Abstract
Due to limited arsenal of systemically available antifungal agents, infections caused by Candida albicans are difficult to treat and the emergence of drug-resistant strains present a major challenge to the clinicians worldwide. Hence further exploration of potential novel and effective antifungal drugs is required. In this study we have explored the potential of a flavonoid, astragalin, in controlling the growth of C. albicans, in both planktonic and biofilm forms by microdilution method; and in regulating the morphological switch between yeast and hyphal growth. Astragalin ability to interfere with membrane integrity, ergosterol synthesis and its role in the regulation of genes encoding for efflux pumps has been addressed. In our study, astragalin treatment produced good antimicrobial and significant antibiofilm activity. Anticandidal activity of astragalin was not related to ERG11 downregulation, neither to direct binding to CYP51 enzyme nor was linked to membrane ergosterol assembly. Instead, astragalin treatment resulted in reduced expression of CDR1 and also affected cell membrane integrity without causing cytotoxic effect on human gingival fibroblast cells. Considering that astragalin-mediated decreased expression of efflux pumps increases the concentration of antifungal drug inside the fungal cells, a combinatorial treatment with this agent could be explored as a novel therapeutic option for candidiasis.
Keywords: astragalin, mode of action, antimicrobial, ergosterol biosynthesis, hyphal transition, membrane integrity
Abbreviations
ROS, reactive oxygen species; ABC, ATP-binding cassette; SDA/SDB, Sabouraud Dextrose Agar/Broth; MIC, Minimum inhibitory concentration; MFC, Minimum fungicidal concentration; CaCYP51, Candida albicans sterol 14α-demethylase; NBT, nitro blue tetrazolium; HGF, human gingival fibroblasts cells; PBS, Phosphate buffered saline; IC50, 50 % of cell viability when compared with untreated control; AmB, amphotericin B; qPCR, Quantitative Real-Time Polymerase Chain Reaction; FC, fold change.
Introduction
There have been increasing incidences of invasive infections caused by Candida spp over the past few years with C. albicans being the most dominant pathogen. Invasive bloodstream infections caused by Candida, also known as candidemia, can result in mortality rates up to 54 % (Xiao et al., 2019[29]). Invasive nature of infection is driven by C. albicans ability to form biofilms - structures with tightly packed group of cells that remain resistant to antifungal treatments (Nett and Andes, 2020[14]). Upon attachment to the host cells, a characteristic feature of C. albicans is the ability to switch from avirulent yeast form of growth to invasive hyphal form. This pathogenic transition is accompanied by increased levels of reactive oxygen species (ROS) and expression of several hyphal specific genes and proteins (Schröter et al., 2000[19]; Thomas et al., 2020[28]). Several drugs are currently in use to combat candidiasis, but Candida spp are able to modify their response to antifungals to be able to survive the treatment. Common adaptation mechanisms of C. albicans in response to antifungals include i) increased expression of CDR1 and CDR2, genes encoding multidrug efflux transporters of the ATP-binding cassette (ABC) transporter family, ii) upregulation of MDR1, a major facilitator transporter gene and iii) induction of ergosterol pathway through enhanced ERG11/ CYP51 expression which is the main target of azoles (Sanglard et al., 2009[17]). All of the above mentioned adaptations by C. albicans present the requirement of higher doses of antifungal drugs for effective control (Kofla et al., 2011[10]). Additionally, high drug concentrations could also result in the development of major side effects among the treated patients (Scorzoni et al., 2017[21]), thereby making it important to find alternative ways to combat candidiasis. Considering the adaptational flexibilities of C. albicans towards antifungal treatment, there is an ever increasing need to search for novel compounds with antifungal properties. Compounds of both natural and synthetic origin have been examined thus far, but for majority of them a mode of action has not yet been revealed (Smiljkovic et al., 2019[23]). Flavonoids are a group of natural compounds found in various plants, teas, wines etc. and have wide range of biological properties, one of them being anticandidal activity (Smiljkovic et al., 2017[24]). Astragalin is a 3-O glucoside of kaempferol and can be found in different plants belonging to Convolvulaceae, Ebenaceae, Rosaceae and Eucommiaceae families. This compound is known to have anti-cancerous, cardioprotective and antioxidative properties (Riaz et al., 2018[16]) while its aglycone kaempferol has been studied for its positive anticandidal activities (Shao et al., 2016[22]). In this study, we investigate the role of astragalin as a novel anticandidal agent and uncover its mode of action towards the inhibition of C. albicans virulence factors.
Material and Methods
Microbial culture conditions
C. albicans was isolated from oral cavities of patients at the Clinic of Otorhinolaryngology, Clinical Hospital Centre Zvezdara, Belgrade, Serbia. Reference strain C. albicans ATCC 10231 was purchased from American Type Culture Collection. Strains were determined on CHROMagar plates (Biomerieux, France), grown on Sabouraud Dextrose Agar/Broth (SDA/SDB) (Merck, Germany) and deposited at the Mycological Laboratory, Department of Plant Physiology, Institute for Biological Research ''Siniša Stanković'', University of Belgrade.
Anticandidal activity
Minimum inhibitory (MIC) and minimum fungicidal concentrations (MFC) of the compounds were determined by the modified microdilution technique (EUCAST, 2002[5]) in 96-well microtiter plates. Briefly, yeast cultures were diluted in sterile saline to a concentration of approximately 1.0 x 105 CFU/per well. To determine the MIC and MFC's, microplates containing fungal cells in SDB medium were incubated with serial dilutions of compounds at 37 °C for 24 h. The MIC values represented the lowest concentrations of the compound at which no microscopic growth was observed. After serial sub-cultivations of 10 µl into microtiter plates containing 100 µl of SDB broth/well, and incubation at 37 °C for 24 h, MFC values were determined as the lowest concentrations with no visible growth, indicating 99.5 % killing of the original inoculum. Ketoconazole (Sigma Aldrich, Germany) was used as a positive control. Flavonoid compound astragalin (kaemferol-3O-glucoside) was commercially bought (Extrasynthese, France).
Since the tested strains exhibited similar sensitivity to anticandidal treatment strain C. albicans 475/15 was selected as reference strain for all the further experiments.
Antibiofilm activity
Impact of astragalin on biofilm formation was determined as described by Cady et al. (2012[3]) with some modifications. C. albicans 475/15 cells were incubated at MIC and subMIC concentrations of the compounds in 96 well microtiter plates with adhesive bottom (Sarstedt, Germany) at 37 ºC. After 24 hours of incubation wells were washed twice with sterile PBS (Phosphate buffered saline, pH 7.4) and cells were fixed with methanol. After fixation methanol was discarded, plate was air dried and stained with 0.1 % crystal violet (Bio-Merieux, France). After 30 min plate was washed with water to remove any unbound stain and air dried. Ethanol 96 % (Zorka, Serbia) was added to dissolve the bound stain. Absorbance was read on Multiskan™ FC Microplate Photometer, Thermo Scientific™ and the percentage of inhibition of biofilm formation was calculated by the formula specified below:
[(A620control- A620sample) / A620control] × 100.
Measurement of membrane permeability
The impact of astragalin on membrane permeability of C. albicans 475/15 was determined according to Tang et al. 2008[27], with some modifications. C. albicans 475/15 was incubated overnight at 37 °C, washed and suspended in 10 mM PBS (pH 7.4). Density of cells was adapted to 108 CFU/mL. C. albicans was incubated with astragalin at 1½ MICs for: 0, 15, 30, 45 and 60 min; astragalin dissolved in PBS was used as blank. The mixture was filtered through 0.22 μm pore size filter and optical density was recorded at 260 nm and 280 nm with Agilent/HP 8453 UV-Visible Spectrophotometer Agilent Technologies, USA, at room temperature.
Ergosterol binding as potential mode of action
Microdilutions of astragalin and amphotericin B (AmB, positive control) were prepared in the same manner as used for determination of antimicrobial activity, except that ergosterol (400 µg/mL) was added to the rows of the plate (Leite et al., 2015[11]). MIC values were observed after incubation at 37 ºC for 24 h, and were compared with the MIC value of samples without ergosterol addition.
Binding affinities of astragalin towards CaCYP51 enzyme
The enzyme sterol 14α-demethylase (CYP51) was previously isolated from C. albicans (CaCYP51) and kindly provided by Laboratory for Molecular Structural Dynamics, National Institute of Chemistry, Ljubljana, Slovenia. Binding properties of astragalin and positive control, ketoconazole (0, 2, 8, 16, 32, 64, 128, 256, 300 µM) were investigated using UV-Visible spectroscopy. Compounds were mixed with CaCYP51 protein and spectra were recorded in 350-500 nm range with potential ligand-induced spectral changes observed as difference type II spectral responses (Zelenko et al., 2014[30]).
RNA isolation and reverse transcription
Total RNA was extracted from 5 mL logarithmic-phase cultures grown in YEPD medium, using RNeasy Protect Mini kit (Qiagen) by a process involving mechanical disruption of the cells with glass beads as previously described (Sanglard et al., 1999[18]). The concentration and purity of the RNA was determined using a UV spectrophotometer (NanoDrop; ThermoFisher Scientific) by measuring the absorbance at 230 (OD230), 260 (OD260) and 280 nm (OD280). The OD260nm/OD280nm of the samples, reflecting the average purity, ranged from 1.80 to 2.05, and the OD260nm/ OD230nm was in the range of 2.00-2.60. For qPCR, 1 µg RNA was reverse transcribed to cDNA using the Transcriptor High Fidelity cDNA synthesis kit (Roche) involving random hexamer as a priming method. Prior to reverse transcription reaction, the total RNA samples were treated with DNase from DNA-freeTM DNA removal kit for 30 min at 37 °C (Invitrogen) according to the manufacturer's instructions.
qPCR
qPCR reactions were performed with 0.2 µM of each primer and 0.2 µM of probe for genes ACT1, CDR1, MDR1 and ERG11 (see Supplementary material), and the iTAQ Supermix with ROX (BioRad, Reinach, Switzerland) according to the manufacturer's instructions using StepOnePlusTM Real Time PCR System (Life Technologies). The expression level of ACT1 was used for normalization, and fold change (FC) values were calculated for CDR1, MDR1 and ERG11 in vitro in the absence and presence of the compounds in their previously determined minimal inhibitory concentrations for 30 min. Technical triplicates were included in each reaction and all the reactions were repeated twice on biological replicates.
Determination of intracellular ROS levels in C. albicans 475/14
The impact of astragalin on levels of intracellular ROS was determined according to the method described by Paez et al. (2010[15]). C. albicans 475/15 was incubated with MIC of astragalin overnight at 37 ºC; 0.5 mL of 1 mg/mL nitro blue tetrazolium (NBT) was added and incubation was continued at 37 ºC for 30 min. After addition of 0.1 mL 0.1 M HCl tubes were centrifuged at 2500 g for 10 min. Dimethyl sulfoxide (0.6 mL) and phosphate saline buffer (0.8 mL) was added to the pellet and absorbance was recorded at 575 nm using Agilent/HP 8453 UV-Visible Spectrophotometer (Agilent Technologies, USA).
Potential of astragalin to interfere with C. albicans yeast to hyphal transition
C. albicans 475/15 cells were incubated with MIC of astragalin in YPD + 10 % FBS. After 4 h of incubation at 37 ºC, cells were observed under microscope (Nikon Eclipse TS2, Netherlands) and number of cells growing in the yeast or hyphal and germ tube form was determined and presented as percentage of hyphal cells. Assay was performed in triplicate.
Cytotoxicity assay
Crystal violet assay was used for the determination of cytotoxic effect, according to the previous protocol (Feoktistova et al., 2016[8]), with slight modifications. We used human gingival fibroblast cells HGF-1 (ATCC® CRL-2014™) for the assay. Cells 4 × 103/well were seeded in a 96-well plate and incubated for 48 h at 37˚C to enable adhesion of cells to the wells. After, the medium was removed from the wells, and 100 µL/well of fresh medium supplemented with different concentrations of astragalin (0.0125 - 0.4 mg/ well) was added to the cells. The cells were treated in triplicate wells for each condition and further incubated for 24 h. The medium was removed and the cells were washed twice with PBS. Then, 100 µL of 0.4 % crystal violet staining solution was added to each well, and incubated for 20 min at room temperature. Crystal violet was removed, the plates were washed in a stream of tap water and left to dry. 100 µL of methanol was added to each well in order to dissolve the dye. Optical density of each well was measured at 570 nm (OD570) with a plate reader. The results were expressed as IC50 value, indicating 50 % of cell viability when compared with untreated control. The solvent was used as a negative control. The criterion used to categorize the cytotoxicity to HGF-1 cells was as follows: IC50 ≤ 20 µg/mL = highly cytotoxic, IC50 ranged between 31 and 200 µg/mL = moderately cytotoxic, IC50 ranged between 201 and 400 µg/mL = weakly cytotoxic, and IC50 > 401 µg/mL = no cytotoxicity.
Results and Discussion
Promising antifungal and antibiofilm activity of astragalin
MIC and MFC values of astragalin and ketoconazole were determined by serial dilutions and incubation for 24 h. We observed that astragalin could inhibit fungal growth. Majority of the strains were identically susceptible to astragalin at MIC value of 0.075 mg/mL, while C. albicans ATCC 10231 had MIC 0.125 mg/mL (Table 1(Tab. 1)). The average MFC values for all strains were twice as high as the MIC values (Table 1(Tab. 1)). In previous studies, ethyl acetate extract of Helichrysum compactum was shown to have promising anticandidal activity (MIC 0.04 mg/mL) which could be linked to the high concentration of astragalin observed in this extract (28 mg/kg) (Süzgeç et al., 2005[25]). Likewise, astragalin extracted from Bellis sylvestris leaves was also able to inhibit more than 20 % growth of C. albicans ATCC 10231 at a concentration of 0.128 mg/mL (Scognamiglio et al., 2016[20]). Surprisingly, some of the earlier studies had reported the lack of antimicrobial activity of astragalin towards ATCC strains 10231 (Süzgeç-Selçuk and Birteksöz et al., 2011[26]) and 14053 even at concentrations as high as 0.5 mg/mL (Fattouch et al., 2007[7]). The differences in these findings could be attributed to diverse methodologies (described by authors) or high diversity among strains selected for testing (Süzgeç et al., 2005[25]; Scognamiglio et al., 2016[20]; Süzgeç-Selçuk and Birteksöz, 2011[26]; Fattouch et al., 2007[7]).
Table 1. Antimicrobial activity of compounds (results represent the MIC and MFC values in mg/mL).
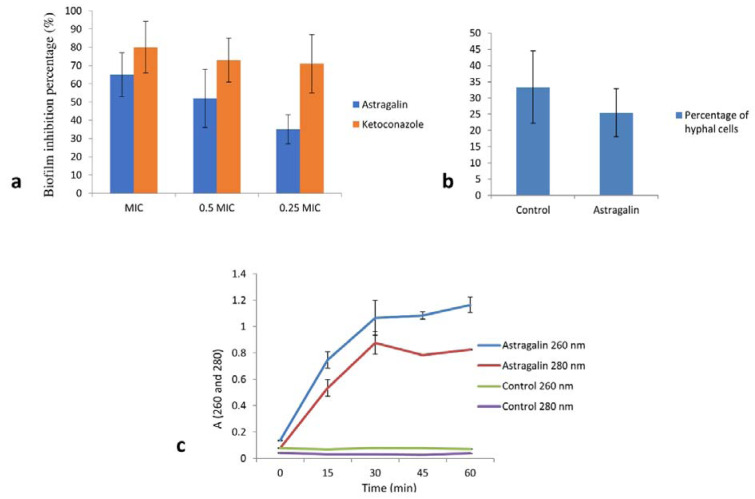
We then assessed the antibiofilm activity of astragalin on strain C. albicans 475/15. In this regard, fungal cells were once again incubated in the absence and presence of astragalin at different concentrations (MIC, 0.5 MIC and 0.25 MIC). Astragalin induced a significant reduction in the ability of C. albicans to form biofilms (Figure 1a(Fig. 1)). The results revealed more than 50 % reduction in the biofilm biomass after incubation of the fungal cells with 0.5 MIC of astragalin, compared to the untreated biofilm and about 65 % reduction with doses equal to MIC (Figure 1a(Fig. 1)). Interestingly, one of previous studies showed no antibiofilm activity of astragalin (resulted in less than 10 % of biofilm inhibition) (Scognamiglio et al., 2016[20]). The effect of astragalin in prevention of biofilm formation found in this study could be explored further since inhibition of this pathogenic trait could lead to lower virulence of fungus. In conclusion, our findings clearly highlighted the antifungal potential of astragalin.
Figure 1. a) Inhibition of biofilm formation after treatment with astragalin and ketoconazole, expressed as inhibition percentage (100 % means no biofilm is established). b) Percentage of hyphal cells after 4 h treatment, control is C. albicans without any treatment. c) Nucleotide leakage detected by measuring the absorbance at wavelengths 260 nm and 280 nm after treatment of C. albicans cells with 1½ MIC of astragalin for 0, 15, 30, 45 and 60 min. All values represent means ± SD of three replicates.
Impact of astragalin on yeast to hyphal transition
In our study, astragalin did not show any significant anti-oxidative activity in lowering the ROS production in C. albicans. We observed only 6 % reduction in ROS production compared to the 40 % inhibition in ROS activity of previously published flavonoids apigenin and apigetrin in an identical assay (Smiljkovic et al., 2017[24]). We also performed the microscopic examination on the growth of fungal cells in the presence and absence of astragalin and determined the number of cells in yeast and hyphal forms. The percentage of hyphal cells was marginally reduced following 4 h treatment with astragalin at MIC (Figure 1b(Fig. 1)). A previous study using honey flavonoid extract containing kaempferol had demonstrated its ability to reduce the number of cells switching from yeast to hyphal growth (Candiracci et al., 2012[4]).
Assessing the binding of astragalin to ergosterol and its ability to interfere with sterol biosynthesis
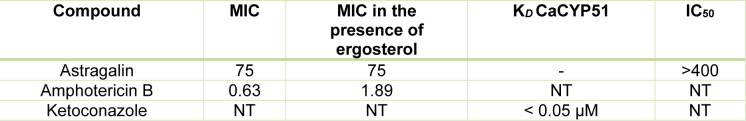
To determine whether astragalin interacts with fungal ergosterol, its MIC against C. albicans 475/15 was determined both with and without the addition of exogenous ergosterol to the culture medium. We found that the MIC of AmB (used as a positive control) was three times higher when ergosterol was added, unlike MIC of astragalin which was not influenced by the presence of ergosterol (Table 2(Tab. 2)).
Table 2. Minimal inhibitory concentrations (µg/mL) of astragalin and amphotericin B towards C. albicans in the absence and presence of ergosterol; dissociation constants (KD) after titration of C. albicans CYP51 (CaCYP51) with tested compounds; cytotoxicity of astragalin towards human gingival fibroblasts (IC50 μg/mL). NT - not tested.
These results suggest that astragalin did not directly interact with ergosterol and that the inhibition of fungal growth was likely caused by other mechanisms. To study the binding potential of astragalin to CYP51 differences in the spectral response was recorded before and after the addition of compound to CYP51 protein. We observed that astragalin did not show any binding affinity for fungal CYP51 (Table 2(Tab. 2)), once again suggesting that antifungal activity of astragalin was not mediated by its interference with the ergosterol biosynthesis. We then assessed the impact of astragalin on ERG11 expression by Quantitative Real-Time Polymerase Chain Reaction (qPCR). Following 30 min treatment of the fungal cells with the compounds, astragalin treatment failed to produce any significant changes in ERG11 mRNA levels (less than 0.5 log2 FC) (Figure 2a(Fig. 2)). Compared to the untreated cells, ketoconazole caused only a slight increase (0.5 log2 FC) in ERG11 levels. We believe that longer treatment period (> 30 min) was probably necessary for stronger induction of the gene. AmB also reduced the expression of ERG11, albeit to a lesser extent (< 1 log2FC). These results suggest that besides binding to ergosterol, AmB reduced the ergosterol content in the cell to contribute to the antifungal activity. Based on the above observations, we believe that astragalin activity is not directly related to the inhibition of ergosterol biosynthesis.
Figure 2. Expression levels of ERG11 (a) and CDR1 (b) after treatment with MIC of astragalin, ketoconazole and amphotericin B; fluphenazine was used as positive control for CDR1 expression. Values are expressed as Log2fold change (log2 FC) of RQ values and presented as an average of two biological replicates.
Impact of astragalin on expression levels of genes coding for efflux pumps
CDR1 and MDR1 expression was tested at the mRNA level after induction of the strain C. albicans 475/15 with the compounds at their MIC concentrations for 30 min. Astragalin reduced CDR1 levels in the fungal cells (> 1.5 log2 FC) (Figure 2b(Fig. 2)). On the other hand, ketoconazole and AmB failed to produce any significant changes to CDR1 expression, possibly since the induction of CDR1 with azoles required longer incubation period to increase the expression of efflux pumps (Liu and Myers, 2017[12]) (Figure 2b(Fig. 2)). Likewise, changes in the expression levels of MDR1 due to astragalin treatment could not be detected since the strain did not have any basal expression of this gene (data not shown). Astragalin was shown to be a potential inhibitor of P-glycoprotein, a protein involved in efflux of drugs (Ammar, 2017[1]). Additionally, other kaempferol derivatives such as kaempferol-3-O-β-d-(6″-E-p-coumaroyl) glucopyranoside and kaempferol 3-O-α-L-(2,4-bis-E-p-coumaroyl) rhamnoside, were shown to inhibit NorA efflux pump of Staphylococcus aureus (Falcão-Silva et al., 2009[6]; Holler et al., 2012[9]). The ability of kaempferol to inhibit bacterial efflux pumps was reported in one of the studies at an IC50 of 19 µg/mL (Brown et al., 2015[2]) which is lower than concentration of astragalin used in this study (75 µg/mL). The downregulation of CDR1, CDR2 and MDR1 by kaempferol has also been well documented (Shao et al., 2016[22]). Astragalin's ability to reduce the expression of CDR1 is of potential interest when developing novel antifungal therapies. A compound that can reduce drug efflux and possess good antifungal effect could be a promising strategy towards candidiasis treatment.
Effect of astragalin on cell membrane integrity
We studied the nucleotide leakage as a measure of astragalin impact on fungal cell membrane integrity by treating the fungal cells with astragalin for varying amounts of time. We observed strong absorbance signals for nucleic acids (A260) and proteins (A280) soon after 30 min treatment (Figure 1c(Fig. 1)). This clearly highlighted a strong effect of astragalin in reducing the fungal membrane integrity, thereby contributing to its antifungal activity. Similarly, astragalin was shown to cause morphological changes to the cell membrane in the parasite Trypanosoma cruzi (Marín et al., 2011[13]). Surprisingly, our finding was not in agreement with an earlier in silico study that predicted astragalin to function as membrane integrity agonist (Ammar, 2017[1]) indicating that in silico docking studies should be followed by at least some in vitro evaluations before predicting biological activities of different compounds.
Astragalin is not cytotoxic to human fibroblast cells
Cytotoxic activity of the compound was tested on HGF-1 cells (Table 2(Tab. 2)). Astragalin did not exhibit any cytotoxicity up to 400 μg/mL. The fact that inhibition of fungal growth is achieved with astragalin in concentration of 75 μg/mL (Table 2(Tab. 2)) makes this flavonoid a promising candidate as safe antifungal compound for potential use in human medicine.
Conclusions
Astragalin, one of the flavonoids found in various plants, was proven as an anticandidal agent. Our results clearly demonstrated promising antimicrobial activities of astragalin and its ability to reduce fungal biofilm formation, which is one important factor promoting Candida virulence, without significantly impacting the yeast to hyphae transition. Furthermore, we explored the astragalin's anticandidal mode of action in more details. Our finding suggested that astragalin acted against the fungal plasma membrane by interfering with the membrane integrity and was able to downregulate the expression of CDR1. These mechanisms could be further investigated in vitro and in vivo in order to classify and develop compound as a therapeutic option. In this perspective, the lack of cytotoxic activity of astragalin on human fibroblasts is of potential interest. Our results confirm that astragalin could be used in combination with other known inhibitors of efflux pumps and commercial antifungals to provide significant antimicrobial effect at lower drug concentrations in the effective treatment of C. albicans infections.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgement
This work is supported by the Serbian Ministry of Education, Science and Technological Development for financial support (Grant number 451-03-68/2020-14/200007). Binding study to CaCYP51 was supported by the Slovenian Research Agency (Grant numbers J1-8145 and P1-0010) and by a program of scientific and technological cooperation between the Republic of Serbia and the Republic of Slovenia “A combined methodology towards the development of novel, selective inhibitors of Candida CYP51”. The authors are grateful to the FEMS for providing FEMS Research and Training Grant (FEMS-GO-2017-015) to Mrs Marija Ivanov for her visit to the Institute of Microbiology, University Hospital Lausanne and University Hospital Center, Rue du Bugnon 48, Lausanne, Switzerland.
Supplementary Material
References
- 1.Ammar O. In silico pharmacodynamics, toxicity profile and biological activities of the Saharan medicinal plant Limoniastrum feei. Braz J Pharm Sci. 2017;53(3):1–10. [Google Scholar]
- 2.Brown AR, Ettefagh KA, Todd D, Cole PS, Egan JM, Foil DH, et al. A mass spectrometry-based assay for improved quantitative measurements of efflux pump inhibition. PLoS One. 2015;10(5):e0124814. doi: 10.1371/journal.pone.0124814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cady NC, McKean KA, Behnke J, Kubec R, Mosier AP, Kasper SH, et al. Inhibition of biofilm formation, quorum sensing and infection in Pseudomonas aeruginosa by natural products-inspired organosulfur compounds. PLoS One. 2012;7(6):e38492. doi: 10.1371/journal.pone.0038492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Candiracci M, Citterio B, Piatti E. Antifungal activity of the honey flavonoid extract against Candida albicans. Food Chem. 2012;131:493–499. [Google Scholar]
- 5.EUCAST (European Committee on Antibiotic Susceptibility) Method for determination of minimal inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Discussion document E. Dis. 7.1. Taufkirchen: European Society of Clinical Microbiology and Infectious Diseases; 2002. [Google Scholar]
- 6.Falcão-Silva VS, Silva DA, Souza Mde F, Siqueira-Junior JP. Modulation of drug resistance in Staphylococcus aureus by a kaempferol glycoside from Herissantia tiubae (Malvaceae) Phytother Res. 2009;23:1367–1370. doi: 10.1002/ptr.2695. [DOI] [PubMed] [Google Scholar]
- 7.Fattouch S, Caboni P, Coroneo V, Tuberoso CI, Angioni A, Dessi S, et al. Antimicrobial activity of Tunisian quince (Cydonia oblonga Miller) pulp and peel polyphenolic extracts. J Agr Food Chem. 2007;55:963–969. doi: 10.1021/jf062614e. [DOI] [PubMed] [Google Scholar]
- 8.Feoktistova M, Geserick P, Leverkus M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb Protoc. 2016;4:pdb.prot087379. doi: 10.1101/pdb.prot087379. [DOI] [PubMed] [Google Scholar]
- 9.Holler JG, Christensen SB, Slotved HC, Rasmussen HB, Gúzman A, Olsen CE, et al. Novel inhibitory activity of the Staphylococcus aureus NorA efflux pump by a kaempferol rhamnoside isolated from Persea lingue Nees. J Antimicrob Chemoth. 2012;67:1138–1144. doi: 10.1093/jac/dks005. [DOI] [PubMed] [Google Scholar]
- 10.Kofla G, Turner V, Schulz B, Storch U, Froelich D, Rognon B, et al. Doxorubicin induces drug efflux pumps in Candida albicans. Med Mycol. 2011;49:132–142. doi: 10.3109/13693786.2010.512022. [DOI] [PubMed] [Google Scholar]
- 11.Leite MC, de Brito Bezerra AP, de Sousa JP, de Oliveira Lima E. Investigating the antifungal activity and mechanism(s) of geraniol against Candida albicans strains. Med Mycol. 2015;53:275–284. doi: 10.1093/mmy/myu078. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Myers LC. Mediator tail module is required for Tac1-activated CDR1 expression and azole resistance in Candida albicans. Antimicrob Agents Ch. 2017;61(11):e01342–e01317. doi: 10.1128/AAC.01342-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marín C, Ramírez-Macías I, López-Céspedes A, Olmo F, Villegas N, Díaz JG, et al. In vitro and in vivo trypanocidal activity of flavonoids from Delphinium staphisagria against Chagas disease. J Nat Prod. 2011;74:744–750. doi: 10.1021/np1008043. [DOI] [PubMed] [Google Scholar]
- 14.Nett JE, Andes DR. Contributions of the biofilm matrix to Candida pathogenesis. J Fungi. 2020;6(1):21. doi: 10.3390/jof6010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paez PL, Becerra MC, Albesa I. Effect of the association of reduced glutathione and ciprofloxacin on the antimicrobial activity in Staphylococcus aureus. FEMS Microbiol Lett. 2010;303:101–5. doi: 10.1111/j.1574-6968.2009.01867.x. [DOI] [PubMed] [Google Scholar]
- 16.Riaz A, Rasul A, Hussain G, Zahoor MK, Jabeen F, Subhani Z, et al. Astragalin: a bioactive phytochemical with potential therapeutic activities. Adv Pharmacol Sci. 2018;2018:9794625. doi: 10.1155/2018/9794625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanglard D, Coste A, Ferrari S. Antifungal drug resistance mechanisms in fungal pathogens from the perspective of transcriptional gene regulation. FEMS Yeast Res. 2009;9:1029–1050. doi: 10.1111/j.1567-1364.2009.00578.x. [DOI] [PubMed] [Google Scholar]
- 18.Sanglard D, Ischer F, Calabrese D, Majcherczyk PA, Bille J. The ATP binding cassette transporter GeneCgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob Agents Ch. 1999;43:2753–2765. doi: 10.1128/aac.43.11.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schröter C, Hipler UC, Wilmer A, Künkel W, Wollina U. Generation of reactive oxygen species by Candida albicans in relation to morphogenesis. Arch Dermatol Res. 2000;292:260–264. doi: 10.1007/s004030050484. [DOI] [PubMed] [Google Scholar]
- 20.Scognamiglio M, Buommino E, Coretti L, Graziani V, Russo R, Caputo P, et al. Phytochemical investigation and antimicrobial assessment of Bellis sylvestris leaves. Phytochem Lett. 2016;17:6–13. [Google Scholar]
- 21.Scorzoni L, de Paula E, Silva AC, Marcos CM, Assato PA, de Melo WC, et al. Antifungal therapy: new advances in the understanding and treatment of mycosis. Front Microbiol. 2017;23(8):36. doi: 10.3389/fmicb.2017.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao J, Zhang M, Wang T, Li Y, Wang C. The roles of CDR1, CDR2, and MDR1 in kaempferol-induced suppression with fluconazole-resistant Candida albicans. Pharm Biol. 2016;54:984–992. doi: 10.3109/13880209.2015.1091483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smiljkovic M, Kostic M, Stojkovic D, Glamoclija J, Sokovic, M Could flavonoids compete with synthetic azoles in diminishing Candida albicans infections? A comparative review based on in vitro studies. Curr Med Chem. 2019;26:2536–2554. doi: 10.2174/0929867325666180629133218. [DOI] [PubMed] [Google Scholar]
- 24.Smiljkovic M, Stanisavljevic D, Stojkovic D, Petrovic I, Marjanovic Vicentic J, Popovic J, et al. Apigenin-7-O-glucoside versus apigenin: Insight into the modes of anticandidal and cytotoxic actions. EXCLI J. 2017;16:795–807. doi: 10.17179/excli2017-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Süzgeç S, Meriçli AH, Houghton PJ, Cubukçu B. Flavonoids of Helichrysum compactum and their antioxidant and antibacterial activity. Fitoterapia. 2005;76:269–272. doi: 10.1016/j.fitote.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Süzgeç-Selçuk S, Birteksöz AS. Flavonoids of Helichrysum chasmolycicum and its antioxidant and antimicrobial activities. S Afr J Bot. 2011;77(1):170–174. [Google Scholar]
- 27.Tang YL, Shi YH, Zhao W, Hao G, Le GW. Insertion mode of a novel anionic antimicrobial peptide MDpep5 (Val-Glu-Ser-Trp-Val) from Chinese traditional edible larvae of housefly and its effect on surface potential of bacterial membrane. J Pharmaceut Biomed. 2008;48:1187–94. doi: 10.1016/j.jpba.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Thomas G, Bain JM, Budge S, Brown AJP, Ames RM. Identifying Candida albicans gene networks involved in pathogenicity. Front Genet. 2020;11:375. doi: 10.3389/fgene.2020.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao Z, Wang Q, Zhu F, An Y. Epidemiology, species distribution, antifungal susceptibility and mortality risk factors of candidemia among critically ill patients: a retrospective study from 2011 to 2017 in a teaching hospital in China. Antimicrob Resist Infect Control. 2019;8:89. doi: 10.1186/s13756-019-0534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zelenko U, Hodošček M, Rozman D, Golič Grdadolnik S. Structural insight into the unique binding properties of pyridylethanol(phenylethyl)amine inhibitor in human CYP51. J Chem Inf Model. 2014;54:3384–3395. doi: 10.1021/ci500556k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.