Abstract
Objectives: The present study aimed to evaluate the effect of curing time and bleaching agents on microhybrid composite resin surface microhardness.
Material and method: A total of 180 microhybrid composite resin disks were divided into two groups in terms of curing time: 40 s, 60 s. Then, each group was divided into three subgroups: control (distilled water), home bleaching (15% carbamide peroxide) and office bleaching (40% hydrogen peroxide). Surface microhardness of the samples was determined by using Vickers hardness test both at baseline and after the completion of the tests. Two-way ANOVA and Tukey HSD tests were used to analyze and compare microhardness changes between groups. Statistical significance was defined at P<0.05.
Results: Based on the two-way ANOVA, curing time, bleaching method, and cumulative effect of these two variables significantly affected composite resin microhardness (P<0.001). Tukey HSD tests showed that microhardness had significantly decreased in the bleaching groups, with the highest decrease when the office bleaching method was used (P<0.001). The samples cured for 40 s exhibited lower microhardness than those cured for 60 s and had an increased reduction in microhardness after bleaching (P<0.001).
Conclusions: Application of both bleaching methods decreased the surface hardness of microhybrid composite resin. An increase in the curing time was associated with a decrease in adverse effects of bleaching agents on microhardness.
Keywords:composite resin, hardness, polymerization, tooth bleaching agent.
INTRODUCTION
Tooth bleaching is one of the most common esthetic procedures in dentistry. Bleaching agents effectively remove stains and discolorations from tooth surfaces. These agents can be used in the office or at home, by the patient (1). Bleaching agents are primarily based on hydrogen peroxide (HP) or its derivatives such as carbamide peroxide (CP) and bleach the teeth through the release of peroxide free radicals. These radicals combine with internal and external pigments of the teeth and remove them through an oxidative reaction (2).
Tooth-colored restorative materials, especially composite resins, comprise an important aspect of modern dentistry. Composite resins are more susceptible to chemical changes than metallic restorative materials and neutral ceramics due to their organic matrix. One of the problems associated with the use of bleaching agents is that they can damage the surface of composite resin restorations, resulting in bacterial adhesion (3). The final effect of these chemical agents on composite resins depends on the type and resistance of the resin matrix, composite resin filler content, the bleaching gel, and the duration of their application (4). Different studies have reported contradictory results on the effect of bleaching agents on composite resin surface microhardness, with some reporting a decrease in composite resin surface microhardness after the use of these agents (5–7). However, some other studies have not reported any changes in the composite resin surface microhardness after applying bleaching agents (8–11).
Composite resin surface microhardness depends on the mechanical properties of the material and its disintegration. Various factors related to composite resin composition, including the monomer type, size and filler content, can affect their mechanical properties (12, 13). Composite resin surface microhardness might be affected by the curing time. Adequate curing time leads to the formation of adequate polymeric chains, bringing about better properties, including microhardness and compressive strength (14, 15).
Considering the effects of various factors, an increase in surface microhardness and an improvement in the physical properties of composite resins through an increase in curing time might improve its clinical longevity and resistance against chemical agents, including bleaching agents. Since no research has been carried out on this topic so far, the present study aimed to evaluate the effect of two bleaching techniques and two curing times on the surface microhardness of microhybrid composite resin.
MATERIALS AND METHODS
The study protocol was approved by the Ethics Committee of Tabriz University of Medical Science (Ir.Tbzmed.rec.1395.527).
Preparation of resin samples
Amelogen Plus (Ultradent, South Jordan, USA) microhybrid composite resin with A3 shade was used in the present study. A total of 180 diskshaped composite resin samples (2 mm in thickness and 8 mm in diameter) were prepared using a round matrix. The circular mold was placed on a glass slab; after placing the composite resin material within the mold, a Mylar transparent matrix band was pressed on the mold to achieve a smooth surface on the composite resin and prevent the formation of an oxygen-inhibited layer. Then, the composite resin samples were light-cured through the Mylar matrix without any distance, using a quartz tungsten halogen (QTH) light-curing unit (Astralis 7, Ivoclar Vivadent FL 9494Schaan, Liechtenstein) at a right angle to the surface and a light intensity of 500 mW/cm² in terms of the following curing times:
1. A curing time of 40 seconds (n=90)
2. A curing time of 60 seconds (n=90)
The light-curing unit`s power density was repeatedly checked during the tests with a radiometer (Demetron, Kerr, USA). The inferior surface of the samples was marked to make a distinction between this side and the surface on which the tests were carried out. The samples were polished with medium to superfine polishing disks (Sof-Lex TM, 3M ESPE, St. Paul, USA) according to the manufacturer`s instructions, followed by rinsing with distilled water and cleaning in an ultrasonic device for two minutes to remove all the debris. The prepared samples were stored in distilled water at 37¢ªC for 24 hours.
Bleaching procedures
The samples were divided into three subgroups (one control group and two test group) (n=30) to carry out the bleaching procedures for two weeks according to the manufacturer’s instructions:
a. Control group; two weeks of storage in distilled water at ambient temperature;
b. At-home bleaching with 15% carbamide peroxide (15% Opalescence PF; Ultradent, South Jordan, USA), four hours a day for two weeks;
c. In-office bleaching with 40% hydrogen peroxide (40% Opalescence Boost, Ultradent, South Jordan, USA), three times during the study, 20 minutes each time, at one-week intervals.
The bleaching agent was applied to the composite surface with a thickness of 1 mm (measured with a scaled periodontal probe) so that it covered the material surface completely. After each bleaching procedure, the samples were irrigated with distilled water and stored in distilled water at ambient temperature until the next procedure. In all groups, fresh distilled water was used every day.
Evaluation of surface microhardness
The surface microhardness of all samples was determined at baseline (24 hours after polymerization) and after the tests, in both the control and test groups, using a Vickers hardness testing machine (UHL Co., Germany) with a Vickers indenter with a 100-gr force and a dwell time of 15 seconds. To this end, the composite resin disks were dried with a piece of gauze, and their upper surface was placed under the indenter; three indentations were produced in each sample .1 mm way from the disk margins and other indentations. The microhardness values at all the three points were measured by determining the diameter of the indented rhombus using the following formula: VH=1.854 F/d2 (16). Finally, the mean of the three points was reported as the surface microhardness of each composite resin sample.
Statistical analysis
SPSS 20 was used to compare the surface microhardness values of the groups. Two-way ANOVA was used to compare the differences in the means before and after intervention in the control, home, and office bleaching groups and the 40- and 60-second light-curing groups. Tukey HSD tests were used for the two-by-two comparisons of the groups. Normal distribution of data was analyzed with Kolmogorov-Smirnov test and Levene’s test was used to evaluate homogeneity of variances among the groups. Statistical significance was set at P<0.05.
RESULTS
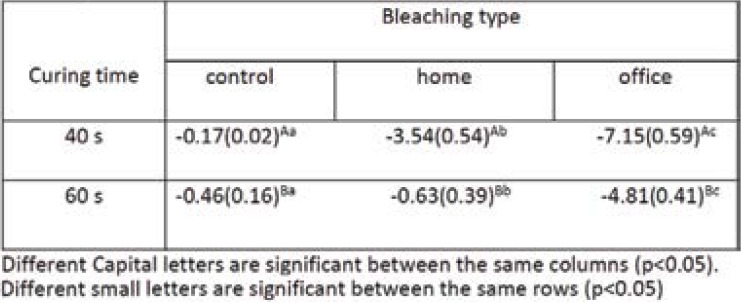
Table 1 summarizes the means and standard deviations (SD) of surface microhardness changes after bleaching intervention on the composite resin groups with 40-second and 60-second curing times. According to the results of two-way ANOVA, the effects of curing time, type of bleaching agent, and their cumulative effect on microhardness were significant [(F=191.03, P<0.001), (F=780.25, p<0.001) and (F=67.83, p<0.001) respectively]; the microhardness change patterns were not the same before and after bleaching in the composite resin groups with 40-second and 60-second curing times in terms of bleaching agent type.
According to Tukey HSD test results for two-by-two comparisons of the changes in mean microhardness values in terms of curing time, the samples cured for 40 seconds exhibited less microhardness than those cured for 60 seconds, and the decrease in microhardness was more marked than that in the 60-second group (P<0.001). Two-by-two comparisons of microhardness changes between the bleached surfaces showed that the highest decrease was related to office bleaching (P<0.001).
DISCUSSION
Since surface microhardness is one of the most important physical properties of composite resins, affecting their surface resistance to abrasion and scratch, the present study evaluated the effects of two bleaching techniques (at-home: 15% CP; in-office 40% HP) and curing time (40 seconds and 60 seconds) on the surface microhardness of microhybrid composite resin.
According to the results of the present study, both in-office and at-home bleaching techniques resulted in a significant decrease in composite resin surface microhardness, which is consistent with the results of a study by Kamangar et al, who reported a decrease in composite rein surface microhardness after bleaching with 15% CP and 40% HP (17). In a study by Taher et al, 15% CP for home bleaching resulted in composite resin surface softening (18).
Carbamide peroxide disintegrates into 1/3 hydrogen peroxide and 2/3 urea in contact with water (19). Hydrogen peroxide produces free radicals, resulting in the separation of polymer chains and breakage of double bonds in the composite resin structure, which might be related to a decrease in surface microhardness. Also, free radicals affect the resin–filler interface and lead to microcracks (11).
In the present study, the use of in-office bleaching with 40% HP resulted in a greater decrease in the surface microhardness of composite resins compared to at-home bleaching with 15% CP, which might be explained by an increase in the disintegration of composite resin material by the higher concentration of HP in the in-office bleaching technique (20, 21). In contrast to the present study, Polydora et al reported that home bleaching with 15% CP (22) and office bleaching with 38% HP (23) did not affect the surface microhardness of composite resins. Yu et al reported that the surface microhardness of composite resins was not affected by 15% CP (24). In a study by Yikilgan et al, 10% CP in a home bleaching technique resulted in a decrease in composite resin surface microhardness, while 45% CP in the office bleaching technique did not affect it, which was attributed to the long-term application of bleaching agents in the home bleaching technique than the in-office bleaching technique; this might have a detrimental effect on composite resin surface (25).
The discrepancies in various study results might be attributed to differences in methodologies and materials used. The effect of the bleaching agents on the surface characteristics of composite resins depends on the type of bleaching agents, their application time, and the substrate (23). Since the bleaching agents affect the resin matrix, composite resins with a higher volume of the matrix are more susceptible to the adverse effects of bleaching agents (23).
Another finding of the present study was the higher microhardness of composite resins cured at a longer time (60 seconds) than composite resins cured for the time recommended by the manufacturer (40 seconds). Besides, there was a smaller decrease in surface microhardness after the bleaching process of composite resins cured for 60 seconds compared to those cured for 40 seconds. Consistent with the present study, a study by Farahat et al showed that an increase in curing time from 20 to 40 seconds with an LED light-curing unit at 1 000 mW/cm² light intensity increased the conversion rate and microhardness of composite resins (26). Also, in a study by Lima et al, an increase in curing time from 20 to 40 seconds with an LED light-curing unit at 800 mW/cm² intensity increased the microhardness of composite resins on the superior and inferior surfaces of the samples (27). Another study showed that, under clinical conditions, the radiant energy released to the inferior surface of composite resin was less than the recommended values, which was affected by the thickness and type of composite resin and the curing conditions. Therefore, under clinical conditions, most composite resins achieve 80% of their microhardness. An increase in curing time might result in higher radiant energy and at an adequate level (28). Based on previous studies, there is a linear relationship between the surface hardness of composite resins and the conversion rate. An increase in curing time increases the photons irradiated on the composite resin, increasing polymerization rate and an improvement in the material’s physical properties (29,30), which might affect the resistance of the resin matrix to bleaching agents in composite resins cured for a longer time. One of the concerns associated with an increase in curing time is an increase in tooth temperature. However, this increase in temperature is at an acceptable level and lower with low-intensity light-curing units (28).
In contrast to the present study, Borges et al reported no differences in the microhardness of low-viscosity composite resins cured for different times (10, 20, 30, 40, 50, and 60 seconds) with an LED light-curing unit at 1 264 mW/cm² intensity (31). Also, in a study by Ozduman et al, different curing times (10, 20, and 30 seconds) did not lead to changes in the microhardness of the inferior surface relative to the superior surface of composite resin samples (32). Various factors, including the size and composition of fillers and monomers in composite resins, the type and light intensity of the light-curing unit, and the duration of curing, affect monomer conversion rate and composite resin microhardness (33). A combination of these factors might explain the differences in results of different studies. It appears that the effect of increasing the curing time on improving microhardness is more prominent in light-curing units with a lower intensity of radiation. In the present study, a QTH light-curing unit with a light intensity of 500 mW/cm² was used to cure the composite resin samples.
Considering the results of the present in vitro study, an increase in microhardness and physical properties of composite resins by increasing the curing time might improve their clinical longevity in the face of chemical agents, including tooth bleaching agents. Even at low concentrations, bleaching agents might adversely affect composite resin surfaces if used for a long time. Since it is difficult to extend these findings to clinical conditions and it is difficult to carry out many procedures ideally in the oral cavity, it is suggested that studies be carried out under conditions very similar to the clinical conditions to evaluate the effects of different curing times with different light-curing units on different composite resin materials.
CONCLUSION
Under the limitations of the present study, it might be concluded that: 1) as a result of using at-home (15% CP) and in-office (40% HP) bleaching agents, the surface microhardness of microhybrid composite resin decreased to varying extents, depending on the method of bleaching; 2) an increase in the curing time of microhybrid composite resin increased its surface microhardness and resistance and, to a lesser extent, led to a decrease in microhardness after bleaching.
Conflicts of interest: none declared.
Financial support: none declared.
Table 1.
The means (standard deaviations) of surface microhardness reduction in the study groups
Contributor Information
Narmin MOHAMMADI, Dental and Periodontal Research Center and Department of Operative Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran.
Fereshteh NASER ALAVI, Department of Operative Dentistry, School of Dentistry, Guilan University of Medical Sciences, Rasht, Iran.
Sahand RIKHTEHGARAN, Department of Operative Dentistry, Tabriz Azad Faculty of Dentistry, Tabriz, Iran.
Mohammad Esmaeel Ebrahimi CHAHAROM, Department of Operative Dentistry, School of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran.
Ashkan SALARI, Department of Periodontics, School of Dentistry, Guilan University of Medical Sciences, Rasht, Iran.
Soodabeh KIMYAI, Dental and Periodontal Research Center and Department of Operative Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran.
Mahmoud BAHARI, Department of Operative Dentistry, School of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran.
References
- 1.Rattacaso RM, da Fonseca Roberti Garcia L, Aguilar FG, et al. Bleaching agent action on color stability, surface roughness and microhardness of composites submitted to accelerated artificial aging. Eur J Dent. 2011;5:143–149. [PMC free article] [PubMed] [Google Scholar]
- 2.Okte Z, Villalta P, García-Godoy F, et al. Surface hardness of resin composites after staining and bleaching. Oper Dent. 2006;31:623–628. doi: 10.2341/05-124. [DOI] [PubMed] [Google Scholar]
- 3.Mor C, Steinberg D, Dogan H, Rotstein I. Bacterial adherence to bleached surfaces of composite resin in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:582–586. doi: 10.1016/s1079-2104(98)90350-x. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Francisconi LF, Atta MT, et al. Effect of bleaching gels on surface roghness of nanofilled composite resins. Eur J Dent. 2011;5:173–179. [PMC free article] [PubMed] [Google Scholar]
- 5.Zuryati AG, Qian OQ, Dasmawati M. Effects of home bleaching on surface hardness and surface roughness of an experimental nanocomposite. J Conserv Dent. 2013;16:356–361. doi: 10.4103/0972-0707.114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lima DANL, De Alexandre RS, Martıns ACM, et al. Effect of curing lights and bleaching agents on physical properties of a hybrid composite resin. J Esthet Restor Dent. 2008;20:266–273. doi: 10.1111/j.1708-8240.2008.00190.x. [DOI] [PubMed] [Google Scholar]
- 7.Malkondu Ö, Yurdagüven H, Say EC, et al. Effect of bleaching on microhardness of esthetic restorative materials. Oper Dent. 2011;36:177–186. doi: 10.2341/10-078-L. [DOI] [PubMed] [Google Scholar]
- 8.García-Godoy F, García-Godoy A, García-Godoy F. Effect of bleaching gels on the surface roughness, hardness and micromorphology of composites. Gen Dent. 2002;50:247–250. [PubMed] [Google Scholar]
- 9.Yap AU, Wattanapayungkul P. Effects of in office tooth whiteners on hardness of tooth-colored restoratives. Oper Dent. 2002;27:137–141. [PubMed] [Google Scholar]
- 10.Basting RT, Fernandéz Y, Fernandez C, et al. Effects of a 10% carbamide peroxide bleaching agent on roughness and microhardness of packable composite resins. J Esthet Restor Dent. 2005;17:256–262. doi: 10.1111/j.1708-8240.2005.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 11.Polydorou O, Mönting JS, Hellwig E, Auschill TM. Effect of in-office tooth bleaching on the microhardness of six dental esthetic restorative materials. Dent Mater. 2007;23:153–158. doi: 10.1016/j.dental.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Beun S, Glorieux T, Devaux J, et al. Characterization of nanofilled compared to universal and microfilled composites. Dent Mater. 2007;23:51–59. doi: 10.1016/j.dental.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Lu H, Lee YK, Oguri M, Powers JM. Properties of a dental resin composite with a spherical inorganic filler. Oper Dent. 2006;31:734–740. doi: 10.2341/05-154. [DOI] [PubMed] [Google Scholar]
- 14.Voltarelli FR, Dos Santos-Daroz CB, Alves MC, et al. Effectiveness of resin composite polymerization when cured at different depths with different curing lights. Gen Dent. 2009;57:314–319. [PubMed] [Google Scholar]
- 15.Alpöz AR, Ertugrul F, Cogulu D, et al. Effects of light curing method and exposure time on mechanical properties of resin based dental materials. Eur J Den. 2008;2:37–42. [PMC free article] [PubMed] [Google Scholar]
- 16.Kimyai S, Bahari M, Naser-Alavi F, Behboodi S. Effect of two different tooth bleaching techniques on microhardness of giomer. J Clin Exp Dent. 2017;9:e249–e253. doi: 10.4317/jced.53290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamangar SS, Kiakojoori K, Mirzaii M, Fard MJ. Effects of 15% Carbamide Peroxide and 40% Hydrogen Peroxide on the Microhardness and Color Change of Composite Resins. J Dent (Tehran) 2014;11:196–209. [PMC free article] [PubMed] [Google Scholar]
- 18.Taher NM. The effect of bleaching agents on the surface hardness of tooth colored restorative materials. J Contemp Dent Pract. 2005;15:6. [PubMed] [Google Scholar]
- 19.Fasanaro TS. Bleaching teeth: history, chemicals, and methods used for common tooth discolorations. J Esthet Dent. 1992;4:71–78. doi: 10.1111/j.1708-8240.1992.tb00666.x. [DOI] [PubMed] [Google Scholar]
- 20.Campos I, Briso AL, Pimenta LA, Ambrosano G. Effects of bleaching with carbamide peroxide gels on microhardness of restoration materials. J Esthet Restor Dent. 2003;15:175–182. doi: 10.1111/j.1708-8240.2003.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee JH, Kim HI, Kim KH, Kwon YH. Effect of bleaching agents on the fluoride release and microhardness of dental materials. J Biomed Mater Res. 2002;63:535–541. doi: 10.1002/jbm.10311. [DOI] [PubMed] [Google Scholar]
- 22.Polydorou O, Hellwig E, Auschill TM. The effect of at-home bleaching on the microhardness of six esthetic restorative materials. J Am Dent Assoc. 2007;138:978–984. doi: 10.14219/jada.archive.2007.0295. [DOI] [PubMed] [Google Scholar]
- 23.Polydorou O, Hellwig E, Auschill TM. The effect of different bleaching agents on the surface texture of restorative materials. Oper Dent. 2006;31:473–480. doi: 10.2341/05-75. [DOI] [PubMed] [Google Scholar]
- 24.Yu H, Li Q, Hussain M, Wang Y. Effects of bleaching gels on the surface microhardness of tooth-colored restorative materials in situ. J Dent. 2008;36:261–267. doi: 10.1016/j.jdent.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Yikilgan İ, Kamak H, Akgul S, et al. Effects of three different bleaching agents on microhardness and roughness of composite sample surfaces finished with different polishing techniques. J Clin Exp Dent. 2017;9:e460–e465. doi: 10.4317/jced.53136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farahat F, Daneshkazemi AR, Hajiahmadi Z. The Effect of Bulk Depth and Irradiation Time on the Surface Hardness and Degree of Cure of Bulk-Fill Composites. J Dent Biomater. 2016;3:284–291. [PMC free article] [PubMed] [Google Scholar]
- 27.Lima AF, de Andrade KM, da Cruz Alves LE, et al. Influence of light source and extended time of curing on microhardness and degree of conversion of different regions of a nanofilled composite resin. Eur J Dent. 2012;6:153–157. [PMC free article] [PubMed] [Google Scholar]
- 28.Par M, Repusic I, Skenderovic H, et al. The effects of extended curing time and radiant energy on microhardness and temperature rise of conventional and bulk-fill resin composites. Clin Oral Investig. 2019;23:3777–3788. doi: 10.1007/s00784-019-02807-1. [DOI] [PubMed] [Google Scholar]
- 29.Agrawal A, Manwar NU, Hegde SG, et al. Comparative evaluation of surface hardness and depth of cure of silorane and methacrylate-based posterior composite resins: An in vitro study. J Conserv Dent. 2015;18:136–139. doi: 10.4103/0972-0707.153070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilie N, Stark K. Effect of different curing protocols on the mechanical properties of low-viscosity bulk fill composites. Clin Oral Investig. 2015;19:271–279. doi: 10.1007/s00784-014-1262-x. [DOI] [PubMed] [Google Scholar]
- 31.Borges BC, Bezerra GV, Mesquita Jde A, et al. Filler morphology of resin-based low-viscosity materials and surface properties after several photoactivation times. Acta Odontol Scand. 2013;71:215–222. doi: 10.3109/00016357.2012.654613. [DOI] [PubMed] [Google Scholar]
- 32.Özduman ZC, Kazak M, Fildisi MA, et al. Effect of Polymerization Time and Home Bleaching Agent on the Microhardness and Surface Roughness of Bulk-Fill Composites: A Scanning Electron Microscopy Study. Scanning vol. 2019;2307305 doi: 10.1155/2019/2307305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston WM, Leung RL, Fan PL. A mathematical model for post-irradiation hardening of photo activated composite resins. Dent Mater. 1985;1:191–194. doi: 10.1016/s0109-5641(85)80017-8. [DOI] [PubMed] [Google Scholar]