Abstract
Polyvinyl acetate (PVAc) and curcumin (Cu) were utilized for preparing new protecting PVAc–Cux (x = 1, 5 and 10) coatings exerting antimicrobial photodynamic activity upon white light irradiation. Toward Salmonella typhimurium or Staphylococcus aureus, the killing efficiency represented the dependence on the Cu concentration and irradiation intensity. Toward S. aureus, the killing efficiency of PVAc–Cu10 coating reached 93% at an energy density of 72 J/cm2. With the change in storage time of coating, the results implied significant stability of photosterilization efficiency within 60 days. Compared with the control experiment, lower total viable counts (TVCs) and total volatile basic nitrogen (TVB-N) values in fresh meat packaged by PVDC films with PVAc–Cu10 coatings during storage at 4 °C demonstrated the practicability of the PVAc–Cux coatings in decontaminating fresh pork. PVAc packed curcumin tightly within polymer chains, thus preventing tautomerization or, more probably, conformational transition, which is advantageous for improving photostability and emission lifetime.
1. Introduction
Curcumin (Cu), as one of the active components of Indian turmeric, has exhibited potential applications as therapeutic agents against several diseases because of its low toxicity, good anti-inflammatory and antibacterial capabilities, and anticancer activities.1−5 However, the low solubility and fast degradation assigned to physiological pH and photoinduced instability in aqueous media restrained numerous applications of curcumin in therapeutic agents and photodynamic inactivation (PDI) against cancerous cells and pathogenic microorganisms.6−9 In addition, the equilibrium corresponding to keto- and enol-tautomers, ultrafast excited-state processes closely associated with salvation, and excited-state intramolecular hydrogen atom transfer (ESIHT) have been considered important factors affecting its antibacterial and antioxidant capacities in solution.10−14 Thus, surfactant utilization15 and encapsulation into delivery systems such as conjugates,16 nanoparticles,17−21 molecular complexes,22 micelles,23 liposomes,24 hydrogels,25 and emulsions26 have been utilized to improve the solubility, stability, and biocompatibility of curcumin in aqueous media.
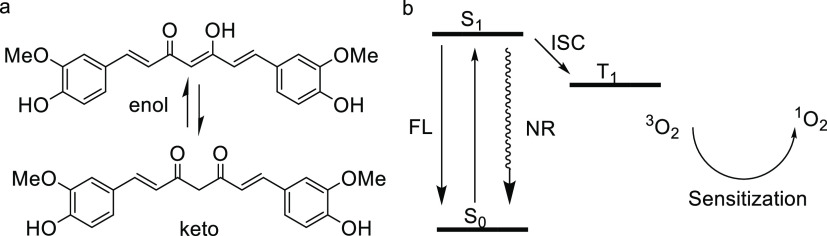
After electrons are excited to higher-energy orbitals from the singlet ground state of curcumin (S0) by means of light illumination, a series of photophysical processes, including the S1 → S0 transition by releasing nonradiative (NR) heat energy or radiative fluorescence (FL), and S1 → T1 intersystem crossing (ISC) will occur. The long lifetime of microseconds of T1 is usually sufficient for curcumin in the T1 state to transfer its energy to adjacent normal oxygen (3O2), generating highly reactive singlet oxygen (1O2, type II reaction), as shown in Figure 1. The toxic singlet oxygen can react with biological molecules such as unsaturated lipids, amino acids of proteins, thus causing cell death and inactivation of microorganisms.27 The inactivation effect is closely associated with the abovementioned photophysical processes and the singlet oxygen generation ability of curcumin. The photoinduced inactivation of bacteria based on curcumin was first reported by Tønnesen et al.28 Haukvik et al. found that curcumin exhibited effective photoinduced antibacterial capacities in aqueous solutions with polyethyleneglycol or Pluronic L35.29,30 Further, Hegge et al. reported that the phototoxic effect of curcumin with either cyclodextrins or PEG 400 in alginate foams proved to be very effective against Gram-positive bacteria.31
Figure 1.
The equilibrium corresponding to keto- and enol-tautomers (a) and the diagram simply describing the energy transfer from T1 of curcumin to singlet oxygen (b) upon light excitation.
Recently, active packaging technologies by means of blending chemicals obtained from plants as antioxidant/antimicrobial components into the packaging material have been considered as effective methods to prolong the shelf life of processed food.32 Owing to its antioxidant/antimicrobial properties, curcumin has been widely used as a food additive in the food processing field and antibacterial films.33 Pang et al. reported efficient antimicrobial activities of chitosan films containing curcumin-loaded silica nanoparticles for food packaging.34 Le et al. showed molecular interactions and antimicrobial activity of curcumin–chitosan blend films.35 Wang et al. fabricated films based on κ-carrageenan incorporated with curcumin for freshness monitoring.36 However, the antibacterial activities of curcumin as a photosensitizer doped into membranes or coatings are still rarely reported in active food-packaging, and the lack of understanding of the photochemical properties and stability of curcumin in a polymer matrix restrained further development of curcumin in photoinduced antibacterial films for food packaging. The blending of curcumin into a polymer matrix as protecting coatings can offer several advantages over its use as an additive in solution. The rigid microenvironment assigned to a polymer matrix can probably stabilize the curcumin by decreasing ultrafast excited-state processes closely associated with solvation and ESIHT under light exposure. In addition, antimicrobial Cu coating on the food surface is preferable over the addition of antimicrobials in food formulation owing to higher microbial contamination at the food surface.37
Polyvinyl acetate (PVAc), with excellent plasticity and strong adhesion, is not soluble with fat and water and utilized as the chewing material of gum sugar and the coating agent of fruit.38 The biodegradability of PVAc with limited hygroscopicity has been proved under certain conditions. It is anticipated that strong interactions may form between the hydroxyl-rich curcumin and the ester linkages abundantly protruding from the polymer backbone. The resulting hydrogen bonds are expected to improve the compatibility between curcumin and PVAc, thus inhibiting phase separation and stabilizing curcumin molecules.
The aims of the present work are (i) to present a simple route to efficiently fabricate photoinduced antibacterial PVAc–Cu coatings against Staphylococcus aureus (Gram-positive) and Salmonella typhimurium (Gram-negative) bacteria, (ii) to investigate the photophysical properties of curcumin using steady-state and time-resolved fluorescence spectroscopy for fundamental understanding of excited state and evaluate the stability of photoinduced antibacterial ability with the increase in storage time of coatings, and (iii) to evaluate the practicability of photoinduced antibacterial PVAc–Cu coatings deposited on PVDC films in decontaminating fresh pork during preservation.
2. Results and Discussion
2.1. Preparation and Photophysical Properties of the PVAc–Cux Coatings
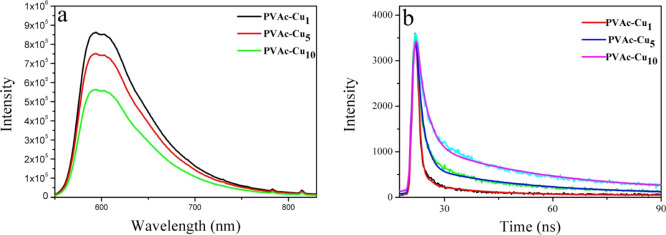
The coating solution containing PVAc and curcumin was, respectively, coated on the surface of PVDC and PET film by utilizing a coating machine. After the wet coat was dried at room temperature for 24 h and vacuum-dried for 6 h to remove ethanol, PVAc–Cux coatings were obtained. The coatings on the surface of stiff PET were utilized for the investigation of photophysical properties, and soft PVDC films containing curcumin coatings were utilized to investigate the capability of decontamination of fresh pork. According to the literature, the phototoxicity of photosensitizers toward pathogenic bacteria was closely associated with the dynamic phenomena at excited state, and long-lived excited-state species showed substantial importance in enhanced antibacterial efficiency.27,39,40 Two fundamental photophysical processes including excited-state hydrogen proton transfer and solvation reorganization will markedly affect the S1 relaxation of curcumin in solvents, thus leading to short fluorescence lifetimes.41 However, it is meaningful to investigate the photophysical properties of curcumin in a solid matrix owing to the lack of understanding on the excited state of curcumin in such systems. Fluorescence lifetimes can be fitted with two exponential decays for PVAc–Cux coatings, as shown in Figure 2. As depicted in Table 1, the average fluorescence lifetime at 20 °C was 0.89, 1.29, and 1.95 ns for PVAc–Cux (x = 1, 5, and 10) coatings, respectively. Interestingly, the lifetime increased when the concentration of curcumin content increased in the range of 1–10%, implying the absence of a severe self-quenching effect of the excited state assigned to the aggregation. Severe self-quenching has been proved to reduce the ability for 1O2 generation, thus lowering the efficiency for photoinduced antibacterial ability.27,39 The comparable curcumin fluorescence lifetimes in water, ethanol, glycerol, and PVA films were, respectively, 0.13, 0.12, 0.51, and 0.99 ns, as have been reported by Gryczynski.10 Sarkar et al. have reported enhanced fluorescence lifetimes of curcumin in nonionic surfactants forming micelles and niosomes, with a maximum of 0.52 ns.41 This clearly indicated a better stabilization of the excited state of curcumin in the PVAc matrix, in comparison with those in solutions or micelles. The polymer film aligned curcumin molecules and packed them tightly within the polymer chains, preventing tautomerization or, more probably, conformational transition. In a hydrophobic PVAc matrix, the fast ESIPT and solvation process in solutions assigned to short fluorescence lifetimes can probably be inhibited, thus leading to increased lifetime.10 The change in the lifetime of curcumin in different concentrations also helped us estimate the radiative (kr) and nonradiative (knr) rate constants using the published equation. As shown in Table 1, knr decreased obviously with the increase in the curcumin concentration, implying the change from the duct of deactivation into the radiative decay channels. The corresponding knr values of curcumin in PVAc–Cu1 and PVAc–Cu10 were 8.66 × 108 and 3.54 × 108 s–1, respectively. In comparison with the nonradiative rate constants of curcumin surrounded by silk biomaterials,8 the values decreased by 1 order of magnitude. When the nonradiative decay of curcumin is reduced, the excited state is saved for fluorescence and ISC, which is probably advantageous to singlet oxygen generation.8,27,39
Figure 2.
PL emission spectrum (a) and time-resolved lifetime decays (b) of curcumin in PVAc–Cux (x = 1, 5, and 10) coatings. The fluorescence decays of curcumin in emission maxima were measured using the excitation at 442 nm.
Table 1. Fluorescence Lifetime (τav), Fluorescence Quantum Yields (Φf), and Radiative (Kr) and Nonradiative Rate Constants (Knr) of Curcumin in PVAc–Cux (x = 1, 5, and 10) Coatings on the Surface of PET Film when Excited at 442 nm.
| coatings | τav (ns) | Φf (%) | Kr × 108 (s–1) | Knr × 108 (s–1) |
|---|---|---|---|---|
| PVAc–Cu1 | 0.89 | 23 | 2.58 | 8.66 |
| PVAc–Cu5 | 1.29 | 28 | 2.17 | 5.58 |
| PVAc–Cu10 | 1.95 | 31 | 1.59 | 3.54 |
2.2. Molecular Simulation
Molecular simulation was used to investigate the interaction between curcumin and PVAc molecules, by modeling the PVAc chain as a molecule containing three repeating monomers (PA3), as shown in Figure 3. The keto and enol forms coexist in the solid state of curcumin. Toward the keto type, the interaction energy mainly attributed to H-bonding between −OH on the benzene of curcumin and C=O of PA3 is 26 kcal/mol (structure a), which is larger than the van der Waals interaction between curcumin and PA3 (16 kcal/mol, structure b). Two hydrogen bonds formed between the hydroxyl of curcumin and the carbonyl group of PA3 with a distance of 1.78 Å and 1.80 Å, respectively. In addition, the interaction energy mainly assigned to van der Waals between curcumin at the enol type and PA3 is 22.98 and 16.15 kcal/mol (structures c and d). The results implied that the polymer chains packed curcumin tightly by the occurrence of H-bonding and van der Waals interaction, probably thus leading to the increased stability of curcumin and emission lifetime in solid state.42
Figure 3.
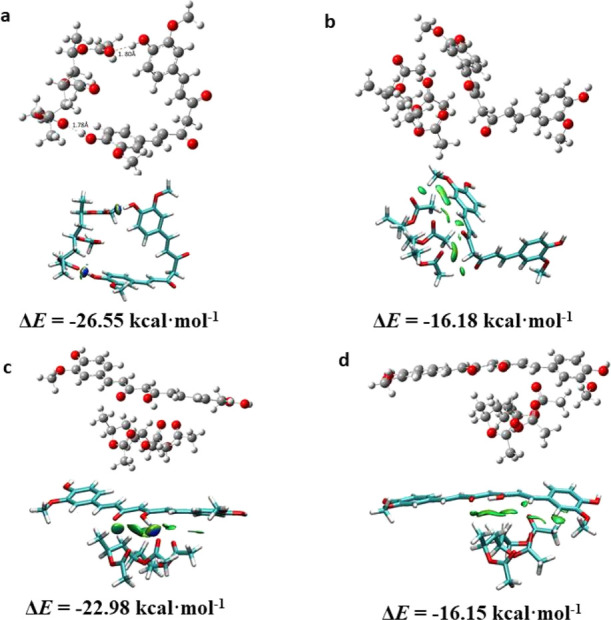
Optimal structures of interaction between PVAc molecules and curcumin and surface plots. Structures (a,b) and (c,d) are PVAc–Cu interaction at keto and enol types, respectively. H bonds are indicated by a dashed line at structure a.
2.3. Antibacterial Photodynamic Inactivation
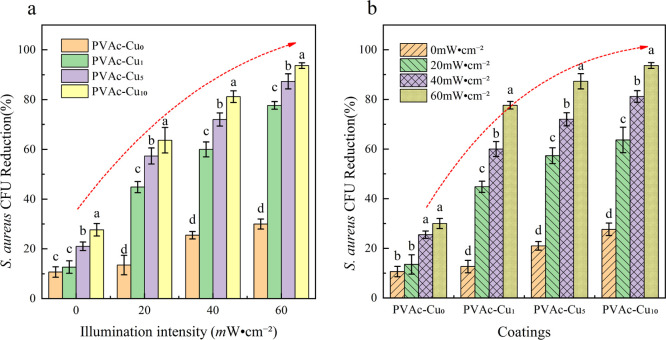
Toward S. aureus, photodynamic inactivation of bacteria was tested by irradiating coatings at different light fluences. As shown in Figure 4, with the increased concentration of curcumin, the killing efficiency was improved for both the light irradiation and dark cases. In comparison to the dark condition, the killing efficiency obviously increased upon white light irradiation. At an irradiation intensity of 20 mW/cm2, the killing efficiency is 10, 14, 20, and 28% for PVAc–Cux (x = 0, 1, 5, and 10, respectively) coatings under dark conditions, obviously lower than 15, 42, 55, and 58% under irradiation conditions. Compared with 42% at 20 mW/cm2, the killing efficiency was increased to 59 and 79%, respectively, for irradiation intensity at 40 and 60 mW/cm2. Toward S. aureus, colony counting showed that the killing efficiency of PVAc–Cux (x = 1, 5, and 10) coatings was 79, 88, and 93% at 60 mW/cm2 irradiation intensity, respectively, which was obviously higher than values (14, 20, and 28%) in the dark, indicating the obvious occurrence of photoinduced antibacterial interaction in coatings. The results displayed the synergistic effects of dark and light toxicity from curcumin coatings, and killing efficiency showed the dependence on the concentration and irradiation intensity. With the increase in light intensity, the bacteriostatic properties of the coating increased significantly. Under the same light intensity, an increased curcumin content also improved the bacteriostatic properties of the coatings, and there was a very significant synergistic effect between them (P < 0.05).
Figure 4.
The change trend of biocidal activities (a,b) of PVAc–Cux (x = 0, 1, 5, and 10) coatings against S. aureus with increased intensity of irradiation (400–800 nm, irradiation intensity: 20, 40, and 60 mW/cm2) at an illumination time of 20 min. Different letters marked in the columns show significant difference (P < 0.05).
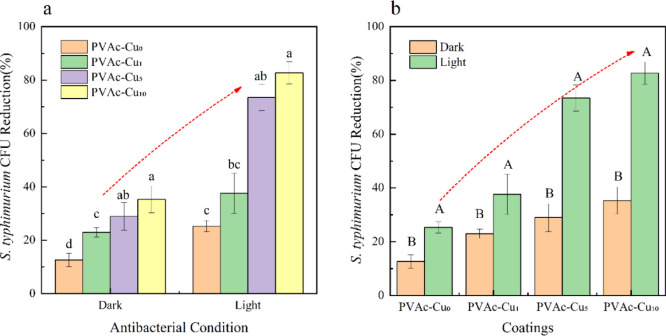
S. typhimurium can also be efficiently inactivated by light irradiation. Bacterial survival experiments were performed in the dark and white light irradiation, as displayed in Figure 5. With the increase in curcumin concentration, the killing efficiency was gradually improved for both the light and dark cases. Toward S. typhimurium, colony counting showed that the killing efficiency of PVAc–Cux (x = 1, 5, and 10) coatings was 38, 73, and 82% at 40 mW/cm2 irradiation intensity, respectively, which was obviously higher than values in the dark, indicating the occurrence of photoinduced antibacterial properties of coatings. It can be seen that PVAc–Cux coatings have both dark toxicity and phototoxicity and the light intensity and curcumin content in coatings have significant synergy effect on antibacterial capacity.
Figure 5.
The change trend of biocidal activity (a,b) of PVAc–Cux (x = 1, 5, and 10) coatings toward S. typhimurium with the increased concentration of curcumin, under white light irradiation with an intensity of 40 mW/cm2 at an illumination time of 20 min. Small and capital letters represent significant differences (P < 0.05) and highly significant differences (P < 0.01), respectively.
2.4. Bacterial Morphology and 1O2 Testing
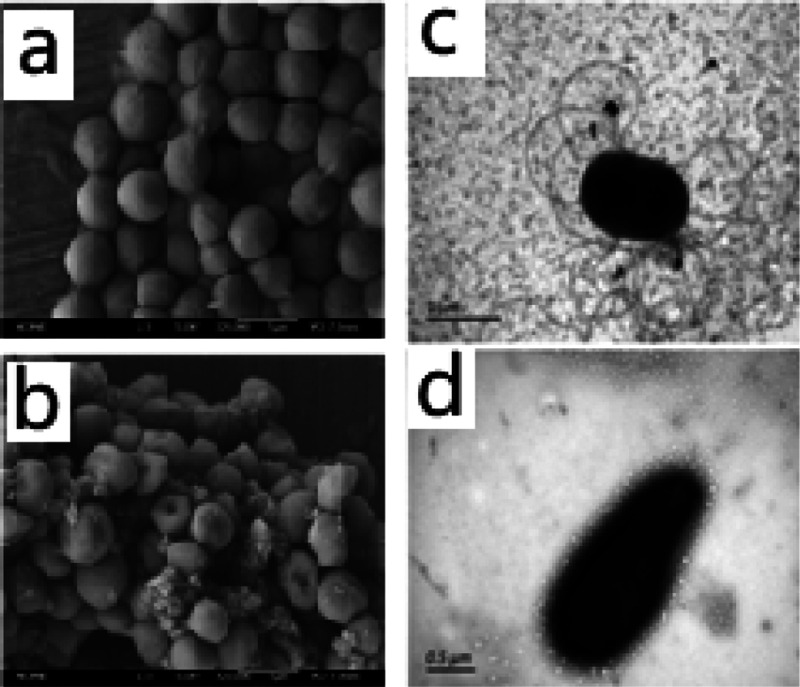
After photodynamic treatment at a radiant exposure of 72 J/cm2, a clear disruption of the outer membrane of S. aureus spores could be observed, probably assigned to 1O2 produced by the curcumin photosensitizer during irradiation. TEM was also used to directly visualize the change of S. typhimurium bacterial morphology, and flagella disappearance and shape change of the bacteria can be observed in Figure 6d. The generation of 1O2 from PVAc–Cu10 coating under white light irradiation can be detected using the SOSG kit. The 1O2 generation of PVAc–Cu10 coating in aqueous solution was measured immediately after light irradiation at 1, 3, and 5 min intervals. It was shown that the total 1O2 generation amount of PVAc–Cu10 at 3 min was appropriately twice as large as that at 1 min, as shown in Figure 7.
Figure 6.
SEM images of S. aureus spores (a,b) and TEM images of S. typhimurium spores (c,d) before and after the photodynamic treatment in the presence of PVAc–Cu10 coating using white light irradiation at a radiant exposure of 72 J/cm2.
Figure 7.
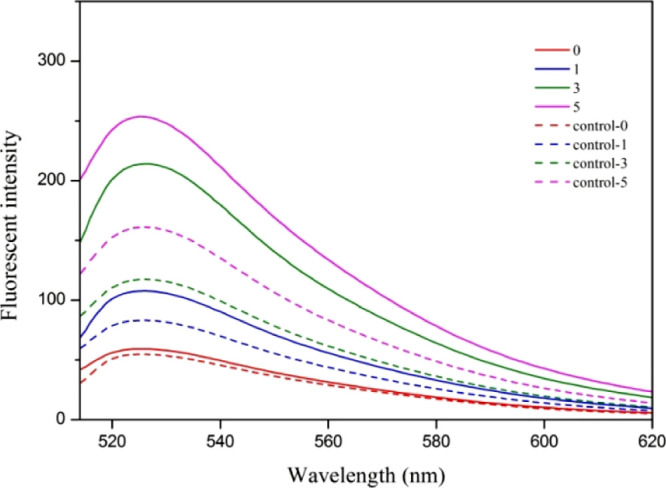
Fluorescence intensity change of SOSG as a function of irradiation time (0, 1, 3, and 5 min) at an excitation of 488 nm, in the absence (control samples) and presence of PVAc–Cu10 coating.
2.5. Oxygen Barrier and Microstructure of PVAc–Cux Coatings
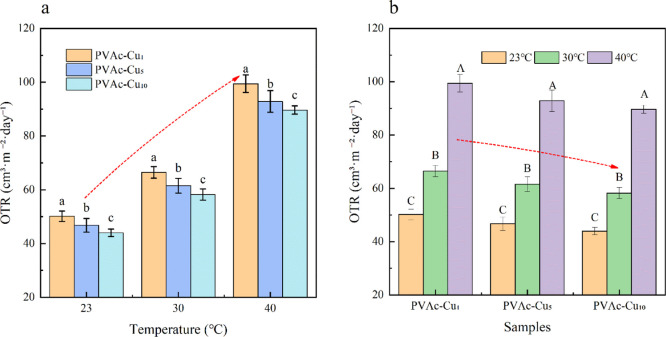
Soft PVAc–Cux coatings were coated on PET film. The oxygen transmission rates for neat PET film at 0% RH condition showed ∼66.29, 85.47, and 122.21 cm3/m2·day at 23, 30, and 40 °C, respectively. In comparison with neat PET film, the oxygen transmission rates for PVAc–Cu0 decreased and showed ∼51.61, 66.79, and 99.85 cm3/m2·day at 23, 30, and 40 °C, respectively. The oxygen transmission rates for PVAc–Cu1 at 0% RH condition showed ∼50.18 cm3/m2·day and decreased with an increase in the curcumin concentration (∼46.75 and 43.96 cm3/m2·day for PVAc–Cu5 and PVAc–Cu10, respectively) at 23 °C, implying the concentration dependence of curcumin in the coatings. The results at 30 and 40 °C represented a similar trend at oxygen transmission rates, as shown in Table 2. The results of factorial design analysis showed that concentration and temperature had a significant effect on the oxygen permeation rate. At the same temperature, the oxygen transmittance of the films decreased significantly (P < 0.05) with the increase in curcumin concentration. At the same concentration, the oxygen transmittance of the films increased significantly with the increase in temperature (P < 0.01), as shown in Figure 8.
Table 2. Oxygen Transmission Rates (OTR, cm3/m2·day) of PET Films Coated by PVAc–Cux (x = 1, 5, and 10) at 0% Relative Humidity and Different Temperatures.
| samples | OTRa | OTRb | OTRc |
|---|---|---|---|
| PET–PVAc–Cu0 | 51.61 ± 1.34 | 66.79 ± 2.58 | 99.85 ± 1.24 |
| PET–PVAc–Cu1 | 50.18 ± 1.58 | 66.45 ± 1.75 | 99.45 ± 2.67 |
| PET–PVAc–Cu5 | 46.75 ± 2.07 | 61.54 ± 2.24 | 92.85 ± 3.27 |
| PET–PVAc–Cu10 | 43.96 ± 1.13 | 58.23 ± 1.70 | 89.66 ± 1.26 |
23 °C.
30 °C.
40 °C.
Figure 8.
Effect of temperature and concentration (a,b) on oxygen transmission rates of PVAc–Cux (x = 0, 1, 5, and 10) coatings at 0% RH. Small and capital letters represent significant differences (P < 0.05) and highly significant differences (P < 0.01), respectively.
With the increased concentration of curcumin, the average roughness of the surface decreased in coatings (PVAc–Cu1, 3.34 nm; PVAc–Cu5, 2.67 nm; and PVAc–Cu10, 2.24 nm), as shown in Figure 9. SEM photographs revealed the smoother surface of coatings when the curcumin concentration increased. Figure 9 showed the static water contact angle of PVAc–Cux coatings (PVAc–Cu1, 59.44°; PVAc–Cu5, 60.70°; and PVAc–Cu10, 64.54°). As shown in Figure 10, the occurrence of a series of 2θ diffraction angles at the range of 5–50 implied the crystallized structure of curcumin powder. No sharp diffraction peaks were observed in PVAc–Cu0 coating because of the amorphous character of PVAc. PVAc–Cux (x = 1, 5, and 10) coatings showed some weak diffraction peaks of curcumin, indicating that the incorporated curcumin was partially crystalline.
Figure 9.
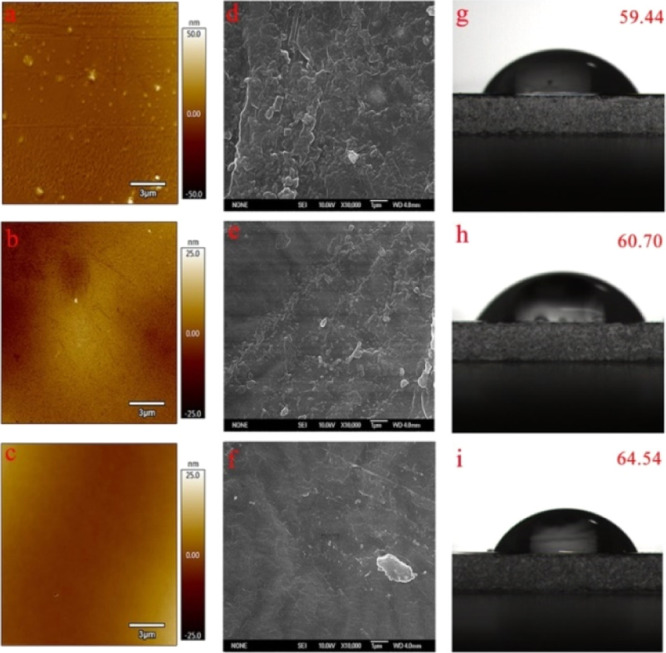
AFM images (a, b, and c for PVAc–Cu1, PVAc–Cu5, and PVAc–Cu10, respectively), SEM images (d, e, and f for PVAc–Cu1, PVAc–Cu5, and PVAc–Cu10, respectively) and static water contact angles on the surfaces of coatings (g, h, and i for PVAc–Cu1, PVAc–Cu5, and PVAc–Cu10, respectively).
Figure 10.
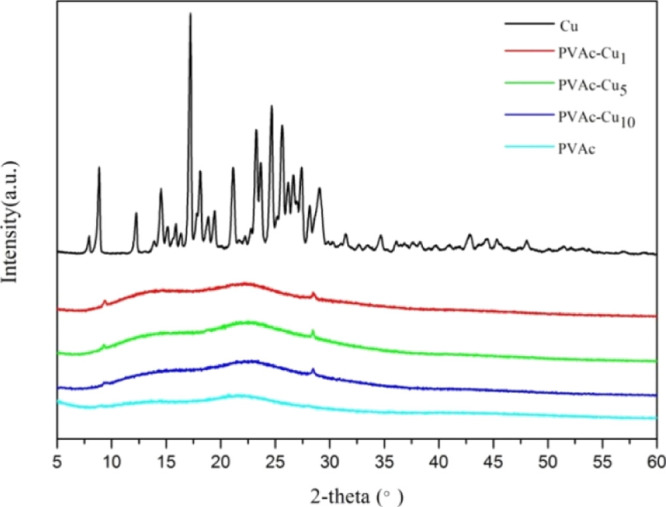
XRD patterns of the curcumin powder and PVAc–Cux (x = 0, 1, 5, and 10) coatings.
2.6. Stability of Coatings in Photoinduced Antibacterials
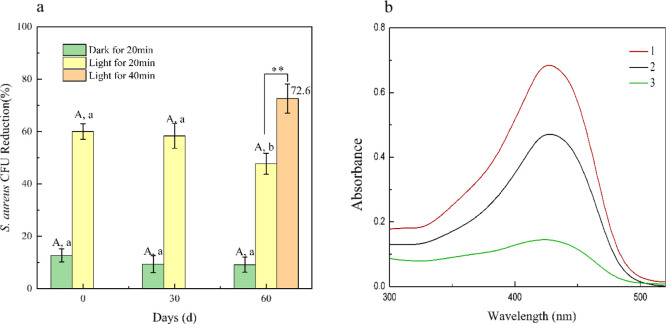
As reported by many research studies, curcumin was apt to rapidly dissociating into vanillin, ferulic acid, and feruloyl methane in alkaline or neutral solution, probably owing to the presence of the unstable β-diketone linker, and almost half of curcumin will degrade in 30 min in aqueous buffer (pH 7.4) solution. As far as we knew, there were no reports on antibacterial stability of coatings utilizing curcumin as an antibacterial agent under white light illumination. In order to evaluate the antibacterial stability of curcumin in coating, we studied the bacterial killing efficiency of PVAc–Cu1 coating in the dark and white light irradiation under different storage times. As shown in Figure 11, the antibacterial efficiency of PVAc–Cu1 against S. aureus under light irradiation was much better than that under dark conditions. The bacteriostatic effect slightly decreased with an increase in the storage time. The bacteriostatic efficiency (58.33%) after 30 days was basically stable in comparison with the initial inhibitory efficiency (60%) at 0 day. After 60 days, the antibacterial efficiency of PVAc–Cu1 decreased significantly from 60 to 47.67% (P < 0.05). The killing efficiency of PVAc–Cu1 coating increased from 47.67 to 72.60% after 40 min illumination, implying that the duration of light induced a significant increase in the antibacterial efficiency of PVAc–Cu1 (P < 0.01). The degradation rate of curcumin in PVAc–Cu1 coating can be estimated by measuring the UV–vis absorption spectrum in solution after the PVAc–Cu1 coating was dissolved at different storage time intervals, as shown in Figure 11b. For PVAc–Cu1 coating stored at room temperature for 60 days, the degradation rate was ∼30% according to the published method8, while the absorbance peak of curcumin in ethanol rapidly decreased after 14 days, implying that curcumin got better stability in coating than in solution. Our results revealed that the PVAc polymeric matrix could act as a good stabilizer for curcumin.
Figure 11.
Changes in biocidal activities (a) of PVAc–Cu1 coating against S. aureus under white light irradiation at a light fluence of 48 J/cm2 (20 min) after storage of 0, 30, and 60 days at 23 °C. The absorption spectrum (b) after PVAc–Cu1 coating was stored for 0 (1) and 60 (2) days and dissolved in ethanol and the absorption spectrum of curcumin in ethanol after a 14-day interval (3). Small letters (P < 0.05) and capital letters and **(P < 0.01).
The same coatings were repeatedly used three times to investigate their abilities to retain the antibacterial activity after multiple challenges with S. aureus bacteria. The change trend of antibacterial efficiency of PVAc–Cux (x = 1, 5 and 10) coatings against S. aureus under white light irradiation at an intensity of 60 mW/cm2 has been shown in Figure 12. With the increased usage times of coatings, the killing rate decreased, probably assigned to the photodegradation of curcumin after UV sterilization and white light irradiation. However, when the concentration of curcumin was higher than 5%, the antibacterial stability was significantly enhanced. Although the antibacterial rate had a downward trend after repeated usages, there was no significant difference (P < 0.05). The killing efficiency of PVAc–Cux (x = 1, 5, and 10, respectively) coatings was 85.92, 87.92, and 97.93% for the first time, slightly higher than 65.41, 73.03, and 89.60% for the third time. When the irradiation time extended to 30 min at an irradiation intensity of 60 mW/cm2, the killing rate of PVAc–Cux (x = 5 and 10) can reach 99.9%. However, the long-time light irradiation can result in the photodegradation of curcumin and reduce their capabilities to retain the antibacterial activity after multiple challenges with bacteria.
Figure 12.
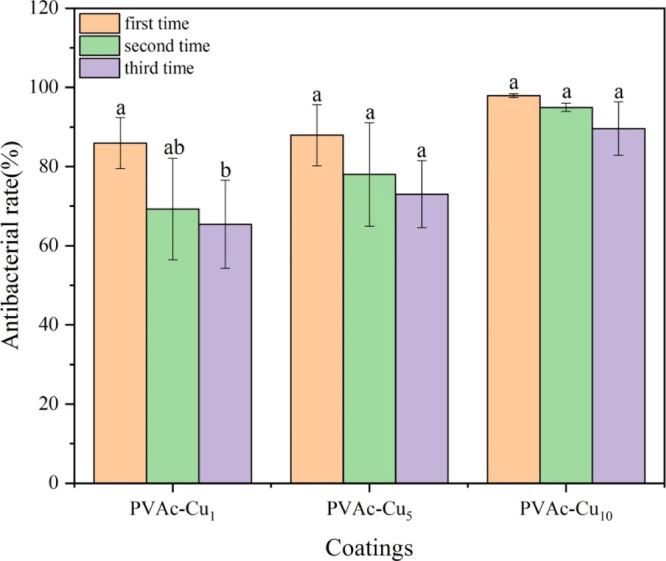
Changes in biocidal activities of PVAc–Cux (x = 1, 5, and 10) coatings against S. aureus under white light irradiation at an intensity of 60 mW/cm2 (20 min) at room temperature, after multiple challenges with bacteria. Small letters (P < 0.05).
2.7. Preservation of Fresh Pork Packaged by PVDC Films with PVAc–Cu10 Coating under White Light Irradiation
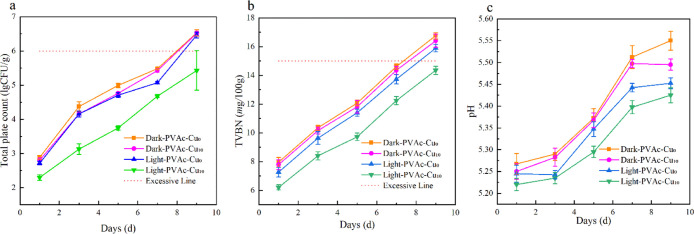
During the storage period, the total viable counts increased in all groups. The TVC value was around 2.2 on the first day, indicating the relatively good quality of pork meat. With the storage time increased, TVC values of pork packaged by PVDC films with PVAc–Cu10 coating under white light irradiation were lower than those in the control groups and dark conditions, thus proving the occurrence of effective photoinduced antibacterial activities. After 9 days, the TVC value at ∼5.4 was still lower than 6.0, which is the critical value of the total bacterial count of meat spoilage. The TVB-N value as an indicator to assess meat freshness is closely in association with the meat decomposition caused by bacteria and enzymes during storage. As shown in Figure 13b, the TVB-N values of the control groups and dark conditions were obviously higher than those of the irradiated groups with PVAc–Cu10 coating, indicating the effective inhibition of meat degradation attributed to the light irradiation during storage. After the ninth day, the TVB-N value at ∼12.5 was still lower than 15.0. However, the TVB-N values in other experimental groups exceeded 15.0. According to the previous report, the TVB-N value below 15 mg/100 g is the acceptable and appropriate limit for the decontamination of pork meat. The change trend of pH values during storage is shown in Figure 13c. All experimental groups exhibited a clear increasing trend of pH values during cold storage. The pH values of the control groups and dark conditions obviously remained higher than those of the irradiated group with PVAc–Cu10 coating, indicating the effective inhibition of the formation of alkaline substances, such as ammonia, biogenic amines, and trimethylamines, caused by microorganisms and endogenous enzymes.
Figure 13.
Effect of white light irradiation (15 min, 40 mW/cm2) on total viable counts [(a) TVCs, log10 CFU/g], total volatile basic nitrogen [(b), TVB-N, mg/100 g] and pH values (c) of packaged pork by means of PVDC films with PVAc–Cu10 coating during storage at 4 °C.
3. Conclusion
In the present work, photoactive PVAc–Cux coatings (x = 1, 5, and 10) were utilized as thin-layer protecting systems for food-packaging purposes. Toward S. typhimurium or S. aureus, the investigation on biocidal activity of PVAc–Cux coatings demonstrated obvious concentration dependence of curcumin, and the killing effect increased with irradiation time. The killing efficiency of PVAc–Cu10 coating against S. aureus reached 93% at 60 mW/cm2 irradiation intensity in 20 min, indicating the occurrence of effective photoinduced antibacterial interaction in coating. The oxygen transmission rate of coatings will vary with the change in temperature and curcumin concentration. The bacteriostatic efficiency at 58.33% after 30 days was basically stable in comparison with the initial inhibitory efficiency at 60% at 0 day, thus implying the stability of photoinduced antibacterial interaction in coating. The average fluorescence lifetime of PVAc–Cux coatings at 20 °C is 0.89, 1.29, and 1.95 ns. In comparison with fluorescence lifetime of curcumin in solution, the much longer lifetimes in coatings were probably assigned to slower conformational change or tautomerization in a rigid microenvironment, thus leading to stronger stability of curcumin. TVC and TVB-N values in meat packaged by PVDC films with PVAc–Cu10 coatings upon white light irradiation were obviously lower than values in control experiments during storage, demonstrating practicability of the coating in decontaminating fresh pork.
4. Materials and Methods
4.1. Materials
Polyvinyl acetate was purchased from Sinopharm Chemical Reagent Co. Ltd. Polyethylene terephthalate (PET) and polyvinylidene chloride (PVDC) film were obtained from Guanjia Co. Ltd. Curcumin powder with a purity of 98% (J&K Scientific Co. Ltd.) was used without further purification. S. aureus and S. typhimurium used for the antibacterial experiments were provided by the School of Food Science and Technology in the Beijing University of Agriculture.
4.2. Molecular Simulation
Discrete Fourier transform calculations were performed based on the Gaussian 09 package. The structural optimization was performed at the B3LYP/6-31+g(d) level with Grimme’s D3 dispersion corrections. The interaction energy (ΔE) was calculated by the following equation: ΔE = ECur–PA3 – (ECur + EPA3), where ECur–PA3, ECur, and EPA3 corresponded to the electronic energy of the curcumin–PA3 complex, curcumin, and PA3, respectively.
4.3. Preparation of the PVAc–Cux Coatings
The abbreviation PVAc–Cux (x = 1, 5, and 10%, the weight ratio of curcumin to PVAc in coatings) was adopted in the manuscript. The general procedure for the fabrication of PVAc–Cu1 coating is as follows: the coating solution was prepared by dissolving PVAc (2.0 g) powder in ethanol (40 mL) in a magnetic stirrer at 45 °C. Afterward, curcumin (0.02 g) was added to the PVAc solution, and the final solution was coated on the surface of PVDC and PET film by utilizing a coating machine, with a thickness of 0.1 and 1 mm, respectively. After the wet coat was dried at room temperature for 24 h and vacuum-dried for 6 h to remove ethanol, PVAc–Cu1 coating was obtained. PVAc–Cu5 and PVAc–Cu10 coatings were obtained by following a similar procedure to prepare PVAc–Cu1.
4.4. Equipment and Methods
Contact angles for water drops on coating surfaces were assessed by means of a measurement system (OCA 15EC, Germany) at room temperature. The surface images of the membranes were recorded using an atomic force microscope (Oxford Cypher VRS, UK). Scanning electron microscopy (JEOL Jsm-6700F, JP) was used to visualize the surface of coatings and morphology of S. aureus. SEM of S. aureus: Bacterial solution irradiated by white light at 60 mW/cm2 for 20 min at the presence of curcumin coating was centrifuged for 3 min at 4 °C. After removing the supernatant, precipitation was washed with PBS solution three times. Then, glutaraldehyde (2.5%) solution was used to cover the surface of the precipitation and stored overnight at 4 °C after being kept for 6 h at room temperature. After rinsing the sample with PBS solution and centrifuging three times, the bacterial sludge was fixed using the mixed solution of osmic acid and PBS solution at a 1:1 ratio until it blackened. Then, the fixed sludge was washed with PBS three times, and centrifugation removed the supernatant. After gradient dehydration using ethanol (30%, 50%, 70%, 90%, anhydrous ethanol and butanol), the bacterial sludge was placed on a filter paper and frozen at −20 °C for 20 min, followed by freeze-drying in a vacuum freeze dryer for 4 h. The dried bacterial sludge was sprayed with gold for SEM observation. At the same time, the control experiment was carried out for comparison. The morphology of S. typhimurium was measured using a Hitachi HT-7650 transmission electron microscope (TEM). Oxygen transmission rate (OTR) values of coatings were obtained using an OX-TRAN model 2/21 instrument at 23 °C and in 0% relative humidity (RH). Fluorescence lifetimes were evaluated using a time-correlated single photon counting spectrometer (TCSPC, Edinburgh instruments FS5). The average fluorescence lifetimes for the decay curves were calculated from the decay times and the relative contributions of the components using the following equation: τav = a1τ1 + a2τ2, where τ1 and τ2 are the first and second components of the decay time of curcumin, respectively, and a1 and a2 are the corresponding relative amplitudes of these components. Fluorescence quantum yields (Φf) were measured in an integrating sphere (IS-080, Labsphere) under the 442 nm line of a HeCd laser.43 The radiative (Kr, s–1) and nonradiative (Knr, s–1) decay rate constants of curcumin in coatings were evaluated using the following equations: Kr = Φf/τav and Knr = [1/τav] – Kr. The generation of 1O2 by coatings upon white light irradiation was determined by the singlet oxygen sensor green reagent (SOSG) method.44SOSG (100 μg) was dissolved in methanol (33 μL) to prepare a solution (5 mM). Then, PVAc–Cu10 coating with 2 × 2 cm2 was put into plates with a diameter of 35 mm. After deionized water (4 mL) was added, SOSG indicator solution (10 μL) was added, followed by white light irradiation at a light intensity of 60 mW/cm2 at different time intervals of 0, 1, 3, and 5 min. Finally, the fluorescence spectrum was recorded using a fluorescence spectrophotometer (Varian Cary Eclipse) at an excitation of 488 nm.
4.5. Antibacterial Assessment
Phototoxicity test of coatings toward S. typhimurium and S. aureus was performed as follows:45 After both sides of the film were sterilized under ultraviolet light for 15 min, the film was cut into tiny squares of 10 × 10 mm2 and placed at the bottom of the well of a 12-well plate with the coated side facing up. A bacterial broth (50 μL, 107 CFU/mL) was inoculated onto each test film, and the bacterial broth cultured in the well in the absence of the test film was used as a control. The 12-well plate was protected by transparent plastic cover to avoid the contamination. The wells containing PVAc–Cux coatings and bacteria were incubated in the dark or irradiated by means of an LED lamp (400–800 nm, PLS-LED100, PL-MW 200 photoradiometer, PerfectLight Co. Ltd. China) at a fluence rate of 20, 40, and 60 mW/cm2 for 20 min, which is corresponding to an energy density of 24, 48, and 72 J/cm2, respectively. The lamp was placed at a distance of 15 cm above the samples. Then, the coatings with bacteria were washed with PBS buffer solution (1 mL, KH2PO4/K2HPO4, 10 mM, pH 7.4) under ultrasonic treatment for 75 s. After gradient dilution of the phosphate buffer with bacteria, a volume (0.1 mL) of the solution was plated on LB agar plates and incubated for 24 h at 37 °C. Viable counts were estimated by a plate count technique. The inhibitory activities (I) under white light irradiation or dark conditions were assessed using the following equation: I (%) = [(N1 – N2)/N1] × 100%, where N1 is the total number of bacteria (CFU/mL) in the control sample in the absence of coatings and N2 is the total number of bacteria of the sample after irradiation or dark conditions in the presence of coatings. The experiment was performed three times.
4.6. Photo-Induced Effect of Curcumin-Containing Coating on TVB-N, TVC and pH Values in the Preservation of Fresh Pork46
Fresh meat was purchased from the supermarket
and kept at 4 °C in an ice bag. The meat was processed into samples
after being preserved in the refrigerator for 1 h. Experimental appliances
such as the chopping board, kitchen knife, analytical balance, disposable
Petri dish, and aseptic bag were placed on an ultra-clean worktable
for ultraviolet sterilization for 30 min. The meat was cut into pieces
with a rectangular shape after removing the fat and skin of the meat.
The experimental samples were weighed and packaged by PVDC films with and without PVAc–Cu10 coating. Both sides of the packaged meat were irradiated for
15 min under white light at an intensity of 40 mW/cm2.
The packaged meat in the absence of irradiation was used as control
samples. Finally, all experimental samples were stored in a refrigerator
at 4 °C. Total volatile basic nitrogen (TVB-N) was
estimated by the microtitration method involving steam distillation
and titration with HCl. First of all, a sample (20.0 g) was minced
and immersed in deionized water (100 mL) for 30 min. The obtained
supernatant (10 mL) was mixed with MgO (5 mL, 10 g/L) and was distilled
using a Kjeldahl nitrogen apparatus. The distillate was collected
with boric acid solution (10 mL, 20 g/L) containing a mixed indicator
consisting of methyl red (1 g/L) and methylene (1 g/L) blue in ethanol.
Then, the distillation solution was titrated with HCl solution (0.01
M). For comparison, distilled water (10 mL) was used instead of the
sample as a blank test. The value of TVBN was calculated
based on the consumption of HCl using the following equation: 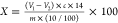 , where V1 is
volume (mL) of HCl used for the sample, V2 is the volume (mL) of HCl used for the blank, C is the concentration of HCl (mol/L), and m is the
weight of the sample (g). Different microorganisms were determined
by the spread plate method. The minced sample (25.0 g) was homogenized
with 225 mL phosphate buffer solution for 2 min. Further serial decimal
dilutions were performed for the determination of microorganisms.
A diluting solution (1 mL) was dropped onto the surface of plate count
agar and coated evenly, and the plates were incubated at 37 °C
for 24 h to calculate total viable counts (TVCs). All
operations were carried out under aseptic conditions. Plate count
agar was selected to determine the total number of spoilage bacteria.
The pH values were assessed by inserting a pH meter probe into the
fresh meat (Tengtu instrument, testo 205).
, where V1 is
volume (mL) of HCl used for the sample, V2 is the volume (mL) of HCl used for the blank, C is the concentration of HCl (mol/L), and m is the
weight of the sample (g). Different microorganisms were determined
by the spread plate method. The minced sample (25.0 g) was homogenized
with 225 mL phosphate buffer solution for 2 min. Further serial decimal
dilutions were performed for the determination of microorganisms.
A diluting solution (1 mL) was dropped onto the surface of plate count
agar and coated evenly, and the plates were incubated at 37 °C
for 24 h to calculate total viable counts (TVCs). All
operations were carried out under aseptic conditions. Plate count
agar was selected to determine the total number of spoilage bacteria.
The pH values were assessed by inserting a pH meter probe into the
fresh meat (Tengtu instrument, testo 205).
4.7. Statistical Analysis
All data were presented as a mean value with their standard deviation (mean ± S.D.). Differences were accepted as significant (P < 0.05) and highly significant (P < 0.01).
Acknowledgments
This work was supported by grants from the National Science Foundation of China (no. 21772014) and the Scientific Research Program of the Beijing Municipal Education Commission (no. KM202010020003).
Author Contributions
L.C., Z.S. are co-first authors.
The authors declare no competing financial interest.
References
- Tsuda T. Curcumin as a functional food-derived factor: degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. 10.1039/c7fo01242j. [DOI] [PubMed] [Google Scholar]
- Chen R.; Wulff J. E.; Moffitt M. G. Microfluidic Processing Approach to Controlling Drug Delivery Properties of Curcumin-Loaded Block Copolymer Nanoparticles. Mol. Pharm. 2018, 15, 4517–4528. 10.1021/acs.molpharmaceut.8b00529. [DOI] [PubMed] [Google Scholar]
- Wang A.; Muhammad F.; Qi W.; Wang N.; Chen L.; Zhu G. Acid-Induced Release of Curcumin from Calcium Containing Nanotheranostic Excipient. ACS Appl. Mater. Interfaces 2014, 6, 14377–14383. 10.1021/am503655z. [DOI] [PubMed] [Google Scholar]
- El-Sherbiny I. M.; Smyth H. D. C. Controlled Release Pulmonary Administration of Curcumin Using Swellable Biocompatible Microparticles. Mol. Pharm. 2012, 9, 269–280. 10.1021/mp200351y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boztas A. O.; Karakuzu O.; Galante G.; Ugur Z.; Kocabas F.; Altuntas C. Z.; Yazaydin A. O. Synergistic Interaction of Paclitaxel and Curcumin with Cyclodextrin Polymer Complexation in Human Cancer Cells. Mol. Pharm. 2013, 10, 2676–2683. 10.1021/mp400101k. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Sanidad K. Z.; Sukamtoh E.; Zhang G. Potential roles of chemical degradation in the biological activities of curcumin. Food Funct. 2017, 8, 907–914. 10.1039/c6fo01770c. [DOI] [PubMed] [Google Scholar]
- Bai F.; Diao J.; Wang Y.; Sun S.; Zhang H.; Liu Y.; Wang Y.; Cao J. A New Water-Soluble Nanomicelle Formed through Self-Assembly of Pectin–Curcumin Conjugates: Preparation, Characterization, and Anticancer Activity Evaluation. J. Agric. Food Chem. 2017, 65, 6840–6847. 10.1021/acs.jafc.7b02250. [DOI] [PubMed] [Google Scholar]
- Panja S.; Behera S.; Kundu S. C.; Halder M. Optical Spectroscopic and Morphological Characterizations of Curcuminized Silk Biomaterials: A Perspective from Drug Stabilization. ACS Omega 2017, 2, 6755–6767. 10.1021/acsomega.7b00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R.; Tønnesen H. H.; Kristensen S.; Berg K. The influence of Pluronics on dark cytotoxicity, photocytotoxicity, localization and uptake of curcumin in cancer cells: studies of curcumin and curcuminoids XLIX. Photochem. Photobiol. Sci. 2013, 12, 559–575. 10.1039/c2pp25249j. [DOI] [PubMed] [Google Scholar]
- Mukerjee A.; Sørensen T. J.; Ranjan A. P.; Raut S.; Gryczynski I.; Vishwanatha J. K.; Gryczynski Z. Spectroscopic Properties of Curcumin: Orientation of Transition Moments. J. Phys. Chem. B 2010, 114, 12679–12684. 10.1021/jp104075f. [DOI] [PubMed] [Google Scholar]
- Harada T.; McTernan H. L.; Pham D.-T.; Lincoln S. F.; Kee T. W. Femtosecond Transient Absorption Spectroscopy of the Medicinal Agent Curcumin in Diamide Linked γ-Cyclodextrin Dimers. J. Phys. Chem. B 2015, 119, 2425–2433. 10.1021/jp507272f. [DOI] [PubMed] [Google Scholar]
- Wu J.; Wang J.; Zhang J.; Zheng Z.; Kaplan D. L.; Li G.; Wang X. Oral Delivery of Curcumin Using Silk Nano- and Microparticles. ACS Biomater. Sci. Eng. 2018, 4, 3885–3894. 10.1021/acsbiomaterials.8b00454. [DOI] [PubMed] [Google Scholar]
- Teng Z.; Li Y.; Wang Q. Insight into Curcumin-Loaded β-Lactoglobulin Nanoparticles: Incorporation, Particle Disintegration, and Releasing Profiles. J. Agric. Food Chem. 2014, 62, 8837–8847. 10.1021/jf503199g. [DOI] [PubMed] [Google Scholar]
- Adhikary R.; Mukherjee P.; Kee T. W.; Petrich J. W. Excited-State Intramolecular Hydrogen Atom Transfer and Solvation Dynamics of the Medicinal Pigment Curcumin. J. Phys. Chem. B 2009, 113, 5255–5261. 10.1021/jp901234z. [DOI] [PubMed] [Google Scholar]
- Song S.; Wang Z.; Qian Y.; Zhang L.; Luo E. The Release Rate of Curcumin from Calcium Alginate Beads Regulated by Food Emulsifiers. J. Agric. Food Chem. 2012, 60, 4388–4395. 10.1021/jf3006883. [DOI] [PubMed] [Google Scholar]
- Safavy A.; Raisch K. P.; Mantena S.; Sanford L. L.; Sham S. W.; Krishna N. R.; Bonner J. A. Design and Development of Water-Soluble Curcumin Conjugates as Potential Anticancer Agents. J. Med. Chem. 2007, 50, 6284–6288. 10.1021/jm700988f. [DOI] [PubMed] [Google Scholar]
- Chen F.-P.; Ou S.-Y.; Tang C.-H. Core–Shell Soy Protein–Soy Polysaccharide Complex (Nano)particles as Carriers for Improved Stability and Sustained Release of Curcumin. J. Agric. Food Chem. 2016, 64, 5053–5059. 10.1021/acs.jafc.6b01176. [DOI] [PubMed] [Google Scholar]
- Liu C.; Cheng F.; Yang X. Fabrication of a Soybean Bowman–Birk Inhibitor (BBI) Nanodelivery Carrier to Improve Bioavailability of Curcumin. J. Agric. Food Chem. 2017, 65, 2426–2434. 10.1021/acs.jafc.7b00097. [DOI] [PubMed] [Google Scholar]
- Wang F.; Yang Y.; Ju X.; Udenigwe C. C.; He R. Polyelectrolyte Complex Nanoparticles from Chitosan and Acylated Rapeseed Cruciferin Protein for Curcumin Delivery. J. Agric. Food Chem. 2018, 66, 2685–2693. 10.1021/acs.jafc.7b05083. [DOI] [PubMed] [Google Scholar]
- Sneharani A. H.; Karakkat J. V.; Singh S. A.; Rao A. G. A. Interaction of Curcumin with β-Lactoglobulin;Stability, Spectroscopic Analysis, and Molecular Modeling of the Complex. J. Agric. Food Chem. 2010, 58, 11130–11139. 10.1021/jf102826q. [DOI] [PubMed] [Google Scholar]
- Bhawana; Basniwal R. K.; Buttar H. S.; Jain V. K.; Jain N. Curcumin Nanoparticles: Preparation, Characterization, and Antimicrobial Study. J. Agric. Food Chem. 2011, 59, 2056–2061. 10.1021/jf104402t. [DOI] [PubMed] [Google Scholar]
- Marcolino V. A.; Zanin G. M.; Durrant L. R.; Benassi M. D. T.; Matioli G. Interaction of Curcumin and Bixin with β-Cyclodextrin: Complexation Methods, Stability, and Applications in Food. J. Agric. Food Chem. 2011, 59, 3348–3357. 10.1021/jf104223k. [DOI] [PubMed] [Google Scholar]
- Banerjee C.; Ghatak C.; Mandal S.; Ghosh S.; Kuchlyan J.; Sarkar N. Curcumin in Reverse Micelle: An Example to Control Excited-State Intramolecular Proton Transfer (ESIPT) in Confined Media. J. Phys. Chem. B 2013, 117, 6906–6916. 10.1021/jp401840z. [DOI] [PubMed] [Google Scholar]
- Peng S.; Zou L.; Liu W.; Liu C.; McClements D. J. Fabrication and Characterization of Curcumin-Loaded Liposomes Formed from Sunflower Lecithin: Impact of Composition and Environmental Stress. J. Agric. Food Chem. 2018, 66, 12421–12430. 10.1021/acs.jafc.8b04136. [DOI] [PubMed] [Google Scholar]
- Harada T.; Lincoln S. F.; Kee T. W. Excited-State dynamics of the medicinal pigment curcumin in a hydrogel. Phys. Chem. Chem. Phys. 2016, 18, 28125–28133. 10.1039/c6cp05648b. [DOI] [PubMed] [Google Scholar]
- Kharat M.; Du Z.; Zhang G.; McClements D. J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. 10.1021/acs.jafc.6b04815. [DOI] [PubMed] [Google Scholar]
- Hu F.; Xu S. D.; Liu B. Photosensitizers with Aggregation-Induced Emission: Materials and Biomedical Applications. Adv. Mater. 2018, 30, 1801350. 10.1002/adma.201801350. [DOI] [PubMed] [Google Scholar]
- Tønnesen H. H.; de Vries H.; Karlsen J.; Beijersbergen van Henegouwen G. Studies on curcumin and curcuminoids. IX: investigation of the photobiological activity of curcumin using bacterial indicator systems. J. Pharm. Sci. 1987, 76, 371–373. 10.1002/jps.2600760506. [DOI] [PubMed] [Google Scholar]
- Haukvik T.; Bruzell E.; Kristensen S.; Tønnesen H. H. Photokilling of bacteria by curcumin in different aqueous preparations. Studies on curcumin and curcuminoids XXXVII. Pharmazie 2009, 64, 666–673. 10.1691/ph.2009.0000. [DOI] [PubMed] [Google Scholar]
- Haukvik T.; Bruzell E.; Kristensen S.; Tønnesen H. H. Photokilling of bacteria by curcumin in selected polyethylene glycol 400 (PEG 400) preparations Studies on curcumin and curcuminoids, XLI. Pharmazie 2010, 65, 600–606. 10.1691/ph.2010.0048. [DOI] [PubMed] [Google Scholar]
- Hegge A. B.; Andersen T.; Melvik J. E.; Bruzell E.; Kristensen S.; Tønnesen H. H. Formulation and bacterial phototoxicity of curcumin loaded alginate foams for wound treatment applications: studies on curcumin and curcuminoides XLII. J. Pharm. Sci. 2011, 100, 174–185. 10.1002/jps.22263. [DOI] [PubMed] [Google Scholar]
- Valdés A.; Mellinas A. C.; Ramos M.; Burgos N.; Jiménez A.; Garrigós M. C. Use of herbs, spices and their bioactive compounds in active food packaging. RSC Adv. 2015, 5, 40324–40335. 10.1039/c4ra17286h. [DOI] [Google Scholar]
- Silva A. C. d.; Santos P. D. d. F.; Palazzi N. C.; Leimann F. V.; Fuchs R. H. B.; Bracht L.; Gonçalves O. H. Production and characterization of curcumin microcrystals and evaluation of the antimicrobial and sensory aspects in minimally processed Carrots. Food Funct. 2017, 8, 1851–1858. 10.1039/c7fo00452d. [DOI] [PubMed] [Google Scholar]
- Wu C.; Zhu Y.; Wu T.; Wang L.; Yuan Y.; Chen J.; Hu Y.; Pang J. Enhanced functional properties of biopolymer film incorporated with curcurmin-loaded mesoporous silica nanoparticles for food packaging. Food Chem. 2019, 288, 139–145. 10.1016/j.foodchem.2019.03.010. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Cai Y.; Jiang X.; Wu J.; Le X. Molecular interactions, characterization and antimicrobial activity of curcumin–chitosan blend films. Food Hydrocolloids 2016, 52, 564–572. 10.1016/j.foodhyd.2015.08.005. [DOI] [Google Scholar]
- Liu J.; Wang H.; Wang P.; Guo M.; Jiang S.; Li X.; Jiang S. Films based on κ-carrageenan incorporated with curcumin for freshness monitoring. Food Hydrocolloids 2018, 83, 134–142. 10.1016/j.foodhyd.2018.05.012. [DOI] [Google Scholar]
- Condat M.; Mazeran P.-E.; Malval J.-P.; Lalevée J.; Morlet-Savary F.; Renard E.; Langlois V.; Abbad Andalloussi S.; Versace D.-L. Photoinduced curcumin derivative-coatings with antibacterial properties. RSC Adv. 2015, 5, 85214–85224. 10.1039/c5ra19499g. [DOI] [Google Scholar]
- Rodriguez N. L. G.; Thielemans W.; Dufresne A. Sisal cellulose whiskers reinforced polyvinyl acetate nanocomposites. Cellulose 2006, 13, 261–270. 10.1007/s10570-005-9039-7. [DOI] [Google Scholar]
- Liu K.; Liu Y.; Yao Y.; Yuan H.; Wang S.; Wang Z.; Zhang X. Supramolecular Photosensitizers with Enhanced Antibacterial Efficiency. Angew. Chem., Int. Ed. 2013, 52, 8285–8289. 10.1002/anie.201303387. [DOI] [PubMed] [Google Scholar]
- Li X.; Lee D.; Huang J.-D.; Yoon J. Phthalocyanine-Assembled Nanodots as Photosensitizers for Highly Efficient Type I Photoreactions in Photodynamic Therapy. Angew. Chem., Int. Ed. 2018, 57, 9885–9890. 10.1002/anie.201806551. [DOI] [PubMed] [Google Scholar]
- Mandal S.; Banerjee C.; Ghosh S.; Kuchlyan J.; Sarkar N. Modulation of the Photophysical Properties of Curcumin in Nonionic Surfactant (Tween-20) Forming Micelles and Niosomes: A Comparative Study of Different Microenvironments. J. Phys. Chem. B 2013, 117, 6957–6968. 10.1021/jp403724g. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; He H.; Dong K.; Fan M.; Zhang S. A DFT study on lignin dissolution in imidazolium-based ionic liquids. RSC Adv. 2017, 7, 12670–12681. 10.1039/c6ra27059j. [DOI] [Google Scholar]
- Aluigi A.; Sotgiu G.; Torreggiani A.; Guerrini A.; Orlandi V. T.; Corticelli F.; Varchi G. Methylene Blue Doped Films of Wool Keratin with Antimicrobial Photodynamic Activity. ACS Appl. Mater. Interfaces 2015, 7, 17416–17424. 10.1021/acsami.5b04699. [DOI] [PubMed] [Google Scholar]
- Delcanale P.; Montali C.; Rodríguez-Amigo B.; Abbruzzetti S.; Bruno S.; Bianchini P.; Diaspro A.; Agut M.; Nonell S.; Viappiani C. Zinc-Substituted Myoglobin Is a Naturally Occurring Photoantimicrobial Agent with Potential Applications in Food Decontamination. J. Agric. Food Chem. 2016, 64, 8633–8639. 10.1021/acs.jafc.6b03368. [DOI] [PubMed] [Google Scholar]
- Kim S.; Fujitsuka M.; Majima T. Photochemistry of Singlet Oxygen Sensor Green. J. Phys. Chem. B 2013, 117, 13985–13992. 10.1021/jp406638g. [DOI] [PubMed] [Google Scholar]
- Sun X.; Guo X.; Ji M.; Wu J.; Zhu W.; Wang J.; Cheng C.; Chen L.; Zhang Q. Preservative effects of fish gelatin coating enriched with CUR/βCD emulsion on grass carp (Ctenopharyngodon idellus) fillets during storage at 4 °C. Food Chem. 2019, 272, 643–652. 10.1016/j.foodchem.2018.08.040. [DOI] [PubMed] [Google Scholar]