Abstract
Introduction
Chronic myeloid leukemia (CML) is a myeloid malignancy characterized by the oncogene BCR-ABL. CML responds well to therapy targeting BCR-ABL in the chronic phase but is resistant to treatment when it progresses to the blast phase (BP). This study attempted to address whether arachidonate 12-lipoxygenase (Alox12) confers to CML drug resistance.
Materials and Methods
We analyzed the expression of Alox12 using Western blotting, ELISA, and RT-PCR methods. Loss of functional analysis was performed using cellular activity assays on CML and normal hematopoietic stem/progenitor cells (HSPCs).
Results
Alox12 and 12-Hydroxyeicosatetraenoic acid (12-HETE) are overexpressed in BP-CML but not HSPCs, and that Alox12-12-HETE axis is regulated by BCR-ABL. The Alox12-12-HETE axis is required for CML. Specific Alox12 inhibitor inhibits colony formation, survival, and self-renewal capacity in BP-CML HSPCs, and to a significantly greater extent than in normal HSPCs. Of note, the Alox12 inhibitor significantly augments dasatinib’s efficacy in BP-CML HSPCs. Mechanism studies show that Alox12 inhibition does not affect activities of essential signaling pathways involved in maintaining stem cell function, such as Wnt, p53, and bone morphogenetic protein (BMP). In contrast, we show that Alox12 inhibition disrupts nicotinamide adenine dinucleotide phosphate (NADPH) homeostasis and induces oxidative stress and damage in CML HSPCs and committed cells.
Conclusion
Alox12-12-HETE axis is a specific and critical regulator of BP-CML HSPCs functions. Pharmacological inhibition of Alox12 may be useful in BP-CML.
Keywords: chronic myeloid leukemia, Alox12, Bcr-Abl, resistance, stem cell
Introduction
Chronic myeloid leukemia (CML) is a lethal myeloproliferative neoplasm resulting from transformation of a hematopoietic stem/progenitor cell (HSPC) by the oncogene BCR-ABL.1 Transformed HSPCs with deregulated tyrosine kinase activity of the BCR-ABL protein have aberrant proliferation and survival advantages over normal hematopoietic cells.2 The remarkable clinical response has been achieved with BCR-ABL tyrosine kinase inhibitors (TKIs), such as imatinib and dasatinib, in CML patients at chronic phase.3 However, these TKIs are not effective when CML progresses to blast phase (BP), suggesting that additional transforming events contribute to the BP phenotype.4 The mechanisms underlying resistance to TKIs in the BP-CML HSPCs involve BCR-ABL-independent mechanisms.5 In this study, we set out to identify factors responsible for proper functions in BP-CML HSPC population that might be druggable.
Lipoxygenases (LOXs) are dioxygenases that catalyze the formation of corresponding hydroperoxides from polyunsaturated fatty acids and have significant mitogenic and chemotactic effects, and stimulate the expression of oncogenes.6,7 LOXs family members, such as Alox15, Alox5 and Alox12, are expressed in hematopoietic cells.8 Particularly, Alox12 is predominantly distributed in blood platelets.9 Alox12 metabolizes arachidonic acid to 12S-hydroxyeicosatetraenoic acid (12S-HETE) by glutathione peroxide.10 Alox12 metabolizes arachidonic acid to 12-hydroxyeicosatetraenoic acid (12-HETE). Alox12-12-HETE has recently garnered attention due to its ability in regulating growth, metastasis, survival, and chemoresistance in many cancers.11–14 Studies have shown that Alox15 and Alox5 are required for myeloid leukemia stem cell to survive and function properly.15,16
We investigated the expression and functions of the Alox12-12-HETE axis in normal and BP-CML HSPCs and its relationship with BCR-ABL activation. Our results show that BCR-ABL regulates the Alox12-12-HETE axis to promote leukemogenesis. In addition, the Alox12-12-HETE axis is required for CML proliferation and survival. Consistent with these findings, we show that the pharmacological inhibitor of Alox12 suppresses HSPC functions and augments BCR-ABL TKI’s efficacy. Mechanism studies show that loss of NADPH homeostasis and induction of oxidative damage are involved in the inhibition of the Alox12-12-HETE axis in CML HSPC and committed cells.
Materials and Methods
Patient Samples, Primary Cell Isolation, Cell Culture, Drugs and Antibodies
BP-CML CD34+ cells (5x10^6 to 10^8 per sample) were purified from bone marrow or peripheral blood of CML patients at blast phase seen at Wuhan Forth Hospital with informed consents approved by the Tongji Medical College Centralised Institutional Review Board (No. 2,017,016). Mononuclear cells were obtained by using Ficoll separation, followed by CD34+ cell selection using CD34 MicroBead kit (Miltenyi Biotec). BP-CML CD34+ and normal bone marrow (NBM) CD34+ cells (StemCell Technologies) were cultured using the same growth factors and cytokines supplemented serum-free medium as described in our previous study.17 Human CML cell lines were cultured in RPMI1640 medium (Life Technologies) supplemented with 10% fetal bovine serum (Hyclone) and 2mM glutamine (Invitrogen). Imatinib (LC laboratories) was reconstituted in PBS. Dasatinib (LC laboratories) and ML355 (Cayman chemical) were dissolved in dimethylis sulfoxidum (DMSO). Antibodies against Bcr-Abl, p-Crkl, and ALOX12 and corresponding secondary HRP-conjugated antibodies were purchased from Santa Cru and standard deviation z Biotechnology.
Cell Transduction and Transfection
NBM CD34+ cells overexpressing Bcr-Abl were generated by retroviral transduction by using NGFR and NGFR P210 (Addgene) vectors as previously described.18 Specific ALOX12 and BCL-ABL knockdown were conducted in CML cells by using nucleofection (Lonza) followed by electroporation. 100 nM scramble siRNA or human ALOX12 or BCR-ABL-specific siRNAs were used for transfection. The target siRNA sequence of BCR-ABL19 and ALOX1220 is the same as previously reported.
Measurement of Proliferation and Apoptosis
Proliferation and apoptosis were measured after 3 days drug treatment or in transfected cells at 72-hour post-transfection. Cell proliferative and apoptotic activities were determined using the same methods as described in our previous study.17
Colony-Forming and Serial Replating Assays
CD34+ cells at 1000/well together with drugs were mixed with HSC-CFU methylcellulose medium (Miltenyi Biotec) and plated onto 6-well plate. After 2 weeks incubation, colonies were formed and counted. Individual colonies formed in colony-forming assay were picked, mixed well with HSC-CFU complete methylcellulose and plated onto 96-well plate. After 2 weeks incubation, wells with colonies were considered as positive. Individual colonies formed in 96-well plate were picked, mixed well with HSC-CFU complete methylcellulose and plated onto 96-well plate again. Serial replating capacity is determined by the percentage of number of positive wells among total number of colonies plated in each serial replating. Total three rounds of serial replating were performed.
Luciferase Reporter Assays
Reporter assays were carried out in CML cells by using nucleofection (Lonza) and electroporation. p53, Wnt or BMP transcriptional activities were assessed by transfecting cells with PG13-luc (wt p53 binding sites), M50 Super 8x TOPFlash or pGL3 BRE plasmid. Cells were treated with ML355 for 24 hours followed by luciferase assays (Promega) according to manufacturer’s instructions to assess β-catenin, p53 and BMP transcriptional activities.
Measurement of SIRT1 Enzyme Activity, NADPH, NADH, 12-Hydroxyeicosatetraenoic Acid (12-HETE) and ALOX12 Levels
After 24 hours drug treatment, cells were lysed using standard protocol. Changes in SIRT1 enzyme activity, NADPH, NADH, 12-HETE and ALOX12 levels were assessed using total cell lysates and were measured using a fluorometric SIRT-1 Activity Assay kit (Abcam), NADPH and NADH colorimetric quantification kits (Abcam, US), 12-HETE ELISA kit (Abcam) and ALOX12 ELISA Kit (MyBioSource).
Measurement of Intracellular Reactive Oxygen Species (ROS) and Oxidative DNA Damage
ROS and oxidative DNA damage were measured after 24 hours drug treatment. Cells were incubated with 10 µM CM-H2DCFDA (Life Technologies, US) at 37°C for 1 h, followed by measuring absorbance at ex/em of 495/525 nm were on Spectramax M5 microplate reader. DNA was extracted using the DNEasy Mini Kit (Qiagen). 8-hydroxy-2ʹ-deoxyguanosine (8-OHdG) levels were quantified using the OxiSelect Oxidative DNA Damage ELISA kit (Cell Biolabs).
Statistical Analyses
All data are obtained from at least three independent experiments and expressed as mean and standard deviation. Student’s t-test was performed to determine statistical significance. Values were considered statistically significant at P < 0.05.
Results
BCR-ABL Regulates Alox12-12-HETE Axis in Normal and Malignant Hematopoietic Cells
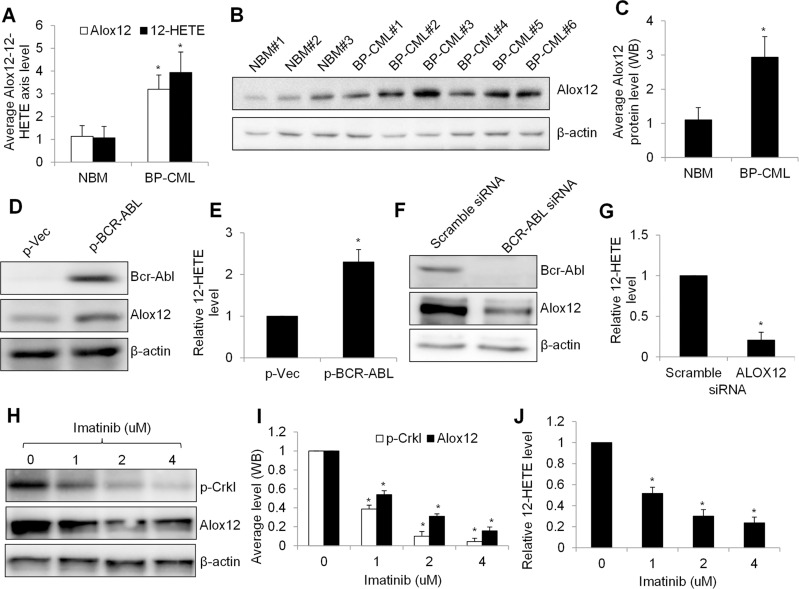
We firstly investigated the expression level of Alox12 and 12-HETE in hematopoietic stem cells isolated from BP-CML patients and compared with normal bone marrow (NBM) counterparts. Among hematopoietic stem/progenitor cell (HSPC) makers, CD34 is well known for its unique expression on HSPCs.21 ELISA analysis showed that the average level of Alox12 protein and 12-HETE was significantly higher in BP-CML (n=8) than NBM (n=5) CD34+ cells (Figure 1A). Western blot analysis showed Alox12 protein level in individual BP-CML (n=6) and NBM (n=3) samples and the quantification of band density confirmed the finding obtained from ELISA assay (Figure 1B and C). In addition, NBM#1 and #2 displayed less Alox12 level compared to all tested BP-CML samples whereas NBM#3 displayed a similar level to BP-CML#1 and #4, suggesting that Alox12 upregulation occurs in some but not all BP-CML patients.
Figure 1.
BCR-ABL regulates Alox12-12-HETE axis in human normal and malignant hematopoietic progenitor cells. (A) Alox12 and 12-HETE levels are higher in BP-CML (n=8) than NBM (n=5) CD34+ cells. (B) WB image shows Alox12 protein level in individual NBM and BP-CML CD34+ cells. (C) Quantification of WB shows average of Alox12 in NBM (n=2) and BP-CML (n=7) CD34+ cells. Overexpression of BCR-ABL in NBM CD34+ cells increased Alox12 protein level (D) and 12-HETE (E). BCR-ABL was transduced to NBM CD34+ cells. BCR-ABL knockdown by siRNA decreases Alox12 expression (F) and 12-HETE (G) in K562 cells. Cells are electroporated with 100 nM scramble or ABL siRNA. Image (H) and quantification (I) of WB shows decreased Alox12 in K562 cells after imatinib treatment. (J) Imatinib significantly decreases 12-HETE in K562 cells. Cells were harvested for WB and ELISA analysis after 24 hours treatment or 72 hours transfection. Densitometry was performed using Image J. *p<0.05, compared to NBM, p-Vec or 0 uM imatinib.
We next investigated whether BCR-ABL expression regulates Alox12 in CML. We transduced NBM CD34+ cells using BCR-ABL retroviral vector NGFR P210 and confirmed the overexpression of BCR-ABL after retroviral transduction (Figure 1D). Of note, Alox12 protein level and 12-HETE level were significantly increased after BCR-ABL overexpression (Figure 1D and E), demonstrating the change of Alox12 expression during BCR-ABL transformation. In contrast, BCR-ABL depletion decreased the Alox12 protein level in CML cells (Figure 1F and G). Consistent with the previous report,22 imatinib decreased p-Crkl (a marker of BCR-ABL function in response to BCR-ABL inhibitors) in CML cells, demonstrating the inhibition of BCR-ABL kinase activity by imatinib (Figure 1H and I). We observed that both Alox12 and 12-HETE levels were decreased in imatinib-treated CML cells (Figure 1H to J). These demonstrate that BCR-ABL regulates Alox12-12-HETE axis in normal and malignant hematopoietic cells.
Alox12-12-HETE Axis is Required for CML Cell Growth and Survival
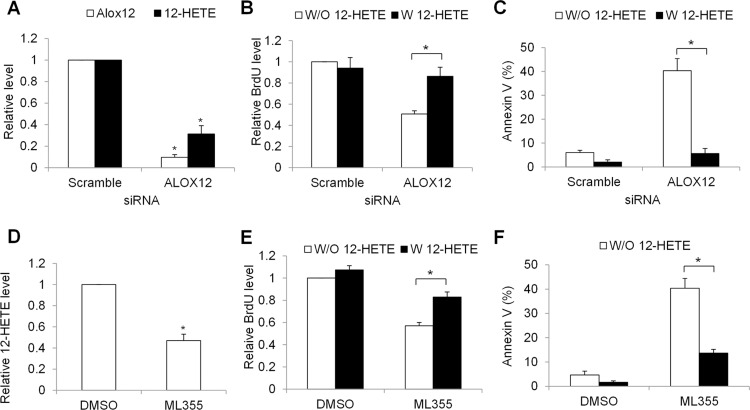
To understand the biological function of Alox12-12-HETE axis in CML cells, we depleted Alox12 in CML cells and examined the growth and survival in the absence and presence of 12-HETE. We observed that specific ALOX12 siRNA resulted in minimal Alox12 protein level and remarkable reduction of 12-HETE in K562 cells (Figure 2A). We further found that Alox12 depletion significantly decreased growth and increased apoptosis in K562 cells, and furthermore that these inhibitory effects were reversed by the addition of 12-HETE (Figure 2B and C). Consistent with siRNA knockdown, pharmacological inhibition of Alox12 using ML355, a potent and selective Alox12 inhibitor,23 led to a significant reduction of growth and survival (Figure 2D–F). In addition, the decreased proliferation and increased apoptosis induced by Alox12 inhibition is not limited to K562 cells; LAMA82 cells responded in a similar manner (Figure S1), suggesting a general regulatory mechanism by Alox12-12-HETE in CML cell growth and survival.
Figure 2.
Alox12-12-HETE axis is required for CML cell growth and survival. (A) ALOX12 knockdown decreases Alox12 and 12-HETE level in K562 cells. (B) Proliferation is decreased and (C) apoptosis is increased in ALOX12- depleted K562 cells, and the effects are reversed by the addition of 12-HETE (1 μM). Cells are electroporated with 100 nM scramble or ALOX12 siRNA and cultured for 24 hours prior to assays. ML355 (10 μM) significantly decreases 12-HETE level (D), inhibits proliferation (E) and induces apoptosis (F) of K562 cells. Proliferation and apoptosis were determined after 72 hours drug treatment. *p<0.05, compared to scramble siRNA or DMSO.
Alox12 Inhibition Selectively Targets BP-CML HSPC Cells, and Acts Synergistically with Dasatinib
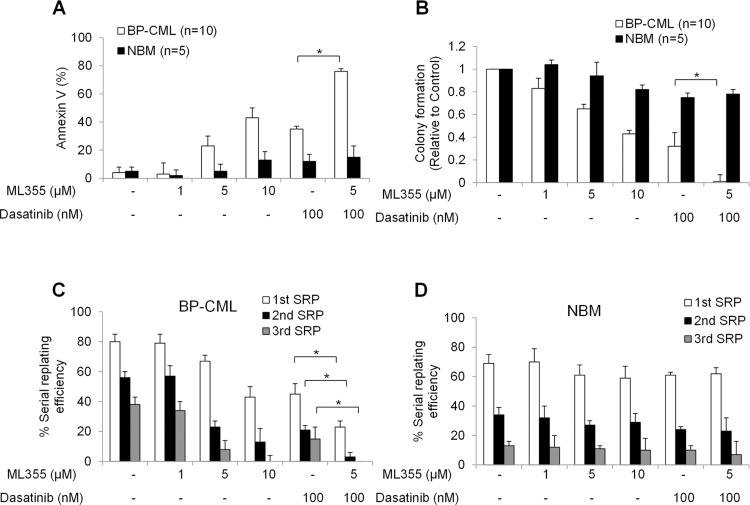
We further investigated the effects of Alox12 inhibition in NMB and BP-CML HSPCs. Apart from apoptosis assay, we performed colony formation and serial replating assays using HSPCs as the hallmark characteristics of HSPCs are to proliferate, differentiate, and self-renew.24 As shown in Figure 3A, ML355 induced apoptosis in BP-CML CD34+ cells in a dose-dependent manner, and to a significantly greater extent than in NBM counterparts (Figure 3A). ML355 also significantly decreased colony formation of BP-CML CD34+ cells and was less effective in NBM counterparts (Figure 3B), suggesting that ML355 preferentially inhibits BP-CML HSPCs growth and differentiation. It is worth noting that the combination of ML355 with dasatinib further enhanced the apoptosis induction and colony formation inhibition in BP-CML but not NBM HSPCs than dasatinib alone (Figure 3A and B).
Figure 3.
Alox12 inhibition selectively targets BP-CML CD34 stem/progenitor cells, and acts synergistically with dasatinib. Combination of ML355 and dasatinib selectively induces apoptosis (A), inhibits colony formation (B) and self-renewal capacity (C and D) in CML CD34 cells (n=10) without affecting NBM CD34 cells (n=5). Colonies were enumerated and individually picked for serial replating. Graphs presented are mean of the results obtained from ten BP-CML patients and five NBM. *p<0.5, compared to control or dasatinib alone.
In serial replating assay, we observed that BP-CML and NBM HSPC control cells were capable of forming colonies up to three rounds of serial replating (equivalent to >8 weeks in vitro) and that the efficiency was reduced with each replating (Figure 2D and E). However, exposure to ML355 significantly impaired the ability of BP-CML but not NBM HSPCs to serially replate (Figure 3C and D). The combination of ML355 and dasatinib completely abolished the self-renewal ability of BP-CML HSPCs during the third replating without affecting NBM HSPCs (Figure 3D).
Alox12 Inhibition Disrupts NADH Homeostasis and Induces Oxidative Damage in CML
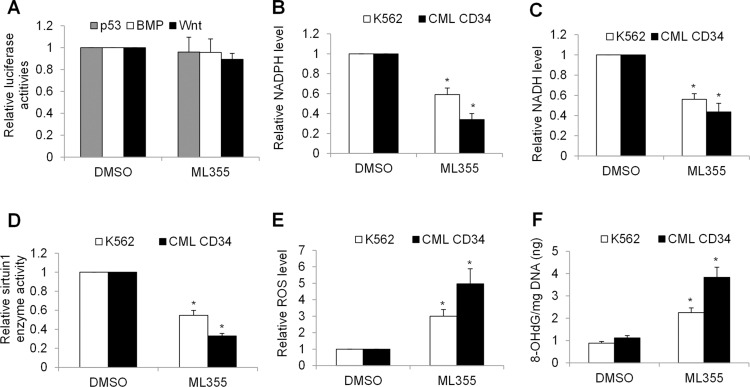
Wnt/β-catenin, p53 and BMP pathways have been reported to play essential roles in maintaining BP-CML HSPC functions, including survival and self-renewal.25–27 Although Alox12 inhibition negatively affected BP-CML HSPCs, Alox12 inhibition by ML355 did not affect transcriptional activities of Wnt/β-catenin, p53, and BMP as shown by luciferase reporter assays (Figure 4A). In contrast, we found that Alox12 inhibition by both ML355 and siRNA knockdown significantly decreased levels of nicotinamide adenine dinucleotide phosphate (NADPH) and its reduced form NADH in K562 and BP-CML HSPCs (Figure 4B and Figure S2A), suggesting that Alox12 inhibition disrupts NADH homeostasis. To further define the biochemical consequences of altered NADH homeostasis, we measured sirtuin1 enzyme activity which is dependent on oxidized NADH. As expected, Alox12 inhibition significantly suppressed sirtuin1 enzyme activity in K562 and BP-CML HSPCs (Figure 4C and Figure S2B). In addition, we found that Alox12 significantly increased ROS and 8-OHdG (an oxidized DNA byproduct). These results indicate that Alox12 inhibition disrupts NADH homeostasis and induces oxidative damage in CML cells.
Figure 4.
Alox12 inhibition decreases levels of NADPH, NADH and sirtuin1 activity, and induces oxidative stress and damage in CML cells. (A) ML533 (10 μM) does not affect Wnt, p53 or BPM transcriptional activity in K562 cells. Cells were transfected with luciferase-based reporter constructs as described in methods. ML533 significantly decreases NADPH (B), NADH (C) and sirtuin1 enzyme activity (D) in K562 cells. ML533 significantly increases ROS (E) and 8-OHdG (F) level in K562 cells. *p<0.05, compared to control.
Discussion
BCR-ABL TKIs are less effective as single agents in targeting leukemia stem cells which represent a reservoir of resistance and source of relapse. The resistance mechanisms are not fully understood. A systematic analysis of potential drug resistance pathways is important to identify potential therapeutic targets. This aims to sensitize BCR-ABL TKI’s efficacy to leukemia stem cells and induce a better patient response. Our prior work had implicated the role of Wnt/β-catenin and ERK/MNK/eIF4E pathways in TKI resistance in CML.17,28 In the present study, we found that Alox12-12-HETE activation is a feature of BP-CML HSPCs and is preferentially required for leukemia HSPCs and committed cells to function properly. The ability of Alox-12-12-HETE inhibition to specifically target leukemia while sparing normal HSPCs is of interest and will contribute to improved control of BP-CML patients.
We demonstrate direct evidence obtained from patient samples that Alox12 and 12-HETE are particularly increased in BP-CML HSPCs compared to normal counterparts (Figure 1A–C), suggesting that BCR-ABL transformation activates the Alox12-12-HETE axis. This conclusion is further supported by our experimental evidence that BCR-ABL activation induces Alox12-12-HETE expression in normal HSPCs or vice versa in CML committed cells (Figure 1D–J). We further noted that Alox12 overexpression is not detected in all but some BP-CML samples (Figure 1B), suggesting that other factors rather than BCR-ABL also mediate Alox12 expression. Alox12 overexpression has been reported in lung, gastric and breast cancers.29–31 Our work supports and further extends the previous findings that apart from cancer committed cells, Alox12-12-HETE axis is also upregulated in primary cancer stem cells, and furthermore that this axis is mediated by oncogenic stress (eg, the presence of BCR-ABL) in cancer. Alox12 elevation has been shown to be positively associated with an advanced stage and poor differentiation in prostate cancer.32 Whether Alox12 expression or mutations can serve as a prognostic marker in CML is worth of future investigation.
We demonstrate that Alox12 inhibition suppresses proliferation and induces apoptosis in CML cells, and its effects are reversed by the addition of 12-HETE (Figure 2 and Figure S1). This data adds to the recent evidence supporting the important role of Alox12-12-HETE in the growth and survival of cancer.29–31,33,34 Notably, we find that Alox12 inhibition impairs CML HSPCs functions (Figure 3). This finding is consistent with the previous reports on the other two ALOX family members that loss of the Alox5 gene impairs leukemia stem cells and prevents chronic myeloid leukemia in mice,35 and Alox15 is required for chronic myeloid leukemia stem cell survival,15 suggesting the important roles of ALOX family in leukemia stem cells. Our work also highlights the selectivity of Alox12 inhibition in BP-CML while sparing normal HSPCs, suggesting the therapeutic window of pharmacological Alox12 inhibitor in CML. The synergistic effect of Alox12 inhibitor with dasatinib in targeting BP-CML stem cells suggests the value of targeting Alox12 in overcoming resistance to BCR-ABL TKIs in CML.
Our work also addresses the mechanisms by which targeting of the Alox12-12-HETE axis inhibits myeloid leukemia. In CML HSPCs and committed cells, we show that Alox12 inhibition does not affect Wnt/β-catenin, BPM, and p53 activities which are well-known signaling pathways playing critical roles in leukemia stem cells (Figure 4A). In contrast, we show that Alox12 inhibition decreases levels of NADPH, NADH and sirtuin1 enzyme activity. In addition, Alox12 inhibition induces oxidative stress and damage (Figure 4B–F and FigureS2). Oxidative stress has emerged as a putative mechanism in the development of BCR-ABL1 (+) and (-) myeloproliferative neoplasms.36–40 Oxidative stress levels are higher in CML patients compared with healthy controls.40 Oxidative stress levels are positively correlated with JAK2V617F mutational status and thrombotic complications in patients with essential thrombocythemia.38 The biological roles of Alox12 in oxidative stress and NADPH homeostasis are complex and are not well understood.41 Our study demonstrates the correlation of Alox12 inhibition with oxidative stress induction and disruption of NADPH homeostasis in CML stem and committed cells. However, the exact mechanisms on how Alox12 inhibition leads to oxidative stress and disrupted metabolic reprogramming in CML are not clear and are worthy of further investigation to identify what are the biologically active lipid mediators involved.
Although BCR-ABL is not the exclusive regulator of the Alox12-12-HETE axis, our findings demonstrate that this axis is preferentially activated in BP-CML stem cells and plays important role in CML growth, survival, differentiation, and self-renewal. We expand the previously known BCR-ABL-regulated molecular network in leukemogenesis. Inhibition of Alox12 is selective and effective in sensitizing BP-CML stem cells to dasatinib treatment. Our results suggest that pharmacological Alox12 inhibitors may have utility in treating BP-CML patients. Pharmacological inhibitors of Alox12 have been developed and shown to have anti-cancer activities in many cancers, such as breast cancer and lung cancer. Our preclinical evidence may accelerate the initiation of clinical trials testing Alox12 inhibitors in combination with BCR-ABL inhibitors for the treatment of BP-CML patients.
Acknowledgments
This work was supported by a research grant (No. WX14B20) provided by Wuhan City Health and Family Planning. Si Gao and Jialin Hu are co-first authors.
Disclosure
All authors declare no conflicts of interest.
References
- 1.Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96(10):3343–3356. doi: 10.1182/blood.V96.10.3343 [DOI] [PubMed] [Google Scholar]
- 2.Braun TP, Eide CA, Druker BJ. Response and resistance to BCR-ABL1-targeted therapies. Cancer Cell. 2020;37(4):530–542. doi: 10.1016/j.ccell.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawyers CL, Druker B. Tyrosine kinase inhibitors in chronic myeloid leukemia. Cancer J Sci Am. 1999;5(2):63–69. doi: 10.3389/fonc.2019.00603 [DOI] [PubMed] [Google Scholar]
- 4.Hehlmann R. How I treat CML blast crisis. Blood. 2012;120(4):737–747. doi: 10.1182/blood-2012-03-380147 [DOI] [PubMed] [Google Scholar]
- 5.Bitencourt R, Zalcberg I, Louro ID. Imatinib resistance: a review of alternative inhibitors in chronic myeloid leukemia. Rev Bras Hematol Hemoter. 2011;33(6):470–475. doi: 10.5581/1516-8484.20110124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merchant N, Bhaskar L, Momin S, Sujatha P, Reddy ABM, Nagaraju GP. 5-Lipoxygenase: its involvement in gastrointestinal malignancies. Crit Rev Oncol Hematol. 2018;127:50–55. doi: 10.1016/j.critrevonc.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 7.Moore GY, Pidgeon GP. Cross-talk between cancer cells and the tumour microenvironment: the role of the 5-lipoxygenase pathway. Int J Mol Sci. 2017;18(2):236. doi: 10.3390/ijms18020236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mashima R, Okuyama T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. 2015;6:297–310. doi: 10.1016/j.redox.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhn H, Banthiya S, van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochim Biophys Acta. 2015;1851(4):308–330. doi: 10.1016/j.bbalip.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–1875. doi: 10.1126/science.294.5548.1871 [DOI] [PubMed] [Google Scholar]
- 11.Liu B, Marnett LJ, Chaudhary A, et al. Biosynthesis of 12(S)-hydroxyeicosatetraenoic acid by B16 amelanotic melanoma cells is a determinant of their metastatic potential. Lab Invest. 1994;70(3):314–323. [PubMed] [Google Scholar]
- 12.Porro B, Songia P, Squellerio I, Tremoli E, Cavalca V. Analysis, physiological and clinical significance of 12-HETE: a neglected platelet-derived 12-lipoxygenase product. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;964:26–40. doi: 10.1016/j.jchromb.2014.03.015 [DOI] [PubMed] [Google Scholar]
- 13.Tang K, Honn KV. 12(S)-HETE in cancer metastasis. Adv Exp Med Biol. 1999;447:181–191. doi: 10.1007/978-1-4615-4861-4_17 [DOI] [PubMed] [Google Scholar]
- 14.Klampfl T, Bogner E, Bednar W, et al. Up-regulation of 12(S)-lipoxygenase induces a migratory phenotype in colorectal cancer cells. Exp Cell Res. 2012;318(6):768–778. doi: 10.1016/j.yexcr.2011.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Peng C, Abraham SA, et al. Arachidonate 15-lipoxygenase is required for chronic myeloid leukemia stem cell survival. J Clin Invest. 2014;124(9):3847–3862. doi: 10.1172/JCI66129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roos J, Oancea C, Heinssmann M, et al. 5-Lipoxygenase is a candidate target for therapeutic management of stem cell-like cells in acute myeloid leukemia. Cancer Res. 2014;74(18):5244–5255. doi: 10.1158/0008-5472.CAN-13-3012 [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Hu J, Song H, Wu T. Antibiotic anisomycin selectively targets leukemia cell lines and patient samples through suppressing Wnt/beta-catenin signaling. Biochem Biophys Res Commun. 2018;505(3):858–864. doi: 10.1016/j.bbrc.2018.09.183 [DOI] [PubMed] [Google Scholar]
- 18.Xiao M, Ai H, Li T, Rajoria P, Shahu P, Li X. Targeting of the BLT2 in chronic myeloid leukemia inhibits leukemia stem/progenitor cell function. Biochem Biophys Res Commun. 2016;472(4):610–616. doi: 10.1016/j.bbrc.2016.03.018 [DOI] [PubMed] [Google Scholar]
- 19.Scherr M, Battmer K, Winkler T, Heidenreich O, Ganser A, Eder M. Specific inhibition of bcr-abl gene expression by small interfering RNA. Blood. 2003;101(4):1566–1569. doi: 10.1182/blood-2002-06-1685 [DOI] [PubMed] [Google Scholar]
- 20.Lin R, Elf S, Shan C, et al. 6-Phosphogluconate dehydrogenase links oxidative PPP, lipogenesis and tumour growth by inhibiting LKB1-AMPK signalling. Nat Cell Biol. 2015;17(11):1484–1496. doi: 10.1038/ncb3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.AbuSamra DB, Aleisa FA, Al-Amoodi AS, et al. Not just a marker: CD34 on human hematopoietic stem/progenitor cells dominates vascular selectin binding along with CD44. Blood Adv. 2017;1(27):2799–2816. doi: 10.1182/bloodadvances.2017004317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu S, Holtz M, Gupta M, Bhatia R. BCR/ABL kinase inhibition by imatinib mesylate enhances MAP kinase activity in chronic myelogenous leukemia CD34+ cells. Blood. 2004;103(8):3167–3174. doi: 10.1182/blood-2003-04-1271 [DOI] [PubMed] [Google Scholar]
- 23.Luci DK, Jameson JB, Yasgar A, et al. Synthesis and structure-activity relationship studies of 4-((2-hydroxy-3-methoxybenzyl)amino)benzenesulfonamide derivatives as potent and selective inhibitors of 12-lipoxygenase. J Med Chem. 2014;57(2):495–506. doi: 10.1021/jm4016476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeishi S, Nakayama KI. Role of Fbxw7 in the maintenance of normal stem cells and cancer-initiating cells. Br J Cancer. 2014. doi: 10.1038/bjc.2014.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashihara E, Takada T, Maekawa T. Targeting the canonical Wnt/beta-catenin pathway in hematological malignancies. Cancer Sci. 2015;106(6):665–671. doi: 10.1111/cas.12655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo YH, Qi J, Cook GJ. Regain control of p53: targeting leukemia stem cells by isoform-specific HDAC inhibition. Exp Hematol. 2016;44(5):315–321. doi: 10.1016/j.exphem.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busch C, Wheadon H. Bone marrow niche crosses paths with BMPs: a road to protection and persistence in CML. Biochem Soc Trans. 2019;47(5):1307–1325. doi: 10.1042/BST20190221 [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Li Y, Lv C, Wang L, Song H. Anthelmintic drug niclosamide enhances the sensitivity of chronic myeloid leukemia cells to dasatinib through inhibiting Erk/Mnk1/eIF4E pathway. Biochem Biophys Res Commun. 2016;478(2):893–899. doi: 10.1016/j.bbrc.2016.08.047 [DOI] [PubMed] [Google Scholar]
- 29.Huang Z, Xia L, Zhou X, Wei C, Mo Q. ALOX12 inhibition sensitizes breast cancer to chemotherapy via AMPK activation and inhibition of lipid synthesis. Biochem Biophys Res Commun. 2019;514(1):24–30. doi: 10.1016/j.bbrc.2019.04.101 [DOI] [PubMed] [Google Scholar]
- 30.Zhong C, Zhuang M, Wang X, et al. 12-Lipoxygenase promotes invasion and metastasis of human gastric cancer cells via epithelial-mesenchymal transition. Oncol Lett. 2018;16(2):1455–1462. doi: 10.3892/ol.2018.8808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Tong W, Liao M, Chen D. Inhibition of arachidonate lipoxygenase12 targets lung cancer through inhibiting EMT and suppressing RhoA and NF-kappaB activity. Biochem Biophys Res Commun. 2020;524(4):803–809. doi: 10.1016/j.bbrc.2020.01.166 [DOI] [PubMed] [Google Scholar]
- 32.Gao X, Porter AT, Honn KV. Involvement of the multiple tumor suppressor genes and 12-lipoxygenase in human prostate cancer. Therapeutic implications. Adv Exp Med Biol. 1997;407:41–53. doi: 10.1007/978-1-4899-1813-0_7 [DOI] [PubMed] [Google Scholar]
- 33.Liu Q, Tan W, Che J, et al. 12-HETE facilitates cell survival by activating the integrin-linked kinase/NF-kappaB pathway in ovarian cancer. Cancer Manag Res. 2018;10:5825–5838. doi: 10.2147/CMAR.S180334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding XZ, Tong WG, Adrian TE. 12-lipoxygenase metabolite 12(S)-HETE stimulates human pancreatic cancer cell proliferation via protein tyrosine phosphorylation and ERK activation. Int J Cancer. 2001;94(5):630–636. doi: 10.1002/ijc.1527 [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Hu Y, Zhang H, Peng C, Li S. Loss of the Alox5 gene impairs leukemia stem cells and prevents chronic myeloid leukemia. Nat Genet. 2009;41(7):783–792. doi: 10.1038/ng.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tofolean IT, Ganea C, Ionescu D, et al. Cellular determinants involving mitochondrial dysfunction, oxidative stress and apoptosis correlate with the synergic cytotoxicity of epigallocatechin-3-gallate and menadione in human leukemia Jurkat T cells. Pharmacol Res. 2016;103:300–317. doi: 10.1016/j.phrs.2015.12.013 [DOI] [PubMed] [Google Scholar]
- 37.Gaman AM, Moisa C, Diaconu CC, Gaman MA. Crosstalk between oxidative stress, chronic inflammation and disease progression in essential thrombocythemia. Rev Chim. 2019;70(10):3486–3489. doi: 10.37358/RC.19.10.7581 [DOI] [Google Scholar]
- 38.Moisa C, Gaman MA, Diaconu CC, Gaman AM. Oxidative stress levels, JAK2V617F mutational status and thrombotic complications in patients with essential thrombocythemia. Rev Chim. 2019;70(8):2822–2825. doi: 10.37358/RC.19.8.7435 [DOI] [Google Scholar]
- 39.Pascu EG, Găman M-A, Moisă C, Assani AD, Găman AM. The involvement of oxidative stress in Chronic Myeloid Leukemia. Rom Biotechnol Lett. 2020;25(1):1267–1274. doi: 10.25083/rbl/25.1/1267.1274 [DOI] [Google Scholar]
- 40.Pascu EG, Gaman MA, Moisa C, Gaman AM. Oxidative stress and BCR-ABL1 transcript levels in Chronic Myeloid Leukemia: an intricate relationship. Rev Chim. 2019;70(9):3193–3196. doi: 10.37358/RC.19.9.7514 [DOI] [Google Scholar]
- 41.Zheng Z, Li Y, Jin G, Huang T, Zou M, Duan S. The biological role of arachidonic acid 12-lipoxygenase (ALOX12) in various human diseases. Biomed Pharmacother. 2020;129:110354. doi: 10.1016/j.biopha.2020.110354 [DOI] [PubMed] [Google Scholar]