Abstract
Aim
This study aimed to evaluate the association between surgeons certified via the Endoscopic Surgical Skill Qualification System (ESSQS) of the Japan Society for Endoscopic Surgery (JSES) and surgical outcomes of laparoscopic distal gastrectomy (LDG) and laparoscopic low anterior resection (LLAR).
Methods
Japanese National Clinical Database data on the patients undergoing LDG and LLAR between 2014‐2016 were analyzed retrospectively. The proportion of cases performed by ESSQS‐certified surgeons was calculated for each procedure, and clinicopathological factors with or without participation of ESSQS‐certified surgeons as an operator were assessed. Then, effects of operations performed by ESSQS‐certified surgeons on short‐term patient outcomes were analyzed using generalized estimating equations logistic regression analysis.
Results
There were 110 610 and 65 717 patients who underwent LDG and LLAR, respectively. The operations performed by ESSQS‐certified surgeons in each procedure totaled 28 467 (35.3%) and 12 866 (31.2%), respectively. A multivariable logistic regression model showed that odds ratios of mortality for LDG and LLAR performed by ESSQS‐certified surgeons were 0.774 (95% CI, 0.566‐1.060, P = 0.108) and 0.977 (0.591‐1.301, P = 0.514), respectively. Odds ratios for secondary endpoints of anastomotic leakage in LDG and LLAR performed by ESSQS‐certified surgeons were 0.835 (95% CI, 0.723‐0.964, P = 0.014) and 0.929 (0.860‐1.003, P = 0.059), respectively, whereas that of ileus/bowel obstruction for LLAR performed by ESSQS‐certified surgeons was 1.265 (1.132‐1.415, P < 0.001). There were no significant associations between the two operations performed by ESSQS‐certified surgeons and other factors such as mortality and overall complications.
Conclusions
ESSQS certification did not affect postoperative mortality following LDG and LLAR, but annual experience of laparoscopic surgery was associated with it. ESSQS certification may contribute to favorable outcomes regarding anastomotic leakage following LDG and LLAR.
Keywords: endoscopic surgical skill qualification system, laparoscopic distal gastrectomy, laparoscopic low anterior resection, National Clinical Database, short‐term outcome
This study aimed to evaluate the association between surgeons certified via the Endoscopic Surgical Skill Qualification System (ESSQS) of the Japan Society for Endoscopic Surgery (JSES) and surgical outcomes of laparoscopic distal gastrectomy (LDG) and laparoscopic low anterior resection (LLAR). The ESSQS contributes to favorable outcomes regarding anastomotic leakage following LDG and LLAR.

1. INTRODUCTION
The use of laparoscopic surgery has continued to increase worldwide ever since laparoscopic distal gastrectomy (LDG) for gastric cancer was introduced in 1994. 1 As a result of previous large randomized studies, laparoscopic surgery has now become a standard surgical treatment for cStage I gastric cancer. 2 , 3 , 4 Based on other randomized clinical trials, as with LDG, laparoscopic low anterior resection (LLAR) has also spread worldwide. 5 , 6 , 7 The National Survey of Endoscopic Surgery conducted by the Japan Society for Endoscopic Surgery (JSES) found that more than 75% of the low anterior resections performed in Japan in 2017 were done laparoscopically. 8 It is noteworthy, however, that these important data on the safety and superiority of laparoscopic surgery were mainly obtained from high‐volume centers or hospitals specializing in gastric and rectal surgery. Thus, whether the usefulness and superiority of laparoscopic surgery for gastric and rectal cancer as indicated by these large studies is applicable in general clinical practice remains unclear. To maintain quality and educate trainers, JSES initiated the Endoscopic Surgical Skill Qualification System (ESSQS) in 2004. 9 , 10 , 11 A few small‐scale studies have shown better clinical outcomes when procedures were performed under the supervision of surgeons certified through the ESSQS by JSES. 12 , 13 , 14 However, few reports have evaluated the effectiveness and usefulness of ESSQS with large‐scale data.
The National Clinical Database (NCD) started as a nationwide registry system in 2011 in Japan. Its database has covered more than 95% of surgeries in Japan and has made it possible to investigate the impact of ESSQS on surgical outcomes.
Thus, in the present study, a large‐scale retrospective cohort analysis of patient data in the Japanese NCD database was performed to evaluate the effectiveness of ESSQS by JSES for LDG and LLAR, which have developed into common and standardized operations in terms of lymph node dissection and reconstruction.
2. METHODS
2.1. ESSQS by JSES
The JSES established the ESSQS and began certification examinations in 2004. 9 , 10 , 11 Two judges assessed unedited videotapes of a procedure in a double‐blinded fashion according to strict common and procedure‐specific criteria. The common criteria were designed to evaluate the surgical set‐up, operator autonomy, display of the surgical field, correct recognition of surgical anatomy, and surgical team co‐operation. Procedure‐specific criteria were used to assess the surgical procedure in a step‐by‐step manner. Of the 1114 surgeons assessed via this qualification system over a 4‐year period, 537 (48.2%) were accredited. To date, the rate of qualification in each surgical field continues to remain at a similar level. During the first year, the inter‐rater agreement of the two judges was low at 0.31, but with revisions of the criteria and the use of consensus meetings, agreement has improved. The surgeons assessed as being qualified by this system experience less frequent complications compared with those who failed the assessment. This system has positively improved and brought standardization to laparoscopic surgery in Japan. 10 , 11 , 12
2.2. NCD registration
Details of the registration of data in the Japanese NCD system were described previously. 15 , 16 Briefly, the NCD began as a nationwide registry system in Japan that was linked with the surgical board certification system in 2011. As of 2018, over 5000 institutions have participated in this system, with approximately 1 400 000 surgical cases being registered annually.
The Japanese Society of Gastroenterological Surgery (JSGS) designated eight main surgical procedures in the gastroenterological section of the NCD as being especially important in terms of medical standards for improving surgical quality: esophagectomy, distal gastrectomy, total gastrectomy, right hemicolectomy, low anterior resection, hepatectomy, pancreaticoduodenectomy, and surgery for acute diffuse peritonitis. All surgical cases are registered in the NCD, and details including morbidities, comorbidities, postoperative complications, and mortalities are input into the system.
2.3. Study population
In total, 110 610 cases of LDG and 65 717 cases of LLAR were registered in the NCD between 2014 and 2016. Among them, benign disease, malignant disease of organs other than the stomach and rectum, open surgery, and emergent surgeries were excluded from this study. Also excluded were 24 cases of LDG and 21 cases of LLAR with data deficits (Figure 1).
FIGURE 1.
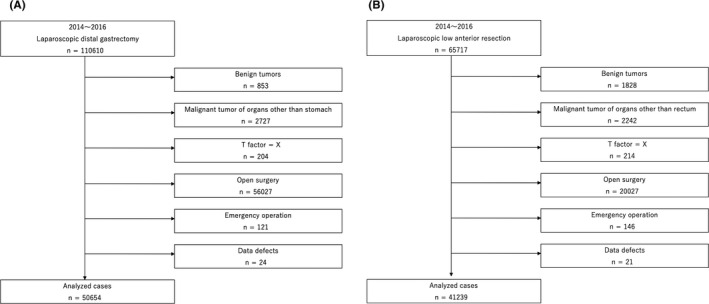
Flowchart detailing the patient selection process in laparoscopic distal gastrectomy (A) and laparoscopic low anterior resection (B)
2.4. Study endpoints
The primary endpoint of the study was mortality (within 30 days after surgery or within 90 days in hospital, respectively). The secondary endpoints included overall postoperative complications (surgical complications: surgical site infection, anastomotic leakage, and pancreatic fistula in LDG; surgical complications: surgical site infection, anastomotic leakage, functional ileus/bowel obstruction, and ileus/bowel obstruction in LLAR; and non‐surgical complications: pneumonia, pulmonary embolism, and urinary tract infection). In particular, anastomotic leakage, pancreatic fistula (Grade B/C), and ileus/bowel obstruction were evaluated as independent complications. An additional endpoint of the study was the incidence of overall postoperative morbidity of ≥grade III according to the Clavien‐Dindo classification, 17 except for pancreatic fistula. Pancreatic fistula was defined according to the ISGPF (International Study Group of Postoperative Pancreatic Fistula). We also assessed the association of the operations performed by ESSQS‐certified surgeons and laparoscopic operation volumes per year with postoperative complications. To determine a trend in operation volumes in terms of various outcomes, we used cut‐off values that divided the study population into three parts, and we created three groups. Finally, the impact of the operations performed by ESSQS‐certified surgeons on postoperative complications was evaluated by multivariable regression modeling.
2.5. Statistical analysis
We used the χ2 test for statistical comparisons between groups. We accounted for clustering at the hospital level by use of generalized estimating equations logistic regression analysis for multivariable analysis of the impact of each clinical factor on the short‐term outcomes. The clinical factors included age at surgery (<65, 65 −75, >75 years), sex (male vs female), body mass index (BMI; <18.5, 18.5 < BMI ≤ 25, ≥25 kg/m2), preoperative chemotherapy (yes vs no), diabetes mellitus (yes vs no), smoking history (Brinkman index: 0, <400, ≥400), habitual alcohol intake (yes vs no), activities of daily living (no vs any assistance), chronic obstructive pulmonary disease (yes vs no), hypertension (yes vs no), ischemic heart disease (yes vs no), need for preoperative dialysis (yes vs no), previous cerebrovascular disease (yes vs no), chronic steroid use (yes vs no), weight loss (yes vs no), bleeding disorder (yes vs no), preoperative blood transfusion (yes vs no), American Society of Anesthesiologists physical status (ASA‐PS: 1, 2, 3‐5), clinical T stage (T0, Tis, T1a, T1b, T2, T3, T4a, T4b), clinical N stage (N0, N1, N2, N3a, N3b, NX), and clinical M stage (M0, M1). The 7th edition of the American Joint Committee on Cancer TNM classification was used to extract representative tumor depth (T), node metastasis (N), and distant metastasis (M). Additionally, a comparative analysis was conducted between groups in terms of operation time, intraoperative blood loss, and the necessity of transfusion. A two‐sided probability level < 0.05 was considered to indicate a significant difference. The software package R version 3.6.0 (2019; R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analysis. The study protocol was approved by the institutional review board of Oita University (approval number B190275).
3. RESULTS
3.1. Patient characteristics
Patient and tumor characteristics are shown in Tables 1A and 1B. There were 110 610 and 65 717 patients who underwent LDG and LLAR, respectively. The proportions of participation of ESSQS‐certified surgeons as the operator were 15 341 (30.3%) and 12 866 (31.2%), respectively (Table 1A: Laparoscopic distal gastrectomy).
TABLE 1A.
Background of patients with laparoscopic distal gastrectomy
| Overall | ESSQS (−) | ESSQS (+) | P | |
|---|---|---|---|---|
| n | 50654 | 35313 | 15341 | |
| Age, years (median [IQR]) | 69.00 [62.00, 76.00] | 70.00 [63.00, 77.00] | 69.00 [62.00, 76.00] | <0.001 |
| Age category, years (%) | ||||
| <65 | 15975 (31.5) | 10858 (30.7) | 5117 (33.4) | <0.001 |
| 65‐75 | 20523 (40.5) | 14360 (40.7) | 6163 (40.2) | |
| >75 | 14156 (27.9) | 10095 (28.6) | 4061 (26.5) | |
| Sex (%) | ||||
| Female | 17621 (34.8) | 12321 (34.9) | 5300 (34.5) | 0.463 |
| Male | 33033 (65.2) | 22992 (65.1) | 10041 (65.5) | |
| Body mass index (%) | ||||
| Normal (18.5 ≤ BMI < 25) | 34830 (68.8) | 24457 (69.3) | 10373 (67.6) | <0.001 |
| Underweight (18.5 > BMI) | 5183 (10.2) | 3733 (10.6) | 1450 (9.5) | |
| Overweight (25 ≤ BMI) | 10641 (21.0) | 7123 (20.2) | 3518 (22.9) | |
| Preoperative chemotherapy (%) | ||||
| No | 50148 (99.0) | 35029 (99.2) | 15119 (98.6) | <0.001 |
| Yes | 506 (1.0) | 284 (0.8) | 222 (1.4) | |
| Diabetes mellitus (%) | ||||
| No | 42158 (83.2) | 29333 (83.1) | 12825 (83.6) | 0.143 |
| Yes | 8496 (16.8) | 5980 (16.9) | 2516 (16.4) | |
| Brinkman index (%) | ||||
| 0 | 27198 (53.7) | 19021 (53.9) | 8177 (53.3) | 0.506 |
| <400 | 6778 (13.4) | 4708 (13.3) | 2070 (13.5) | |
| ≥400 | 16678 (32.9) | 11584 (32.8) | 5094 (33.2) | |
| Habitual alcohol intake | ||||
| No | 23238 (45.9) | 16297 (46.2) | 6941 (45.2) | 0.062 |
| Yes | 27416 (54.1) | 19016 (53.8) | 8400 (54.8) | |
| COPD (%) | ||||
| No | 48228 (95.2) | 33717 (95.5) | 14511 (94.6) | <0.001 |
| Yes | 2426 (4.8) | 1596 (4.5) | 830 (5.4) | |
| Hypertension (%) | ||||
| No | 31134 (61.5) | 21636 (61.3) | 9498 (61.9) | 0.175 |
| Yes | 19520 (38.5) | 13677 (38.7) | 5843 (38.1) | |
| Ischemic heart disease (%) | ||||
| No | 49024 (96.8) | 34146 (96.7) | 14878 (97.0) | 0.098 |
| Yes | 1630 (3.2) | 1167 (3.3) | 463 (3.0) | |
| Dialysis (%) | ||||
| No | 50356 (99.4) | 35105 (99.4) | 15251 (99.4) | 1 |
| Yes | 298 (0.6) | 208 (0.6) | 90 (0.6) | |
| Cerebrovascular disease (%) | ||||
| No | 49259 (97.2) | 34313 (97.2) | 14946 (97.4) | 0.111 |
| Yes | 1395 (2.8) | 1000 (2.8) | 395 (2.6) | |
| Steroid (%) | ||||
| No | 50162 (99.0) | 34962 (99.0) | 15200 (99.1) | 0.459 |
| Yes | 492 (1.0) | 351 (1.0) | 141 (0.9) | |
| Weight loss (%) | ||||
| No | 49781 (98.3) | 34690 (98.2) | 15091 (98.4) | 0.302 |
| Yes | 873 (1.7) | 623 (1.8) | 250 (1.6) | |
| Bleeding disorder (%) | ||||
| No | 48819 (96.4) | 34041 (96.4) | 14778 (96.3) | 0.727 |
| Yes | 1835 (3.6) | 1272 (3.6) | 563 (3.7) | |
| Preoperative transfusion (%) | ||||
| No | 50302 (99.3) | 35064 (99.3) | 15238 (99.3) | 0.718 |
| Yes | 352 (0.7) | 249 (0.7) | 103 (0.7) | |
| ASA‐PS (%) | ||||
| 1 | 14865 (29.3) | 10579 (30.0) | 4286 (27.9) | <0.001 |
| 2 | 31636 (62.5) | 21775 (61.7) | 9861 (64.3) | |
| 3‐5 | 4153 (8.2) | 2959 (8.4) | 1194 (7.8) | |
| T factor (%) | ||||
| T0 | 211 (0.4) | 140 (0.4) | 71 (0.5) | <0.001 |
| T1a | 15130 (29.9) | 11040 (31.3) | 4090 (26.7) | |
| T1b | 22447 (44.3) | 15906 (45.0) | 6541 (42.6) | |
| T2 | 5765 (11.4) | 3796 (10.7) | 1969 (12.8) | |
| T3 | 4069 (8.0) | 2563 (7.3) | 1506 (9.8) | |
| T4a | 2464 (4.9) | 1474 (4.2) | 990 (6.5) | |
| T4b | 199 (0.4) | 114 (0.3) | 85 (0.6) | |
| Tis | 369 (0.7) | 280 (0.8) | 89 (0.6) | |
| N factor (%) | ||||
| N0 | 41824 (82.6) | 29641 (83.9) | 12183 (79.4) | <0.001 |
| N1 | 4553 (9.0) | 2998 (8.5) | 1555 (10.1) | |
| N2 | 2487 (4.9) | 1561 (4.4) | 926 (6.0) | |
| N3a 7 | 1291 (2.5) | 794 (2.2) | 497 (3.2) | |
| N3b | 432 (0.9) | 268 (0.8) | 164 (1.1) | |
| NX | 67 (0.1) | 51 (0.1) | 16 (0.1) | |
| M factor (%) | ||||
| M0 | 50118 (98.9) | 34977 (99.0) | 15141 (98.7) | <0.001 |
| M1 | 536 (1.1) | 336 (1.0) | 200 (1.3) | |
| Participation of ESSQS‐certified surgeons (%) | ||||
| No | 35313 (69.7) | — | — | |
| Yes | 15341 (30.3) | — | — | |
| Participation of JSGS‐certified surgeons (%) | ||||
| No | 22187 (43.8) | 19751 (55.9) | 2436 (15.9) | <0.001 |
| Yes | 28467 (56.2) | 15562 (44.1) | 12905 (84.1) | |
| Laparoscopic operation volume (median [IQR]) | 26.00 [13.00, 48.00] | 21.00 [11.00, 41.00] | 40.00 [20.00, 66.00] | <0.001 |
| C1 (1‐17 cases) | 18083 (35.7) | 14894 (42.2) | 3189 (20.8) | <0.001 |
| C2 (18‐41 cases) | 16386 (32.3) | 11658 (33.0) | 4728 (30.8) | |
| C3 (≥42 cases) | 16185 (32.0) | 8761 (24.8) | 7424 (48.4) | |
Abbreviations: ASA‐PS, American Society of Anesthesiologists physical status; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ESSQS, Endoscopic Surgical Skill Qualification System; IQR, interquartile range; JSGS, Japanese Society of Gastroenterological Surgery.
TABLE 1B.
Background of patients with laparoscopic low anterior resection
| Overall | ESSQS (−) | ESSQS (+) | P | |
|---|---|---|---|---|
| n | 41239 | 28373 | 12866 | |
| Age, yrs (median [IQR]) | 67.00 [60.00, 74.00] | 68.00 [61.00, 75.00] | 66.00 [58.00, 73.00] | <0.001 |
| Age category, yrs (%) | ||||
| <65 | 15986 (38.8) | 10433 (36.8) | 5553 (43.2) | <0.001 |
| 65‐75 | 16577 (40.2) | 11565 (40.8) | 5012 (39.0) | |
| >75 | 8676 (21.0) | 6375 (22.5) | 2301 (17.9) | |
| Sex (%) | ||||
| Male | 14179 (34.4) | 9781 (34.5) | 4398 (34.2) | 0.574 |
| Female | 27060 (65.6) | 18592 (65.5) | 8468 (65.8) | |
| BMI (%) | ||||
| Normal (18.5 ≤ BM I < 25) | 27539 (66.8) | 18968 (66.9) | 8571 (66.6) | 0.074 |
| Underweight (18.5> BMI) | 4326 (10.5) | 3026 (10.7) | 1300 (10.1) | |
| Overweight (25 ≤ BMI) | 9374 (22.7) | 6379 (22.5) | 2995 (23.3) | |
| Preoperative chemotherapy (%) | ||||
| No | 38398 (93.1) | 26959 (95.0) | 11439 (88.9) | <0.001 |
| Yes | 2841 (6.9) | 1414 (5.0) | 1427 (11.1) | |
| Diabetes mellitus (%) | ||||
| No | 33825 (82.0) | 23149 (81.6) | 10676 (83.0) | 0.001 |
| Yes | 7414 (18.0) | 5224 (18.4) | 2190 (17.0) | |
| Brinkman index (%) | ||||
| 0 | 22804 (55.3) | 16011 (56.4) | 6793 (52.8) | <0.001 |
| <400 | 5568 (13.5) | 3645 (12.8) | 1923 (14.9) | |
| ≥400 | 12867 (31.2) | 8717 (30.7) | 4150 (32.3) | |
| Habitual alcohol intake | ||||
| No | 18825 (45.6) | 13081 (46.1) | 5744 (44.6) | 0.006 |
| Yes | 22414 (54.4) | 15292 (53.9) | 7122 (55.4) | |
| COPD (%) | ||||
| No | 39744 (96.4) | 27343 (96.4) | 12401 (96.4) | 0.958 |
| Yes | 1495 (3.6) | 1030 (3.6) | 465 (3.6) | |
| Hypertension (%) | ||||
| No | 26344 (63.9) | 17902 (63.1) | 8442 (65.6) | <0.001 |
| Yes | 14895 (36.1) | 10471 (36.9) | 4424 (34.4) | |
| Ischemic heart disease (%) | ||||
| No | 40063 (97.1) | 27538 (97.1) | 12525 (97.3) | 0.105 |
| Yes | 1176 (2.9) | 835 (2.9) | 341 (2.7) | |
| Dialysis (%) | ||||
| No | 41061 (99.6) | 28237 (99.5) | 12824 (99.7) | 0.035 |
| Yes | 178 (0.4) | 136 (0.5) | 42 (0.3) | |
| Cerebrovascular disease (%) | ||||
| No | 40153 (97.4) | 27558 (97.1) | 12595 (97.9) | <0.001 |
| Yes | 1086 (2.6) | 815 (2.9) | 271 (2.1) | |
| Steroid (%) | ||||
| No | 40891 (99.2) | 28114 (99.1) | 12777 (99.3) | 0.027 |
| Yes | 348 (0.8) | 259 (0.9) | 89 (0.7) | |
| Weight loss (%) | ||||
| No | 40376 (97.9) | 27767 (97.9) | 12609 (98.0) | 0.383 |
| Yes | 863 (2.1) | 606 (2.1) | 257 (2.0) | |
| Bleeding disorder (%) | ||||
| No | 39911 (96.8) | 27447 (96.7) | 12464 (96.9) | 0.477 |
| Yes | 1328 (3.2) | 926 (3.3) | 402 (3.1) | |
| Preoperative transfusion (%) | ||||
| No | 40969 (99.3) | 28153 (99.2) | 12816 (99.6) | <0.001 |
| Yes | 270 (0.7) | 220 (0.8) | 50 (0.4) | |
| ASA‐PS (%) | ||||
| 1 | 12461 (30.2) | 8606 (30.3) | 3855 (30.0) | <0.001 |
| 2 | 25298 (61.3) | 17201 (60.6) | 8097 (62.9) | |
| 3‐5 | 3480 (8.4) | 2566 (9.0) | 914 (7.1) | |
| T factor (%) | ||||
| T0 | 217 (0.5) | 136 (0.5) | 81 (0.6) | <0.001 |
| T1a | 8024 (19.5) | 5492 (19.4) | 2532 (19.7) | |
| T1b | 8405 (20.4) | 5793 (20.4) | 2612 (20.3) | |
| T2 | 18848 (45.7) | 12908 (45.5) | 5940 (46.2) | |
| T3 | 4075 (9.9) | 2861 (10.1) | 1214 (9.4) | |
| T4a | 859 (2.1) | 544 (1.9) | 315 (2.4) | |
| T4b | 811 (2.0) | 639 (2.3) | 172 (1.3) | |
| Tis | 25662 (62.2) | 17691 (62.4) | 7971 (62.0) | 0.042 |
| N factor (%) | ||||
| N0 | 5880 (14.3) | 4094 (14.4) | 1786 (13.9) | |
| N1 | 4461 (10.8) | 3073 (10.8) | 1388 (10.8) | |
| N2 | 122 (0.3) | 79 (0.3) | 43 (0.3) | |
| N3a 7 | 3279 (8.0) | 2234 (7.9) | 1045 (8.1) | |
| N3b | 1780 (4.3) | 1165 (4.1) | 615 (4.8) | |
| NX | 55 (0.1) | 37 (0.1) | 18 (0.1) | |
| M factor (%) | ||||
| M0 | 38322 (92.9) | 26404 (93.1) | 11918 (92.6) | 0.145 |
| M1a | 2292 (5.6) | 1535 (5.4) | 757 (5.9) | |
| M1b | 625 (1.5) | 434 (1.5) | 191 (1.5) | |
| Performance by ESSQS‐certified surgeons (%) | ||||
| No | 28373 (68.8) | — | — | |
| Yes | 12866 (31.2) | — | — | |
| Performance by JSGS‐certified surgeons (%) | ||||
| No | 18720 (45.4) | 16823 (59.3) | 1897 (14.7) | <0.001 |
| Yes | 22519 (54.6) | 11550 (40.7) | 10969 (85.3) | |
| Laparoscopic operation volume (median [IQR]) | 18.00 [10.00, 32.00] | 15.00 [8.00, 26.00] | 28.00 [17.00, 50.00] | <0.001 |
| C1 (1‐5 cases) | 12609 (30.6) | 10759 (37.9) | 1850 (14.4) | <0.001 |
| C2 (6‐11 cases) | 12450 (30.2) | 9347 (32.9) | 3103 (24.1) | |
| C3 (≥12 cases‐) | 16180 (39.2) | 8267 (29.1) | 7913 (61.5) | |
Abbreviations: ASA‐PS, American Society of Anesthesiologists physical status; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ESSQS, Endoscopic Surgical Skill Qualification System; IQR, interquartile range; JSGS, Japanese Society of Gastroenterological Surgery.
LDG performed by the ESSQS‐certified surgeons group tended to include a higher proportion of patients with high BMI, preoperative chemotherapy, advanced clinical disease stage including clinical T, N, and M, operations performed by JSGS‐certified surgeons, and laparoscopic operation volumes per year (>42 cases). However, the proportion of patients with chronic obstructive pulmonary disease was lower in the non‐ESSQS‐certified surgeons group. In the ESSQS‐certified surgeons group, the proportion of patients with PS 2 was higher and the proportions of patients with PS 1 and PS 3‐5 were lower than those in the non‐ESSQS‐certified surgeons group (Table 1B: Laparoscopic low anterior resection).
LLAR performed by ESSQS‐certified surgeons tended to include a higher proportion of patients with preoperative chemotherapy, high Brinkman index, habitual alcohol intake, advanced clinical disease stage including clinical T and N, operations performed by JSGS‐certified surgeons, and laparoscopic operation volumes per year (>12 cases). However, diabetes mellitus, hypertension, dialysis, cerebrovascular disease, and preoperative transfusion were lower in the patients undergoing surgery performed by the ESSQS‐certified surgeons group than those by the non‐ESSQS‐certified surgeons group. The proportion of patients with PS 2 was higher and the proportions of patients with PS 1 and PS 3‐5 were lower in the ESSQS‐certified surgeons group than those in the non‐ESSQS‐certified surgeons group.
3.2. Surgical outcomes
The operative outcomes are shown in Tables 2A and 2B (Table 2A: Laparoscopic distal gastrectomy).
TABLE 2A.
Surgical outcomes of laparoscopic distal gastrectomy
| Overall | ESSQS (−) | ESSQS (+) | P | |
|---|---|---|---|---|
| n | 50654 | 35313 | 15341 | |
| Operation time, min (median [IQR]) | 289.00 [239.00, 348.00] | 293.00 [243.00, 352.00] | 279.00 [230.00, 336.00] | <0.001 |
| Intraoperative blood loss, mL (median [IQR]) | 33.00 [10.00, 100.00] | 40.00 [10.00, 104.00] | 25.00 [5.00, 70.00] | <0.001 |
| Intraoperative transfusion (%) | ||||
| (−) | 49527 (97.8) | 34464 (97.6) | 15063 (98.2) | <0.001 |
| (+) | 1127 (2.2) | 849 (2.4) | 278 (1.8) | |
| Overall complications (%) | ||||
| (−) | 47255 (93.3) | 32985 (93.4) | 14270 (93.0) | 0.112 |
| (+) | 3399 (6.7) | 2328 (6.6) | 1071 (7.0) | |
| Surgical complication (%) | ||||
| (−) | 47907 (94.6) | 33448 (94.7) | 14459 (94.3) | 0.034 |
| (+) | 2747 (5.4) | 1865 (5.3) | 882 (5.7) | |
| Non‐surgical complication (%) | ||||
| (−) | 49802 (98.3) | 34712 (98.3) | 15090 (98.4) | 0.623 |
| (+) | 852 (1.7) | 601 (1.7) | 251 (1.6) | |
| Superficial incisional SSI (%) | ||||
| (−) | 50130 (99.0) | 34933 (98.9) | 15197 (99.1) | 0.175 |
| (+) | 524 (1.0) | 380 (1.1) | 144 (0.9) | |
| Deep incisional SSI (%) | ||||
| (−) | 50483 (99.7) | 35199 (99.7) | 15284 (99.6) | 0.432 |
| (+) | 171 (0.3) | 114 (0.3) | 57 (0.4) | |
| Organ/space SSI (%) | ||||
| (−) | 49545 (97.8) | 34561 (97.9) | 14984 (97.7) | 0.173 |
| (+) | 1109 (2.2) | 752 (2.1) | 357 (2.3) | |
| Anastomotic leakage (%) | ||||
| (−) | 49692 (98.1) | 34609 (98.0) | 15083 (98.3) | 0.02 |
| (+) | 962 (1.9) | 704 (2.0) | 258 (1.7) | |
| Pancreatic fistula (%) | ||||
| (−) | 49875 (98.5) | 34801 (98.6) | 15074 (98.3) | 0.016 |
| (+) | 779 (1.5) | 512 (1.4) | 267 (1.7) | |
| Ileus (%) | ||||
| (−) | 50402 (99.5) | 35146 (99.5) | 15256 (99.4) | 0.261 |
| (+) | 252 (0.5) | 167 (0.5) | 85 (0.6) | |
| Bowel obstruction (%) | ||||
| (−) | 50557 (99.8) | 35248 (99.8) | 15309 (99.8) | 0.639 |
| (+) | 97 (0.2) | 65 (0.2) | 32 (0.2) | |
| Pneumonia (%) | ||||
| (−) | 49963 (98.6) | 34810 (98.6) | 15153 (98.8) | 0.083 |
| (+) | 691 (1.4) | 503 (1.4) | 188 (1.2) | |
| Pulmonary embolism (%) | ||||
| (−) | 50621 (99.9) | 35293 (99.9) | 15328 (99.9) | 0.342 |
| (+) | 33 (0.1) | 20 (0.1) | 13 (0.1) | |
| Urinary tract infection (%) | ||||
| (−) | 50504 (99.7) | 35217 (99.7) | 15287 (99.6) | 0.151 |
| (+) | 150 (0.3) | 96 (0.3) | 54 (0.4) | |
| Mortality (%) | ||||
| (−) | 50439 (99.6) | 35154 (99.5) | 15285 (99.6) | 0.2 |
| (+) | 215 (0.4) | 159 (0.5) | 56 (0.4) | |
Abbreviations: IQR, interquartile range; SSI, surgical site infection.
TABLE 2B.
Surgical outcomes of laparoscopic low anterior resection
| Overall | ESSQS (−) | ESSQS (+) | P | |
|---|---|---|---|---|
| n | 41239 | 28373 | 12866 | |
| Operation time, min (median [IQR]) | 283.00 [224.00, 360.00] | 286.00 [227.00, 361.00] | 277.00 [217.00, 360.00] | <0.001 |
| Intraoperative blood loss, mL (median [IQR]) | 30.00 [6.00, 100.00] | 30.00 [8.00, 100.00] | 20.00 [5.00, 75.00] | <0.001 |
| Intraoperative transfusion (%) | ||||
| (−) | 40095 (97.2) | 27510 (97.0) | 12585 (97.8) | <0.001 |
| (+) | 1144 (2.8) | 863 (3.0) | 281 (2.2) | |
| Overall complications (%) | ||||
| (−) | 35290 (85.6) | 24362 (85.9) | 10928 (84.9) | 0.014 |
| (+) | 5949 (14.4) | 4011 (14.1) | 1938 (15.1) | |
| Surgical complication (%) | ||||
| (−) | 35691 (86.5) | 24607 (86.7) | 11084 (86.1) | 0.115 |
| (+) | 5548 (13.5) | 3766 (13.3) | 1782 (13.9) | |
| Non‐surgical complication (%) | ||||
| (−) | 40551 (98.3) | 27929 (98.4) | 12622 (98.1) | 0.017 |
| (+) | 688 (1.7) | 444 (1.6) | 244 (1.9) | |
| Superficial incisional SSI (%) | ||||
| (−) | 40372 (97.9) | 27757 (97.8) | 12615 (98.0) | 0.159 |
| (+) | 867 (2.1) | 616 (2.2) | 251 (2.0) | |
| Deep incisional SSI (%) | ||||
| (−) | 40890 (99.2) | 28111 (99.1) | 12779 (99.3) | 0.013 |
| (+) | 349 (0.8) | 262 (0.9) | 87 (0.7) | |
| Organ/space SSI (%) | ||||
| (−) | 38906 (94.3) | 26737 (94.2) | 12169 (94.6) | 0.162 |
| (+) | 2333 (5.7) | 1636 (5.8) | 697 (5.4) | |
| Anastomotic leakage (%) | ||||
| (−) | 37697 (91.4) | 25901 (91.3) | 11796 (91.7) | 0.19 |
| (+) | 3542 (8.6) | 2472 (8.7) | 1070 (8.3) | |
| Ileus (%) | ||||
| (−) | 40175 (97.4) | 27719 (97.7) | 12456 (96.8) | <0.001 |
| (+) | 1064 (2.6) | 654 (2.3) | 410 (3.2) | |
| Bowel obstruction (%) | ||||
| (−) | 40833 (99.0) | 28098 (99.0) | 12735 (99.0) | 0.68 |
| (+) | 406 (1.0) | 275 (1.0) | 131 (1.0) | |
| Pneumonia (%) | ||||
| (−) | 40986 (99.4) | 28178 (99.3) | 12808 (99.5) | 0.005 |
| (+) | 253 (0.6) | 195 (0.7) | 58 (0.5) | |
| Pulmonary embolism (%) | ||||
| (−) | 41195 (99.9) | 28346 (99.9) | 12849 (99.9) | 0.367 |
| (+) | 44 (0.1) | 27 (0.1) | 17 (0.1) | |
| Urinary tract infection (%) | ||||
| (−) | 40818 (99.0) | 28128 (99.1) | 12690 (98.6) | <0.001 |
| (+) | 421 (1.0) | 245 (0.9) | 176 (1.4) | |
| Mortality (%) | ||||
| (−) | 41107 (99.7) | 28274 (99.7) | 12833 (99.7) | 0.148 |
| (+) | 132 (0.3) | 99 (0.3) | 33 (0.3) | |
Abbreviations: IQR, interquartile range; SSI, surgical site infection.
Operation time, intraoperative blood loss, amount of intraoperative transfusion, and occurrence of anastomotic leakage were significantly lower in the operations performed by ESSQS‐certified surgeons than in those performed by non‐ESSQS‐certified surgeons. The incidences of the surgical complication and pancreatic fistula were significantly more frequent in the operations performed by ESSQS‐certified surgeons than in those performed by non‐ESSQS‐certified surgeons. Mortality was not significantly different between the ESSQ‐ and non‐ESSQ‐certified surgeons groups (0.4% vs 0.5%, P = 0.2) (Table 2B: Laparoscopic low anterior resection).
Operation time, intraoperative blood loss, amount of intraoperative transfusion, deep incisional surgical site infection, and pneumonia were significantly lower in the operations performed by ESSQS‐certified surgeons than in those performed by non‐ESSQS‐certified surgeons. In contrast, the incidences of overall complications, non‐surgical complications, ileus/bowel obstruction, and urinary tract infection were significantly more frequent in the operations performed by the ESSQS‐certified surgeons than in those performed by the non‐ESSQS‐certified surgeons. Mortality was not significantly different between the ESSQ‐ and non‐ESSQ‐certified surgeons groups (0.3% vs 0.3%, P = 0.148).
3.3. Association between the operation performed by ESSQS‐certified surgeons and postoperative complications
(Table 3A: Laparoscopic distal gastrectomy). The multivariable logistic regression model showed that the operations performed by ESSQS‐certified surgeons were an independent predictor of anastomotic leakage. The odds ratio (OR) of anastomotic leakage for the operations performed by the ESSQS‐certified surgeons was 0.835 (95% confidence interval [CI], 0.723‐0.964, P = 0.014). There was no correlation between the operations performed by ESSQS‐certified surgeons and mortality. The regression analysis showed that a higher laparoscopic operation volume per year (>42) was an independent predictor of mortality, overall complications, and anastomotic leakage. The ORs of mortality and anastomotic leakage for the higher laparoscopic operation volume per year (>42) group were 0.406 (95% CI, 0.280‐0.588, P < 0.001) and 0.777 (95% CI, 0.659‐0.915, P = 0.003), respectively. In contrast, the ORs of overall complications and pancreatic fistula for the higher laparoscopic operation volume per year (>42) group were 1.184 (95% CI, 1.084‐1.292, P < 0.001) and 1.534 (95% CI, 1.278‐1.840, P < 0.001), respectively (Table 3B: Laparoscopic low anterior resection).
TABLE 3A.
Multivariable analysis of surgical outcomes of laparoscopic distal gastrectomy
| OR | 2.5% | 97.5% | P | |
|---|---|---|---|---|
| ESSQS +/− | ||||
| Mortality (within 30 d after surgery and within 90 d in hospital) | ||||
| ESSQS (−) | Reference | |||
| ESSQS (+) | 0.774 | 0.566 | 1.060 | 0.108 |
| Overall complications | ||||
| ESSQS (−) | Reference | |||
| ESSQS (+) | 1.042 | 0.967 | 1.122 | 0.283 |
| Pancreatic fistula | ||||
| ESSQS (−) | Reference | |||
| ESSQS (+) | 1.141 | 0.979 | 1.330 | 0.091 |
| Anastomotic leakage | ||||
| ESSQS (−) | Reference | |||
| ESSQS (+) | 0.835 | 0.723 | 0.964 | 0.014 |
| Laparoscopic operation volume per year | ||||
| Mortality (within 30 d after surgery and within 90 d in hospital) | ||||
| C1 (1‐17 cases) | Reference | |||
| C2 (18‐41 cases) | 0.587 | 0.428 | 0.806 | 0.001 |
| C3 (≥42 cases) | 0.406 | 0.280 | 0.588 | 0.000 |
| Overall complications | ||||
| C1 (1‐17 cases) | Reference | |||
| C2 (18‐41 cases) | 1.024 | 0.939 | 1.118 | 0.591 |
| C3 (≥42 cases) | 1.184 | 1.084 | 1.292 | <0.001 |
| Pancreatic fistula | ||||
| C1 (1‐17 cases) | Reference | |||
| C2 (18‐41 cases) | 1.291 | 1.072 | 1.555 | 0.007 |
| C3 (≥42 cases) | 1.534 | 1.278 | 1.840 | <0.001 |
| Anastomotic leakage | ||||
| C1 (1‐17 cases) | Reference | |||
| C2 (18‐41 cases) | 0.911 | 0.781 | 1.061 | 0.231 |
| C3 (≥42 cases) | 0.777 | 0.659 | 0.915 | 0.003 |
All models are risk adjusted.
Abbreviation: OR, odds ratio.
TABLE 3B.
Multivariable analysis of surgical outcomes of laparoscopic low anterior resection
| OR | 2.5% | 97.5% | P | |
|---|---|---|---|---|
| ESSQS +/− | ||||
| Mortality (within 30 d after surgery and within 90 d in hospital) | ||||
| ESSQS (−) | Reference | |||
| ESSQS (+) | 0.877 | 0.591 | 1.301 | 0.514 |
| Overall complication | ||||
| ESSQS (−) | Reference | |||
| ESSQS (+) | 1.028 | 0.969 | 1.092 | 0.362 |
| Anastomotic leakage | ||||
| ESSQS (−) | Reference | |||
| ESSQS (+) | 0.929 | 0.860 | 1.003 | 0.059 |
| Ileus/bowel obstruction | ||||
| ESSQS (−) | Reference | |||
| ESSQS (+) | 1.265 | 1.132 | 1.415 | <0.001 |
| Laparoscopic operation volume per year | ||||
| Mortality (within 30 d after surgery and within 90 d in hospital) | ||||
| C1 (1‐5 cases) | Reference | |||
| C2 (6‐11 cases) | 0.762 | 0.512 | 1.130 | 0.180 |
| C3 (≥12 cases) | 0.344 | 0.209 | 0.565 | <0.001 |
| Overall complication | ||||
| C1 (1‐5 cases) | Reference | |||
| C2 (6‐11 cases) | 1.089 | 1.012 | 1.172 | 0.023 |
| C3 (≥12 cases) | 1.113 | 1.037 | 1.195 | 0.003 |
| Anastomotic leakage | ||||
| C1 (1‐5 cases) | Reference | |||
| C2 (6‐11 cases) | 0.994 | 0.909 | 1.087 | 0.893 |
| C3 (≥12 cases) | 0.921 | 0.844 | 1.006 | 0.066 |
| Ileus/bowel obstruction | ||||
| C1 (1‐5 cases) | Reference | |||
| C2 (6‐11 cases) | 1.214 | 1.050 | 1.403 | 0.009 |
| C3 (≥12 cases) | 1.484 | 1.293 | 1.702 | <0.001 |
All models are risk adjusted.
Abbreviation: OR, odds ratio.
The multivariable logistic regression model indicated that the operations performed by ESSQS‐certified surgeons showed a tendency for a lower incidence of anastomotic leakage. The OR of anastomotic leakage for the operations performed by ESSQS‐certified surgeons was 0.929 (95% CI, 0.860‐1.003, P = 0.059), whereas that of ileus/bowel obstruction was 1.265 (95% CI, 1.132‐1.415, P < 0.001). There was no correlation between the operations performed by ESSQS‐certified surgeons and mortality. The regression analysis showed that a higher laparoscopic operation volume per year (>12) was an independent predictor of mortality and anastomotic leakage. The ORs of mortality and anastomotic leakage for the higher laparoscopic operation volume per year (>12) group were 0.344 (95% CI, 0.209‐0.565, P < 0.001) and 0.921 (95% CI, 0.844‐1.006, P = 0.066), respectively. In contrast, the ORs of overall complications and ileus/bowel obstruction for the higher laparoscopic operation volume per year (>12) group were 1.113 (95% CI, 1.037‐1.195, P = 0.003) and 1.484 (95% CI, 1.293‐1.702, P < 0.001), respectively. There was a tendency for the level of each OR to be similar between the ESSQS‐certified surgeons group and the laparoscopic operation volume per year (>12) group.
4. DISCUSSION
To the best of our knowledge, the present study is the first and largest to investigate the effectiveness of ESSQS for LDG and LLAR through a large‐scale retrospective cohort analysis of patient data in the Japanese NCD. Although the present study did not reveal a contribution of ESSQS to lower mortality, it did show that ESSQS might contribute to a favorable outcome in terms of anastomotic leakage following LDG and LLAR. In terms of the primary endpoint of the present study, the mortality from LDG and LLAR was not significantly affected regardless of whether non‐ESSQ‐ or ESSQ‐certified surgeons performed the procedures. We speculated that the very low mortality rate for LDG and LLAR might have obscured the impact of ESSQ between the two procedures. Although the P value for the incidence of anastomotic leakage with LLAR in the ESSQS‐certified surgeons group was 0.059 by multivariate analysis, we thought that at least the ESSQS‐certified surgeons group showed a tendency for a lower incidence of anastomotic leakage, which was considered a meaningful indicator clinically.
In the 8‐year period since the NCD was initiated in 2011, over 5 million cases from more than 4200 facilities have been registered. The efforts of Japanese surgeons have clearly resulted in the successful establishment of this nationwide surgical database. Risk models for 30‐day and operative mortality for several procedures were created, and outcomes for each procedure have been retrospectively published. 18 , 19 Additionally, Kanaji et al reported the high quality of the NCD database for gastroenterological surgery in 2019. 20 During the first audit of the 2015 data, verification of case registration accuracy undertaken on 338 patients (99.4% of extracted cases) showed data accuracy with the maximum number of postoperative variables to be> 95%. The accuracy of mortality and patient status 30 days postoperatively was similarly high at> 99%. The sensitivity was 1.00, and the specificity was 1.00. These results indicated the high accuracy of data entry in the gastroenterological section of the NCD. We analyzed NCD data from more than 250 000 patients registered from 2014 through 2016 in the present study. Many studies have investigated surgeon quality through the evaluation of surrogate markers of superiority such as surgical experience and skill. 21 , 22 , 23 , 24 From this viewpoint, surgeons certified by ESSQS may be considered to have achieved a certain level of surgical expertise. However, in the past, because of the small number of cases examined, evaluation of the relation between operations performed by ESSQS‐certified surgeons and surgical outcomes has been difficult, 12 , 13 , 14 but the NCD database now makes the investigation of the effect of ESSQS on surgical outcomes possible.
We considered the higher occurrence of ileus/bowel obstruction following LLAR performed by ESSQS‐certified surgeons group and in the laparoscopic operation volume per year (>12) group to be acceptable and natural because of the transitional period for the spread of LLAR, which meant the expansion of indications for highly advanced disease. The background of the patients in the present study showed that more ESSQS‐certified surgeons performed operations for patients undergoing neoadjuvant therapy than those without ESSQS certification. According to the JSES survey, drastic changes in the indication for laparoscopic surgery from early‐stage disease to highly advanced disease, some of which required preoperative treatment, occurred during the period of 2014‐2016. 8 Although we focused on highly advanced disease and preoperative treatment in the ESSQS‐certified surgeons group, we must recognize that several patient background factors might favorably or unfavorably affect clinical outcomes in the ESSQS‐certified surgeons group such as age, diabetes mellitus, Brinkman index, habitual alcohol intake, cerebrovascular disease, and steroid use, which could not be explained individually. So far, Koh et al have reported that the Centers for Medicare & Medical Services (CMS) high‐stars rating of a hospital did not always signify improved patient outcomes for advanced laparoscopic abdominal operations including gastric cancer and rectal cancer compared to the CMS low‐stars rating. 25 Additionally, Gambhir et al reported that surgical volume did not appear to be associated with improved patient outcomes owing to the complexity of patients such as those with highly advanced disease and worse general condition through the US News & World Report top ranking for gastroenterology and gastrointestinal operations. 26 Although leading hospitals such as high‐volume hospitals and the CMS high‐stars hospitals generally might be thought to have better clinical outcomes in terms of all clinical parameters compared to other hospitals, this did not appear to be reflected in clinical practice. In the present study, the operations performed by ESSQS‐certified surgeons correlated significantly with the lower occurrence of the important adverse event of anastomotic leakage. It is considered that anastomotic leakage is associated with basic skill and technique, and ileus/bowel obstruction might result from wider dissection of the peritoneum and lymph nodes, such as pelvic lateral lymph node dissection, for highly advanced disease. Of course, it is important to recognize that there is no data on the level of lymphadenectomy in the NCD database.
Laparoscopic operation volume per year was also a good predictor of postoperative complications such as mortality and anastomotic leakage in LDG and mortality in LLAR. In terms of higher overall complications (P < 0.001) and pancreatic fistula (P < 0.001) in LDG in institutions treating> 42 patients and higher overall complications (P < 0.001) and ileus/bowel obstruction (P < 0.001) in LLAR in institutions treating> 12 patients, these unfavorable outcomes showed an almost similar tendency as those in the ESSQS‐certified surgeons group. We speculated that these favorable and unfavorable outcomes might be attributed to the correlation of more ESSQS‐certified surgeons with higher‐volume institutions. In clinical practice, it is reasonable to make clinical decisions based on both the ESSQS and laparoscopic operation volume per year. According to the Japanese Gastric Cancer Association guideline, laparoscopic surgery is recommended for gastric cancer (grade 2, evidence level B), and in the JSCCR (Japanese Society for Cancer of the Colon and Rectum) guideline, laparoscopic surgery for gastric cancer and colorectal cancer (grade 2, evidence level B) is recommended as a standard treatment. 4 , 27 Additionally, it is stated that the indication for surgery should be determined at each surgical team level. However, determining the indication for laparoscopic gastrectomy in each hospital is nonobjective and difficult. Therefore, the outcomes shown in the present study could help surgeons to determine the indications for laparoscopic surgery in each hospital because there are no criteria for the evaluation of surgical team level. An operation performed by ESSQS‐certified surgeons is one of the most valuable predictors of optimal clinical outcome in terms of anastomotic leakage.
There are several limitations in the present study. First, it is a retrospective, observational study. Potential bias due to heterogeneity of the surgical quality or performance by each hospital cannot be excluded. Second, this analysis did not overcome all of the uncertainties associated with the details of the surgical procedures, such as the degree of lymphadenectomy, methods or technique of reconstruction, type of approach (e.g. robotic surgery, single‐port surgery, or transanal total mesorectal excision), or types of energy devices used. Third, the oncological and long‐term outcomes are not available from the Japanese NCD. Only data regarding baseline characteristics and short‐term outcomes were allowed to be input. A definitive conclusion as to the oncological validity of this surgery must also depend on the data from other clinical trials. Fourth, the clinical impact of operations in which ESSQS‐certified surgeons participated as an assistant could not be evaluated.
In conclusion, ESSQS certification did not affect postoperative mortality following LDG and LLAR, but annual experience of laparoscopic surgery was associated with it. ESSQS certification may contribute to favorable outcomes regarding anastomotic leakage following LDG and LLAR.
DISCLOSURE
Funding: Author YK received lecture fees and was supported by a grant from TAIHO PHARMACEUTICAL CO, LTD. and CHUGAI PHARMACEUTICAL CO, LTD. Authors HE, HY, and HM were supported by grants from the National Clinical Database, Johnson & Johnson K.K., and Nipro Co.
Conflict of Interest: Authors declare no conflicts of interest for this article.
Akagi T, Endo H, Inomata M, et al. Clinical impact of Endoscopic Surgical Skill Qualification System (ESSQS) by Japan Society for Endoscopic Surgery (JSES) for laparoscopic distal gastrectomy and low anterior resection based on the National Clinical Database (NCD) registry. Ann Gastroenterol Surg. 2020;4:721–734. 10.1002/ags3.12384
REFERENCES
- 1. Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy‐assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4(2):146–8. [PubMed] [Google Scholar]
- 2. Katai H, Mizusawa J, Katayama H, Morita S, Yamada T, Bando E, et al. Survival outcomes after laparoscopy‐assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non‐inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5(2):142–51. [DOI] [PubMed] [Google Scholar]
- 3. Kim H‐H, Han S‐U, Kim M‐C, Kim W, Lee H‐J, Ryu SW, et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long‐term survival among patients with stage i gastric cancer: The KLASS‐01 randomized clinical trial. JAMA Oncol. 2019;5(4):506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jeong S‐Y, Park JW, Nam BH, Kim S, Kang S‐B, Lim S‐B, et al. Open versus laparoscopic surgery for mid‐rectal or low‐rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open‐label, non‐inferiority, randomised controlled trial. Lancet Oncol. 2014;15(7):767–74. [DOI] [PubMed] [Google Scholar]
- 6. Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MHGM, de Lange‐de Klerk ESM, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372(14):1324–32. [DOI] [PubMed] [Google Scholar]
- 7. Fleshman J, Branda ME, Sargent DJ, Boller AM, George VV, Abbas MA, et al. Disease‐free survival and local recurrence for laparoscopic resection compared with open resection of stage ii to iii rectal cancer: Follow‐up results of the ACOSOG Z6051 randomized controlled trial. Ann Surg. 2019;269(4):589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inomata M, Shiroshita H, Uchida H, Bandoh T, Akira S, Yamaguchi S, et al. Current status of endoscopic surgery in Japan: The 14th National Survey of Endoscopic Surgery by the Japan Society for Endoscopic Surgery. Asian J Endosc Surg. 2020;13(1):7–18. [DOI] [PubMed] [Google Scholar]
- 9. Mori T, Kimura T, Kitajima M. Skill accreditation system for laparoscopic gastroenterologic surgeons in Japan. Minim Invasive Ther Allied Technol. 2010;19(1):18–23. [DOI] [PubMed] [Google Scholar]
- 10. Kimura T, Mori T, Konishi F, Kitajima M. Endoscopic surgical skill qualification system in Japan: five years of experience in the gastrointestinal field. Asian J Endosc Surg. 2010;3:66–70. [Google Scholar]
- 11. Yamakawa T, Kimura T, Matsuda T, Konishi F, Bandai Y. Endoscopic Surgical Skill Qualification System (ESSQS) of the Japanese Society of Endoscopic Surgery (JSES). BH Surgery. 2013;3:6–8. [Google Scholar]
- 12. Ichikawa N, Homma S, Yoshida T, Ohno Y, Kawamura H, Kamiizumi Y, et al. Supervision by a technically qualified surgeon affects the proficiency and safety of laparoscopic colectomy performed by novice surgeons. Surg Endosc. 2018;32(1):436–42. [DOI] [PubMed] [Google Scholar]
- 13. Ichikawa N, Homma S, Yoshida T, Ohno Y, Kawamura H, Wakizaka K, et al. Mentor tutoring: an efficient method for teaching laparoscopic colorectal surgical skills in a general hospital. Surg Laparosc Endosc Percutan Tech. 2017;27(6):479–84. [DOI] [PubMed] [Google Scholar]
- 14. Homma S, Kawamata F, Yoshida T, Ohno Y, Ichikawa N, Shibasaki S, et al. The balance between surgical resident education and patient safety in laparoscopic colorectal surgery: surgical resident's performance has no negative impact. Surg Laparosc Endosc Percutan Tech. 2017;27(4):295–300. [DOI] [PubMed] [Google Scholar]
- 15. Miyata H, Mori M, Kokudo N, Gotoh M, Konno H, Wakabayashi GO, et al. Association between institutional procedural preference and in‐hospital outcomes in laparoscopic surgeries; Insights from a retrospective cohort analysis of a nationwide surgical database in Japan. PLoS One. 2018;13(3):e0193186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kakeji Y, Takahashi A, Udagawa H, Unno M, Endo I, Kunisaki C, et al. Surgical outcomes in gastroenterological surgery in Japan: report of national clinical database 2011–2016. Ann Gastroenterol Surg. 2018;2(1):37–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hata T, Ikeda M, Miyata H, et al. Frequency and risk factors for venous thromboembolism after gastroenterological surgery based on the Japanese National Clinical Database (516 217 cases). Ann Gastroenterol Surg. 2019;3(5):534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haga Y, Miyata H, Tsuburaya A, Gotoh M, Yoshida K, Konno H, et al. Development and validation of grade‐based prediction models for postoperative morbidity in gastric cancer resection using a Japanese web‐based nationwide registry. Ann Gastroenterol Surg. 2019;3(5):544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kanaji S, Takahashi A, Miyata H, Marubashi S, Kakeji Y, Konno H, et al. Initial verification of data from a clinical database of gastroenterological surgery in Japan. Surg Today. 2019;49(4):328–33. [DOI] [PubMed] [Google Scholar]
- 21. Hiki N, Honda M, Etoh T, Yoshida K, Kodera Y, Kakeji Y, et al. Higher incidence of pancreatic fistula in laparoscopic gastrectomy. Real‐world evidence from a nationwide prospective cohort study. Gastric Cancer. 2018;21(1):162–70. [DOI] [PubMed] [Google Scholar]
- 22. Yoshida K, Honda M, Kumamaru H, Kodera Y, Kakeji Y, Hiki N, et al. Surgical outcomes of laparoscopic distal gastrectomy compared to open distal gastrectomy: A retrospective cohort study based on a nationwide registry database in Japan. Ann Gastroenterol Surg. 2017;2(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maruthappu M, Gilbert BJ, El‐Harasis MA, Nagendran M, McCulloch P, Duclos A, et al. The influence of volume and experience on individual surgical performance: a systematic review. Ann Surg. 2015;261(4):642–7. [DOI] [PubMed] [Google Scholar]
- 24. Schmidt CM, Turrini O, Parikh P, et al. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single‐institution experience. Arch Surg. 2010;145(7):634–40. [DOI] [PubMed] [Google Scholar]
- 25. Koh CY, Inaba CS, Sujatha‐Bhaskar S, Nguyen NT. Association of Centers for Medicare & Medicaid Services Overall Hospital Quality Star Rating With Outcomes in Advanced Laparoscopic Abdominal Surgery. JAMA Surg. 2017;152(12):1113–7. [DOI] [PubMed] [Google Scholar]
- 26. Gambhir S, Daly S, Grigorian A, et al. Association of US News and World Report top ranking for gastroenterology and gastrointestinal operation with patient outcomes in abdominal procedures. JAMA Surg. 2019;154(9):861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hashiguchi Y, Muto K, Saito Y, et al. Japanese Society for the Colon and Rectum (JSCCR) guideline 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25(1):1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]


