SUMMARY
Transplant-associated thrombotic microangiopathy (TA-TMA) is a complication of allogeneic transplantation (allo-HCT). The incidence and risk factors associated with TA-TMA are not well known. A retrospective analysis from the Center for International Blood and Marrow Transplant Research (CIBMTR) was conducted including patients receiving allo-HCT between 2008 and 2016 with the primary objective of evaluating the incidence of TA-TMA. Secondary objectives included identification of risk factors associated with TA-TMA and the impact of TA-TMA on overall survival and the need for renal replacement therapy (RRT). Among 23,665 allo-HCT recipients, the 3-year cumulative incidence of TA-TMA was 3%. Variables independently associated with development increased incidence of TA-TMA included female sex, prior autologous transplant, primary disease (acute lymphoblastic leukemia and severe aplastic anemia), donor type (mismatched or unrelated donor), conditioning intensity (myeloablative), GVHD prophylaxis (sirolimus + calcineurin inhibitor), pre-transplant kidney dysfunction, and acute GVHD (time-varying effect). TA-TMA was associated with higher mortality (HR=3.1, 95%Confidence Interval [CI]=2.8-16.3) and RRT requirement (HR=7.1, 95%CI=5.7-311.6). This study provides epidemiologic data on TA-TMA and its impact on transplant outcomes. Increased awareness of the risk factors will enable providers to be vigilant of this uncommon but serious transplant complication. The results will also provide benchmarking for future study designs and comparisons.
Keywords: thrombotic microangiopathy, TA-TMA, allogeneic transplantation, allo-HCT
INTRODUCTION
Thrombotic microangiopathy belongs to the family of thrombotic endothelial disorders that also includes atypical hemolytic uremic syndrome (aHUS) and thrombotic thrombocytopenic purpura (TTP). Transplant-associated thrombotic microangiopathy (TA-TMA) occurs when endothelial injury in the context of allogeneic hematopoietic cell transplantation (allo-HCT) causes microangiopathic hemolytic anemia and platelet consumption, resulting in microvascular thrombosis and fibrin deposition in the microcirculation.1,2 In its most severe form, TA-TMA is associated with high mortality (60-90%)3; while milder cases may increase the risk of developing chronic kidney disease (CKD).2
The incidence of and risk factors for TA-TMA continue to be debated. The previously reported incidence of TA-TMA has ranged widely (0.5-76%), reflecting variable awareness among institutions, variable diagnostic criteria, and limited data within single institutions4. High-dose chemotherapy, radiation, calcineurin inhibitor (CNI) exposure, graft versus host disease (GVHD), and infections have all been suggested as causative factors for the development of TA-TMA, but the exact pathogenesis of TA-TMA remains unknown4.
With the increasing number of allo-HCT performed each year, there is a need to understand the epidemiology of TA-TMA and its impact on kidney function and mortality. The CIBMTR database provides an opportunity to study rare post-transplant complications. We sought to evaluate the incidence and risk factors for provider-reported TA-TMA.
METHODS
Data sources
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a working group of more than 500 transplant centers worldwide that contribute detailed data on HCT to a statistical center at the Medical College of Wisconsin (MCW) in Milwaukee, WI and the National Marrow Donor Program (NMDP)/ Be the Match in Minneapolis, MN. Participating centers are required to report all consecutive transplants and compliance is monitored by on-site audits. Computerized checks for discrepancies, physicians’ review of submitted data ensure compliance and data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. The NMDP Central Institutional Review Board reviewed and approved this study.
Patients
Patients who underwent a first allo-HCT for malignant or non-malignant diseases between 2008 and 2016 were included in this analysis. Graft sources included peripheral blood, bone marrow and cord blood. Patients receiving identical twin transplants were excluded. Patients with a diagnosis of paroxysmal nocturnal hemoglobinuria (PNH) and those with non-malignant diseases with previous autologous HCT were excluded. Of note, among patients with aplastic anemia (n=1123) included in the study, 181 patients were known to have a PNH clone and were kept in the analysis. Additional exclusion criteria are detailed in Supplemental Table S1.
Definition of variables and study endpoints
In our study, we assessed the presence of TA-TMA as recorded by the individual centers as a binary outcome for “post-transplant microangiopathy-thrombotic thrombocytopenic purpura (TTP), hemolytic uremic syndrome (HUS), or similar syndrome.” The CIBMTR does not stipulate a specific set of diagnostic criteria, so the presence or absence of TA-TMA was judged by the treating physicians. The intensity of conditioning regimen was defined using the consensus criteria.5 Acute GVHD was graded using standard criteria.6 Estimated glomerular filtration rate (eGFR) was used as a surrogate for pre-transplant kidney function. For adults, eGFR was calculated using the CKD-EPI method and for pediatric cases, it was calculated using the Bedside Schwartz method.7–9 The primary endpoint was to evaluate cumulative incidence of provider-reported TA-TMA. Secondary endpoints included identification of risk factors associated with TA-TMA and evaluating the impact of TA-TMA on overall survival (OS) measured from time of transplant until the date of death from any cause and estimating the risk of requiring renal replacement therapy (RRT) as identified by center as a binary response question on follow-up forms
Statistical analysis
The risk of TA-TMA was estimated using the cumulative incidence method to account for death without TA-TMA as a competing risk. Patients were censored at the time of second allo-HCT or last follow-up. Associations among baseline patient-, disease-, and transplantation-related variables and TA-TMA were evaluated using Cox proportional hazards regression model. A forward stepwise model building approach was used to identify covariates that influenced outcomes. Covariates with a p<0.05 were considered statistically significant. The variables considered in multivariate analysis were age, race, performance score (Karnofsky/Lansky scale), baseline kidney function, disease type, disease status, donor type, prior auto-HCT, use of anti-thymocyte globulin (ATG)/alemtuzumab, year of allo-HCT, GVHD prophylaxis, conditioning intensity, and graft type (Supplemental Table S2). Test of interaction and assumption for proportional hazards were performed in all models. To test the association between GVHD and TA-TMA, acute GVHD was tested as a time-varying covariate in the final model after adjusting for all other significant baseline covariates. For analyses of OS and need for RRT, extended Cox regression models were used to assess the impact of TA-TMA onset as a time-varying covariate. Kaplan-Meier curves were used to depict patient survival after the diagnosis of TMA. Landmark analysis at 6 months following allo-HCT was performed to compare patient survival with and without TMA.10 All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Baseline Characteristics
A total of 23,665 patients were included in the study (see Supplemental Table S1). The baseline patient-, disease- and transplant-related characteristics are shown in Table 1. The median age was 49 years (range: 1-83 years). Most of the patients were Caucasians (79%), with a male (59%) predominance and good performance status (KPS ≥90%, 65%). Malignant diseases comprised 88% of the study population, with acute myeloid leukemia (36%) and myelodysplastic syndrome (19%) as the most common diseases. The most common graft source used for allo-HCT was peripheral blood (60%). The majority of patients received myeloablative conditioning regimen (56%). The most common type of GVHD prophylaxis included CNI-based regimens (92%). The median time from transplant to TA-TMA was 3 months (range: 1.5–6.5 months). Median follow-up of survivors was 37 months (range 3–110 months).
Table 1.
Baseline characteristics of patients who underwent allo-HCT between 2008-2016
| Variable | N=23665 (%) |
|---|---|
| Median age at HCT, years (range) | 49 (1-83) |
| Male gender | 13928 (59) |
| Race | |
| Caucasian | 18752 (79) |
| African-American | 2148 (9) |
| Others | 1835 (8) |
| Missing | 930 (4) |
| Karnofsky score | |
| ≥90 | 15272 (65) |
| <90 | 7909 (33) |
| Missing | 484 (2) |
| HCT-CI (excluding renal condition) | |
| 0 | 8648 (37) |
| 1 | 3222 (14) |
| 2 | 2724 (12) |
| ≥3 | 8322 (35) |
| Missing | 749 (3) |
| Pre-conditioning kidney function | |
| Decreased (GFR<60) | 1848 (8) |
| Normal (GFR ≥60) | 20667 (87) |
| Missing | 1150 (5) |
| Disease* | |
| Malignant | 20847 (88) |
| Non-malignant | 2818 (12) |
| Donor/recipient CMV status | |
| Both negative | 8652 (37) |
| Any positive | 14824 (63) |
| Missing | 189 (<1) |
| Type of donor | |
| HLA-identical sibling | 5963 (25) |
| Other related | 2439 (10) |
| Matched URD (8/8) | 8022 (34) |
| Partially matched URD (7/8) | 1919 (8) |
| Mismatched URD (<7/8) | 119 (<1) |
| URD, HLA match unknown | 413 (2) |
| CB 6/6 | 538 (2) |
| CB 5/6 | 1860 (8) |
| CB <5/6 | 1672 (7) |
| CB, HLA match unknown | 692 (3) |
| Missing | 28 (<1) |
| Donor-recipient ABO match | |
| Matched | 11836 (50) |
| Minor Mismatch | 4922 (21) |
| Major Mismatch | 6018 (25) |
| CB - recipient A | 85 (<1) |
| CB - recipient B | 37 (<1) |
| CB - recipient AB | 15 (<1) |
| CB - recipient O | 111 (<1) |
| CB - recipient ABO unknown | 10 (<1) |
| Missing | 631 (3) |
| Prior auto-HCT | |
| No | 22450 (95) |
| Yes | 1089 (5) |
| Missing | 116 (<1) |
| Graft type | |
| Bone marrow | 4732 (20) |
| Peripheral blood | 14171 (60) |
| CB | 4762 (20) |
| Conditioning regimen and intensity | |
| Myeloablative TBI | 5522 (23) |
| Myeloablative Bu based | 7132 (30) |
| Other myeloablative | 701 (3) |
| RIC/NMA | 10279 (43) |
| Missing | 31 (<1) |
| GVHD prophylaxis** | |
| CNI + post-Cy | 1412 (6) |
| CNI + Siro (no post-Cy) | 1791 (8) |
| Siro (no CNI or post-Cy) | 166 (<1) |
| Csa (no Tac, Siro, or post-Cy) | 6452 (27) |
| Tac (no Csa, Siro, or post-Cy) | 12112 (51) |
| Other prophylaxis strategies | 1732 (7) |
| ATG/ alemtuzumab | |
| No ATG or alemtuzumab | 15041 (64) |
| ATG alone | 7462 (32) |
| Alemtuzumab alone | 1146 (5) |
| ATG + alemtuzumab | 16 (<1) |
| Year of transplant | |
| 2008-2012 | 11222 (47) |
| 2013-2016 | 12443 (53) |
| Median follow-up of survivors (range), months | 37 (3-110) |
Abbreviations: HCT- hematopoietic cell transplantation, HCT-CI- HCT comorbidity index, GFR- glomerular filtration rate, CMV – cytomegalovirus, HLA - human leukocyte antigen, URD-unrelated donor, CB- cord blood, auto- autologous, Bu-busulfan, TBI-total body irradiation, RIC/NMA- reduced-intensity conditioning/non-myeloablative conditioning, GVHD- graft-versus-host disease, Cy-Cyclophosphamide, CNI-calcineurin inhibitor, Tac- tacrolimus, Csa-cyclosporine, Siro-sirolimus, ATG- anti-thymocyte globulin
Disease breakdown is shown in Supplemental Table S4
Cumulative incidence of TA-TMA
The cumulative incidence of TA-TMA was 2% (95% CI=2-2%) at 1 year, and 3% (95% CI= 3-3%) at 2-years and 3-years post allo-HCT. Because these estimates were lower than those reported in several prior reports, we re-evaluated our data in several ways. First, we performed a sensitivity analysis to address potential under-reporting by examining the incidence per center and excluded centers that contributed 50 or fewer patients during the study period and centers that reported no cases of TMA. In this scenario, the 1-year cumulative incidence increased slightly to 3%. Second, we ranked the incidence by site and noted the highest 1-year incidence in a single center was 12% (95% CI=9-15%).
Risk factors for the development of TA-TMA
On multivariable analysis, the factors that were associated with decreased risk of TA-TMA included male sex (relative to females, HR=0.70, 95%CI=0.59-0.81), use of ATG or alemtuzumab (compared to no ATG/alemtuzumab, HR=0.69, 95%CI=0.56-0.85) and reduced intensity/non-myeloablative conditioning (vs. busulfan-based myeloablative conditioning, HR=0.73, 95%CI=0.59-0.90) (Figure 1; Supplemental Table S3). In contrast, African-American race (relative to Caucasians, HR=1.46, 95%CI=1.13-1.89), a diagnosis of acute lymphoblastic leukemia (ALL) or aplastic anemia (relative to acute myeloid leukemia, HR=1.33, 95%CI=1.03-1.71 for ALL and HR=1.83, 95%CI=1.22-2.74 for aplastic anemia), poor renal function (GFR<60 relative to GFR≥60, HR=1.47, 95%CI=1.08-1.99), CNI with sirolimus as GVHD prophylaxis (relative to CNI + others, HR=2.77, 95%CI=1.78-4.31), and prior auto-HCT (relative to no prior auto-HCT, HR=1.84, 95%CI=1.30-2.61) (Figure 1; Supplemental Table S3) were associated with increased risk of TA-TMA. Compared to HLA-identical sibling donor, all other donor types had increased risk for TA-TMA development; those conferring a significantly higher risk of TA-TMA were matched unrelated donor (URD) (HR=1.54, 95%CI=1.23-1.93), partially matched (7/8-matched) URD (HR=1.85, 95%CI=1.35-2.52), mismatched unrelated donor (MMUD) (<7/8-matched) (HR=4.01, 95%CI=2.02-7.95), other related donor (HR=1.51, 95%CI=1.06-2.15) and cord blood (≤4/6-match, HR=2.17, 95%CI=1.58-2.96) (Figure 1; Supplemental Table S3). After adjusting for all these risk factors, patients who developed acute GVHD post-transplant had a significantly higher risk of TA-TMA (grade II, HR=2.19, 95%CI=1.78-2.69; grade III-IV, HR=4.79, 95%CI=3.94-5.81) relative to those with grade I or no acute GVHD (Figure 1; Supplemental Table S3).
Figure 1.
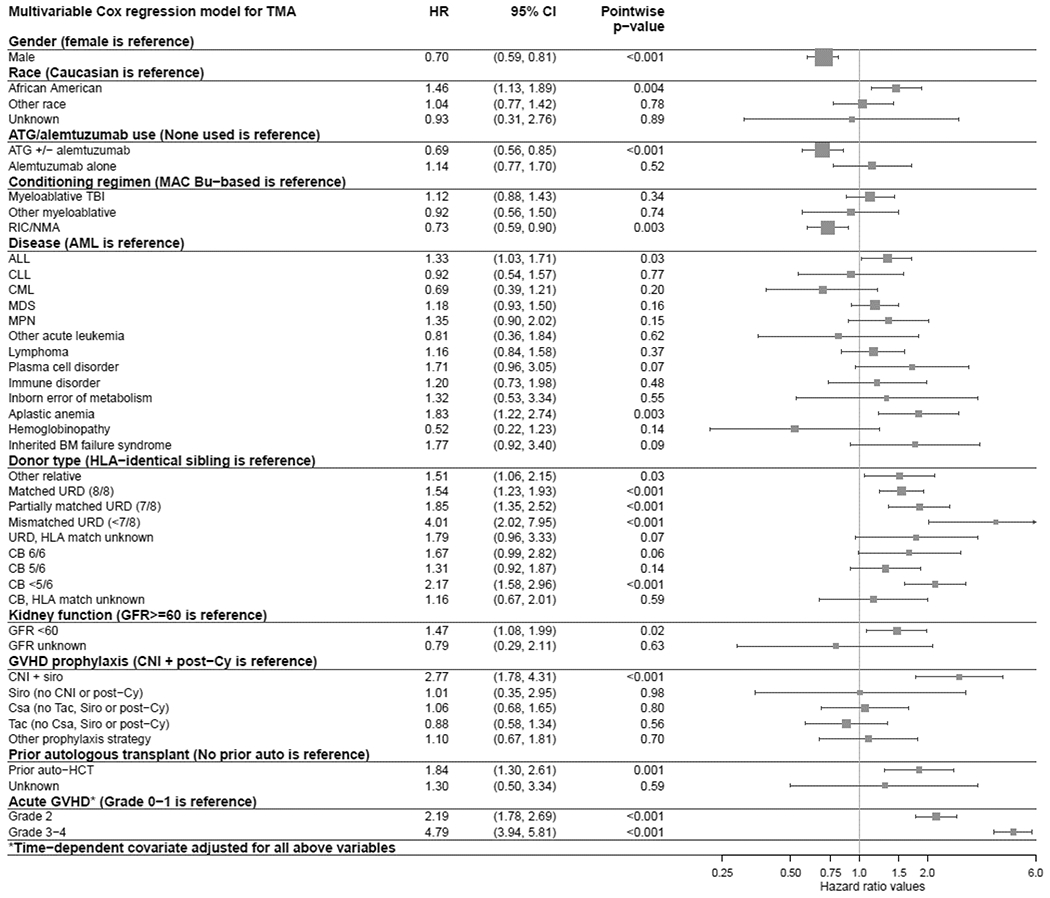
Forest plot of multivariate model for TA-TMA
Impact of TA-TMA on survival and need for renal replacement therapy
In multivariate analysis, patients who developed TA-TMA had a significantly increased risk for mortality compared to those without TA-TMA considering TA-TMA as a time-dependent covariate (HR=3.09, 95%CI=2.79-16.34, p<0.001). In an unadjusted subgroup analysis, there was a higher risk of mortality in patients with TA-TMA receiving plasmapheresis relative to no plasmapheresis (HR=4.14, 95%CI=3.44-4.99, p<0.001) (Table 2). The probability of OS after diagnosis of TA-TMA was 52% at 6 months, and 42% at 1 year (Figure 2). A six-months landmark analysis showed a significantly decreased survival in patients with TA-TMA compared to those without TA-TMA (Figure 3).
Table 2.
Cox regression models of the impact of TA-TMA on overall survival and renal failure/need for dialysis, using TA-TMA as time-dependent covariate
| Outcome | Level | N | HR | Lower 95% CI | Upper 95% CI | Pwp | Overall p-value |
|---|---|---|---|---|---|---|---|
| Overall survival | <0.001 | ||||||
| No TA-TMA | 22944 | 1.00 | |||||
| TA-TMA, all patients | 653 | 3.09 | 2.79 | 16.34 | <0.001 | ||
| TA-TMA, no plasmapheresis | 499 | 2.47 | 2.20 | 2.78 | <0.001 | ||
| TA-TMA, plasmapheresis | 154 | 4.14 | 3.44 | 4.99 | <0.001 | ||
| Need for RRT | <0.001 | ||||||
| No TA-TMA | 21939 | 1.00 | |||||
| TA-TMA, all patients | 619 | 7.12 | 5.74 | 311.64 | <0.001 | ||
| TA-TMA, no plasmapheresis | 467 | 3.81 | 2.87 | 5.05 | <0.001 | ||
| TA-TMA, plasmapheresis | 152 | 14.92 | 10.90 | 20.42 | <0.001 |
Abbreviations: HR - hazard ratio, CI - confidence interval, TA-TMA - transplant associated thrombotic microangiopathy; N - number; RRT - renal replacement therapy, pwp - pairwise p-value
Adjusted for age, race, KPS, kidney function at baseline, disease, disease status, donor type, prior auto-HCT, use of ATG/ alemtuzumab, year of TX, GVHD prophylaxis, conditioning, and graft type.
Figure 2.
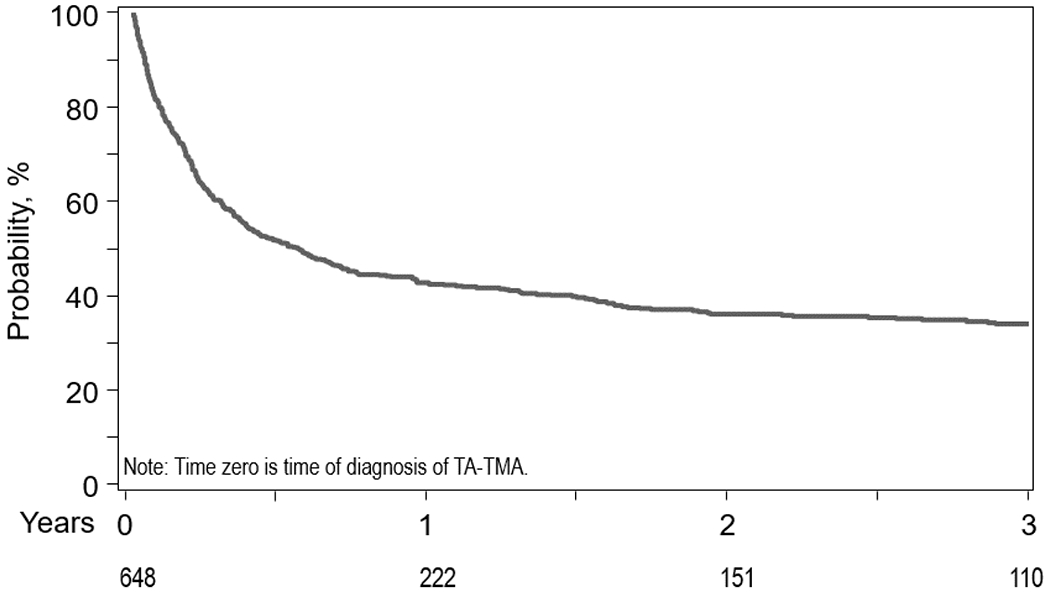
Overall Survival after TA-TMA diagnosis
Figure 3.
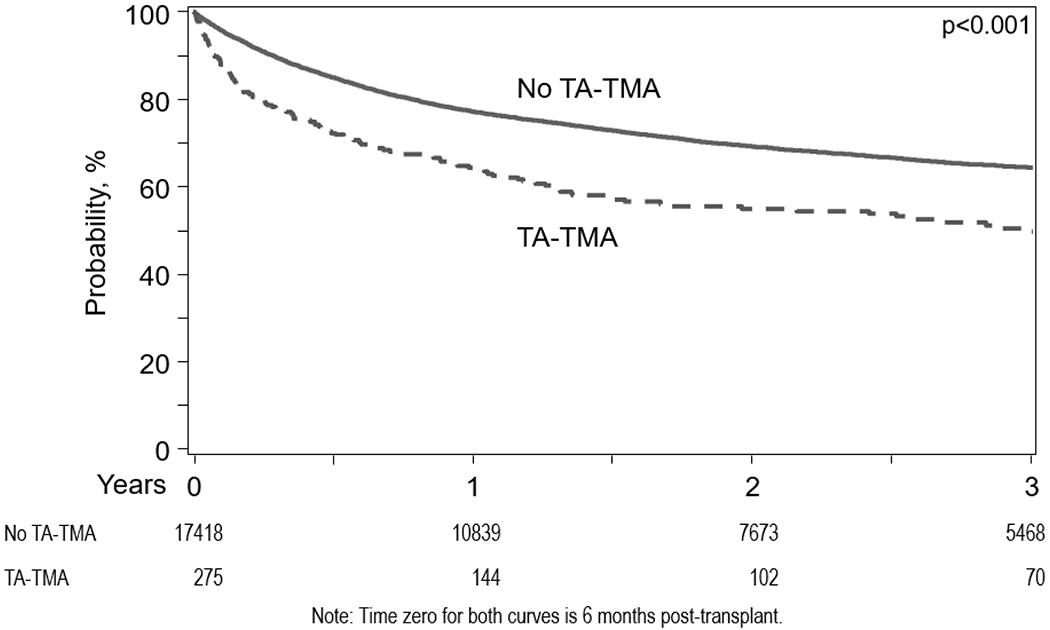
Landmark Analysis at 6 months following allo-HCT showing survival between TA-TMA and no TA-TMA
Patients who developed TA-TMA had a significantly higher risk of RRT requirement compared to those without TA-TMA considering TA-TMA as a time-dependent covariate (HR=7.12, 95%CI=5.74-311.64, p<0.001) (Table 2). The median time from TA-TMA to RRT was 2.01 months (range: 0.6-2.01 months). In an unadjusted subgroup analysis, there was a significantly higher risk of RRT requirement among patients with TA-TMA receiving plasmapheresis compared to no plasmapheresis (HR=14.92, 95%CI=10.90-20.42, p<0.001) (Table 2).
Causes of Death
At last follow-up, 65% (429/661) of patients with TA-TMA and 46% (10,644/22,994) of those without TA-TMA had died (Table 3). Among TA-TMA patients, the most common cause of death was organ failure (24%), followed by primary disease (22%), infection (20%), GVHD (19%), and hemorrhage/vascular (8%). In contrast, among non-TMA patients, the most common cause of death was primary disease (45%), followed by organ failure (17%), infection (16%), and GVHD (12%), and hemorrhage/vascular (3%). The distribution of type of organ failure was similar in both groups.
Table 3:
Causes of Death
| Causes of death, N (%) | No TMA (n=10644) | TMA (n=429) |
|---|---|---|
| Organ failure | 1848 (17) | 104 (24) |
| Multisystem organ failure | 359 | 25 |
| Pulmonary failure | 413 | 23 |
| ARDS | 228 | 15 |
| IPS | 117 | 7 |
| Pneumonitis | 65 | 1 |
| DAD or other pulmonary syndrome | 23 | 1 |
| Cardiac failure | 220 | 12 |
| Liver failure | 62 | 3 |
| VOD/SOS | 99 | 4 |
| Renal failure | 38 | 6 |
| CNS failure | 37 | 1 |
| Other organ failure or not specified | 187 | 6 |
| Primary Disease | 4738 (45) | 96 (22) |
| Infection | 1715 (16) | 87 (20) |
| Graft versus Host disease | 1275 (12) | 81 (19) |
| Hemorrhage or vascular | 266 (3) | 35 (8) |
| Second malignancy | 224 (2) | 3 (<1) |
| Graft rejection/failure | 151 (1) | 2 (<1) |
| Other/unknown | 427 (4) | 21 (5) |
Abbreviations: IPS - Idiopathic pulmonary syndrome, DAD – diffuse alveolar damage, ARDS – acute respiratory distress syndrome, VOD – veno-occlusive disease, SOS - sinusoidal obstruction syndrome, CNS – central nervous system
DISCUSSION
In this registry-based retrospective cohort study, we identified 661 cases of provider-reported TA-TMA among 23,665 pediatric and adult allo-HCT recipients between 2008 and 2016. To our knowledge, this is the largest study to date to describe the epidemiology of this uncommon post-transplant complication. The overall provider-reported incidence of TA-TMA in the study was approximately 2-3%. We further explored the incidence of this complication over time which demonstrated no specific trend during the period of the study. Several risk factors were shown to be associated with the development of TA-TMA Patients with TA-TMA experienced substantially higher mortality with a 3-fold increased risk of death and an even higher risk of requiring renal replacement therapy of over 7-fold, compared to those without TA-TMA, confirming though rare, this is a devastating complication of allo-HCT.
It is important to contrast the incidence of provider-reported TA-TMA in the CIBMTR database with that of laboratory-detected TA-TMA reported in other studies. A recent study from the European Society for Blood and Marrow Transplantation (EBMT) revealed significant inter-center variability in adoption of laboratory criteria used for TA-TMA diagnosis and in agreement on schistocyte recognition and counting.11 Among 17 centers surveyed, 41% used the International Working Group criteria12, 41% used the overall-TMA criteria13, and 18% used physician’s decision. Due to non-standardized diagnostic criteria and multifactorial etiology of TA-TMA, as many as 64% of laboratory-detected TA-TMA cases may not be clinically recognized or reported.14 It is likely that the under-reporting is proportionate to the severity of disease with mild TA-TMA less likely to be captured.
Given this discrepancy between laboratory-based and provider-reported TMA diagnosis, we examined other single-center studies published during the same period as our study (2008-2016). Whereas one prospective study of predominantly pediatric patients reported a 1-year cumulative incidence of 39%,15 the remaining studies of adult patients reported a 6-month to 1-year cumulative incidence ranging from 4 to 16%.14,16–20 These incidences are higher to what was reported to the CIBMTR, which are likely a subset of mostly more severe cases. The CIBMTR captures follow up in calendar forms and center summarizes all events that occurred in the previous reporting period. It is likely that cases of transient TA-TMA that are treated by discontinuation of CNI, for example are not clinically severe enough to be captured systematically in a retrospective matter. This is indeed a pitfall of this analysis, however the strong association between the reported TA-TMA with post-transplant outcomes strengthens the point that these provider-defined TA-TMA events were clinically significant. Our study highlights the need for uniform consensus diagnostic criteria that are easily implementable and highly specific for TA-TMA, preferably incorporating the use of laboratory biomarkers to compliment non-specific hemolysis parameters. We recommend assessing the incidence of both laboratory-detected and provider-reported TA-TMA. We acknowledge that the former may be a surrogate for the latter and note that provider-reported TMA probably represents merely a subset of all TA-TMA which are clinically significant.
In our study, significant risk factors for TA-TMA included ALL and aplastic anemia (compared to AML), mismatched donor source (URD or CB), prior autologous transplant, CNI plus sirolimus as GVHD prophylaxis, and grade II-IV GVHD. The risk factors associated with TA-TMA in our study are consistent with the previously published studies.13,14,16–28. Several other risk factors were also identified in this study, including African-American race, aplastic anemia, decreased baseline GFR, and non-ATG containing conditioning. We hypothesize that patients with ALL, especially pediatric, had a higher risk of developing TA-TMA due to more frequent use of total body irradiation (TBI)-based myeloablative conditioning, which could be associated with more endothelial injury, thereby predisposing to TA-TMA. Among patients with severe aplastic anemia with a reported PNH clone, the incidence of TA-TMA was not higher than in those who received an allo-HCT without PNH (p=0.88). The use of ATG or alemtuzumab was also associated with reduced risk for TA-TMA compared to other GVHD prophylaxis regimens, as these regimens are less likely to use CNI.
The relationship between TA-TMA and acute GVHD had a gradient effect where more severe presentations of acute GVHD were associated with a higher risk for TA-TMA (HR 4.79, 95% CI=3.94-5.81 for grade III-IV vs grade ≤I). It is hypothesized that this phenomenon may be a function of ongoing endothelial injury as the causal link. Luft et al. previously reported that both steroid-refractory GVHD and TA-TMA correlate with markers of endothelial cell dysfunction29,30. Wall et al. demonstrated that steroid-refractory gastrointestinal GVHD has a strong relationship with TA-TMA and proposed that complement activation serves as the mechanistic link between the two 31.
Since the majority of patients received CNI-based GVHD prophylaxis, we could not draw conclusions regarding the specific risks associated with CNI vs. no CNI. However, there was no significant difference between tacrolimus and cyclosporine-based GVHD prophylaxis as a predictor of TA-TMA. Although CNI alone did not seem to be associated with TA-TMA, we did observe a significantly increased risk of TA-TMA in patients receiving both CNI and sirolimus (HR 2.77, 95%CI=1.78-4.31). It is plausible that these two drugs act synergistically to trigger TA-TMA or perhaps sirolimus alone is sufficient to cause TA-TMA. Understanding the association between immunosuppressant drugs and TA-TMA is crucial - The Bone Marrow Transplant Clinical Trials Network Toxicity Committee consensus summary recommends CNI cessation as the first-line management option,32 although many large cohort studies to-date reported no benefit to this approach.14,21,27 Based on the results of the study and other recent reports,14,19 sirolimus cessation for a patient with CNI plus sirolimus regimen is a reasonable choice but CNI cessation in patients on monotherapy should be carefully considered, given the strong association between severe GVHD and TA-TMA.
We observed a significantly higher risk of mortality and the need for RRT in patients with TA-TMA. The cause of death among TA-TMA patients was more frequently a transplant-related complication rather than disease relapse. Indeed, several prior studies have shown that TA-TMA with concurrent GVHD or infection had worse survival than TA-TMA alone.14,19,20 Since the survival analysis was not adjusted for these time-varying complications that often occur concurrently with TA-TMA, we could not conclude whether TA-TMA onset was an independent predictor of outcomes or simply a surrogate for other complications. Similarly, the higher risk of mortality and need for RRT among the patients with TA-TMA receiving (vs. not receiving) plasmapheresis was likely reflective of providers’ bias in using plasmapheresis in more severe cases of TA-TMA rather than milder cases. In addition, based on published reports, we recognize that plasmapheresis is an ineffective treatment for TA-TMA.32,33 The study was also limited by not capturing information on other novel potential treatment modalities such as complement blockade, anti-VWF or anti-endothelial targeting.
While the study confirms that developing TA-TMA following allo-HCT carries a high risk of complications, OS appears to be better than historic cohorts. A comprehensive systematic review published in 2004 by George et al. reported that patients with TA-TMA had median cumulative mortality of 75%, with 82% of deaths occurring within 3 months of diagnosis.3 The median OS in our study was 6 months and the cumulative mortality was 35% at 3 months and 60% during longer follow-up. This result is consistent with several recently published single-center cohort studies; the reasons are unclear, particularly given the lack of substantive improvements in supportive care for this condition and the lack of improvement in survival over time (data not shown). However, given the strong link between TA-TMA and severe GVHD, it is plausible that the improvement in OS in the TA-TMA cohort is largely related to the advances in treatment of GVHD and allied supportive care in the current era.34
In conclusion, this study describes the epidemiology of TA-TMA, risk factors, the impact of TA-TMA and its treatment on subsequent HCT outcomes. As an observational cohort for TA-TMA, these results may be useful for benchmarking for future comparative studies and clinical trial designs.
Supplementary Material
ACKNOWLEDGMENTS
Collaborators (Other members of the Writing Committee):
Allistair A. Abraham20, Vaibhav Agrawal21, Mahmoud Aljurf22, Medhat Askar23, Pere Barba24, Alice Bertaina25, Jean-Yves Cahn26, Jan Cerny27, Hannah K. Choe28, Miguel Angel Diaz29, Christopher Dvorak30, Nosha Farhadfar31, Shahinaz M. Gadalla32, Usama Gergis33, Siddhartha Ganguly34, Shahrukh Hashmi35, 22, Kimberly A. Kasow36, Sunita Nathan37, Roomi Nusrat38, Sachiko Seo39, Niketa C. Shah40.
20Division of Blood and Marrow Transplantation, Center for Cancer and Blood Disorders, Children’s National Medical Center, Washington, DC; 21Division of Hematology-Oncology, Indiana University School of Medicine, Indianapolis, IN; 22Department of Oncology, King Faisal Specialist Hospital Center & Research, Riyadh, Saudi Arabia; 23Baylor University Medical Center, Dallas, TX; 24Hospital Vall d’Hebron, Barcelona, Spain; 25Stanford University School of Medicine, Stanford, CA; 26Department of Hematology, CHU Grenoble Alpes, Grenoble, France; 27Division of Hematology/Oncology, Department of Medicine, University of Massachusetts Medical Center, Worcester, MA; 28Ohio State Medical Center, James Cancer Center, Columbus, OH; 29Department of Hematology/Oncology, Hospital Infantil Universitario Nino Jesus, Madrid, Spain; 30Division of Pediatric Allergy, Immunology & Bone Marrow Transplantation, Benioff Children’s Hospital, University of California San Francisco, San Francisco, CA; 31Division of Hematology/Oncology, University Florida College of Medicine, Gainesville, FL; 32Division of Cancer Epidemiology & Genetics, NIH-NCI Clinical Genetics Branch, Rockville, MD; 33Hematolgic Malignancies & Bone Marrow Transplant, Department of Medical Oncology, New York Presbyterian Hospital/Weill Cornell Medical Center, New York, NY; 34Division of Hematological Malignancy and Cellular Therapeutics, University of Kansas Health System, Kansas City, KS; 35Department of Internal Medicine, Mayo Clinic, Rochester, MN; 36University of North Carolina, Chapel Hill, NC; 37Rush University Medical Center, Chicago, IL; 38Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ; 39Department of haematology and Oncology, Dokkyo Medical University, Tochigi, Japan; 40Yale New Haven Hospital, Yale University , New Haven, CT
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement U24HL138660 from NHLBI and NCI; Grant U24CA233032 from the NCI; Grant OT3HL147741 from NHLBI; Grant R21HL140314 from NHBLI; Grant U01HL128568 from NHLBI; a contract HHSH250201700006C with Health Resources and Services Administration (HRSA/DHHS); Grants N00014-18-1-2888 and N00014-17-1-2850 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; *Anthem, Inc.; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; Boston Children’s Hospital; *Bristol Myers Squibb Co.; *Celgene Corp.; Children’s Hospital of Los Angeles; *Chimerix, Inc.; *CSL Behring; *CytoSen Therapeutics, Inc.; Dana Farber Cancer Institute; *Daiichi Sankyo Co., Ltd.; Fred Hutchinson Cancer Research Center; *Gamida-Cell, Ltd.; Gilead Sciences, Inc.; *GlaxoSmithKline (GSK); HistoGenetics, Inc.; Immucor; Incyte Corporation; Janssen Biotech, Inc.; *Janssen Pharmaceuticals, Inc.; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Karius, Inc.; Karyopharm Therapeutics, Inc.; *Kite, a Gilead Company; *Magenta Therapeutics; Medac GmbH; The Medical College of Wisconsin; Mediware; Merck & Company, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; Mundipharma EDO; National Marrow Donor Program; Novartis Oncology; Novartis Pharmaceuticals Corporation; *Omeros Corporation; *Oncoimmune, Inc.; PCORI; *Pfizer, Inc.; *Phamacyclics, LLC; PIRCHE AG; *Regeneron Pharmaceuticals, Inc.; REGiMMUNE Corp.; *Sanofi Genzyme; *Seattle Genetics; *Shire; Sobi, Inc.; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; Swedish Orphan Biovitrum, Inc.; *Takeda Oncology; University of Minnesota; University of Pittsburgh; University of Texas-MD Anderson; University of Wisconsin - Madison and Viracor Eurofins. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Funding: Not applicable
LIST OF ABBREVIATIONS
- TA-TMA
transplant-associated thrombotic microangiopathy
- Allo-HCT
Allogeneic hematopoietic cell transplantation
- TED
Transplant Essential Data
- CRF
Comprehensive Report Form
- HCT-CI
HCT comorbidity index
- Cy
cyclophosphamide
- GFR
glomerular filtration rate
- GVHD
graft-versus-host disease
- URD
unrelated donor
- CNI
calcineurin inhibitor
- TAC
tacrolimus
- CSA
cyclosporine
- ATG
anti-thymocyte globulin
- CB
cord blood
- OS
overall survival
- PFS
progression-free survival
- HR
hazard ratio
- CI
confidence interval
- TA-TMA
transplant associated thrombotic microangiopathy
- RRT
renal replacement therapy
- KPS
Karnofsky performance status
Footnotes
Ethics approval and consent to participate: Not applicable
Consent for publication: Not applicable
Availability of data and material: Please contact author for data requests
Competing interests: The authors declare that they have no competing interests pertaining to the manuscript.
REFERENCES
- 1.Siami K, Kojouri K, Swisher KK, Selby GB, George JN, Laszik ZG. Thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation: an autopsy study. Transplantation. 2008;85(1):22–28. doi: 10.1097/01.tp.0000297998.33418.7e [DOI] [PubMed] [Google Scholar]
- 2.Changsirikulchai S, Myerson D, Guthrie KA, McDonald GB, Alpers CE, Hingorani SR. Renal thrombotic microangiopathy after hematopoietic cell transplant: role of GVHD in pathogenesis. Clin J Am Soc Nephrol. 2009;4(2):345–353. doi: 10.2215/CJN.02070508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George JN, Li X, McMinn JR, et al. Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome following allogeneic HPC transplantation: A diagnostic dilemma. Transfusion. 2004;44(2):294–304. doi: 10.1111/j.1537-2995.2004.00700.x [DOI] [PubMed] [Google Scholar]
- 4.Laskin BL, Goebel J, Davies SM, Jodele S. Small vessels , big trouble in the kidneys and beyond : hematopoietic stem cell transplantation – associated thrombotic microangiopathy. Blood. 2011;118(6):1452–1462. doi: 10.1182/blood-2011-02-321315. [DOI] [PubMed] [Google Scholar]
- 5.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. http://www.ncbi.nlm.nih.gov/pubmed/7581076. [PubMed] [Google Scholar]
- 7.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4(11):1832–1843. doi: 10.2215/CJN.01640309 [DOI] [PubMed] [Google Scholar]
- 10.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710–719. doi: 10.1200/JCO.1983.1.11.710 [DOI] [PubMed] [Google Scholar]
- 11.Moiseev IS, Tsvetkova T, Aljurf M, et al. Clinical and morphological practices in the diagnosis of transplant-associated microangiopathy: a study on behalf of Transplant Complications Working Party of the EBMT. Bone Marrow Transplant October 2018. doi: 10.1038/s41409-018-0374-3 [DOI] [PubMed] [Google Scholar]
- 12.Ruutu T, Barosi G, Benjamin RJ, et al. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. Haematologica. 2007;92(1):95–100. doi: 10.3324/haematol.10699 [DOI] [PubMed] [Google Scholar]
- 13.Cho B-S, Yahng S-A, Lee S-E, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;90(8):918–926. doi: 10.1097/TP.0b013e3181f24e8d [DOI] [PubMed] [Google Scholar]
- 14.Li A, Wu Q, Davis C, et al. Transplant-Associated Thrombotic Microangiopathy Is a Multifactorial Disease Unresponsive to Immunosuppressant Withdrawal. Biol Blood Marrow Transplant. 2019;25(3):570–576. doi: 10.1016/j.bbmt.2018.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jodele S, Davies SM, Lane A, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124(4):645–653. doi: 10.1182/blood-2014-03-564997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labrador J, López-Corral L, López-Godino O, et al. Risk factors for thrombotic microangiopathy in allogeneic hematopoietic stem cell recipients receiving GVHD prophylaxis with tacrolimus plus MTX or sirolimus. Bone Marrow Transplant. 2014;49(5):684–690. doi: 10.1038/bmt.2014.17 [DOI] [PubMed] [Google Scholar]
- 17.Sakellari I, Gavriilaki E, Boussiou Z, et al. Transplant-associated thrombotic microangiopathy: an unresolved complication of unrelated allogeneic transplant for hematologic diseases. Hematol Oncol. 2017;35(4):932–934. doi: 10.1002/hon.2346 [DOI] [PubMed] [Google Scholar]
- 18.Ye Y, Zheng W, Wang J, et al. Risk and prognostic factors of transplantation-associated thrombotic microangiopathy in allogeneic haematopoietic stem cell transplantation: a nested case control study. Hematol Oncol. 2017;35(4):821–827. doi: 10.1002/hon.2310 [DOI] [PubMed] [Google Scholar]
- 19.Postalcioglu M, Kim HT, Obut F, et al. Impact of Thrombotic Microangiopathy on Renal Outcomes and Survival after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2018;24(11):2344–2353. doi: 10.1016/j.bbmt.2018.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraft S, Bollinger N, Bodenmann B, et al. High mortality in hematopoietic stem cell transplant-associated thrombotic microangiopathy with and without concomitant acute graft-versus-host disease. Bone Marrow Transplant. 2019;54(4):540–548. doi: 10.1038/s41409-018-0293-3 [DOI] [PubMed] [Google Scholar]
- 21.Uderzo C, Bonanomi S, Busca A, et al. Risk factors and severe outcome in thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Transplantation. 2006;82(5):638–644. doi: 10.1097/01.tp.0000230373.82376.46 [DOI] [PubMed] [Google Scholar]
- 22.Willems E, Baron F, Seidel L, Frère P, Fillet G, Beguin Y. Comparison of thrombotic microangiopathy after allogeneic hematopoietic cell transplantation with high-dose or nonmyeloablative conditioning. Bone Marrow Transplant. 2010;45(4):689–693. doi: 10.1038/bmt.2009.230 [DOI] [PubMed] [Google Scholar]
- 23.Shayani S, Palmer J, Stiller T, et al. Thrombotic Microangiopathy Associated with Sirolimus Level after Allogeneic Hematopoietic Cell Transplantation with Tacrolimus/Sirolimus-Based Graft-versus-Host Disease Prophylaxis. Biol Blood Marrow Transplant. 2013;19(2):298–304. doi: 10.1016/j.bbmt.2012.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamae H, Yamane T, Hasegawa T, et al. Risk factor analysis for thrombotic microangiopathy after reduced-intensity or myeloablative allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2006;81(7):525–531. doi: 10.1002/ajh.20648 [DOI] [PubMed] [Google Scholar]
- 25.Cutler C, Henry NL, Magee C, et al. Sirolimus and thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(7):551–557. doi: 10.1016/j.bbmt.2005.04.007 [DOI] [PubMed] [Google Scholar]
- 26.Shimoni A, Yeshurun M, Hardan I, Avigdor A, Ben-Bassat I, Nagler A. Thrombotic microangiopathy after allogeneic stem cell transplantation in the era of reduced-intensity conditioning: The incidence is not reduced. Biol Blood Marrow Transplant. 2004;10(7):484–493. doi: 10.1016/j.bbmt.2004.03.002 [DOI] [PubMed] [Google Scholar]
- 27.Oran B, Donato M, Aleman A, et al. Transplant-associated microangiopathy in patients receiving tacrolimus following allogeneic stem cell transplantation: risk factors and response to treatment. Biol Blood Marrow Transplant. 2007;13(4):469–477. doi: 10.1016/j.bbmt.2006.11.020 [DOI] [PubMed] [Google Scholar]
- 28.Martinez MT, Bucher C, Stussi G, et al. Transplant-associated microangiopathy (TAM) in recipients of allogeneic hematopoietic stem cell transplants. Bone Marrow Transplant. 2005;36(11):993–1000. doi: 10.1038/sj.bmt.1705160 [DOI] [PubMed] [Google Scholar]
- 29.Luft T, Dietrich S, Falk C, et al. Steroid-refractory GVHD: T-cell attack within a vulnerable endothelial system. Blood. 2011;118(6):1685–1692. doi: 10.1182/blood-2011-02-334821 [DOI] [PubMed] [Google Scholar]
- 30.Zeisbrich M, Becker N, Benner A, et al. Transplant-associated thrombotic microangiopathy is an endothelial complication associated with refractoriness of acute GvHD. Bone Marrow Transplant. 2017;52(10):1399–1405. doi: 10.1038/bmt.2017.119 [DOI] [PubMed] [Google Scholar]
- 31.Wall SA, Zhao Q, Yearsley M, et al. Complement-mediated thrombotic microangiopathy as a link between endothelial damage and steroid-refractory GVHD. Blood Adv. 2018;2(20):2619–2628. doi: 10.1182/bloodadvances.2018020321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho VT, Cutler C, Carter S, et al. Blood and Marrow Transplant Clinical Trials Network Toxicity Committee consensus summary: Thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(8):571–575. doi: 10.1016/j.bbmt.2005.06.001 [DOI] [PubMed] [Google Scholar]
- 33.Li A, Makar RS, Hurwitz S, et al. Treatment with or without plasma exchange for patients with acquired thrombotic microangiopathy not associated with severe ADAMTS13 deficiency: a propensity score-matched study. Transfusion. 2016;56(8):2069–2077. doi: 10.1111/trf.13654 [DOI] [PubMed] [Google Scholar]
- 34.Khoury HJ, Wang T, Hemmer MT, et al. Improved survival after acute graft-versus-host disease diagnosis in the modern era. Haematologica. 2017;102(5):958–966. doi: 10.3324/haematol.2016.156356 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


