Abstract
Aim
The prognostic value of the stage III subclassification system based on the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma has not yet been clarified. This study aimed to develop a modified system with optimal risk stratification and compare its performance with the current staging systems.
Methods
Clinicopathological data from 6855 patients with stage III colorectal cancers who underwent D3 dissection were collected from a nationwide multicenter database. After determining patient survival rates across 13 divisions based on pathological N stage (N1, N2a, and N2b/N3) and tumor depth (T1, T2, T3, T4a, and T4b), except for T1N2a and T1N2b/N3 due to the small number, we categorized patients into three groups and developed a trisection staging system according to the Akaike information criterion. We then compared the Akaike information criterion of the developed system with those of the current staging systems.
Results
The T1N1[rank, 1] division (98.5%) had the most favorable prognosis in terms of 5‐year cancer‐specific survival, followed by T2N1[2] (93.9%), T2N2a[3] (92.0%), T3N1[4] (87.0%), T3N2a[5] (78.8%), T4aN1[6] (78.7%), T2N2b/N3[7] (77.8%), T4aN2a[8] (75.2%), T4bN1[9] (73.5%), T3N2b/N3[10] (64.7%), T4aN2b/N3[11] (61.5%), T4bN2b/N3[12] (43.0%), and T4bN2a[13] (42.5%). Compared to the categorizations of the Japanese and tumor‐node‐metastasis systems (Akaike information criterion, 22 684.6 and 22 727.1, respectively), the following stage categorizations were proven to be the most clinically efficacious: T1N1[1 ]‐T3N1[4], T3N2a[5 ]‐T4bN1[9], and T3N2b/N3[10 ]‐T4bN2a[13] (Akaike information criterion, 22 649.2).
Conclusion
The proposed modified system may be useful in the risk stratification of patients with stage III colorectal cancer who had undergone D3 dissection.
Keywords: AIC, prognosis, stage III colorectal cancer, subclassification, tumor‐node‐metastasis
The present study retrospectively analyzed data from 6855 patients with colorectal cancer who underwent resection with D3 dissection and showed the presence of prognostic heterogeneity in each subclassification category (i.e. stage IIIa, IIIb, and IIIc). Subsequently, we created a modified staging system primarily considering prognostic stratification power.

1. INTRODUCTION
D3 dissection, which includes complete dissection of regional lymph nodes comprising the main lymph nodes (i.e. around the root of the feeding artery) in all cases and the lateral pelvic lymph nodes in lower rectal cancer, has been the standard practice for stage II/III colorectal cancer (CRC) in Japan. 1 , 2 In a retrospective analysis using a multi‐institutional database, we had previously reported the outstanding value of the presence of metastatic disease in the main or lateral lymph nodes, 3 which is denoted as N3 in the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma verified by the Japanese Society for Cancer of the Colon and Rectum (JSCCR). 4 , 5 Subsequently, we structured a modified lymph node metastasis (N) staging system where N3‐positive cancers were merged with those in the N2b category (seven or more metastatic lymph nodes) and showed that the modified N staging performed better than both of the tumor‐node‐metastasis (TNM) and JSCCR (8th) N staging systems. 5 , 6 , 7 , 8 Accordingly, the modified N classification has been formally adopted by the JSCCR (9th) N classification. 4
Currently, studies have repeatedly shown that the TNM classification (7th or 8th), whose stage III subclassification is minutely determined by tumor depth (T) and N status, shows considerably better prognostic value compared to other systems. 9 , 10 , 11 On the other hand, the JSCCR (8th) stage III subclassification system, which is solely based on N category, has been replaced by the new stage III classification (9th) based on the combination of the JSCCR (9th) N and T status. 4 The trisection subclassification of the JSCCR (9th) (stage IIIa, b, and c) had been set to be similar to the TNM subclassification (stage IIIA, B, and C) considering interchangeability, except for N3‐positive cancers (Figure 1A,B ). However, the prognostic value of this system and adequacy of adopting the TNM staging system have not been clearly examined through a large cohort study. Accordingly, a superior stage III subclassification could possibly be derived from a different staging scheme.
FIGURE 1.
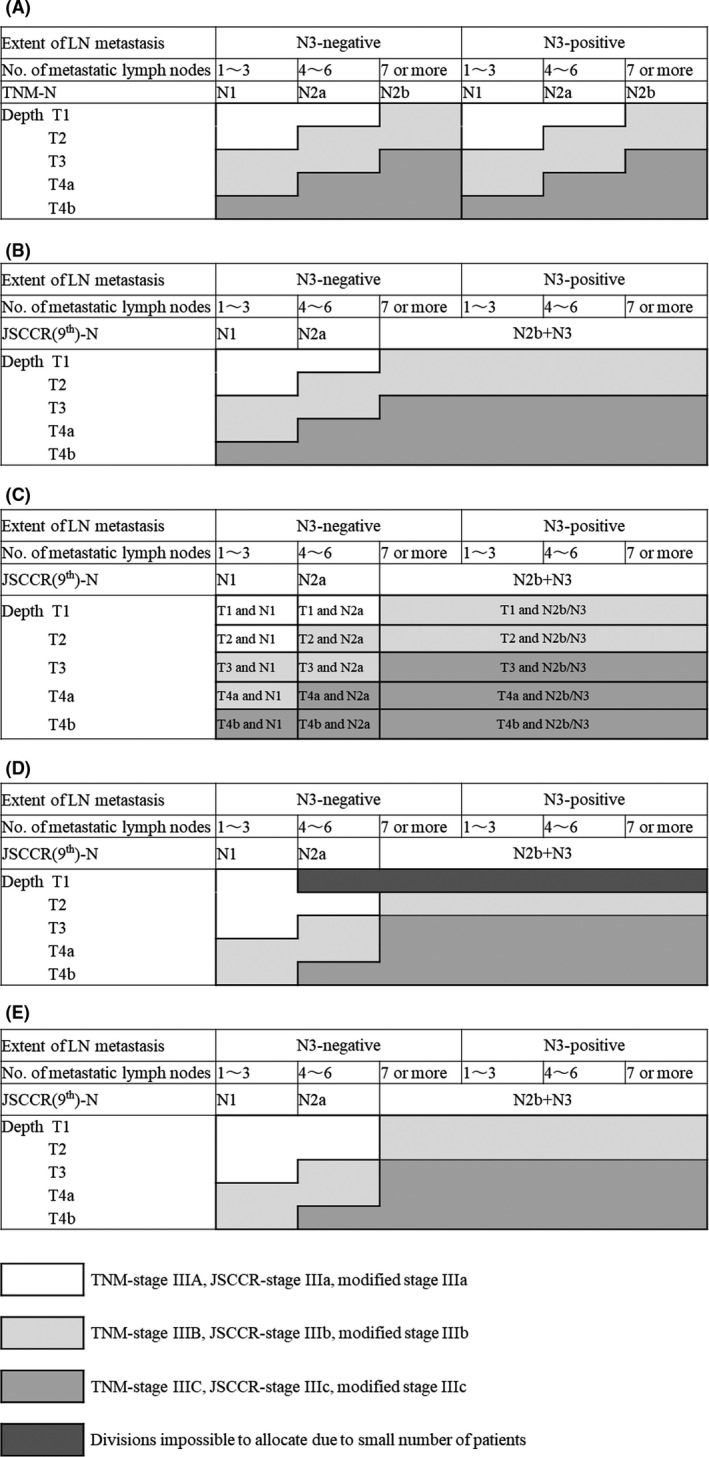
The TNM classification is determined according to the number of metastatic lymph nodes and tumor depth (A), whereas the JSCCR (9th) subclassification status is determined by tumor depth and JSCCR (9th) N classification, including both the number and extent of metastatic lymph nodes (B). Metastasis to the main or lateral lymph nodes is denoted as N3, which is ranked equally to N2b in the JSCCR (9th) guidelines. Patients were temporarily divided into 15 divisions according to the JSCCR (9th) N status and tumor depth (C). The modified stage III subclassification system based on risk stratification power is described in (D), which can be revised considering SEER data (E). TNM, tumor‐node‐metastasis; JSCCR, Japanese Society for Cancer of the Colon and Rectum; SEER, Surveillance, Epidemiology, and End Results
The present study aimed to develop a modified stage III sub‐staging system using the JSCCR (9th) N classification and compare its performance with the current staging systems. We initially determined the presence of prognostic heterogeneity in each subclassification category (i.e. stage IIIa, b, and c). Subsequently, we attempted to create the best system for trisection classification primarily considering prognostic stratification power based on the Akaike information criterion (AIC), which has been used to generally assess prognostic scoring systems. 12 , 13 Finally, the qualities of the new classification system were compared to those of the TNM and JSCCR (9th) systems.
2. METHODS
2.1. Patients
Clinical and pathological data from 6866 patients with histologically confirmed lymph node metastasis who underwent curative surgery (R0) with D3 dissection for primary CRC at 127 institutions between 1995 and 2006 were obtained from the multi‐institutional database of the JSCCR. The subjects were the same as those included in a preceding study, the characteristics of whom have been listed herein. 3 Patients with distant metastases or multiple primary cancers, those who received preoperative adjuvant therapy, and those with appendiceal or anal canal cancer were excluded. Additionally, cases with unknown or missing data on tumor depth, location and extent of lymph node dissection, number of metastatic lymph nodes, N3 status, and/or survival were excluded.
Information on adjuvant chemotherapy was obtained from data collected between 2002 and 2006 given that no such data had been recorded in the registry before 2001. Accordingly, 60.6% (1402/2312) of the patients received postoperative adjuvant chemotherapy. 5‐Fluorouracil (5‐FU)‐based adjuvant chemotherapy was administered to patients considering that oxaliplatin had not yet been approved for use in Japan as adjuvant chemotherapy until 2008.
Written informed consent was obtained from all patients in accordance with the respective institutional regulations. The study protocol was approved by the ethics committee of the National Defense Medical College Hospital.
2.2. New substage arrangement
Patients with stage III disease were divided into 15 divisions according to the JSCCR (9th) N and T status (Figure 1C). At minimum, a division required 10 patients to generate a proper survival curve; hence, divisions with fewer patients were excluded from analyses. Survival curves for each division in each of the three subclassification categories (i.e. IIIa, b, and c) were composed and compared to determine intra‐subcategorical heterogeneity. Thereafter, divisions showing similar outcomes were combined into a single group. These connected groups were further integrated to create the best trisection subclassification that yielded the best risk stratification power based on the AIC.
2.3. Statistical analysis
Cancer‐specific survival (CSS) was defined as duration from surgery to death due to CRC recurrence. Patient survival curves were generated using the Kaplan‐Meier method and were compared using the log‐rank test. All statistical analyses were performed using JMP12 software (SAS Institute). P values <.05 were considered statistically significant. AIC was analyzed using the Cox proportional hazards regression model to identify the grading system with the highest ability to stratify patients by survival outcome. The model that had the lowest AIC value was considered optimum (i.e. the simplest effective model with the least information loss when predicting outcome). 14
3. RESULTS
3.1. Cancer‐specific survival heterogeneity in stage III subcategories
Figure 1C outlines the 15 divisions according to JSCCR(9th) N and T status, while Figure 2A‐C describes their corresponding survival curves per stage III subcategory. Given that the number of patients in two divisions (i.e. T1 and N2a and T1 and N2b/N3) did not reach 10 (n = 8 and 3, respectively), such patients were excluded from analyses. The characteristics of the remaining 6855 patients are listed in Table 1.
FIGURE 2.
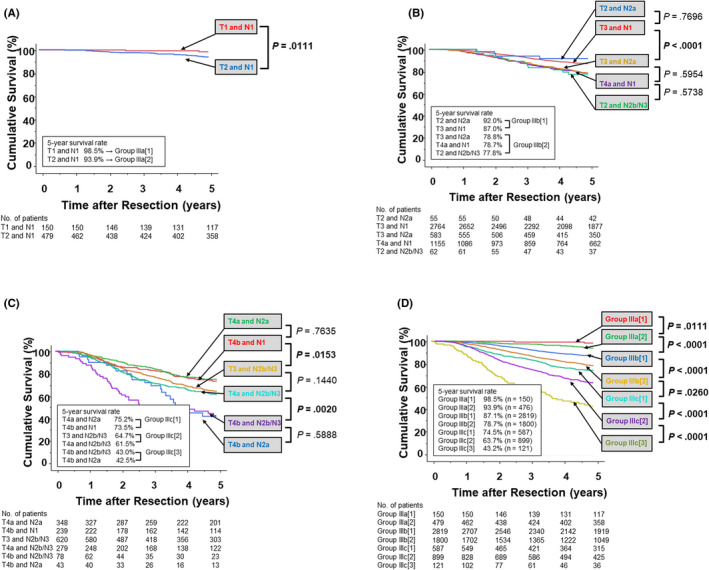
Cumulative cancer‐specific survival curves for each division are described in patients with JSCCR (9th) stage IIIa (A), IIIb (B), IIIc (C). Based on the statistical difference and similarity, divisions in stage IIIa, IIIb, and IIIc were integrated into two, two, and three groups, respectively, whose cumulative cancer‐specific survival curves are displayed in (D). JSCCR, Japanese Society for Cancer of the Colon and Rectum
TABLE 1.
Patient distribution
| No of cases (%) | P‐value | ||||
|---|---|---|---|---|---|
| Modified stage | |||||
| Total | Stage IIIa | Stage IIIb | Stage IIIc | ||
| n = 6855 | n = 3448 | n = 2387 | n = 1020 | ||
| Age a | |||||
| ≤59 y | 2414 | 1219 (50.5) | 798 (33.1) | 397 (16.5) | .0001 |
| 60‐69 y | 2354 | 1237 (52.6) | 791 (33.6) | 326 (13.9) | |
| ≥70 y | 2064 | 976 (47.3) | 792 (38.4) | 296 (14.3) | |
| Sex b | |||||
| Male | 3140 | 1571 (50.0) | 1111 (35.4) | 458 (14.6) | .66 |
| Female | 3703 | 1870 (50.5) | 1274 (34.4) | 559 (15.1) | |
| Tumor location | |||||
| Right‐side | 2058 | 977 (47.5) | 790 (38.4) | 291 (14.1) | <.0001 |
| Left‐side | 1992 | 1097 (55.1) | 683 (34.3) | 212 (10.6) | |
| Rectum | 2805 | 1374 (49.0) | 914 (32.6) | 517 (18.4) | |
| Depth | |||||
| T1 | 150 | 150 (100) | 0 (0) | 0 (0) | <.0001 |
| T2 | 596 | 534 (89.6) | 62 (10.4) | 0 (0) | |
| T3 | 3967 | 2764 (69.7) | 583 (14.7) | 620 (15.6) | |
| T4a | 1782 | 0 (0) | 1503 (84.3) | 279 (15.7) | |
| T4b | 360 | 0 (0) | 239 (66.4) | 121 (33.6) | |
| TNM‐N status | |||||
| N1 | 5020 | 3393 (67.6) | 1421 (28.3) | 206 (4.1) | <.0001 |
| N2a | 1168 | 55 (4.7) | 941 (80.6) | 172 (14.7) | |
| N2b | 667 | 0 (0) | 25 (3.8) | 642 (96.3) | |
| N3 status | |||||
| N3− | 6302 | 3448 (54.7) | 2341 (37.2) | 513 (8.1) | <.0001 |
| N3+ | 553 | 0 (0) | 46 (8.3) | 507 (91.7) | |
| JSCCR(9th)‐N status | |||||
| N1 | 4787 | 3393 (70.9) | 1394 (29.1) | 0 (0) | <.0001 |
| N2a | 1029 | 55 (5.3) | 931 (90.5) | 43 (4.2) | |
| N2b/N3 | 1039 | 0 (0) | 62 (6.0) | 977 (94.0) | |
| TNM‐stage | |||||
| IIIA | 656 | 629 (95.9) | 27 (4.1) | 0 (0) | <.0001 |
| IIIB | 6864 | 2819 (58.0) | 1773 (36.5) | 272 (5.6) | |
| IIIC | 1335 | 0 (0) | 587 (44.0) | 748 (56.0) | |
| JSCCR(9th)‐stage | |||||
| IIIa | 629 | 629 (100) | 0 (0) | 0 (0) | <.0001 |
| IIIb | 4619 | 2819 (61.0) | 1800 (39.0) | 0 (0) | |
| IIIc | 1607 | 0 (0) | 587 (36.5) | 1020 (63.5) | |
| Number of LNs examined c | |||||
| ≤11 | 1590 | 892 (56.1) | 532 (33.5) | 166 (10.4) | <.0001 |
| ≥12 | 4910 | 2367 (48.2) | 1730 (35.2) | 813 (16.6) | |
| Adjuvant‐chemotherapy d | |||||
| Chemotherapy | 1838 | 869 (47.3) | 655 (35.6) | 314 (17.1) | .0023 |
| Surgery alone | 924 | 500 (54.1) | 296 (32.0) | 128 (13.9) | |
Abbreviation: TNM, tumor‐node‐metastasis; JSCCR, Japanese Society for Cancer of the Colon and Rectum.
Information of age is not available for 23 patients.
Information of sex is not available for 12 patients.
Information of number of LNs examined is not available for 355 patients.
Information of adjuvant chemotherapy is not available for 4093 patients.
For stage IIIa (Figure 2A ), two divisions (T1 and N1 and T2 and N1) showed a significant difference in CSS (P = .011) and were thus allocated to group IIIa[1] and IIIa[2], respectively. For stage IIIb (Figure 2B), T2 and N2a had the most favorable prognosis in terms of 5‐year CSS (92.0%), followed by T3 and N1 (87.0%), T3 and N2a (78.8%), T4a and N1 (78.7%), and T2 and N2b/N3 (77.8%). No significant difference in survival was observed between the former two divisions and between the latter three divisions, whereas a marked difference was observed between T3 and N1 and T3 and N2a (P < .0001). Accordingly, the former two divisions were designated to group IIIb[1] and the latter three to group IIIb[2]. For stage IIIc (Figure 2C), T4a and N2a had the most favorable prognosis (75.2%), followed by T4b and N1 (73.5%), T3 and N2b/N3 (64.7%), T4a and N2b/N3 (61.5%), T4b and N2b/N3 (43.0%), and T4b and N2a (42.5%). No significant difference in survival was observed between the first two divisions, between the next two divisions, and between the last two divisions, whereas a marked difference was observed between T4b and N1 and T3 and N2b/N3 (P = .015), and between T4a and N2b/N3 and T4b and N2b/N3 (P = .0020). Accordingly, the first two divisions were designated to group IIIc[1], the next two to group IIIc[2], and the last two to group IIIc[3]. Survival curve comparisons in each respective group revealed significant differences among all groups (Figure 2D).
3.2. Rearrangement of stage III subclassification
The upper half of Table 2 shows each AIC score according to a single boundary. Our results revealed that the smallest AIC was achieved when stage III was classified as {Group IIIa[1], IIIa[2], and IIIb[1] (5‐year CSS, 88.5%)} and {IIIb[2], IIIc[1], IIIc[2], and IIIc[3] (5‐year CSS, 72.8%)}. The lower half of Table 2 shows the AIC scores of the three categories based on two boundaries, including the aforementioned one. Our results showed that the best performance was achieved when stage III was classified as {Group IIIa[1], IIIa[2], and IIIb[1] (5‐year CSS, 88.5%)}, {IIIb[2] and IIIc[1] (5‐year CSS, 77.7%)}, and {IIIc[2] and IIIc[3] (5‐year CSS, 61.3%)}, which was considered the most efficient stage III trisection subclassification (Figure 1D).
TABLE 2.
Exploration of the best staging system
| Grouping system | 5‐year cancer specific survival [No of cases] | AIC | Ranking | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bisection | ||||||||||
| Group IIIa[1] vs Group IIIa[2]b[1]b[2]c[1]c[2]c[3] | 98.5% | [150] | vs | 80.5% | [6705] | 22 953.3 | 6 | |||
| Group IIIa[1]a[2] vs Group IIIb[1]b[2]c[1]c[2]c[3] | 95.0% | [629] | vs | 79.4% | [6226] | 22 884.5 | 4 | |||
| Group IIIa[1]a[2]b[1] vs Group IIIb[2]c[1]c[2]c[3] | 88.5% | [3448] | vs | 72.8% | [3407] | 22 735.0 | 1 | |||
| Group IIIa[1]a[2]b[1]b[2] vs Group IIIc[1]c[2]c[3] | 85.2% | [5248] | vs | 66.1% | [1607] | 22 756.0 | 2 | |||
| Group IIIa[1]a[2]b[1]b[2]c[1] vs Group IIIc[2]c[3] | 84.2% | [5835] | vs | 61.3% | [1020] | 22 769.7 | 3 | |||
| Group IIIa[1]a[2]b[1]b[2]c[1]c[2] vs Group IIIc[3] | 81.5% | [6734] | vs | 43.2% | [121] | 22 925.1 | 5 | |||
| Three divisions | ||||||||||
| Group IIIa[1] vs a[2]b[1] vs b[2]c[1]c[2]c[3] | 98.5% | [150] | vs | 88.1% | [3298] | vs | 72.8% | [3407] | 22 709.3 | 4 |
| Group IIIa[1]a[2] vs b[1] vs b[2]c[1]c[2]c[3] | 95.0% | [629] | vs | 87.1% | [2819] | vs | 72.8% | [3407] | 22 695.5 | 3 |
| Group IIIa[1]a[2]b[1] vs b[2] vs c[1]c[2]c[3] | 88.5% | [3448] | vs | 78.7% | [1800] | vs | 66.1% | [1607] | 22 710.8 | 5 |
| Group IIIa[1]a[2]b[1] vs b[2]c[1] vs c[2]c[3] | 88.5% | [3448] | vs | 77.7% | [2387] | vs | 61.3% | [1020] | 22 649.2 | 1 |
| Group IIIa[1]a[2]b[1] vs b[2]c[1]c[2] vs c[3] | 88.5% | [3448] | vs | 73.9% | [3286] | vs | 43.2% | [121] | 22 688.5 | 2 |
Abbreviation: AIC, Akaike information criterion
Survival curves and AIC scores of the modified stage III, TNM stage III, and JSCCR (9th) stage III classifications are described in Figure 3. The modified stage III staging system yielded a more favorable survival (AIC = 22,649.2) compared to the TNM (AIC, 22,727.1) and JSCCR (9th) (AIC = 22,684.6) classification systems.
FIGURE 3.
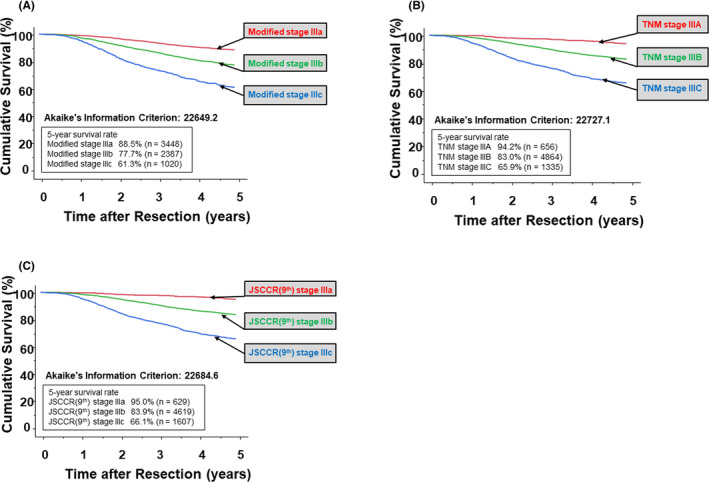
A, The cumulative cancer‐specific survival curves for patients with stage III colorectal cancer stratified by the modified stage classification show the most favorable AIC (22,649.2). B, The cumulative cancer‐specific survival curves for patients stratified by the TNM classification had an AIC of 22,727.1. C, The cumulative cancer‐specific survival curves for patients stratified by the JSCCR (9th) classification had an AIC of 22,684.6. AIC, Akaike information criterion; TNM, tumor‐node‐metastasis; JSCCR, Japanese Society for Cancer of the Colon and Rectum
Additionally, we tried to construct the optimal stage III quadrisection subclassification. When stage III was classified as {Group IIIa[1], IIIa[2] (5‐year CSS, 95.0%)}, {IIIb[1] (5‐year CSS, 87.1%)} {IIIb[2] and IIIc[1] (5‐year CSS, 77.7%)}, and {IIIc[2] and IIIc[3] (5‐year CSS, 61.3%)}, the AIC score was the smallest. This subclassification was the most efficient and had JSCCR (9th) stage IIIa as a constituent substage.
4. DISCUSSION
Cancer staging systems need to maintain prognostic power, simplicity, and continuity. However, their most essential goal is, undoubtedly, discriminative power of prognosis and/or recurrence risk. Based on the present data, divisions in each stage III subclassification category showed diverse survival, which supposedly worsens the quality of the present system. This implies sufficient grounds for controversy on the propriety of the subclassification systems. In this context, we herein managed to create a new system based on the AIC that has improved prognostic‐discriminative power.
The JSCCR (9th) system has several issues. First, stage IIIa patients accounted for only 9.2% of all patients, despite specifically possessing excellent prognosis (5‐year CSS, 95.0%). Second, no less than 67.3% of all stage III patients were categorized as stage IIIb, which had the largest (T3 and N1) and second largest (T4a and N1) divisions whose survivals showed marked difference (5‐year CSS, 87.0% vs 78.7%, respectively; P < .0001). Third, stage IIIc had conspicuous heterogeneity in 5‐year CSS ranging from 75.2% (T4a and N2a) to 42.5% (T4b and N2a). In contrast, the newly arranged subclassification system included the T3 and N1 division in stage IIIa, which comprised 50.2% of all stage III patients, while the T4a and N1 division remained in stage IIIb. In consequence, the modification basically resolved the issue of the enlarged stage IIIb population. At this point, the 5‐year survival of the newly proposed stage IIIa was 88.5%, which was still excellent considering the node‐positive population. Additionally, T4a and N2a and T4b and N1 were removed from stage IIIc, while the new stage IIIc was transformed into a small population (14.9% of all patients) with much poorer survival (5‐year CSS, 61.3%), which indicates a definite target for intensive adjuvant chemotherapy. Actually, the rearrangement of the T3 and N1 division has already been suggested by Kusumoto et al, 15 whose exploratory analysis of data from Adjuvant Chemotherapy Trial of TS‐1 for Colon Cancer (ACTS‐CC) clearly demonstrated a similarity of patient survival rates between the T3 and TNM‐N1 division and the TNM stage IIIA category. Our present study using a nationwide database notedly yielded similar results highlighting the fundamental necessity to modify the system.
Large‐scale studies using stage III populations have shown that postoperative adjuvant chemotherapy effectively prolongs patient survival. 16 , 17 , 18 5‐FU‐based chemotherapies without oxaliplatin have been widely used for this purpose in Japan, whereas 6‐month use of oxaliplatin‐containing regimens has been common in Western countries. A recent notable study, the International Duration Evaluation of Adjuvant Therapy (IDEA) collaboration, revealed that 3 months of an oxaliplatin regimen was as effective as 6 months of the same regimen for low‐risk stage III patients. 19 Notably, however, the low‐risk group in the IDEA trial differed from TNM stage IIIA or JSCCR (9th) stage IIIa but was more similar to the newly arranged stage IIIa, wherein the largest division (T3 and N1) was included. In clinical practice, a patient‐suited regimen should be faithfully selected according to expected recurrence risk and/or recommendations based on clinical trial results. Given that the TNM and JSCCR (9th) staging are neither consistent with the patient subgrouping adopted in the essential clinical research nor the best predictors of prognosis, determinants of treatment choice could possibly be dissociated from these formal stagings. Therefore, establishing a new stage III subclassification that is strongly associated with recurrence risk and can help decide treatment strategies should be recognized as a pressing need.
We should discuss the relevance of the modified system for the selection of adjuvant chemotherapy regimens. The T3 and N1 division of our cohort showed 87.0% of 5‐year CSS, and T3 and TNM‐N1a division showed 90.7% of 5‐year overall survival and 78.4% of 5‐year disease‐free survival in ACTS‐CC trial. 15 Considering that favorable survivals were demonstrated among these patients with oxaliplatin‐free regimens and that survival rates of other divisions in modified stage IIIa were superior to that of T3 and N1, 5‐FU‐based regimens seem highly acceptable as adjuvant chemotherapy in modified stage IIIa patients. Moreover, based on the IDEA trial, 19 we believe that 3‐month oxaliplatin administration is an option. 19 In contrast, T4 and N1 division in our cohort showed 78.7% of 5‐year CSS, and T4 and TNM‐N1a division indicated 82.5% of 5‐year overall survival and 55.2% of 5‐year disease‐free survival in ACTS‐CC trial, 15 implying that this category has a considerable number of patients who needed more intensive chemotherapy. T4 and N1 was the maximum component of the modified stage IIIb, and divisions in this substage did not widely differ in the survival rates. Thus, oxaliplatin‐containing regimens are regularly recommended in this category. Patients in modified stage IIIc showed 61.3% of 5‐year CSS, close to the overall survival rate in patients who had undergone hepatectomy for liver metastasis (range, 46.7%–69.5%), 20 and > 50% developed recurrence. 15 Thus, it is necessary to administer oxaliplatin‐based chemotherapy without delay and implement an intensive follow‐up plan.
This study has several potential limitations worth noting. First, we could not allocate two divisions (i.e. T1 and N2a and T1 and N2b/N3) into the subcategories because of the insufficient number of patients. However, based on data from the Surveillance, Epidemiology, and End Results cancer registry of the United States National Cancer Institute, T1 and TNM‐N2a had a better prognosis than T3 and TNM‐N1, while T1 and TNM‐N2b had a prognosis worse than T4a and TNM‐N1 but better than T4b and TNM‐N1. 9 Hence, we believe it appropriate that these two divisions be included in the modified stage IIIa and IIIb, respectively (Figure 1E). Second, effects of adjuvant chemotherapy were not determined. However, we believe their effects to be limited given that none of the patients received oxaliplatin as adjuvant chemotherapy. Third, the modified stage is limited in that it cannot select patients with extremely good prognosis similar to the JSCCR (9th) stage IIIa. If the four‐division classification is adopted, JSCCR (9th) stage IIIa becomes an independent substage. The clinical value of this quadrisection subclassification should be clarified in future studies.
In conclusion, the present study established a modified staging system for stage III patients according to the JSCCR (9th) N and T status, which performed better than current staging systems. Despite the widely variable prognosis of patients with node‐positive CRC, the modified system could contribute to improved understanding of recurrence risk and subsequent decision‐making for adequate adjuvant therapy.
CONFLICT OF INTEREST
Authors declare no conflicts of interest for this article.
ACKNOWLEDGEMENTS
This work was supported by the Japanese Society for Cancer of the Colon and Rectum (JSCCR).
Shinto E, Ike H, Hida J-I, et al. Proposal of a modified subclassification system for stage III colorectal cancer: A multi‐institutional retrospective analysis. Ann Gastroenterol Surg. 2020;4:667–675. 10.1002/ags3.12375
REFERENCES
- 1. Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25(1):1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23(1):1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shinto E, Hida JI, Kobayashi H, Hashiguchi Y, Hase K, Ueno H, et al. Prominent information of jN3 positive in stage iii colorectal cancer removed by D3 dissection: retrospective analysis of 6866 patients from a multi‐institutional database in Japan. Dis Colon Rectum. 2018;61(4):447–53. [DOI] [PubMed] [Google Scholar]
- 4. Japanese Society for Cancer of the Colon and Rectum . Japanese Classification of colorectal, appendiceal, and anal carcinoma, 9th edn Tokyo: Kanehara & Co., Ltd.; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Japanese Society for Cancer of the Colon and Rectum . Japanese Classification of colorectal, appendiceal, and anal carcinoma, 8th edn Tokyo: Kanehara & Co., Ltd.; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shinto E, Hida JI, Ike H, Kobayashi H, Hashiguchi Y, Hase K, et al. A new N staging system for colorectal cancer in the era of extended lymphadenectomy. Ann Surg Oncol. 2018;25(13):3891–7. [DOI] [PubMed] [Google Scholar]
- 7. Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumors, 7th edn Hoboken, NJ: Wiley‐Blackwell; 2009. [Google Scholar]
- 8. Brierley J, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumors, 8th edn Oxford: Wiley‐Blackwell; 2017. [Google Scholar]
- 9. Hashiguchi Y, Hase K, Kotake K, Ueno H, Shinto E, Mochizuki H, et al. Evaluation of the seventh edition of the tumour, node, metastasis (TNM) classification for colon cancer in two nationwide registries of the United States and Japan. Colorectal Dis. 2012;14(9):1065–74. [DOI] [PubMed] [Google Scholar]
- 10. Gao P, Song YX, Wang ZN, Hui‐Mian X. Is the prediction of prognosis not improved by the seventh edition of the TNM classification for colorectal cancer? Analysis of the surveillance, epidemiology, and end results (SEER) database. BMC Cancer. 2013;13:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim KH, Yang SS, Yoon YS, Lim S‐B, Yu CS, Kim JC. Validation of the seventh edition of the American Joint Committee on Cancer tumor‐node‐metastasis (AJCC TNM) staging in patients with stage II and stage III colorectal carcinoma: analysis of 2511 cases from a medical centre in Korea. Colorectal Dis. 2011;13(8):e220–e226. [DOI] [PubMed] [Google Scholar]
- 12. Wang SJ, Lemieux A, Kalpathy‐Cramer J, Ord CB, Walker GV, Fuller CD, et al. Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. J Clin Oncol. 2011;29(35):4627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ueno H, Mochizuki H, Akagi Y, Kusumi T, Yamada K, Ikegami M, et al. Optimal colorectal cancer staging criteria in TNM classification. J Clin Oncol. 2012;30(13):1519–26. [DOI] [PubMed] [Google Scholar]
- 14. Information AH. Theory and Extension of the Maximum Likelihood Principle. In: Kotz S, Johnson NL, eds. Breakthroughs in Statistics Foundations and Basic Theory, vol. 1 New York: Springer‐Verlag; 1973. p. 610–24. [Google Scholar]
- 15. Kusumoto T, Ishiguro M, Nakatani E, Yoshida M, Inoue T, Nakamoto Y, et al. Updated 5‐year survival and exploratory T x N subset analyses of ACTS‐CC trial: a randomised controlled trial of S‐1 versus tegafur‐uracil/leucovorin as adjuvant chemotherapy for stage III colon cancer. ESMO Open. 2018;3(6):e000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gill S, Loprinzi CL, Sargent DJ, Thomé SD, Alberts SR, Haller DG, et al. Pooled analysis of fluorouracil‐based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22(10):1797–806. [DOI] [PubMed] [Google Scholar]
- 17. Mamounas E, Wieand S, Wolmark N, Bear HD, Atkins JN, Song K, et al. Comparative efficacy of adjuvant chemotherapy in patients with Dukes' B versus Dukes' C colon cancer: results from four National Surgical Adjuvant Breast and Bowel Project adjuvant studies (C‐01, C‐02, C‐03, and C‐04). J Clin Oncol. 1999;17(5):1349–55. [DOI] [PubMed] [Google Scholar]
- 18. Wilkinson NW, Yothers G, Lopa S, Costantino JP, Petrelli NJ, Wolmark N, et al. Long‐term survival results of surgery alone versus surgery plus 5‐fluorouracil and leucovorin for stage II and stage III colon cancer: pooled analysis of NSABP C‐01 through C‐05. A baseline from which to compare modern adjuvant trials. Ann Surg Oncol. 2010;17(4):959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378(13):1177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shinto E, Takahashi K, Yamaguchi T, Hashiguchi Y, Kotake K, Itabashi M, et al. Validation and Modification of the Japanese Classification System for Liver Metastases from Colorectal Cancer: A Multi‐institutional Study. Ann Surg Oncol. 2015;22(12):3888–95. [DOI] [PubMed] [Google Scholar]


