Abstract
Although tuberculosis is a major underlying cause of pulmonary mycetoma due to Scedosporium apiospermum, little is known about coinfection with nontuberculous mycobacteria (NTM) and S. apiospermum. A 67‐year‐old man with NTM presented with recurrent haemoptysis. Computed tomography of the chest revealed pulmonary mycetoma in the left upper lobe of the lung, and culture of bronchial washing fluid yielded S. apiospermum. Oral voriconazole therapy ameliorated both haemoptysis and mycetoma findings. As far as we are aware, this is the first reported case of S. apiospermum lung disease in a patient with NTM but without tuberculosis. The possibility of S. apiospermum infection should thus be considered in the differential diagnosis of pulmonary mycetoma. Although S. apiospermum mycetoma resembles aspergilloma, antifungal strategies for these two conditions differ, with the collection of culture specimens such as by bronchoscopy being compulsory for accurate diagnosis and appropriate management of S. apiospermum infection.
Keywords: Haemoptysis, nontuberculous mycobacteria, pulmonary mycetoma, Scedosporium apiospermum, voriconazole
Although tuberculosis has been identified as a major underlying cause of pulmonary mycetoma due to Scedosporium apiospermum infection, little is known about coinfection with nontuberculous mycobacteria (NTM) and S. apiospermum. We now report a case of S. apiospermum lung disease in a patient with NTM infection, but with neither tuberculosis nor an immunosuppressed condition. This case reinforces the fact that clinicians should be aware of the possibility of S. apiospermum infection even in patients who are immunocompetent or free from tuberculosis.
Introduction
Scedosporium apiospermum is a widely distributed saprophytic fungus. It infects the lungs only rarely, with pulmonary manifestations ranging from simple colonization to mycetoma formation and invasive disease [1]. Clinical and radiological features of pulmonary S. apiospermum infection are similar to those of disease caused by Aspergillus spp., but it is essential that these two fungal infections be distinguished to allow for appropriate treatment, given the differences in antifungal resistance between Scedosporium and Aspergillus spp. [2].
Tuberculosis has been identified as a major predisposing factor for pulmonary S. apiospermum mycetoma [1]. Although aspergilloma develops readily in individuals infected with nontuberculous mycobacteria (NTM) as well as in those with tuberculosis, little is known about the relation of S. apiospermum mycetoma to NTM infection. We now report a case of haemoptysis due to fungus balls caused by S. apiospermum in a patient with NTM‐related lung disease.
Case Report
A 67‐year‐old Japanese man presented at our hospital with recurrent haemoptysis that he had experienced for 10 months. He had been undergoing management for NTM lung disease due to Mycobacterium kansasii and Mycobacterium avium—including the administration of several antibiotics (such as rifampicin, ethambutol, clarithromycin, and sitafloxacin)—for more than 15 years. He thus initially received successful antibiotic treatment over two years for M. kansasii lung disease. Five years later, he developed M. avium lung disease. Administration of antibiotic therapy over another two years ameliorated the disease, but it worsened again six months after the end of the therapy. Despite continuous antibiotic treatment for the past six years, the NTM lesions had grown slowly. Three years before presentation at our hospital, he also underwent segmentectomy as a diagnostic treatment for a tumour‐mimicking nodule in the right upper lobe of the lung, and the resected specimen revealed massive caseous necrosis with M. avium pathogens. He had no other history of serious infection, immunodeficiency disorder, or taking of immunosuppressive medication, and he had a negative human immunodeficiency virus serostatus and a normal glycated haemoglobin concentration. He was a 4.5 pack‐year former smoker, but had refrained from smoking for 42 years. His occupation had been a steelworker until he retired eight years before admission.
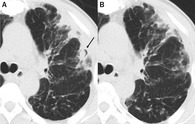
Physical examination revealed that the patient was afebrile and had an oxygen saturation of 97% on room air. There were no abnormal breath sounds. Blood analysis showed an elevated serum level of C‐reactive protein (3.71 mg/dL). An interferon‐γ release assay for tuberculosis, β‐d‐glucan assay, and Aspergillus galactomannan enzyme‐linked immunosorbent assay were all negative. Computed tomography (CT) of the chest revealed cavitary lesions with intracavitary nodules, with the typical appearance of fungus balls, in the left upper lobe of the lung (Fig. 1A). No pathogens other than NTM were detected by Gram staining, acid‐fast staining, or culture of sputum.
Figure 1.
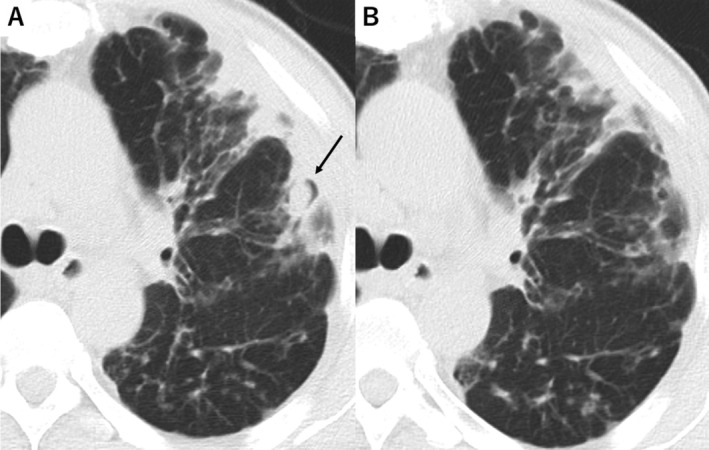
Computed tomography findings of the chest. (A) Cavitary lesions with fungus balls (arrow) were detected in the left upper lobe of the lung on admission. (B) Treatment with voriconazole for six months ameliorated the cavitary lesions.
Bronchoscopy revealed purulent secretion throughout the trachea and bronchi. Culture of bronchial washing fluid from the left superior lobar bronchus yielded S. apiospermum (Fig. 2), leading to a diagnosis of pulmonary S. apiospermum mycetoma. Oral treatment with voriconazole was initiated at a dose of 400 mg/day, which was subsequently increased to 500 mg/day on the basis of therapeutic drug monitoring. Taking into account the interactions between rifampicin and voriconazole, we stopped rifampicin before starting voriconazole therapy. The frequency of haemoptysis declined, and the cavitations and fungus balls in the CT scan were ameliorated at six months after the initiation of the antifungal agent (Fig. 1B).
Figure 2.
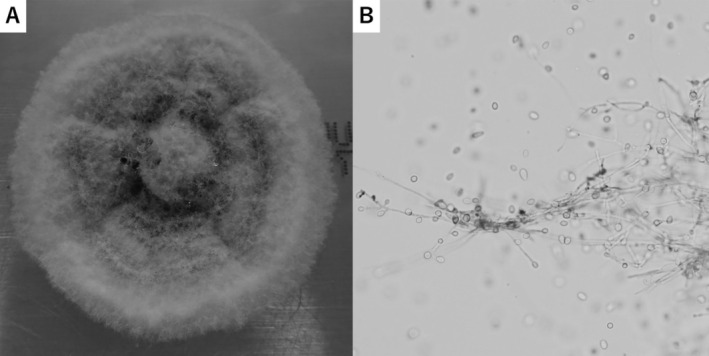
Scedosporium apiospermum cultured from bronchial washing fluid collected from the left superior lobar bronchus. (A) Macroscopic findings for culture on Sabouraud dextrose agar. (B) Lactophenol cotton blue staining (original magnification: 400×).
Discussion
Scedosporium apiospermum is a rare fungal cause of pulmonary infection. A literature review revealed the presence of cavitary lesions containing fungus balls, often referred to as pulmonary mycetoma, in 24.3% of reported cases of pulmonary S. apiospermum infection. It also indicated that pulmonary mycetoma due to S. apiospermum occurred predominantly in individuals with tuberculosis or in those with an immunocompromised condition such as is associated with solid organ transplantation, haematological malignancy, or chronic corticosteroid use [1]. Pulmonary mycetoma due to Aspergillus spp. infection, or aspergilloma, is frequently accompanied by either tuberculosis or NTM infection. However, in contrast to tuberculosis, there have been only two reported cases of coinfection with S. apiospermum and NTM in the lung, both of which were in patients with a history of tuberculosis [3, 4]. Although the reason for the rareness of coinfection with NTM and S. apiospermum is unknown, it might be at least in part due to the lower frequency of cavitations in individuals with NTM than in those with tuberculosis [5]. In addition, patients with cavitary NTM pulmonary disease were found to be less likely to have underlying comorbidities than were those with cavitary tuberculosis [5], which might influence the tendency to develop S. apiospermum coinfection. On the other hand, an in vitro study showed that M. avium infection attenuated lymphocyte immune responses via the programmed cell death‐1 pathway, which might be expected to facilitate the development of S. apiospermum infection [6]. As far as we are aware, the present case is the first reported for S. apiospermum lung disease in a patient with NTM infection, but with neither tuberculous infection nor an immunosuppressed condition.
As in the present case, haemoptysis is reported to be a common and potentially life‐threatening symptom of pulmonary S. apiospermum mycetoma, as it is also in pulmonary aspergilloma. In addition, the radiological findings of pulmonary S. apiospermum mycetoma mimic those of aspergilloma [1]. Although it is difficult to distinguish between these two conditions, a definitive diagnosis of scedosporiosis is important for clinical management because it presents unique treatment challenges, with S. apiospermum being resistant to several antifungal agents including amphotericin B [2]. Given that aspergilloma has been identified as a risk factor for haemoptysis among patients with mycobacterial disease [7], S. apiospermum infection may have a similar unfavourable effect on the development of haemoptysis in this population.
Voriconazole is the first‐line antifungal agent for the treatment of scedosporiosis [2]. However, the required duration of this therapy is unknown [1, 2]. The present patient was not a candidate for surgical treatment because of his extensive NTM lung disease, with careful monitoring of such patients thus being necessary.
With regard to the differential diagnosis of pulmonary mycetoma, clinicians should be aware of the possibility of S. apiospermum infection even in patients who are immunocompetent or free from tuberculosis. The increasing prevalence of NTM infection worldwide will likely result in an increase in the number of cases of simultaneous respiratory infection with S. apiospermum and NTM. Given that pathogen identification is critical for accurate diagnosis of S. apiospermum infection, the collection of specimens for culture such as by bronchoscopy is necessary for the appropriate management and treatment of this disease.
Disclosure Statement
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Acknowledgments
We thank Assistant Chief Technologist Makiko Kiyosuke and the staff of the Department of Clinical Chemistry and Laboratory Medicine, Kyushu University Hospital, for bacterial examination and detection of S. apiospermum.
Ogata, H , Harada, E , Okamoto, I . (2020) Scedosporium apiospermum lung disease in a patient with nontuberculous mycobacteria. Respirology Case Reports, 9(1), e00691 10.1002/rcr2.691
Associate Editor: Wei Shen Lim
References
- 1. Kantarcioglu AS, de Hoog GS, and Guarro J. 2012. Clinical characteristics and epidemiology of pulmonary pseudallescheriasis. Rev. Iberoam. Micol. 29:1–13. [DOI] [PubMed] [Google Scholar]
- 2. Tortorano AM, Richardson M, Roilides E, et al. 2014. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin. Microbiol. Infect. 20:27–46. [DOI] [PubMed] [Google Scholar]
- 3. Murayama T, Amitani R, Tsuyuguchi K, et al. 1998. Polypoid bronchial lesions due to Scedosporium apiospermum in a patient with Mycobacterium avium complex pulmonary disease. Eur. Respir. J. 12:745–747. [DOI] [PubMed] [Google Scholar]
- 4. Sunagawa K, Yagoshi M, Suzuki A, et al. 2018. Cytological and molecular detection of Scedosporium apiospermum in a patient treated for a Mycobacterium avium complex infection. Diagn. Cytopathol. 46:642–644. [DOI] [PubMed] [Google Scholar]
- 5. Shu CC, Lee CH, Hsu CL, et al. 2011. Clinical characteristics and prognosis of nontuberculous mycobacterial lung disease with different radiographic patterns. Lung 189:467–474. [DOI] [PubMed] [Google Scholar]
- 6. Shu CC, Wang JY, Wu MF, et al. 2017. Attenuation of lymphocyte immune responses during Mycobacterium avium complex‐induced lung disease due to increasing expression of programmed death‐1 on lymphocytes. Sci. Rep. 7:42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shin BS, Jeon GS, Lee SA, et al. 2011. Bronchial artery embolisation for the management of haemoptysis in patients with pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 15:1093–1098. [DOI] [PubMed] [Google Scholar]


