the APN paradox is poorly understood. However, it was described that the down-regulation of APN receptor, AdipoR1 might be attributed to insulin/APN resistances [37]. Thus, given the comorbidity of AD with type 2 diabetes mellitus (T2DM) [38], it is possible that hyperadiponectinemia due to APN and insulin coresistance in T2DM might underlie the APN paradox.
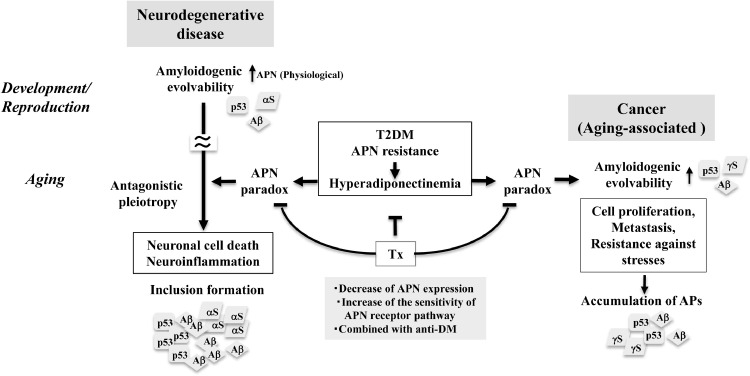
Fig. 3.
APN paradox as a therapeutic target of cancer: a hypothetic view. It is assumed that aging-associated neurodegenerative disease featured with neuronal cell death and neuroinflammation, might be an antagonistic phenomena phenomenon derived from amyloidogenic evolvability physiologically regulated by APN in development/reproduction. Supposing that amyloidogenic evolvability of cancer occurs in aging, it is predicted that APN paradox caused by hyperadiponectinemia under the diabetic conditions might lead to enhanced amyloidogenic evolvability associated with APs fibrillar formation. Accordingly, the APN paradox might be a therapeutic target by either decreasing APN expression or increasing the sensitivity of APN receptor signaling pathways. Furthermore, supposing that T2DM may be situated upstream of hyperadiponectinemia, combined therapy with a T2DM anti-diabetic agent might be more effective (Tx). AP, amyloidogenic protein; APN, adiponectin; T2DM, type 2 diabetes mellitus.
Emerging evidence suggests that the APN paradox is also the case with other aging-associated diseases, including neurodegenerative disease and cancer. In the nervous system, APN may be involved in evolvability in reproduction, which might later be manifest as neurodegenerative disease, such as AD through the antagonistic pleiotropy mechanism in aging [36]. Supporting this, hyperadiponectinemia was correlated with amyloid imaging of brain in normal aging [39], suggesting that the APN paradox might play an important role including aggregation of APs in the early stage, such as mild cognitive impairment, in AD. In cancers, a recent prospective cohort study showed that significantly higher serum APN concentrations were observed in incident cancers, and were independently associated with cancer-related deaths in T2DM, indicating that the APN paradox might be manifest in cancer comorbid with T2DM [40].
Given that common APs are involved in both neurodegeneration and cancer, the APN paradox might also occur in cancer pathogenesis during aging. It is generally believed that APN may exert its anticarcinogenic effects including regulating cell survival, apoptosis, and metastasis via a plethora of signaling pathways [41]. Similar to the stimulating effect of the APN paradox on APs aggregation in AD, APs evolvability in cancer might be promoted by the APN paradox (Figure 3).
Targeting the APN paradox for cancer therapy
Accumulating evidence suggests that hyperadiponectinemia due to APN resistance under the diabetic conditions might result in APN paradox, leading to aging-associated chronic diseases, including neurodegenerative diseases [36] and cancers [40]. Therefore, it is reasonable to predict that reducing the APN paradox by preventing APN signaling might be beneficial to the therapy of cancer (Figure 3). For this, one possible therapeutic strategy is a dose reduction of APN either at the mRNA level by ASO [32] or at the protein level by immunotherapy might reduce formation of AP protofibrils, might prove effective.
Alternatively, APN signaling might be improved at the level of the APN receptors to potentially relieve APN resistance. As such, it is worth noting that resveratrol up-regulates renal AdipoR1 and -R2 expression in diabetic db/db mice, improving complications such as diabetic nephropathy [42]. Furthermore, it is also possible the sensitivity of APN receptor signaling pathway might be increased through modification of signaling molecules thereby. In this context, APPL1, adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif, might be interesting [43].
It is also worth noting that the use of metformin, anti-T2DM drug, has been associated with a reduced risk of developing cancer and an improvement in overall cancer survival rates in meta-analyses, indicating that metformin can be used as an adjuvant treatment for various cancers associated with T2DM, including hepatocellular carcinoma, pancreatic, endometrial, breast, and colorectal cancer [44,45]. Collectively, we predict that suppression of the APN paradox through decrease of APN expression, combined with a treatment of T2DM, might be a therapeutic strategy against cancers associated with T2DM (Figure 3).
Concluding remarks
Given that the common APs might be involved in the pathogenesis of neurodegenerative disease and cancer through evolvability, both mechanisms and therapeutic strategies may be similar between the 2 disorders. Thus, findings relevant to either neurodegenerative disease or aging-associated cancer might be collectively beneficial to a mutual understanding both types of conditions. In AD, despite the apparent neurotoxicity of Aβ supported by many experimental models, the outcomes of multiple recent clinical studies of Aβ immunotherapy have been unsatisfactory [35]. In this regard, tau might be additionally important beyond Aβ alone. Alternatively, it might be possible that suppression of protein aggregation of APs may be not sufficient for the therapy of AD. If the same is applied to cancer, suppressing expression of individual APs, such as p53, γS, tau and CT family of peptides, might be not efficient for the therapy of cancer.
As discussed above, accumulating evidence suggests that the APN paradox might be critical not only for circulatory diseases but also neurodegenerative disease and cancer, in which T2DM may play a stimulatory role. Therefore, it is predicted that suppressing the APN paradox by decreasing APN expression, combined with a T2DM anti-diabetic treatment, might be considered for the therapeutic strategy against both neurodegenerative disease and cancer associated with T2DM mechanisms. Since human aging is distinct from those of animals, there are currently no appropriate experimental models to evaluate our hypothetic view that APN paradox might be a potential target for the therapy of aging-associated diseases, including neurodegenerative disease and cancer. Thus, better understanding of this issue might be a central issue in GeroScience.
Author contribution
Regarding the paper ‘Adiponectin paradox as a therapeutic target of the cancer evolvability in aging’, MH had an idea, MH, YT, and GH wrote the manuscript, and all authors discussed and agreed to submit the paper.
Acknowledgments
We are grateful for the continuous encouragement of Drs. Kaori Hashimoto (Tokyo Metropolitan Institute of Medical Science, Tokyo) and Maria del Carmen Ruiz de la Cruz (University of Chicago, USA).
Footnotes
Abbreviations: APs, amyloidogenic proteins; APN, adiponectin; Aβ, amyloid-β; AD, Alzheimer's disease; αS, α-synuclein; PD, Parkinson's disease; γS, γ-synuclein; CT, calcitonin; AM, adrenomedullin; HD, Huntington's disease; ASO, antisense oligonucleotide; T2DM, type 2 diabetes mellitus.
Declaration of Competing Interest: The authors declare that they have no competing interests.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Wickner RB. Yeast and fungal prions. Cold Spring Harb Perspect Biol. 2016;8:a02531. doi: 10.1101/cshperspect.a023531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashimoto M, Ho G, Sugama S, Takamatsu Y, Shimizu Y, Takenouchi T, Waragai M, Masliah E. Evolvability of amyloidogenic proteins in human brain. J Alzheimers Dis. 2018;62:73–83. doi: 10.3233/JAD-170894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashimoto M, Ho G, Takamatsu Y, Shimizu Y, Sugama S, Takenouchi T, Waragai M, Masliah E. Evolvability and neurodegenerative disease: antagonistic pleiotropy phenomena derived from amyloid aggregates. J Parkinsons Dis. 2018;8:405–408. doi: 10.3233/JPD-181365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takamatsu Y, Ho G, Hashimoto M. Amyloid Evolvability and Cancer. Trends Cancer. 2020;6:624–627. doi: 10.1016/j.trecan.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad M, Attoub S, Singh MN, Martin FL, El-Agnaf OM. Gamma-synuclein and the progression of cancer. FASEB J. 2007;21:3419–3430. doi: 10.1096/fj.07-8379rev. [DOI] [PubMed] [Google Scholar]
- 6.Khurana R, Agarwal A, Bajpai VK, Verma N, Sharma AK, Gupta RP, Madhusudan KP. Unraveling the amyloid associated with human medullary thyroid carcinoma. Endocrinology. 2004;145:5465–5470. doi: 10.1210/en.2004-0780. [DOI] [PubMed] [Google Scholar]
- 7.Jin WS, Bu XL, Liu YH, Shen LL, Zhuang ZQ, Jiao SS, Zhu C, Wang QH, Zhou HD, Zhang T. Plasma Amyloid-Beta Levels in Patients with Different Types of Cancer. Neurotox Res. 2017;31:283–288. doi: 10.1007/s12640-016-9682-9. [DOI] [PubMed] [Google Scholar]
- 8.Thomas CM, Asa SL, Ezzat S, Sawka AM, Goldstein D. Diagnosis and pathologic characteristics of medullary thyroid carcinoma-review of current guidelines. Curr Oncol. 2019;26:338–344. doi: 10.3747/co.26.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warrington JI, Richards GO, Wang N. The role of the calcitonin peptide family in prostate cancer and bone metastasis. Curr Mol Biol Rep. 2017;3:197–203. doi: 10.1007/s40610-017-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martínez A, Kapas S, Miller MJ, Ward Y, Cuttitta F. Coexpression of receptors for adrenomedullin, calcitonin gene-related peptide, and amylin in pancreatic beta-cells. Endocrinology. 2000;141:406–411. doi: 10.1210/endo.141.1.7261. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran V, Arumugam T, Hwang RF, Greenson JK, Simeone DM, Logsdon CD. Adrenomedullin is expressed in pancreatic cancer and stimulates cell proliferation and invasion in an autocrine manner via the adrenomedullin receptor, ADMR. Cancer Res. 2007;67:2666–2675. doi: 10.1158/0008-5472.CAN-06-3362. [DOI] [PubMed] [Google Scholar]
- 12.Ding X, Flatt PR, Permert J, Adrian TE. Pancreatic cancer cells selectively stimulate islet beta cells to secrete amylin. Gastroenterology. 1998;114:130–138. doi: 10.1016/s0016-5085(98)70641-9. [DOI] [PubMed] [Google Scholar]
- 13.Cao P, Abedini A, Raleigh DP. Aggregation of islet amyloid polypeptide: from physical chemistry to cell biology. Curr Opin Struct Biol. 2013;23:82–89. doi: 10.1016/j.sbi.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rigoldi F, Metrangolo P, Redaelli A, Gautieri A. Nanostructure and stability of calcitonin amyloids. J Biol Chem. 2017;292:7348–7357. doi: 10.1074/jbc.M116.770271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krammer C, Kryndushkin D, Suhre MH, Kremmer E, Hofmann A, Pfeifer A, Scheibel T, Wickner RB, Schätzl HM, Vorberg I. The yeast Sup35NM domain propagates as a prion in mammalian cells. Proc Natl Acad Sci U S A. 2009;106:462–467. doi: 10.1073/pnas.0811571106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szybinska A, Lesniak W. P53 dysfunction in neurodegenerative diseases - the cause or effect of pathological changes? Aging Dis. 2017;8:506–518. doi: 10.14336/AD.2016.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang JR, Ghafouri M, Mukerjee R, Bagashev A, Chabrashvili T, Sawaya BE. Role of p53 in neurodegenerative diseases. Neurodegener Dis. 2012;9:68–80. doi: 10.1159/000329999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jazvinscak Jembrek M, Slade N, Hof PR, Simic G. The interactions of p53 with tau and Ass as potential therapeutic targets for Alzheimer's disease. Prog Neurobiol. 2018;168:104–127. doi: 10.1016/j.pneurobio.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Qi X, Davis B, Chiang YH, Filichia E, Barnett A, Greig NH, Hoffer B, Luo Y. Dopaminergic neuron-specific deletion of p53 gene is neuroprotective in an experimental Parkinson's disease model. J Neurochem. 2016;138:746–757. doi: 10.1111/jnc.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bae BI, Xu H, Igarashi S, Fujimuro M, Agrawal N, Taya Y, Hayward SD, Moran TH, Montell C, Ross CA. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington's disease. Neuron. 2005;47:29–41. doi: 10.1016/j.neuron.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Kawashima M, Suzuki SO, Doh-ura K, Iwaki T. alpha-Synuclein is expressed in a variety of brain tumors showing neuronal differentiation. Acta Neuropathol. 2000;99:154–160. doi: 10.1007/pl00007419. [DOI] [PubMed] [Google Scholar]
- 22.Fung KM, Rorke LB, Giasson B, Lee VM, Trojanowski JQ. Expression of alpha-, beta-, and gamma-synuclein in glial tumors and medulloblastomas. Acta Neuropathol. 2003;106:167–175. doi: 10.1007/s00401-003-0718-x. [DOI] [PubMed] [Google Scholar]
- 23.Matsuo Y, Kamitani T. Parkinson's disease-related protein, alpha-synuclein, in malignant melanoma. PLoS One. 2010;5:e10481. doi: 10.1371/journal.pone.0010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garni R, Segura-Collar B, Sánchez-Gómez P. Novel functions of the neurodegenerative-related gene tau in cancer. Front Aging Neurosci. (Lausanne) 2019;11:231. doi: 10.3389/fnagi.2019.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Souter S, Lee G. Tubulin-independent tau in Alzheimer's disease and cancer: Implications for disease pathogenesis and treatment. Curr Alzheimer Res. 2010;7:697–707. doi: 10.2174/156720510793611637. [DOI] [PubMed] [Google Scholar]
- 26.Rossi G, Redaelli V, Contiero P, Fabiano S, Tagliabue G, Perego P, Benussi L, Bruni AC, Filippini G, Farinotti M. Tau mutations serve as a novel risk factor for cancer. Cancer Res. 2018;78:3731–3739. doi: 10.1158/0008-5472.CAN-17-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez AP, Masa JS, Guedan MA, Futch HS, Martinez-Murillo R. Adrenomedullin expression in Alzheimer's brain. Curr Alzheimer Res. 2016;13:428–438. doi: 10.2174/1567205013666160229112725. [DOI] [PubMed] [Google Scholar]
- 28.Raimundo AF, Ferreira S, Martins IC, Menezes R. Islet amyloid polypeptide: a partner in crime with a beta in the pathology of Alzheimer's disease. Front Mol Neurosci. 2020;13:35. doi: 10.3389/fnmol.2020.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belfiore M, Cariati I, Matteucci A, Gaddini L, Macchia G, Fioravanti R, Frank C, Tancredi V, D'Arcangelo G, Diociaiuti M. Calcitonin native prefibrillar oligomers but not monomers induce membrane damage that triggers NMDA-mediated Ca(2+)-influx, LTP impairment and neurotoxicity. Sci Rep. 2019;9:5144. doi: 10.1038/s41598-019-41462-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matafora V, Farris F, Restuccia U, Tamburri S, Martano G, Bernardelli C, Sofia A, Pisati F, Casagrande F, Lazzari L. Amyloid aggregates accumulate in melanoma metastasis modulating YAP activity. EMBO Rep. 2020;21:e50446. doi: 10.15252/embr.202050446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian L, Zhao Y, Truong MJ, Lagadec C, Bourette RP. Synuclein gamma expression enhances radiation resistance of breast cancer cells. Oncotarget. 2018;9:27435–27447. doi: 10.18632/oncotarget.25415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rinaldi C, Wood MJA. Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat Rev Neurol. 2018;14:9–21. doi: 10.1038/nrneurol.2017.148. [DOI] [PubMed] [Google Scholar]
- 33.Costa DC, de Oliveira GA, Cino EA, Soares IN, Rangel LP, Silva JL. Aggregation and prion-like properties of misfolded tumor suppressors: is cancer a prion disease? Cold Spring Harb Perspect Biol. 2016;8 doi: 10.1101/cshperspect.a023614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva JL, Cino EA, Soares IN, Ferreira VF, APdO G. Targeting the prion-like aggregation of mutant p53 to combat cancer. Acc Chem Res. 2018;51:181–190. doi: 10.1021/acs.accounts.7b00473. [DOI] [PubMed] [Google Scholar]
- 35.Waragai M, Ho G, Takamatsu Y, Wada R, Sugama S, Takenouchi T, Masliah E, Hashimoto M. Adiponectin paradox as a therapeutic target in Alzheimer's disease. J Alzheimers Dis. 2020;76:1249–1253. doi: 10.3233/JAD-200416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waragai M, Ho G, Takamatsu Y, Wada R, Sugama S, Takenouchi T, Masliah E, Hashimoto M. Adiponectin paradox in Alzheimer's disease; relevance to amyloidogenic evolvability? Front Endocrinol (Lausanne) 2020;11:108. doi: 10.3389/fendo.2020.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engin A. Adiponectin-resistance in obesity. Adv Exp Med Biol. 2017;960:415–441. doi: 10.1007/978-3-319-48382-5_18. [DOI] [PubMed] [Google Scholar]
- 38.Chatterjee S, Mudher A. Alzheimer's disease and type 2 diabetes: a critical assessment of the shared pathological traits. Front Neurosci. 2018;12:383. doi: 10.3389/fnins.2018.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wennberg AM, Gustafson D, Hagen CE, Roberts RO, Knopman D, Jack C, Petersen RC, Mielke MM. Serum adiponectin levels, neuroimaging, and cognition in the Mayo clinic study of aging. J Alzheimers Dis. 2016;53:573–581. doi: 10.3233/JAD-151201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee CH, Lui DTW, Cheung CYY, Fong CHY, Yuen MMA, Chow WS, Woo YC, Xu A, Lam KSL. Higher circulating adiponectin concentrations predict incident cancer in type 2 diabetes - the adiponectin paradox. J Clin Endocrinol Metab. 2020;105:dgaa075. doi: 10.1210/clinem/dgaa075. [DOI] [PubMed] [Google Scholar]
- 41.Katira A, Tan PH. Evolving role of adiponectin in cancer-controversies and update. Cancer Biol Med. 2016;13:101–119. doi: 10.28092/j.issn.2095-3941.2015.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park HS, Lim JH, Kim MY, Kim Y, Hong YA, Choi SR, Chung S, Kim HW, Choi BS, Kim YS. Resveratrol increases AdipoR1 and AdipoR2 expression in type 2 diabetic nephropathy. J Transl Med. 2016;14:176. doi: 10.1186/s12967-016-0922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim R-Y. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2016;14:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 44.Zhou PT, Li B, Liu FR, Zhang MC, Wang Q, Li YY, Xu C, Liu YH, Yao Y, Li D. Metformin is associated with survival benefit in pancreatic cancer patients with diabetes: a systematic review and meta-analysis. Oncotarget. 2017;8:25242–25250. doi: 10.18632/oncotarget.15692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zi F, Zi H, Li Y, He J, Shi Q, Cai Z. Metformin and cancer: an existing drug for cancer prevention and therapy. Oncol Lett. 2018;15:683–690. doi: 10.3892/ol.2017.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Dai Q, Park D, Deng X. Targeting DNA replication stress for cancer therapy. Genes (Basel) 2016;7:51. doi: 10.3390/genes7080051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker C, Mojares E, del Río Hernández A. Role of extracellular matrix in development and cancer progression. Int J Mol Sci. 2018;19:3028. doi: 10.3390/ijms19103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mantovani F. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019;26:199–212. doi: 10.1038/s41418-018-0246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaczorowski A, Tolstov Y, Falkenstein M, Vasioukhin V, Prigge E-S, Geisler C, Kippenberger M, Nientiedt C, Ratz L, Kuryshev V. Laboratory-prostate cancer. Rearranged ERG confers robustness to prostate cancer cells by subverting the function of p53. Urol Oncol. 2020;38 doi: 10.1016/j.urolonc.2020.06.016. 736.e731-736.e710. [DOI] [PubMed] [Google Scholar]
- 50.Farmer KM, Ghag G, Puangmalai N, Montalbano M, Bhatt N, Kayed R. P53 aggregation, interactions with tau, and impaired DNA damage response in Alzheimer's disease. Acta Neuropathologica Communications. 2020;8:132. doi: 10.1186/s40478-020-01012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J, Jiao L, Xu C, Yu Y, Zhang Z, Chang Z, Deng Z, Sun Y. Neural protein gamma-synuclein interacting with androgen receptor promotes human prostate cancer progression. BMC Cancer. 2012;12:593. doi: 10.1186/1471-2407-12-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galvin JE, Uryu K., Lee VM-Y, Trojanowski JQ. Axon pathology in Parkinson's disease and Lewy body dementia hippocampus contains α-, β-, and γ-synuclein. Proc Natl Acad Sci U S A. 1999;96:13450–13455. doi: 10.1073/pnas.96.23.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matrone MA, Whipple RA, Thompson K, Cho EH, Vitlo MI, Bazler EM, Yoon JR, Ioffe OB, Tuttle KC, Tan M, et al.(2010) Metastatic breast tumors express increased tau, which promotes microtentacle formation and the reattachment of detached breast tumor cells. 29, 3217–3227. [DOI] [PMC free article] [PubMed]
- 54.Waragai M, Adame A, Trinh I, Sekiyama K, Takamatsu Y, Une K, Masliah E, Hashimoto M. Possible involvement of adiponectin, the anti-diabetes molecule, in the pathogenesis of Alzheimer's disease. J Alzheimers Dis. 2016;52:1453–1459. doi: 10.3233/JAD-151116. [DOI] [PubMed] [Google Scholar]
- 55.Ferrero H, Larrayoz IM, Martisova E. Increased levels of brain adrenomedullin in the neuropathology of Alzheimer's disease. Mol Neurobiol. 2018;55:5177–5183. doi: 10.1007/s12035-017-0700-6. [DOI] [PubMed] [Google Scholar]