Abstract
Background:
Organ transplant recipients are vulnerable to multiple infectious agents and in a world with a circulating SARS-CoV-2 virus, it would be expected that patients who are immunosuppressed would have higher mortality.
Objective:
To determine the COVID-19 mortality in transplant recipients.
Methods:
We conducted a search in PubMed and Google scholar databases using the keywords for COVID-19 and transplantation. All related studies between January 1, 2020 and May 7, 2020 were reviewed. All relevant published articles related to COVID-19 in transplant recipients were included.
Results:
46 articles were included; they studied a total of 320 transplant patients—220 kidney transplant recipients, 42 liver, 19 heart, 22 lung, 8 HSCT, and 9 dual organ transplant recipients. The overall mortality rate was 20% and was variable among different organs and different countries. 65 transplant recipients died of complications attributable to COVID-19; 33 were males (15% of males in this cohort), 8 females (8% of females in this cohort), and 24 whose sex was not determined. They had a median age of 66 (range: 32–87) years. The median transplantation duration was 8 years (range: 30 days to 20 years). The most frequent comorbidity reported was hypertensions followed by diabetes mellitus, obesity, malignancy, ischemic heart disease, and chronic obstructive pulmonary disease. The most frequent cause of death reported was acute respiratory distress syndrome.
Conclusion:
Transplant recipients in our cohort had a high mortality rate. However, outcomes were not the same in different countries based on outbreak settings. Mortality was noted in elder patients with comorbidities.
Key Words: COVID-19, Transplant recipients, Kidney transplant, Liver transplant, Heart transplant
INTRODUCTION
Organ transplantation is currently an established line of treatment for end-stage organ disease. As the recipients are under chronic immunosuppression, they become vulnerable to multiple infectious agents, particularly the emerging infectious diseases. Coronavirus disease 2019 (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is an emerging pandemic with over 6,000,000 confirmed cases worldwide and more than 390,000 deaths (reported case fatality 4%–14% in developed countries) [1]. Assessment of severity and outcome of SARS-CoV-2 infection in organ transplant recipients is required. Organ transplant recipients on chronic immunosuppression may alter the clinical presentation revealing atypical findings in such population [2]. Besides that, the course of the disease in the transplant patient population is unknown and it is unclear whether immunosuppression results in a worse prognosis compared to the general population or not. On the other hand, immunosuppressive medication should be maintained in such patients to avoid transplant rejection.
Since the start of COVID-19 pandemic, several guidelines have recommended withdrawal of calcineurin inhibitors in transplant patients with severe SARS-CoV-2 infection [3, 4]. Yet, emerging evidence suggests that the severe form of the disease and the leading cause of death in such infection is a hyperinflammatory state and cytokine storm [5]. Therefore, theoretically immunosuppressive therapy could have a positive effect on transplant patients infected with SARS-CoV-2.
The objective of this review was to study the available information following almost 100 days since COVID-19 was reported and assess the risk of mortality of SARS-CoV-2 infection in transplant recipients.
MATERIALS AND METHODS
We conducted a PubMed search using the words “COVID-19 AND transplant”[All Fields] OR “severe acute respiratory syndrome coronavirus 2 AND transplant”[All Fields] OR “2019-nCoV AND transplant”[All Fields] OR “SARS-CoV-2 AND transplant”[All Fields] OR (“coronavirus”[MeSH Terms] OR “coronavirus”[All Fields])) AND transplant [All Fields]). We also included relevant articles from the references of manuscripts studied.
Google Scholar was also systematically searched, using the terms COVID-19 or SARS-CoV-2 AND transplant. Any reference list from eligible articles was reviewed to include any potential relevant article. The last search conducted was on May 7, 2020.
Retrospective studies, systematic and narrative reviews, case-series and case-reports were included in the review. Studies published in languages other than English were excluded. Three reviewers (MA, MM, and MN) independently screened the titles, abstracts, and full texts of the retrieved articles to assess the eligibility of studies for inclusion. Duplicate references were removed and a final list of articles was generated.
Among 605 articles found, 46 met the eligibility criteria. Included studies were published case of solid organ transplant recipients that were infected by SARS-CoV-2 confirmed by RT-PCR.
Patients were categorized as mild, moderate, and severe according to what was reported; when not reported, we categorized patients as either mild/asymptomatic according to symptoms with no evidence of pneumonia, moderate when there was evidence of pneumonia, and severe when SpO2 was ≤93% on breathing room air or required intensive care unit (ICU) admission.
RESULTS
Forty-six of 605 retrieved articles were included; they studied a total of 320 transplant recipients; 218 (68.1%) were males and 99 (30.9%) were females; sex was not reported in three pediatric patients. In terms of epidemiology, 161 (50.3%) were reported from the USA, 52 (16.3%) from Spain, 43 (13.4) from Italy, 31 (9.7%) from China, 12 (3.8%) from Iran, 7 (2.2%) from UK, 7 (2.2%) from France, 2 (0.6%) from Germany, 2 (0.6%) from Korea, 1 (0.3%) from Brazil, 1 (0.3%) from Netherlands, and 1 (0.3%) from Turkey. Regarding the organs transplanted, 220 (69%) underwent kidney transplantation, 42 (13%) liver transplantation, 22 (7%) lung transplantation, 19 (6%) heart transplantation, 8 (3%) hematopoietic stem cell transplantation (HSCT), and 9 (3%) dual organ transplantation. Sixty-nine (21.7%) of cases were asymptomatic or mildly infected, 123 (38.7%) had moderate infection, and 126 (39.6%) had severe infection; two patients in one study had no severity report. Among the 320 transplant recipients studied, 64 (20%) died, all but two of them had severe SARS-CoV-2 infection (Tables 1 and 2).
Table 1.
Characteristics of transplant recipients with COVID-19 in the included case reports
| Reference | Country | No | Organ | Median age | Mild/Asympt-omatic | Moderate | Severe | Sex | Alive |
|---|---|---|---|---|---|---|---|---|---|
| Mathies et al [6] | Germany | 1 | Heart | 77 | 1 | M | 1 (100%) | ||
| Chen et al [7] | China | 1 | Kidney | 49 | 1 | M | 1 (100%) | ||
| Arpali et al [8] | Turkey | 1 | Kidney | 28 | 1 | F | 1 (100%) | ||
| Guillen at el [2] | Spain | 1 | Kidney | 50 | 1 | M | 1 (100%) | ||
| Zhong et al [9] | China | 2 | 1 Kidney 1 liver |
48, 37 | 1 | 1 | 2M | 2 (100%) | |
| Zhu et al [10] | China | 10 | Kidney | 44.5 | 2 | 8 | 8M/2F | 9 (90%) | |
| Akalin et al [11] | USA | 36 | Kidney | 60 | 8 | 17 | 11 | 26M/ 10F | 26 (72%) |
| Lagana et al [12] | USA | 1 | Liver | 1 | 1 | F | 1 (100%) | ||
| Hsu et al [13] | USA | 1 | Heart/ Kidney | 39 | 1 | M | 1 (100%) | ||
| Wang et al [14] | China | 1 | Kidney | 49 | 1 | M | 1 (100%) | ||
| D’Antiga [15] | Italy | 3 | Liver | pediatric | 3 | 3 (100%) | |||
| Donato et al [16] | Italy | 8 | Liver | 70 | 6 | 2 | 6M/2F | 8 (100%) | |
| Marx et al [17] | Italy | 1 | Kidney | 58 | 1 | M | 1 (100%) | ||
| Huang et al [18] | China | 1 | Liver | 59 | 1 | M | 0 | ||
| TCUKTP [19] | USA | 15 | Kidney | 51 | 5 | 6 | 4 | 10M/ 5F | 13 (86%) |
| Kates et al [20] | USA | 4 | 1 Kidney 1 Liver 1 Heart 1 Lung |
62.5 | 2 | 1 | 1 | 3M/1F | 4 (100%) |
| Fontana et al [21] | Italy | 1 | Kidney | 61 | 1 | M | 1 (100%) | ||
| Periera et al [22] | USA | 90 | 46 Kidney 13 Liver 9 Heart 17 Lung 5 Dual |
57 | 22 | 41 | 27 | 53 M/ 37 F | 74 (82%) |
| Fernandez-Ruiz et al [23] | Spain | 18 | 8 Kidney 6 Liver 4 Heart |
71 | 5 | 8 | 5 | 13M/ 5F | 13 (72%)* |
| Huang et al [24] | China | 2 | 1 Kidney 1 HSCT |
55 | 2 | 2M | 0 | ||
| Bhoori et al [25] | Italy | 3** | Liver | >65*** | 3 | 3M | 0 | ||
| Chen et al [26] | China | 3 | Lung╪ | 66 | 3 | 3M | 2 (66%) | ||
| Alberici et al [27 | Italy | 20 | Kidney | 59 | 11 | 9 | 16M/ 4F | 15 (75%) | |
| Ning et al [28] | China | 1 | Kidney | 29 | 1 | M | 1 (100%) | ||
| Qin et al [29] | China | 1 | Liver | 37 | 1 | M | 1 (100%) | ||
| Seminari et al [30] | Italy | 1 | Kidney | 50 | 1 | M | 1 (100%) | ||
| Zhu et al [31] | China | 1 | Kidney | 52 | 1 | M | 1 (100%) | ||
| Liu et al [32] | China | 1 | Liver | 50 | 1 | M | 1 (100%) | ||
| Maggi et al [33] | Italy | 2 | Liver | 61, 69 | 2M | 1 (50%) | |||
| Bartiromo et al [34] | Italy | 1 | Kidney | 36 | 1 | F | 1 (100%) | ||
| Zhang et al [35] | China | 5 | Kidney | 45 | 5 | 4M/1F | 5 (100%) | ||
| Li et al [36] | China | 2 | Heart | 51, 43 | 1 | 1 | 2M | 2 (100%) | |
| Gandolfini et al [37] | Italy | 2 | Kidney | 75, 52 | 2 | 1M/1F | 1 (50%) | ||
| Banerjee et al [38] | UK | 7 | Kidney | 54 | 2 | 5 | 4M/3F | 6 (85%) | |
| Kim et al [39] | Korea | 2 | Kidney | 56, 36 | 2 | 2M | 2 (100%) | ||
| Nair et al [40] | USA | 10 | Kidney | 57 | 3 | 2 | 5 | 6M/4F | 7 (70%) |
| Meziyerh et al [41] | Nether-lands | 1 | Kidney | 35 | 1 | M | 1 (100%) | ||
| Hammami et al [42] | USA | 1 | Liver | 63 | 1 | M | 1 (100%) | ||
| Aigner et al [43] | Germany | 1 | Lung | 59 | 1 | F | 1 (100%) | ||
| Billah et al [44] | USA | 1 | Kidney | 44 | 1 | M | 1 (100%) | ||
| Machado et al [45] | Brazil | 1 | Kidney/ Liver | 69 | 1 | M | 1 (100%) | ||
| Bussalino et al [46] | Italy | 1 | Kidney | 32 | 1 | M | 1 (100%) | ||
| Abrishami et al [47] | Iran | 12 | Kidney | 47 | 12 | 9M/ 3F | 4 (25%) | ||
| Malard et al [48] | France | 7 | HSCT | 61 | 4 | 3 | 5M/2F | 6 (86%) | |
| Montagud-Marrahi et al [49] | Spain | 33 | 31 Kidney 2 Dual |
57 | 7 | 13 | 13 | 19M/14F | 31 (94%) |
| Holzhauser et al [50] | USA | 2 | Heart | 67 | 2 | M/F | 1 (50%) |
*Mortality 2 Kidney, 2 Lung, 1 Heart
**148 patient data was not available (only report 3 male patients were reported)
*** This was the age reported
╪ Patients received lung transplants for COVID-19
F: Female; M: Male; NA, not available; TCUKTP: The Columbia University Kidney Transplant Program
Table 2.
Detailed number of patients in different organs transplanted and their mortality percentages
| Total | 320 | 174* | 29* | 10* | 5* | 8 | 4* |
|---|---|---|---|---|---|---|---|
| Total mortality | 64 (20%) | 36 (20%) | 5 (17.2%) | 2 (20%) | 3 (60%) | 2 (25%) | 0 |
*These are the total number of patients excluding the patients described in Periera et al as these details were not mentioned
In our cohort, 65 transplant recipients died of complication attributable to COVID-19; 33 were males (15% of males in this cohort), 8 females (8% of females in this cohort), and 24 whose sex was not determined [11, 22]. Periera, et al [22], reported 16 deaths without mentioning the age of patients, clinical characteristics or cause of death. The median age of the remaining patients was 66 (range: 32–87) years. Regarding the time since transplantation to the time of COVID-19, there was no information for 27 (41%) patients; one had lung transplantation 40 days after COVID-19; the remaining cases had a median transplantation duration of 8 years (range: 30 days to 20 years). Comorbidities were reported in 38 cases (58% of total mortality), and included hypertension in 22 patients (58%), diabetes mellitus in 11 (29%), obesity in 5 (13%), malignancy in 5 (13%), ischemic heart disease in 4 (11%), chronic obstructive pulmonary disease in 2 (5%), hepatitis B in 2 (5%), asthma in 1 (3%), hepatitis C in 1 (3%), HIV in 1 (3%), and chronic kidney disease in 1 (3%). Nine (14%) patients did not have comorbidities.
The most frequent cause of death reported was acute respiratory distress syndrome (ARDS). None of the patients faced issues regarding hospital resource availability that may have affect cause of death. All the deceased cases with reported detailed characteristics had reduced or stopped their immunosuppressive therapy apart from steroids. None had graft rejection (Table 3). There were no any post-mortem exams reported in our cohort.
Table 3.
Clinical characteristics of deceased transplant recipients infected by COVID-19
| Reference | Organ | No | Age | Sex | Time since Tx | Comorbities | COVID-19 severity | Radiologic features and relevant labs | Therapeutic approach | Length of hospital stay | Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhu et al [10] China |
Kidney | 1/10 (10%) | 59 | M | ND | HTN, HHD, COPD | Severe (ICU) | -CT chest: Multiple bilateral ground glass opacities -Significant serum creatinine elevation -Significant decrease in urine volume |
-MPA cessation -CNI cessation -IV MP -IVIG -Antiviral (not specified) -Mechanical Vent. |
6 days | -Acute renal allograft failure -Sudden acute respiratory failure |
| Akalin et al [11] #1* USA |
Kidney | 10/36 (28%) | 60 | M | 5 weeks | None | Mild (home isolation) | ND | MMF cessation | None | -Sudden death |
| Akalin et al [11] #2* USA |
Kidney | 10/36 (28%) | 72 | M | 3 months | DM, HTN | Mild (home isolation | ND | Decrease IS (not specified) | None | -Sudden death |
| Huang et al [18] China |
Liver | 1/1 (100%) | 59 | M | 3 years | History of HBV and HCC | Mild Complicated in day 4 by nosocomial infection | -Chest CT scan showed bilateral ground-glass opacities -Day 4: Marked lung inflammation, blood culture positive for candida albicans, alveolar lavage positive for Ps. |
At admission: lopinavir/ritonavir , piperacillin tazobactam Decrease in MMF, CNI -ECMO at later period - Cefperazone-sulbactam and caspofungin |
45 days | Multiple organ failure |
| TCUKTP [19] #1 USA |
Kidney | 2/15 (13%) | 70 | M | 5 years | ND | Severe | Lymphopenia (500) CRP=100mg/dL IL-6=89.5pg/mL |
Held MPA, postponed belatacept HQ, azithromycin |
ND | Severe acute respiratory distress syndrome |
| TCUKTP [19] #2 USA |
Kidney | 78 | M | 10 years | ND | Severe | CXR: bilateral patchy opacity Lymphocytes=860 CRP=208mg/dL IL-6=10 pg/mL |
Held MMF HQ, azithromycin |
ND | Severe acute respiratory distress syndrome | |
| Periera et al [22] USA |
ND | 16/90 (17.8%) | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Fernandez-Ruiz et al [23] #1 Spain |
Kidney | 5/18 (27.8%) | 78 | M | 8.3 years | HTN, prostatic adeno-carcinoma | Severe | CXR; Unilateral diffuse consolidation | Lopinavir/ritonavir Reduction of tacrolimus dose High flow O therapy |
5 days | ARDS |
| Fernandez-Ruiz et al [23] #2 Spain |
Kidney | 71 | F | 6 years | HTN | Severe | CXR: Bilateral interstitial pneumonia, patchy consolidations |
Lopinavir/ritonavir HQ, Reduction of tacrolimus dose, discontinuation of MPA and prednisone Metilprednisolone (day +10), IVIg (day +10) |
16 days | ARDS | |
| Fernandez-Ruiz et al [23] #3 Spain | Liver | 72 | M | 5.5 years | HTN, DM, obesity | Severe | CXR: Multifocal consolidation | HCQ (day +1), LPV/r, IFN-β, HFOT, Transitory conversion from MMF to tacrolimus |
7 days | Progressive respiratory failure with ARDS, renal failure, |
|
| Fernandez-Ruiz et al [23] #4 Spain |
Liver | 73 | M | 16.4 years | HBV cirrhosis, diabetes, asthma, bronchiectasis, splenectomy |
Severe (ICU) | CXR: multifocal consolidation | Discontinuation of MMF Mechanical vent. |
24 days | ARDS, refractory shock |
|
| Fernandez-Ruiz et al [23] #5 Spain |
Heart | 63 | M | 17.9 years | IHD,, HTN, DMs, lung cancer, peripheral artery disease | Severe | CXR: bilateral diffuse consolidation | Discontinuation of cyclosporine and MMF LPV/r, HQ, IFN-β (day +6) |
10 days | ARDS | |
| Huang et al24 #1 China |
HSCT | 2/2 (100%) | 51 | M | 2 years | History of acute myeloid leukemia | Severe | Chest CT: multiple patchyground glass opacities bilaterally. Lymphopenia (258) |
Cessation of IS (Cs) LPV/r, MP, Mechanical vent. linezolid, meropenem, and caspofungin for nosocomial infection |
22 days | ARDS |
| Huang et al24 #2 China |
Kidney | 58 | M | 12 years | None | Severe | Chest CT: multiple patchy ground glass opacities Lymphopenia (376) |
Cessation of IS (MMF), LPV/r Methylprednisolone, Mechanical vent. linezolid, meropenem, and caspofungin when nosocomial infection ECMO |
40 days | Multiorgan failure | |
| Bhoori et al25 Italy |
Liver | 3/3 (100%) | >65** | 3M | The 3 cases transplanted more than 10 years ago | The 3 had HTN, DM, obesity | 3 severe | ND | All three patients their IS regimen had been gradually tapered off, with very low trough concentrations of CI (two patients receiving Cs [28 and 35 ng/mL, respectively] and one receiving tacrolimus [2 1ng/mL]). |
3 to 12 days | ARDS in the 3cases |
| Feng et al [26] China |
Lung | 1/3 (33%) | 66 | M | Lung transplanted after COVID-19 infection by 42 days | HTN | Severe | ND | ND | ND | Death post operative by 1 day |
| Alberici et al [27] #1 Italy |
Kidney | 5/20 (25%) | 71 | M | 13 years | IHD | Severe | ND | MMF/CNI/low-dose steroids Dexamethasone |
Overall, patients died after a median of 11 days from admission | ARDS |
| Alberici et al [27] #2 Italy |
57 | M | 2 years | HCV infection | Severe | ND | MMF/CNI/low-dose steroids, combination of lopinavir and ritonavir, HQ, Dexamethasone Tocilizumab | ARDS | |||
| Alberici et al [27] #3 Italy |
59 | M | 5 years | HTN | Severe | ND | MMF/CNI/low-dose steroids, combination of lopinavir and ritonavir, HQ, Dexamethasone | ARDS | |||
| Alberici et al [27] #4 Italy |
70 | F | 16 years | HTN | Severe | ND | MMF/CNI/low-dose steroids, combination of lopinavir and ritonavir, HQ, Dexamethasone | ARDS | |||
| Alberici et al [27] #5 Italy |
63 | M | 16 years | HTN | Severe | ND | MMF/CNI combination of lopinavir and ritonavir, HQ, Dexamethasone Tocilizumab | ||||
| Maggi et al [33] Italy |
Liver | 1/2 (50%) |
69 | M | 30 days | HIV | Severe | ND | ND | ND | ARDS |
| Gandolfini et al [37] Italy |
Kidney | 1/2 (50%) | 75 | M | 10 years | COPD, heart disease, HTN, obesity | Severe | Chest CT: 40% lung involvement CRP=180mg/dL Lymphocytes=880 |
Reduction of MMF and CNI dose lopinavir and ritonavir, HQ Antibiotics |
5 days | ARDS |
| Banerjee et al [38] UK |
Kidney | 1/7 (14%) | 76 | F | 1 year | DM, HTN | Severe (ICU) | CXR: revealed bilateral patchy consolidation Lymphocyts=800 CRP=83mg/dL d-dimer>6000µg/l |
MMF stopped, CNI reduced Mechanical ventilator Antibiotics |
12 days | ARDS, AKI |
| Nair et al [40] #1 USA |
Kidney | 3/10 (30%) | 56 | M | 20 years | DM, HTN | Severe (ICU) | CXR: multifocal patchy opacity Lymphocyts=320 CRP=30.6mg/dL Ferritin=2871ng/mL |
Cessation of MMF and CNI HQ, azithromycin, ceftriaxone Mechanical vent. |
5days | ARDS AKI |
| Nair et al [40] #2 USA |
74 | F | 8 years | HTN, malignancy (not specified) | Severe (ICU) | CXR: multifocal patchy opacity Lymphocyts=440 Ferritin=817ng/mL |
Machanical vent. Cessation of CNI, HQ, Azithromycin |
21 days | ARDS | ||
| Nair et al [40] #3 USA |
56 | F | 3 years | DM, HTN | Severe (ICU) | CXR: multifocal patchy opacity Ferritin=994ng/mL CRP=23.5mg/dL |
Mechanical vent. Cessation of MMF HQ, azithromycin Levofloxacin, ceftriaxone |
8 days | ARDS | ||
| Abrishami et al [47] Iran |
Kidney | 8/12 (67%) | 32-66 (median 56) | 7M/1F | 3-18 years (median 14.5) |
1 HTN, IHD 7 none |
8 Severe (ICU) |
Lymphopenia (6/8), Elevated CRP (6/8), elevated creatinine (3/8) | ND | ND | 8 ARDS |
| Malard et al [48] France |
HSCT | 1/7 (14%) |
65 | M | ND | HTN | Severe | ND | ND | 17 days | ARDS |
| Montagud-Marrahi et al [49] Spain |
31 Kidney 2 Dual |
2/33 (6%) | 87 72 |
F M |
ND | ND | Both severe (ICU) | ND | ND | 13 days 22 days |
ARDS |
| Holzhauser et al [50] USA |
Heart | 1/2 (50%) |
59 | F | 8 years | DM, HTN, CKD | Severe | CXR: bilateral diffuse bronchial wall thickening and patchy peribronchial ground-glass opacities CRP=110 mg/dL Ferritin 4342ng/mL (day 6) |
HQ, doxycyclin, IVIG, lopinavir/ritonavir CVVHD Heparin drip |
10 days | ARDS AKI |
*Only two from ten patients were reported in details
**This is the age reported in this article
AKI: acute kidney injury; ARDS: acute respiratory distress syndrome; CKD: chronic kidney disease; CNI: calcineurin inhibitor; COPD: chronic obstructive pulmonary disease; CRP: C reactive protein; Cs: cyclosporine; CVVHD: continuous veno‐venous hemodialysis ; CXR: chest x ray; ECMO: extracorporeal membrane oxygenation; F: female; HBV: hepatitis B virus; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; HHD: hypertensive heart disease; HSCT: Haematopoeitic Stem cell Transplant; HFOT: high flow O therapy; HIV: human immunodeficiency virus; HQ: hydroxychloroquine; HTN: hypertension; ICU: intensive care unit; IHD: ischemic heart disease;IS: immunosuppressive; LPV/r: lopinavir/ritonavir; M: male; MMF: mycophenolate mofetil; MP: methylprednisolone; MPA: mycophenolic acid; ND: not determined; Ps: pseudomonas; TCUKTP: The Columbia University Kidney Transplant Program; Tx: transplant
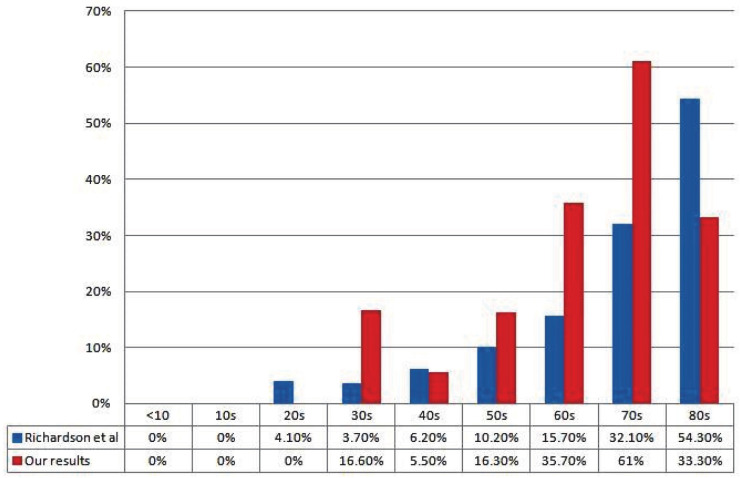
We compared transplant mortality by 10-year age intervals to data from Richardson, et al [51], on mortality in New York similar to most of our patients who were reported from same city. It was evident that mortality increased with increasing age among transplant recipients (Fig 1).
Figure 1.
Comparison of mortality rates in transplant recipients and mortality rates reported by Richardson, et al [51].
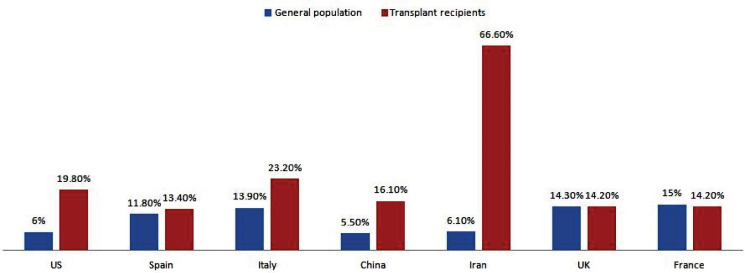
Our transplant recipient cohort mortality was categorized into different countries reported from, and contrasted to the general population mortality at the same country (Fig 2).
Figure 2.
Mortality rates in transplant recipients infected with COVID-19 in selected countries compared to the corresponding mortalities in general population. Data of general population adapted from Dong, et al [1]
DISCUSSION
In this review, we studied the cases of SARS-CoV-2 infection in solid organ transplant recipients. All reported patients received various regimens of immunosuppression which theoretically would lead to more severe infections or increased mortality.
The percentage of patients with asymptomatic or mild disease was 21.7%; 38.7% had moderate severity and 39.6% had severe disease. The total mortality was 20%; 35.7% of the recipients who had severe infection died. We can thus conclude that solid organ transplant recipients who receive immunosuppressive are at greater risk for presenting with severe manifestations of COVID-19 and subsequent mortality.
There is over-activation of the complement system and prolonged inflammatory response due to discordant expression of type I and type II cytokines resulting in cytokine storm and unfavorable outcome in SARS-CoV-2 infection [52].
Corticosteroids have a role in reducing systemic symptoms and decreasing alveolar exudation that results from cytokine storm [53].
Calcineurin inhibitors (CNIs) could inhibit the viral replication in vitro [54]. Cyclosporin could also inhibit replication of several coronaviruses in vitro independent of its immunosuppressive effect and at noncytotoxic concentrations [55].
These findings may support continuing the use of immunosuppressive therapy in selected transplant recipients with SARS-CoV-2 infection.
Alberici, et al [27], described their experience with managing patients with kidney disease including patients with renal transplantation during the current COVID-19 pandemic in Brescia city in the Lombardy region of Italy and provided preliminary outcome data on 20 kidney transplant recipients. Among them, 5 (25%) patients died. Their treatment approach included two phases: the first phase considered antiviral drugs (e.g., lopinavir/ritonavir, darunavir/cobicistat darunavir/ritonavir, and chloroquine-hydroxychloroquine,) that could manage the first phase of the disease, which is associated with viral replication and cytopathic effect. In the second phase of the disease, which begins 7–10 days after the onset of symptoms, they have considered immunosuppressive and immunomodulatory drugs that may be of benefit during this phase, which is characterized by hyperinflammatory and cytokine release syndromes that ultimately leads to increased risk of death with the progressive lung involvement and escalating needs of oxygen supplementation and ventilatory support. They postulated that use of glucocorticoid and the interleukin-6 inhibitor, tocilizumab, in this phase could be beneficiary [27].
Akalin, et al [11], described 36 consecutive adult kidney-transplant recipients; 97% of patients were receiving tacrolimus, 94% prednisone, and 86% mycophenolate mofetil or mycophenolic acid; 78% of patients were hospitalized, 39% of whom required intubation. The total fatality rate was 28% (n=10). They managed the kidney transplant recipients infected by SARS-CoV-2 by withdrawal of an antimetabolite in 86% of patients. In addition, tacrolimus was withheld in 21% of severely ill patients. They administered hydroxychloroquine to 86% of patients and apixaban to patients with D-dimer levels >3.0 µg/mL; two patients received tocilizumab.
The Columbia University Kidney Transplant Program reported a series of 15 kidney transplant recipients infected with SARS CoV-2 [19]. At time of diagnosis, all but one were taking tacrolimus; 80% were also taking either mycophenolate mofetil or mycophenolic acid; 67% were taking prednisone.
They have managed the immunosuppressive therapy in infected patients by complete cessation of antimetabolites or leflunomide in 10 out of 14 (71%) patients while continuing the tacrolimus and the baseline prednisone in those who were on maintenance prednisone. Two (13%) patients died in this series.
Fernández-Ruiz, et al [23], reported a cohort of 18 solid organ transplant recipients infected with SARS-CoV-2 (8 kidneys [44%], 6 livers [33%], and 3 hearts [22%]) at a tertiary-care center in Madrid. They managed the patients by administering lopinavir/ritonavir (usually associated with hydroxychloroquine in 50% of patients, hydroxylchloroquine as monotherapy in 27.8% of patients, and interferon-β in 16.7% of recipients. None of the patients described stopped their immunosuppressive therapy. The case fatality rate was 28% (5 of 18).
Pereira, et al [22], reported the largest cohort in this review. It included 90 patients with a median age of 57 years; 46 were kidney recipients, 17 lung, 13 liver, 9 heart, and 5 dual-organ transplants; 24% of patients had mild disease, 46% had moderate and 30% had severe disease. In accordance with our results, most of the organ transplant recipients had moderate-to-severe disease. However, in the cohort of Pereira, et al (n=68), 12% required non-re-breather and only 35% required intubation. Fatality of SARS-CoV-2 infection reported in this cohort was 18% (24% of hospitalized patients and 52% of patients admitted to the ICU). Yet, details regarding mortality with each organ transplant were not mentioned in the study. The treatment approach in this cohort was based upon expert opinion. They decreased or held the antimetabolite while dosing of other agents was less uniformly decreased [22].
Montagud-Marrahi, et al [49], had 33 kidney transplant recipients in Spain; two of them also had a pancreatic transplantation. They had a favorable outcome; only two patients died, 31(94%) survived. Their policy for immune suppression was to discontinue mycophenolate and/or mTOR-i in all patients; if patients were on CNIs, it would also be stopped if lopinavir/ritonavir is prescribed (due to interactions); steroids were maintained as in many programs; 78.8% of their patients had ≥1 immunosuppressants withdrawn.
Malard, et al [48], studied patients with hematological diseases. In their cohort, they had seven patients who received HSCT. None of these patients was on immunesuppression with CNIs or antimetabolites; they had a favorable outcome; only one patient died, six survived.
Abrishami, et al [47], described a case series of 12 kidney transplant recipients. All patients included in this series were on steroids, calcineurin inhibitors/sirolimus, and mycophenolate mofetil/azathioprine at the time of admission. They have changed the regimen of immunosuppressive therapy by reducing the dose of immunosuppressive agents and changing the oral to intravenous steroid. However, only four patients survived, a fatality rate of 75%.
Calculating the number of patients who died among those who stopped or continued immunosuppressive therapy was not easy, as this was not detailed in all articles.
Chen, et al [26], had reported three lung transplant recipients following COVID-19 infection with extreme high sequential organ failure assessment scores. They have postulated that performing lung transplantation in end-stage patients with respiratory failure due to COVID-19-related pulmonary fibrosis could help in reducing mortality rate in such patients. Two of the three recipients survived post-lung transplantation.
We did not include study of Agnes, et al [56], as their results reported in Italian registry might have been included in other studies. In their report, they had 24 liver transplant recipients with COVID-19 of whom 19 were survived (79%), which is similar to other studies.
The concern that immunosuppression may be associated with poor virologic control is present, increasing the risk of developing a more severe disease and more prolonged viral shedding than the general population. On the other hand, reducing immunosuppression may lead to acute rejection and may cause an immune reconstitution-like reaction with a paradoxical worsening of the disease.
In the described cohorts in this review, transplant recipients with SARS-CoV-2 infection appeared to have mostly a moderate-to-severe disease, although testing limitations and reporting bias could likely make undercounting of mild/asymptomatic cases.
Our results showed a higher mortality among organ transplant recipients with SARS-Cov-2 infection (20%) if compared to the reported 4%–14% mortality rate among patients with COVID-19 in the general population [1]. The median age for our transplant cohort was 52 years; when the mortality was compared to the cohort from New York, it was clear that younger ages had lower mortality and older ages had higher mortality (Fig 1). We hypothesize that at younger ages immunosuppression may offer survival benefit given that the pathogenesis of COVID-19 is the development of cytokine storm. Yet, with advancing age transplant recipients would have more comorbidities which may lead to increased mortality.
Variation in mortality among different countries (Fig 2) was also noted which could be related to the overwhelming nature of this disease to the health care systems that could affect mortality; for certain countries, the mortality was similar to the general population. Some countries had few cases, hence, we excluded them from the analyses.
Many of the included studies were case-reports and it would be reasonable to assume that there might be some reporting bias due to reporting cases with favorable outcomes—most of these case reports showed no mortalities. Cohorts may have shown better representation of the true mortality. However, another confounder was that most of these cohorts were from areas where the health care system was overwhelmed (e.g., New York and Iran). These might overestimate the mortality.
In conclusion, our results showed a higher mortality rate among organ transplant recipients with SARS-CoV-2 infection compared with COVID-19 patients in the general population. Most of the studies documented similar presentation to the general population and outcomes were different in various countries based on outbreak settings and if the country’s health care system was overwhelmed. Further research is needed to guide immunosuppression regimens and understand long-term complications of COVID-19 in transplant recipients and on transplanted organs.
CONFLICTS OF INTEREST:
None declared.
FINANCIAL SUPPORT:
None.
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–4. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guillen E G J, Pineiro, I, et al. Case report of COVID-19 in a kidney transplant recipient: Does immunosuppression alter the clinical presentation? Am J Transplant. 2020 doi: 10.1111/ajt.15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberici F, Delbarba E, Manenti C, et al. Management of patients on dialysis and with kidney transplant during COVID-19 coronavirus infection. Parma, Italy, European Renal Association – European Dialysis and Transplant Association. 2020. [(Accessed May 10, 2020)]. Available from www.era-edta.org/en/wpcontent/uploads/2020/03/COVID_guidelines_finale_eng-GB.pdf.
- 4.López-Oliva MO, González E, Miranda RJ, Jiménez C. Management of kidney transplant immunosuppression in positive coronavirus infection requiring hospital admission. Parma, Italy, European Renal Association European Dialysis and Transplant Association. 2020. [ (Accessed May 10, 2020)]. Available from www.eraedta.org/en/wpcontent/uploads/2020/03/ManagementofkidneytransplantimmunosuppressionLaPaz.Pdf.
- 5.Mehta P, McAuley DF, Brown M, et al. HLH Across Speciality Collaboration, UK: COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathies D, Rauschning D, Wagner U, et al. A Case of SARS-CoV-2-pneumonia with successful antiviral therapy in a 77-year-old male with heart transplant [published online ahead of print, 2020 Apr 21] Am J Transplan . 2020 doi: 10.1111/ajt.15932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S, Yin Q, Shi H, et al. A familial cluster, including a kidney transplant recipient, of Coronavirus Disease 2019 (COVID-19) in Wuhan, China [published online ahead of print, 2020 Apr 3] Am J Transplant . 2020 doi: 10.1111/ajt.15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arpali E, Akyollu B, Yelken B, et al. Case report: A kidney transplant patient with mild COVID-19 [published online ahead of print, 2020 Apr 16] Transpl Infect Dis. 2020:e13296. doi: 10.1111/tid.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong Z, Zhang Q, Xia H, et al. Clinical characteristics and immunosuppressant management of coronavirus disease 2019 in solid organ transplant recipients [published online ahead of print, 2020 Apr 13] Am J Transplant . 2020 doi: 10.1111/ajt.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu L, Gong N, Liu B, et al. Coronavirus Disease 2019 Pneumonia in Immunosuppressed Renal Transplant Recipients: A Summary of 10 Confirmed Cases in Wuhan, China [published online ahead of print, 2020 Apr 18] Eur Urol. 2020;S0302-2838:30214–1. doi: 10.1016/j.eururo.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akalin E, Azzi Y, Bartash R, et al. COVID-19 and Kidney Transplantation PT. N Engl J Med . 2020;382:2475–7. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagana SM, De Michele S, Lee MJ, et al. COVID-19 Associated Hepatitis Complicating Recent Living Donor Liver Transplantation [published online ahead of print, 2020 Apr 17] Arch Pathol Lab Med. 2020:10.5858/arpa.2020–0186-SA. doi: 10.5858/arpa.2020-0186-SA. [DOI] [PubMed] [Google Scholar]
- 13.Hsu JJ, Gaynor P, Kamath M, et al. COVID-19 in a High-Risk Dual Heart and Kidney Transplant Recipient [published online ahead of print, 2020 Apr 21] Am J Transplant. 2020 doi: 10.1111/ajt.15936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Li X, Cao G, et al. COVID-19 in a Kidney Transplant Patient [published online ahead of print, 2020 Apr 6] Eur Urol. 2020;S0302-2838:30211–6. doi: 10.1016/j.eururo.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Antiga L. Coronaviruses and Immunosuppressed Patients: The Facts During the Third Epidemic [published online ahead of print, 2020 Mar 20] Liver Transpl . 2020 doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 16.Donato MF, Invernizzi F, Lampertico P, Rossi G. Health Status of Patients Who Underwent Liver Transplantation During the Coronavirus Outbreak at a Large Center in Milan, Italy [published online ahead of print, 2020 Apr 22] Clin Gastroenterol Hepatol. 2020;S1542-356:30538–3. doi: 10.1016/j.cgh.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marx D, Moulin B, Fafi-Kremer S, et al. First case of COVID-19 in a kidney transplant recipient treated with belatacept [published online ahead of print, 2020 Apr 13] Am J Transplant . 2020 doi: 10.1111/ajt.15919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang JF, Zheng KI, George J, et al. Fatal outcome in a liver transplant recipient with COVID-19 [published online ahead of print, 2020 Apr 10] Am J Transplant . 2020 doi: 10.1111/ajt.15909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Columbia University Kidney Transplant Program. Early Description of Coronavirus 2019 Disease in Kidney Transplant Recipients in New York [published online ahead of print, 2020 Apr 21] J Am Soc Nephrol. 2020:ASN.2020030375–10. doi: 10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olivia S, Kates , Cynthia E, Fisher , Helen C, et al. Earliest cases of coronavirus disease 2019 (COVID-19) identified in solid organ transplant recipients in the United States. Am J Transplant. 2020 doi: 10.1111/ajt.15944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontana F, Alfano G, Mori G, et al. COVID-19 pneumonia in a kidney transplant recipient successfully treated with Tocilizumab and Hydroxychloroquine [published online ahead of print, 2020 Apr 23] Am J Transplant . 2020 doi: 10.1111/ajt.15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in Solid Organ Transplant Recipients: Initial Report from the US Epicenter [published online ahead of print, 2020 Apr 24] Am J Transplant . 2020 doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain [published online ahead of print, 2020 Apr 16] Am J Transplant . 2020 doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, et al. COVID-19 in posttransplant patients—report of 2 cases. Am J Transplant. 2020;20:1879–81. doi: 10.1111/ajt.15896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy [published online ahead of print, 2020 Apr 9] Lancet Gastroenterol Hepatol . 2020;S2468-1253:30116–3. doi: 10.1016/S2468-1253(20)30116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen JY, Qiao K, Liu F, et al. Lung transplantation as therapeutic option in acute respiratory distress syndrome for COVID-19-related pulmonary fibrosis [published online ahead of print, 2020 Apr 1] Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000839. oi: 10.1097/CM9.0000000000000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia [published online ahead of print, 2020 Apr 9] Kidney Int . 2020:S0085–2538 . doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ning L, et al. Novel coronavirus (SARS-CoV-2) infection in a renal transplant recipient: Case report. Am J Transplant. 2020;20:1864–8. doi: 10.1111/ajt.15897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin J, Wang H, Qin X, et al. Perioperative Presentation of COVID-19 Disease in a Liver Transplant Recipient [published online ahead of print, 2020 Mar 27] Hepatology . 2020 doi: 10.1002/hep.31257. doi: 10.1002/hep.31257. [DOI] [PubMed] [Google Scholar]
- 30.Seminari E, Colaneri M, Sambo M, et al. the COVID19 IRCCS San Matteo Pavia Task Force SARS Cov-2 infection in a renal-transplanted patients A case report. Am J Transplant . 2020 doi: 10.1111/ajt.15902. [Google Scholar]
- 31.Zhu L, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020 doi: 10.1111/ajt.15869. doi: 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu B, Wang Y, Zhao Y, Shi H, Zeng F, Chen Z. Successful treatment of severe COVID-19 pneumonia in a liver transplant recipient [published online ahead of print, 2020 Apr 3] Am J Transplant . 2020 doi: 10.1111/ajt.15901. doi: 10.1111/ajt.15901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maggi U. The impact of the COVID-19 outbreak on Liver Transplantation programmes in Northern Italy. Am J Transplant Accepted \ 2019 doi: 10.1111/ajt.15948. doi: 10.1111/ajt.15948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartiromo M, Borchi B, Botta A. Threatening drug-drug interaction in a kidney transplant patient with Coronavirus Disease 2019 (COVID-19) Transpl Infect Dis. 2020 doi: 10.1111/tid.13286. doi: 10.1111/tid.13286 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Chen Y, Yuan Q, et al. Identification of Kidney Transplant Recipients with Coronavirus Disease 2019 [published online ahead of print, 2020 Apr 2] Eur Urol . 2020:S0302–283830205-0. doi: 10.1016/j.eururo.2020.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li F, Cai J, Dong N. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant . 2020;39:496–7. doi: 10.1016/j.healun.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gandolfini I, Delsante M, Fiaccadori E, et al. COVID-19 in kidney transplant recipients. Am J Transplant. 2020 doi: 10.1111/ajt.15891. doi: 10.1111/ajt.15891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banerjee D, Popoola J, Shah S, et al. COVID-19 infection in kidney transplant recipients [published online ahead of print, 2020 Apr 9] Kidney. 2020;S0085-2538:30361–6. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Y, Kwon O, Paek JH, et al. Two distinct cases with COVID-19 in kidney transplant recipients. Am J Transplant . 2020 doi: 10.1111/ajt.15947. doi: 10.1111/ajt.15947 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nair V, Jandovitz N, Hirsch JS, et al. COVID-19 in kidney transplant recipients. Am J Transplant . 2020 doi: 10.1111/ajt.15967. doi: 10.1111/ajt.15967 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meziyerh S, Zwart TC, van Etten RW, et al. Severe COVID-19 in a renal transplant recipient: A focus on pharmacokinetics. Am J Transplant . 2020 doi: 10.1111/ajt.15943. doi: 10.1111/ajt.15943 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hammami MB, Garibaldi B, Shah P, et al. Clinical Course of COVID-19 in a Liver Transplant Recipient on Hemodialysis and Response to Tocilizumab Therapy: A Case Report. Am J Transplant . 2020 doi: 10.1111/ajt.15985. doi: 10.1111/ajt.15985 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aigner C, Dittmer U, Kamler M, et al. COVID-19 in a lung transplant recipient [published online ahead of print, 2020 Apr 13] J Heart Lung Transplant. 2020:S1053–249831511. doi: 10.1016/j.healun.2020.04.004. doi: 10.1016/j.healun.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Billah M, Santeusanio A, Delaney V, et al. A Catabolic State in a Kidney Transplant Recipient with COVID-19. Transpl Int. 2020 doi: 10.1111/tri.13635. doi: 10.1111/tri.13635 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Barros Machado DJ, Ianhez LE. COVID-19 pneumonia in kidney transplant recipients- where we are? Transpl Infect Dis. 2020:e13306. doi: 10.1111/tid.13306. doi: 10.1111/tid.13306 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bussalino E, De Maria A, Russo R, Paoletti E. Immunosuppressive therapy maintenance in a kidney transplant recipient with SARS-CoV-2 pneumonia: A case report [published online ahead of print, 2020 Apr 13] Am J Transplant. 2020 doi: 10.1111/ajt.15920. doi: 10.1111/ajt.15920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abrishami A, Samavat S, Behnam B, et al. Clinical Course, Imaging Features, and Outcomes of COVID-19 in Kidney Transplant Recipients. European Urology. 2020 doi: 10.1016/j.eururo.2020.04.064. doi: 10.1016/j.eururo.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malard F, Genthon A, Brissot E, et al. COVID-19 outcomes in patients with hematologic disease [published online ahead of print, 2020 May 6] Bone Marrow Transplant. 2020:1–5. doi: 10.1038/s41409-020-0931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montagud-Marrahi E, Cofan F, Torregrosa JV, et al. Preliminary data on outcomes of SARS-CoV-2 infection in a Spanish single centre cohort of kidney recipients. Am J Transplant . doi: 10.1111/ajt.15970. doi: 10.1111/ajt.15970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holzhauser L, Lourenco L, Sarswat N, et al. Early Experience of COVID-19 in Two Heart Transplant Recipients: Case Reports and Review of Treatment Options. Am J Transplant . doi: 10.1111/ajt.15982. doi: 10.1111/ajt.15982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area [published online ahead of print, 2020 Apr 22] JAMA. 2020;e206775 :10. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smits SL, A de Lang, JM van den Brand, et al. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6:e1000756. doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lansbury LE, Rodrigo C, Leonardi-Bee J, et al. Corticosteroids as Adjunctive Therapy in the Treatment of Influenza: An Updated Cochrane Systematic Review and Meta-analysis. Crit Care Med. 2020;48:e98–e106. doi: 10.1097/CCM.0000000000004093. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka Y, Sato Y. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses . 2013 doi: 10.3390/v5051250. May 22. doi: 10.3390/v5051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pfefferle S, Schöpf J, Kögl M, et al. The SARS-coronavirus-host interactome: identification of cyclophilins as target for pancoronavirus inhibitors. PLoS Pathog. 2011:e1002331. doi: 10.1371/journal.ppat.1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agnes S, Andorno E, Avolio AW, et al. Preliminary Analysis of the Impact of COVID-19 Outbreak on Italian Liver Transplant Programs [published online ahead of print, 2020 May 6] Liver Transpl. 2020 doi:10.1002/lt.25790. [Google Scholar]