Abstract
Background: Longer time between symptom onset and treatment of Lyme disease has been associated with poor outcomes. Reducing time-to-treatment requires knowledge of risks for treatment delays. We conducted a population-based study to evaluate factors associated with delayed treatment of Lyme disease and the relation between delayed treatment and post-treatment Lyme disease syndrome (PTLDS).
Methods: We mailed questionnaires to 5,314 individuals with a Lyme disease diagnosis or blood test followed by an antibiotic order in the medical record of a Pennsylvania health system from 2015 to 2017. Analyses were confined to 778 respondents who reported that they were treated for Lyme disease within the past 5 years and reported a rash and/or a positive blood test for Lyme disease. Time-to-treatment was calculated as the sum of two windows before and after seeking care for Lyme disease symptoms: time to first medical contact and time under care. We used logistic regression to evaluate factors associated with delayed time-to-treatment in each time window (>14 days vs. ≤14 days) and the association between total time-to-treatment (>30 days vs. ≤30 days) and PTLDS. We used inverse probability weighting to calculate estimates for the study's source population (5,314 individuals sent questionnaires).
Results: In the source population, 25% had time to first contact >14 days, 21% had time under care >14 days, and 31% had a total time-to-treatment >30 days. Being uninsured and attributing initial symptoms to something other than Lyme disease were positively associated with delayed time to first medical contact, while seeking care at an urgent care or emergency setting (vs. primary care) was negatively associated. Diagnoses between November and April, and the absence of rash were positively associated with delays. Individuals whose treatment was delayed, defined as time-to treatment >30 days had 2.26 (95% confidence interval: 1.25, 4.05) times the odds of PTLDS as those who were treated within 30 days of symptom onset.
Conclusions: In a population-based study in Pennsylvania, one-third of Lyme disease patients reported delayed treatment, which was associated with PTLDS. To improve Lyme disease outcomes, prevention efforts should aim to reduce the time before and after seeking care.
Keywords: Lyme disease, treatment delays, post-treatment Lyme disease syndrome, time-to-treatment, disparities
Introduction
Lyme disease is on the rise in the United States, with almost 30,000 confirmed and over 13,000 probable cases in 2017 (1). Delayed treatment can lead to disseminated infection and serious complications (2, 3). Longer time between symptom onset and treatment (time-to-treatment) has been associated with poor Lyme disease outcomes (4–7). Post-treatment Lyme disease syndrome (PTLDS) is characterized by persistent or recurrent symptoms, lasting 6 months or more of fatigue, musculoskeletal pain, and cognitive complaints leading to decline in physical and social functioning (3, 8). The role of time-to-treatment in PTLDS remains unknown. Timely treatment may be important in preventing PTLDS and other long-term consequences of Lyme disease. Strategies to ensure timely treatment require a better understanding of the risk factors for treatment delays.
Of the few studies of time-to-treatment in Lyme disease, most have been confined to individuals with Lyme neuroborreliosis, a neurological manifestation of disseminated Lyme disease that occurs in about 12% of Lyme disease cases (4–7, 9). These studies have reported that longer time-to-treatment is associated with poor outcomes, including persistent Lyme disease symptoms and poor quality-of-life. No studies have evaluated the role of time-to-treatment in PTLDS, a condition that occurs in an estimated 10 to 20% of Lyme disease cases (10). PTLDS is a well-defined condition that is distinct from chronic Lyme disease, a non-specific term that has been used to describe illness in individuals with Lyme disease and around which there is ongoing debate (11). The biological basis for PTLDS is not well-understood, and no evidence-based treatment has been identified (8). Thus, exploring options for prevention is critical.
Evidence-based strategies for reducing time-to-treatment of Lyme disease are lacking, in part due to limited understanding of related risk factors. Prior studies have generally measured time-to-treatment of Lyme disease as a single time period (5–7). However, the General Model of Total Patient Delay, a widely used model that describes stages of treatment delay, differentiates the time before and after a patient sees a medical professional (12). The time between symptom onset and seeing a medical professional (hereafter, “time to first medical contact”) and the time while under the care of a medical professional until receiving treatment (hereafter, “time under care”) involve different actors and occur in different settings. Thus, these stages may have distinct risk factors that require different approaches for promoting timely treatment.
We conducted a retrospective cohort study of time-to-treatment among a general population sample of individuals treated for Lyme disease at Geisinger, a health system in Pennsylvania, the state with the most confirmed Lyme disease cases in the United States (13). Using self-administered questionnaire data, we characterized respondents' experiences with Lyme disease symptoms, care-seeking, diagnosis, and treatment; measured risk factors for delays in time to first medical contact and time under care; and examined associations between time-to-treatment and PTLDS.
Methods
Study Population
Participants were identified through the Geisinger electronic health record (EHR). Geisinger serves patients across 45 Pennsylvania counties. The primary care population represents the age and sex distribution of the region's population (14). We mailed questionnaires to 5,314 adult patients who met previously described EHR-based criteria for Lyme disease between 2015 and 2017 (15). Briefly, individuals had to have a Lyme disease diagnostic code (International Classification of Diseases, 9th Revision, code 088.81) or both a Current Procedural Terminology code for a Lyme disease serologic test (enzyme immunoassay or Western blot) and an antibiotic order appropriate for Lyme disease, regardless of length of treatment, within 30 days after the sample draw. Appropriate treatment was defined by the Infectious Disease Society of America's (IDSA) recommended first or second line antibiotics (3) and three antibiotics either closely related to recommended treatments or that were historical treatments (15). We excluded antibiotic orders if the diagnosis codes linked to the medication orders were for respiratory disease, since these are common diagnoses treated with the same antibiotics as Lyme disease. A $1 bill was included with the questionnaire. Non-respondents were re-sent a questionnaire 6 weeks after the original mailing. Geisinger's Institutional Review Board approved the study.
Questionnaire Development
We developed a questionnaire to measure time-to-treatment for Lyme disease and potential related factors and outcomes, informed by interviews with Lyme disease patients and physicians (16). Based on findings from this formative work, a panel of experts specializing in epidemiology, survey research, infectious disease, and rheumatology developed the questionnaire. Questions were derived from existing instruments or created de novo based on scientific literature.
Time-to-Treatment
Time-to-treatment was measured (in days) as the sum of two time windows: time to first medical contact and time under care. Time to first medical contact was based on response to the question, “About how long did you wait after your first symptom of Lyme disease before contacting a medical professional?” Time under care was based on response to the question, “How long was it from your first contact with a medical provider to when you were treated for Lyme disease?”
PTLDS
PTLDS was defined based on criteria developed by Aucott et al. (8), consistent with guidelines from the IDSA (3). Participants were classified as having PTLDS if they had received antibiotic treatment for Lyme disease and reported persistent symptoms and functional deficit. Respondents were classified as having persistent symptoms if they reported that one of the following symptoms had not changed, had worsened, or had newly occurred in the 6 months after completing antibiotic treatment for Lyme disease: fatigue, muscle pain, joint pain, memory changes, difficulty finding words, or difficulty focusing. Functional deficit was defined as a standardized T score <45 of the mean of the following subscales from the 36-Item Short Form Health Survey: role limitations due to physical health, energy/fatigue, emotional well-being, or role limitations due to emotional health (10, 17). Consistent with IDSA guidelines, a participant could not be classified as having PTLDS if they reported a prior diagnosis of fibromyalgia or chronic fatigue syndrome (CFS) (3).
Lyme Disease Symptoms, Care-Seeking, Diagnosis, and Treatment
The questionnaire captured the respondents' experiences related to Lyme disease symptoms, care-seeking, diagnosis, and treatment. Items related to Lyme disease symptoms included whether the respondent observed a tick bite or a rash, whether the rash was a bull's-eye rash, the constancy of symptoms, and to what condition respondents initially attributed their Lyme disease symptoms. Items related to care-seeking included specialty of the first medical professional contacted for Lyme disease symptoms, reason for contacting the medical professional, and barriers to contacting a medical professional. The questionnaire also assessed diagnosis received at the first medical visit, whether an antibiotic was prescribed, number of medical professionals seen before receiving a diagnosis of Lyme disease, and blood testing results.
Coping
Coping was assessed using the John Henry Active Coping Scale, a 12-item scale that assesses a personality pre-disposition to cope with psychosocial stressors (18). Items were summed for a total score ranging from 12 to 60, then dichotomized at the median to categorize respondents into low and high active coping groups.
Clinical and Demographic Characteristics
Through the questionnaire, we assessed history of a diagnosis prior to Lyme disease of cancer, fibromyalgia, CFS, rheumatoid arthritis, migraine, depression, and anxiety, as well as marital status, income, education, occupation, and insurance status at the time of Lyme disease diagnosis. Age and sex were obtained from the EHR.
Statistical Analysis
The goals of the analysis were to describe time-to-treatment in a population-based sample of individuals treated for Lyme disease, to identify risk factors for the two time-to-treatment delay windows, and to evaluate associations between time-to-treatment and PTLDS. Analyses were confined to respondents who self-reported a Lyme disease diagnosis within the past 5 years, completed questions related to time-to-treatment and rash, whose Lyme disease was confirmed based on self-report of a rash and/or a positive blood test for Lyme disease, who reported being prescribed antibiotics, and for whom time-to-treatment was plausible (i.e., less than their age) (n = 778). We used inverse probability weighting based on EHR-based characteristics available on responders and non-responders to calculate estimates for the source population of the study (the 5,314 individuals sent questionnaires).
We conducted chi-square tests to evaluate the proportion of individuals with delays in time to first medical contact and time under care by the following variables: season of diagnosis (November–April, May–October); presence of rash (yes, no); symptom attribution (Lyme disease, other condition); first medical professional contacted [primary care, urgent care, emergency department, other (e.g., inpatient or specialist)]; self-reported diagnosis of cancer, fibromyalgia, CFS, rheumatoid arthritis, migraine, depression, and anxiety (yes, no); age (18–39, 40–49, 50–59, 60–69, ≥ 70 years); sex (male, female); insurance at time of diagnosis (private insurance, Medicaid, no health insurance, Medicare); education (less than high school, high school graduate, some college, associate degree, bachelor's degree, graduate degree); and marital status (never married, separated/divorced/widowed, married or living with a partner). For each time window a delay was described as a period lasting more than 14 days. Next, we used logistic regression to evaluate factors associated with treatment delays, separately for time to first medical contact and time under care (>14 days vs. ≤ 14 days). All models controlled for age (continuous), sex, insurance status, rash, and season of diagnosis. Age was tested for linearity. Additional variables that demonstrated a bivariate association with the treatment delay were added to models individually. The final models retained variables that remained associated with the treatment delay using a threshold of p < 0.05. We used robust standard errors, calculated using the Huber–White sandwich estimator. Model diagnostics were performed to confirm the validity of multivariable models. Hosmer–Lemeshow tests and F-tests were used to assess goodness-of-fit, while scatterplots of standardized residual vs. predicted probability of outcome were used to look for influential observations (Supplementary Material).
We used logistic regression to evaluate the association between time-to-treatment (sum of time to first medical contact and time under care, >30 days vs. ≤ 30 days) and PTLDS (yes vs. no). The base model included age-centered, age-centered squared, sex, insurance, and time-to-treatment. We evaluated the following variables for confounding: self-reported prior diagnosis of cancer, migraine, rheumatoid arthritis, depression, or anxiety; education; occupation; marital status; and coping score (< median vs. ≥ median). Variables were retained if adding the variable to the model changed the estimate of the association between time-to-treatment and PTLDS by at least 10%. We evaluated whether depression, anxiety, rash, and coping modified the association between time-to-treatment and PTLDS by adding cross-product terms (separately for each interaction) to the model. The same model diagnostics described above were performed. Analyses were conducted using Stata 14.1 (19).
Results
Demographic and Clinical Characteristics
Of the 5,314 individuals who received a questionnaire, 1,364 returned a completed questionnaire, of whom 778 met the inclusion criteria for the analysis (Figure 1). Because weighted analysis accounts for potential participation bias, only weighted results are described in the text; both unweighted and weighted results are presented in tables. A little less than half of the study population was female and the mean age was 51 years (Table 1). At the time of Lyme disease diagnosis, 78% of the study population had private health insurance, 3% were not insured, and the remaining were insured with Medicare or Medicaid. An estimated 11.5% of the study population met the criteria for PTLDS.
Figure 1.
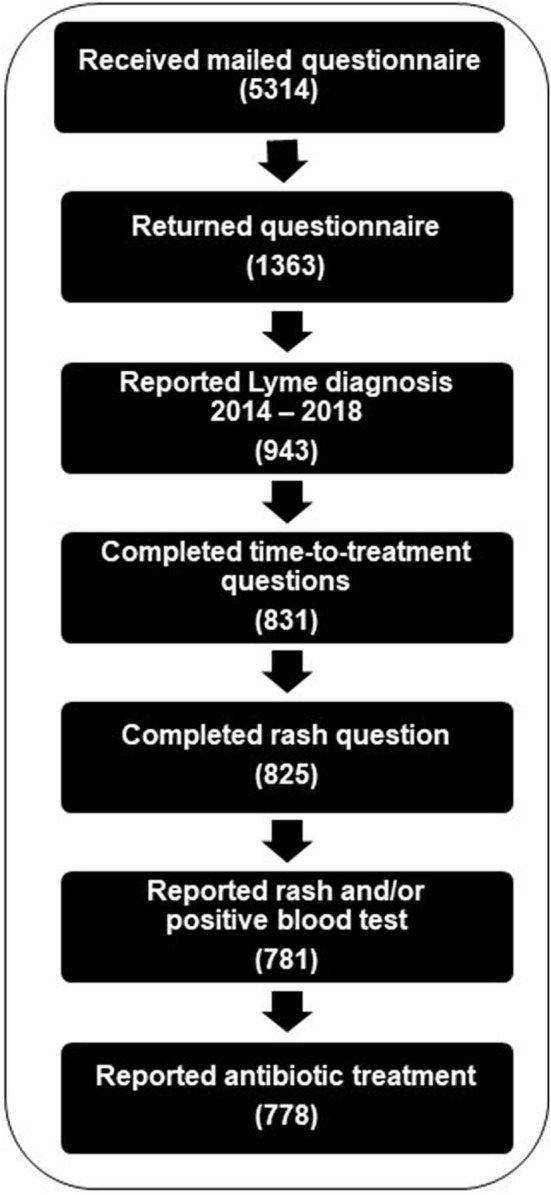
Creation of analytic dataset of respondents to the Lyme disease time-to-treatment questionnaire, with inclusion based on responses to questionnaire items regarding date of Lyme disease diagnosis, completion of time-to-treatment and rash questions with plausible response, report of rash and/or blood test, and report of antibiotic treatment.
Table 1.
Characteristics of study population, with unweighted and weighted percentages.
| Frequencies unless otherwise noted | |||
|---|---|---|---|
| N | Unweighted % | Weighted %a | |
| Total respondents | 778 | 100 | n/a |
| Age in years, mean | 57 | 51 | |
| 18–39 | 131 | 17 | 29 |
| 40–49 | 107 | 14 | 16 |
| 50–59 | 152 | 20 | 19 |
| 60–69 | 236 | 30 | 22 |
| ≥70 | 152 | 20 | 13 |
| Female | 401 | 52 | 48 |
| Education | |||
| Less than high school | 53 | 7 | 9 |
| High school graduate | 233 | 30 | 28 |
| Some college | 141 | 18 | 20 |
| Associate degree | 84 | 11 | 11 |
| Bachelor's degree | 139 | 18 | 18 |
| Graduate degree | 128 | 16 | 15 |
| Marital status | |||
| Never married | 83 | 11 | 16 |
| Separated, divorced, or widowed | 107 | 14 | 13 |
| Married or living with a partner | 588 | 76 | 71 |
| Self-reported health insurance statusb | |||
| Medicaid (with or without Medicare) | 52 | 7 | 10 |
| Medicare only | 91 | 12 | 8 |
| No health insurance | 22 | 3 | 3 |
| Private insurance | 613 | 79 | 78 |
| Self-reported diagnoses prior to Lyme diseasec | |||
| Cancer | 74 | 10 | 8 |
| Fibromyalgia | 26 | 3 | 3 |
| Chronic fatigue syndrome | 18 | 2 | 2 |
| Rheumatoid arthritis | 54 | 7 | 6 |
| Migraine | 88 | 11 | 11 |
| Depression | 137 | 18 | 17 |
| Anxiety | 142 | 18 | 19 |
| PTLDSd | |||
| Yes | 75 | 10 | 12 |
| No | 693 | 89 | 87 |
| Missing | 10 | 1 | 1 |
PTLDS, post-treatment Lyme disease syndrome.
Weighted by participation rates.
Self-reported insurance coverage at time of Lyme diagnosis.
Self-reported diagnosis by a doctor that occurred prior to Lyme disease.
PTLDS based on self-reported new or persistent symptoms and functional impairment after treatment, excluding those with prior diagnosis of chronic fatigue syndrome or fibromyalgia.
Time-to-Treatment
Median time-to-treatment was 13 days (Table 2). An estimated 31% of study population had time-to-treatment >30 days. One-quarter reported time-to-first contact with a medical professional >14 days and 21% reported time under care >14 days. Among those with total time-to-treatment >30 days, the average ratio of time to first medical contact to time under care was 1:1, with an equal contribution of time from both delay windows.
Table 2.
Symptom, care-seeking, diagnostic, and treatment experiences for Lyme disease among survey respondents (n = 778), with unweighted and weighted proportions.
| Frequencies unless otherwise noted | |||
|---|---|---|---|
| N | Unweighted % | Weighted % | |
| Time to treatment for Lyme disease | |||
| Days from first symptoms to contacting a medical professional, median (range) | 7 (0, 5,479) | ||
| 0–14 days | 601 | 77 | 75 |
| >14 days | 177 | 23 | 25 |
| Days from healthcare contact to treatment, median (range) | 2 (0, 13,880) | ||
| 0–14 days | 634 | 81 | 79 |
| >14 days | 144 | 19 | 21 |
| Total days from first symptoms to treatment, median (range) | 13 (0, 13,890) | ||
| 0–4 days | 203 | 26 | 24 |
| >4–14 days | 215 | 28 | 27 |
| >14–30 days | 142 | 18 | 18 |
| >30 days−6 months | 149 | 19 | 21 |
| >6 months | 69 | 9 | 10 |
| Experiences with Lyme disease symptoms | |||
| Observed a tick bite | 214 | 28 | 28 |
| Reported rasha | |||
| Experienced a typical bull's-eye rash | 372 | 48 | 46 |
| Experienced a rash (not bull's-eye) | 163 | 21 | 20 |
| No rash | 239 | 31 | 33 |
| Constancy of symptomsa | |||
| Symptoms were constant | 242 | 31 | 31 |
| Symptoms would come and go | 92 | 12 | 11 |
| Some constant, some would come and go | 375 | 48 | 51 |
| Attributed first symptoms to Lyme disease | 167 | 21 | 21 |
| Misattributed first symptoms to other conditionsb | |||
| Flu or virus | 251 | 32 | 34 |
| Bug bite, allergy, or skin problem | 127 | 16 | 15 |
| Muscle or joint strain/injury | 89 | 11 | 12 |
| Arthritis or bursitis | 80 | 10 | 10 |
| Dehydration, overexertion, stress, old age | 22 | 3 | 3 |
| Other | 49 | 6 | 8 |
| Did not know | 41 | 5 | 5 |
| Experiences seeking medical care for Lyme disease symptoms | |||
| Did not wait to contact a medical professional | 421 | 54 | 51 |
| Barriers to contacting a medical professionalb | |||
| Symptoms perceived to not be serious or were attributed to another cause | 321 | 41 | 43 |
| Socioeconomic barriers (e.g., cost, transportation, caregiving duties) | 41 | 5 | 7 |
| Immediate healthcare not accessible (e.g., appointments unavailable, traveling) | 21 | 3 | 4 |
| Reason for contacting a doctorb | |||
| Suspected Lyme disease (e.g., tick bite, bull's-eye rash, previous experience) | 95 | 12 | 11 |
| New symptoms appeared | 152 | 20 | 22 |
| Symptoms did not go away | 340 | 44 | 44 |
| Symptoms got more severe | 315 | 40 | 43 |
| Symptoms interfered with work or daily tasks | 175 | 22 | 27 |
| Family or friend said to go | 146 | 19 | 19 |
| Experiences with diagnosis and treatment for Lyme disease symptoms | |||
| First medical professional contacted about symptomsa | |||
| Urgent care | 190 | 24 | 25 |
| Emergency department | 85 | 11 | 12 |
| Primary care | 477 | 61 | 61 |
| Otherc | 25 | 3 | 3 |
| Diagnosis received at first medical visita | |||
| Lyme disease or suspected Lyme diseased | 455 | 58 | 56 |
| Flu or other viral infection | 50 | 6 | 6 |
| Skin rash, allergic reaction, shingles | 47 | 6 | 6 |
| Muscle or joint injury | 30 | 4 | 5 |
| Cellulitis or other skin infection | 23 | 3 | 3 |
| Insect bite | 22 | 3 | 3 |
| Arthritis | 5 | 1 | 1 |
| Other | 36 | 5 | 5 |
| None | 97 | 12 | 13 |
| Number of medical professionals seen for Lyme disease symptoms before receiving a Lyme disease diagnosisa | |||
| 0–1 | 423 | 54 | 52 |
| 2 | 140 | 18 | 19 |
| ≥3 | 91 | 12 | 13 |
| Medical care provider who diagnosed respondent's Lyme diseasea | |||
| Urgent care clinic doctor | 154 | 20 | 19 |
| Emergency department doctor | 72 | 9 | 9 |
| Primary care or family doctor | 432 | 56 | 55 |
| Specialist (e.g., rheumatologist, cardiologist, neurologist, infectious disease doctor) | 75 | 10 | 10 |
| Lyme specialist | 25 | 3 | 4 |
| Self-diagnosis or other non-medical diagnosis | 10 | 1 | 1 |
| Diagnosis seasona | |||
| May–October | 582 | 75 | 74 |
| November–April | 136 | 17 | 18 |
| Blood testinga | |||
| First test was positive | 501 | 64 | 63 |
| First test was negative, second test was positive | 102 | 13 | 16 |
| Blood tests only negative | 47 | 6 | 6 |
| Blood never tested | 110 | 14 | 13 |
| Received antibiotic treatment at first medical visit | |||
| Yes | 542 | 70 | 68 |
| No | 236 | 30 | 32 |
| Lyme disease treatment receiveda | |||
| 1 oral antibiotic | 556 | 71 | 70 |
| 1 intravenous antibiotic or 2 oral antibiotics | 135 | 17 | 17 |
| >2 antibiotics | 76 | 10 | 11 |
Categories do not add to 100% of sample due to missing data.
Categories are not mutually exclusive.
“Other” includes specialists (e.g., dermatologist) and inpatient/hospital.
Respondent indicated there was no diagnosis, but blood testing was ordered.
Experiences With Lyme Disease Symptoms, Care-Seeking, Diagnosis, and Treatment
Forty-six percent of the study population reported having a bull's-eye rash, and 20% reported a rash without central clearing. About one-fifth (21%) of the population attributed their initial symptoms to Lyme disease; the remaining attributed initial symptoms to flu or a virus (34%); a bug bite, allergy, or skin problem (15%); a muscle or joint strain/injury (12%); bursitis (10%); or a mix of other conditions (Table 2). Nearly half of the study population reported they did not immediately contact a medical professional largely because initial symptoms were not perceived to be serious.
The majority of the study population reported initially seeking care from a primary care provider (61%). Urgent care was the first contact for an estimated 25% of the population. An estimated 56% received a diagnosis of Lyme disease at their initial medical visit (Table 2), though 68% of the study population reported receiving antibiotic treatment at their first visit. Most diagnoses (74%) occurred between May and October.
Factors Associated With Delayed Time to First Medical Contact
In bivariate analyses, factors associated with delayed time to first contact with a medical professional (>14 days) included younger age, no rash, Lyme disease diagnosis between November and April, misattribution of symptoms, being uninsured, first medical contact in an urgent care or emergency department setting, and self-reported diagnosis of cancer. In a model adjusted for age, sex, presence of rash, and diagnosis season, the odds of delayed time to first medical contact among those who reported being uninsured was 3.49 [95% confidence interval (CI): 1.19, 10.21] times the odds of those with private insurance. The odds of delayed time to first medical contact among those who initially attributed their symptoms to something other than Lyme disease was 3.51 (95% CI: 1.79, 6.89) times the odds of those who initially attributed symptoms to Lyme disease (Table 3). Odds of delay among individuals who initially sought care in an urgent care or emergency department setting were 0.33 (95% CI: 0.17, 0.64) and 0.37 (0.17, 0.81), respectively, times the odds of those who sought care from a primary care provider. The odds of delay among who reported a rash was 0.44 (95% CI: 0.27, 0.71) times the odds among those without rash.
Table 3.
Logistic regression analysis of factors related to delays in contacting a medical professionala for Lyme disease.
| Study sample (n = 717b) unweighted | Source population weightedc | |
|---|---|---|
| Respondent characteristic | Odds ratio (95% CI) | Odds ratio (95% CI) |
| Age | 0.98 (0.97, 1.00) | 0.97 (0.96, 0.99) |
| Sex, female | 0.73 (0.50, 1.08) | 0.70 (0.44, 1.10) |
| Insuranced | ||
| Privately insured | Ref | Ref |
| Medicaid only or with Medicare | 1.03 (0.51, 2.07) | 1.26 (0.61, 2.62) |
| No health insurance | 3.09 (1.21, 7.86) | 3.49 (1.19, 10.21) |
| Medicare only | 1.50 (0.77, 2.92) | 1.84 (0.92, 3.69) |
| Presence of rash | 0.39 (0.26, 0.58) | 0.44 (0.27, 0.71) |
| Diagnosis season | ||
| May–October | Ref | Ref |
| November–April | 2.20 (1.42, 3.41) | 2.60 (1.60, 4.21) |
| Attributed first symptoms to Lyme disease | ||
| Yes | Ref | Ref |
| No | 2.93 (1.67, 5.14) | 3.51 (1.79, 6.89) |
| First medical provider contacted about Lyme disease symptoms | ||
| Primary care/family doctor | Ref | Ref |
| Urgent care clinic | 0.38 (0.22, 0.66) | 0.33 (0.17, 0.64) |
| Emergency department | 0.49 (0.27, 0.89) | 0.37 (0.17, 0.81) |
| Othere | 1.48 (0.61, 3.60) | 1.23 (0.44, 3.44) |
Delay characterized as >14 days (vs. ≤ 14 days) from first symptoms of Lyme disease to contacting a medical professional, as reported by respondents.
Data on rash, diagnosis season, and first medical provider contacted about Lyme disease symptoms missing for 61 respondents.
Weighted by participation rates.
Self-reported insurance coverage at time of Lyme diagnosis.
“Other” includes specialists (e.g., dermatologist) and inpatient/hospital.
Factors Associated With Time Under Care
In bivariate analyses, factors associated with delayed treatment while under care of a medical professional (>14 days) included younger age; never married; unable to work/disabled; no rash; Lyme disease diagnosis between November and April; first medical contact in an emergency department or “other” setting; and self-reported diagnosis of fibromyalgia, CFS, or migraine prior to Lyme disease. In models adjusted for age, sex, and insurance status, rash was associated with nearly half the odds of delay under care (Table 4). The odds of the delay among those diagnosed between November and April was 2.36 (95% CI: 1.37, 4.07) times the odds of those diagnosed at other times of the year. The odds of delay among those with a diagnosis of chronic fatigue syndrome was 5.02 (95% CI: 1.79, 14.12) times the odds among those without a diagnosis.
Table 4.
Logistic regression analysis of factors related to delays between healthcare contact and treatmenta for Lyme disease.
| Study sample (n = 718b) unweighted | Source population weightedc | |
|---|---|---|
| Respondent characteristic | Odds Ratio (95% CI) | Odds Ratio (95% CI) |
| Age | 0.98 (0.97, 1.00) | 0.98 (0.96, 1.00) |
| Sex, female | 0.96 (0.64, 1.43) | 1.06 (0.66, 1.71) |
| Insuranced | ||
| Privately insured | Ref | Ref |
| Medicaid only or with Medicare | 1.43 (0.72, 2.84) | 1.09 (0.48, 2.50) |
| No health insurance | 1.13 (0.41, 3.18) | 1.13 (0.40, 3.21) |
| Medicare only | 0.51 (0.22, 1.17) | 0.75 (0.25, 2.28) |
| Rash accompanied Lyme disease | 0.52 (0.34, 0.78) | 0.56 (0.34, 0.91) |
| Diagnosis season | ||
| May–October | Ref | Ref |
| November–April | 2.07 (1.32, 3.25) | 2.36 (1.37, 4.07) |
| Chronic fatigue syndromee | 5.03 (1.90, 13.29) | 5.02 (1.79, 14.12) |
Delay characterized as >14 days (vs. ≤ 14 days) from first contact with a medical provider to treatment for Lyme disease, as reported by respondents.
Data on rash and diagnosis season missing for 60 respondents.
Weighted by participation rates.
Self-reported insurance coverage at time of Lyme diagnosis.
Self-reported diagnosis (yes vs. no) by a doctor that occurred prior to Lyme disease.
Time-to-Treatment and PTLDS
The odds of PTLDS among those with time-to-treatment >30 days was 2.26 (95% CI: 1.25, 4.05) times the odds of those treated within 30 days, adjusting for age (centered and centered-squared), sex, and insurance status. Depression, anxiety, presence of rash, and coping did not modify the association between time-to-treatment and PTLDS.
Discussion
In this first population-based study of time-to-treatment of Lyme disease, we characterized experiences with Lyme disease symptoms, care-seeking, diagnosis, and treatment among individuals in Pennsylvania, a state highly endemic to Lyme disease; identified common and unique factors associated with delays before and after contacting a medical professional; and evaluated long-term consequences of delayed treatment. In a novel finding, we observed that time-to-treatment was associated with PTLDS, demonstrating the potential long-term consequences of delayed treatment. Several factors—including insurance status, the presence of a rash, diagnosis season, attribution of initial symptoms to Lyme disease, the first medical provider contacted about the symptoms, and a diagnosis of chronic fatigue syndrome prior to Lyme disease—were related to treatment delays. These findings have important implications for strategies to reduce time-to-treatment in Lyme disease and the potential of these efforts to improve long-term outcomes.
We found that delayed treatment was associated with higher risk of PTLDS. Although our study is the first to evaluate time-to-treatment in relation to PTLDS, the findings are consistent with prior studies that examined persistent symptoms. Rebman et al. (11) found that in a sample of individuals with PTLDS, 45% reported time-to-treatment >30 days. Negative consequences of delayed treatment for Lyme disease have been previously reported, with longer time-to-treatment associated with persistent symptoms, poor quality-of-life, and Lyme neuroborreliosis, but none, to our knowledge, have demonstrated an association with PTLDS specifically (4–7, 20). The benefit of shorter time-to-treatment has been attributed to the prevention of pathogen dissemination, resulting from earlier eradication of the Borrelia burgdorferi bacterium (5). Alternative hypotheses for the benefit of early antibiotic treatment include early interruption of the immune response, which may prevent secondary autoimmune reactions (5). While the pathogenesis of PTLDS remains unknown, an autoimmune response is one of the hypothesized causes (8).
Averting treatment delays in Lyme disease may be a key strategy for preventing PTLDS and other serious complications. Prior studies of Lyme disease have defined treatment delay as the time between symptom onset and treatment, with definitions of delay ranging from >30 days to >6 weeks (4, 5, 7, 11). We found that time-to-treatment >30 days has potentially important implications for Lyme disease outcomes, as this delay was associated with more than twice the odds of PTLDS. Of concern, 31% of our study population reported time-to-treatment exceeding 30 days. Other studies have also reported a large proportion of individuals with time-to-treatment longer than 30 days (7, 11). Thus, there remains a substantial delay in Lyme disease care that, if closed, could improve Lyme disease outcomes.
We found that the two time windows comprising time-to-treatment (both before and after contacting a medical professional) contributed equally to Lyme disease treatment delays. One prior study used similar time-to-treatment windows to evaluate individuals with Lyme neuroborreliosis, observing even longer delays than in our study, with a median time from symptom onset to first hospital contact of 20 days and a median time from first hospital contact to treatment of 24 days (4). Thus, there are opportunities to shorten time-to-treatment both before and after an infected individual engages with the healthcare system.
The absence of a rash was a strong factor in delayed treatment for Lyme disease, as it was associated with both delay windows, signifying its importance to both individual and provider behavior. The association of rash with delayed time to medical contact aligns with a prior qualitative study that revealed patients with treatment delays ruled out the possibility of Lyme disease because they did not observe a bull's-eye rash (16). Similar to our findings, past reports indicate that up to 30% of people with Lyme disease do not present with erythema migrans (21) and a subset of these individuals do not present with the characteristic bull's-eye appearance (21). On the healthcare side, misdiagnosis reportedly occurs more commonly among patients with Lyme disease that do not present with erythema migrans (22). This work suggests that efforts to reduce time-to-treatment should include educational campaigns targeting patients and healthcare providers on alternative clinical presentations of Lyme disease and erythema migrans (22).
Delays before and after contacting a medical professional were also more common for Lyme disease diagnosed between November and April compared to other times of the year. A prior study of Lyme neuroborreliosis similarly reported longer time-to-treatment when Lyme disease occurred in winter and early spring (4). This is the time of year when Lyme disease is least commonly contracted (1), thus patients and medical professionals may be less likely to attribute symptoms to Lyme disease in this time period. Though less common in these months, thousands of confirmed cases of Lyme disease are reported from November to April (1). Building awareness among patients and medical providers of the risk of Lyme disease throughout the year in endemic regions provides another opportunity for reducing time-to-treatment.
Uninsured individuals in our study were more likely to delay contacting a medical professional for their symptoms than were individuals with private insurance. This finding aligns with a prior qualitative study of treatment delays in Lyme disease, which highlighted the symptoms that individuals endured while waiting to obtain health insurance, including debilitating joint pain and dangerously high fevers (16). Treatment delays due to lack of insurance occur for a range of conditions, from myocardial infarction (23, 24) to cancer (25), and improving accessibility of health insurance is a critical goal in efforts to provide timely treatment. Considering that the costs of diagnosing and treating acute and uncomplicated Lyme disease are relatively inexpensive (26), diagnostic tests and treatment should be made accessible and affordable for those with and without health insurance.
Most participants reported initially contacting a primary care provider for their Lyme disease symptoms. However, these individuals were at greater risk of delayed treatment than individuals who sought care in an urgent care or emergency department setting. Wait times for primary care appointments can be lengthy, and many primary care clinics do not offer evening or weekend hours (27). In our study, the inability to obtain care outside of work hours or while traveling away from home, and responsibilities such as caregiving duties were noted as barriers to seeking prompt care for Lyme disease symptoms. Urgent care clinics offer an important option for individuals who might otherwise delay contacting a medical professional. Increasing use of urgent care clinics for Lyme disease symptoms may require public health campaigns to inform the general population of the importance of prompt treatment for Lyme disease.
A self-reported diagnosis of CFS prior to Lyme disease increased the odds of delay while under care. Considering the similarity in some symptoms in the two conditions, health care providers may not have initially recognized the onset of Lyme disease symptoms as a new condition, resulting in delayed treatment. Alternatively, CFS may have been later misdiagnosed as Lyme disease, or Lyme disease may have been initially misdiagnosed as CFS (28). Given the small number of individuals in our sample with CFS, these findings should be considered preliminary.
The strengths of this study include a population-based sample from a Lyme endemic state, identification of separate risk factors associated with two time windows of treatment delays that potentially require unique approaches to reducing delays, and evaluation of the association between time-to-treatment and PTLDS using guideline-based criteria that includes persistent symptoms and functional deficit. This study had some limitations. First, we did not require a positive blood test when identifying Lyme disease cases. It is possible that some study respondents did not have Lyme disease, though unlikely given the combination of EHR data—which has demonstrated utility in identifying Lyme disease cases (15)—and self-reported data to identify cases. Confining the study to individuals with a positive blood test would have excluded individuals who were promptly treated with antibiotics or tested before antibodies developed, resulting in an overestimation of time-to-treatment. Second, individuals with longer time-to-treatment or with persistent symptoms may have been more likely to respond to the questionnaire, potentially resulting in an overestimation of time-to-treatment and its association with PTLDS. To mitigate participation bias, we employed inverse probability weighting. Third, the study population was diagnosed with Lyme disease at Geisinger, a single integrated health system. However, Geisinger has more than 44 community practice sites, 12 hospital campuses, and more than 20 urgent care clinics across a large geographic region; thus, the findings reflect the practices of Lyme disease diagnosis across a range of clinical settings. Moreover, questionnaires captured information on experiences within and outside of Geisinger. Finally, our findings may be subject to same-source bias due to the use of self-reported data for both exposures and outcomes.
Conclusions
In a population-based study of Lyme disease in Pennsylvania, treatment delays, defined as time-to-treatment >30 days, were reported by nearly one-third of individuals with Lyme disease. Delays before and after contacting a medical professional had common and unique risk factors. Delayed treatment was associated with PTLDS. To improve long-term outcomes of Lyme disease, strategies for preventing delayed treatment should aim to reduce both the time before and after contacting a medical professional.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Geisinger Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
AH, MP, and BS conceived and designed the analysis. CN performed the data analysis. AH, BS, MP, KM, AR, JA, CH, and BS contributed to the interpretation of results. All authors contributed to the writing and final review of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dione Mercer and Allison Fielder for their assistance in data collection.
Footnotes
Funding. This study was funded by the Steven & Alexandra Cohen Foundation. The funder did not have a role in the study design, collection, analysis, interpretation of data, or writing of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.560018/full#supplementary-material
References
- 1.Centers for Disease Control Climate Change Increases the Number and Geographic Range of Disease-Carrying Insects and Ticks. (2019). Available online at: https://www.cdc.gov/climateandhealth/pubs/vector-borne-disease-final_508.pdf (accessed October 15, 2019).
- 2.Mead PS. Epidemiology of lyme disease. Infect Dis Clin North Am. (2015) 29:187–210. 10.1016/j.idc.2015.02.010 [DOI] [PubMed] [Google Scholar]
- 3.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the infectious diseases society of America. Clin Infect Dis. (2007) 45:941 10.1086/508667 [DOI] [PubMed] [Google Scholar]
- 4.Knudtzen FC, Anderson NS, Jensen TG, Skarphedinsson S. Characteristics and clinical outcome of Lyme neuroborreliosis in a high endemic area, 1995–2014: a retrospective cohort study in Denmark. Clin Infect Dis. (2017) 65:1489–95. 10.1093/cid/cix568 [DOI] [PubMed] [Google Scholar]
- 5.Ljøstad U, Myglanda A. Remaining complaints 1 year after treatment for acute lyme neuroborrelisos; frequency, pattern, and risk factors. Eur J Neurol. (2010) 17:118–23. 10.1111/j.1468-1331.2009.02756.x [DOI] [PubMed] [Google Scholar]
- 6.Shadik NA, Phillips CB, Sangha O, Logigian EL, Kaplan RF, Wright EA, et al. Musculoskeletal and neurologic outcomes in patients with previously treated Lyme disease. Ann Intern Med. (1999) 131:91–926. 10.7326/0003-4819-131-12-199912210-00003 [DOI] [PubMed] [Google Scholar]
- 7.Eikeland R, Mygland A, Herlofson K, Ljøstad U. Risk factors for a non-favorable outcome after treated European neuroborreliosis. Acta Neurol Scandinavica. (2013) 127:154–60. 10.1111/j.1600-0404.2012.01690.x [DOI] [PubMed] [Google Scholar]
- 8.Aucott JN, Crowder LA, Kortte KB. Development of a foundation for a case definition of post-treatment lyme disease syndrome. Int J Infect Dis. (2013) 17:e443–9. 10.1016/j.ijid.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 9.Koedel U, Fingerle V, Pfister HW. Lyme neuroborreliosis-epidemiology, diagnosis and management. Nat Rev Neurol. (2015) 11:446–56. 10.1038/nrneurol.2015.121 [DOI] [PubMed] [Google Scholar]
- 10.Marques A. Chronic lyme disease: a review. Infect Dis Clin North Am. (2008) 22:341–60. 10.1016/j.idc.2007.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rebman AW, Bechtold K, Yang T, Mihm EA, Soloski MJ, Novak CB, et al. The clinical, symptom and quality-of-life characterization of a well-defined group of patients with posttreatment lyme disease syndrome. Front Med. (2017) 4:224. 10.3389/fmed.2017.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen BL, Cacioppo JT. Delay in seeking a cancer diagnosis: delay stages and psychophysiological comparison processes. Br J Soc Psychol. (1995) 34:33–52. 10.1111/j.2044-8309.1995.tb01047.x [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Lyme Disease Data Tables: Most Recent Year. 2018. (2018). Available online at: https://www.cdc.gov/lyme/datasurveillance/tables-recent.html (accessed January 15, 2019).
- 14.Casey JA, Savitz DA, Rasmussen SG, Ogburn EL, Pollak J, Mercer DG, et al. Unconventional natural gas development and birth outcomes in Pennsylvania, USA. Epidemiology. (2016) 27:163–72. 10.1097/EDE.0000000000000387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon KA, Pollack J, Hirsch AG, Aucott JN, Nordberg C, Heaney CD, et al. Epidemiology of lyme disease in Pennsylvania 2006-2014 using electronic health records. Tick Tick Borne Dis. (2018) 10:241–50. 10.1016/j.ttbdis.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 16.Hirsch AG, Herman RJ, Rebman A, Moon KA, Aucott J, Heaney C, et al. Obstacles to diagnosis and treatment of lyme disease in the USA: a qualitative study. BMJ Open. (2018) 8:e021367. 10.1136/bmjopen-2017-021367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.RAND Corporation 36-Item Short Form Survey (SF-36). (2019). Available online at: https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html (accessed October 15, 2019).
- 18.Watson JM, Logan HL, Tomar SL. The influence of active coping and perceived stress on health disparities in a multi-ethnic low income sample. BMC Public Health. (2008) 8:41. 10.1186/1471-2458-8-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.StataCorp Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; (2015). [Google Scholar]
- 20.Shadik NA, Phillips CB, Logigian EL, Steere AC, Kaplan RF, Berardi VP, et al. The long-term clinical outcomes of lyme disease: a population-based retrospective cohort study. Ann Intern Med. (1994) 121:560–7. 10.7326/0003-4819-121-8-199410150-00002 [DOI] [PubMed] [Google Scholar]
- 21.Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA. (2007) 297:2617–27. 10.1001/jama.297.23.2617 [DOI] [PubMed] [Google Scholar]
- 22.Aucott J, Morrison C, Munoz B, Rowe PC, Schwarzwalder A, West SK. Diagnostic challenges of early Lyme disease: lessons from a community case series. BMC Infect Dis. (2009) 9:79. 10.1186/1471-2334-9-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen HL, Saczynski JS, Gore JM, Goldberg RJ. Age and sex differences in duration of prehospital delay in patients with acute myocardial infarction: a systematic review. Circ Cardiovasc Qual Outcomes. (2010) 3:82–92. 10.1161/CIRCOUTCOMES.109.884361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smolderen KG, Spertus JA, Nallamothu BK, Krumholz HM, Tang F, Ross JS, et al. Health care insurance, financial concerns in accessing care, and delays to hospital presentation in acute myocardial infarction. JAMA. (2010) 303:1392–400. 10.1001/jama.2010.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rauscher GH, Ferrans CE, Kaiser K, Campbell RT, Calhoun EE, Warnecke RB. Misconceptions about breast lumps and delayed medical presentation in urban breast cancer patients. Cancer Epidemiol Biomarkers Prev. (2010) 19:640–7. 10.1158/1055-9965.EPI-09-0997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adrion ER, Aucott J, Lemke KW, Weiner JP. Health care costs, utilization and patterns of care following Lyme disease. PLoS ONE. (2015) 0:e0116767. 10.1371/journal.pone.0116767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang MC, Ryan G, McGlynn EA, Mehrotra A. Why do patients seek care at retail clinics and what alternatives did they consider? Am J Med Qual. (2010) 25:128–34. 10.1177/1062860609353201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steere AC, Taylor E, McHugh GL, Logigian EL. The overdiagnosis of lyme disease. JAMA. (1993) 269:1812–6. 10.1001/jama.1993.03500140064037 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.


