Abstract
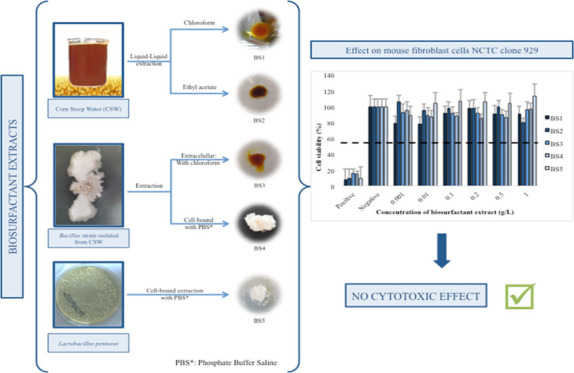
In this work, five biosurfactant extracts, obtained from different sources, all of them with demonstrated antimicrobial properties, were characterized and subjected to a cytotoxic study using mouse fibroblast cells (NCTC clone 929). Biosurfactant extracts obtained directly from corn steep water (CSW) showed similar surfactant characteristics to those of the extracellular biosurfactant extract produced by Bacillus isolated from CSW and grown in tryptic soy broth, observing that they are amphoteric, consisting of viscous and yellowish liquid with no foaming capacity. Contrarily, cell-bound biosurfactant extracts produced from Lactobacillus pentosus or produced by Bacillus sp isolated from CSW are nonionic, consisting of a white powder with foaming capacity. All the biosurfactants possess a similar fatty acid composition. The cytotoxic test revealed that the extracts under evaluation, at a concentration of 1 g/L, were not cytotoxic for fibroblasts (fibroblast growth > 90%). The biosurfactant extract obtained from CSW with ethyl acetate, at 1 g/L, showed the highest cytotoxic effect but above the cytotoxicity limit established by the UNE-EN-ISO10993-5. It is remarkable that the cell-bound biosurfactant produced by L. pentosus, at a concentration of 1 g/L, promoted the growth of the fibroblast up to 113%.
1. Introduction
Synthetic surfactants are amphiphilic molecules composed of hydrophobic and hydrophilic moieties. This structure allows them to improve the solubility of different types of compounds. As a consequence, the use of surfactants is widely extended in broad fields such as environmental, food, cosmetic, or pharmaceutical industries.1−3 However, synthetic detergents have been demonstrated to present negative effects or low compatibility. In particular, in cosmetic or pharmaceutical formulations, the relationship between the use of certain surfactants and the development of dermatitis and skin and ocular irritation, among others, has been reported.4−7 In this regard, biosurfactants have arisen as an alternative to petrochemical surfactants. In contrast to synthetic tensides, biosurfactants are surface-active compounds produced by microorganisms composed of natural molecules consisting of lipids, sugars, and/or proteins. This singular composition confers them advantageous properties such as better biodegradability or less toxicity, maintaining similar surface properties to their synthetic counterparts.8−10 Some authors have catalogued biosurfactants as the molecules of the 21st century with multifunctional properties.11,12 Nevertheless, their high production cost represents their main disadvantage. Consequently, many authors have proposed alternatives such as the use of renewable materials as carbon sources. For instance, canola oil, soybean oil, waste frying oil, or hydrolyzates from cellulosic or hemicellulosic fractions of vine-trimming wastes have been proposed as a less expensive option compared to traditional culture medias.13−17
Biosurfactants not only stand out for being natural homologous of synthetic surfactants. They are also known for their multiple applications in food, cosmetic, pharmaceutical, or environmental industries. For instance, the potential use of a lipopeptide produced by Bacillus cereus UCP 1615 as a seawater bioremediation agent was demonstrated.18 This biosurfactant has also been studied by Ostendorf et al.,14 demonstrating its efficacy in removing oil adsorbed to rock. In addition to environmental applications, some biosurfactants have been demonstrated to have interesting properties in cosmetic and pharmaceutical fields. For instance, the biosurfactant produced by Lactobacillus paracasei is able to maintain the emulsion stability of a formulation with almond oil, after 7 days, with a percentage of emulsion volume about 80%.19 Another biosurfactant, classified as a sophorolipid and produced by Starmerella bombicola, has been proved to be a good adjuvant in creams for wound infections.20 On its part, the biosurfactant extract obtained from the corn-milling industry stream has been employed in sunscreen formulations, and its influence in different drug permeation through silicone membranes with positive effects was also proven.3,21 This biosurfactant extract, obtained from corn steep water (CSW), is capable of reducing the surface tension of water up to 39 mN/m, and it possesses a critical micellar concentration (CMC) between 0.075 and 0.200 g/L, depending on the grade of purity achieved.22,23
Moreover, it is also used as antimicrobial agents. Thus, Rodríguez-López et al.24 have demonstrated the antimicrobial activity of the biosurfactant extract obtained from the residual stream of the corn-milling industry, observing in addition that it is nonirritant, contrarily to synthetic surfactants. Vecino and collaborators25 have also tested the potential use of glycolipopeptides, similar to the biosurfactant produced by Lactobacillus pentosus, against skin pathogens such as Streptococcus agalactiae.25 However, few assays exist about the cytotoxic effect of biosurfactants. Following this, the few studies related with the cytotoxic effect of biosurfactants are revised.
Burgos-Díaz et al.26 evaluated a biosurfactant produced by Sphingobacterium detergents, observing that the fractions tested reduced cell proliferation and induced apoptosis. Moreover, the results achieved with this biosurfactant were better in comparison with those of dodecyl sodium sulfate, a synthetic surfactant commonly used in cosmetic formulations. Another example of less cytotoxicity effect of biosurfactants, in comparison with synthetic detergents, has been reported by Patowary and collaborators.27 These authors demonstrated the antibiotic activity of a biological detergent produced by Pseudomonas aeruginosa PG1 against pathogenic bacteria and fungus, as well as its noncytotoxic effect on mouse fibroblasts (NCTC clone 929). As it can be observed, the studies dealing with cytotoxic studies of biosurfactants are scarce, and considering that microorganisms produce biosurfactants with unique bioactive properties, it should be interesting to evaluate the cytotoxic effect of biosurfactants from different sources. Moreover, as it was previously described, some biosurfactants, including those evaluated in the current work, possess antimicrobial activity. Therefore, cytotoxic analyses should be mandatory to discharge any possible alteration on cells produced by their inclusion in cosmetic or pharmaceutical formulations.
In this regard, the aim of this work was to characterize and study the effect of five biosurfactant extracts, all of them with antimicrobial properties and obtained from different sources, on fibroblasts. The samples used in this study include two extracellular biosurfactant extracts obtained directly from CSW, using different extraction methods; two biosurfactants (extracellular or cell-bound) produced by an endospore-forming Bacillus strain, isolated from CSW; and one cell-bound biosurfactant extract produced by L. pentosus. These last three extracts were all obtained in controlled fermentations.
2. Materials and Methods
2.1. Biosurfactant Extract Production
2.1.1. Extraction of Biosurfactants from CSW
Biosurfactants were extracted from CSW (FEED Stimulants, Netherlands), a residual stream from the corn-milling industry fermented spontaneously by lactic acid bacteria and other Bacilli strains, following the protocol reported by Vecino et al.17 Therefore, CSW was subjected to 2 different extraction processes, using chloroform or ethyl acetate, obtaining two biosurfactant extracts, BS1 and BS2, respectively. The extraction of the biosurfactant with chloroform was carried out at 56 °C for 1 h using a 2:1 v/v chloroform/CSW ratio. On the other hand, the extraction with ethyl acetate was carried out at 25 °C for 1 h with a ethyl acetate/CSW ratio of 3:1 v/v. After extraction, both organic solvents were evaporated by vacuum distillation with a rotavapor R-120 (Büchi Labortechnik, Switzerland), obtaining two oily extracts, BS1 (extracted with chloroform) and BS2 (extracted with ethyl acetate).
2.1.2. Production and Extraction of the Biosurfactant from the Bacillus Strain Isolated from CSW
The endospore-forming Bacillus strain, isolated from CSW in previous works, has been identified as Aneurinibacillus aneurinilyticus and corresponds to the strain deposited in the Spanish Type Culture Collection (CECT) (Valencia, Spain), which was identified as CECT 9939.29,30 This microorganism was grown at 37 °C for 48 h in 200 mL of tryptic soy broth (TSB), previously sterilized at 121 °C for 15 min. The biomass was separated from the culture media by centrifugation at 5000 rpm in a ROTINA 380R (Hettich, Germany) in order to extract two different biosurfactants, one from the fermented broth (BS3) and other from Bacillus cells (BS4). For obtaining BS3, the culture medium was subjected to extraction with chloroform, following the same procedure previously explained.17 However, for obtaining BS4, the biomass was washed twice with distilled water. This step allowed to eliminate any compound present in the culture media. In this case, the extraction of the cell-bound biosurfactant (BS4) was carried out by stirring the biomass with 100 mL of phosphate-buffered saline (PBS) (10 mM KH2PO4/K2HPO4 with 150 mM NaCl) for 2 h at 25 °C. The supernatant, containing the biosurfactant, was submitted to a dialysis process at 4 °C for 48 h in a Spectra/Pore dialysis membrane (molecular weight cutoff of 6000–8000 Da; Spectrum Laboratories, Inc, Rancho Dominguez, CA). Finally, the extract (BS4) was lyophilized using a LyoQuest HT40 (Telstar).
2.1.3. Production and Extraction of the Biosurfactant Produced by L. pentosus (BS5)
L. pentosus CECT 4023T (ATCC-8041) was obtained from the CECT (Valencia, Spain). The lyophilized microorganism was grown in a 250 mL Erlenmeyer flask for 24 h, at 31 °C and 200 rpm, containing 100 mL of MRS Broth (de Man, Rogosa and Sharpe medium), previously sterilized by filtration through a 0.22 μm polyvinylidene fluoride membrane. For biosurfactant production, this inoculum was added in a 1000 mL Erlenmeyer flask, containing 600 mL of MRS broth, which was fermented at 31 °C and 200 rpm. After 72 h, the stationary phase was achieved and the fermentation stopped. It should be remarked that L. pentosus is a lactic acid bacterium, which has been demonstrated to only produce cell-bound biosurfactants.31,32 Following this, the biomass was separated from the culture medium by centrifugation and the biosurfactant extracted from cells with PBS, following the same procedure described above for BS4 (Table 1).
Table 1. Summary of Production and Extraction Conditions of the Biosurfactant Extracts under Study.
| biosurfactant | BS1 | BS2 | BS3 | BS4 | BS5 |
| type of biosurfactant | extracellular | extracellular | extracellular | cell-bound | cell-bound |
| fermentation process | spontaneous | spontaneous | controlled | controlled | controlled |
| nutritional medium | CSW | CSW | TSB | TSB | MRS broth |
| microorganism | lactic acid bacteria and other Bacilli strains associated with the steeping process | lactic acid bacteria and other Bacilli strains associated with the steeping process | Bacillus strain isolated from CSW | Bacillus strain isolated from CSW | L. pentosus |
| extraction process | chloroform | ethyl acetate | chloroform | PBSa | PBSa |
Buffer phosphate saline.
2.2. Characterization of Biosurfactant Extracts
2.2.1. Critical Micellar Concentration
The CMC represents the concentration of the surfactant in which the molecules acquire a micelle conformation. Below the CMC exists a linear relationship between the concentration of the biosurfactant and the reduction in the surface tension produced.33 Thus, the CMCs of all biosurfactant extracts under study were determined by preparing several solutions at concentrations from 0 to 1 g/L in the case of BS1, BS2, BS3, and BS4 and from 0 to 10 g/L in the case of BS5. The surface tension of these solutions were measured using a tensiometer KRÜSS (K20 EasyDyne) and by applying the Wilhelmy plate methodology. Measurements were made in triplicate and the value of demineralized water (72 mN/m) was used as a negative control (absence of biosurfactant).
2.2.2. Ionic Charge
An interesting property of surface-active compounds is their ionic charge as it will determine their future application. The ionic charge of the biosurfactant extracts under study was determined using cationic and anionic resins. For that, 1 g of IR 120 (cationic exchange resin) or IRA 400 (anionic exchange resin) were put in contact with 10 mL of each biosurfactant extract, according to the protocol previously described by Rodríguez-López et al.34 After 30 min, the supernatant was separated from the resin and its surface tension measured, in order to evaluate the presence or absence of the biosurfactant in the remaining solutions. This protocol was carried out in triplicate.
2.2.3. Chemical Characterization of Biosurfactant Extracts
Biosurfactant extracts were chemically characterized in order to elucidate their main structure and composition. Elemental analyses, in which total organic C, N, and H were included, were carried out in triplicate by thermal conductivity detection. For these analyses, the sample decomposition by combustion was conducted, followed by thermal conductivity detection on a Carlo Erba EA-1108CHN-O element analyzer. The N % was converted in protein content, following the methodology previously described by Mariotti et al.35
A colorimetric analysis of sugars was also carried out in order to quantify the amount of carbohydrates present in the biosurfactant extracts. With this purpose, the methodology described by Dubois et al was applied.36 A sample of 7 mg of the biosurfactant extract was measured and mixed with 0.05 mL of 80% phenol in water. Finally, 3 mL of concentrated sulfuric acid was added. The tubes were allowed to stand 10 min and, then, shaken for 20 min in a water bath at 25 °C. The colored solutions were measured 480 nm in a V-650 spectrophotometer in triplicate (Jasco, Canada).
In addition, also in triplicate, the lipid content was determined employing gas chromatography coupled to mass spectrometry (GC–MS). In this case, the biosurfactant extracts were methylated and submitted to a transesterification of fatty acids into fatty acid methyl esters (FAMEs). These previous steps were carried out following the protocol defined by the European Standard, EN-ISO-12966-3:2009.
After methylation and transesterification, FAMEs were separated in a ZB-WAX column (60 m × 0.25 mm i.d. × 0.25 μm film thickness) with an oven temperature gradient of 60 °C of 2 min, followed by 60–200 °C at a 10 °C/min rate maintained for 27 min. Then, the temperature increased to 240 °C at 5 °C/min and was held for 20 min. The carrier gas employed was helium with a flow rate of 1 mL/min, the temperature of both injector inlet and the transfer line of the detector being 250 °C. The lipid mass spectra were registered by a mass selective detector under electron impact ionization at 70 eV of voltage. The data were acquired over a m/z range of 40–400. FAMEs were identified using a mass spectra library from the GC–MS system and comparing retention times. The FAME standard mix was also injected under the same conditions.
Finally, in order to compare all the masses present in the biosurfactant extracts in this study, an electrospray ionization mass spectrometry/collision-induced dissociation (ESI-MS/CID) was employed. For that, a current of electrons was used to ionize the molecules of 1 mg of the biosurfactant extracts, previously dissolved in chloroform and volatilized under vacuum. The fragmentation pattern was recorded on a mass spectrometer Bruker FTMS APEXIII (Fremont, CA) in positive mode.
2.3. Evaluation of Cytotoxicity
2.3.1. Cell Culture Conditions
The cytotoxicity assay was performed with the NCTC clone 929 (ECACC 88102702) mouse fibroblast cell line. Cells were cultured in DMEM medium (Lonza), supplemented with 10% of fetal bovine serum (HyClone); 1% of a combination of penicillin, streptomycin, and amphotericin B (Lonza) at 37 °C; and 5% of CO2 in a humidified atmosphere. Cells with their exponential growth were trypsinized and used in the cytotoxicity assay.
2.3.2. Cytotoxicity Assay
To evaluate the cytotoxicity each biosurfactant was weighed and dissolved in the same culture medium to obtain concentrations of 1, 0.5, 0.2, 0.1, 0.01, and 0.001 g/L. These concentrations were tested in order to determine the biosurfactant behavior below and above their CMC, with the exception of L. pentosus, which was evaluated at the same concentrations for comparative purposes. Following the UNE-EN-ISO 10993-5:2009, a suspension in DMEM of fibroblasts of 1 × 105 cells/mL was seeded on a 96-well microplate in a volume of 100 μL per well. After 72 h of incubation at 37 °C and 5% of CO2 in a humidified atmosphere, a sub-confluent layer was formed and the cell medium was replaced by the already prepared dilutions of the biosurfactants. Four replicates per concentration were incubated with the cells for 24 h. As a positive control, a phenol solution of 6.4 g/L was employed, whereas the negative control was the culture medium.
After that time, the cellular viability was quantified by using the MTS Cell Proliferation Assay Kit (Abcam). This colorimetric assay is based on the reduction of the MTS tetrazolium compound only by viable cells to generate a colored formazan dye that is soluble in the culture medium. A volume of 10 μL of MTS reactive was added to each well. At the end of 45 min of incubation (37 °C and 5% CO2), the absorbance of the resulting solutions was read at a wavelength of 490 nm in a microplate spectrophotometer (Bio-Rad). The test was performed in triplicated and results were expressed in the percentage of viability compared to the negative control, which was the same medium without the biosurfactant extract (0 g/L).
Then, a quantitative evaluation of these percentages was also carried out by using one scale of cytotoxicity (Adapted from Xian and the UNE-EN-ISO 10993-5:2009), where levels of cytotoxicity were associated with the percentages of viability obtained.37,38 Where the value 0 was attributed to 90% of cell viability and the highest level of cytotoxicity (<10% cell viability) was attributed to 4 in the scale (Table 2).
Table 2. Equivalence between Cell Viability and Cytotoxicity28,29.
| cell viability (%) | >90% | 80–90% | 50–80% | 30–50% | <30% |
| cytotoxic scale | 0 (zero) | 1 (mild) | 2 (moderate) | 3 (intense) | 4 (severe) |
2.3.3. Statistical Analysis
Data are presented as means ± standard error, with n = 4. All obtained data underwent statistical analysis using IBM SPSS Statistics 23.0 (IBM Corporation, NY, USA) software package. The nonparametric Mann–Whitney U test was used to determine the difference between the values and the limit (70%) of cytotoxicity (according to UNE-EN-ISO 10993:5). Statistical significance was determined to be p ≤ 0.05 at the 95% confidence level.
3. Results and Discussion
3.1. Characterization of Biosurfactant Extracts under Evaluation
The main chemical and physical properties of the five biosurfactant extracts in this study are summarized in Table 3. As it can be observed, BS1 and BS2 possess similar surfactant properties, with their CMC being 0.139 and 0.176 g/L, respectively. These data are in concordance with the results previously reported.24,34,39−41 The differences between BS1 and BS2 can be explained based on the different organic solvents used during extraction, that produce variations between both biosurfactant extracts in terms of the composition (see elemental analyses). Therefore, a higher % of C in BS1 was observed, consistent with a higher content in fatty acids as well. Moreover, as it was already mentioned, BS2 possess a higher CMC than BS1, which allows to speculate that chloroform gives a more active surfactant extract, although both solvents used were able to extract biosurfactants with similar surface-active characteristics. Regarding BS3, the CMC value obtained was similar to BS2, although with a lower reduction of surface tension (43.4 mN/m).
Table 3. Composition and Main Physicochemical Properties of the Biosurfactant Extracts under Studya.
| biosurfactant | BS1 | BS2 | BS3 | BS4 | BS5 |
| Physicochemical Properties | |||||
| CMC (g/L) | 0.139 ± 0.003 | 0.176 ± 0.005 | 0.174 ± 0.004 | 0.320 ± 0.010 | 1.81 ± 0.21 |
| minimum surface tension (mN/m) | 39.3 ± 0.6 | 37.7 ± 0.6 | 43.4 ± 1.6 | 42.8 ± 3.0 | 49.5 ± 0.7 |
| foam capacity | negative | negative | negative | positive | positive |
| appearance | oily | oily | oily | white powder | white powder |
| ionic behavior | amphoteric | amphoteric | amphoteric | nonionic | nonionic |
| Elemental Analyses | |||||
| N (%) | 1.10 ± 0.01 | 0.82 ± 0.02 | 7.98 ± 1.55 | 6.77 ± 3.01 | 7.11 ± 0.04 |
| H (%) | 11.29 ± 0.12 | 6.68 ± 0.11 | 4.82 ± 0.21 | 3.73 ± 0.11 | 2.97 ± 0.02 |
| C (%) | 74.70 ± 0.10 | 42.51 ± 1.05 | 32.05 ± 1.91 | 24.33 ± 0.23 | 19.83 ± 0.01 |
| Polymeric Analyses | |||||
| carbohydrates (%) | 0.36 ± 0.01 | 0.60 ± 0.03 | 2.20 ± 0.03 | 1.34 ± 0.11 | 7.80 ± 1.01 |
| protein (%) | 6.87 | 5.12 | 49.88 | 42.31 | 44.44 |
| C16 | 20.6 | 21.0 | 38.5 | 37.6 | 14.8 |
| C16:1 | 1.9 | ||||
| C18 | 2.8 | 5.1 | 61.5 | 62.4 | 10.4 |
| C18:1 | 24.5 | 18.1 | 27.4 | ||
| C18:2 | 48.4 | 41.4 | 47.5 | ||
| C18:3 | 2.7 | 11 | |||
| C20 and C24 | 1 | 1.5 | |||
Not determined.
It is known that lactic acid bacteria and other microorganisms are able to grow in CSW; thus, it can be speculated that BS1 and BS2 are a mixture of biosurfactants produced by different microorganisms, including the endosporing forming Bacillus sp used to produce BS3. The carbohydrate content of BS1 and BS2 was negligible, confirming that the biosurfactants contained in BS1 and BS2 should be lipopeptides; whereas in BS3, 2.2% of sugar was observed suggesting that the biosurfactant extract could be a mixture of lipopeptides and glycopeptides.
On the other hand, BS4 and BS5 correspond with cell-bound biosurfactant extracts produced by a Bacillus strain isolated from CSW (BS4) and by L. pentosus (BS5), both produced in controlled fermentations using TSB and MRS broths, respectively. Regarding CMC of the cell-bound biosurfactants, BS4 showed a CMC value of 0.320 g/L, whereas BS5 showed 1.81 g/L, observing that lactic acid bacteria produced biosurfactants with lower surface-active capacity than the Bacillus sp strain isolated form CSW. Moreover, CMC of cell-bound biosurfactants are higher than CMCs of extracellular ones, being this difference particularly accentuated in the biosurfactant extract produced by L. pentosus. This value is not surprising, thus, in general, cell-bound biosurfactants present lower surface activity than extracellular biosurfactants.42,43 For instance, Bustos et al.44 studied the capacity of L. pentosus for producing biosurfactants after various fermentative processes, reporting a CMC of 1.95 g/L, which is in consonance with the results obtained in this work.44 Moreover, regarding carbohydrate content, BS5 showed the highest concentration of the five extracts under evaluation. This value, in addition to the N % associated with a higher amount of proteins, suggested that BS5 was a biosurfactant extract mainly composed of carbohydrates and peptides/proteins. Consequently, it can be speculated that the biosurfactants categorized were a glycoprotein or glycolipopeptides. Furthermore, the composition of the biosurfactant extracts obtained from L. pentosus fermentations has been already reported.44−46 For instance, Vecino et al.45 determined the optimal extraction conditions, observing independently of the time or temperature; the biosurfactant extract produced by L. pentosus was identified as a glycoprotein or a glycolipopeptide. Contrarily, based on the composition listed in Table 3, BS1, BS2, BS3, and BS4 are consistent with the presence of lipopeptides. This is in consonance with the results previously reported, in which similar surfactant properties were observed in the same extracts BS1, BS2, BS3, and BS4,25 in comparison with surfactin and other biosurfactants produced by Bacillus subtilis reported in the literature.24,29
Regarding cell-bound biosurfactants, Gudiña et al.47 measured the CMC of the cell-bound biosurfactant produced by L. paracasei, resulting in a value of 2.5 g/L, which is comparable to the CMC of the biosurfactant produced by L. pentosus, evaluated in this work (BS5). Moreover, these authors have also reported that Lactobacillus agilis CCUG31450 possess a CMC of 7.5 g/L, which is much higher than the one obtained for BS5.
In terms of their ionic behavior, BS1, BS2, and BS3 were entrapped by IRA 400 and IR120, which means that they possess both cationic and anionic charges. For this reason, they can be considered amphoteric. This result is in concordance with the ionic behavior previously observed by Rodríguez-López et al.34 Contrarily, surfactin, one of the most studied biosurfactants, was defined as an anionic lipopeptide; in this case, surfactin was produced by B. subtilis consisting of a cyclic heptapeptide (Gly-Leu-d-Leu-Val-Asp-d-Leu-Leu).48 Regarding the ionic behavior of BS4 and BS5, neither the anionic nor cationic resins were able to entrap them. Therefore, both cell-bound biosurfactant extracts, BS4 and BS5, can be considered as nonionic.
Despite that ionic behavior of biosurfactants is going to conditioned their further applications, this property is not widely studied. Therefore, anionic surfactants have been reported to be more aggressive and irritant than cationic. The biosurfactant extracts under evaluation were nonionic or amphoteric. Usually amphoteric surfactants are widely used in mild formulations because they are more compatible not only with other components of the formulation but also with cell membranes.49,50 This is in consonance with the results obtained by Rodríguez-López et al.,24 observing that the biosurfactant extract was obtained from CSW and extracted with chloroform (BS1), and the one produced by L. pentosus (BS5) is not eliminate irritant, after analysis, the protocol established by ISO 11930:201 was followed.
Regarding the fatty acid analyses, the presence of C16 and C18 fatty acids in all the extracts was detected. In the case of BS1 and BS2, it was observed that the major fatty acids were C18:2 (representing 48.4 and 41.4% of the total fatty acids, respectively); whereas, the fatty acids contained in BS3 and BS4 were 61.5 and 62.4% C18, respectively, followed by a 38.5 and 37.6% C16. It is interesting to remark that these fatty acids are also present in corn oil, one of the sources used to obtain the biosurfactants.51 Moreover, the fatty acids found in these extracts are similar to the fatty acids found in other lipopeptide biosurfactants, like surfactin, fengycin, and iturin, which are composed also of C16 and C18 fatty acids.52−54 Regarding BS5, produced by L. pentosus, the presence of C16 and C18 fatty acids was also detected but to a lower extent, deduced by the lower % of C and the highest concentration of proteins (deduced by the higher % of N). The main fatty acid present in BS5 was C18:2, followed by C18:1 and C16 fatty acids. These results are in good agreement with previous studies.55 C16 fatty acids were also detected by Sharma et al.56 in a glycolipid biosurfactant produced by Enterococcus faecium. Furthermore, Sharma et al.57 reported the production of a glycolipid biosurfactant produced by Lactobacillus helveticus, also composed of C16 fatty acids.
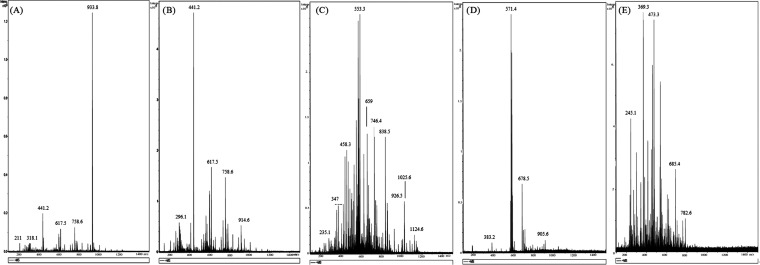
The biosurfactant extracts used in the study were also characterized using mass spectrometry. Figure 1 records the main biomarkers present in the biosurfactant samples. In the cases of BS1 (Figure 1A), BS2 (Figure 1B), BS3 (Figure 1C), and BS4 (Figure 1D), the signals detected were similar to those found in the literature for other lipopeptide biosurfactants like surfactin.22,29,34 Traditionally, m/z signals observed between 900 and 1200 Da, the same range as the biomarkers detected in the biosurfactant extracts in this study, BS1, BS2, BS3, and BS4, are associated with the presence of lipopeptide tensides.53,58−60 For instance, 933, 914, and 905 m/z signals were detected in BS1, BS2, and BS4, respectively, whereas, in BS3, the biomarkers observed within the range associated with lipopeptides were 1124, 1025, and 926 Da. The differences could be explained not only because of the extraction methodologies employed but also taking into account the culture media in each case. In this sense, it is usually to find variations in the m/z signals of lipopeptides produced by the same microorganisms when growth conditions are modified. Therefore, Bartal et al.,58 who studied the effect of different cultivation parameters on surfactin, determined that all the surfactin homologous possess a molecular weight within 993 and 1049 Da. Some authors also described other lipopeptides produced by B. subtilis, namely, Iturin and Fengycin, whose biomarkers were located around 1000 and 1400 Da, respectively.61 In addition, the presence of other signals in lipopeptide extracts is common in literature. For instance, Barbachano-Torres and collaborators62 described lipopeptides with biomarkers at 608, 776, 850, and 924 m/z obtained after several purification steps.
Figure 1.
ESI spectra of biosurfactant extracts in this study BS1 (A), BS2 (B), BS3 (C), BS4 (D), and BS5 (E).
In this sense, it is usual to find mass spectra of lipopeptide extracts with different molecular sizes, derived not only from the presence of biosurfactants but also from other secondary metabolites or molecules extracted from the culture media, such as antioxidants, phospholipids, or free fatty acids.41 In this regard, these secondary metabolites are usually associated with biomarkers with m/z below 400 Da. For instance, Rodríguez-López et al.41 identified signals of 219 m/z present in biosurfactant extracts derived from CSW, as a peptide chain composed of glutamic acid and alanine. Similarly, another biomarker, 317 m/z was described as a chain of glutamic acid/glutamine, glycine, and aspartic acid/asparagine. These two peptide chains could be compared with biomarkers within 318–211 m/z, as recorded in Figure 1. However, it is important to highlight that in the biosurfactant extract from CSW, the presence of free fatty acids was also detected, with m/z under 300 Da. Consequently, it can be speculated that some of these signals correspond to these lipidic molecules.
Relating to the values detected for BS5 (Figure 1E), the signals recorded were similar to those found in glycolipids or glycolipopeptides. Therefore, Dziwornu et al.63 isolated a glycolipid produced by Laurencia alfredensis, obtaining a molecular ion at m/z 793 after purification using HPLC. Other authors described a rhamnolipid produced by P. aeruginosa, recording m/z signals between 475 and 677. Some authors64 report that the differences between biomarkers can be related with the different sizes of the lipidic fraction. In general, the signals associated with glycolipids and glycolipopeptides are usually in the lower ranges in comparison to lipopeptides, although they can be derived from higher masses being more unstable, which have been broken during the primary ionization process producing multiple-charged ions.
3.2. Cytotoxicity
Nowadays, the exposure of humans to synthetic surfactants is widening. Consequently, the cases of people who present side effects have increased as well. In this regard, EU countries have declared it to be mandatory to evaluate the irritant potential and the cell damage caused by new products or ingredients for pharmaceuticals.65
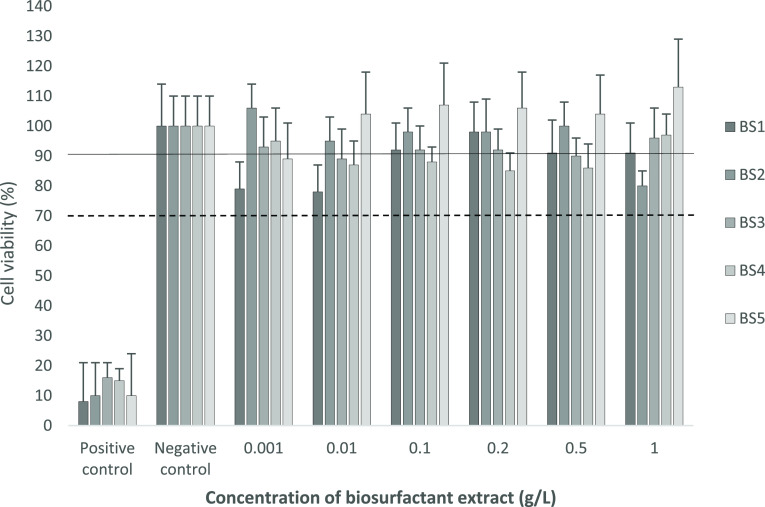
For this reason, the cytotoxicity evaluation of the biosurfactant extracts was carried out following the MTS assay suggested by the UNE-EN-ISO 10993-5:2009. Five biosurfactant extracts were tested in a wide concentration range from 0 to 1 g/L, achieving different viability percentages, as shown in Figure 2. All the experiments were compared to a negative control, which was the same medium without a biosurfactant extract (0 g/L), and a positive control, represented by phenol. The dotted line indicated the limit (70%) of cytotoxicity according to the UNE-EN-ISO 10993-5 standard. In Table 2, the equivalences between cellular viability and values in cytotoxicity scale were summarized, 0 being the lowest toxicity value and 4 the highest.
Figure 2.
Cell viability detected in mouse fibroblast cells (NCTC clone 929) in the presence of different concentrations (g/L) of BS1, BS2, BS3, BS4, and BS5, compared to the positive control (phenol) and negative control (DMEM, 0 g/L biosurfactant). Results were expressed in percentage compared to the negative control. The dotted line indicated the limit (70%) of cytotoxicity according to UNE-EN-ISO 10993-5 standard, whereas the solid line marked 90% of cell viability, indicating the mild cytotoxicity.
As it was reflected in Figure 2, the five biosurfactant extracts at the concentrations studied are above the cytotoxicity limit. Going into detail, BS1 presented cellular viability closer than the negative control for all the concentrations tested except for 0.001 and 0.01 g/L. It can be highlighted that all concentrations are above the cytotoxicity limit (dotted line), the cytotoxicity being at 1 g/L zero. These results are consistent with those previously obtained by Rodríguez-López et al.,24 in which it was reflected that the same extract studied in this work (BS1), at 1 g/L, has no eye irritant capacity in the chorioallantoic membrane (HET-CAM test), being perfectly suitable for their use in cosmetic products.
On the other hand, comparatively, for BS2, the results obtained were better than those of BS1, reaching identic or higher values for cell viability at all concentrations except for the maximum tested. In the same way, the cytotoxicity was 0 in all the concentrations tested with one exception, at 1 g/L. In this last case, at 1 g/L, the cytotoxicity was categorized as mild, obtaining a value of 1. Moreover, the cellular viability was lower than the negative control and BS1. This fact could be explained by the differences found in the biomarkers detected in BS1 and BS2 and by the lower C content of BS2, which is consistent with a lower amount of fatty acids in BS2 in comparison with BS1.
Similarly, BS3, with comparable physicochemical properties to BS1 and BS2, gave cytotoxicity values of 0 at all the concentrations tested except for 0.01 g/L. According to the results obtained, when BS3 was tested at 0.01 g/L it gave a cell viability of 89%, which is considered as a mild cytotoxic substance. However, at 1 g/L, the cell viability determined was 96%. On the other hand, BS4, despite the fact of being produced by the same microorganism as BS3, reflected a slightly higher cytotoxicity, although the values are not significant. In fact, in this particular case, at 0.001 and 1 g/L, the cytotoxicity was categorized as zero, whereas the other concentrations tested were classified as mild, with a value of 1.
These results should be pointed out, especially in comparison to those found in the literature (Table 4). For instance, Burgos-Díaz and collaborators26 tested two fractions of biosurfactant extracts produced by Sphingobacterium detergens, using the MTT methods. These two extracts consisted of a phospholipid fraction and a polar lipid mixture. Both portions showed a decrease in cell viability, reaching values below 25% in the case of phospholipids and 75% for the lipid mixture at concentrations of 1 g/L. Moreover, Basit et al.66 have found a lipopeptide produced by Bacillus cereus, which presented cell viability from 63 to 92% at concentrations tested from 10 to 5 g/L, respectively. However, in that case, the biosurfactants produced by B. cereus were tested on baby hamster kidney (BHK-21) cell lines, different than the fibroblast employed in this work. Other lipopeptides produced in this case by Bacillus stratosphericus FLU5 have been demonstrated to maintain the viability of human embryonic kidney cell line (HEK293) after 24 h of incubation in a 96% cell viability, at a concentration of the biosurfactant of 1 g/L.67
Table 4. Overview of the Effect of Biosurfactants on Cell Viability Reported in the Literature.
| biosurfactant | concentration (g/L) | cell viability (%) | references |
|---|---|---|---|
| BS1 | 1 | 91 | under study |
| BS2 | 1 | 80 | under study |
| BS3 | 1 | 96 | under study |
| BS4 | 1 | 97 | under study |
| BS5 | 1 | 113 | under study |
| lipopeptide from Bacillus cereus | 10 | 63 | 66 |
| lipopeptide from Bacillus stratosphericus | 1 | 96 | 67 |
| glycolipid from Enterococcus faecium | 6.25 | 90 | 56 |
| glycolipid from Cyberlindnera saturnus | 1 | 70 | 68 |
Related to the biosurfactant produced by L. pentosus, BS5, results have shown its positive effect on fibroblasts, achieving a 113% of cell viability at 1 g/L, although no significant differences were observed between the values of the different concentrations. This behavior was similar in all the concentrations tested, increasing the number of viable cells in comparison to the control. In fact, the percentage of viable cells decreased as long as the concentration decreased. As a consequence, it can be speculated that BS5 presents benefits for the growth of fibroblasts, being a potential growth factor. A similar behavior has been observed for other biosurfactants, particularly in the lipopeptides produced by Streptosporangium amethystogenes sub sp. fukuiense Al-23456. This biosurfactant showed a proliferative effect on bone marrow cells from BALB/c female mice.68 Other lipopeptides, like a type of surfactin produced by B. subtilis, have been proven to have a proliferative and a differentiation effect on mammalian cells.69 In the same way, as it occurred with BS1, these results are in concordance with previous works, in which the irritancy response of the biosurfactant extract produced by L. pentosus was negligible.24
In comparison to other biosurfactants produced by lactic acid bacteria, it has been found that the biosurfactant produced by E. faecium, characterized as a glycolipid, reduced the cell viability of fibroblasts up to 90% at 6.25 g/L. In that work, it was also compared to a commercial rhamnolipid, which showed a cell viability of 35.33%.56 In this sense, BS5 would have a more intense effect, due to the fact that at lower concentrations it was able to increase the cell concentration after 24 h. Moreover, a glycolipid produced by Cyberlindnera saturnus SBPN-27, named cybersan gave a negative effect on fibroblasts 3T3, reducing their viability up to 70% at 1 g/L.70
Although the biosurfactant extracts tested in this work (BS1, BS2, BS3, BS4, and BS5) are not the same type of molecules, the slight differences in their cytotoxicity could be caused not only by their structure but also because of the extraction process. It is important to remember that BS1, BS2, and BS3 are obtained through an organic solvent extraction, whereas BS4 and BS5 are extracted with a saline aqueous solution. Nevertheless, it is remarkable that the biosurfactant extracts under this study evidenced lower cytotoxicity against mammalian cells in comparison to other well-known lipopeptide biosurfactants such as surfactin or glycolipids.71−73
Statistical analysis performed at all concentrations of each biosurfactant show that there are significant differences (p ≤ 0.05) in all cases with respect to the cytotoxicity limit (70%) established by the UNE-EN-ISO10993-5. These observations confirm the biocompatibility of the biosurfactant extracts under this study, and their nontoxicity toward fibroblasts (NCTC clone 929). Furthermore, they represent an interesting alternative to synthetic surfactants, particularly in those areas such as cosmetics or pharmaceutics, in which the contact between the surface-active compound and the cells are unavoidable.
4. Conclusions
In the current work, five biosurfactant extracts, three of them secreted extracellularly and the other two obtained from the cell membrane of microorganisms (cell-bound biosurfactants) were characterized and evaluated in terms of cytotoxicity on fibroblasts (NCTC clone 929). It has been demonstrated that these five biosurfactant extracts at the concentrations studied are above the cytotoxicity limit according to the UNE-EN-ISO 10993-5 standard. Moreover, at 1 g/L, the biosurfactant extracts tested had no cytotoxicity. In this sense, although at lower concentrations, some of the samples evaluated gave mild toxicity, the least cell viability percentage achieved was 78%, detected for BS2 and still much higher than those reported in the literature with other biosurfactants. Furthermore, it was even detected that the biosurfactant extract produced by L. pentosus was able to increase the cell concentration, what could have interesting applications in skin regeneration for cosmetic or pharmaceutical purposes.
In general terms, this work proves the reliability of all the biosurfactants under study in cosmetic, pharmaceutical, or healthcare applications because their presence does not affect fibroblast (NCTC clone 929) growth.
Acknowledgments
This research was partially supported by the European Union projects 0245_IBEROS_1_E and 0302_CVMAR_I_1_P, both from Interreg (POCTEP 2015). Moreover, regional funds for Competitive Reference Groups (GRC) ED431C 2017_51, Research networks ED431D 2017/13 both from Xunta de Galicia (Spain). This study was also supported by the Spanish Ministry of Economy and Competitiveness (MINECO) under the project RTI-2018-093610-B-100. S.P.-D. is grateful for funding support from a Xunta de Galicia pre-doctoral grant (ED481A 2019/314). A.L.-P. also acknowledges to the University of Vigo for their pre-doctoral fellowships.
The authors declare no competing financial interest.
References
- Gharaei-Fa E. Biosurfactants in Pharmaceutical Industry: A Mini-Review. Am. J. Drug Discovery Dev. 2010, 1, 58–69. 10.3923/ajdd.2011.58.69. [DOI] [Google Scholar]
- López-Prieto A.; Rodríguez-López L.; Rincón-Fontán M.; Moldes A. B.; Cruz J. M. Effect of Biosurfactant Extract Obtained from the Corn-Milling Industry on Probiotic Bacteria in Drinkable Yogurt. J. Sci. Food Agric. 2019, 99, 824–830. 10.1002/jsfa.9251. [DOI] [PubMed] [Google Scholar]
- Rincón-Fontán M.; Rodríguez-López L.; Vecino X.; Cruz J. M.; Moldes A. B. Design and Characterization of Greener Sunscreen Formulations Based on Mica Powder and a Biosurfactant Extract. Powder Technol. 2018, 327, 442–448. 10.1016/j.powtec.2017.12.093. [DOI] [Google Scholar]
- Kosumi H.; Yanagi T.; Izumi K.; Ito T.; Shimizu H. Hair Colour Shampoo Dermatitis. Contact Dermatitis 2017, 77, 419–421. 10.1111/cod.12851. [DOI] [PubMed] [Google Scholar]
- Swak J. G.; Herbert K. L. Optical Damage and Recovery of the in Vitro Bovine Ocular Lens for Alcohols, Surfactants, Acetates, Ketones, Aromatics, and Some Consumer Products: A Review. J. Toxicol., Cutaneous Ocul. Toxicol. 1997, 16, 173–187. 10.3109/15569529709048894. [DOI] [Google Scholar]
- Warshaw E. M.; Goodier M. C.; DeKoven J. G.; Maibach H. I.; Taylor J. S.; Sasseville D.; Belsito D. V.; Fowler J. F.; Fransway A. F.; DeLeo V. A.; Marks J. G.; Pratt M. D.; Mathias T.; Zirwas M. J.; Zug K. A. Contact Dermatitis Associated with Skin Cleansers. Dermatitis 2018, 29, 32–42. 10.1097/DER.0000000000000330. [DOI] [PubMed] [Google Scholar]
- Mehling A.; Kleber M.; Hensen H. Comparative Studies on the Ocular and Dermal Irritation Potential of Surfactants. Food Chem. Toxicol. 2007, 45, 747–758. 10.1016/j.fct.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Rodríguez-López L.; Rincón-Fontán M.; Vecino X.; Cruz J. M.; Moldes A. B. Biological Surfactants vs. Polysorbates: Comparison of Their Emulsifier and Surfactant Properties. Tenside, Surfactants, Deterg. 2018, 55, 273–280. 10.3139/113.110574. [DOI] [Google Scholar]
- Paulino B. N.; Pessôa M. G.; Mano M. C. R.; Molina G.; Neri-Numa I. A.; Pastore G. M. Current Status in Biotechnological Production and Applications of Glycolipid Biosurfactants. Appl. Microbiol. Biotechnol. 2016, 100, 10265–10293. 10.1007/s00253-016-7980-z. [DOI] [PubMed] [Google Scholar]
- Rodríguez-López L.; Rincón-Fontán M.; Vecino X.; Moldes A. B.; Cruz J. M. Biodegradability Study of the Biosurfactant Contained in a Crude Extract from Corn Steep Water. J. Surfactants Deterg. 2020, 23, 79–90. 10.1002/jsde.12338. [DOI] [Google Scholar]
- De S.; Malik S.; Ghosh A.; Saha R.; Saha B. A Review on Natural Surfactants. RSC Adv. 2015, 5, 65757–65767. 10.1039/C5RA11101C. [DOI] [Google Scholar]
- Santos D.; Rufino R.; Luna J.; Santos V.; Sarubbo L. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. 10.3390/ijms17030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Armendáriz B.; Cal-y-Mayor-Luna C.; El-Kassis E. G.; Ortega-Martínez L. D. Use of Waste Canola Oil as a Low-Cost Substrate for Rhamnolipid Production Using Pseudomonas Aeruginosa. AMB Express 2019, 9, 61. 10.1186/s13568-019-0784-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostendorf T. A.; Silva I. A.; Converti A.; Sarubbo L. A. Production and Formulation of a New Low-Cost Biosurfactant to Remediate Oil-Contaminated Seawater. J. Biotechnol. 2019, 295, 71–79. 10.1016/j.jbiotec.2019.01.025. [DOI] [PubMed] [Google Scholar]
- Patowary K.; Das M.; Patowary R.; Kalita M. C.; Deka S. Recycling of Bakery Waste as an Alternative Carbon Source for Rhamnolipid Biosurfactant Production. J. Surfactants Deterg. 2019, 22, 373–384. 10.1002/jsde.12242. [DOI] [Google Scholar]
- Cortés-Camargo S.; Pérez-Rodríguez N.; Oliveira R. P. d. S.; Huerta B. E. B.; Domínguez J. M. Production of Biosurfactants from Vine-Trimming Shoots Using the Halotolerant Strain Bacillus Tequilensis ZSB10. Ind. Crops Prod. 2016, 79, 258–266. 10.1016/j.indcrop.2015.11.003. [DOI] [Google Scholar]
- Santos E. F.; Teixeira M. F. S.; Converti A.; Porto A. L. F.; Sarubbo L. A. Production of a New Lipoprotein Biosurfactant by Streptomyces Sp. DPUA1566 Isolated from Lichens Collected in the Brazilian Amazon Using Agroindustry Wastes. Biocatal. Agric. Biotechnol. 2019, 17, 142–150. 10.1016/j.bcab.2018.10.014. [DOI] [Google Scholar]
- Durval I. J. B.; Mendonça A. H. R.; Rocha I. V.; Luna J. M.; Rufino R. D.; Converti A.; Sarubbo L. A. Production, Characterization, Evaluation and Toxicity Assessment of a Bacillus Cereus UCP 1615 Biosurfactant for Marine Oil Spills Bioremediation. Mar. Pollut. Bull. 2020, 157, 111357. 10.1016/j.marpolbul.2020.111357. [DOI] [PubMed] [Google Scholar]
- Ferreira A.; Vecino X.; Ferreira D.; Cruz J. M.; Moldes A. B.; Rodrigues L. R. Novel Cosmetic Formulations Containing a Biosurfactant from Lactobacillus Paracasei. Colloids Surf., B 2017, 155, 522–529. 10.1016/J.COLSURFB.2017.04.026. [DOI] [PubMed] [Google Scholar]
- Lydon H. L.; Baccile N.; Callaghan B.; Marchant R.; Mitchell C. A.; Banat I. M. Adjuvant Antibiotic Activity of Acidic Sophorolipids with Potential for Facilitating Wound Healing. Antimicrob. Agents Chemother. 2017, 61, e02547 10.1128/AAC.02547-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-López L.; Shokry D. S.; Cruz J. M.; Moldes A. B.; Waters L. J. The Effect of the Presence of Biosurfactant on the Permeation of Pharmaceutical Compounds through Silicone Membrane. Colloids Surf., B 2019, 176, 456–461. 10.1016/j.colsurfb.2018.12.072. [DOI] [PubMed] [Google Scholar]
- Rincón-Fontán M.; Rodríguez-López L.; Vecino X.; Cruz J. M.; Moldes A. B. Influence of Micelle Formation on the Adsorption Capacity of a Biosurfactant Extracted from Corn on Dyed Hair. RSC Adv. 2017, 7, 16444–16452. 10.1039/C7RA01351E. [DOI] [Google Scholar]
- Rodríguez-López L.; Vecino X.; Barbosa-Pereira L.; Moldes A. B.; Cruz J. M. A Multifunctional Extract from Corn Steep Liquor: Antioxidant and Surfactant Activities. Food Funct. 2016, 7, 3724–3732. 10.1039/C6FO00979D. [DOI] [PubMed] [Google Scholar]
- Rodríguez-López L.; Rincón-Fontán M.; Vecino X.; Cruz J. M.; Moldes A. B. Preservative and Irritant Capacity of Biosurfactants from Different Sources: A Comparative Study. J. Pharm. Sci. 2019, 108, 2296. 10.1016/j.xphs.2019.02.010. [DOI] [PubMed] [Google Scholar]
- Vecino X.; Rodríguez-López L.; Ferreira D.; Cruz J. M.; Moldes A. B.; Rodrigues L. R. Bioactivity of Glycolipopeptide Cell-Bound Biosurfactants against Skin Pathogens. Int. J. Biol. Macromol. 2018, 109, 971–979. 10.1016/j.ijbiomac.2017.11.088. [DOI] [PubMed] [Google Scholar]
- Burgos-Díaz C.; Martín-Venegas R.; Martínez V.; Storniolo C. E.; Teruel J. A.; Aranda F. J.; Ortiz A.; Manresa Á.; Ferrer R.; Marqués A. M. Vitro Study of the Cytotoxicity and Antiproliferative Effects of Surfactants Produced by Sphingobacterium Detergens. Int. J. Pharm. 2013, 453, 433–440. 10.1016/J.IJPHARM.2013.06.029. [DOI] [PubMed] [Google Scholar]
- Patowary K.; Patowary R.; Kalita M. C.; Deka S. Characterization of Biosurfactant Produced during Degradation of Hydrocarbons Using Crude Oil as Sole Source of Carbon. Front. Microbiol. 2017, 8, 1–14. 10.3389/fmicb.2017.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecino X.; Barbosa-Pereira L.; Devesa-Rey R.; Cruz J. M.; Moldes A. B. Optimization of Liquid–Liquid Extraction of Biosurfactants from Corn Steep Liquor. Bioprocess Biosyst. Eng. 2015, 38, 1629–1637. 10.1007/s00449-015-1404-9. [DOI] [PubMed] [Google Scholar]
- López-Prieto A.; Martínez-Padrón H.; Rodríguez-López L.; Moldes A. B.; Cruz J. M. Isolation and Characterization of a Microorganism That Produces Biosurfactants in Corn Steep Water. CyTA--J. Food 2019, 17, 509–516. 10.1080/19476337.2019.1607909. [DOI] [Google Scholar]
- López-Prieto A.; Rodríguez-López L.; Rincón-Fontán M.; Cruz J. M.; Moldes A. B. Characterization of Extracellular and Cell Bound Biosurfactants Produced by Aneurinibacillus Aneurinilyticus Isolated from Commercial Corn Steep Liquor. Microbiol. Res. 2021, 242, 126614. 10.1016/j.micres.2020.126614. [DOI] [PubMed] [Google Scholar]
- Vecino X.; Barbosa-Pereira L.; Devesa-Rey R.; Cruz J. M.; Moldes A. B. Optimization of Extraction Conditions and Fatty Acid Characterization of Lactobacillus Pentosus Cell-Bound Biosurfactant/Bioemulsifier. J. Sci. Food Agric. 2015, 95, 313–320. 10.1002/jsfa.6720. [DOI] [PubMed] [Google Scholar]
- Moldes A. B.; Torrado A. M.; Barral M. T.; Domínguez J. M. Evaluation of Biosurfactant Production from Various Agricultural Residues by Lactobacillus Pentosus. J. Agric. Food Chem. 2007, 55, 4481–4486. 10.1021/jf063075g. [DOI] [PubMed] [Google Scholar]
- Lange R. K.Surfactants: A Practical Handbook; Lange K. R., Ed.; Hanser Gardner Publications, 1999. [Google Scholar]
- Rodríguez-López L.; Rincón-Fontán M.; Vecino X.; Cruz J. M.; Moldes A. Ionic Behavior Assessment of Surface-Active Compounds from Corn Steep Liquor by Exchange Resins. J. Surfactants Deterg. 2017, 20, 207–217. 10.1007/s11743-016-1897-5. [DOI] [Google Scholar]
- Mariotti F.; Tomé D.; Mirand P. P. Converting Nitrogen into Protein - Beyond 6.25 and Jones’ Factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. 10.1080/10408390701279749. [DOI] [PubMed] [Google Scholar]
- Dubois M.; Gilles K. A.; Hamilton J. K.; Rebers P. A.; Smith F. Colorimetric Method for the Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. 10.1021/ac60111a017. [DOI] [PubMed] [Google Scholar]
- Xian W.Module III: Biocompatibility Testing: Cytotoxicity and Adhesion. A Laboratory Course in Biomaterials; CRC Press-Taylor & Francis: Boca Raton, Florida, 2009; pp 99–128. [Google Scholar]
- UNE-EN ISO 10993-5:2009 Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity.
- Rincón-Fontán M.; Rodríguez-López L.; Vecino X.; Cruz J. M.; Moldes A. B. Study of the Synergic Effect between Mica and Biosurfactant to Stabilize Pickering Emulsions Containing Vitamin E Using a Triangular Design. J. Colloid Interface Sci. 2019, 537, 34–42. 10.1016/j.jcis.2018.10.106. [DOI] [PubMed] [Google Scholar]
- Rincón-Fontán M.; Rodríguez-López L.; Vecino X.; Cruz J. M.; Moldes A. B. Adsorption of Natural Surface Active Compounds Obtained from Corn on Human Hair. RSC Adv. 2016, 6, 63064–63070. 10.1039/C6RA13823C. [DOI] [Google Scholar]
- Rodríguez-López L.; Rincón-Fontán M.; Vecino X.; Cruz J. M.; Moldes A. B. Extraction, Separation and Characterization of Lipopeptides and Phospholipids from Corn Steep Water. Sep. Purif. Technol. 2020, 248, 117076. 10.1016/j.seppur.2020.117076. [DOI] [Google Scholar]
- Vecino X.; Rodríguez-López L.; Ferreira D.; Cruz J. M.; Moldes A. B.; Rodrigues L. R. Bioactivity of Glycolipopeptide Cell-Bound Biosurfactants against Skin Pathogens. Int. J. Biol. Macromol. 2018, 109, 971–979. 10.1016/j.ijbiomac.2017.11.088. [DOI] [PubMed] [Google Scholar]
- Cornea C. P.; Roming F. I.; Sicuia O. A.; Voaideş C.; Zamfir M.; Grosu-Tudor S. S. Biosurfactant Production by Lactobacillus Spp. Strains Isolated from Romanian Traditional Fermented Food Products. Rom. Biotechnol. Lett. 2016, 21, 11312–11320. [Google Scholar]
- Bustos G.; Arcos U.; Vecino X.; Cruz J. M.; Moldes A. B. Recycled Lactobacillus Pentosus Biomass Can Regenerate Biosurfactants after Various Fermentative and Extractive Cycles. Biochem. Eng. J. 2018, 132, 191–195. 10.1016/j.bej.2018.01.021. [DOI] [Google Scholar]
- Vecino X.; Barbosa-Pereira L.; Devesa-Rey R.; Cruz J. M.; Moldes A. B. Optimization of Extraction Conditions and Fatty Acid Characterization of Lactobacillus Pentosus Cell-Bound Biosurfactant/Bioemulsifier. J. Sci. Food Agric. 2015, 95, 313–320. 10.1002/jsfa.6720. [DOI] [PubMed] [Google Scholar]
- Moldes A. B.; Paradelo R.; Vecino X.; Cruz J. M.; Gudiña E.; Rodrigues L.; Teixeira J. A.; Domínguez J. M.; Barral M. T. Partial Characterization of Biosurfactant from Lactobacillus Pentosus and Comparison with Sodium Dodecyl Sulphate for the Bioremediation of Hydrocarbon Contaminated Soil. BioMed Res. Int. 2013, 2013, 1–6. 10.1155/2013/961842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudiña E. J.; Teixeira J. A.; Rodrigues L. R. Isolation and Functional Characterization of a Biosurfactant Produced by Lactobacillus Paracasei. Colloids Surf., B 2010, 76, 298–304. 10.1016/j.colsurfb.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Liu F.; Xiao J.; Garamus V. M.; Almásy L.; Willumeit R.; Mu B.; Zou A. Interaction of the Biosurfactant, Surfactin with Betaines in Aqueous Solution. Langmuir 2013, 29, 10648–10657. 10.1021/la400683u. [DOI] [PubMed] [Google Scholar]
- Oleochemicals Oils & Fats . Surfactants and Skin. International News on Fats, Oils, and Related Materials, 2016. [Google Scholar]
- Martínez-González M. I.; González-Pérez R.; García-Rio I.; Heras-González S. Allergic Contact Dermatitis Caused by Benzoic Acid and Lauryl Glucoside in a Sunscreen. Contact Dermatitis 2017, 77, 186–187. 10.1111/cod.12810. [DOI] [PubMed] [Google Scholar]
- Kim H.-S.; Kim Y.-B.; Lee B.-S.; Kim E.-K. Sophorolipid Production by Candida Bombicola ATCC 22214 from a Corn-Oil Processing Byproduct. J. Microbiol. Biotechnol. 2005, 15, 55–58. [Google Scholar]
- Vanittanakom N.; Loeffler W.; Koch U.; Jung G. Fengycin-A Novel Antifungal Lipopeptide Antibiotic Produced by Bacillus Subtilis F-29-3. J. Antibiot. 1986, 39, 888–901. 10.7164/antibiotics.39.888. [DOI] [PubMed] [Google Scholar]
- Gudiña E. J.; Fernandes E. C.; Rodrigues A. I.; Teixeira J. A.; Rodrigues L. R. Biosurfactant Production by Bacillus Subtilis Using Corn Steep Liquor as Culture Medium. Front. Microbiol. 2015, 6, 59. 10.3389/fmicb.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimkić I.; Stanković S.; Nišavić M.; Petković M.; Ristivojević P.; Fira D.; Berić T. The Profile and Antimicrobial Activity of Bacillus Lipopeptide Extracts of Five Potential Biocontrol Strains. Front. Microbiol. 2017, 8, 925. 10.3389/fmicb.2017.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecino X.; Barbosa-Pereira L.; Devesa-Rey R.; Cruz J. M.; Moldes A. B. Optimization of Extraction Conditions and Fatty Acid Characterization of Lactobacillus Pentosus Cell-Bound Biosurfactant/Bioemulsifier. J. Sci. Food Agric. 2015, 95, 313–320. 10.1002/jsfa.6720. [DOI] [PubMed] [Google Scholar]
- Sharma D.; Saharan B. S.; Chauhan N.; Procha S.; Lal S. Isolation and Functional Characterization of Novel Biosurfactant Produced by Enterococcus Faecium. Springerplus 2015, 4, 4. 10.1186/2193-1801-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D.; Saharan B. S.; Chauhan N.; Bansal A.; Procha S. Production and Structural Characterization of Lactobacillus Helveticus Derived Biosurfactant. Sci. World J. 2014, 2014, 493548. 10.1155/2014/493548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartal A.; Vigneshwari A.; Bóka B.; Vörös M.; Takács I.; Kredics L.; Manczinger L.; Varga M.; Vágvölgyi C.; Szekeres A. Effects of Different Cultivation Parameters on the Production of Surfactin Variants by a Bacillus Subtilis Strain. Molecules 2018, 23, 2675. 10.3390/molecules23102675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bóka B.; Manczinger L.; Kecskeméti A.; Chandrasekaran M.; Kadaikunnan S.; Alharbi N. S.; Vágvölgyi C.; Szekeres A. Ion Trap Mass Spectrometry of Surfactins Produced by Bacillus Subtilis SZMC6179J Reveals Novel Fragmentation Features of Cyclic Lipopeptides. Rapid Commun. Mass Spectrom. 2016, 30, 1581–1590. 10.1002/rcm.7592. [DOI] [PubMed] [Google Scholar]
- Pecci Y.; Rivardo F.; Martinotti M. G.; Allegrone G. LC/ESI-MS/MS Characterisation of Lipopeptide Biosurfactants Produced by the Bacillus Licheniformis V9T14 Strain. J. Mass Spectrom. 2010, 45, 772–778. 10.1002/jms.1767. [DOI] [PubMed] [Google Scholar]
- Farias B. C. S.; Hissa D. C.; do Nascimento C. T. M.; Oliveira S. A.; Zampieri D.; Eberlin M. N.; Migueleti D. L. S.; Martins L. F.; Sousa M. P.; Moyses D. N.; Melo V. M. M. Cyclic Lipopeptide Signature as Fingerprinting for the Screening of Halotolerant Bacillus Strains towards Microbial Enhanced Oil Recovery. Appl. Microbiol. Biotechnol. 2018, 102, 1179–1190. 10.1007/s00253-017-8675-9. [DOI] [PubMed] [Google Scholar]
- Barbachano-Torres A.; López-Ortega M. A.; Delgado-García M.; González-García Y.; Rodríguez J. A.; Kirchmayr M. R.; Camacho-Ruíz R. M. Production and Characterization of Surface-Active Lipopeptides by Haloalkaliphilic Bacteria Salibacterium Sp. 4CTb. J. Surfactants Deterg. 2020, 23, 67–78. 10.1002/jsde.12336. [DOI] [Google Scholar]
- Dziwornu G. A.; Caira M. R.; Mare J.-A. d. l.; Edkins A. L.; Bolton J. J.; Beukes D. R.; Sunassee S. N. Isolation, Characterization and Antiproliferative Activity of New Metabolites from the South African Endemic Red Algal Species Laurencia Alfredensis. Molecules 2017, 22, 513. 10.3390/molecules22040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabturani N.; Latif J.; Radiman S.; Hamzah A. Spectroscopic Analysis of Rhamnolipid Produced by Pseudomonas Aeruginosa UKMP14T. Malaysian J. Anal. Sci. 2016, 20, 31–43. 10.17576/mjas-2016-2001-04. [DOI] [Google Scholar]
- Benavides T.; Mitjans M.; Martínez V.; Clapés P.; Infante M. R.; Clothier R. H.; Vinardell M. P. Assessment of Primary Eye and Skin Irritants by in Vitro Cytotoxicity and Phototoxicity Models: An in Vitro Approach of New Arginine-Based Surfactant-Induced Irritation. Toxicology 2004, 197, 229–237. 10.1016/j.tox.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Basit M.; Hidayat Rasool M.; Ali Raza Naqvi S.; Waseem M.; Aslam B. Biosurfactants Production Potential of Native Strains of Bacillus Cereus and Their Antimicrobial, Cytotoxic and Antioxidant Activities. Pak. J. Pharm. Sci. 2018, 31, 251. [PubMed] [Google Scholar]
- Hentati D.; Chebbi A.; Hadrich F.; Frikha I.; Rabanal F.; Sayadi S.; Manresa A.; Chamkha M. Production, Characterization and Biotechnological Potential of Lipopeptide Biosurfactants from a Novel Marine Bacillus Stratosphericus Strain FLU5. Ecotoxicol. Environ. Saf. 2019, 167, 441–449. 10.1016/J.ECOENV.2018.10.036. [DOI] [PubMed] [Google Scholar]
- Cameotra S. S.; Makkar R. S. Recent Applications of Biosurfactants as Biological and Immunological Molecules. Curr. Opin. Microbiol. 2004, 7, 262–266. 10.1016/j.mib.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Rodrigues L.; Banat I. M.; Teixeira J.; Oliveira R. Biosurfactants: Potential Applications in Medicine. J. Antimicrob. Chemother. 2006, 57, 609–618. 10.1093/jac/dkl024. [DOI] [PubMed] [Google Scholar]
- Senthil Balan S.; Ganesh Kumar C.; Jayalakshmi S. Physicochemical, Structural and Biological Evaluation of Cybersan (Trigalactomargarate), a New Glycolipid Biosurfactant Produced by a Marine Yeast, Cyberlindnera Saturnus Strain SBPN-27. Process Biochem. 2019, 80, 171–180. 10.1016/J.PROCBIO.2019.02.005. [DOI] [Google Scholar]
- Hirata Y.; Ryu M.; Oda Y.; Igarashi K.; Nagatsuka A.; Furuta T.; Sugiura M. Novel Characteristics of Sophorolipids, Yeast Glycolipid Biosurfactants, as Biodegradable Low-Foaming Surfactants. J. Biosci. Bioeng. 2009, 108, 142–146. 10.1016/j.jbiosc.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Vollenbroich D.; Özel M.; Vater J.; Kamp R. M.; Pauli G. Mechanism of Inactivation of Enveloped Viruses by the Biosurfactant Surfactin from Bacillus Subtilis. Biologicals 1997, 25, 289–297. 10.1006/biol.1997.0099. [DOI] [PubMed] [Google Scholar]
- Stipcevic T.; Piljac T.; Isseroff R. Di-Rhamnolipid from Pseudomonas Aeruginosa Displays Differential Effects on Human Keratinocyte and Fibroblast Cultures [3]. J. Dermatol. Sci. 2005, 40, 141–143. 10.1016/j.jdermsci.2005.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]