Abstract
Purpose
To investigate the association between 1-year myopia progression and subsequent 2-year myopia progression among myopic children in the Singapore Cohort Study of the Risk Factors for Myopia.
Methods
This retrospective analysis included 618 myopic children (329 male), 7 to 9 years of age (mean age, 8.0 ± 0.8) at baseline with at least two annual follow-up visits. Cycloplegic autorefraction was performed at every visit. Receiver operating characteristic (ROC) curves from multiple logistic regressions were derived for future fast 2-year myopia progression.
Results
Children with slow progression during the first year (slower than –0.50 diopter [D]/y) had the slowest mean subsequent 2-year myopia progression (–0.41 ± 0.33 D/y), whereas children with fast progression (faster than –1.25 D/y) in year 1 had the fastest mean subsequent 2-year myopia progression (–0.82 ± 0.30 D/y) (P for trend < 0.001). Year 1 myopia progression had the highest area under the curve (AUC) for predicting fast subsequent 2-year myopia progression (AUC = 0.77; 95% confidence interval [CI], 0.73–0.80) compared to baseline spherical equivalent (AUC = 0.70; 95% CI, 0.66–0.74) or age of myopia onset (AUC = 0.66; 95% CI, 0.61–0.70) after adjusting for confounders. Age at baseline alone had an AUC of 0.65 (95% CI, 0.61–0.69).
Conclusions
One-year myopia progression and age at baseline were associated with subsequent 2-year myopia progression in children 7 to 9 years of age.
Translational Relevance
Myopia progression and age at baseline may be considered by eye care practitioners as two of several factors that may be associated with future myopia progression in children.
Keywords: myopia, progression, age at baseline, children
Introduction
Myopia has become a significant global public health and socioeconomic problem.1–3 Severe cases of myopia are associated with risk of irreversible vision impairment and blindness due to pathological changes in the retina, choroid, and sclera. Evidence from previous surveys indicates that the prevalence of myopia is high in East Asian and Singapore children,4–10 and the prevalence has been shown to be higher among schoolchildren in Singapore (62.2%, 2002) than in Australia (11.9%, 2001).6,10 Additionally, the annual progression rate of myopia among schoolchildren was found to be higher in urban children compared to rural children in East Asia.5,11–15
A meta-analysis showed that myopia progressed at –0.55 diopter (D) (95% confidence interval [CI], –0.39 to –0.72) after 1 year of follow-up in children with a mean age of 9.3 years for urban Europeans and at –0.82 D (95% CI, –0.71 to –0.93) for urban East Asians.16
Several longitudinal studies have identified various risk factors for subsequent myopia progression, such as younger age at baseline, greater myopic baseline spherical equivalent (SE), parental myopia, and age of myopia onset.16–21 Being able to estimate faster future myopia progression is important for clinicians seeking to identify an appropriate strategy to control myopia progression. A survey of pediatric ophthalmologists reported that the most common indication for initiating myopia control treatment was prior rate of progression.22 By association, this criterion has been used to predict faster future progression, but there is limited information available to link myopia progression during a given year with subsequent myopia progression in a longitudinal cohort study.
The purpose of this study was to investigate the association between 1-year myopia progression and subsequent 2-year myopia progression amongst myopic children in the Singapore Cohort Study of the Risk Factors for Myopia (SCORM).
Methods
Study Population
The SCORM study was initiated in three schools in Singapore from 1999 to 2001.11,23,24 The methodology has been described previously. Children 7 to 9 years of age were recruited. Children who had serious medical conditions, eye disorders, or allergy to eye drops were excluded. Of the 1979 participants, our study included 618 children who were myopic (SE ≤ –0.50 D) at baseline and had at least two follow-up visits. Annual examinations until 2004 (the fourth visit) were included for the analysis. Written informed consent was obtained from the parents after an explanation of the nature and possible consequences of the study. Approval was obtained from the Ethics Committee at the Singapore Eye Research Institute, and the study was conducted according to the tenets of the Declaration of Helsinki.
Eye Measurements
Cycloplegia was used in both eyes. At least 30 minutes after the last drop, the averages of five consecutive refraction and keratometry readings were obtained by using table-mounted Canon RK-5 Autorefractor Keratometers (Canon, Inc., Tokyo, Japan). The same procedures were performed by trained staff annually.
Questionnaire Data
Parents completed detailed questionnaires during the baseline visit. For parents who were not conversant in English, Chinese and Malay versions were provided. Parents were asked to quantify their child's near-work activity as the number of books read per week.25 Outdoor time of the child was the number of hours spent outdoors per week.26 Age of myopia onset was assessed by asking when the child first wore spectacles for nearsightedness. For children who were newly diagnosed with myopia at baseline, age of myopia onset was recorded as the age at the baseline visit. Parents were also asked whether they were wearing spectacles or contact lenses (CLs) to see clearly at far distance, and a positive response was used to classify the parent as myopic. Maternal education level was asked.
Data Analysis and Definition
SE was defined as spherical power plus half negative cylinder power. Myopia was defined as SE of less than –0.50 D, and the worse eye was used in the analysis. If SE was the same for both eyes, one of the two eyes was selected randomly. The main exposure was myopia progression in the first year (year 1 myopia progression), which was the difference in SE at the second visit and the baseline visit. The primary outcome variable was 2-year myopia progression (year 2 to 3), which was the difference in SE between the fourth visit and the second visit. Multiple linear regression analysis was performed, with subsequent 2-year myopia progression as the dependent variable and year 1 myopia progression as the main exposure, with adjustment of the potentially most prognostic covariates: age, gender, ethnicity, maternal education, parental myopia, baseline SE, near work, and outdoor time, all chosen because of their known relationship to the progression of myopia.16–20 We also defined a binary outcome for predicting future fast myopia progression (based on the median cutoff).
The estimated probabilities of prognostic covariates for predicting future fast myopia progression were obtained from the logistic regression that included each of the main covariates of interest and other covariates in the model. These estimated probabilities were used to construct the receiver operating characteristics (ROC) curve and compute the area under the curve (AUC), sensitivity (Sens), specificity (Spec), and positive predictive value (PPV) for predicting future fast myopia progression. Due to natural slowing progression by age, it may not be appropriate to use the same definition to evaluate the progression rate in each year; thus, we used the median cutoff of each year's progression. Similar analyses were performed for the prediction of fast year 3 annual myopia progression, including myopia progression in the prior 1 year and cumulative myopia progression for the 2 years at year 3. Finally, we evaluated the association between year 1 myopia progression and subsequent 2-year myopia progression using both multiple linear regression and AUC analyses within subgroups of age, baseline SE, near work, and parental myopia. As the age-stratified analysis was conducted using data collected based on the age at the time of visits, data from the same child could appear in more than one subgroup. MedCalc 18.5 (MedCalc Software, Ltd., Ostend, Belgium) was used for the ROC curve analysis. Other data analyses were conducted using SPSS Statistics 24.0 (IBM, Armonk, NY). P < 0.05 was considered statistically significant.
Results
The characteristics of the study subjects are shown in Table 1. There were 618 children, 329 boys and 289 girls, with a mean age (± SD) of 8.0 ± 0.8 years at baseline. There were 509 Chinese (82.4%), and 109 non-Chinese children (11.0% Malays, 6.1% Indians, and 0.5% children of other races). Annual myopia progression rates in year 1 (n = 618), year 2 (n = 618), and year 3 (n = 555) were –0.88 ± 0.50 D/y, –0.68 ± 0.46 D/y, and –0.48 ± 0.38 D/y (P for trend < 0.001), respectively, showing that annual SE progression declined with age. Chinese children had slightly higher myopia progression at year 1 (–0.88 ± 0.50 vs. –0.76 ± 0.49 D/y; P = 0.018).
Table 1.
Demographics and Parental Characteristics of Myopic Singapore Children in SCORM Study (N = 618)
| Characteristics | Mean (SD) or n (%)a |
|---|---|
| Age (y) | 8.01 ± 0.84 |
| 7 | 212 (34.3) |
| 8 | 185 (29.9) |
| 9 | 221 (35.8) |
| Gender | |
| Male | 329 (53.2) |
| Female | 289 (46.8) |
| Race | |
| Chinese | 509 (82.4) |
| Non-Chinese | 109 (17.6) |
| Baseline SE (D) | –2.28 ± 1.68 |
| –0.50 to –1.49 | 261 (42.2) |
| –1.50 to –2.99 | 193 (31.2) |
| –3.00 or worse | 164 (26.5) |
| Number of parents with myopia | |
| None | 183 (29.6) |
| One | 255 (41.3) |
| Both | 180 (29.1) |
| Myopia progression | |
| Year 1 (D/y) | –0.86 ± 0.50 |
| Year 1 fast (≤–1.00 D/y) | 249 (40.3) |
| Age of myopia onset (y) | 7.18 ± 1.14 |
| ≤6 | 120 (19.4) |
| 7 | 276 (44.7) |
| 8 | 144 (23.3) |
| 9 | 78 (12.6) |
Mean for continuous variables and percentages for categorical variables.
Year 1 Myopia Progression and Subsequent 2-Year Myopia Progression
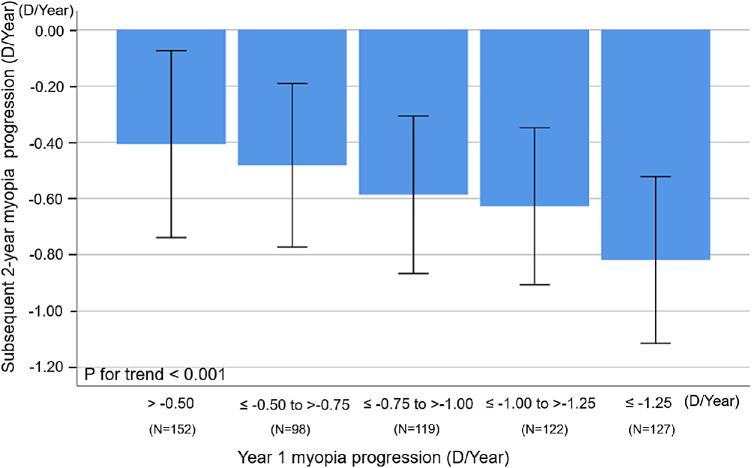
The relationship between year 1 and subsequent 2-year myopia progression is shown in the Figure. Children with slow myopia progression during the first year (>–0.50 D/y) had the smallest mean subsequent 2-year myopia progression (–0.41 ± 0.33 D/y), whereas children with fast myopia progression (<–1.25 D/y) in year 1 had the fastest mean subsequent 2-year progression (–0.82 ± 0.30 D/y), and, albeit with relatively large SDs, there was a statistically significant dose–response relationship (P for trend < 0.001) between categories of year 1 myopia progression and mean subsequent 2-year myopia progression.
Figure.
Relationship between myopia progression during the first year and subsequent 2-year myopia progression (year 2 to year 3) among myopic Singapore children 7 to 9 years of age at baseline in the SCORM study (N = 618). Error bars represent 1 SD.
Subsequent 2-year myopia progression was significantly associated with myopia progression in year 1 (β = 0.28; 95% CI, 0.23–0.33; P < 0.001) and age at baseline (β = 0.06; 95% CI, 0.03–0.09; P < 0.001). There was no significant association with baseline SE (P = 0.07), age of myopia onset (P = 0.27), parental myopia (P = 0.56), near work (P = 0.89), or outdoor time (P = 0.91). Year 1 myopia progression alone had the highest AUC to predict fast 2-year myopia progression (AUC = 0.72; 95% CI, 0.68–0.75) compared to age alone (AUC = 0.65; 95% CI, 0.61–0.69), baseline SE alone (AUC = 0.53; 95% CI, 0.49–0.58), age of myopia onset alone (AUC = 0.54; 95% CI, 0.50–0.58), and parental myopia alone (AUC = 0.57; 95% CI, 0.53–0.61). After adjusting for confounders, the AUCs of year 1 myopia progression, baseline SE, and age of myopia onset to predict 2-year myopia progression were 0.77, 0.70, and 0.66, respectively (Table 2).
Table 2.
ROC Curve Parameters for Predicting Fast Subsequent 2-Year Myopia Progression Using Baseline Parameters Among Myopic Singapore Children 7 to 9 Years Old at Baseline in SCORM Study (N = 618)
| Fast Subsequent 2-Year Myopia Progression (<–0.58 D/y) | |||||
|---|---|---|---|---|---|
| Parameter | N | AUC (95% CI) | Sensitivity (%) | Specificity (%) | PPV (95% CI) |
| Year 1 myopia progression (D/y)a | 528 | 0.77 (0.73–0.80) | 62.0 | 72.2 | 72.1 (67.2–76.6) |
| Baseline SE (D)b | 528 | 0.70 (0.66–0.74) | 66.2 | 63.8 | 64.4 (60.2–68.5) |
| Age of myopia onset (y)c | 528 | 0.66 (0.61–0.70) | 63.5 | 61.9 | 62.3 (58.0–66.4) |
Model includes age, gender, ethnicity, maternal education, parental myopia, year 1 myopia progression, baseline SE, near work, and outdoor time.
Model includes age, gender, ethnicity, maternal education, parental myopia, baseline SE, near work, and outdoor time.
Model includes age, gender, ethnicity, maternal education, parental myopia, year 1 myopia progression, age of myopia onset, near work, and outdoor time.
In subgroup analysis, we examined the association between myopia progression in year 1 and 2-year myopia progression by age groups of 7 years (n = 190), 8 years (n = 334), and 9 years (n = 341), respectively. The adjusted regression coefficients (β) were similar across age groups: for 7 year olds, β = 0.26 (95% CI, 0.18–0.34; P < 0.001); for 8 year olds, β = 0.28 (95% CI, 0.20–0.35; P < 0.001); and for 9 year olds, β = 0.25 (95% CI, 0.18–0.31; P < 0.001). The AUCs for year 1 myopia progression, adjusted for confounders, to predict fast 2-year myopia progression were similar within each age strata: for 7 year olds, AUC = 0.76 (95% CI, 0.69–0.82; Sens, 72.5%; Spec, 72.5%; PPV, 90.1%); for 8 years olds, AUC = 0.70 (95% CI, 0.64–0.74; Sens, 43.5%; Spec, 85.1%; PPV, 73.9%); and for 9 year olds, AUC = 0.72 (95% CI, 0.67–0.77; Sens, 66.3%; Spec, 73.5%; PPV, 70.0%).
In another subgroup analysis, we examined the association by baseline SE groups. From multiple linear regression analyses, the regression coefficients for the association between year 1 myopia progression and 2-year myopia progression were β = 0.27 (95% CI, 0.19–0.35; P < 0.001) for SE between –0.50 and –1.49 D; β = 0.29 (95% CI, 0.18–0.40; P < 0.001) for SE between –1.50 and –2.99 D; and β = 0.30 (95% CI, 0.20–0.39; P < 0.001) for SE of –3.00 D or worse. The AUCs for year 1 myopia progression, adjusted for confounders, to predict fast 2-year myopia progression were as follows: AUC = 0.79 (95% CI, 0.73–0.84; Sens, 57.9%; Spec, 84.8%; PPV, 80.5%) for SE between –0.50 and –1.49 D; AUC = 0.78 (95% CI, 0.71–0.84; Sens, 76.1%; Spec, 67.1%; PPV, 71.3%) for SE between –1.50 and –2.99 D; and AUC = 0.80 (95% CI, 0.72–0.86; Sens, 55.7%; Spec, 89.7%; PPV, 81.0%) for SE of –3.00 D or worse. The effect of year 1 progression on 2-year myopia progression was similar for the different baseline SE groups.
Subgroup analysis by near work and parental factors showed similar year 1 progression effects on 2-year myopia progression across groups of near work, either ≤2 books per week (β = 0.25; 95% CI, 0.18–0.31; P < 0.001) or 3+ books per week (β = 0.31; 95% CI, 0.23–0.39; P < 0.001), as well as across groups of parental myopia, either no parental myopia (β = 0.26; 95% CI, 0.18–0.35; P < 0.001) or parental myopia (β = 0.29; 95% CI, 0.22–0.35; P < 0.001).
The AUCs for year 1 myopia progression, adjusted for confounders, to predict fast 2-year myopia progression were similar within each near work and parental strata. The AUCs were 0.77 (95% CI, 0.72–0.82; Sens, 86.5%; Spec, 55.0%; PPV, 63.7%) for ≤2 books per week and 0.77 (95% CI, 0.70–0.83; Sens, 60.8%, Spec, 81.9%; PPV, 79.3%) for 3+ books per week. The AUC for no parental myopia was 0.79 (95% CI, 0.72–0.85; Sens, 69.7%; Spec, 73.1%; PPV, 64.8%) compared with 0.76 (95% CI, 0.71–0.80; Sens, 59.4%; Spec, 78.5%; PPV, 76.0%) for parental myopia.
Year 1 Myopia Progression and the Association with Myopia Progression in Years 2 and 3
Multiple regression analysis (adjusted for age, gender, ethnicity, maternal education, parental myopia, baseline SE, near work, and outdoor time) indicated that myopia progression rates in years 2 and 3 increased by 0.37 D (95% CI, 0.31–0.44; P < 0.001) and 0.20 D (95% CI, 0.13–0.27; P < 0.001), respectively, for every 1-D increase in year 1 myopia progression. The AUCs for year 1 myopia progression to predict fast year 2 (≤–0.75 D) and year 3 (≤–0.48 D) progression were 0.77 (95% CI, 0.73–0.80; Sens, 49.8%; Spec, 87.7%; PPV, 79.4%) and 0.69 (95% CI, 0.65–0.73; Sens, 77.3%; Spec, 52.3%; PPV, 61.8%), respectively.
Cumulative Myopia Progression and Fast Myopia Progression
The regression coefficients for the association between year 3 progression with preceding single-year (year 2) progression and preceding 2-year progression (year 1 to year 2) were β = 0.20 D (95% CI, 0.12–0.27; P < 0.001) and β = 0.14 D (95% CI, 0.10–0.18; P < 0.001), respectively. The AUC for fast year 3 myopia progression using the preceding 2-year progression was 0.71 (95% CI, 0.67–0.75; Sens, 73.9%; Spec, 61.4%; PPV, 65.7%), which was similar to that of a single preceding year progression (β = 0.69; 95% CI, 0.64–0.73; Sens, 69.3%; Spec, 64.8%; PPV, 66.3%).
Discussion
We found that 1-year annual myopia progression and age at baseline were associated with subsequent 2-year myopia progression and that children with fast progression in the previous year were at higher risk of fast myopia progression in the subsequent 2 years. There was a dose–response relationship between categories of fast myopia progression and the mean myopia progression in the subsequent 2 years. Annual myopia progression in year 1 is a better clinical indicator of subsequent 2-year myopia progression than baseline SE and age of myopia onset in children 7 to 9 years of age. However, annual myopia progression as a stand-alone factor cannot fully predict subsequent 2-year myopia progression, as multiple factors are involved.
Myopia Progression in the First Year and Subsequent 2-Year Myopia Progression
Our results show that there was a relationship between past and future progression, and we quantified the value of using myopia progression when compared to other indices, such as SE or age at baseline, age of myopia onset, near work, and parental myopia. To our knowledge, there are no previous longitudinal studies using more than 2 years of data to compare myopia progression during a given year with subsequent 2-year progression. To date, no evidence-based clinical management guideline for myopic children has been established to tailor treatment based on expected future myopia progression. Past progression has been the most favored indicator among pediatric ophthalmologists for initiating myopia progression therapy, and the average threshold used is –1 D.22 However, using past progression has obvious pitfalls. Children with slow progression (>–0.50 D/y) in the first year of our study had a mean (± SD) progression of –0.41 ± 0.33 D/y, indicating that about 16% of children (one in six) that progress by less than 0.50 in year 1 will progress by –0.74 D in the subsequent 2 years. A similar proportion of subjects with measured progression between –0.50 and –1.00 D in the first year of the study would show more than –1.00 D of annual progression in the subsequent 2 years. Past progression may be inadequate as a standalone indicator of whether to treat. Our results show that even a child with slow myopia progression in the first year may have faster progression that requires treatment in the following year. Generally, children with fast progression are advised to start myopia control treatment (e.g., optical devices including CLs, novel spectacles, or pharmacological agents as appropriate). However, myopia progression in the previous year does not seem to predict accurately subsequent 2-year myopia progression. A limiting factor in using past refractive change as a measure of future progression is repeatability of refractive error measurement. Estimates of repeatability limits for the autorefractor under cycloplegia were ±0.32 D in previous studies.27,28 The repeatability limits are therefore of the order of annual progression in young myopes. The modest value of using past progression as an indicator of future progression may therefore be more related to accuracy of measurement than consistency of progression in individuals. The decision to start treatment must be made carefully, taking into account multiple risk factors such as younger age, age of myopia onset, higher baseline SE, and parental myopia rather than one single factor. Further research is necessary to establish accurate prediction models of future myopia progression and to determine the best therapeutic strategy for each individual.
Myopia Progression in the Preceding Year Is a Better Clinical Parameter Compared with Other Factors
Although the AUCs were not good enough for accurate prediction, the AUCs for year 1 myopia progression for fast subsequent 2-year myopia progression were highest when compared to age at baseline, baseline SE, and age of myopia onset, indicating that myopia progression in the first year was a better clinical parameter of future myopia progression. Previous longitudinal studies reported that younger age at baseline, myopic SE at baseline, and parental myopia were associated with myopia progression in school-aged children.17,21,29,30 Hsu et al.15 reported that children with fast myopia progression had higher myopia at baseline (odds ratio, 0.67; 95% CI, 0.61–0.72) when compared with children with slow myopia progression among 3256 myopic children (mean age, 7.49 years). Pärssinen et al.20 reported that myopia progression was significantly associated with female gender, age of myopia onset, and baseline myopic SE in a 3-year follow-up study among 238 schoolchildren. The Guangzhou Twin Eye Study revealed that myopia progressed faster in children with parental myopia among 1831 school-aged children.31 However, these studies did not examine whether these factors predicted future myopia progression better than myopia progression in the previous year.
Our results have revealed that annual myopia progression is linked to subsequent 2-year myopia progression but the prediction is at best moderate. This is likely due to multiple other factors that can affect myopia progression. Children with multiple risk factors, especially fast myopia progression, younger age, early myopia onset, and parental myopia, may require more intensive management. Age at baseline was associated with subsequent 2-year myopia progression and seems to be an important factor to consider, particularly when clinicians do not have access to refractive history data. Previous studies have shown that myopic progression is faster in younger children. In a retrospective study that was based on the Atropine for the Treatment of Myopia trial and included 182 children 6 to 12 years of age, higher myopic SE and younger age at baseline were associated with myopia progression.21 In another trial, younger age and higher baseline myopia were associated with myopia progression among 135 myopic Canadian–Chinese children 8 to 13 years of age.32 In a recent study, age at baseline was the most significant factor contributing to the 3-year progression of myopia in both Singaporean and Finnish children.33 Overall, our results seem to indicate that myopia progression can be determined by faster myopia progression at a younger age; however, age of myopia onset was not associated with 2-year myopia progression in our study. Further longitudinal studies on myopia progression from childhood to adulthood are necessary to uncover the link among age, age of myopia onset, and myopia progression. The age-stratified analysis showed that the AUCs for year 1 myopia progression to predict subsequent 2-year myopia progression were similar in all age groups. This may be due to the narrow age range at baseline in our study. In our stratified analysis of baseline SE, the AUC of year 1 myopia progression to subsequent 2-year myopia progression was slightly better in higher myopic SE, but the difference was small. Further studies with a wider range of baseline SE values are necessary to confirm this trend. Subgroup analysis across near work and parental myopia groups also showed a similar year 1 progression effect on 2-year myopia progression.
We found that the AUC of myopia progression in the previous year to predict progression 2 years ahead was lower than that to predict subsequent-year progression. Our results support the suggestion that all myopic children should be seen at least every year, as progression rates are variable and fast progressors may be difficult to detect with only one visit. However, more studies are necessary to understand the usefulness of annual visits compared with an approached based on overall history rather than 1 year.
Our results have also confirmed that cumulative myopia progression during the past few years is associated with subsequent-year myopia progression but does not improve the accuracy of prediction. This suggests that doctors may not need to review more than the previous 1 year of a patient's data to predict future myopia progression; however, future studies with longer visits are required to further establish this observation.
Strengths and Limitations
The major strengths of the current study are the research design, which enabled us to analyze the natural progression of untreated myopic eyes, and the standardized measurements of cycloplegic refractive error taken at annual intervals over several years in a large cohort. The limitations of our study include the lack of precise data on the age of onset of myopia before the start of the study and the drop-out rate in the main cohort. Among the myopic children examined at baseline, 93 children (13.1%) dropped out at subsequent follow-up visits. There were no significant differences in age, gender, baseline SE, age of myopia onset, or myopia progression rate in year 1 between the children who were included and the children who were lost to follow-up. The children who remained in the study had a larger percentage of non-myopic parents (29.6% vs. 14.0%) compared to the children who dropped out. This may have reduced the proportion of higher myopia progression within our sample and may have underestimated the observed association. Additionally, selecting the worse eye for the analysis might have introduced a bias because the worse eye might regress myopia progression more frequently than the better eye. Another limitation is that the results are mostly applicable to this cohort and may not be reproducible in other cohorts. The preliminary prediction findings may be overfitted, so future work is needed. Further studies with larger sample sizes are required to explore a better predictive model. In addition, our results may not be generalized to older populations, as we only included participants 7 to 9 years of age at baseline.
Conclusions
One-year annual myopia progression and age at baseline were associated with subsequent 2-year myopia progression in children 7 to 9 years of age, but the ability to predict future myopia progression was modest. A personalized myopia management strategy for a given individual should be determined based on multiple patient-specific factors, including myopia progression in the previous year, younger age, higher initial SE, younger age of myopia onset, and parental myopia. Further studies are needed to explore the role of annual myopia progression as an estimate of how much a child's myopia will progress in the future.
Acknowledgments
The authors thank Chye-Fong Peck for data collection.
Supported by a grant from the Singapore Government under the Industry Alignment Fund–Industry Collaboration Projects (I1901E0038), by a National Medical Research Council Individual Research Grant (NMRC/0975/2005), and by Johnson & Johnson Vision.
Disclosure: S. Matsumura, None; C. Lanca, None; H.M. Htoon, None; N. Brennan, Johnson & Johnson Vision (E); C.-S. Tan, None; B. Kathrani, Johnson & Johnson Vision (E); A. Chia, None; D. Tan, None; C. Sabanayagam, None; S.-M. Saw, None
References
- 1. Dolgin E. The myopia boom. Nature. 2015; 519(7543): 276–278. [DOI] [PubMed] [Google Scholar]
- 2. Zheng Y-F, Pan C-W, Chay J, Wong TY, Finkelstein E, Saw S-M. The economic cost of myopia in adults aged over 40 years in Singapore. Invest Ophthalmol Vis Sci. 2013; 54(12): 7532–7537. [DOI] [PubMed] [Google Scholar]
- 3. Liang YB, Wong TY, Sun LP, et al.. Refractive errors in a rural Chinese adult population: the Handan Eye Study. Ophthalmology. 2009; 116(11): 2119–2127. [DOI] [PubMed] [Google Scholar]
- 4. He M, Zeng J, Liu Y, Xu J, Pokharel GP, Ellwein LB. Refractive error and visual impairment in urban children in southern China. Invest Ophthalmol Vis Sci. 2004; 45(3): 793–799. [DOI] [PubMed] [Google Scholar]
- 5. Fan DSP, Lam DSC, Lam RF, et al.. Prevalence, incidence, and progression of myopia of school children in Hong Kong. Invest Ophthalmol Vis Sci. 2004; 45(4): 1071–1075. [DOI] [PubMed] [Google Scholar]
- 6. Ip JM, Huynh SC, Robaei D, et al.. Ethnic differences in refraction and ocular biometry in a population-based sample of 11–15-year-old Australian children. Eye. 2008; 22(5): 649–656. [DOI] [PubMed] [Google Scholar]
- 7. Murthy GVS, Gupta SK, Ellwein LB, et al.. Refractive error in children in an urban population in New Delhi. Invest Ophthalmol Vis Sci. 2002; 43(3): 623–631. [PubMed] [Google Scholar]
- 8. Sapkota YD, Adhikari BN, Pokharel GP, Poudyal BK, Ellwein LB. The prevalence of visual impairment in school children of upper-middle socioeconomic status in Kathmandu. Ophthalmic Epidemiol. 2008; 15(1): 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao Z, Meng N, Muecke J, et al.. Refractive error in school children in an urban and rural setting in Cambodia. Ophthalmic Epidemiol. 2012; 19(1): 16–22. [DOI] [PubMed] [Google Scholar]
- 10. Saw S-M, Cheng A, Fong A, Gazzard G, Tan DTH, Morgan I. School grades and myopia. Ophthalmic Physiol Opt. 2007; 27(2): 126–129. [DOI] [PubMed] [Google Scholar]
- 11. Saw S-M, Shankar A, Tan S-B, et al.. A cohort study of incident myopia in Singaporean children. Invest Ophthalmol Vis Sci. 2006; 47(5): 1839–1844. [DOI] [PubMed] [Google Scholar]
- 12. Lam CS, Edwards M, Millodot M, Goh WS. A 2-year longitudinal study of myopia progression and optical component changes among Hong Kong schoolchildren. Optom Vis Sci. 1999; 76(6): 370–380. [DOI] [PubMed] [Google Scholar]
- 13. You X, Wang L, Tan H, et al.. Near work related behaviors associated with myopic shifts among primary school students in the Jiading District of Shanghai: a school-based one-year cohort study. PLoS One. 2016; 11(5): e0154671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsai D-C, Fang S-Y, Huang N, et al.. Myopia development among young schoolchildren: the Myopia Investigation Study in Taipei. Invest Ophthalmol Vis Sci. 2016; 57(15): 6852–6860. [DOI] [PubMed] [Google Scholar]
- 15. Hsu C-C, Huang N, Lin P-Y, et al.. Risk factors for myopia progression in second-grade primary school children in Taipei: a population-based cohort study. Br J Ophthalmol. 2017; 101(12): 1611–1617. [DOI] [PubMed] [Google Scholar]
- 16. Donovan L, Sankaridurg P, Ho A, Naduvilath T, Smith EL, Holden BA. Myopia progression rates in urban children wearing single-vision spectacles. Optom Vis Sci. 2012; 89(1): 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu Y, Ding X, Long W, He M, Yang X. Longitudinal changes in spherical equivalent refractive error among children with preschool myopia. Invest Ophthalmol Vis Sci. 2019; 60(1): 154–160. [DOI] [PubMed] [Google Scholar]
- 18. Ma Y, Zou H, Lin S, et al.. Cohort study with 4-year follow-up of myopia and refractive parameters in primary schoolchildren in Baoshan District, Shanghai. Clin Experiment Ophthalmol. 2018; 46(8): 861–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. French AN, Morgan IG, Burlutsky G, Mitchell P, Rose KA. Prevalence and 5- to 6-year incidence and progression of myopia and hyperopia in Australian schoolchildren. Ophthalmology. 2013; 120(7): 1482–1491. [DOI] [PubMed] [Google Scholar]
- 20. Pärssinen O, Lyyra AL. Myopia and myopic progression among schoolchildren: a three-year follow-up study. Invest Ophthalmol Vis Sci. 1993; 34(9): 2794–2802. [PubMed] [Google Scholar]
- 21. Loh K-L, Lu Q, Tan D, Chia A. Risk factors for progressive myopia in the atropine therapy for myopia study. Am J Ophthalmol. 2015; 159(5): 945–949. [DOI] [PubMed] [Google Scholar]
- 22. Leshno A, Farzavandi SK, Gomez-de-Liaño R, Sprunger DT, Wygnanski-Jaffe T, Mezer E. Practice patterns to decrease myopia progression differ among paediatric ophthalmologists around the world. Br J Ophthalmol. 2020; 104(4): 535–540. [DOI] [PubMed] [Google Scholar]
- 23. Saw S-M, Tong L, Chua W-H, et al.. Incidence and progression of myopia in Singaporean school children. Invest Ophthalmol Vis Sci. 2005; 46(1): 51–57. [DOI] [PubMed] [Google Scholar]
- 24. Saw SM, Hong CY, Chia KS, Stone RA, Tan D. Nearwork and myopia in young children. Lancet. 2001; 357(9253): 390. [DOI] [PubMed] [Google Scholar]
- 25. Saw S-M, Chua W-H, Hong C-Y, et al.. Nearwork in early-onset myopia. Invest Ophthalmol Vis Sci. 2002; 43(2): 332–339. [PubMed] [Google Scholar]
- 26. Dirani M, Tong L, Gazzard G, et al.. Outdoor activity and myopia in Singapore teenage children. Br J Ophthalmol. 2009; 93(8): 997–1000. [DOI] [PubMed] [Google Scholar]
- 27. Chat SW, Edwards MH. Clinical evaluation of the Shin-Nippon SRW-5000 autorefractor in children. Ophthalmic Physiol Opt. 2001; 21(2): 87–100. [PubMed] [Google Scholar]
- 28. Zadnik K, Mutti DO, Adams AJ. The repeatability of measurement of the ocular components. Invest Ophthalmol Vis Sci. 1992; 33(7): 2325–2333. [PubMed] [Google Scholar]
- 29. Wu P-C, Yang Y-H, Fang P-C. The long-term results of using low-concentration atropine eye drops for controlling myopia progression in schoolchildren. J Ocul Pharmacol Ther. 2011; 27(5): 461–466. [DOI] [PubMed] [Google Scholar]
- 30. Saw S-M, Javier Nieto F, Katz J, Schein OD, Levy B, Chew S-J. Familial clustering and myopia progression in Singapore school children. Ophthalmic Epidemiol. 2001; 8(4): 227–236. [DOI] [PubMed] [Google Scholar]
- 31. Liao C, Ding X, Han X, et al.. Role of parental refractive status in myopia progression: 12-year annual observation from the Guangzhou Twin Eye Study. Invest Ophthalmol Vis Sci. 2019; 60(10): 3499–3506. [DOI] [PubMed] [Google Scholar]
- 32. Cheng D, Woo GC, Drobe B, Schmid KL. Effect of bifocal and prismatic bifocal spectacles on myopia progression in children. JAMA Ophthalmol. 2014; 132(3): 258–264. [DOI] [PubMed] [Google Scholar]
- 33. Pärssinen O, Soh ZD, Tan C-S, Lanca C, Kauppinen M, Saw S-M. Comparison of myopic progression in Finnish and Singaporean children [published online ahead of print July 24, 2020]. Acta Ophthalmol, 10.1111/aos.14545. [DOI] [PubMed] [Google Scholar]