Abstract
Ipilimumab plus nivolumab (Ipi/Nivo) has revolutionized advanced renal cell carcinoma (RCC) treatment. However, it encompassed fatal immune-related adverse events (irAEs). Myocarditis with concomitant myasthenia gravis (MG) has a mortality rate of 50%, and a high dose of methylprednisolone (mPSL) should be administered with careful attention to MG exacerbation. We present the case of a 59-year-old man with progressing lung metastasis of RCC. After one cycle of Ipi/Nivo, he experienced myocarditis and MG, managed by mPSL pulse therapy, plasma exchange, and high-dose intravenous immunoglobulin. We share the therapeutic course, aiming to contribute to the limited literature on rare but aggressive irAEs.
Keywords: Ipilimumab, Nivolumab, Myocarditis, Myasthenia gravis
Abbreviations: RCC, renal cell carcinoma; MG, myasthenia gravis; ICI, immune checkpoint inhibitor; Ipi/Nivo, ipilimumab plus nivolumab; irAE, immune-related adverse event; mPSL, methylprednisolone; PE, plasma exchange; IVIg, intravenous immunoglobulin; CAVB, complete atrioventricular block; CPK, creatine phosphokinase
Highlights
-
•
Ipilimumab plus nivolumab is associated with fatal immune-related adverse events.
-
•
Myocarditis concomitant with myasthenia gravis (MG) is rare, but has a high mortality rate.
-
•
In the comorbid condition, prompt intervention for myocarditis is essential for survival.
-
•
Although high-dose methylprednisolone may worsen MG, myocarditis should be prioritized.
Introduction
The development of immune checkpoint inhibitors (ICIs) revolutionized cancer treatment. In renal cell carcinoma (RCC), although combined ipilimumab and nivolumab (Ipi/Nivo) resulted in a complete response for metastatic status in up to 9% of cases,1 some immune-related adverse events (irAEs) lead to high mortality rates. Because irAEs are rare but treatable by early diagnosis and proper intervention, clinicians should be familiar with various initial manifestations and clinical courses through case reports. Myocarditis is one of the fatal irAEs and coexists with simultaneous myasthenia gravis (MG) in 50% of the cases. In the comorbid condition, methylprednisolone (mPSL) needs to be carefully administered because mPSL may temporarily exacerbate MG.2
We report a case of severe myocarditis and MG following Ipi/Nivo treatment for advanced RCC. As mPSL pulse therapy did not improve MG, plasma exchange (PE) and high-dose intravenous immunoglobulin (IVIg) therapy were added to the treatment, resulting in successful management.
Case presentation
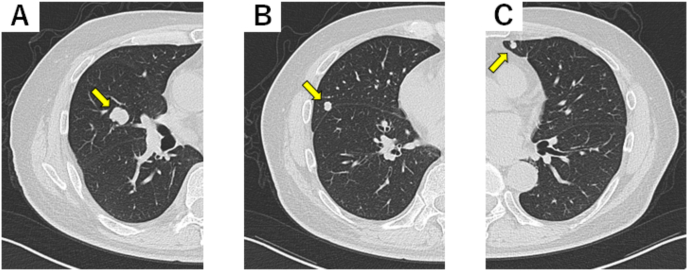
A 59-year-old man with no previous history of autoimmune and cardiac diseases was diagnosed with bilateral RCC. The patient subsequently underwent right nephrectomy and left partial nephrectomy. The pathological diagnosis was pT3a G2 and pT1 G2 clear cell carcinomas for the right and left RCCs, respectively. One year later, multiple lung metastases progressing rapidly were detected (Fig. 1). One cycle of combination immunotherapy with ipilimumab (1 mg/kg) and nivolumab (240 mg) was administered. Twenty-one days later, he experienced bilateral ptosis and malaise, and left eyeball adduction, descent, and taste were also impaired.
Fig. 1.
Computed tomography scan of the thorax. The yellow arrows denote multiple pulmonary metastases before therapy with ipilimumab and nivolumab. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Electrocardiogram findings revealed a complete atrioventricular block (CAVB). Levels of serum troponin I, creatine phosphokinase (CPK), and creatinine phosphokinase-myocardial band were 3.78 ng/mL, 8944 ng/mL, and 180 ng/mL, respectively. His acetylcholine receptor antibody level was 18 U/L, and anti-muscle-specific kinase antibodies were normal. Computed tomography of the chest did not reveal any thymoma or thymic hyperplasia, suggesting MG. All other laboratory tests were normal. Myocardial escape enzyme increased, but coronary angiography revealed no significant vascular stenosis. Cardiac biopsy was not performed, but his symptoms suggested drug-induced myocarditis with MG. The cardiology and neurology departments were consulted to consider steroid administration.
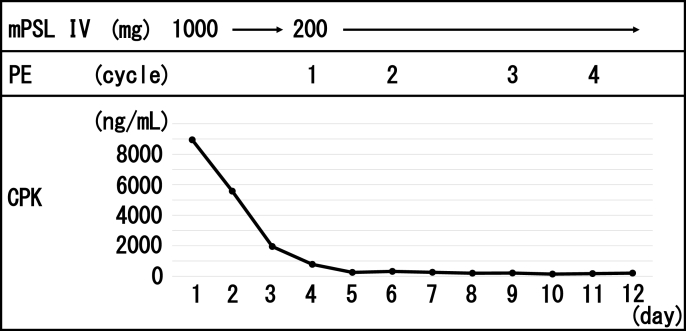
The patient was treated in the intensive care unit in case of MG exacerbation or cardiac arrest. Intravenous mPSL pulse therapy was initiated at a dose of 1000 mg/day for three days. On day two, the CAVB disappeared on the electrocardiogram, and the patient's cardiac rhythm returned to its sinus rhythm. On day three, CPK levels steadily decreased to 778 ng/mL, and mPSL was reduced to 200 mg on day four of the treatment. In contrast, neurological symptoms poorly improved. PE was then added every other day for four days (Fig. 2), and the ptosis resolved. CPK decreased within normal limits. Four weeks after admission, CPK values rose again to 1014 ng/mL. Along with the normal ECG and troponin T value, this suggested that MG, not myocarditis, was flared. IVIg therapy was administered at a dose of 400 mg/kg for four days, normalizing CPK. Since then, steroids were gradually reduced. Six months after therapy, myocarditis and MG have not been reactivated despite the cessation of steroid, with partial response of metastatic lung lesions.
Fig. 2.
Treatment with changes in serum creatine phosphokinase levels during hospitalization.
Abbreviations: mPSL, methylprednisolone; PE, plasma exchange; IVIg, intravenous immunoglobulin; CPK, creatine phosphokinase.
Discussion
ICIs led to a wide range of irAEs not experienced with conventional tyrosine kinase or mTOR inhibitors. The frequency of myocarditis with Ipi/Nivo is less than 1%, but the mortality rate may reach up to 50%, especially when it is concomitant with MG.3 Although the guidelines for each irAE are still being established, these concomitant conditions have yet to be suggested. Therefore, an accumulation of these case reports is essential.
While a higher dose of initial mPSL cause less major adverse cardiac events in cases of myocarditis,4 it has a greater possibility of exacerbating MG. In our case, priority was given to treating myocarditis, which is more lethal. Because some reports showed delay or failure of myocarditis with a lower dose of mPSL,2 1000 mg of mPSL was administered on the first day of the onset. Although the acute exacerbation of MG after steroid administration has been described as transient, it occurs in 50% of patients including progression to respiratory failure.5 The use of PE or IVIg has led to favorable outcomes in most patients with severe MG, and their early use is recommended simultaneously with steroids to overcome the risk of a transient worsening, especially in patients with severe disease.5 Although our case did not lead to severe MG crisis, PE and IVIg were mandatory to manage MG.
Conclusion
We report a case of MG and myocarditis caused by Ipi/Nivo treatment for metastatic RCC. Although a high dose of steroids may worsen MG, the treatment priority should be placed on myocarditis, and mPSL pulse therapy should be administered.
Consent
Written informed consent was obtained from the patient for publication of this case report.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Acknowledgements
We would like to thank Editage (http://www.editage.com) for editing and reviewing this manuscript for English language.
References
- 1.Motzer J., Tannir N., McDermott D. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Salem J.E., Cohen J. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimura T., Fukushima S., Miyashita A. Myasthenic crisis and polymyositis induced by one dose of nivolumab. Canc Sci. 2016;107:30. doi: 10.1111/cas.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahmood S., Fradley M., Cohen J. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safa H., Johnson D.H., Tirnh V.A. Immune checkpoint inhibitor related myasthenia gravis: single center experience and systematic review of the literature. J Immunother Cancer. 2019;7:319. doi: 10.1186/s40425-019-0774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]