Abstract
mascRNA is a small cytoplasmic RNA derived from the lncRNA MALAT1. After being processed by the tRNA processing enzymes RNase P and RNase Z, mascRNA undergoes CCA addition like tRNAs and folds into a tRNA‐like cloverleaf structure. While MALAT1 functions in multiple cellular processes, the role of mascRNA was largely unknown. Here, we show that mascRNA binds directly to the multi‐tRNA synthetase complex (MSC) component glutaminyl‐tRNA synthetase (QARS). mascRNA promotes global protein translation and cell proliferation by positively regulating QARS protein levels. Our results uncover a role of mascRNA that is independent of MALAT1, but could be part of the molecular mechanism of MALAT1's function in cancer, and provide a paradigm for understanding tRNA‐like structures in mammalian cells.
Keywords: MALAT1, mascRNA, multi‐tRNA synthetase complex, translation, tRNA‐like structure
Subject Categories: RNA Biology, Protein Biosynthesis & Quality Control
tRNA‐like noncoding RNA mascRNA increases the cytosolic protein levels of multi‐tRNA synthetase complex (MSC) component glutaminyl‐tRNA synthetase (QARS) by interacting with it and promoting its stability, thereby upregulating global protein translation.
Introduction
Eukaryotic genomes transcribe a broad spectrum of protein‐coding RNAs and noncoding RNAs, including primary and processed RNAs (Djebali et al, 2012). Much is understood about infrastructural ncRNAs including tRNAs, rRNAs, and snoRNAs, but only a small portion of other regulatory ncRNAs such as long noncoding RNA (lncRNA) and Piwi‐associated RNAs have been well studied (Ponting et al, 2009). Over the past decade, increasing evidence has indicated that ncRNAs play significant roles in diverse biological processes. Among all the lncRNAs, the metastasis‐associated lung adenocarcinoma transcript 1 (MALAT1) is one of the most studied. It has been reported to be a crucial regulator of cancer metastasis and a candidate therapeutic target for the treatment of breast cancer (Gutschner et al, 2013; Mendell, 2016). Interestingly, processing of MALAT1 transcript by RNase P and RNase Z that are also involved in tRNA biogenesis generates a small noncoding RNA mascRNA. mascRNA undergoes CCA addition and folds into a tRNA‐like structure (Wilusz et al, 2008). Unlike most lncRNAs that tend to be poorly conserved, MALAT1 and its processing product mascRNA are highly conserved in vertebrates (Quinn & Chang, 2016; Zhang et al, 2017a).
tRNA‐like structures have long been studied in plant viruses. They regulate plant virus replication and virus translation by mimicking tRNAs or acting as a 3′‐translational enhancer (Barends et al, 2004; Matsuda & Dreher, 2004; Dreher, 2009). By contrast, tRNA‐like structures in eukaryotes are not well investigated. The reported tRNA‐like ncRNAs in eukaryotes include mascRNA and MEN β tRNA‐like small RNA, which are processed from lncRNA MALAT1 and MEN β, respectively. Unlike mascRNA that undergoes CCA addition and can be easily detected in multiple cell lines, MEN β tRNA‐like small RNA undergoes CCACCA addition and becomes undetectable by Northern blotting due to degradation (Sunwoo et al, 2009; Wilusz et al, 2011; Kuhn et al, 2015).
MALAT1 has been extensively studied and is believed to promote cell proliferation and cancer cell metastasis by multiple mechanisms (Tripathi et al, 2010; Li et al, 2014; Zhang et al, 2017b). However, less attention has been given to understanding the function of mascRNA and its mechanism. The 3′ fragments of MALAT1 that contains the mascRNA sequence have been shown to be sufficient to promote proliferation, migration, and invasion of SW480 colon cancer cells (Xu et al, 2011). The possible involvement of mascRNA itself in such processes, however, has not been carefully explored. mascRNA has also been reported to be involved in cardiovascular innate immunity with the underlying mechanism still unknown (Gast et al, 2016). Whether the function of mascRNA is related to MALAT1 or the tRNA‐like structure has not been examined. Here, we show that mascRNA interacts with the multi‐tRNA synthetase complex and promotes global protein translation by increasing glutaminyl‐tRNA synthetase (QARS) levels and that the function is independent of MALAT1.
Results
mascRNA interacts with the multi‐tRNA synthetase complex (MSC) component QARS
ncRNAs function through interacting with genome, mRNAs, and RNA‐binding proteins (Marchese et al, 2017). To examine whether such an interaction is involved in mascRNA function, a gel shift assay was performed with the cytosol of HEK293 cells or the postnuclear membrane lysate. A higher mascRNA band was observed with the cytosol but not the postnuclear membrane lysate (Fig 1A). No similar bands were observed with 5S rRNA, suggesting that the interactions are specific to mascRNA (Fig 1A). Subcellular fractionation also showed no association of mascRNA with the postnuclear membrane fraction in either HEK293 cells or HepG2 cells, consistent with the gel shift assay (Fig 1B). However, a membrane‐associated pool of mascRNA was observed in the mouse kidney, suggesting that translocation of the RNA to a membrane compartment or organelle may occur in certain cells or under some specific circumstances (Fig EV1A).
Figure 1. mascRNA forms a stable complex with cytosolic components.
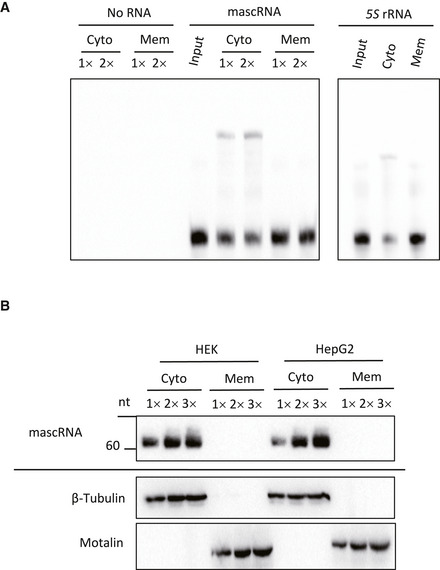
- Gel shift assay of biotin‐labeled mascRNA and 5S rRNA with the cytosol (Cyto) and the postnuclear membrane lysate (Mem).
- Northern blotting of mascRNA (top panel) and Western blotting of β‐Tubulin and Mortalin (bottom two panels) in the cytosolic fractions and the postnuclear membrane fractions of HEK293 and HepG2 cells.
Figure EV1. Expression and subcellular localization of mouse mascRNA in tissues, and expression and immunoprecipitation of FLAG‐tagged ARSs.
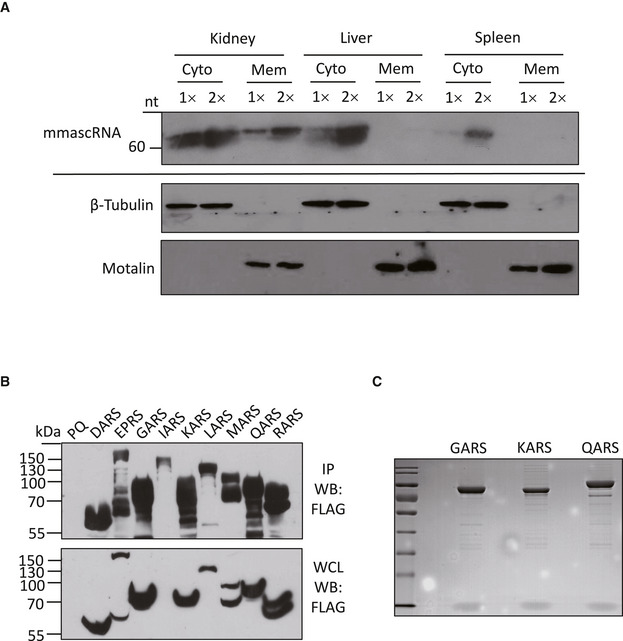
- Northern blotting of mouse mascRNA (mmascRNA; top panel) and Western blotting of β‐Tubulin and Mortalin (bottom 2 panels) in the cytosolic fractions and the postnuclear membrane fractions of different tissues.
- Western blot detection of FLAG‐tagged proteins in whole cell lysates (WCL) and immunoprecipitation samples (IP) from cells overexpressing DARS, EPRS, GARS, IARS, KARS, LARS, MARS, QARS or RARS, or the control cells (PQ). The expected size of each FLAG‐tagged protein: DARS 61.9 kDa, EPRS 175.4 kDa, GARS 88.0 kDa, IARS 149.4 kDa, KARS 76.3 kDa, LARS 139.3 kDa, MARS 105.9 kDa, QARS 92.6 kDa, and RARS 80.2 kDa.
- SDS–PAGE analysis and Coomassie staining of the proteins in anti‐FLAG pulldown samples.
To examine whether the interaction in the cytosol is with proteins, we performed biotinylated RNA pulldown and mass spectrometry analysis. In vitro transcribed mascRNA and its antisense RNA were labeled with biotin and incubated with the cell lysate in a buffer containing excess yeast tRNAs. The proteins in complex with the biotinylated RNA were isolated with streptavidin agarose beads. The mascRNA interacting proteins were then analyzed with SDS–PAGE and mass spectrometry (MS). The results show that mascRNA binds to several proteins with a higher affinity compared with its antisense transcript (Fig 2A). MS analysis of these proteins identified eleven components of the multi‐tRNA synthetase complex (MSC; Fig 2B). The MSC is a hub for protein synthesis (Robinson et al, 2000). The core components of eight aminoacyl‐tRNA synthetases (DARS, EPRS, IARS, KARS, LARS, MARS, QARS, and RARS) that attach amino acids to their cognate tRNAs and three accessory proteins were prominent in mascRNA pulldown samples. The aminoacyl‐tRNA synthetases (ARS) that are not in the MSC were not detected. An exception was threonyl‐tRNA synthetase‐like protein 2 (TARSL2), but it was detected at a much lower level (Dataset EV1). TARSL2 has previously been reported to be a potential component of the MSC by affinity purification MS using KARS as a bait protein (Kim et al, 2013). Identification of TARSL2 in our system could be due to weak interaction between TARSL2 and KARS. The existence of EPRS and QARS in the pulldown was further validated by Western blotting, and the non‐interacting aminoacyl‐tRNA synthetase GARS was not detected (Fig 2C). In contrast, another tRNA‐like ncRNA menRNA did not pulldown QARS (Fig 2C). Comparison of our results with the reported MALAT1 RNA pulldown data showed that the protein interactome of mascRNA is completely different from that of MALAT1 (Chen et al, 2017; Spiniello et al, 2018), suggesting that mascRNA and MALAT1 may have separate functions.
Figure 2. mascRNA interacts with the multi‐tRNA synthetase complex (MSC) component QARS.
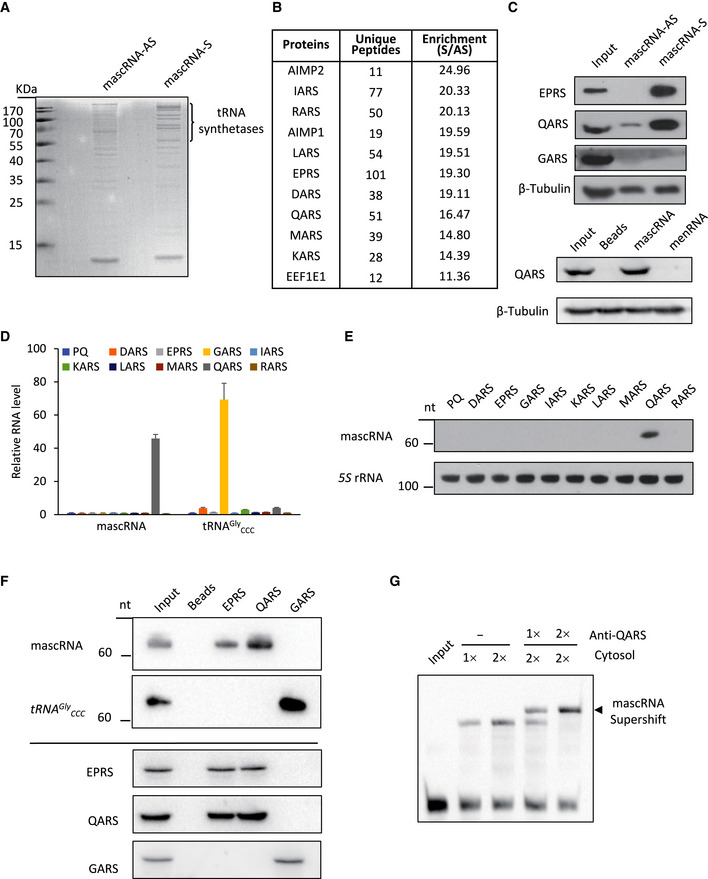
- SDS–PAGE analysis and Coomassie staining of the proteins in the RNA pulldown samples. Biotin‐labeled mascRNA (S) and the antisense RNA (AS) were used to pull down the RNA‐protein complexes.
- Representative data of mass spectrometry analysis of the RNA pulldown samples. Proteins most enriched in mascRNA (S) RNA pulldown sample are listed.
- Western blots of the proteins in mascRNA S and AS pulldown samples (top 4 panels), and mascRNA and menRNA pulldown samples (bottom 2 panels). β‐Tubulin was used as a loading control.
- qRT–PCR analysis of RNAs extracted from ARS immunoprecipitation (RIP) samples (error bars: mean ± SD; biological replicates n = 3). Eight complex‐forming ARSs (DARS, EPRS, IARS, KARS, LARS, MARS, QARS, and RARS) and GARS tagged with FLAG were overexpressed in HEK cells. ARS‐RNA complexes were immunoprecipitated with anti‐FLAG Magnetic Beads. The cell line constructed with PQCXIP vector (PQ) was used as a negative control. GAPDH was used as an internal control for qRT–PCR.
- Northern blot validation of mascRNA in the RIP samples. 5S rRNA from the RIP samples was used as an internal control.
- Northern blot (top 2 panels) and Western blot (bottom 3 panels) analysis of ARS immunoprecipitation samples (RIP). RIP was performed using EPRS, QARS, and GARS antibodies.
- Gel shift assay of biotin‐labeled mascRNA with the cytosol in the presence or the absence of a QARS antibody.
mascRNA interacting with ARSs is consistent with the remarkable similarities between mascRNA and tRNAs in their processing and cloverleaf structures. In general, the interaction between a tRNA and its ARS is highly specific, which is required for accurate protein translation (Schimmel & Soll, 1979). If mascRNA mimics tRNAs in protein interaction, it should also bind to certain ARS in the MSC instead of binding nonspecifically to all complex‐forming ARSs and the other MSC components. To find the mascRNA interacting ARS, we constructed eight stable cell lines overexpressing each complex‐forming ARS with a FLAG tag. ARS‐RNA complexes were affinity isolated by immunoprecipitation using the anti‐FLAG Magnetic Beads. Expression and immunoprecipitation of the FLAG‐tagged ARSs were examined by Western blotting (Fig EV1B). ARS interacting RNAs were then extracted from the immunoprecipitated samples and analyzed by qRT–PCR after treatment with DNase I. qRT–PCR results indicate that mascRNA binds specifically to QARS (Fig 2D). Copurification of mascRNA with QARS was further validated by Northern blotting (Fig 2E).
That mascRNA was detected only with the overexpressed QARS but not the other overexpressed ARSs in the RNA immunoprecipitation (RIP) assay suggests that the majority of the overexpressed ARSs are not incorporated into MSC. Indeed, Coomassie staining of the FLAG immunoprecipitation samples showed that the ratio of the other MSC components to the FLAG‐tagged protein is very low (Fig EV1C). To examine whether the interaction also occurs without QARS overexpression, immunoprecipitation was performed using antibodies against the endogenous QARS, EPRS, and GARS, and lysates of HEK cells. mascRNA was detected in the immunoprecipitation samples of QARS and EPRS, but not the sample of GARS that unlike the other two, is not a subunit of MSC (Fig 2F). The immunoprecipitation assays with the overexpressed FLAG‐tagged proteins and the endogenous proteins suggest that the interaction between mascRNA and QARS is more direct than the interactions between mascRNA and the other MSC forming ARSs.
To further verify the interaction between mascRNA and QARS, the gel shift assay was performed in the presence and absence of a QARS antibody. A supershift band was observed with the QARS antibody, suggesting formation of a super‐complex between mascRNA, QARS, and the QARS antibody (Fig 2G).
tRNAs physically interact with ARS at both the acceptor stem and the anticodon stem‐loop (Qin et al, 2014). Nucleotides in both regions have been shown to be major identity elements in Escherichia coli (Jahn et al, 1991). To examine whether mascRNA also mimics tRNAs in ARS binding, we constructed a series of mascRNA mutants with mutations at either the acceptor stem or the anticodon stem‐loop and performed the pulldown assay (Fig 3A–E). Even though the same amount of RNA was used, some mutants appeared to bind the streptavidin beads less efficiently (Fig 3A and B). However, enough mutants bound the beads with similar efficiency as the wild‐type mascRNA did, and all these acceptor stem mutants but not the anticodon stem‐loop mutants retained the capacity to co‐precipitate QARS (Fig 3A–E). In addition, a band similar to the wild‐type mascRNA gel shift band was observed in the assay with the acceptor stem mutant (Mut1) but not the anticodon stem‐loop mutant (Anti‐Mut1; Fig 3F). These results suggest that the anticodon stem‐loop but not the acceptor stem is required for mascRNA‐QARS interaction. Therefore, even though mascRNA and tRNA Gln UUG have many sequence similarities (Fig 3G), their interactions with QARS do not appear to be the same.
Figure 3. The anticodon stem‐loop is required for mascRNA‐QARS interaction.
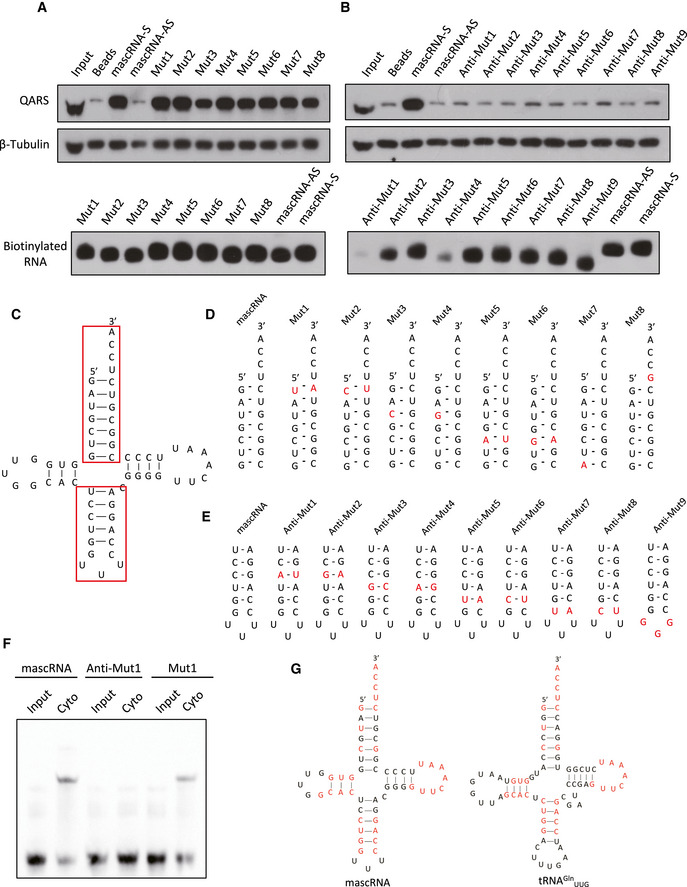
- Biotin‐labeled mascRNA (sense [S] and antisense [AS]) and the acceptor stem mutants (Mut1–8) were used to pulldown the RNA–protein complexes. Top panel: Western blotting of QARS in the pulldown samples. β‐Tubulin was used as an cytosolic input control for the pulldown. Bottom panel: biotin detection of the biotin‐labeled mascRNA and the mutants.
- Biotin‐labeled mascRNA (sense (S) and antisense [AS]) and the anticodon stem‐loop mutants (Anti‐Mut1–9) were used to pulldown the RNA–protein complexes. Top panel: Western blotting of QARS in the pulldown samples. β‐Tubulin was used as an cytosolic input control for the pulldown. Bottom panel: biotin detection of the biotin‐labeled mascRNA and the mutants.
- The secondary structure of mascRNA with the acceptor stem and the anticodon stem‐loop in red boxes.
- The acceptor stems of mascRNA and the mutants with the mutated nucleotides in red.
- The anticodon stem‐loops of mascRNA and the mutants with the mutated nucleotides in red.
- Gel shift assay of biotin‐labeled mascRNA or the mutants (Anti‐Mut1 and Mut1) with the cytosol.
- Comparison of human mascRNA and tRNAGln UUG secondary structures. The identical nucleotides were marked in red.
We also did not observe any significant effect of tRNAGln UUG on mascRNA‐QARS interaction or vice versa, as excess tRNAGln UUG had no effect on the formation of mascRNA gel shift band and neither did excess mascRNA affect copurification of QARS by tRNAGln UUG (Fig EV2A–C), suggesting that mascRNA and tRNAGln UUG bind to QARS at different sites. That the acceptor stem of mascRNA is not required for mascRNA‐QARS interaction is also consistent with the previous findings that mascRNA is not aminoacylated in HeLa cells even though it also has CCA addition (Wilusz et al, 2008). Similarly, in our system of HEK293 cells, we did not observe any aminoacylation of mascRNA either (Fig EV2D). mascRNA also lacks a conserved anticodon loop (Fig 3G), which makes it hard for mascRNA to transfer amino acids to accurate sites. Therefore, mascRNA does not appear to function by competing with tRNAGln UUG for QARS binding or by transferring amino acids.
Figure EV2. mascRNA has no effect on tRNAG ln UUG‐QARS interaction, does not transfer amino acids, and does not affect tRNAG ln UUG levels, but has an effect on protein degradation.
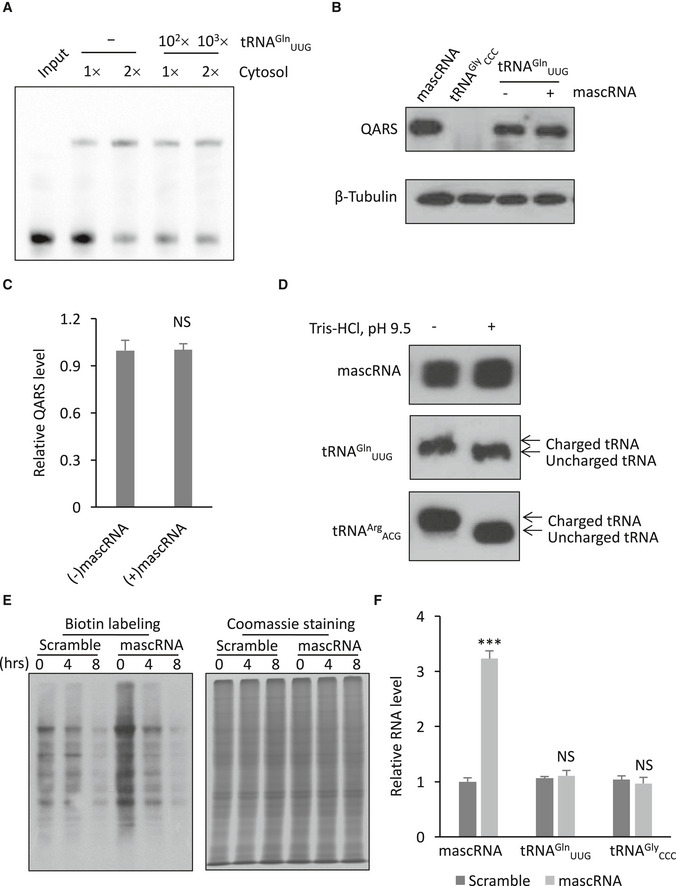
- Gel shift assay of biotin‐labeled mascRNA with the cytosol in the presence (102× or 103× the amount of mascRNA) or absence (–) of excess unlabeled tRNAGln UUG.
- Western blot detection of QARS copurified with biotin‐labeled tRNAGln UUG with (+) or without (−) excessive mascRNA. Biotin‐labeled mascRNA was used as a positive control for QARS copurification, and biotin‐labeled tRNAGly ccc was used as a negative control.
- Quantification of the levels of tRNAGln UUG‐binding QARS in Panel B.
- Aminoacylation analysis of mascRNA and tRNAs by polyacrylamide gel electrophoresis under acidic conditions. To deaminoacylate charged tRNAs, RNAs were treated with pH 9.5 Tris–HCl buffer for 30 min before polyacrylamide gel electrophoresis. tRNAGln UUG and tRNAArg ACG were used as positive controls.
- Decay of the pulse biotin‐labeled proteins in HEK cells stably expressing mascRNA (mascRNA) or the scrambled RNA (Scramble). Biotin labeling represents newly synthesized proteins, while Coomassie staining represents total proteins.
- qRT–PCR analysis of mascRNA, tRNAGln UUG, and tRNAGly ccc expression in mascRNA overexpressing (mascRNA) and the scrambled RNA expressing (Scramble) cells.
mascRNA promotes global protein translation
ARSs function in global protein translation by charging appropriate amino acids to their cognate tRNAs. Recent studies have also revealed non‐canonical functions of ARSs in inflammation, tumorigenesis, and other biological processes (Yannay‐Cohen et al, 2009; Park et al, 2012; Lee et al, 2016; Arif et al, 2017). mascRNA interacting with QARS suggests that it may function in one or more of these QARS‐regulated processes. To examine whether mascRNA functions in protein translation, we measured the newly synthesized protein levels in HEK cells stably overexpressing mascRNA or expressing the scrambled RNA. Northern blot analysis showed that mascRNA levels increased to threefold of the endogenous levels in the mascRNA overexpressing cell line (Fig 4A). The expression of mascRNA precursor MALAT1 was not affected by mascRNA overexpression (Fig 4B). A protein labeling and detection method using the non‐canonical amino acid 4‐Azido‐l‐homoalanine (L‐AHA) and click chemistry was then used to examine the newly synthesized proteins (Dieterich et al, 2007; Presolski et al, 2011). The levels of newly synthesized proteins were significantly higher in cells stably overexpressing mascRNA (Fig 4C and D). In vitro transcribed mascRNA or eGFP mRNA fragment was also used to transfect HEK cells, and a similar increase in newly synthesized proteins was observed with the transient transfection of mascRNA but not the eGFP mRNA fragment (Fig 4E–G). The acceptor stem mutant (Mut1) that binds to QARS as efficiently as the wild‐type mascRNA also showed a similar effect on the levels of newly synthesized proteins, while the mutation in the anticodon stem‐loop region (Anti‐Mut1) that abolishes the interaction eliminated the effect, suggesting the interaction between mascRNA and QARS is vital for this effect (Fig 4H–J). The increase in the newly synthesized protein levels, however, could also be due to a decrease in the protein degradation rate. Therefore, we performed the chase assay to examine the decay of the newly synthesized proteins. The degradation rate appeared to be higher with mascRNA overexpression (Figs 4K and EV2E), confirming that the increase in the newly synthesized protein levels is due to an elevated protein synthesis rate. The increase in the degradation rate also suggests a feedback to counteract the increase in the protein synthesis rate and maintain stable protein levels.
Figure 4. mascRNA promotes global protein translation.
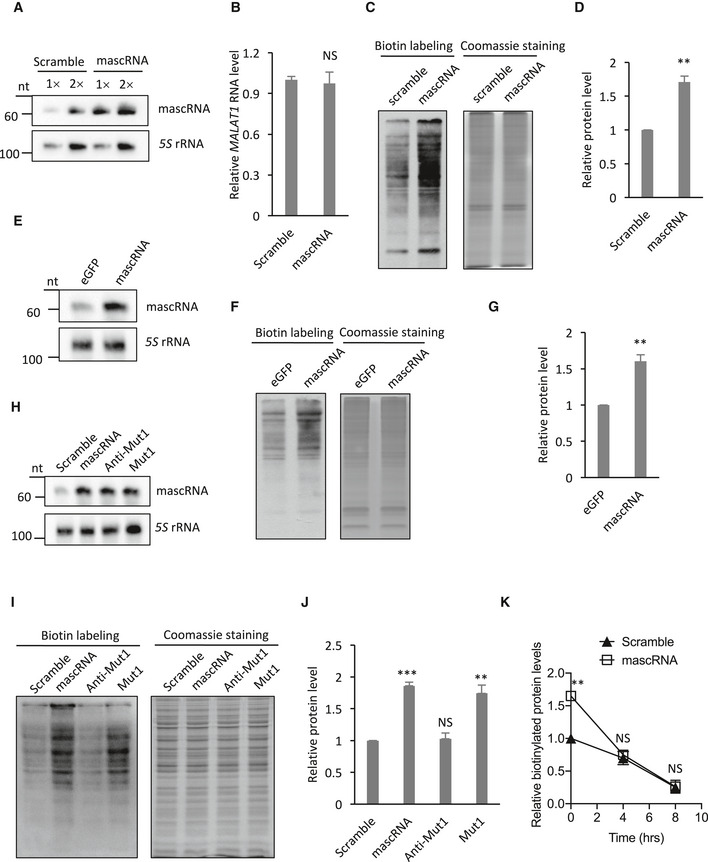
- Northern blot analysis of mascRNA in mascRNA overexpressing HEK cells (mascRNA) and the cells expressing the scrambled RNA (Scramble). 5S rRNA was used as an internal control.
- qRT–PCR analysis of MALAT1 (total RNAs used as templates) in mascRNA overexpressing (mascRNA) and the scrambled RNA expressing (Scramble) cells.
- Detection of the newly synthesized proteins in HEK cells stably expressing mascRNA (mascRNA) or the scrambled RNA (Scramble). Left: biotin detection of the labeled nascent proteins; right: Coomassie staining of total proteins.
- Quantification of the levels of the labeled nascent proteins in Panel C.
- Northern blot analysis of mascRNA in HEK cells transiently transfected with in vitro synthesized eGFP RNA fragment or mascRNA. 5S rRNA was used as an internal control.
- Detection of the newly synthesized proteins in HEK cells transiently transfected with in vitro synthesized eGFP RNA fragment or mascRNA. Left: biotin detection of the labeled nascent proteins; right: Coomassie staining of total proteins.
- Quantification of the levels of the labeled nascent proteins in Panel F.
- Northern blot analysis of mascRNA in mascRNA overexpressing (mascRNA), and the mutants (Anti‐Mut1 and Mut1) or the scrambled RNA (Scramble) expressing HEK cells. 5S rRNA was used as an internal control.
- Detection of the newly synthesized proteins in HEK cells stably expressing mascRNA (mascRNA), the mutants (Anti‐Mut1 and Mut1), or the scrambled RNA. (Scramble). Left: biotin detection of the labeled nascent proteins; right: Coomassie staining of total proteins.
- Quantification of the levels of the labeled nascent proteins in Panel I.
- Decay of the pulse biotin‐labeled proteins in HEK cells stably expressing mascRNA (mascRNA) or the scrambled RNA (Scramble).
mascRNA promotes global protein translation by increasing QARS protein levels
Considering the similarities between mascRNA and tRNAGln UUG, we first explored the possibility of mascRNA functioning on global protein translation by affecting tRNAGln UUG levels, since no interference of QARS‐tRNAGln UUG interaction by mascRNA was observed. qRT–PCR results showed that tRNAGln UUG levels are not affected by mascRNA overexpression (Fig EV2F), suggesting that the molecular mechanism may be mostly based on mascRNA's effect on QARS instead of tRNAGln UUG.
Aminoacyl‐tRNA synthetases catalyze aminoacylation of cognate tRNAs. Amino acids and tRNAs have also been shown to regulate ARSs’ synthesis in return (Johnson et al, 1977). For example, higher level of uncharged tRNAHis increases histidyl‐tRNA synthetase (HARS) translation and leads to a higher rate of global protein translation in Saccharomyces cerevisiae (Levi & Arava, 2019). To examine whether mascRNA has a similar effect on QARS, we compared the QARS protein levels in mascRNA overexpressing cells with those in control cells. More QARS protein was detected in mascRNA overexpressing cells, while the other ARSs remained the same (Fig 5A and B). The expression of the acceptor stem mutant (Mut1) that binds to QARS as efficiently as the wild‐type mascRNA but not the binding‐deficient anticodon stem‐loop mutant (Anti‐Mut1) showed a similar positive effect on QARS levels, while reducing mascRNA levels with shRNA for MALAT1 had an opposite effect (Fig 5C–H).
Figure 5. mascRNA increases QARS protein stability.
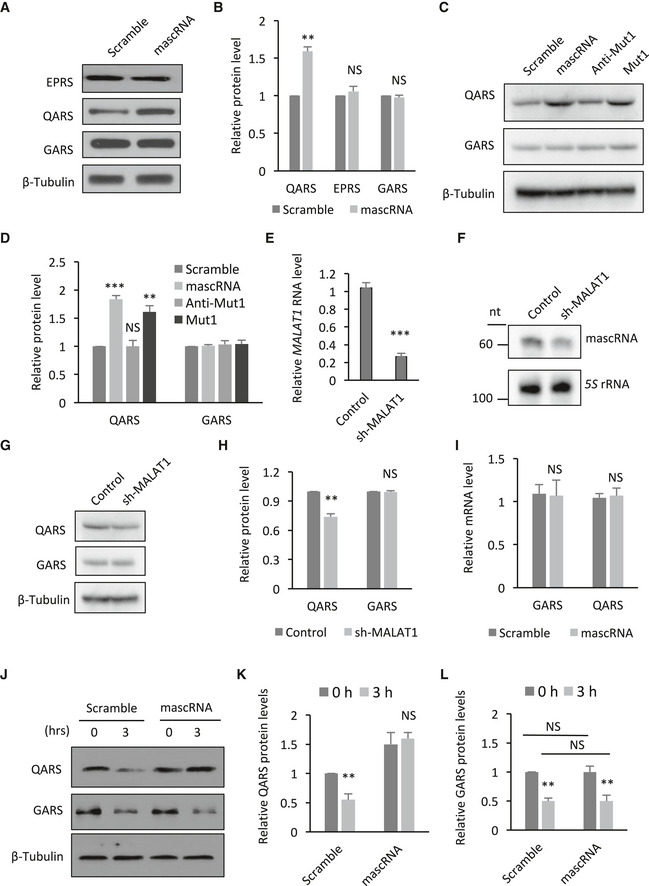
- Western blot detection of ARS proteins in mascRNA overexpressing (mascRNA) or the scrambled RNA expressing (Scramble) cells. β‐Tubulin was used as a loading control.
- Quantification of the protein levels in Panel A.
- Western blot detection of ARS proteins in HEK cells stably expressing mascRNA (mascRNA), the mutants (Anti‐Mut1 and Mut1), or the scrambled RNA (Scramble). β‐Tubulin was used as a loading control.
- Quantification of the protein levels in Panel C.
- qRT–PCR analysis of MALAT1 RNA levels in MALAT1 knockdown and the control cells.
- Northern blot analysis of mascRNA in MALAT1 knockdown and the control cells. 5S rRNA was used as an internal control.
- Western blot detection of ARS proteins in MALAT1 knockdown and the control cells.
- Quantification of the protein levels in Panel G.
- qRT–PCR analysis of GARS and QARS mRNAs in mascRNA overexpressing (mascRNA) or the scrambled RNA expressing (Scramble) cells.
- Western blot detection of ARS proteins in mascRNA overexpressing (mascRNA) or the scrambled RNA expressing (Scramble) cells, before and after emetine and cycloheximide treatment. β‐Tubulin was used as a loading control.
- Quantification of the relative QARS protein levels in Panel J.
- Quantification of the relative GARS protein levels in Panel J.
The levels of QARS mRNA, however, were not affected by changes in mascRNA levels (Fig 5I), suggesting that QARS might be regulated by mascRNA at a post‐transcriptional level. The decay of QARS was examined after the protein synthesis was inhibited with emetine and cycloheximide. An increase in QARS protein stability with mascRNA overexpression was observed (Fig 5J and K), consistent with the direct interaction between mascRNA and QARS. The regulation appeared to be QARS specific, as no similar effect of mascRNA on GARS was observed (Fig 5J and L).
To investigate whether mascRNA promotes global protein translation by increasing QARS protein levels, QARS was overexpressed in HEK cells (Fig 6A). Global protein translation levels in these cells were measured by L‐AHA labeling of the nascent proteins. As expected, overexpression of QARS led to higher global protein translation levels (Fig 6B and C). Overexpression of GARS also had a similar effect (Fig 6A–C). Interestingly, overexpression of QARS in mascRNA overexpressing cells did not further increase global protein translation, suggesting a saturation point for QARS‐regulated translation enhancement (Fig 6D–F). Indeed, when different expression levels of mascRNA were examined, a positive correlation between QARS levels and mascRNA levels was observed, but the positive effect on global protein translation quickly reached a plateau (Fig EV3). Unlike QARS overexpression, GARS overexpression in mascRNA overexpressing cells further increased global protein translation (Fig 6D–F), indicating a similar but mostly independent mechanism for GARS regulation of global translation. The expression of QARS binding‐deficient anticodon stem‐loop mutant (Anti‐Mut1) has no effect on global protein translation or QARS levels (Figs 4I and J, and, 5C and D), and simultaneous overexpression of QARS became effective again on upregulating global protein translation (Fig 6G–I). The positive effect of QARS overexpression became even more prominent with MALAT1 knockdown that by itself decreases QARS protein levels and downregulates global protein translation (Figs 5E–G and 6J–L). Reducing QARS or GARS levels with shRNAs both had a negative impact on global protein translation, but only the QARS knockdown effect could be partially reversed by mascRNA overexpression (Figs 6M and N, and EV4). That only a partial but not full rescue was achieved is likely because mascRNA overexpression did not fully restore QARS levels (Fig EV4B). These results provide further evidence that mascRNA functions through QARS and that GARS regulates translation independently.
Figure 6. mascRNA promotes global protein translation by increasing QARS protein levels.
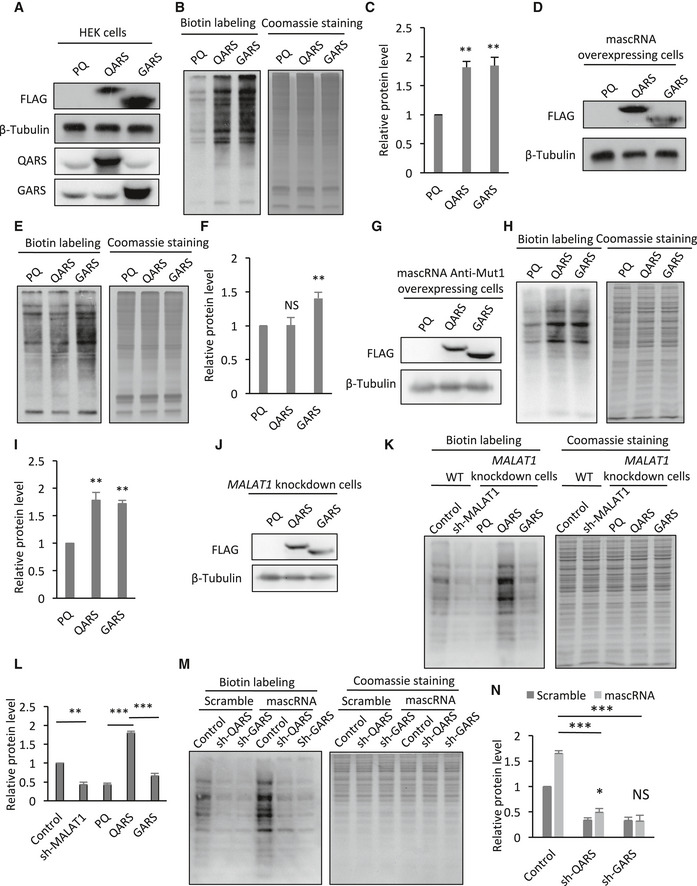
- Western blot detection of endogenous and FLAG‐tagged ARS proteins in HEK cells transiently transfected with PQCXIP vector (PQ), QARS expressing (QARS), or GARS expressing (GARS) plasmid. β‐Tubulin was used as a loading control.
- Detection of the newly synthesized proteins in HEK cells transiently transfected with PQCXIP vector (PQ), QARS expressing (QARS), or GARS expressing (GARS) plasmid. Left, biotin detection of the labeled nascent proteins; right, Coomassie staining of total proteins.
- Quantification of the labeled nascent protein levels in Panel B.
- Western blot detection of FLAG‐tagged ARS proteins in mascRNA overexpressing cells transiently transfected with PQCXIP vector (PQ), QARS expressing (QARS), or GARS expressing (GARS) plasmid. β‐Tubulin was used as a loading control.
- Detection of the newly synthesized proteins in mascRNA overexpressing cells transiently transfected with PQCXIP vector (PQ), QARS expressing (QARS), or GARS expressing (GARS) plasmid. Left, biotin detection of the labeled nascent proteins; right, Coomassie staining of total proteins.
- Quantification of the labeled nascent protein levels in Panel E.
- Western blot detection of FLAG‐tagged ARS proteins in mascRNA anticodon stem‐loop mutant Anti‐Mut1 expressing cells transiently transfected with PQCXIP vector (PQ), QARS expressing (QARS), or GARS expressing (GARS) plasmid. β‐Tubulin was used as a loading control.
- Detection of the newly synthesized proteins in mascRNA Anti‐Mut1 expressing cells transiently transfected with PQCXIP vector (PQ), QARS expressing (QARS), or GARS expressing (GARS) plasmid. Left, biotin detection of the labeled nascent proteins; right, Coomassie staining of total proteins.
- Quantification of the labeled nascent protein levels in Panel H.
- Western blot detection of FLAG‐tagged ARS proteins in MALAT1 knockdown cells with stable QARS (QARS) or GARS (GARS) overexpression or without (PQ). β‐Tubulin was used as a loading control.
- Detection of the newly synthesized proteins in wild‐type HEK cells (WT) and MALAT1 knockdown cells (sh‐MALAT1), or MALAT1 knockdown cells with stable QARS (QARS) or GARS (GARS) overexpression or without (PQ). Left, biotin detection of the labeled nascent proteins; right, Coomassie staining of total proteins.
- Quantification of the labeled nascent protein levels in Panel K.
- Detection of the newly synthesized proteins in QARS (shQARS) or GARS (shGARS) knockdown cells and the control cells stably overexpressing mascRNA (mascRNA) or expressing the scrambled RNA (Scramble). Left, biotin detection of the labeled nascent proteins; right, Coomassie staining of total proteins.
- Quantification of the labeled nascent protein levels in Panel M.
Figure EV3. The effect of mascRNA on global protein translation is dose dependent.
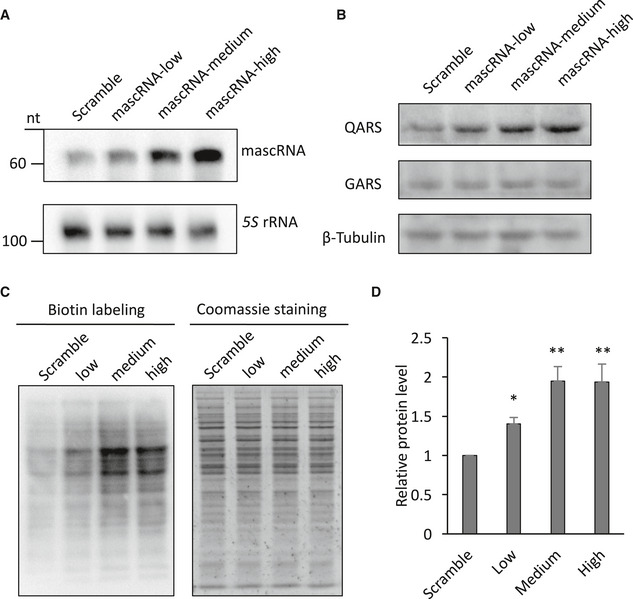
- Northern blot detection of mascRNA in HEK cells expressing different levels of mascRNA (low, medium, and high) or the scrambled RNA (Scramble). 5S rRNA was used as an internal control.
- Western blot detection of QARS and GARS protein levels in cells expressing different levels of mascRNA or the scrambled RNA. β‐Tubulin was used as a loading control.
- Detection of the newly synthesized proteins in HEK cells expressing different levels of mascRNA (low, medium, and high) or the scrambled RNA (Scramble). Left, biotin detection of the labeled nascent proteins; right, Coomassie staining of total proteins.
- Quantification of the labeled nascent protein levels in Panel C.
Figure EV4. Partial recue of QARS protein levels by mascRNA overexpression in QARS knockdown cells.
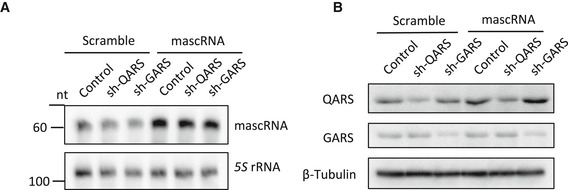
- Northern blot analysis of mascRNA in QARS (shQARS) or GARS (shGARS) knockdown cells and the control cells stably overexpressing mascRNA (mascRNA) or expressing the scrambled RNA (Scramble). 5S rRNA was used as an internal control.
- Western blot detection of ARS proteins in QARS (shQARS) or GARS (shGARS) knockdown cells and the control cells stably overexpressing mascRNA (mascRNA) or expressing the scrambled RNA (Scramble). β‐Tubulin was used as a loading control.
mascRNA regulates cell proliferation
Global protein translation in general is positively correlated with cell proliferation (Morita et al, 2015). A correlation of mascRNA levels with cell proliferation potentials was observed. In both the serum‐starved HEK293 cells and the differentiated human embryonic stem cells, mascRNA levels dropped (Fig 7A and B). The decrease in mascRNA levels in serum‐starved cells and differentiated stem cells could be the result of a reduction in MALAT1 levels (Fig EV5A and B). To further investigate whether mascRNA is involved in cell proliferation regulation, the proliferation rates of mascRNA overexpressing HEK, HeLa, and MCF‐7 cells and the corresponding control cells were examined by soft agar colony formation assay. All these mascRNA overexpressing cells generated significantly more colonies than the control cells (Fig 7C and D). Once again, the acceptor stem mutant but not the anticodon stem‐loop mutant showed similar effects on cell proliferation as wild‐type mascRNA (Figs 7E and EV5C). Reducing QARS levels with shRNAs abolished the positive effect of mascRNA overexpression (Figs 7F and EV5D). QARS overexpression, however, did not fully reverse the negative effect of MALAT1 knockdown as it did with global protein translation (Figs 7G and EV5E), which is not surprising as MALAT1 has been shown to promote cell proliferation and the mechanism might be mascRNA and QARS independent (Tripathi et al, 2010; Li et al, 2017; Zhang et al, 2017b; Liang et al, 2018).
Figure 7. mascRNA promotes cell proliferation.
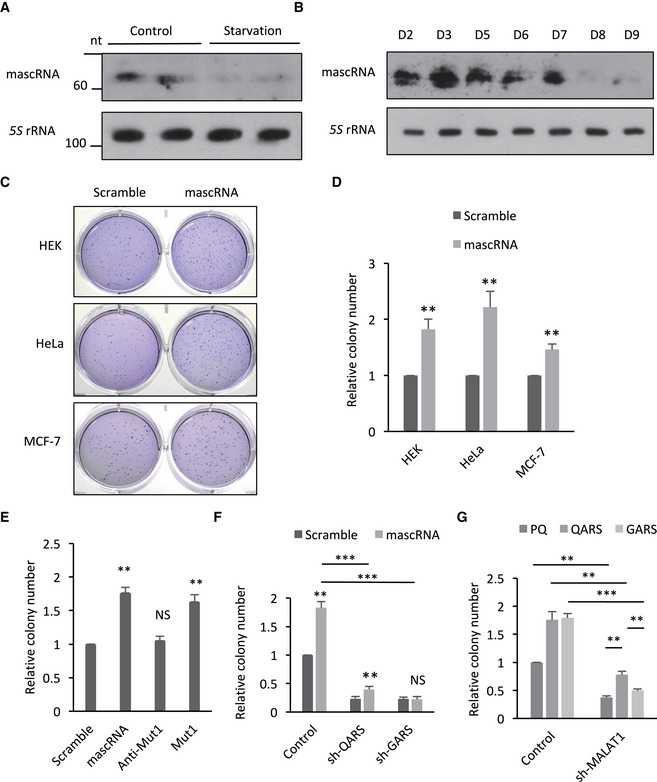
- Northern blotting of mascRNA and 5S rRNA in the lysates of HEK cells before and after FBS starvation.
- Northern blotting of mascRNA and 5S rRNA in the lysates of human embryonic stem cells on different days of differentiation.
- Soft agar colony formation assay of HEK, HeLa, or MCF‐7 cells overexpressing mascRNA or expressing the scrambled RNA.
- Quantification of stained colonies in Panel C.
- Soft agar colony formation assay of HEK cells overexpressing mascRNA, or expressing the mutants (Anti‐Mut1 and Mut1) or the scrambled RNA (Scramble).
- Soft agar colony formation assay of QARS (shQARS) or GARS (shGARS) knockdown cells and the control cells stably overexpressing mascRNA (mascRNA) or expressing the scrambled RNA (Scramble).
- Soft agar colony formation assay of MALAT1 knockdown cells (shMALAT1) and the control cells (Control) with stable QARS (QARS) or GARS (GARS) overexpression or without (PQ).
Figure EV5. mascRNA promotes cell proliferation through QARS .
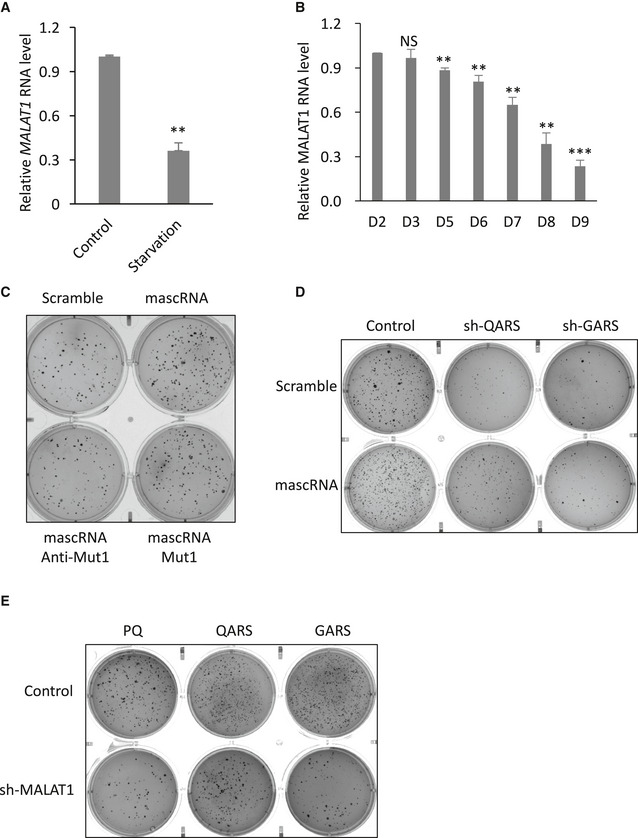
- qRT–PCR analysis of MALAT1 levels in the lysates of HEK cells before (Control) and after (Starvation) FBS starvation.
- qRT–PCR analysis of MALAT1 levels in the human embryonic stem cells on different days of differentiation (D2, D3, D5, D6, D7, D8, D9).
- Soft agar colony formation assay of HEK cells overexpressing mascRNA, or expressing the mutants (Anti‐Mut1 and Mut1) or the scrambled RNA (Scramble).
- Soft agar colony formation assay of QARS (shQARS) or GARS (shGARS) knockdown cells and the control cells stably overexpressing mascRNA (mascRNA) or expressing the scrambled RNA (Scramble).
- Soft agar colony formation assay of MALAT1 knockdown cells (shMALAT1) and the control cells (Control) with stable QARS (QARS) or GARS (GARS) overexpression or without (PQ).
Discussion
mascRNA is the processing product of a long ncRNA MALAT1 and folds into a tRNA‐like cloverleaf structure. It has been postulated to function by affecting tRNA‐related proteins or regulating MALAT1, but the exact functions and their molecular mechanisms were unclear. Here, we provide the first evidence that mascRNA directly interacts with the MSC component glutaminyl‐tRNA synthetase QARS and promotes global protein translation. Since mascRNA and tRNAGln UUG are similar in sequence and structure, the interaction results seem like a natural outcome. However, our results also suggest that mascRNA and tRNAGln UUG do not compete with each other for QARS, suggesting a more complex regulation mechanism. This noncompetitive binding model is also more consistent with the translation enhancement function of mascRNA.
We also show that mascRNA enhances global protein translation by increasing QARS protein levels. It was recently reported that HARS regulates its own translation by binding to a tRNAHis anticodon‐like structure in its own mRNA and that an increase in uncharged tRNAHis levels reduces the association, increasing the translation of HARS (Levi & Arava, 2019). We have shown that mascRNA does not affect the transcription of the QARS gene, so the regulation has to be at a post‐transcriptional level. Our results show that mascRNA specifically increases QARS protein stability, providing a different mechanism for tRNA‐like structures to regulate protein levels.
Extensive evidence shows that MALAT1 promotes cell proliferation and cancer metastasis. Mechanisms including transcriptional regulation and post‐transcriptional regulation such as regulation of alternative splicing have been proposed (Tripathi et al, 2010; Li et al, 2014; Zhang et al, 2017b). It has also been proposed that some regulatory roles of MALAT1 may be conferred by mascRNA. Overexpression of 3′ MALAT1 fragments that contain the mascRNA sequence promotes cell proliferation, while 5′ MALAT1 fragments do not have a similar effect (Xu et al, 2011). Our data show that mascRNA overexpression does not affect MALAT1 levels, consistent with a previous study (Gast et al, 2016). The effects of cytosolic mascRNA are likely independent of MALAT1 in the nucleus. However, since mascRNA is the processing product of MALAT1, changes on MALAT1 levels would have a direct impact on mascRNA levels, leading to the overlap of some functions. Our findings that mascRNA promotes global protein translation and cell proliferation indicate that the functions of MALAT1 are partially conferred by its processing product mascRNA.
Enrichment of mascRNA in CD14+ monocytic cells has previously been reported, and an antiviral role of mascRNA was proposed (Gast et al, 2016). Our findings that mascRNA interacts with QARS and promotes global protein translation may also provide a molecular mechanism for these functions. Monocytes can divide into dendritic cells, hence the requirement for an increase in global protein translation. Functional roles of mascRNA in immunity may also be conferred by the non‐canonical functions of ARSs. For example, the MSC component EPRS is a regulator of immune responses to viral infection (Lee et al, 2016). It is possible that EPRS function is indirectly affected by mascRNA or that QARS is also involved in immunity. More studies are needed to validate these hypotheses.
Recent studies have revealed important roles of small RNAs derived from tRNAs, including 3′ and 5′ tRNA fragments (tRFs). tRFs may inhibit translation initiation, regulate ribosome biogenesis, or regulate cell proliferation (Ivanov et al, 2011; Kim et al, 2017; Zhou et al, 2017). Notably, a 5′ tRF derived from glutaminyl tRNAGln associates with the MSC and stimulates translation of ribosomal and RNA‐binding proteins (Keam et al, 2017), indicating that the MSC is capable of interacting with small RNAs other than tRNAs. In another study, a 5′ tRF derived from tRNAGln inhibits protein translation independent of complementary target sites (Sobala & Hutvagner, 2013). It would be of interest to examine whether a similar process occurs for mascRNA or whether mascRNA could regulate the tRF‐mediated processes.
tRNA‐like structures have long been studied in many plant viruses, but not so well in eukaryotes. Transcriptomic studies have revealed the existence of many tRNA‐like structures in eukaryotes with functions still unknown. One hundred‐thirty MALAT1‐like genomic loci with tRNA‐like structure were identified in vertebrate genomes (Zhang et al, 2017a). In another study, 18 tRNA‐like structures were identified in murine lens (Khan et al, 2016). The rat mitochondrial genome also encodes a tRNA‐like structure (Yu et al, 2008). In addition, some mRNAs contain tRNA‐like domains that may function as cis‐regulatory elements of their own translation (Daher & Rueda, 2012; Levi & Arava, 2019). Our results provide a paradigm for understanding the functions of tRNA‐like structures in eukaryotes.
Materials and Methods
Cell lines and plasmid
Cell lines used include HEK293, HeLa, and stable cell lines generated with these cells. Plasmids used include PQCXIP, PQCXIP‐DARS-FLAGHis, PQCXIP‐EPRS-FLAGHis, PQCXIP‐GARS-FLAGHis, PQCXIP‐IARS-FLAGHis, PQCXIP‐KARS-FLAGHis, PQCXIP‐LARS-FLAGHis, PQCXIP‐MARS-FLAGHis, PQCXIP‐QARS-FLAGHis, PQCXIP‐RARS-FLAGHis, PQsuper, PQsuper‐mascRNA, PQsuper‐mascRNA‐Anti-Mut1, PQsuper‐mascRNA‐Mut1, pLKO, pLKO‐sh-MALAT1, pLKO‐sh-QARS, and pLKO‐sh-GARS.
To construct PQCXIP‐DARS-FLAGHis, DARS was PCR amplified from cytosolic cDNA using primers 5′‐ATATggatccATGCCCAGCGCCAGCGCCA‐3′ and 5′‐TATAgcggccgcaAGGAGTGAGTCGTTTGGGATCA‐3′, and inserted into PQCXIP‐FLAGhis with NotI and BamHI.
To construct PQCXIP‐EPRS-FLAGHis, EPRS was PCR amplified from cytosolic cDNA using primers 5′‐ATATgcggccgcATGGCGACGCTCTCTCTGAC‐3′ and 5′‐ACTGACCGGTGTAGCTGCGACCAAATAAGGTGTAG‐3′, and inserted into PQCXIP‐FLAGhis with NotI and AgeI.
To construct PQCXIP‐GARS-FLAGHis, GARS was PCR amplified from cytosolic cDNA using primers 5′‐ATATggatccATGCCCTCTCCGCGTCCAG‐3′ and 5′‐ACTAgcggccgcTTCCTCGATTGTCTCTTTTTTACCAG‐3′, and inserted into PQCXIP‐FLAGhis with NotI and BamHI.
To construct PQCXIP‐IARS-FLAGHis, IARS was PCR amplified from cytosolic cDNA using primers 5′‐CGTCggatccATGCTTCAACAAGTTCCAGAAAACAT‐3′ and 5′‐GCGCTTAATTAAGAAGTCTGCTGTTGTTGGTAACAC‐3′, and inserted into PQCXIP‐FLAGhis with BamHI and PacI.
To construct PQCXIP‐KARS-FLAGHis, KARS was PCR amplified from cytosolic cDNA using primers 5′‐ATATgcggccgcATGGCGGCCGTGCAGGCG‐3′ and 5′‐ACATctcgagGACAGAAGTGCCAACTGTTGTG‐3′, and inserted into PQCXIP‐FLAGhis with NotI and XhoI.
To construct PQCXIP‐LARS-FLAGHis, LARS was PCR amplified from cytosolic cDNA using primers 5′‐AGTCggatccATGGCGGAAAGAAAAGGAACAGC‐3′ and 5′‐CAGTctcgagATGAACCAGATAGATTATTGTATCGCC‐3′, and inserted into PQCXIP‐FLAGhis with BamHI and XhoI.
To construct PQCXIP‐MARS-FLAGHis, MARS was PCR amplified from cytosolic cDNA using primers 5′‐AGTCggatccATGAGACTGTTCGTGAGTGATGG‐3′ and 5′‐CTGActcgagCTTTTTCTTCTTGCCTTTAGGGGC‐3′, and inserted into PQCXIP‐FLAGhis with BamHI and XhoI.
To construct PQCXIP‐QARS-FLAGHis, QARS was PCR amplified from cytosolic cDNA using primers 5′‐ATATGCGGCCGCATGGCGGCTCTAGACTCCC‐3′ and 5′‐CGCGTTAATTAACACCTTTCCTGGGTCTTCCTTC‐3′, and inserted into PQCXIP‐FLAGhis with NotI and PacI.
To construct PQCXIP‐RARS-FLAGHis, RARS was PCR amplified from cytosolic cDNA using primers 5′‐ACATggatccATGGACGTACTGGTGTCTGAGT‐3′ and 5′‐CTGActcgagCATCCTTTGGACAGGTTTTATTCCCA‐3′, and inserted into PQCXIP‐FLAGhis with BamHI and XhoI.
To construct PQsuper‐mascRNA, mascRNA was PCR amplified from cytosolic cDNA using primers 5′‐AGATCTGATGCTGGTGGTTGGCACTCC‐3′ and 5′‐CTCGAGAGACGCCGCAGGGATTTGAAC‐3′, and inserted into PQsuper with BglII and XhoI.
To construct PQsuper‐mascRNA‐Mut1 and PQsuper‐mascRNA‐Anti-Mut1, mascRNA mutants with sticky ends were synthesized (Invitrogen) and inserted into PQsuper with BglII and XhoI.
To construct PQsuper‐mascRNA‐Mut1 and PQsuper‐mascRNA‐Anti-Mut1, mascRNA mutants with sticky ends were synthesized (Invitrogen) and inserted into PQsuper with BglII and XhoI.
pLKO-sh‐MALAT1, pLKO‐sh-QARS, and pLKO‐sh-GARS were obtained from shRNA Library at Tsinghua University.
sh‐MALAT1 was designed to target 5′‐ACGGAAGTAATTCAAGATCAA‐3′.
Cell culture and transfection
HEK293 cells, HeLa, HepG2, and MCF‐7 cells were cultured in DMEM supplemented with 10% fetal bovine serum. All cell lines used were tested for mycoplasma contamination. To generate HEK293 stable cell lines, HEK293T cells were transfected with VSVg and Hit60 packaging vectors and the plasmid of interest using TurboFect (Thermo). Harvested retroviruses were used to infect the cells of interest, followed by selection with 5 μg/ml puromycin. For transient transfections, HEK cells were transfected with 3 μg in vitro transcribed mascRNA and eGFP RNA fragment (first 61 nts) using Lipofectamine RNAiMAX transfection reagent (Invitrogen). For cell starvation treatment, cells were cultured in DMEM medium without fetal bovine serum for 12 h.
In vitro transcription
RNAs were synthesized using MEGAscript SP6 Kit (Ambion). For biotin‐labeled RNA synthesis, biotin RNA labeling mix (Roche) was used. RNAs were purified using TRIzol reagent (Invitrogen). In vitro transcribed RNAs were treated with RNA 5′ Pyrophosphohydrolase (RppH; NEB) to remove pyrophosphate from the RNAs, and then purified by ethanol precipitation.
Subcellular fractionation
Cells were collected, washed with PBS, and resuspended in homogenization buffer (0.225 M mannitol, 0.075 M sucrose, and 20 mM HEPES, pH 7.4; ~ 3 × 107 cells/ml). Resuspended cells were processed in a Dounce homogenizer 30 times on ice. Nuclei were pelleted twice at 800 g for 5 min at 4°C. Supernatants were then centrifuged at 21 kg for 30 min at 4°C. The pellets were washed three times with 1.5 ml homogenization buffer and kept as the postnuclear membranes (PNM). The supernatants were kept as the cytosol. For isolation of the cytoplasm, washed cells were lysed with Buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, and 0.5% NP‐40) on ice for 20 min and then centrifuged at 15 kg for 10 min at 4°C. The supernatants were kept as the cytoplasm. Protease inhibitors (Thermo) and Ribolock RNase inhibitors (Thermo) were added during subcellular fractionation.
RNA isolation
RNAs were purified using 400 μl TRIzol reagent (Invitrogen) and treated with 500 U RNase‐free DNase I (Thermo) in 50 μl DNase buffer for 25 min at 37°C. DNase I was inactivated with the addition of 5 mM EDTA and incubation at 70°C for 10 min. RNAs were then purified with TRIzol. For RNAs used in aminoacylation assay, RNA isolation was performed in an acidic condition and RNA samples were kept on ice. For purification of mouse tissue RNAs, mouse liver, kidney, and spleen were prepared under the guidance of the Institution's Animal Care and Use Committee and mouse. Tissues were sheared into small pieces and then homogenized with a dounce homogenizer, before the TRIzol RNA purification steps. Total RNAs for differentiated embryonic stem cells were gifts from Dr Qianran Qi at Tsinghua University.
RNA pulldown
RNA pulldown procedures were adapted from a published protocol (Marin‐Bejar & Huarte, 2015). 107 HEK cells were harvested and lysed on ice with Buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, and 0.5% NP‐40) containing protease inhibitors and Ribolock RNase inhibitors. The cytosol was collected and incubated with washed streptavidin agarose beads to clear internal biotinylated proteins. Three microgram biotin‐labeled mascRNA (S) and mascRNA antisense (AS) were first incubated at 65°C for 10 min, cooled down slowly to room temperature, and then incubated with equivalent biotin‐cleared cytosol for 1 h at 4°C and then incubated with 30 μl washed streptavidin beads for 45 min. Protein‐biotinylated RNA complexes were isolated with streptavidin agarose beads. RNA interacting proteins were then dissolved in SDS lysis buffer and analyzes by Western blot or mass spectrometry.
Western blotting
One hundred microgram protein lysates were resolved by SDS–PAGE, transferred to nitrocellulose membranes, and incubated for 1 h with 5% milk TBS‐T and 1 h with primary antibodies in 5% BSA at room temperature. Antibodies used include anti‐β‐Tubulin (1:2,000; Abcam, ab6060), anti‐Mortalin (1:2,000; Sigma‐Aldrich, G4045), anti‐QARS (1:1,000; ProteinTech, 12645‐1‐AP), anti‐EPRS (1:1,000; Genetex, GTX130848‐S), anti‐GARS (1:1,000; Proteintech, 15831‐1‐AP), and anti‐FLAG (1:2,000; Sigma‐Aldrich, SAB4301135). Secondary antibodies include goat anti‐mouse and goat anti‐rabbit antibodies (1:10,000; Sigma‐Aldrich, A0545, A5795).
RNA immunoprecipitation
RNA immunoprecipitation procedures were adapted from a published protocol (Knuckles et al, 2012). HEK cells overexpressing FLAG‐tagged ARSs were collected, washed with PBS, and lysed on ice with IP lysis buffer (50 mM HEPES, pH 7.5, 0.4 M NaCl, 1 mM EDTA, 1 mM DTT, 0.5% Triton X‐100, and 10% glycerol) containing protease inhibitors and Ribolock RNase inhibitor. Cytosolic lysates were then incubated with blocked Anti‐FLAG M2 Magnetic Beads (Sigma‐Aldrich) for 2 h at 4°C. The beads were then washed four times with IP lysis buffer. For RIP using antibodies against ARSs, cytosolic lysates of HEK cells were incubated with anti‐EPRS antibody (Genetex), anti‐QARS antibody (ProteinTech) or anti‐GARS antibody (ProteinTech) for 2 h at 4°C. Protein A/G Agarose Beads (Thermo Scientific) were then added and incubated for 1 h at 4°C. Proteins bound to the bead and RNAs that interact with the proteins were eluted with SDS lysis buffer. RNAs were then extracted with TRIzol reagent, while a fraction of the immunoprecipitated protein sample was used as a control.
qRT–PCR
qRT–PCR was performed using the AccessQuick RT‐PCR kit (Promega). cDNA was synthesized using the Superscript First Strand Synthesis System (Invitrogen). 0.3 μl of the 20 μl cDNA was used for a 10 μl SYBR green qPCR.
RNAs were extracted from the cytosol or total cells. Two microliter of the final 45 μl RNA was used for a 20 μl cDNA synthesis reaction using the Superscript First Strand Synthesis System (Invitrogen) and specific reverse primers for RNAs of interest. 0.3 μl of the 20 μl cDNA was used for a 10 μl SYBR green qPCR. GAPDH was used as an internal control. Primers 5′‐GAGTACGTCGTGGAGTC‐3′ and 5′‐GGTCCACCACCCTGTTG‐3′ were used for GAPDH. Primers 5′‐GATGCTGGTGGTTGGCACTC‐3′ and 5′‐AGACGCCGCAGGGATTTGAA‐3′ were used for mascRNA. Primers 5′‐AGGCGTTTTCCAAGAGTGGG‐3′ and 5′‐TGGCAAAATGGCGGACTTTC‐3′ were used for MALAT1. Primers 5′‐CTGAGGTGCCTGGTGGATTT‐3′ and 5′‐GCTTTCCCTGATGGCTGTCT‐3′ were used for QARS. Primers 5′‐GCCAGCAGGGAGATCTTGTG‐3′ and 5′‐TAAAGTGCTGCCTCCAGGTC‐3′ were used for GARS. Primers 5′‐GGTTCCATGGTGTAATGGTtAGCAC‐3′ and 5′‐TGGAGGTTCCACCGAGATTTGAAC‐3′ were used for tRNAGln UUG. Primers 5′‐GCATTGGTGGTTCAGTGGTAGA‐3′ and 5′‐TTGCATTGGCCGGGAATTGAAC‐3′ were used for tRNAGly ccc.
Northern blotting
RNAs were extracted from the RIP sample, the cytosol, or the soluble fraction of HEK cells using TRIzol reagent. Six microgram RNAs were resolved on a 6% polyacrylamide‐8 M urea gel, transferred to a Amersham Hybond N+ membrane (GE), and hybridized with biotin‐labeled RNA probes. For mascRNA detection, biotin‐labeled mascRNA antisense RNA probe was used. For 5S rRNA, biotin‐labeled full‐length 5S antisense RNA probe was used. For tRNAs, biotin‐labeled tRNA antisense RNA probes were used. Experiments were performed using North2South Hybridization and Detection Kit (Thermo). For detection of tRNA aminoacylation by polyacrylamide gel electrophoresis under acidic conditions, Northern blotting was performed as described in published literature (Varshney et al, 1991).
mascRNA probe sequence:
5′AGACGCCGCAGGGATTTGAACCCCGTCCTGGAAACCAGGAGTGCCAACCACCAGCATC3′
5S rRNA probe sequence:
5′AAAGCCTACAGCACCCGGTATTCCCAGGCGGTCTCCCATCCAAGTACTAACCAGGCCCGACCCTGCTTAGCTTCCGAGATCAGACGAGATCGGGCGCGTTCAGGGTGGTATGGCCGTAGAC3′
tRNAGly CCC probe sequence:
5′TGCATTGGCCGGGAATTGAACCCGGGtCTCCCGCGTGGGAGGCGAGAATTCTACCACTGAACCACC3′
tRNAGln UUG probe sequence:
5′AGGTCCCACCGAGCTCGGATCGCTGGATTCAAAGTCCAGAGTGCTAACCATTACACCATGGGACC3′
tRNAArg ACG probe sequence:
5′CGAGCCAGCCAGGAGTCGAACCTGGAaTCTTCTGATCCGTAGTCAGACGCGTTaTCCATTGCGCCACTGGCCC’
Electrophoretic mobility shift assay
Five nanogram biotin‐labeled RNAs were incubated at 65°C for 10 min and then cooled down to 4°C slowly. The cytosol or the postnuclear membrane lysates were incubated with biotin‐labeled RNAs in a binding buffer (25 mM Tris–HCl (pH 8.0), 150 mM KCl, 5 mM EDTA (pH 8.0), 0.5 mM DTT, 0.5% NP‐40, and 100 U/ml RNase inhibitor) for 1 h at 4°C. For supershift EMSA, the anti‐QARS antibody was added after co‐incubation of mascRNA with the cytosol and then incubated for another 1 h at 4°C. For competitive EMSA, biotin‐labeled mascRNA and 100× or 1,000× unlabeled tRNAGln UUG were incubated with the cytosolic proteins for 1 h at 4°C. The 6% polyacrylamide gel was first run without any sample at 150 V for 30 min in 0.5× TBE buffer. The samples were then mixed with RNA loading buffer, and resolved on the pre‐run gel. Free RNAs and protein–RNA complexes were detected using biotin detection reagents (Thermo).
Protein degradation assay
Cells were cultured to ~ 70–80% confluency in six‐well plates. 100 μg/ml emetine and 50 μg/ml cycloheximide were added to the medium, and the cells were grown for three more hours. The cells were then collected, and the cytosols were extracted. The samples were then resolved on a 12% SDS–PAGE and analyzed by Western blotting.
AHA labeling of newly synthesized proteins
Experimental procedures for AHA labeling of newly synthesized proteins were adapted from a published protocol (Dieterich et al, 2007). Cells were cultured to ~ 70–80% confluency in a six‐well plate. Before AHA labeling, cells were cultured in a medium without methionine (Gibco, cat21013024) for 5 min. The medium was then changed to one with 0.5 mM L‐AHA (Jena Bioscience) for 2 h. Labeled cells were harvested and lysed with 1% SDS in PBS and treated with Benzonase (Bestbio). Cell lysates were incubated at 95°C for 10 min. Click reaction was then performed after the samples were cooled to 37°C in a reaction system containing 50 μM Acetylene‐PEG4‐Biotin (Jena Bioscience), 0.1 mM GuSO4, 0.5 mM THPTA (Jena Bioscience), and 5 mM sodium ascorbate (Solarbio) for 2 h. The reaction was stopped with 1 mM EDTA. The labeled proteins were analyzed with SDS–PAGE and biotin detection reagents.
Soft agar colony formation assay
Experimental procedures of soft agar colony formation assay were adapted from a published protocol (Borowicz et al, 2014). 104 cells were cultured in a cell culture medium containing 0.3% agar on the upper layer and 0.6% agar on the bottom layer. The cells were cultured for 2 weeks, and 0.2 ml medium was added twice a week to prevent desiccation. Cells were stained with crystal violet (Solarbio). Stained colonies were photographed and analyzed with ImageJ.
Author contributions
XL, QX and GW conceptualized the study; GW involved in funding acquisition; XL, JH, SW, QZ, PL, HF, XS and HF investigated the study and contributed to methodology; XL and GW wrote the manuscript; GW supervised the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figure PDF
Dataset EV1
Review Process File
Acknowledgements
This research was supported by the Priority Research Program of the Ministry of Science and Technology of the People's Republic of China 2017YFA0504600, National Natural Science Foundation of the People's Republic of China grant 91949103, 91649103, and Ministry of Education of the People's Republic of China 1000 Talents Youth program.
EMBO Reports (2020) 21: e49684
Data availability
This study includes no data deposited in external repositories.
References
- Arif A, Terenzi F, Potdar AA, Jia J, Sacks J, China A, Halawani D, Vasu K, Li X, Brown JM et al (2017) EPRS is a critical mTORC1‐S6K1 effector that influences adiposity in mice. Nature 542: 357–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barends S, Rudinger‐Thirion J, Florentz C, Giege R, Pleij CW, Kraal B (2004) tRNA‐like structure regulates translation of Brome mosaic virus RNA. J Virol 78: 4003–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowicz S, Van Scoyk M, Avasarala S, Karuppusamy Rathinam MK, Tauler J, Bikkavilli RK, Winn RA (2014) The soft agar colony formation assay. J Vis Exp 92: e51998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Liu Y, Zhuang H, Yang B, Hei K, Xiao M, Hou C, Gao H, Zhang X, Jia C et al (2017) Quantitative proteomics reveals that long non‐coding RNA MALAT1 interacts with DBC1 to regulate p53 acetylation. Nucleic Acids Res 45: 9947–9959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher M, Rueda D (2012) Fluorescence characterization of the transfer RNA‐like domain of transfer messenger RNA in complex with small binding protein B. Biochemistry 51: 3531–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich DC, Lee JJ, Link AJ, Graumann J, Tirrell DA, Schuman EM (2007) Labeling, detection and identification of newly synthesized proteomes with bioorthogonal non‐canonical amino‐acid tagging. Nat Protoc 2: 532–540 [DOI] [PubMed] [Google Scholar]
- Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F et al (2012) Landscape of transcription in human cells. Nature 489: 101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher TW (2009) Role of tRNA‐like structures in controlling plant virus replication. Virus Res 139: 217–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gast M, Schroen B, Voigt A, Haas J, Kuehl U, Lassner D, Skurk C, Escher F, Wang X, Kratzer A et al (2016) Long noncoding RNA MALAT1‐derived mascRNA is involved in cardiovascular innate immunity. J Mol Cell Biol 8: 178–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutschner T, Hämmerle M, Diederichs S (2013) MALAT1 — a paradigm for long noncoding RNA function in cancer. J Mol Med (Berl) 91: 791–801 [DOI] [PubMed] [Google Scholar]
- Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P (2011) Angiogenin‐induced tRNA fragments inhibit translation initiation. Mol Cell 43: 613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn M, Rogers MJ, Soll D (1991) Anticodon and acceptor stem nucleotides in tRNA(Gln) are major recognition elements for E. coli glutaminyl‐tRNA synthetase. Nature 352: 258–260 [DOI] [PubMed] [Google Scholar]
- Johnson RC, Vanatta PR, Fresco JR (1977) Metabolic regulation of aminoacyl‐tRNA synthetase biosynthesis in bakers’ yeast. J Biol Chem 252: 878–882 [PubMed] [Google Scholar]
- Keam SP, Sobala A, Ten Have S, Hutvagner G (2017) tRNA‐derived RNA fragments associate with human multisynthetase complex (MSC) and modulate ribosomal protein translation. J Proteome Res 16: 413–420 [DOI] [PubMed] [Google Scholar]
- Khan SY, Hackett SF, Riazuddin SA (2016) Non‐coding RNA profiling of the developing murine lens. Exp Eye Res 145: 347–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Park SJ, Na S, Kim JS, Choi H, Kim YK, Paek E, Lee C (2013) Reinvestigation of aminoacyl‐tRNA synthetase core complex by affinity purification‐mass spectrometry reveals TARSL2 as a potential member of the complex. PLoS ONE 8: e81734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Fuchs G, Wang S, Wei W, Zhang Y, Park H, Roy‐Chaudhuri B, Li P, Xu J, Chu K et al (2017) A transfer‐RNA‐derived small RNA regulates ribosome biogenesis. Nature 552: 57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuckles P, Vogt MA, Lugert S, Milo M, Chong MM, Hautbergue GM, Wilson SA, Littman DR, Taylor V (2012) Drosha regulates neurogenesis by controlling neurogenin 2 expression independent of microRNAs. Nat Neurosci 15: 962–969 [DOI] [PubMed] [Google Scholar]
- Kuhn CD, Wilusz JE, Zheng Y, Beal PA, Joshua‐Tor L (2015) On‐enzyme refolding permits small RNA and tRNA surveillance by the CCA‐adding enzyme. Cell 160: 644–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EY, Lee HC, Kim HK, Jang SY, Park SJ, Kim YH, Kim JH, Hwang J, Kim JH, Kim TH et al (2016) Infection‐specific phosphorylation of glutamyl‐prolyl tRNA synthetase induces antiviral immunity. Nat Immunol 17: 1252–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi O, Arava Y (2019) mRNA association by aminoacyl tRNA synthetase occurs at a putative anticodon mimic and autoregulates translation in response to tRNA levels. PLoS Biol 17: e3000274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Chen P, Qu J, Shi L, Zhuang W, Fu J, Li J, Zhang X, Sun Y, Zhuang W (2014) Activation of LTBP3 gene by a long noncoding RNA (lncRNA) MALAT1 transcript in mesenchymal stem cells from multiple myeloma. J Biol Chem 289: 29365–29375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Song Y, Liu F, Liu D, Miao H, Ren J, Xu J, Ding L, Hu Y, Wang Z et al (2017) Long non‐coding RNA MALAT1 promotes proliferation, angiogenesis, and immunosuppressive properties of mesenchymal stem cells by inducing VEGF and IDO. J Cell Biochem 118: 2780–2791 [DOI] [PubMed] [Google Scholar]
- Liang J, Xu L, Zhou F, Liu AM, Ge HX, Chen YY, Tu M (2018) MALAT1/miR‐127‐5p regulates osteopontin (OPN)‐mediated proliferation of human chondrocytes through PI3K/Akt pathway. J Cell Biochem 119: 431–439 [DOI] [PubMed] [Google Scholar]
- Marchese FP, Raimondi I, Huarte M (2017) The multidimensional mechanisms of long noncoding RNA function. Genome Biol 18: 206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin‐Bejar O, Huarte M (2015) RNA pulldown protocol for in vitro detection and identification of RNA‐associated proteins. Methods Mol Biol 1206: 87–95 [DOI] [PubMed] [Google Scholar]
- Matsuda D, Dreher TW (2004) The tRNA‐like structure of Turnip yellow mosaic virus RNA is a 3′‐translational enhancer. Virology 321: 36–46 [DOI] [PubMed] [Google Scholar]
- Mendell JT (2016) Targeting a long noncoding RNA in breast cancer. N Engl J Med 374: 2287–2289 [DOI] [PubMed] [Google Scholar]
- Morita M, Gravel SP, Hulea L, Larsson O, Pollak M, St‐Pierre J, Topisirovic I (2015) mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle 14: 473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MC, Kang T, Jin D, Han JM, Kim SB, Park YJ, Cho K, Park YW, Guo M, He W et al (2012) Secreted human glycyl‐tRNA synthetase implicated in defense against ERK‐activated tumorigenesis. Proc Natl Acad Sci USA 109: E640–E647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting CP, Oliver PL, Reik W (2009) Evolution and functions of long noncoding RNAs. Cell 136: 629–641 [DOI] [PubMed] [Google Scholar]
- Presolski SI, Hong VP, Finn MG (2011) Copper‐catalyzed azide‐alkyne click chemistry for bioconjugation. Curr Protoc Chem Biol 3: 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Hao Z, Tian Q, Zhang Z, Zhou C, Xie W (2014) Cocrystal structures of glycyl‐tRNA synthetase in complex with tRNA suggest multiple conformational states in glycylation. J Biol Chem 289: 20359–20369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Chang HY (2016) Unique features of long non‐coding RNA biogenesis and function. Nat Rev Genet 17: 47–62 [DOI] [PubMed] [Google Scholar]
- Robinson JC, Kerjan P, Mirande M (2000) Macromolecular assemblage of aminoacyl‐tRNA synthetases: quantitative analysis of protein‐protein interactions and mechanism of complex assembly. J Mol Biol 304: 983–994 [DOI] [PubMed] [Google Scholar]
- Schimmel PR, Soll D (1979) Aminoacyl‐tRNA synthetases: general features and recognition of transfer RNAs. Annu Rev Biochem 48: 601–648 [DOI] [PubMed] [Google Scholar]
- Sobala A, Hutvagner G (2013) Small RNAs derived from the 5′ end of tRNA can inhibit protein translation in human cells. RNA Biol 10: 553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiniello M, Knoener RA, Steinbrink MI, Yang B, Cesnik AJ, Buxton KE, Scalf M, Jarrard DF, Smith LM (2018) HyPR‐MS for multiplexed discovery of MALAT1, NEAT1, and NORAD lncRNA protein interactomes. J Proteome Res 17: 3022–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunwoo H, Dinger ME, Wilusz JE, Amaral PP, Mattick JS, Spector DL (2009) MEN epsilon/beta nuclear‐retained non‐coding RNAs are up‐regulated upon muscle differentiation and are essential components of paraspeckles. Genome Res 19: 347–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA et al (2010) The nuclear‐retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell 39: 925–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney U, Lee CP, RajBhandary UL (1991) Direct analysis of aminoacylation levels of tRNAs in vivo. Application to studying recognition of Escherichia coli initiator tRNA mutants by glutaminyl‐tRNA synthetase. J Biol Chem 266: 24712–24718 [PubMed] [Google Scholar]
- Wilusz JE, Freier SM, Spector DL (2008) 3′ end processing of a long nuclear‐retained noncoding RNA yields a tRNA‐like cytoplasmic RNA. Cell 135: 919–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz JE, Whipple JM, Phizicky EM, Sharp PA (2011) tRNAs marked with CCACCA are targeted for degradation. Science (New York, NY) 334: 817–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Yang M, Tian J, Wang X, Li Z (2011) MALAT‐1: a long non‐coding RNA and its important 3′ end functional motif in colorectal cancer metastasis. Int J Oncol 39: 169–175 [DOI] [PubMed] [Google Scholar]
- Yannay‐Cohen N, Carmi‐Levy I, Kay G, Yang CM, Han JM, Kemeny DM, Kim S, Nechushtan H, Razin E (2009) LysRS serves as a key signaling molecule in the immune response by regulating gene expression. Mol Cell 34: 603–611 [DOI] [PubMed] [Google Scholar]
- Yu CH, Liao JY, Zhou H, Qu LH (2008) The rat mitochondrial Ori L encodes a novel small RNA resembling an ancestral tRNA. Biochem Biophys Res Comm 372: 634–638 [DOI] [PubMed] [Google Scholar]
- Zhang B, Mao YS, Diermeier SD, Novikova IV, Nawrocki EP, Jones TA, Lazar Z, Tung CS, Luo W, Eddy SR et al (2017a) Identification and characterization of a class of MALAT1‐like genomic loci. Cell Rep 19: 1723–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Hamblin MH, Yin KJ (2017b) The long noncoding RNA Malat 1: its physiological and pathophysiological functions. RNA Biol 14: 1705–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K, Diebel KW, Holy J, Skildum A, Odean E, Hicks DA, Schotl B, Abrahante JE, Spillman MA, Bemis LT (2017) A tRNA fragment, tRF5‐Glu, regulates BCAR3 expression and proliferation in ovarian cancer cells. Oncotarget 8: 95377–95391 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figure PDF
Dataset EV1
Review Process File
Data Availability Statement
This study includes no data deposited in external repositories.


