Abstract
Introduction: Hemolytic uremic syndrome is the most frequent cause of acute renal failure in children, commonly after gastrointestinal infections with E. coli or Salmonella, and it is characterized by progressive renal failure associated with microangiopathic hemolytic anemia and thrombocytopenia. Cardiac involvement is frequently encountered and can be potentially fatal in hemolytic uremic syndrome. It is usually determined by overhydration, hypertension, anemia, diselectrolytemia, acid-base disorders and tendency to form thrombi, and it consists in the following conditions: pericarditis, myocardial infarction, dilated cardiomyopathy, cardiac failure, and arrythmias.
Objective: The aim of this study is to observe the incidence of cardiovascular complications in children with acute hemolytic uremic syndrome, underline which are the most useful tools in establishing an accurate diagnosis, and discover the treatment protocol that has the most powerful impact upon the cardiovascular manifestations.
Materials and methods: We studied a number of 50 children who checked in the Nephrology Department of “M. S. Curie” Emergency Clinical Hospital in Bucharest, Romania, between January 2016 and August 2020. We performed the clinical examination of all patients as well as several paraclinical tests such as electrocardiogram, transthoracic echocardiography, arterial blood pressure monitorization, and vascular Doppler ultrasound. Patients included in the study were aged between five and 40 months.
Discussion and results: The majority of these children were diagnosed with arterial hypertension and some of them with cardiac failure and profound venous thrombosis. Transthoracic echocardiography revealed pathological aspects such as left ventricular hypertrophy, diastolic dysfunction, systolic dysfunction of the left ventricle, mitral regurgitation, aortic regurgitation, and pericarditis. Cardiac ultrasound findings were reversible in the majority of patients, most of them being treated with ACE inhibitors (eventually in association with other antihypertensive drugs).
Keywords:hemolytic uremic syndrome, renal failure, cardiovascular complication, thrombocytopenia.
INTRODUCTION
Hemolytic uremic syndrome (HUS) is a clinical syndrome characterized by the following triad: acute renal injury, associated with thrombocytopenia and microangiopathic (non-immune, negative Coombs test) hemolytic anemia. It is the most frequent cause of acute kidney injury among pediatric population (1, 2). It is usually caused by the “Shiga” toxin produced by Escherichia coli, but it may have a multitude of other etiologies (Figure 1) (3, 4). Older clinical classifications divided the HUS into two groups: diarrhea positive HUS (D+HUS), found in 90% of patients, and diarrhea negative HUS (D-HUS). Recent international studies have led to the conclusion that genetic mutations in the alternative complement pathway are also involved in the ethiopathogenic mechanisms of this syndrome (4, 6).
There are several histopathological findings characteristic to the hemolytic uremic syndrome, including thickening of arterioles and capillary walls, endothelial swelling and detachment along with deposits of fibrin and platelet-rich thrombi that lead to obstruction in the vascular lumen. This pathological mechanism affects various organs, but mainly the kidney. The extrarenal targets are the neurological, respiratory, and digestive systems. The cardiovascular system is also affected, both in the acute phase and during the chronic phase of the HUS (7-9).
OBJECTIVES
The aim of our study is to assess the possible cardiovascular complications of the hemolytic uremic syndrome and develop the most efficient investigation protocol and treatment regimen.
MATERIALS AND METHODS
This retrospective descriptive study was carried out in the Departments of Nephrology Cardiology of “Marie Sklodowska Curie” Emergency Hospital for Children, Bucharest, Romania. Fifty patients diagnosed with the hemolytic uremic syndrome, who had been hospitalized for years, between 2016 and 2020, were included in the study. The mean age of the subjects was 18 months.
Laboratory investigations included thrombocytopenia (less than 100.000 thrombocytes), hemolytic anemia (Hb less than 10.5 g/dL), high level of LDH (more than 500 U/L), serum creatinine higher than 35 mmol/L in patients under one year of age and serum creatinine higher than 80 mmol/L in patients above one year of age. The peripheral blood smear showed the presence of schistocytes. All patients underwent cardiological examination including EKG, arterial blood pressure monitorization over 24 hours, echocardiography, and venous Doppler examination. A complete cardiological evaluation was done during the acute phase of HUS, one month after discharge from the hospital, three months after checking out of the hospital, and one year after.
RESULTS
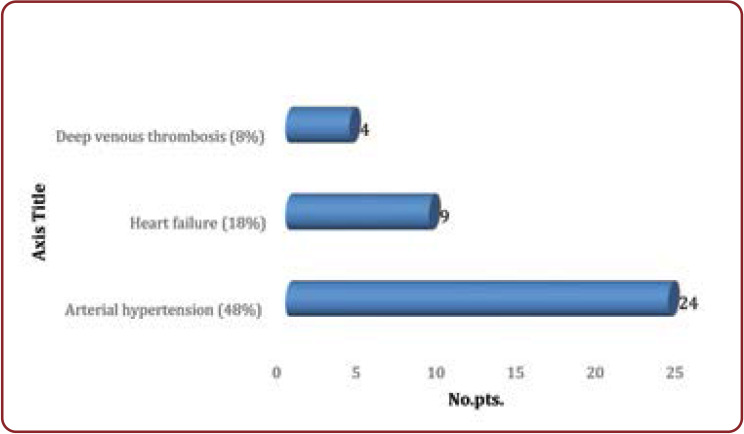
Among the clinical manifestations regarding the cardiovascular consequences of HUS, we encountered: arterial hypertension (48% of patients), cardiac failure (18% of patients), and acute venous thrombosis of the inferior limbs (8% of patients) (Figure 2).
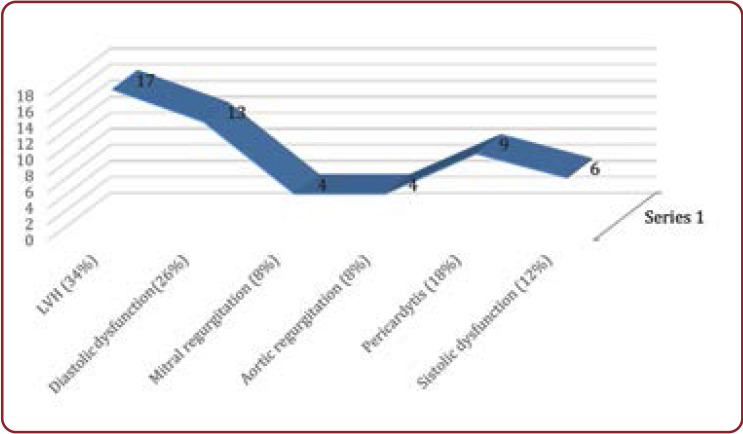
Echocardiographic findings among patients who had cardiovascular consequences of HUS comprised (5): left ventricular (LV) hypertrophy (34% of patients), diastolic dysfunction (26% of patients), mitral regurgitation (8%), aortic regurgitation (8%), pericarditis (18%), and systolic dysfunction (12% of patients) (Figure 3).
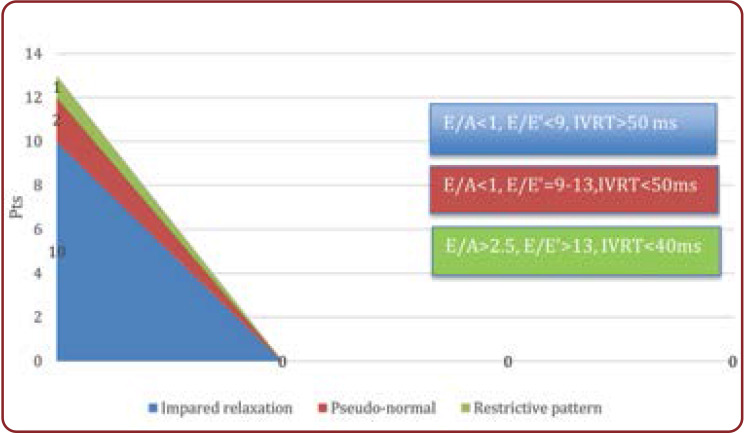
Tissue Doppler imaging was an important tool in the assessment of incipient diastolic dysfunction of the left ventricle. Inverted E/A ratio (E<A), increased isovolumetric relaxation time (IVRT) over 50 ms and E/E’ ratio < 9 indicated impaired LV relaxation (grade 1 diastolic dysfunction) in 60% of patients; E/E’ ratio between 9 and 13 associated with IVRT <50 ms and E/A >1 showed a pseudo-normal filling pattern (grade 2 diastolic dysfunction) in 35% of patients; E/A ratio >2.5, along with a short IVRT <40 ms; and E/E’ >13 indicated a restrictive pattern (grade 3 diastolic dysfunction) in 13% of patients (Figure 4) (10-12). The reversibility of restrictive pattern of LV diastolic filling was difficult to assess using Valsalva maneuvers in these small children.
All hypertensive patients (24 of all the 50 patients included in this study) had been treated with 1-5 antihypertensive drugs for an efficient blood pressure control, and the majority of them (18 patients) received ACE inhibitors. Only those who had clear contraindications for ACE inhibitor treatment (hiperkalemia, worsened renal failure with increased creatinine level, allergic reactions) did not receive this class of medication. Arterial hypertension was clearly more frequent in patients who needed dialysis (63% of all dialyzed patients were hypertensive instead of only 13% of the non-dialyzed ones). Two out of six patients with systolic disfunction have died.
At three-month cardiologic reevaluation after acute HUS, 20 patients presented arterial hypertension (67% from initially hypertensive patients). Interestingly, four of them had been normotensive during the acute episode of HUS and became hypertensive after the resolution of the acute phase. Regarding the echocardiography findings, only seven patients presented LV hypertrophy and six had LV diastolic dysfunction.
At one year follow-up, only eight patients continued to be hypertensive, and two subjects presented residual non-occlusive deep vein thrombosis without clinical signs. Echocardiography revealed LV hypertrophy in five patients and LV impaired relaxation in two subjects (Figure 5).
DISCUSSION
Patients who suffered from severe arterial hypertension had been treated with 3-5 different antihypertensive agents: ACE inhibitors, beta- blockers, alpha-blockers, loop diuretics, calcium channel blockers. Six patients had stage 2 arterial hypertension (13) (>99th percentile for age + 5 mm Hg) with media of BP over 24 hours up to 153/95 mm Hg (continuous BP monitoring/ 24 h). The first choice of treatment were ACE inhibitors. Twenty patients from those with arterial hypertension received ACE inhibitor treatment (83%), this medication being avoided only in those who presented contraindications.
Patients who had venous thrombosis needed increased attention regarding the administration of low molecular weight heparin (enoxaparin) because of the simultaneous thrombocytopenia (less than 100.000 thrombocytes) and reduced renal function (GFR less than 30 mL/min). The anticoagulant treatment was maintained for six months.
CONCLUSION
Arterial hypertension was more frequently encountered in patients who underwent dialysis. The majority of those who developed arterial hypertension in the acute phase of HUS did not have a substantial decrease in arterial blood pressure levels measured at the three-month check-up.
All patients with LV hypertrophy had arterial hypertension, but not all subjects with arterial hypertension had LV hypertrophy. The reversibility of LV hypertrophy did not always correlate with the normalization of arterial blood pressure (there were a few patients with persistent mild LVH despite BP normalization).
Our study underlines the importance of arterial blood pressure monitorization and echocardiography both in the acute phase of HUS and long after checking out of the hospital, taking into consideration the effects of the remaining renal lesions in the further clinical evolution of the child and the possibility of arterial hypertension onset late after acute HUS.
Last but not least, our study reflects the high incidence of arterial hypertension (46% of patients) and global cardiovascular complications (69% of patients) among children suffering from HUS, with a mortality of 4%.
A child who is diagnosed with HUS needs long term follow up, which has to be done by a multidisciplinary team that includes both a nephrologist and a cardiologist, due to the cardiorenal pathology which is related to acute HUS and remanent kidney injury.
Conflict of interests: none declared
Financial support: none declared.
Informed consent was obtained from all patients included in the study.
All procedures and experiments of this study respect the ethical standards in the Helsinki Declaration of 1975, as revised in 2008(5), as well as the national law.
FIGURE 1.
Etiological factors for the hemolytic uremic syndrome (HUS) (1)
FIGURE 2.
Cardiovascular consequences of HUS – clinical manifestations
FIGURE 3.
Echocardiographic findings in the acute phase of HUS
FIGURE 4.
Left ventricular diastolic dysfunction in acute HUS
FIGURE 5.
Clinical and echocardiography parameters durnig the first year after acute UHS
Contributor Information
Cristina FILIP, Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; Department of Pediatric Cardiology, “M. S. Curie” Emergency Clinical Hospital for Children, Bucharest, Romania.
Alin NICOLESCU, Department of Pediatric Cardiology, “M. S. Curie” Emergency Clinical Hospital for Children, Bucharest, Romania.
Eliza CINTEZA, Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; Department of Pediatric Cardiology, “M. S. Curie” Emergency Clinical Hospital for Children, Bucharest, Romania.
Gabriela DUICA, Department of Pediatric Cardiology, “M. S. Curie” Emergency Clinical Hospital for Children, Bucharest, Romania.
Georgiana NICOLAE, Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; Department of Pediatric Cardiology, “M. S. Curie” Emergency Clinical Hospital for Children, Bucharest, Romania.
Daniela SAFTA-BASCHIERU, Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; Department of Pediatric Cardiology, “M. S. Curie” Emergency Clinical Hospital for Children, Bucharest, Romania.
Ioana MIHALACHE, Department of Pediatric Cardiology, “M. S. Curie” Emergency Clinical Hospital for Children, Bucharest, Romania.
Loredana POPA, Department of Pediatric Cardiology, “M. S. Curie” Emergency Clinical Hospital for Children, Bucharest, Romania.
Mariana COSTIN, Department of Pediatric Cardiology, “M. S. Curie” Emergency Clinical Hospital for Children, Bucharest, Romania.
Georgiana MATEI, Department of Pediatric Cardiology, “M. S. Curie” Emergency Clinical Hospital for Children, Bucharest, Romania.
Mihaela RUSU, Department of Pediatric Cardiology, “M. S. Curie” Emergency Clinical Hospital for Children, Bucharest, Romania.
Mihaela BALGRADEAN, Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; Department of Pediatric Cardiology, “M. S. Curie” Emergency Clinical Hospital for Children, Bucharest, Romania.
References
- 1.Canpolat N. Hemolytic uremic syndrome. Turk Pediatri Ars. 2015;2:73–82. doi: 10.5152/tpa.2015.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanasescu MD, Dorin D, Georgescu A. Evolutia si tratamentul in sindromul hemolitic uremic la copil. Romanian Journal of Pediatrics. 2008;4:375–380. [Google Scholar]
- 3.Falup-Pecurariu O, Lixandru RI, Cojocaru E, et al . Shiga toxin producing Escherichia coli-associated diarrhea and hemolytic uremic syndrome in young children in Romania. Gut Pathog. 2019;11:46. doi: 10.1186/s13099-019-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noris M, Mescia F, Remuzzi G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Neophrol. 2012;11:622–633. doi: 10.1038/nrneph.2012.195. [DOI] [PubMed] [Google Scholar]
- 5.Mele C, Remuzzi G, Noris M. Hemolytic uremic syndrome. Semin Immunopathol. 2014;36:399–420. doi: 10.1007/s00281-014-0416-x. [DOI] [PubMed] [Google Scholar]
- 6.Campistol JM, Arias M, Ariceta G, et al. An update for atypical hemolytic uremic syndrome: diagnosis and treatment document. Nefrologia. 2015;5:421–427. doi: 10.1016/j.nefro.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Balgradean M, Croitoru A, Leibovitz E. An outbreak of hemolytic uremic syndrome in southern Romania during 2015-2016: Epidemiologic, clinical, laboratory, microbiologic, therapeutic and outcome characteristics. Pediatrics & Neonatology. 2019;60:87–94. doi: 10.1016/j.pedneo.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Noris M, Remuzzi G. Cardiovascular complications of hemolytic uremic syndrome. Nat Rev Nephrol. 2014;3:174–180. doi: 10.1038/nrneph.2013.280. [DOI] [PubMed] [Google Scholar]
- 9.Noris M, Remuzzi G. Cardiovascular complications in atypical haemolytic uraemic syndrome. Nat Rev Nephrol. 2014;3:174–180. doi: 10.1038/nrneph.2013.280. [DOI] [PubMed] [Google Scholar]
- 10.Dallaire F, Slorach C, Hui W, et al. Reference Values for Pulse Wave Doppler and Tissue Doppler Imaging in Pediatric Echocardiography. Circulation: Cardiovas Imaging. 2015;2:e002167. doi: 10.1161/CIRCIMAGING.114.002167. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz L, Koch H, Bein G, Brockmeier K. Ventricular Diastolic Function in Infants, Children, and Adolescents. Reference Values and Analysis of Morphologic and Physiologic Determinants of Echocardiographic Doppler Flow Signals During Growth and Maturation. JACC. 1998;5:1441–1448. doi: 10.1016/s0735-1097(98)00379-9. [DOI] [PubMed] [Google Scholar]
- 12.Paris G, Gorla SR, Arenas-Morales AJ, et al. Comparison of echocardiographic changes in children with primary hypertension and hypertension due to mild to moderate chronic kidney disease. Pediatr Nephrol. 2019;3:487–494. doi: 10.1007/s00467-018-4096-y. [DOI] [PubMed] [Google Scholar]
- 13.Lurbe E, Cifkova R, Cruickshank JK, et al. Management of high blood pressure in children and adolescents: recommendations of the European Society of Hypertension. J Hypertens. 2009;9:1719–1742. doi: 10.1097/HJH.0b013e32832f4f6b. [DOI] [PubMed] [Google Scholar]
- 14.Spinale JM, Ruebner RL, Copelovitch L, Kaplan BS. Long-term outcomes of Shiga toxin hemolytic uremic syndrome. Pediatr Nephrol. 2013;11:2097–2105. doi: 10.1007/s00467-012-2383-6. [DOI] [PubMed] [Google Scholar]