Abstract
Background
The adenoma-carcinoma sequence in thyroid nodules is an enigmatic phenomenon. Genomics is the only definitive modality to resolve this hypothesis. Adenomas and papillary carcinomas tend to have mutations in RAS and highly specific BRAF gene respectively. In this context, we set out study the prevalence and clinical significance of these somatic mutations in surgical tissue samples.
Material and methods
This retrospective study was conducted on surgically managed thyroid nodule patients. Institutional ethical committee approval was obtained. Diagnosis was based on biochemical confirmation, imaging, fine needle aspiration cytology and later confirmed by histopathology. We selected 100 benign thyroid adenomas (BTA) and 100 papillary thyroid carcinoma (PTC) cases. Archived tumour tissue samples of selected cases were retrieved. After appropriate processing of samples, DNA extraction, cDNA preparation, PCR amplification, application of 4 sets of Primers were performed as part of mutational analysis of RAS (H-,K-,N-) and BRAF genes.
Results
Homozygous mutations in N-RAS were found in 36/100 (36%) of BTA and 7/100 (7%) of PTC cases. No H-RAS or K-RAS mutations were found in both groups. Homozygous mutations were found in BRAF gene in 4/100 (4%) of BTA cases and 52/100 (52%) of PTC cases. The differences were statistically significant.
Conclusions
Similar N-RAS and BRAF mutations were prevalent in both benign and malignant thyroid nodules giving some evidence for linkage between them. Though not robust, we opine that there is possibility of adenoma-carcinoma sequence in thyroid nodules.
Keywords: Papillary thyroid cancer, Thyroidectomy, BRAF gene, RAS gene, Adenoma
Highlights
-
•
Targeted genetic analysis of BRAF and RAS genes, genetic analysis in histopathologically confirmed tissues rather than cytology specimen.
-
•
Simultaneous similar mutations in BTA and PTC areas in mixed tumours.
-
•
First of its kind study from India.
-
•
Utility of doing genetic analysis of BRAF and RAS genes and their significance in dictating the tumour biology of thyroid adenomas and papillary thyroid cancer.
-
•
The results gives a potential insight into the phenomenon of adenoma to carcinoma sequence in thyroid tumourigenesis.
1. Background
Goiter and thyroid nodules due to benign adenomas (BTA) and papillary thyroid cancer (PTC) are very common endocrine surgical disorders [[1], [2], [3]]. The issue of adenoma-carcinoma sequence (ACS) is a well established phenomenon in colorectal adenocarcinoma [4,5]. But, this ACS is an enigmatic and debatable issue in thyroid nodules [6,7]. There are indirect evidences such as mixed simultaneous tumours, hostage theory, collision hypothesis etc., to justify this ACS [[8], [9], [10]]. Genomics is the only definitive modality to resolve this hypothesis of ACS. BTAs tend to have higher prevalence of RAS gene mutations and PTC tends to have higher prevalence of BRAF gene mutations, respectively [11,12]. Genetic studies looking into their role in ACS are scanty, especially in the Indian population. In this context, we set out to study the prevalence and biological/clinical significance of these somatic mutations in our study population.
2. Material and methods
This is a retrospective, multi-institutional collaborative study conducted between departments of Endocrine surgery, biochemistry, genetics and anatomy. One hundred cases of BTA and 100 cases of PTC operated between 2011 and 2015 with minimum followup of 5 years, were included as study cohorts. All clinical, investigative, pathological, treatment and follow-up details were analysed systematically. Inclusion criteria was all BTA and PTC cases with histopathology of well differentiated papillary cancer and benign thyroid adenomas. Exclusion criteria are colloid nodules, cysts, Hashimoto's thyroiditis, follicular thyroid cancer, poorly differentiated thyroid cancer, anaplastic cancer, medullary thyroid cancer and Hurthle cell cancer; cases operated elsewhere; those with incomplete records and lost to follow-up cases. This study complied with the international ethical norms of the Helsinki Declaration — Ethical Principles for Medical Research Involving Human Subjects, 2004 [13]. Institutional ethical committee approval was obtained. This study has been reported in line with the STOCSS guidelines [14]. The study has been registered in Research Registry with UIN: researchregistry6239 (https://www.researchregistry.com/registernow#home/registrationdetails/5fa9ab4b72f9bb0016578a9c/). Informed consent was obtained from all the included members of the cohort.
2.1. Definitions and standards employed for this study
-
(1)
TNM staging of AJCC 6th edition was applied to stage all cases [15].
-
(2)
AMES (Age, Metastasis, Extrathyroidal invasion, Tumour size) of Cady's risk grouping was used [16].
-
(3)
WHO classification was used as a guide to define and classify pathological types [17].
-
(4)
Synchronous distant metastases was defined as either clinically detected metastases within 6 months of surgery, confirmed by ultrasonography, computerized tomography (CT scan) or diagnosed on post radio-iodine ablation scan and metachronous metastases was defined as metastasis detected after 6 months or at follow-up whole body post radio-iodine scans (WBRI)
2.2. Abbreviations used in this study
BTA = benign thyroid adenomas; DTC = follicular cell derived differentiated thyroid cancer; PTC = Papillary thyroid cancer; ETI = Extrathyroidal invasion; EFS = Event free survival; OS = Overall survival.
2.3. Genetic methodology
Archived thyroidectomy tissue samples of selected cases were retrieved for genetic analysis. After appropriate processing of thyroid tissue samples, DNA extraction and cDNA preparation was done. DNA quality was checked by agarose gel electrophoresis for any degradation or RNA contamination. DNA was quantified by spectrophotometric method with absorbance at 260 nm and 280 nm. The isolated DNA samples were stored at 4 °C for genetic analysis later. Polymerase chain reaction (PCR) was used to amplify the fragments of target genes from the isolated DNA. Cycling conditions were 95 °C for 5 min (one cycle); at 95 °C for 40 s, at 55 °C for 40 s, at 72 °C for 60 s (for 35 cycles), and final extension at 72 °C for 10 min using one pair of primers annealing at regions of interest. We used four sets of primers depending on number of screened single nucleotide polymorphisms (SNPs) – 1 set for BRAF gene and three sets for RAS gene (N-, H-, K-). Each set consisted forward and reverse reading frames. Full set of primer sequences are shown in Table 1. The quality of PCR products was checked with agarose gel electrophoresis. Nucleotide sequences of all amplified PCR products were determined in both orientations by direct sequencing with an Applied Biosystems 3730XL Sequencer (Macrogen, Seoul, South Korea). The results were analyzed using Bio-Edit (version 7.1.3), (Ibis Biosciences, Carlsbad, CA, USA for Bio-Edit Applied Biosystems Co. for Sequence scanner NCBI. NLM). NIH [National Library of Medicine. National Institute of Health] and Nucleotide database for Nucleotide blast program. Two types of mutations were looked for – known (mutations already reported in the database of SNPs) and unknown (mutations never reported before). Known mutations and single nucleotide polymorphisms (SNPs) were analyzed with restriction fragment length polymorphism analysis. Transcriptomic sequencing of exonic segments of above genes, are analyzed. For unknown or novel mutations, we planned to select hotspots on sequencing and study them. The structural and functional analysis of the gene segments consisting mutations, was performed.
Table 1.
Details of Primer sequences.
| Gene | Primers | Annealing Temperature | Size of the PCR product |
|---|---|---|---|
| BRAF | FP: 5′GCTTGCTCTGATAGGAAAATGAG-3′ | 56 °C | 237 bp |
| RP: 5′GATACTCAGCAGCATCTCAGG-3′ | |||
| KRAS | FP: 5′-GGCCTGCTGAAAATGACTGA-3′ | 52 °C | 81 bp |
| RP: 5′-TAGCTGTATCGTCAAGGCAC-3′ | |||
| HRAS | FP: 5′-TGA GGA GCG ATG ACG GAA-3′ | 52 °C | 133 bp |
| RP: 5′-GCG CTAGGC TCA CCT CTA T-3′ | |||
| NRAS | FP: 5′-CCT GTT TGTTGG ACA TAC TG-3 | 52 °C | 143 bp |
| RP: 5′-CCT GTA GAG GTT AAT ATCCG-3′ |
2.4. Statistical analysis
Statistical analysis was performed using IBM SPSS software. Descriptive statistics were analyzed with t-test and Chi-square tests. Univariate and multivariate analysis were done using general linear model. P value of <0.05 was taken as statistically significant probability cutoff.
3. Results
Mean follow up in PTC and BTA were 62 ± 21.5 months (60–123) and 54.5 ± 19 months, respectively. Mean age and gender ratio were 41 ± 12 years (19–57); M:F = 1:1.3 and 43 ± 10.5 years (26–65); M:F = 1:1.6 in PTC and BTA, respectively. Total thyroidectomy was performed in 96% and hemithyroidectomy in 4% cases of PTC. Hemithyroidectomy and total thyroidectomy was performed in 80% and 20% cases of BTA, respectively. No cases of permanent hypoparathyroidism or recurrent laryngeal nerve palsy were noted in postoperative period. Frequency distribution and statistical significance of various clinical, investigative and treatment parameters are detailed in Table 2. The number of subjects according to TNM group staging were 49%, 19%, 23% and 8% in PTC. According to AMES risk stratification, high risk to low risk cases ratio in PTC was 1.7:1.
Table 2.
Comparative significance and frequency of clinical, treatment variables.
| Variable | PTC | BTA | P value |
|---|---|---|---|
| Age (in years) | 41 ± 12 (19–57) | 43 ± 10.5 (26–65) | 0.115 |
| Sex ratio (M:F) | 1:1.3 | 1:1.6 | 0.626 |
| Lymphadenopathy | 30% | NA | NA |
| Tumour size (in cm) | 3.9 ± 0.9 | 4.3 ± 1.2 | NA |
| Total thyroidectomy | 96% | 80% | 0.08 |
| Hemithyroidectomy | 80% | 20% | 0.034 |
NA = Not applicable.
Histopathology was classical, follicular variant and tall cell variant in 82%, 12% and 6% of PTC cases, respectively. In BTA, all were benign follicular adenomas. There was a tiny subset of four cases of PTC, there was co-occurrence of adenoma in the excised nodular goiter. Radio-iodine ablation was utilized in 45% (PTC) of cases. Nodal recurrence (PTC = 3%) and systemic metastasis was 3% in PTC (synchronous = 3%; metachronous = 0%). Five year event free survival (EFS) and overall survival (OS) was 95% and 100% in DTC, respectively. Among AMES and TNM variables, only metastases affected EFS (P value = < 0.05) and none affected OS. As shown in Table 3 - age of the patient, presence of distant metastasis and tumour size, but not ETI had statistically significant effect on overall survival and recurrence free survival.
Table 3.
Analysis of prognostic factors in PTC.
| Variable | UVA | MVA |
|---|---|---|
| Age | 0.04 | 0.08 |
| Sex | 0.913 | 0.755 |
| Tumour size | 0.01 | 0.08 |
| ETI | 0.07 | 0.978 |
| Metastases | 0.009 | 0.01 |
| Lymphadenopathy | 0.387 | 0.623 |
UVA = Univariate analysis; MVA = Multivariate analysis.
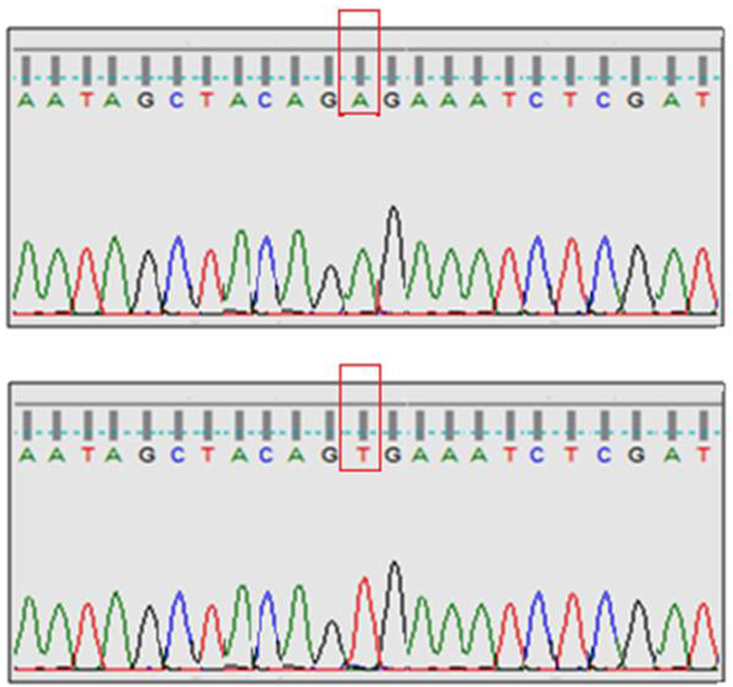
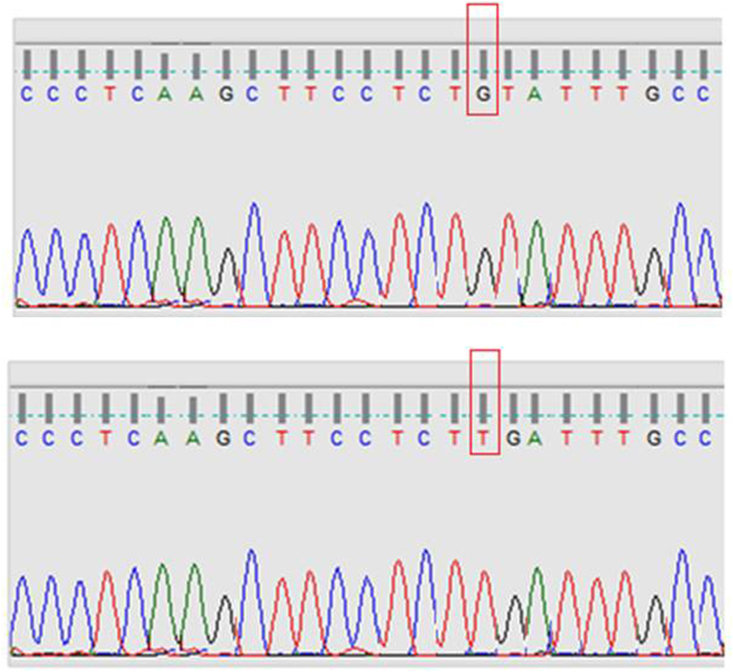
Homozygous mutations in N-RAS gene were found in 36/100 (36%) of BTA and 7/100 (7%) of PTC cases. No H-RAS or K-RAS gene mutations were found in both groups. Homozygous mutations were found in BRAF gene in 4/100 (4%) of BTA cases and 52/100 (52%) of PTC cases. The differences were statistically significant. In the subgroup with both BTA and PTC, similar type of N-RAS, BRAF gene mutations was found from both areas in three cases of this subgroup. As detailed in Table 4, the extrathyroidal invasion, lymphadenopathy rate and mean tumour size were more in BRAF positive > BRAF negative PTC cases, reaching statistical significance, suggestive of an aggressive tumour biology in BRAF positive PTC. Prognostic parameters such as age of the patient, metastasis rate, recurrence rate and survival rates were not statistically significant between BRAF positive and BRAF negative PTC cases. Representative illustrations of gene mutation sequences are shown in Fig. 1, Fig. 2. No novel mutations were found in our study.
Table 4.
Prognostic significance of BRAF gene mutations in PTC.
| Variable | BRAF positive PTC | BRAF negative PTC | P value |
|---|---|---|---|
| Age (in years) | 39 ± 10 (19–57) | 42 ± 9.5 (20–58) | 0.109 |
| Sex ratio (M:F) | 1:1.3 | 1:1.35 | 0.933 |
| Lymphadenopathy | 37% | 22% | 0.04 |
| Tumour size (in cm) | 4.1 ± 0.8 | 2.9 ± 1.3 | 0.03 |
| ETI | 41% | 19% | 0.02 |
| Metastasis rate | 9.6% | 6.2% | 0.262 |
| RFS | 4% | 2% | NA |
| OS | 100% | 100% | NA |
NA = Not applicable; RFS = recurrence survival rate; OS = overall survival rate.
Fig. 1.
BRAF gene mutation found in our study - upper panel shows normal sequence; lower panel shows mutant sequence.
Fig. 2.
NRAS gene mutation found in our study – upper panel shows normal sequence; lower panel shows mutant sequence.
4. Discussion
Goiter and thyroid nodules constitute a significant epidemiological burden, globally [18,19]. Though, there are protean causes of nodular goiters such as endemic goiter, colloid nodule, Hashimoto's thyroiditis (HT) etc., benign adenoma (BTA) and papillary thyroid cancer (PTC) are the most common endocrine surgical disorders. The social, clinical impact of BTA and PTC on the patient and their family are manifold. The treatment of BTA is either surgical thyroidectomy or conservative observation and followup depending on various sonographic, cytological gradings [20]. One of the primary concern in the management of BTA is when and how to intervene. The main reason for surgical intervention in BTA is the potential threat of its transformation/progression to malignancy. PTC is the commonest endocrine and thyroid malignancy. The treatment of PTC is primarily surgical thyroidectomy with or without adjuvant radioiodine therapy, based on clinico-pathological stage/grade [20].
The clinicians, endocrinologists, oncologists, surgeons, pathologists and endocrine surgeons are intrigued with the comparative tumour biology between BTA and PTC. Evolution of genetic studies and ready availability of targeted genetic analysis opened up a new avenue to plug the lacunae in cancer tumour biology [21]. Genomics is one of the definitive modalities to resolve this conundrum [21,22]. Moreover, poorly differentiated cancer (PDC) and anaplastic cancer (ATC) are thought to arise from preexisting PTCs based on their frequent co-occurrence in the same tumour specimen, where they consistently share a driver mutations in BRAF and RAS genes [23,24]. Thyroid differentiation score studies showed thatATC had profoundly lower mRNA levels of genes involved in iodine metabolism, justifying the hypothesis of progressive tumour biology [25,26].
The transformation and progression of BTA in to PTC is an enigmatic and debatable issue. The phenomenon of Adenoma-to-carcinoma sequence (ACS) in thyroid, has been speculated and investigated, since a long time [6,7,22]. The phenomenon of ACS is a well established theory in colonic adenocarcinoma. This is proven by the genetic analysis and in relation with familial colonic cancer syndromes such as Gardner, Cowden, Turcot syndromes etc., [4,5]. For instance, adenomatous polyposis coli (APC) gene mutation confirmed this ACS in colonic cancer related to familial adenomatous polyposis [27]. But, there is dearth of robust evidence about ACS in thyroid tumour biology. There are indirect evidences of ACS operating in thyroid such as presence of mixed simultaneous tumours, common stem cell theory, hostage theory, collision hypothesis etc., [6,7]. Collision theory postulates that simultaneous multifocal origin from different cell clones, or hostage theory which states that adenomatous areas are sequestrated by another tumour type, though the exact etiology is elusive [8,9].
Genetic studies looking into their role in ACS are scanty, especially in the Indian population [28]. In this context, we set out to study the prevalence and biological/clinical significance of these somatic mutations in our study population. BTAs tend to have higher prevalence of RAS gene mutations and PTC tends to have higher prevalence of BRAF gene mutations, respectively. RAS gene mutations are relatively rare (0–8%) in PTC [29], though very few studies showed a higher incidence of up to 45%. But, this higher frequency of RAS gene mutations were found only follicular variant of PTC [29,30]. In our study, we have not evaluated mutation frequency in various subtypes of PTC. Eighty two percent of our PTC cases belonged to classical variant in our cohort. The most common subtype of RAS gene mutation in the literature was NRAS, though KRAS and HRAS are found with variable frequency [29,30]. In our study, only NRAS gene mutation was found in all positive cases. The reported frequency of BRAF gene mutations in PTC was 27–65%, making it the most common mutation [31,32]. Our study cohort had 52% frequency of BRAF mutation. The frequency of BRAF gene mutations is lower in BTA and it is proven to be specific for PTC. This fact was confirmed in our study, as shown by the statistically significant differences in RAS, BRAF gene mutation frequencies in BTA and PTC. We opine that one of main reasons for wide variations in frequency and pattern of mutations is the geographical and ethnic factors, apart from study design, genetic methodology and study cohort etc., We opine that BRAF gene mutation portends an aggressive tumour biology and worser prognosis in PTC. Further, the significant difference in tumour size, ETI, metastases rate, lymphadenopathy rates between BRAF positive and BRAF negative PTC cases shows that they are biologically different. Exisiting literature also supports the phenomenon, that the BRAF positive PTC are more aggressive with higher recurrence rates [33,34]. Strengthening this speculation is also the extrapolative evidence of genetic studies suggestive of transition from DTC to PDC. They show increasing mutation rates of RAS, BRAF, RET-PTC, PPAR/PAX8, WNT/B catenin genes [[35], [36], [37]]. Several additional studies concluded that, RAS was a marker of poor prognosis, although sample size in each study was small and mutation testing was performed in selected patients. Another reason for aggressive impact of RAS in those studies was selection of already biologically aggressive PDC and anaplastic cancers. We cannot comment on the prognostic impact of RAS, because of its low frequency, selection of only PTC cases and shorter followup of cases in our study.
Inspite of various surrogate markers such as age, ETI, immunohistochemistry, thyroglobulin and radioiodine avidity, we opine that the ultimate marker of prognostic outcome is tumour biology dictated by rapidity of growth, presence of distant metastasis and response to treatment. Few studies showed that BRAF mutations had larger tumour size, lymph node metastasis and poor prognosis, but RAS mutations had no statistically significant on prognostic outcome in PTC. Detailed prognostic influence of mutations was not studied in this paper, because the primary focus of our study is ACS and not genotype-phenotype correlations. However, BRAF positivity had better RFS and OS than RAS, but this difference was statistically not significant in this study. Our study, shows that specific oncoprints correlated with tumour biology and may in future help in predicting the phenotypes of BTA, which may progress to PTC through ACS pathway.
Thus studying the relevant gene mutations in BTA and PTC, might help in better inter-institutional comparision of data, auditing and prognostication. Clear prognostic grading based on targeted genetic information can also lead to optimal management and follow-up protocols. The key take away messages of this study are targeted genetic analysis of BRAF and RAS genes, genetic analysis in histopathologically confirmed tissues rather than cytology specimen, simultaneous similar mutations in BTA and PTC areas in mixed tumours, comparision of various clinical parameters, emphasis on possible ACS, first of its kind study from India. The challenges encountered in this study are retrospective design, shorter follow-up, no reliable preoperative prognostic markers necessitating individualized treatment on case to case basis. But, these drawbacks are ubiquitous with any thyroid nodule/cancer studies. We tried to minimise their impact by performing targeted gene analysis in histopathologically confirmed tissues with specific emphasis on ACS. Thus we need larger studies (preferably prospective randomised controlled trials) with longer follow-up from widely different geographical areas to establish and utilize our findings. Though, the confirmation of progressive ACS is sub-optimal through this study, our genetic data provisionally hints at continuous ACS operating in transformation of BTA to PTC, coded by these chronologically accumulating molecular signatures.
5. Conclusions
Similar N-RAS and BRAF gene mutations were prevalent in both benign and malignant thyroid nodules giving some evidence for linkage between them. K-RAS and H-RAS gene mutations were not found in both the groups. Thus, we opine that there is possibility of a potential adenoma-carcinoma sequence in thyroid nodules.
Declaration of competing interest
Authors declare no potential conflicts of interest related to this work. There is no funding source or sponsorship for this work.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.amsu.2020.11.069.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Song Y.S., Lim J.A., Park Y.J. Mutation profile of well-differentiated thyroid cancer in asians. Endocrinol. Metab. (Seoul). 2015;30:252–262. doi: 10.3803/EnM.2015.30.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Zaher N., Al-Salam S., El Teraifi H. Thyroid carcinoma in the United Arab Emirates: perspectives and experience of a tertiary care hospital. Hematol. Oncol. Stem. Cell. Ther. 2008;1:14–21. doi: 10.1016/s1658-3876(08)50055-0. [DOI] [PubMed] [Google Scholar]
- 3.Popoveniuc G., Jonklaas J. Thyroid nodules. Med. Clin. 2012;96:329–349. doi: 10.1016/j.mcna.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bortz J.H., Friedrich-Nel H. The adenoma–carcinoma sequence, management and treatment of colon cancer. In: Bortz J., Ramlaul A., Munro L., editors. CT Colonography for Radiographers. Springer.; Cham: 2009. [DOI] [Google Scholar]
- 5.Nguyen H.T., Duong H. The molecular characteristics of colorectal cancer: implications for diagnosis and therapy (Review) Oncology. Letters. 2018;16:9–18. doi: 10.3892/ol.2018.8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhargav P.R.K. Enigma of adenoma-carcinoma sequence in thyroid gland: an interesting case report of multiple pathologies with literature review. World J. Endocr. Surg. 2016;8:172–174. [Google Scholar]
- 7.Bhargav P., Gayathri K.B. Synchronous occurrence of anaplastic, follicular and papillary carcinomas with follicular adenoma in thyroid gland. Indian J. Pathol. Microbiol. 2011;54:41. doi: 10.4103/0377-4929.81600. [DOI] [PubMed] [Google Scholar]
- 8.Matias- Guiu X. Compound medullary-papillary carcinoma of the thyroid: ture mixed versus collision tumour. Histopathology. 1994;25:183–185. doi: 10.1111/j.1365-2559.1994.tb01578.x. [DOI] [PubMed] [Google Scholar]
- 9.Mizukami Y., Michigishi T., Nonomura A., Nakamura S., Noguchi M., Hashimoto T. Mixed medullary-follicular carcinoma of the thyroid occurring in familial form. Histopathology. 1993;22:284–287. doi: 10.1111/j.1365-2559.1993.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 10.Volante M., Papotti M., Roth J., Saremasiani P., Speel E.J., Lloyd R.V. Mixed medullary-follicular thyroid carcinoma,Molecular evidence for a dual origin of tumor components. Am. J. Pathol. 1999;155:1499–1509. doi: 10.1016/S0002-9440(10)65465-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung C.K., Little M.P., Lubin J.H., Brenner A.V., Wells S.A., Jr., Sigurdson A.J. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J. Clin. Endocrinol. Metab. 2014;99:E276–E285. doi: 10.1210/jc.2013-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Censi S., Cavedon E., Bertazza L., Galuppini F., Watutantrige-Fernando S., De Lazzari P. Frequency and significance of ras, tert promoter, and braf mutations in cytologically indeterminate thyroid nodules: a monocentric case series at a tertiary-level endocrinology unit. Front. Endocrinol. 2017;8:273. doi: 10.3389/fendo.2017.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Medical Organization Declaration of Helsinki. B. M. J. 2004;313:1448–1449. [Google Scholar]
- 14.Agha R., Abdall-Razak A., Crossley E., Dowlut N., Iosifidis C., Mathew G. For the STROCSS group. The STROCSS 2019 guideline: strengthening the reporting of cohort studies in surgery, international. Journal. Of. Surgery. 2019;72:156–165. doi: 10.1016/j.ijsu.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Döbert N., Menzel C., Oeschger S., Grünwald F. Differentiated thyroid carcinoma: the new UICC 6th edition TNM classification system in a retrospective analysis of 169 patients. AJCC. Thyroid. 2004;14:65–70. doi: 10.1089/105072504322783867. [DOI] [PubMed] [Google Scholar]
- 16.Sanders L.E., Cady B. Differentiated thyroid cancer: reexamination of risk groups and outcome of treatment. Arch. Surg. 1998;133:419–425. doi: 10.1001/archsurg.133.4.419. [DOI] [PubMed] [Google Scholar]
- 17.Sobrinho-Simões M., Albores-Saavedra J., Tallini G., Santoro M., Volante M., Pilotti S. Poorly differentiated carcinoma. In: DeLellis R.A., Lloyd R.V., Heitz P.U., Eng C., editors. In World Health Organization Classification of Tumors: Pathology and Genetics of Tumours of Endocrine Organs. IARC Press; Lyon: 2004. pp. 73–76. [Google Scholar]
- 18.Zheng L., Yan W., Kong Y., Liang P., Mu Y. An epidemiological study of risk factors of thyroid nodule and goiter in Chinese women. Int. J. Clin. Exp. Med. 2015;8:11379–11387. [PMC free article] [PubMed] [Google Scholar]
- 19.Carle A., Krejbjerg A., Laurberg P. Epidemiology of nodular goitre. Influence of iodine intake. Best Pract. Res. Clin. Endocrinol. Metabol. 2014;28:465–479. doi: 10.1016/j.beem.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Haugen B.R., Alexander E.K., Bible K.C. American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyman D.M., Taylor B.S., Baselga J. Implementing genome-driven oncology. Cell. 2017;168:584–599. doi: 10.1016/j.cell.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Younis E. Oncogenesis of thyroid cancer. Asian Pac. J. Cancer Prev. APJCP. 2017;18:1191–1199. doi: 10.22034/APJCP.2017.18.5.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikiforova M.N. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J. Clin. Endocrinol. Metab. 2003;88:5399–5404. doi: 10.1210/jc.2003-030838. [DOI] [PubMed] [Google Scholar]
- 24.Nikiforov Y.E. Genetic alterations involved in the transition from well-differentiated to poorly differentiated and anaplastic thyroid carcinomas. Endocr. Pathol. 2004;15:319–327. doi: 10.1385/ep:15:4:319. [DOI] [PubMed] [Google Scholar]
- 25.Landa I., Ibrahimpasic T., Boucai L. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Invest. 2016;126:1052–1066. doi: 10.1172/JCI85271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He H. The role of microRNA genes in papillary thyroid carcinoma. Proc. Natl. Acad. Sci. U.S.A. 2005;102:19075–19080. doi: 10.1073/pnas.0509603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nallamilli B.R.R., Hegde M. Detecting APC gene mutations in familial adenomatous polyposis (FAP) Curr. Protoc. Hum. Genet. 2017;92:1–16. doi: 10.1002/cphg.29. [DOI] [PubMed] [Google Scholar]
- 28.George N., Agarwal A., Kumari N., Agarwal S., Krisnani N., Gupta S.K. Mutational profile of papillary thyroid carcinoma in an endemic goiter region of north India. Indian. J. Endocrinol. Metab. 2018;22:505–510. doi: 10.4103/ijem.IJEM_441_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howell G.M., Hodak S.P., Yip L. RAS mutations in thyroid cancer. Oncol. 2013;18:926–932. doi: 10.1634/theoncologist.2013-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park J.Y., Kim W.Y., Hwang T.S., Lee S.S., Kim H., Han H.S. BRAF and RAS mutations in follicular variants of papillary thyroid carcinoma. Endocr. Pathol. 2013;24:69–76. doi: 10.1007/s12022-013-9244-0. [DOI] [PubMed] [Google Scholar]
- 31.Goutas N., Vlachodimitropoulos D., Bouka M., Lazaris A.C., Nasioulas G., Gazouli M. BRAF and K-RAS mutation in a Greek papillary and medullary thyroid carcinoma cohort. Anticancer Res. 2008;28:305–308. [PubMed] [Google Scholar]
- 32.Naito H., Pairojkul C., Kitahori Y., Yane K., Miyahara H H., Konishi N. Different ras gene mutational frequencies in thyroid papillary carcinomas in Japan and Thailand. Canc. Lett. 1998;131:171–175. doi: 10.1016/s0304-3835(98)00149-9. [DOI] [PubMed] [Google Scholar]
- 33.Nikiforov Y.E., Ohori N.P., Hodak S.P., Carty S.E., LeBeau S.O., Ferris R.L. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J. Clin. Endocrinol. Metab. 2011;96:3390–3397. doi: 10.1210/jc.2011-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kebebew E., Weng J., Bauer J., Ranvier G., Clark O.H., Duh Q.Y., Shibru D. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann. Surg. 2007;246:466–470. doi: 10.1097/SLA.0b013e318148563d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volante M., Rapa I., Gandhi M., Bussolati G., Giachino D., Papotti M. RAS mutations are the predominant molecular alteration in poorly differentiated thyroid carcinomas and bear prognostic impact. J. Clin. Endocrinol. Metab. 2009;94:4735–4741. doi: 10.1210/jc.2009-1233. [DOI] [PubMed] [Google Scholar]
- 36.Nikiforov Y.E., Nikiforova M.N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011;7:569–580. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 37.Wreesmann V.B., Ghossein R.A., Patel S.G., Harris C.P., Schnaser E.A., Shaha A.R. Genome-wide appraisal of thyroid cancer progression. Am. J. Pathol. 2002;161:1549–1556. doi: 10.1016/S0002-9440(10)64433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.