Abstract
Objective
To investigate the effect of therapeutic suggestions played to patients through earphones during surgery on postoperative pain and opioid use.
Design
Blinded randomised controlled study.
Setting
Five tertiary care hospitals in Germany.
Participants
385 of 400 patients consecutively recruited from January to December 2018 who were to undergo surgery for 1-3 hours under general anaesthesia. In the per protocol analysis 191 patients were included in the intervention group and 194 patients in the control group.
Intervention
The intervention comprised an audiotape of background music and positive suggestions based on hypnotherapeutic principles, which was played repeatedly for 20 minutes followed by 10 minutes of silence to patients through earphones during general anaesthesia. Patients in the control group were assigned to a blank tape.
Main outcome measures
The main outcome was dose of opioid administered by patient controlled analgesia or nurse controlled analgesia within the first postoperative 24 hours, based on regular evaluation of pain intensity on a numerical rating scale (range 0-10, with higher scores representing more severe pain).
Results
Compared with the control group, the intervention group required a significantly (P=0.002) lower opioid dose within 24 hours after surgery, with a median of 4.0 mg (interquartile range 0-8) morphine equivalents versus 5.3 (2-12), and an effect size (Cohen’s d) of 0.36 (95% confidence interval 0.16 to 0.56). The number of patients who needed opioids postoperatively was significantly (P=0.001) reduced in the intervention group: 121 of 191 (63%, 95% confidence interval 45% to 70%) patients in the intervention group versus 155 of 194 (80%, 74% to 85%) in the control group. The number needed to treat to avoid postoperative opioids was 6. Pain scores were consistently and significantly lower in the intervention group within 24 hours after surgery, with an average reduction of 25%. No adverse events were reported.
Conclusions
Therapeutic suggestions played through earphones during general anaesthesia could provide a safe, feasible, inexpensive, and non-drug technique to reduce postoperative pain and opioid use, with the potential for more general use. Based on the finding of intraoperative perception by a considerable number of patients, surgeons and anaesthetists should be careful about background noise and conversations during surgery.
Trial registration
German Clinical Trial Register DRKS00013800.
Introduction
Anaesthesia is usually considered a state of no sensations, yet several observations suggest that the central auditory pathway stays intact during anaesthesia.1 2 3 Intraoperative awareness, for instance, has been reported in a small number of patients and can lead to severe sequelae, such as post-traumatic stress disorder.4 5 6 Higher frequencies of wakefulness without explicit memory have been observed in patients during surgery as well as reactions to meaningful events such as a simulated complication during surgery.7 Because of the mainly negative effects of such intraoperative perceptions, attempts have been made to avoid “inadequate” anaesthesia.8 9 In contrast, several studies have tried to use the intact perception of words and sounds in a positive way and tested the use of taped therapeutic suggestions during general anaesthesia.10 11 12 A recent meta-analysis of 32 randomised controlled trials (n=2102 patients) of adequate quality on the efficacy of therapeutic suggestions under general anaesthesia found no effect on pain intensity or mental distress but did find small but statistically significant positive effects on postoperative drug use and recovery.13 These results raised hopes that a non-drug approach such as therapeutic suggestions might be beneficial for surgical patients. However, the identified randomised controlled trials were relatively old (1986-2001), small, and heterogeneous in design. Moreover, treatment and prophylaxis regimens have since changed—in these studies the management and depth of anaesthesia were not standardised and the applied suggestions varied and often contained negations.
Adequate analgesia is a major goal and challenge of postoperative care, both for patients’ comfort and healing and for recovery and outcome. Opioids are primarily used for this purpose, although these drugs have severe side effects and complications.14 As most of these side effects and complications are dose related, opioid dose sparing strategies are desirable.15 Such strategies include use of co-analgesics, such as non-steroidal anti-inflammatory drugs, which themselves are associated with side effects and risks.16 Therefore, non-drug means to reduce opioid use are in demand as part of a multimodal opioid sparing regimen. In light of the recent opioid epidemic and the pivotal role of postoperative pain,17 supplementary non-drug approaches are needed. We hypothesised that an audiotape of therapeutic suggestions played to patients during surgery would lead to a reduced need for opioid drugs in the 24 hours after surgery.
Methods
Study design and patients
The study was a blinded randomised placebo controlled trial in five tertiary care hospitals in Germany: University Hospital Knappschaftskrankenhaus Bochum, University Hospital of Regensburg, University Hospital of LMU Munich, University Hospital of Cologne, and Klinikum Kassel (University Hospital of the Faculty of Medicine, University of Southampton, UK). The study was conducted in accordance with the consolidated standards of reporting trials (CONSORT) guidelines and the principles of Good Clinical Practice, as well as with the study protocol (http://anaesthesie.rub.de/files/files/protocols/intraop_sugg_study_protocol_1.6.pdf) and the statistical analysis plan (http://anaesthesie.rub.de/files/files/protocols/intraop_sugg_SAP_1.2.pdf). The protocol did not change during the study.
Patients were considered eligible for enrolment if they were aged 18 to 70 years, to undergo elective surgery requiring general anaesthesia with a planned duration of 1-3 hours, and at risk of postoperative pain and nausea. We excluded patients who had severe pre-existing health conditions representing a constant threat to life (American Society of Anesthesiologist score of ≥4),18 required postoperative mechanical ventilation, or received an epidural catheter or other kinds of regional anaesthesia. Eligible patients were included after written informed consent had been obtained.
Randomisation and masking
The patients were randomly assigned in a 1:1 ratio to intervention or control group. After anaesthetic induction and intubation the responsible anaesthetist drew an envelope from a box and accordingly connected one of two identical MP3 players marked A or B to earphones in the patient’s ears and started to play the audio recording at a standard volume. A medical student blinded to group allocation collected the data. The leading investigator terminated blinding after 24 hours. As a result, neither the patient nor the medical staff involved with patient care (anaesthetist, nurse, data collector) had knowledge about group allocation.
Procedures
Patients in the intervention group were assigned to an audiotape containing background music and therapeutic suggestions, including indirect and positive messages (see appendix text A) for 20 minutes followed by silence for 10 minutes. The tape played continually during surgery. At the end of surgery, a different audiotape was played to prepare the patients for emergence from anaesthesia, starting when volatile anaesthesia was stopped (see appendix text B). The tape was stopped and the earphones removed before extubation.
Two of the authors (EH, NZ) developed and recorded the text, which was based on hypnotherapeutic principles and dealt with topics such as competence and care of the surgical and anaesthesiology team, pain regulation, dissociation to a safe place, affirmation, anxiety control, and confidence.19 The background music was from the CD Trancemusik (Hypnos Verlag, Stuttgart, Germany). Patients in the control group received an audiotape with no auditory output. Study staff in both anaesthesia and postoperative care received comprehensive instructions and training into use of the equipment. General anaesthesia comprised balanced anaesthesia with volatile anaesthetics at 1.0 ± 0.2 minimum alveolar concentration. Depth of anaesthesia was controlled by electroencephalography based monitoring (Bispectral Index, Medtronic, Meerbusch, Germany, or Narcotrend, Narcotrend Group, Hannover, Germany), with a target index of 40 to 60. Both defined minimum alveolar concentration and bispectral index measure the adequacy of anaesthesia to avoid intraoperative awareness.9 10 Patients who scored 3 or more for postoperative pain on a numerical rating scale (NRS, range 0-10,20 with higher scores indicating more severe pain) received an intravenous opioid bolus (piritramide) administered either by the attending nurse (nurse controlled analgesia) or by the patient (patient controlled analgesia, using a bolus with lockout interval). Optional non-opioid drugs were used according to local protocol and the patient’s medical history. Before surgery the patients’ susceptibility to verbal suggestions was tested using a five item modified Harvard group scale of hypnotic susceptibility,21 and level of anxiety was tested using the state trait anxiety inventory.22
Outcomes
Our primary endpoint was requirement for opioids within 24 hours after surgery as delivered by nurse controlled or patient controlled analgesia. As one centre used oral opioids tilidine or oxycodone in addition to piritramide, we calculated morphine milligram equivalents (MME) as the sum of mg piritramide×0.7, mg tilidine×0.2, and mg oxycodone×0.8.23 24 We evaluated opioid consumption within the first two postoperative hours, reflecting the closely controlled analgesia used in the post-anaesthesia care unit.
To improve the validity of assessing opioid consumption, application was linked to a defined pain level. Therefore, we asked patients to rate the intensity of their pain on a NRS (range 0-10, with higher scores indicating more severe pain),25 and linked opioid application to a score of 3 or more. After baseline assessment of patients before surgery, pain was measured on admission to the post-anaesthesia care unit shortly after extubation and repeated at 15 minute intervals for two hours to calculate the mean pain score within that period. The patients were then tested after 24 hours and asked about maximal pain scores within the two hour and 24 hour periods. Other secondary outcomes were use of non-opioid drugs, comfort, mental orientation, anxiety levels, postoperative nausea and vomiting, use of antiemetics, and anaesthesia wake-up time. We converted the dosage of non-opioid drugs to percentage of maximum daily dose (MDD) to take into account the differing half lives of analgesics (MDD of metamizole=4000 mg, paracetamol=4000 mg, ibuprofen=2400 mg, diclofenac=150 mg, etoricoxib=120 mg; from information provided by the manufacturers). The appendix presents the results for the other secondary outcomes and related preoperative variables.
Statistical analysis
Our sample size was based on a power analysis of seven studies from a meta-analysis on intraoperative suggestions and analgesic use,13 which were comparable to our study for study design and quality. The effect sizes in these studies ranged from 0.244 to 0.459. For our sample size calculation, we conservatively assumed an effect size of 0.3, which was achieved by five of these studies. Based on a 1:1 randomisation ratio, we calculated that we would need a total of 368 patients to obtain about 80% power to detect a difference in postoperative opioid dosage at a two sided α level of 0.05. We present continuous, non-normally distributed variables as medians and interquartile ranges and categorical variables as frequencies and percentages. In subgroup analyses we stratified surgeries according to expected pain within 24 hours postoperatively (high pain surgeries: gynaecology, orthopaedics, abdominal general surgery; low pain surgeries: non-abdominal general surgery, vascular, urology)24 and performed separate analyses for each subgroup. Subgroup analysis was also done on patients who received patient controlled and nurse controlled analgesia. Linear or logistic mixed effects model was used to evaluate the influence of therapeutic suggestions on postoperative opioid dose. Fixed effects of this model were treatment group, expected high versus low postoperative pain levels, and intraoperative opioid, non-opioid, and clonidine dosage; allocation to study centre was treated as a random effect. For comparison of fixed effects estimates, we normalised covariates before model calculation. Possible differences between surgeries with high or low expected postoperative pain levels on primary outcome were assessed in addition to subgroup analysis by creating a separate mixed effects model that additionally included an interaction term between expected pain levels and treatment group allocation. Comparison of variables between groups in secondary outcomes, which were not related to pain (see appendix) was performed using Mann-Whitney U test for non-normally distributed continuous variables and Pearson’s χ2 test for categorical variables. Effect sizes for outcome variables were described by Cohen’s d along with 95% confidence intervals.26 For dichotomous outcome variables, we calculated point estimates with 95% confidence intervals for treatment group specific proportions, difference in proportions between the intervention and control group with corresponding 95% confidence intervals, and number needed to treat (NNT). For graphical presentation of non-parametric numerical pain rating scale scores and MME, we performed bootstrapping with resampling and calculated the means and 95% confidence intervals. Statistical analysis was performed with GraphPad Prism 8 (GraphPad Software, San Diego, CA) and The R Project for Statistical Computing (The R Foundation for Statistical Computing, Vienna, Austria). A two sided P value of less than 0.05 was considered to be statistically significant.
Patient and public involvement
Patients were not involved in designing the research question, outcome measures, or interpretation or writing up of results of this study. Patient representatives in our ethics committee were asked for comments on general comprehensibility. The patient representatives of each participating hospital were informed about the study and its start. Results will be presented to patients and the public as part of regular information events.
Results
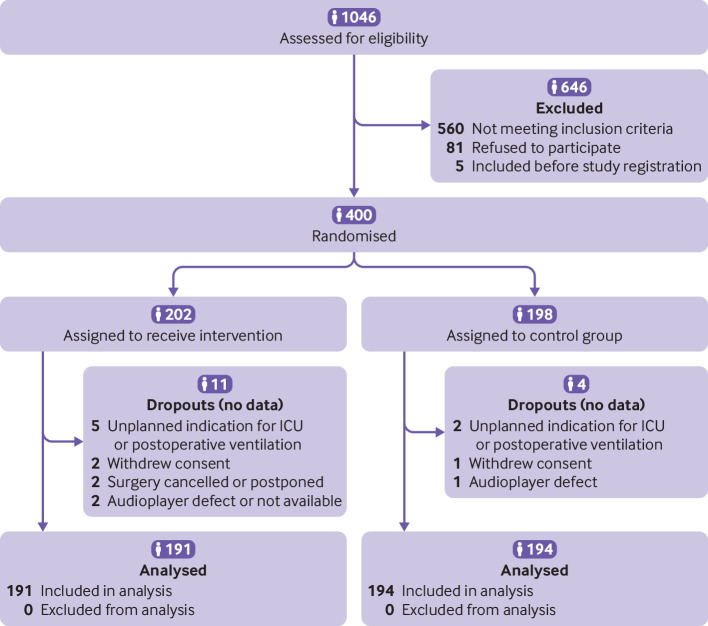
A total of 400 patients were recruited and randomised (80 in each study site, 202 in the intervention group and 198 in the control group) from January to December 2018 (fig 1). One hundred and ninety one patients in the intervention group and 194 patients in the control group were included in the per protocol analysis.
Fig 1.
Flow of patients through the study. No postoperative data were collected for dropouts and they were excluded from analysis before unblinding of the study. ICU=intensive care unit
Table 1 presents the baseline characteristics and pain and anxiety scores of the patients. Duration of surgery, preoperative pain, and intraoperative use of analgesics were equally distributed between the groups, as were the surgical procedures (P=0.32), which comprised 27 types; table 1 lists the 10 most common types of surgery together with the proportion of patients who underwent high pain surgeries. Three centres used nurse controlled analgesia and two used patient controlled analgesia. The proportions of patients with high pain surgeries or patient controlled analgesia did not differ between the groups. In one centre, 14 patients in the intervention group and 20 in the control group received oral opioids in addition to intravenous piritramide, accounting for 14.0% of total MME. No patients remembered having earphones or listening to music and verbal suggestions or were able to tell whether they had or not. No side effects were recorded.
Table 1.
Baseline characteristics of participants assigned to therapeutic suggestions by audiotape during surgery (intervention) or to a blank audiotape (n=385). Values are numbers (percentages) unless stated otherwise
| Characteristics | Intervention group (n=191) | Control group (n=194) |
|---|---|---|
| Median (interquartile range) age (years) | 52 (43-62) | 54 (46-62) |
| Women | 115 (60) | 110 (57) |
| Median (interquartile range) preoperative score: | ||
| NRS (0-10) | 0 (0-1) | 0 (0-2) |
| STAI-S (range 20-80) | 41.0 (33-51) | 39.5 (33-50) |
| Type of surgery: | ||
| Thyroid gland | 36 (19) | 30 (15) |
| Abdominal hernia | 23 (12) | 24 (12) |
| Spinal* | 19 (10) | 23 (12) |
| Cholecystectomy | 19 (10) | 17 (9) |
| Laparoscopy | 17 (9) | 13 (7) |
| Hysterectomy | 17 (9) | 9 (5) |
| Colorectal | 9 (5) | 13 (7) |
| Adrenalectomy | 7 (4) | 6 (3) |
| Fundoplication | 7 (4) | 15 (8) |
| Pelvic floor repair | 6 (3) | 1 (0.5) |
| Other† | 31 (16) | 43 (22) |
| High pain surgeries‡ | 136 (71) | 147 (76) |
| Patient controlled analgesia | 78 (40) | 79 (40) |
| Median (interquartile range) duration of surgery (mins) | 95 (69-140) | 106 (74-141) |
| Intraoperative drugs: | ||
| Median (interquartile range) fentanyl (mg), n=85/93 | 0.5 (0.4-0.5) | 0.5 (0.5-0.6) |
| Median (interquartile range) sufentanil (µg), n=106/101 | 50 (40-64) | 50 (40-70) |
| Median (interquartile range) non-opioids (% of MDD§) | 33 (25-50) | 31 (25-50) |
| Patients with non-opioids | 165 (86) | 161 (83) |
| Patients with clonidine | 22 (1) | 35 (18) |
NRS=numerical rating scale; STAI-S=state trait anxiety inventory scale22; MMD=maximum daily dose.
Herniated intervertebral disc, lumbar spinal stenosis.
Inter alia prostatectomy, oophorectomy, nephrectomy, living kidney donation, hemorrhoidectomy, gastrectomy, vaginal surgery, and skin and soft tissue surgery.
Gynaecology, orthopaedics, abdominal general surgery, according to Gerbershagen et al.24
Calculated to correct for different non-opioid analgesics with various half-lives (MDD of metamizole=4000 mg, paracetamol=4000 mg, ibuprofen=2400 mg, diclofenac=150 mg, etoricoxib=120 mg, from information provided by manufacturers).
Primary outcome: postoperative opioids
Opioid use in the first 24 postoperative hours was significantly lower in intervention patients compared with control patients (fig 2 and table 2). On average, the dose of opioids was reduced by 2.8 MME (95% confidence interval 1.2 to 4.3 MME) (point estimate for difference between population means after bootstrapping), corresponding to a saving of 34%. The reduction in opioid requirement was already significant within the first two hours after surgery at the post-anaesthesia care unit (P<0.001), the period when opioid administration was closely linked to the regular query of the pain level (evaluated every 15 minutes). The dose of opioids in this early postoperative period was reduced by 28% in the intervention group (3.3 MME, 95% confidence interval 2.6 to 3.9 MME) compared with the control group (4.6 MME, 3.9 to 5.2 MME), calculated by bootstrapping: mean difference 1.3 mg (95% confidence interval 0.4 to 2.2), and 61% more patients without opioids were observed. Significantly fewer patients in the intervention group than control group needed opioids within 24 hours postoperatively: 121 out of 191 (63%) v 155 of 194 (80%) participants. In the control group, 26% more patients needed opioids (when calculated for equal group size). The number needed to treat to avoid postoperative opioids was 6 (fig 2 and table 2).
Fig 2.
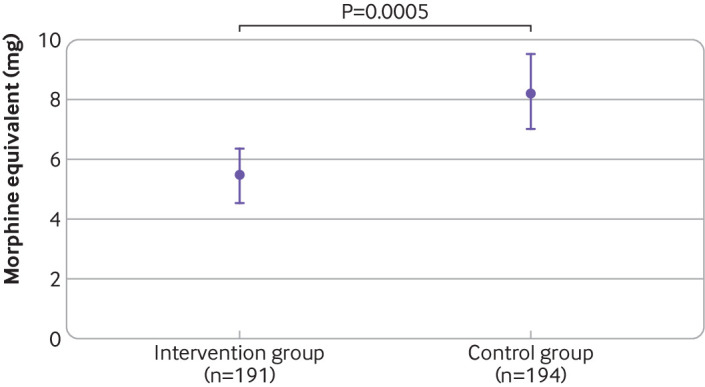
Postoperative dose of opioids within 24 hours after surgery. Data are calculated by bootstrapping owing to non-normally distributed outcome variables. Doses are in morphine milligram equivalents (MME) to account for different types of opioids (intravenous morphine=1.0, piritramide=0.7, tilidine=0.2, oxycodone=0.8)23 24 used in one centre
Table 2.
Requirement for analgesia and pain and after surgery in participants assigned to therapeutic suggestions by audiotape during surgery (intervention) or to a blank audiotape (n=385). Values are medians (interquartile ranges) unless stated otherwise
| Intervention group (n=191) | Control group (n=194) | Absolute difference (95% CI) | P value* | Cohen’s d (95% CI) | NNT | |
|---|---|---|---|---|---|---|
| Postoperative opioids: | ||||||
| MME† | 4.0 (0-8) | 5.3 (2-12) | 0.002 | 0.36 (0.2 to 0.6) | ||
| None, No/% (95% CI) | 70/37(30 to 44) | 39/20 (157 to 26) | 16.5 (8 to 25) | <0.001 | 0.46 (0.2 to 0.7) | 6.0 |
| No high dose (MME ≥10), No/% (95% CI) | 153/80 (74 to 86) | 129/66 (59 to 73) | 13.6 (5 to 22) | 0.014 | 0.39 (0.1 to 0.7) | 7.3 |
| Postoperative non-opioids: | ||||||
| % of MDD‡ | 50 (6-100) | 75 (25-100) | 0.0135 | 0.25 (0.1 to 0.5) | ||
| Postoperative pain, NRS (01-10): | ||||||
| Average within 2 hours | 2 (1-3) | 3 (1-4) | <0.001 | 0.40 (0.2 to 0.6) | ||
| Maximum within 24 hours | 4 (3-6) | 5 (4-7) | <0.001 | 0.45 (0.3 to 0.7) | ||
| Patients with NRS <3: | ||||||
| At 0 minutes (admission to PACU), No/% (95% CI) | 145/76 (70 to 82) | 122/63(56 to 70) | 13.4 (4 to 23) | 0.003 | 0.35 (0.1 to 0.6) | 7.7 |
| Average within 2 hours, No/% (95% CI) | 119/62 (55 to 69) | 84/43 (36 to 51) | 19.0 (9 to 29) | <0.001 | 0.43 (0.2 to 0.7) | 5.3 |
| At 24 hours, No/% (95% CI) | 110/57.6 (50.2 to 64.7) | 75/38.7 (31.8 to 45.9) | 18.9 (9.1 to 28.7) | 0.001 | 0.42 (0.2 to 0.7) | 5.3 |
NNT=number needed to treat of pain; MME=morphine milligram equivalents; MDD=maximum daily dose; NRS=numerical rating scale; PACU=post-anaesthesia care unit.
Mixed effect.
MDD calculated to correct for different non-opioid analgesics with various half-lives (metamizole=4000 mg, paracetamol=4000 mg, ibuprofen=2400 mg, diclofenac=150 mg, etoricoxib=120 mg, from information provided by manufacturers).
In subgroup analyses by expected pain intensity from surgery (high or low) and by type of controlled analgesia (nurse or patient), the intervention was associated with lower opioid use postoperatively (see results in appendix table s1). Opioid consumption within 24 hours of surgery was not significantly different between patients receiving nurse controlled analgesia and those using patient controlled analgesia (P=0.72 for the intervention and P=0.93 for the control).
In the mixed effects model for analysis of contributing factors, group allocation was the main determinant of postoperative opioid dose (table 3). Furthermore, expected pain level by surgery type (high or low) and individual level pain during surgery (shown by dosage of opioids delivered intraoperatively) showed an effect. In an additional analysis, no interaction was observed between expected pain levels by surgery type and treatment group allocation on postoperative opioid dose (standardised estimate −0.88, 95% confidence interval −4.1 to 2.4; P=0.60). The variance in the primary outcome between study sites was noticeably lower than the random effects within the location.
Table 3.
Mixed effects model on postoperative opioid dose within 24 hours of surgery. Values are standardised estimates (95% confidence intervals) unless stated otherwise
| Variables | Estimates | P value |
|---|---|---|
| Group allocation (intervention v control) | −2.26 (−3.7 to 0.8) | 0.002 |
| Surgery related pain levels (expected high v expected low) | 1.90 (0.1 to 3.7) | 0.04 |
| Intraoperative opioid dose | 1.91 (1.1 to 2.7) | <0.001 |
| Intraoperative non-opioid dose | −0.46 (−1.3 to 0.4) | 0.29 |
| Intraoperative clonidine dose | 0.36 (−0.4 to 1.1) | 0.37 |
| Random effects (study centre): | ||
| Within group variance | 50.00 | |
| Between group variance | 6.72 | |
| Intraclass correlation coefficient* | 0.12 |
For comparison of fixed effects estimates, with variables normalised before model calculation.
Secondary outcome: postoperative pain
Although patients in both groups had similar pain levels preoperatively, the postoperative course of pain differed between the groups (fig 3). The first postoperative evaluation of pain on admission to the post-anaesthesia care unit, before any postoperative opioid was given, showed significantly lower mean pain scores in the intervention group (1.4 (SD 2.2) v 2.2 (2.7), P=0.002), and more patients had a score of less than 3 (3 being the common threshold for pain treatment). The average pain score remained 25% lower in the intervention group and was less than 3 within 24 hours, in contrast with the control group with a score above the threshold of 3 (fig 3). Moreover, despite a significantly higher opioid consumption in the control group, after 24 hours 61% (95% confidence interval 54% to 68%) of these patients (119 of 194) had a postoperative pain score of 3 or more, indicative of ongoing clinically relevant pain and need for analgesics, compared with 81 of 191 (42%, 35% to 50%) patients in the intervention group (P<0.001). The number needed to treat to save one patient from relevant pain (NRS score ≥3) was 5.3 (table 2).
Fig 3.
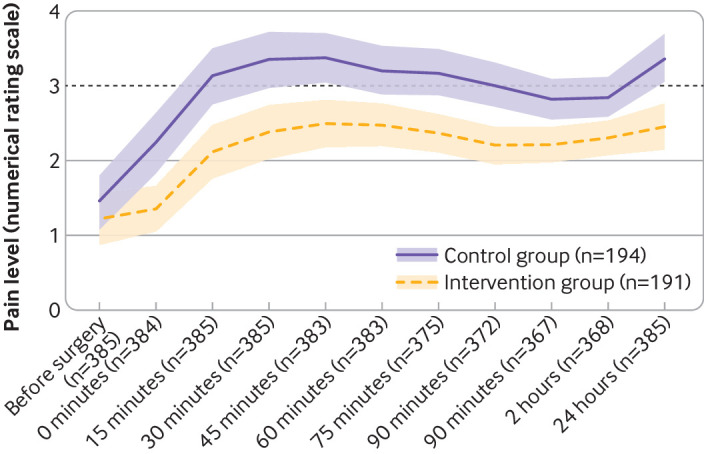
Course of preoperative and postoperative pain levels. Data are calculated from mean values by bootstrapping owing to non-normally distributed outcome variable. Dashed line represents the numerical rating scale threshold of 3 for pain treatment. Whiskers represent 95% confidence intervals.
Discussion
This study found a statistically significant reduction in use of postoperative opioids in patients who received therapeutic suggestions by audiotape during surgery, which comprised background music and mindful text. Furthermore, the number of patients who requested and received opioids was significantly lower after the intervention. This addressed number of affected patients are relevant for two reasons. Firstly, any report of an effect on medication requirement needs information on the involved portion of patients to distinguish dose reduction in most patients from reduction in the number of patients with medication requirement. Secondly, the phenomenon of “intraoperative awareness”, the common explanation for intraoperative perception and the reaction of only a few patients,5,6 cannot account for the observed portion of affected patients (26% more abstinence from opioids).
A mean saving of 2.8 MME for each patient might seem unimportant; however, in most pain studies the focus is generally on relative saving and not the absolute dose, and an opioid dose reduction of 30%, as reported here, is considered relevant.16 Moreover, a mean saving does not reflect benefit for individual patients.27 When patients with a high need for postoperative analgesia (≥10 MME, corresponding to 10 doses of piritramide triggered in patient controlled analgesia) were taken into account, the number decreased by 41% after intervention (see table 2). In addition, the saving of about 33 patients from exposure to postoperative opioids (when normalised to 200 participants in each group) is of clinical interest.
Opioid consumption is one of the most appropriate and widely established measures to evaluate postoperative pain, especially when standardised and regulated such as in patient controlled analgesia (to a numerical pain rating scale score ≥3).20 However, pain intensity also has to be considered, as the two variables pain and analgesia are interdependent and inseparably connected. Furthermore, pain scoring in this study was part of a defined opioid treatment. Starting from admission to the post-anaesthesia care unit to 24 hours after surgery, postoperative pain was significantly lower in the intervention group (fig 3). For interpretation of the observed relatively high pain scores it is important to take into account that the pain score and dosage of opioids were not matched—that is, the regular evaluation of pain could have taken place immediately before, immediately after, or at some time after a dose of the opioid.
Several studies have shown that particular words might be processed by patients to their disadvantage—words such “pain” might increase or even induce pain.19 28 29 For this reason we avoided negative words and negations in the text, and used connotations such as “increased comfort” instead of “no pain,” and with suggestions for regulation and reframing. Moreover, support, care, self-healing power, and meaning were communicated. Remarkably, we found analgesic effects without addressing the topic “pain” or “analgesia.” The tested suggestions were associated with the general therapeutic goal of keeping postoperative pain below a score of 3 and with a number needed to treat of 6 to avoid postoperative opioids.
Comparison with other studies
Table 4 compares the effect sizes of different verbal interventions for postoperative pain relief between our study and two other studies. Hypnosis is the induction of a trance state and the use of enhanced suggestibility. Evidence suggests that preoperative hypnosis is effective at reducing postoperative side effects after medical interventions, including pain.31 A recent meta-analysis of 26 randomised controlled trials on the effectiveness of suggestive techniques in reducing adverse reactions to surgery and anaesthesia found a statistically significant reduction in pain and a non-significant reduction in consumption of analgesics after surgery.30 Studies on wake therapeutic communication—suggestions given without induction of hypnosis, based on the observation that surgical patients enter a natural trance state through preoperative anxiety and stress and behave as though hypnotised32—showed low effect on pain and no significant effect on drug use.33
Table 4.
Effect sizes of different verbal interventions for postoperative pain relief
| Hypnosis (Kekecs 2014)30 | Wake suggestions (Kekecs 2014)30 | Intraoperative suggestions (Rosendahl 2016)13 | Intraoperative suggestions (current study) | |
|---|---|---|---|---|
| Pain | 0.35* | 0.13* | 0.04* | 0.45† |
| Opioids | 0.23* | 0.09* | 0.16* | 0.36† |
Hedges’s g provides a correction of Cohen’s d when groups differ considerably in sample size. With similar sample size of the two groups in the present study, Hedges’s g and Cohen’s d are directly comparable.
Hedges’s g.
Cohen’s d.
In our study we found even higher effects, but with much less effort (no preparation, no specialist, inexpensive). We consider a reduced resistance to suggestions after loss of critical, rational thinking and an access to the subconscious to be responsible. An early review about the efficacy of therapeutic suggestions during general anaesthesia found mixed results,10 and a recent meta-analysis showed small but statistically significant effects on use of analgesics but not on pain level (table 4).13 This was a further reason we chose opioid requirement as the primary outcome in this study. In contrast, the effects reported here are much stronger than observed in previous and smaller studies and involve both analgesic requirement and pain level.
Strengths and limitations of this study
That we found a stronger effect of therapeutic suggestions during surgery than in previous studies might relate to our study design, with analgesic requirement based on a NRS score for pain instead of arbitrarily administered analgesia. Generally, with a liberal pain management and high opioid dosage, pain levels are low and differed little between study groups. Conversely, restricted analgesia leads to high pain scores with high discriminatory power. Only by using a well defined strategy for pain treatment and by considering both pain level and opioid consumption, induced changes in pain and request for opioids can show. A further reason for more pronounced effects could be associated with the highly developed text we chose for the audiotape. In this text, negative expressions such as “feel no pain” or “absence of nausea” were replaced by positive ones such as “increased comfort.” The fundamental structure of the text for the therapeutic communication was based on themes derived from basic psychological needs and positive suggestions set against traumatic stressors.19 Covered topics included support, contact, comfort, control, information, instructions, respect, safety, confidence, and healing. The hypnotic interventions included dissociation to a safe place of wellbeing, reframing of disturbing sensations and noises, reinforcement of self-confidence, affirmation, and indirect suggestions (“He looks like he is really doing well” in the third person, instead of a direct “You will be doing great.”) (see text in appendix). Several of these components have been used in previous studies, but not in such a concentrated and structured way.10-12 Moreover, former studies on this topic included fewer than 100 patients in each group and were performed more than 19 years ago, during which time considerable changes and developments in anaesthesia and pain management have occurred.
A limitation of our study is that the contribution of factors other than the therapeutic suggestions remains unclear—for example, positive effects can be expected from the background music.34 Several beneficial effects have been described for perioperative music, among others, on postoperative pain and analgesia.35 36 However, most work on music used in a medical setting is done on conscious patients before, during, or after surgery, and some meta-analyses have concluded that the effect of music is lower when given during general anaesthesia.37 A recent randomised controlled trial on music given under controlled general anaesthesia found no effect on postoperative neurohormonal stress response and opioid dosage.37 The combination of music with therapeutic communication has been studied as well. In our meta-analysis of 32 studies with intraoperative suggestions we identified seven that included background music.13 The overall saving of postoperative opioids described there cannot be explained solely by an effect of music, and the demonstrated lack of pain reduction was despite the additional music. Moreover, a beneficial effect can be expected from shielding through earphones against intraoperative noises and careless talk, including negative suggestions.19
The use of two methods for administering postoperative opioids—patient controlled analgesia and nurse controlled analgesia—might be considered a limitation. However, in the respective study centres either one was applied uniformly in both groups, and the effect of the intervention was shown with either type of analgesia. The heterogeneity in surgical procedures included in our study does not necessarily represent a disadvantage or limitation, when wider clinical impact and application is considered, but it does argue for a strong and robust effect. With higher homogeneity of samples, effect size usually increases, among other reasons because of smaller variance, but generalisability or external validity decreases.38 Rather, it presented a problem in previous studies as the results from particular types of surgery such as hysterectomy cannot be easily transferred to other types of surgery, such as orthopaedic, or to predominantly male populations. However, there were some limitations in the overall invasiveness, extent, and durationof the surgeries, and to enable the results to be transferable to more invasive operations, such as cardiac surgery and other procedures with indication for postoperative intensive care, further studies are necessary. Our subgroup analysis shows a comparable effect of the verbal intervention in surgeries that result in both high and low pain. Moreover, in a mixed effect model (table 3), painfulness of surgery shows an effect on requirement for opioids postoperatively but not on the effect of the intervention. This indicates that intraoperative suggestions might be helpful in different types of surgery. Finally, the mechanism and mediators of the treatment effects were not the focus of this study and need further research. The intraoperative suggestions might have an analgesic effect postoperatively but might also modulate the development of pain during surgery.
Meaning of the study and clinical implications
As median values are hardly affected by single events, the significant effects observed in this study cannot be explained by merely the response of a few patients, such as with intraoperative awareness.5 6 Also the calculated number needed to treat is only compatible with the response of a considerable portion of patients. Explicit memory of perception and words is no prerequisite for effectiveness of suggestions in unconscious patients but rather meaningfulness, such as in experiments when a complication is simulated during surgery,7 or as in the therapeutic audio suggestions used in this study. Based on our finding of intraoperative perception, surgeons and anaesthetists should be careful about background noise and conversations during surgery and instead use the patient’s perception for positive suggestions.
Various factors are linked to opioid consumption. Our analysis in a mixed effects model (table 3) shows that therapeutic suggestions during surgery as an intervention remain an independent determinant in this interplay of influencing factors. Surgery stratified according to expected postoperative pain level was not a determinant of the opioid saving effect of positive suggestions. Rather, individual sensitivity to pain and course of the surgery reflected by the wide range of opioid dose required during surgery could play a role. This is also indicated by the high variance of pain level by type of surgery.24 Therefore, our results might indicate a wide application of intraoperative therapeutic suggestions, especially in patients with high sensitivity to pain.
With a saving of one third of postoperative opioids and noticeably fewer patients using opioids, the observed effect of the tested non-drug intervention not only reached statistical significance but is also of clinical interest. Validation by further and larger studies could lead to a call for a more general use of therapeutic suggestions in surgical patients. The efficacy of intraoperative therapeutic suggestions shown here, together with the low effort and costs necessary for implementation and with no side effects observed or expected, makes it hard to argue against using this simple method for reduction of postoperative pain and opioid use. Earlier studies on intraoperative suggestions failed to encourage wider clinical use because of low quality and lack of corresponding reviews. Our recent meta-analysis and the present study should help to draw attention to this topic in surgery and anaesthesia. The reported significant opioid saving effect of therapeutic suggestions during surgery might encourage wider use and help to solve the social problems of opioid misuse, addiction, and overdose.17
Future research
Therapeutic suggestions during anaesthesia and surgery need further evaluation, particularly in more invasive and painful surgical procedures. Efforts to optimise the therapeutic suggestions are desirable and promising. For instance, evidence from meta-analysis indicates that therapeutic suggestions are more effective when delivered at least in part before the medical procedure rather than solely during the procedure.33 Indication of perception and positive effects in anaesthetised patients could encourage a similar evaluation and application of taped positive suggestions for non-drug support in other unconscious patients—for example, during resuscitation, intensive care, or coma..
What is already known on this topic
Perception during general anaesthesia has been reported, mostly with negative consequences such as with “intraoperative awareness”
Studies on perception during anaesthesia have been undertaken with the intention of using it positively
A recent meta-analysis of older trials indicates improvements in postoperative recovery
What this study adds
This study found a reduction in postoperative pain and need for opioids after delivery of therapeutic suggestions during surgery, with a number needed to treat of 6 to avoid postoperative opioids
The underlying intraoperative perception suggests that surgical teams should be aware of background noise or negative conversations during surgery
Therapeutic suggestions during surgery could provide a safe, feasible, inexpensive, and non-drug technique to reduce postoperative pain and opioid use, with the potential for more general use
Acknowledgments
We thank the patients for participation in the study; Bharat Jasani (Institute of Cancer and Genetics, Cardiff University, UK) for English proofreading of the manuscript; Anita Jung (hypnotherapist, Austin, TX) for English proofreading of the tested texts for intraoperative suggestions; and Mark Jensen (vice chair for research in Rehabilitation Medicine at the University of Washington, WA) for critical review of the manuscript.
Web extra.
Extra material supplied by authors
Supplementary information: additional tables and text of therapeutic suggestions
Contributors: HN and NZ contributed equally to the study. EH, NZ, and GO conceived and designed the study. EH and NZ developed and taped the intervention text. HN and NZ had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. AM and GO gave administrative support, including ethics committee approval and study registration. LG, MM, KG, CM, AZ, and KL acquired, analysed, and interpreted the data. NZ, SA, GO, MT, JH, and TS supervised the study in the five study centres. HN, NZ, TR, and KS did the statistical analysis and produced tables and figures. EH, NZ, and HN drafted the manuscript. All authors critically revised the manuscript for scientific content and approved the final version of the article. EH, HN, and NZ are the guarantors of the study. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study received no direct funding. Statistical analysis was partly supported by de.NBI, a project of the German Federal Ministry of Education and Research (BMBF) (grant No FKZ 031 A 534A), without any role in study design, data collection, and publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was approved by the ethics committee of the Medical Faculty of the Ruhr-University Bochum (registration No 17-5957-BR). All ethics committees of the participating centres approved the study. All patients gave written informed consent.
Data sharing: The raw trial data after deidentification can be shared on individual request to the first author at hartmuth.nowak@kk-bochum.de. To gain access, data access agreement needs to be signed. Proposals will be considered up to 36 months after article publication.
The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned and registered have been explained.
Dissemination to participants and related patient and public communities: The results of the research will be disseminated to the public through press release, broadcasts, popular science articles, and newspapers.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Cheek DB. The anesthetized patient can hear and can remember. Am J Proctol 1962;13:287-90. [PubMed] [Google Scholar]
- 2. Clark DL, Rosner BS. Neurophysiologic effects of general anesthetics. I. The electroencephalogram and sensory evoked responses in man. Anesthesiology 1973;38:564-82. 10.1097/00000542-197306000-00011 [DOI] [PubMed] [Google Scholar]
- 3. Madler C, Keller I, Schwender D, Pöppel E. Sensory information processing during general anaesthesia: effect of isoflurane on auditory evoked neuronal oscillations. Br J Anaesth 1991;66:81-7. 10.1093/bja/66.1.81 [DOI] [PubMed] [Google Scholar]
- 4. Hajat Z, Ahmad N, Andrzejowski J. The role and limitations of EEG-based depth of anaesthesia monitoring in theatres and intensive care. Anaesthesia 2017;72(Suppl 1):38-47. 10.1111/anae.13739 [DOI] [PubMed] [Google Scholar]
- 5. Tasbihgou SR, Vogels MF, Absalom AR. Accidental awareness during general anaesthesia - a narrative review. Anaesthesia 2018;73:112-22. 10.1111/anae.14124 [DOI] [PubMed] [Google Scholar]
- 6. Samuelsson P, Brudin L, Sandin RH. Late psychological symptoms after awareness among consecutively included surgical patients. Anesthesiology 2007;106:26-32. 10.1097/00000542-200701000-00009 [DOI] [PubMed] [Google Scholar]
- 7. Levinson BW. States of awareness during general anaesthesia. Preliminary communication. Br J Anaesth 1965;37:544-6. 10.1093/bja/37.7.544 [DOI] [PubMed] [Google Scholar]
- 8. Gao WW, He YH, Liu L, Yuan Q, Wang YF, Zhao B. BIS Monitoring on Intraoperative Awareness: A Meta-analysis. Curr Med Sci 2018;38:349-53. 10.1007/s11596-018-1886-1 [DOI] [PubMed] [Google Scholar]
- 9. Messina AG, Wang M, Ward MJ, et al. Anaesthetic interventions for prevention of awareness during surgery. Cochrane Database Syst Rev 2016;10:CD007272. 10.1002/14651858.CD007272.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Merikle PM, Daneman M. Memory for unconsciously perceived events: evidence from anesthetized patients. Conscious Cogn 1996;5:525-41. 10.1006/ccog.1996.0031 [DOI] [PubMed] [Google Scholar]
- 11. Nilsson U, Rawal N, Uneståhl LE, Zetterberg C, Unosson M. Improved recovery after music and therapeutic suggestions during general anaesthesia: a double-blind randomised controlled trial. Acta Anaesthesiol Scand 2001;45:812-7. 10.1034/j.1399-6576.2001.045007812.x [DOI] [PubMed] [Google Scholar]
- 12. Dawson P, Van Hamel C, Wilkinson D, Warwick P, O’Connor M. Patient-controlled analgesia and intra-operative suggestion. Anaesthesia 2001;56:65-9. 10.1046/j.1365-2044.2001.01763-5.x [DOI] [PubMed] [Google Scholar]
- 13. Rosendahl J, Koranyi S, Jacob D, Zech N, Hansen E. Efficacy of therapeutic suggestions under general anesthesia: a systematic review and meta-analysis of randomized controlled trials. BMC Anesthesiol 2016;16:125. 10.1186/s12871-016-0292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain 2002;3:159-80. 10.1054/jpai.2002.123652 [DOI] [PubMed] [Google Scholar]
- 15. Kehlet H. Postoperative opioid sparing to hasten recovery: what are the issues? Anesthesiology 2005;102:1083-5. 10.1097/00000542-200506000-00004 [DOI] [PubMed] [Google Scholar]
- 16. Marret E, Kurdi O, Zufferey P, Bonnet F. Effects of nonsteroidal antiinflammatory drugs on patient-controlled analgesia morphine side effects: meta-analysis of randomized controlled trials. Anesthesiology 2005;102:1249-60. 10.1097/00000542-200506000-00027 [DOI] [PubMed] [Google Scholar]
- 17. Chen Q, Larochelle MR, Weaver DT, et al. Prevention of Prescription Opioid Misuse and Projected Overdose Deaths in the United States. JAMA Netw Open 2019;2:e187621. 10.1001/jamanetworkopen.2018.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Owens WD, Felts JA, Spitznagel EL., Jr ASA physical status classifications: a study of consistency of ratings. Anesthesiology 1978;49:239-43. 10.1097/00000542-197810000-00003 [DOI] [PubMed] [Google Scholar]
- 19. Hansen E, Zech N. Nocebo effects and negative suggestions in daily clinical practice - Forms, impact and approaches to avoid them. Front Pharmacol 2019;10:77. 10.3389/fphar.2019.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McNicol ED, Ferguson MC, Hudcova J. Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain. Cochrane Database Syst Rev 2015;6:CD003348. 10.1002/4651858.CD003348pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riegel B, Tönnies S, Hansen E, Zech N, Eck S, Batra A, Peter B German norms of the Harvard Group Scale of Hypnotic Susceptibility, Form A (HGSHS:A) and proposal of a 5-item short version (HGSHS-5G). Int J Clin Exp Hypn 2000; (in press). 10.1080/00207144.2021.1836645. [DOI] [PubMed] [Google Scholar]
- 22. Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol 1992;31:301-6. 10.1111/j.2044-8260.1992.tb00997.x [DOI] [PubMed] [Google Scholar]
- 23. Roessler M, Eulitz N. Notarzt und Palliativmedizin. Anästh Intensivmed. 2018;59:430-8. [Google Scholar]
- 24. Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology 2013;118:934-44. 10.1097/ALN.0b013e31828866b3 [DOI] [PubMed] [Google Scholar]
- 25. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S240-52. 10.1002/acr.20543 [DOI] [PubMed] [Google Scholar]
- 26. Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc 2007;82:591-605. 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- 27. Frampton JE. Tapentadol immediate release: a review of its use in the treatment of moderate to severe acute pain. Drugs 2010;70:1719-43. 10.2165/11204470-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 28. Häuser W, Hansen E, Enck P. Nocebo phenomena in medicine: their relevance in everyday clinical practice. Dtsch Arztebl Int 2012;109:459-65. 10.3238/arztebl.2012.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richter M, Eck J, Straube T, Miltner WHR, Weiss T. Do words hurt? Brain activation during the processing of pain-related words. Pain 2010;148:198-205. 10.1016/j.pain.2009.08.009 [DOI] [PubMed] [Google Scholar]
- 30. Kekecs Z, Nagy T, Varga K. The effectiveness of suggestive techniques in reducing postoperative side effects: a meta-analysis of randomized controlled trials. Anesth Analg 2014;119:1407-19. 10.1213/ANE.0000000000000466 [DOI] [PubMed] [Google Scholar]
- 31. Häuser W, Hagl M, Schmierer A, Hansen E. The Efficacy, Safety and Applications of Medical Hypnosis: A Systematic Review of Meta-analyses. Dtsch Arztebl Int 2016;113:289-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheek DB. Importance of recognizing that surgical patients behave as though hypnotized. Am J Clin Hypn 1962;4:227-36. 10.1080/00029157.1962.10401905 [DOI] [PubMed] [Google Scholar]
- 33. Schnur JB, Kafer I, Marcus C, Montgomery GH. Hypnosis to manage distress related to medical procedures: a meta-analysis. Contemp Hypn 2008;25:114-28. 10.1002/ch.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsota P, Christodoulopoulou T, Smyrnioti ME, et al. Music’s use for anesthesia and analgesia. J Altern Complement Med 2013;19:298-307. 10.1089/acm.2010.0235 [DOI] [PubMed] [Google Scholar]
- 35. Hole J, Hirsch M, Ball E, Meads C. Music as an aid for postoperative recovery in adults: a systematic review and meta-analysis. Lancet 2015;386:1659-71. 10.1016/S0140-6736(15)60169-6 [DOI] [PubMed] [Google Scholar]
- 36. Kühlmann AYR, de Rooij A, Kroese LF, van Dijk M, Hunink MGM, Jeekel J. Meta-analysis evaluating music interventions for anxiety and pain in surgery. Br J Surg 2018;105:773-83. 10.1002/bjs.10853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Migneault B, Girard F, Albert C, et al. The effect of music on the neurohormonal stress response to surgery under general anesthesia. Anesth Analg 2004;98:527-32. 10.1213/01.ANE.0000096182.70239.23 [DOI] [PubMed] [Google Scholar]
- 38. Rief W, Nestoriuc Y, Weiss S, Welzel E, Barsky AJ, Hofmann SG. Meta-analysis of the placebo response in antidepressant trials. J Affect Disord 2009;118:1-8. 10.1016/j.jad.2009.01.029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: additional tables and text of therapeutic suggestions