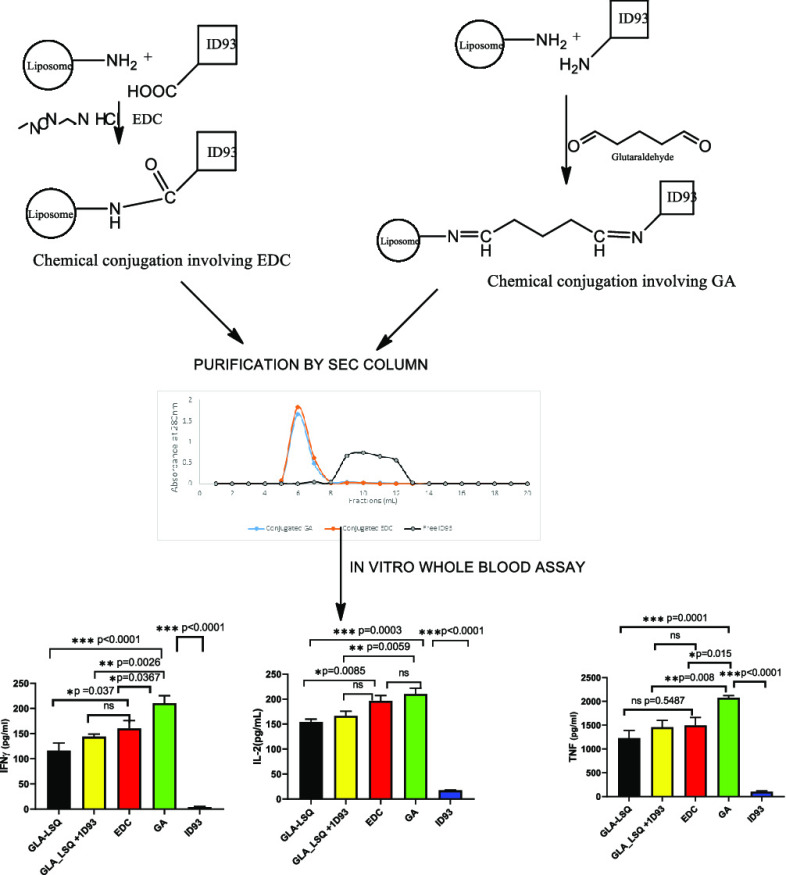
Abstract
Tuberculosis (TB) remains a foremost poverty-related disease with a high rate of mortality despite global immunization with Bacille Calmette–Guérin (BCG). Several adjuvanted recombinant proteins are in clinical development for TB to protect against the disease in infants and adults. Nevertheless, simple mixing of adjuvants with antigens may not be optimal for enhancing the immune response due to poor association. Hence, co-delivery of adjuvants with antigens has been advocated for improved immune response. This report, therefore, presents a strategy of using chemical conjugation to co-deliver an adjuvanted recombinant protein TB vaccine (ID93 + GLA-LSQ). Chemical conjugation involving glutaraldehyde (GA) or 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) was used to associate the antigen (ID93) to the modified liposome (mGLA-LSQ). The physicochemical stability of the formulations was evaluated using high-performance liquid chromatography (HPLC) (adjuvant content), dynamic light scattering (DLS, particle size analysis), and sodium dodecyl sulfate-polyacrylamide gel (SDS) electrophoresis (protein analysis). The bioactivity was assessed by cytokine stimulation using fresh whole blood from 10 healthy donors. The conjugates of ID93 + mGLA_LSQ maintained liposomal and protein integrity with the two protein chemistries. The GLA and QS21 content of the vaccine were also stable for 3 months. However, only the glutaraldehyde conjugates provoked significant secretion of interleukin-2 (210.4 ± 11.45 vs 166.7 ± 9.15; p = 0.0059), interferon-gamma (210.5 ± 14.79 vs 144.1 ± 4.997; p = 0.0011), and tumor necrosis factor alpha (2075 ± 46.8 vs 1456 ± 144.8; p = 0.0082) compared to simple mixing. Conjugation of recombinant protein (ID93) to the liposome (mGLA_LSQ) through chemical conjugation resulted in a stable vaccine formulation, which could facilitate co-delivery of the subunit vaccine to promote a robust immune response.
Introduction
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis. According to the 2019 WHO annual report, TB remains one of the top 10 causes of deaths worldwide, with an estimated 10.4 million cases worldwide in 2018.1 Six countries (India, Indonesia, China, Nigeria, Pakistan, and South Africa) accounted for 60% of the new cases, and there were also an estimated 480,000 new cases of multidrug-resistant TB (MDR-TB).
The Bacille Calmette–Guérin (BCG) vaccine, which was developed in 1921, is currently the only vaccine available for TB. BCG is a live, attenuated vaccine that is widely used to prevent severe disseminated disease in newborns and young children. Unfortunately, the vaccine is only partially effective: it provides some protection against severe forms of pediatric TB but is not completely protective against the disease in infants and fails to protect against pulmonary TB in adults.2,3 It is also not possible to “boost” the protection offered by initial BCG vaccination with a subsequent BCG shot later in life. High mortality and morbidity continue to be associated with M. tuberculosis infections despite the widespread use of BCG.3 Therefore, there is an urgent need for new, affordable, safe, and effective vaccines that can prevent all forms of TB, including drug-resistant strains. Such vaccines should, preferably, be suitable for all age groups, including those with HIV, and be protective in infancy and early childhood.1,2
As of the last count, there are 14 TB vaccine candidates in various stages of clinical development: 11 in phase II or phase III trials and 3 in phase I trials.1 These include recombinant BCGs, whole-cell derived vaccines, recombinant viral-vectored platforms, protein and adjuvant combinations, and mycobacterial extracts.1,3 They are developed to either prevent infection (pre-exposure) or primary progression to disease or reactivation of latent tuberculosis infection (LTBI).1
Subunit vaccines are generally highly purified recombinant protein or peptide antigens consisting of defined pathogenic4 units devoid of danger signals.5 These vaccines are poorly immunogenic and typically do not elicit responses that could confer protection on their own. They usually require an adjuvant to stimulate adaptive immune responses.6 Some of the vaccine candidates for the prevention of TB under clinical trials are recombinant proteins in combination with adjuvants.1 These include M72/AS01E (GSK and IAV),7 H56:IC31 (SSI, Valneva, and Aeras),8 and ID93/GLA-SE9 or GLA-LSQ (IDRI, Wellcome Trust, IAVI).
Adjuvant delivery systems such as liposomes facilitate vaccine delivery to antigen-presenting cells (APC), resulting in enhanced humoral and cell-mediated immunity to a broad spectrum of bacterial, protozoan, and viral antigens.10 However, in some cases, it has been observed that simply mixing adjuvants with the antigens can result in less optimal immune responses compared to compositions where the antigen and adjuvant are associated.11,12 Co-delivery of adjuvants with antigens using a suitable system has been shown to enable the adjuvants to stimulate more specific and potent immune response while also reducing off-target effects, thus potentially producing a safer vaccine.13 Co-delivery of antigens and liposomes or other adjuvants containing TLR agonists to APCs ensure that both molecules are co-localized to the identical endosome or phagosome within the same APC, which may enhance the antigen presentation and processing efficiency.14 Several antigen-adjuvant association strategies have been employed, including covalent conjugation, encapsulation, and entrapping antigens in lipid-based vesicles.13,14
ID93 is a recombinant antigen protein that composes of three M. tuberculosis immune-dominant antigens (Rv2608, Rv3619, and Rv3620) and one M. tuberculosis latency-associated antigen (Rv1813).15 In the presence of an appropriate adjuvant, the protein can stimulate Th1 immune responses known to confer protective immunity that is required for an effective TB vaccine.16 ID93 has been efficiently combined with either GLA-SE (emulsion) or GLA-LSQ (liposome) for the stimulation of high antibody production and Th1 immune responses that have been linked with protective immunity in TB.10,15,17 Both ID93 + GLA-SE and ID93 + GLA-LSQ have been evaluated in phase I/II clinical trials.9 The glucopyranosyl lipid A (GLA) adjuvant is a synthetic toll-like receptor (TLR) 4 ligand that is formulated as either a stable emulsion (GLA-SE)9 or as a liposomal formulation containing QS-21 saponin (GLA-LSQ).18 The current composition is composed of two vials, one containing the antigen (ID93) and one containing the adjuvant (GLA-SE or GLA-LSQ), which are “bedside-mixed” immediately before immunization.
In the present study, we hypothesized that chemical conjugation of ID93 to the surface of GLA-LSQ using chemical conjugation could facilitate the co-delivery of the antigen and adjuvants, thereby improving bioactivity. We explored the use of two chemical covalent conjugation approaches bearing in mind that conjugation chemistry could also affect the immune response. The stimulation of memory T-cell cytokine recall responses was performed based on a previously published in vitro method using whole blood from humans presumed to have been previously immunized with BCG.19 The stimulation of the three cytokines,20 interferon-gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin-2 by the conjugates, were evaluated using the in vitro human whole blood assay. The physicochemical stability of the formulations was also assessed for approximately 3 months.
Results
Incorporation of DOPE into the GLA-LSQ
The GLA-LSQ configuration was successfully altered to include the phospholipid dioleoylphosphatidylethanolamine (DOPE) to provide the amino group for covalent conjugation by combining dipalmitoylphosphatidylcholine (DOPC), cholesterol, and DOPE in a molar ratio of 3:2:1. The manual preparation using the sonication method gave a single-phase translucent formulation with an average particle size of 100.3 ± 0.1 nm within 2 h of sonication at 60 °C and was stable for a minimum of 6 months. However, volumes greater than 20 mL required an extended sonication time and were not stable for more than a week. On the other hand, by using a Microfluidics processor, it was possible to manufacture a bulk volume of liposome with a particle size of 70.6 ± 0.5 nm and also maintain liposomal integrity based on particle size when stored at 2–8 °C.
Conjugation of the Protein (ID93) to the Liposome
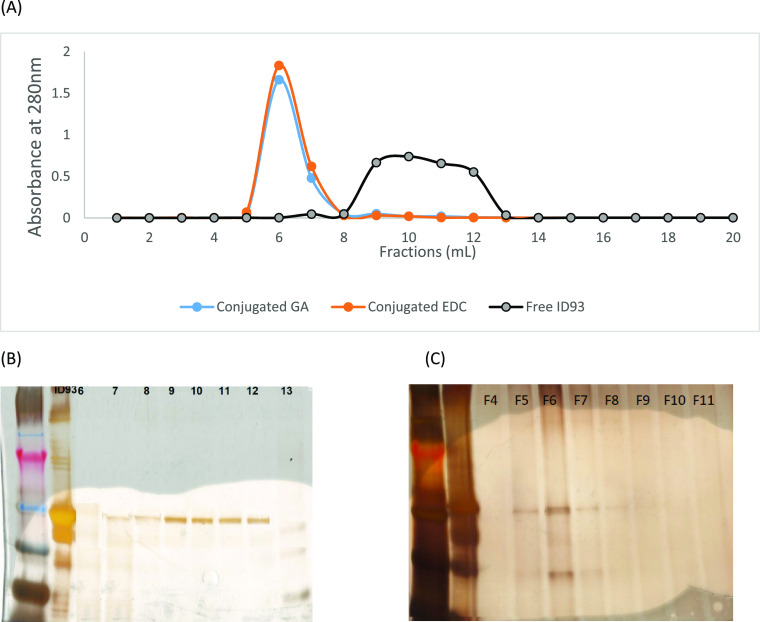
The conjugated liposome was eluted on an SEC column packed with Sepharose CL-4B in fractions between 5 and 7 mL, while free ID93 was eluted in fractions between 9 and 12 mL (Figure 1). The conjugation efficiency of the two methods (Table 1) showed that there was no significant difference in the mean percentage of conjugated ID93 (EDC vs GA − 92.0 ± 0.1 vs 90.0 ± 1.0; p = 0.15). The pH of the EDC conjugates (pH = 6.3) was lower than that of the GA conjugates (pH = 7.23). Following conjugation, the particle size of the EDC conjugate liposomal formulation increased by 10.6% while that of the GA increased by 13.6%.
Figure 1.
Characterization of the conjugates using size exclusion gel chromatography (SEC), SDS-PAGE, and UV analysis. (A) Plots of absorbance measured at 280 nm of the eluted fractions from the SEC column packed with Sepharose CL-4B for conjugated liposome GA (glutaraldehyde), EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride) and unconjugated or free ID93. (B) Typical SDS-PAGE gel of the fractions from the SEC column when ID93 alone was loaded. (C) Typical SDS-PAGE gel of the fractions from the SEC column when conjugated liposome was loaded on the column.
Table 1. GLA and QS-21 Contents of EDC and GA Conjugates at T0.
| EDC conjugates | GA conjugates | |
|---|---|---|
| ID93 conjugation efficiencya | 92.0 ± 0.1% | 90.0 ± 1.0% |
| GLA contentb | 35.8 ± 1.1 μg/mL | 36.8 ± 0.9 μg/mL |
| QS21b | 29.0 ± 0.8 μg/mL | 30.9 ± 3.1 μg/mL |
The data obtained from the UV analysis.
The data from the HPLC-ELSD data for GLA and QS21.
Physicochemical Stability of the Conjugates
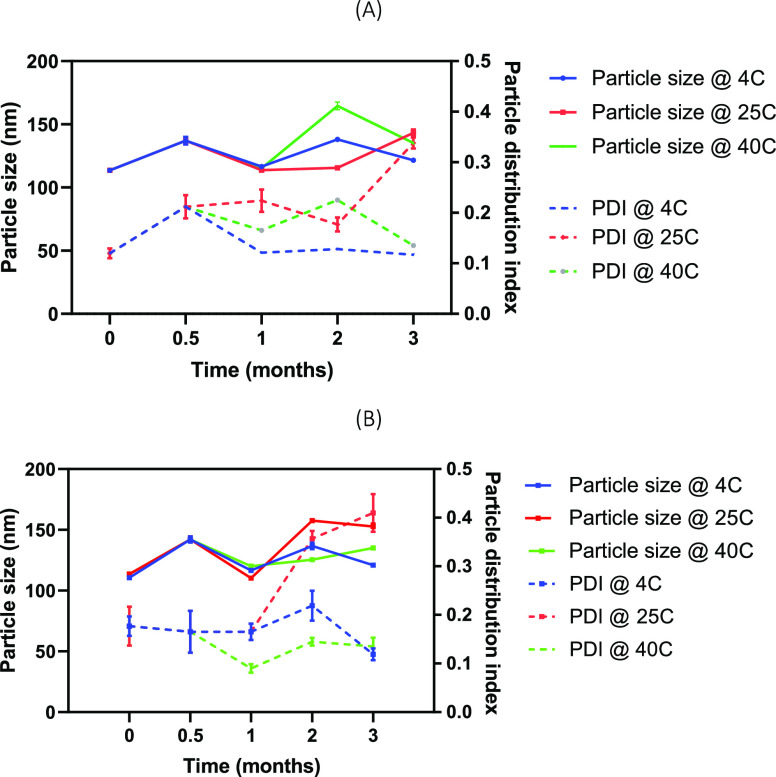
The data from the HPLC-ELSD analysis indicated that the change in the GLA and QS-21 content of both EDC and GA formulations was <20% when observed at 4 °C for 2 months. As presented in Table 2, the GLA content was reduced by 15.9 and 11.7% in EDC and GA formulations, whereas 4.1 and 3.2% reductions were observed for QS-21 in EDC and GA formulations, respectively. The osmolality data of the two formulations monitored over 3 months at three different temperatures (4, 25, and 40 °C), as shown in Table 3, indicate that there were no appreciable changes in the osmolality across the temperature. However, the osmolality of EDC formulation was a little higher than that of the GA formulation. As shown in Figure 2, there was <20% growth in the particle size and PDI of the two formulations when stored at 4 °C for 3 months, whereas the particle size grew 25–40% at 25 and 40 °C with somewhat higher growth in the EDC formulation.
Table 2. Stability of the GLA and QS-21 Content in the Conjugates Stored at 4 °C for 2 Months.
| parameters | GA (μg/mL) | EDC (μg/mL) |
|---|---|---|
| GLA contenta | ||
| day 0 | 36.8 ± 0.9 | 35.8 ± 1.1 |
| 0.5 months | 35.1 ± 0.1 | 31.6 ± 0.6 |
| 1 month | 33.2 ± 1.0 | 30.5 ± 1.2 |
| 2 months | 32.5 ± 0.1 | 30.1 ± 1.2 |
| QS-21a | ||
| day 0 | 30.9 ± 3.1 | 29.0 ± 0.8 |
| 0.5 months | 30.9 ± 1.2 | 28 .0 ± 1.8 |
| 1 month | 31.1 ± 1.7 | 28.5 ± 2.2 |
| 2 months | 29.9 ± 1.2 | 27.8 ± 1.9 |
The data from the HPLC-ELSD determinations for the GLA and QS21 content in the formulations.
Table 3. Osmolality of the Conjugates at Different Temperature for 3 Months.
| 4 °C |
25 °C |
40 °C |
||||
|---|---|---|---|---|---|---|
| parameters | GA | EDC | GA | EDC | GA | EDC |
| osmolality | ||||||
| 0 month | 96.3 ± 6.5 | 156.7 ± 1.2 | 96.3 ± 6.5 | 156.7 ± 1.2 | 96.3 ± 6.5 | 156.7 ± 1.2 |
| 1 month | 96.0 ± 6.6 | 163.7 ± 1.5 | 106.0 ± 7.9 | 161.3 ± 5.5 | 102.3 ± 5.5 | 158.3 ± 1.5 |
| 2 months | 99.0 ± 2.7 | 160.7 ± 2.3 | 102.3 ± 3.2 | 156.0 ± 1.7 | 93.7 ± 2.1 | 167.7 ± 2.5 |
| 3 months | 107.7 ± 5.7 | 164.7 ± 0.6 | 104.0 ± 3.6 | 164.3 ± 2.1 | 101.0 ± 2.6 | 168.0 ± 3.5 |
Figure 2.
Particle size and particle distribution index of the (A) GA conjugate and (B) EDC conjugate at 4, 25, and 40 °C indicative of the conjugates at accelerated temperature.
Biological Activity Using Whole Blood Assay
The assays for three cytokines were linear in the concentration range of 0–7500 (IFN-γ), 0–1500 (IL-2), and 0–6000 pg/mL (TNFα) with R2 > 0.99. When the IFN-γ production was compared across the tested formulations, as shown in Figure 3, the GA conjugate formulation was observed to stimulate a significantly higher amount of IFN-γ than the same amount of mGLA-LSQ + ID93 (210.5 ± 46.8 pg/mL vs 144.1 ± 15.8 pg/mL; p = 0.0026). However, there was no significant difference in the amount produced by EDC conjugates when compared to the mixed vaccine (160.2 ± 49.7 pg/mL vs 144.1 ± 15.8 pg/mL; p = 0.873). In addition, there was a significant difference between the GA and EDC conjugates in stimulation of IFN-γ production (210.5 ± 46.8 pg/mL vs 160.2 ± 49.7 pg/mL; p = 0.0367). On the other hand, IL-2 production by the EDC conjugate was not significantly different from the mixed formulation (196.6 ± 33.44 pg/mL vs 166.97 ± 28.93 pg/mL; p = 0.1107), whereas there was a significant difference between the GA conjugate and the mixed vaccine (210.4 ± 36.53 pg/mL vs 166.97 ± 28.93 pg/mL; p = 0.0059). There was no statistically significant difference between GA and EDC conjugates in terms of IL-2 production (210.4 ± 36.53 pg/mL vs 196.6 ± 33.44 pg/mL; p = 0.7762).
Figure 3.
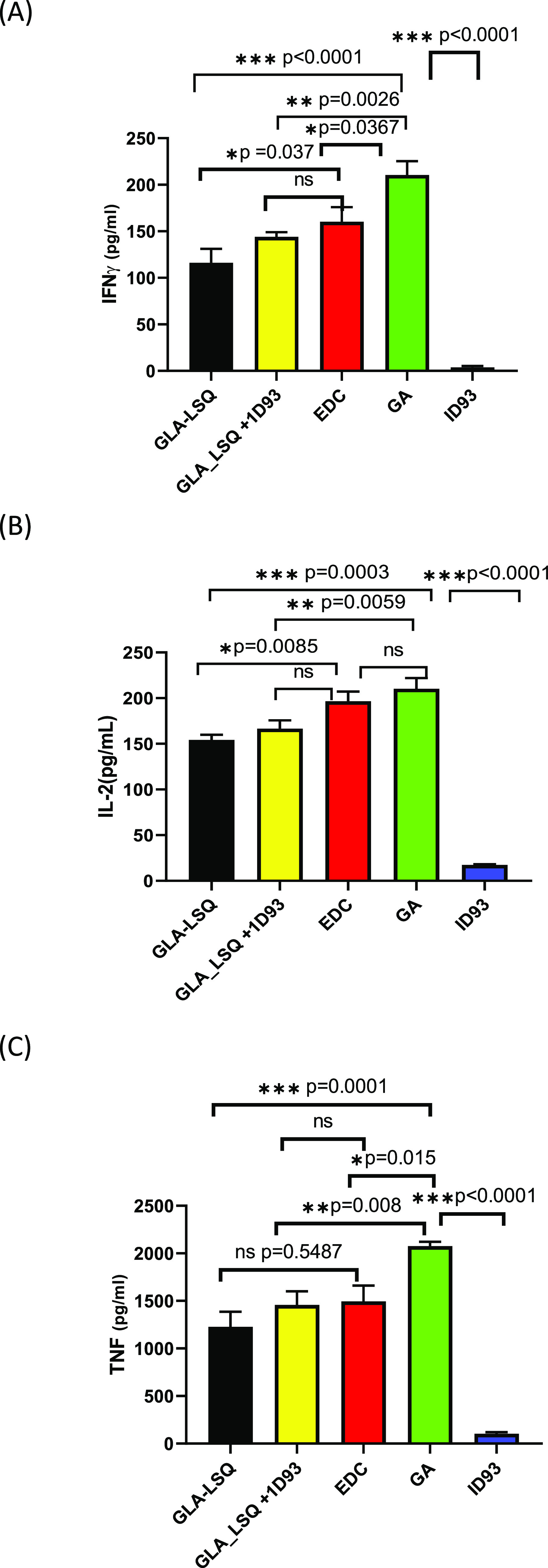
Immunological response after stimulation of the whole blood of 10 healthy volunteers with formulations. The measurements of (A) interferon-gamma (IFN-γ), (B) interleukin-2 (IL-2), and (C) tumor necrosis factor (TNF-α) were determined using ELISA plates read at 450 nm.
For the stimulation of TNFα by the formulations, it was observed that GA conjugates produced a significantly higher TNFα than the vaccine mixture (2075 ± 148.0 pg/mL vs 1456 ± 458.1 pg/mL; p = 0.0082) and also than the EDC conjugates (2075 ± 148.0 pg/mL vs 1495 ± 527.6 pg/mL; p = 0.015). However, there was no difference between the EDC conjugates’ TNF production when compared to the mixed vaccine (1495 ± 527.6 pg/mL vs 1456 ± 458.1 pg/mL; p = 0.9994).
Discussion
Adjuvants containing GLA and QS-21 have been shown both at preclinical and clinical studies to synergistically induce both innate immune response and antigen-specific Th1 cellular immunity.7,10,21 When co-administered with the vaccine antigen ID93, GLA_SE has also been demonstrated to reduce the bacterial burden of M. tuberculosis in the lung and spleen, thereby preventing extensive lung pathology.20 In this study, ID93 was covalently attached to a modified GLA-LSQ, and the formulation demonstrated a superior stimulation of Th1-type cytokine recall responses.
For a successful covalent conjugation, the assemblage, composition, and correct ratio of the lipid constituents of the liposome as well as the size and nature of the outer surface group of the liposome are critical.10,22 In this study, to facilitate the conjugation using GA and EDC, DOPE (to provide amino group) was introduced into the composition of GLA-LSQ formulation. Incorporation of DOPE into the liposome is challenging because DOPE is a non-bilayer prone lipid with the cross-sectional area of the head group being smaller than that of the acyl chain. Therefore, in solution, they tend to form an aggregate of negative curvature structures.23 However, with a combination of DOPE with DOPC (a bilayer prone lipid) in a molar ratio of 1:3, a stable formulation of modified liposome (mGLA-LSQ) was formed. This formulation had a comparable particle size and polydispersity index of unmodified GLA-LSQ. The concentration of the liposomal contents (GLA and QS-21) and particle size was also maintained for a minimum of 6 months at 4 °C.
Various conjugation chemistries have been described in the literature for coupling of protein molecules to liposomes.22 These include homo- or heterobifunctional cross-linkers with varying spacer lengths that can create linkages between amine, carboxyl, and sulfhydryl groups. In this study, glutaraldehyde (a homobifunctional cross-linking agent) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) (a heterobifunctional cross-linker) were used to attach ID93 protein to mGLA-LSQ. Glutaraldehyde was used for covalent linkage of the amino group on the protein to the amino group on the liposome surfaces. A two-step dialysis method with GA was used to avoid vesicle aggregation and protein polymerization, as observed in previous reports using a one-step reaction or two steps with direct interaction with GA.24 This method yielded an excellent conjugation efficiency and did not affect the integrity of the protein or that of the liposomes since there was minimal growth in the particle size after conjugation. The nature and content of the active constituents of the formulation, i.e., GLA and QS-21, were also maintained. Heterobifunctional cross-linking chemistry based on the coupling of the carboxyl group on the protein to the amino group on the liposome was also explored using EDC.25 In this study, successful conjugation using EDC was observed to be dependent on the EDC concentration. EDC concentration higher than 200 μg/mL of the reaction mixture caused degradation of the protein. This observation was in agreement with earlier reports that excess EDC can cause degradation of protein.26 There was no significant difference in the two conjugation chemistries when the conjugation efficiency, integrity of protein, and formulation stability at 4 °C were compared. This is important for taking the formulation forward since conjugation chemistry has been shown to influence the characteristics of macromolecular structures formed and also the immunogenicity of the conjugate.27
Several studies have shown that covalent antigen conjugation results in superior antibody induction,5,27−29 which is not unanticipated since B-cell receptors can identify intact antigen on the adjuvant’s (liposome) surface.28 Moreover, antigens coupled to the surface of liposomes consisting of unsaturated fatty acids have also been reported to be pinocytosed by APCs, loaded onto the class I MHC processing pathway, and presented to both CD4+ and CD8+ T cells.30,31 Thus, adjuvant-coupled antigens are anticipated to be appropriate for the development of vaccines that induce humoral and cellular immunity.31 The T-cell-dependent manner in which conjugate vaccines have been described to work could significantly boost immunogenicity compared to unconjugated antigens.32
In this study, as seen from the results, both conjugation methods consistently produced enhanced stimulation of T-cell recall responses. However, the responses with the GA conjugate were statistically significant. The difference in the biological activity of the two conjugates shows that comparable conjugation efficiency and stability of the conjugates may not translate to equivalent biological activity. This indicates that some other factors such as the quantity and quality of T-cell epitopes provided by the conjugates can influence the immune response stimulated by the conjugate. The study also demonstrates that selective use of GA could produce a conjugate with enhanced immunogenic response despite the shortcomings that have been associated with conjugation using glutaraldehyde.
Many new vaccine candidates with adjuvants are presented in separate vials to enable bedside mixing of the antigen with the adjuvant. However, this results in the potential for mixing errors and added cost. Effective conjugation of the antigen to the adjuvant could enable the presentation of the vaccine in one vial and therefore prevent wastage and mixing errors. Thus, apart from enhanced immunogenicity, the stability of the formulation at 4 °C indicates the potential to change the configuration of the ID93 + GLA-LSQ vaccine to a single vial.
In conclusion, controlled conjugation of ID93 to the liposome produced an enhanced memory T-cell cytokine recall response with GA in a whole blood in vitro assay, whereas EDC conjugates did not enhance bioactivity. The conjugated formulations are physicochemically stable, and the integrity of the protein can be maintained for at least 3 months when stored at 4 °C, although there was limited loss in the GLA content over this time. This approach also presents the opportunity of having a one-vial formulation to avoid dispensing errors or wastage. However, there is a need to extend stability monitoring and evaluate bioactivity to validate the findings of this study in in vivo animal models and human studies.
Experimental Procedure
Formulation of Liposome for Conjugation
Liposomes were prepared by combining DOPC, cholesterol, and DOPE at different molar ratios in chloroform. The final GLA concentration of the liposomal formulation was 0.1 mg/mL. The chloroform was evaporated using a Genevac EZ-2 centrifugal evaporator until a film was formed. A hydrating solution, phosphate-buffered saline (pH 7.2) containing 0.04 mg/mL of QS21, was added to the film. It was sonicated at ∼60 °C with a Crest Powersonic CP230D (Trenton, NJ) water bath ultrasonicator for approximately 2 h or until it appeared to be a single-phase, translucent formulation. For a larger volume, >20 mL, the solution was homogenized for 5 min at 3500 rpm and microfluidized using a Microfluidics M110P (Newton, MA) for five passes at 20,000 psi and 10 °C. QS21 dissolved in phosphate-buffered saline was added to the microfluidized solution to have final solutions containing 0.04 mg/mL of QS21 and 0.1 mg/mL of GLA.
Conjugation of ID93 to GLA-LSQ Liposome
Conjugation of ID93 to Liposome Using 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC)
In this study, the modified method used by Bartczak and Kanaras33 was adopted. mGLA-LSQ was mixed with ID93 (200 μg/mL) in a reaction vessel containing 200 μg of EDC/mL of the reaction mixture. The mixture was then vortex-mixed for 30 s and incubated at room temperature for 2 h. The resulting conjugates were purified using size exclusion gel chromatography (SEC). Phosphate-buffered saline (pH 7.2) was used as a mobile phase on an SEC column packed with Sepharose CL-4B (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). ID93 solution without an adjuvant, ID93 mixed with GLA-LSQ, and conjugates were introduced onto the SEC column and eluted with PBS. PBS (20 mL) was used, and 1 mL of fractions was collected. The column fractions were also monitored using SDS electrophoresis with ProteoSilver as the staining kit, according to the manufacturer’s protocol. The efficiency of the conjugation process was determined by measuring the amount of free ID93 in the pooled fractions containing free ID93 using a validated UV method at 280 nm.
Conjugation of ID93 to Liposome Using 25% Glutaraldehyde
A method earlier described by Zegers et al.24 for controlled conjugation using dialyzed GA was modified for this study. mGLA-LSQ (2 mL) was dispensed into a dialysis cassette (Slide-A-Lyzer, Thermo Scientific, UK) and dialyzed against 200 mL of 0.2% of 25% glutaraldehyde (1600 μL of fresh 25% glutaraldehyde in 200 mL of PBS) for 20 h at 4 °C. The activated liposome was dialyzed against 100 mL of PBS three times for several hours to remove excess GA. The activated liposome was transferred to the reaction vessel and 800 μg of ID93 in 1000 μL of PBS and incubated for 20 h at 4 °C. Tris HCL (600 μL, pH 7.2) was added to the mixture to get rid of excess glutaraldehyde, and the mixture was incubated at room temperature for 2 h. The characterization of the conjugates and efficiency of the reaction was determined, as described above for EDC.
Physicochemical Stability of the Antigen-Conjugated Liposomes
The physicochemical stability of the antigen-conjugated liposomes was assessed at zero time and after 1 and 3 months. The particle size, visual appearance, pH, and osmolality of the samples stored at 4, 25 and 40 °C were examined at above-mentioned time points, while GLA (HPLC-CAD/ELSD) and QS21 (HPLC-CAD) concentration and ID93 integrity (SDS-PAGE) of the sample stored at 4 °C were determined at 0, 0.5, 1, and 2 months.
Particle Size Determination
The particle mean hydrodynamic diameter (Z-average diameter) and polydispersity index (PDI) of the liposome and conjugated liposome-antigen were measured by dynamic light scattering (DLS) at a 90° angle using a Zetasizer Nano-S (Malvern Instruments, Worcestershire, UK). The formulations were prepared at 1:100 dilutions using ultrapure water in a 1.5 mL polystyrene disposable cuvette. The measurements were taken in triplicates.
GLA Quantitation by Reversed-Phase HPLC
The concentration of GLA was determined using a modified high-performance liquid chromatography (HPLC) method, as described by Kramer et al.34 The chromatographic system consisting of S600 series liquid chromatography (Skyam, Germany) was fitted with a quaternary pump, autoinjector, and evaporative light scattering detector (ELSD). Chromatographic separation was achieved at 25 °C on a reverse-phase Agilent Zorbax XDB C-18 column (5 μm, 150 × 4.6 mm i.d.; Agilent Technologies, Palm Alto, USA) at a flow rate of 1 mL/min. Gradient elution was adopted within 25 min with 100% mobile phase A (10 mM ammonium acetate and 1% acetic acid in methanol: chloroform and water (65:30:5)) and mobile phase B (10 mM ammonium acetate and 1% acetic acid in methanol: chloroform and isopropanol (50:30:20)). The effluents were monitored with an ELSD detector nebulized with nitrogen gas at a temperature of 70 °C and a pressure of 3.5 bar. The injection volume was 100 μL. Under the conditions described, a plot standard peak area response versus GLA concentration was used to generate a standard curve in the concentration range of 5–25 μg/mL. Triplicate samples of the conjugated liposomes were diluted 1:50 in mobile phase B, and 100 μL of the sample was injected onto the C-18 column.
QS21 Quantitation by Reversed-Phase HPLC
The concentration of QS21 was determined using a validated high-performance liquid chromatography (HPLC) method. The chromatographic system consisting of S600 series liquid chromatography (Skyam, Germany) was fitted with a quaternary pump, autoinjector, and evaporative light scattering detector. Chromatographic separation was achieved at 25 °C on a reverse-phase Alltech Vydac 214TP54 C4 column, 4.6 × 250 mm, 5 μm at a flow rate of 1 mL/min. Gradient elution was adopted with mobile phase A (water with 0.1% trifluoroacetic acid) and mobile phase B (acetonitrile with 0.1% trifluoroacetic acid). The effluents were monitored with an ELSD detector nebulized with nitrogen gas at a temperature of 80 °C and a pressure of 3.5 bar. The injection volume was 100 μL. Under the conditions described, a plot standard peak area response versus QS21 concentration was used to generate a standard curve in the concentration range of 5–30 μg/mL. Triplicate samples of the conjugated liposomes were diluted 1:50 in mobile phase B, and 100 μL of the sample was injected onto the column. The concentration of QS21 was calculated from the standard curve equation.
Biological Activity of the Antigen-Conjugated Liposomes Using Human Whole Blood Assay
The ethics committee of the Institute of Public Health, Obafemi Awolowo University, approved the study. Ten healthy adults with no history of exposure to active TB were enrolled, and all the subjects were provided a written informed consent before participation. The subjects recruited have prior exposure to BCG vaccines or tuberculin skin test (TST) and did not have any exposure to any anti-infective drugs in the last 2 months before participation. Pregnant women or lactating mothers and TB patients were excluded from this study. Blood samples were collected into heparinized blood collection tubes and processed within 2 h after collection. The whole venous blood was diluted 10-fold with a serum-free complete synthetic cell culture medium. The formulations (conjugates, ID93, mixture of ID93 + mGLA-LSQ, mGLA-LSQ,) and controls (serum-free media alone, negative control; 10 μg/mL of phytohemagglutinin, positive control) were prepared using serum-free media as the diluent. Each sample (100 μL) was dispensed per well in U-bottom 96-well tissue culture plates. Each sample was dispensed into at least six wells, and 100 μL of a 10-fold diluted fresh blood sample was added to each sample well. The mixture was incubated at 37 °C for 12 days in a 5% CO2 humidified incubator. The cultured plate was removed from the incubator at the end of the 12th day and centrifuged at 1100 rpm (218g) for 1 min. The supernatants were pooled together for each sample and stored at −80 °C until the day of analysis. IFN-γ, IL-2, and TNFα enzyme-linked immunosorbent assays (ELISAs) were conducted using ELISA kits (RayBiotech USA) following the manufacturer’s instructions. ELISA plates were read at 450 nm using a Multiskan FC microplate photometer (Thermo Scientific, China).
Data Analysis and Statistical Analysis
IFN-γ, IL-2, and TNFα ELISA data were analyzed using Skanlt software 5.0.0.42 (Thermo Scientific). The background production of IFN-γ, IL-2, and TNFα for each individual was determined by calculating the average concentration of the negative control, and these were subtracted from the formulation-stimulated wells. Statistical analysis was done using GraphPad Prism 8.0.1. One-way analysis of variance (ANOVA) was performed, and Tukey’s multiple comparison test with a single pooled variance was performed. The p values <0.05 were considered significant.
Acknowledgments
An EDCTP career development grant (TMA2017CDF-1860) to B.A.A. financially supported this study.
The authors declare no competing financial interest.
References
- WHO . WHO | Global Tuberculosis Report 2019, 2020. 1037//0033-2909.I26.1.78 [Google Scholar]
- World Health Organization . WHO consolidated guidelines on drug-resistant tuberculosis treatment. WHO Consol Guidel drug-resistant Tuberc Treat, 2019. [PubMed]
- Andersen P.; Kaufmann S. H. E. Novel vaccination strategies against tuberculosis. Cold Spring Harb Perspect Med. 2014, 4, a018523 10.1101/cshperspect.a018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle P. M. Progress in vaccine development. Curr Protoc Microbiol. 2015, 36, 18.1.1–18.1.26. [DOI] [PubMed] [Google Scholar]
- Moyle P. M.; Toth I. Modern Subunit Vaccines: Development, Components, and Research Opportunities. ChemMedChem 2013, 8, 360–376. 10.1002/cmdc.201200487. [DOI] [PubMed] [Google Scholar]
- Sivakumar S. M.; Safhi M. M.; Kannadasan M.; Sukumaran N. Vaccine adjuvants - Current status and prospects on controlled release adjuvancity. Saudi Pharm J. 2011, 4, 197–206. 10.1016/j.jsps.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenhoff T. H. M.; Tait D. R.; Van Der Meeren O.; Hatherill M. A trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med. 2020, 381, 2429–2439. 10.1056/NEJMc2001364. [DOI] [PubMed] [Google Scholar]
- Suliman S.; Luabeya A. K. K.; Geldenhuys H.; Tameris M.; Hoff S. T.; Shi Z.; Tait D.; Kromann I.; Ruhwald M.; Rutkowski K. T.; Shepherd B.; et al. Dose optimization of H56:IC31 vaccine for tuberculosis-endemic populations a double-blind, placebo-controlled, dose-selection trial. Am J Respir Crit Care Med. 2019, 99, 220–231. 10.1164/rccm.201802-0366OC. [DOI] [PubMed] [Google Scholar]
- Penn-Nicholson A.; Tameris M.; Smit E.; Day T. A.; Musvosvi M.; Jayashankar L.; Vergara J.; Mabwe S.; Bilek N.; Geldenhuys H.; et al. Safety and immunogenicity of the novel tuberculosis vaccine ID93 + GLA-SE in BCG-vaccinated healthy adults in South Africa: a randomised, double-blind, placebo-controlled phase 1 trial. Lancet Respir Med. 2018, 6, 287–298. 10.1016/S2213-2600(18)30077-8. [DOI] [PubMed] [Google Scholar]
- Abhyankar M. M.; Orr M. T.; Lin S.; Suraju M. O.; Simpson A.; Blust M.; Pham T.; Guderian J. A.; Tomai M. A.; Elvecrog J.; Pedersen K.; Petri W. A.; Fox C. B. Adjuvant composition and delivery route shape immune response quality and protective efficacy of a recombinant vaccine for Entamoeba histolytica. npj Vaccines. 2018, 3, 22. 10.1038/s41541-018-0060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. S.; Endsley A. N.; Huang L. Design considerations for liposomal vaccines: Influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine. 2012, 30, 2256–2272. 10.1016/j.vaccine.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutz M.; Giquel B.; Hu Q.; Abuknesha R.; Uematsu S.; Akira S.; Nestle F. O.; Diebold S. S. Antibody-antigen-adjuvant conjugates enable co-delivery of antigen and adjuvant to dendritic cells in cis but only have partial targeting specificity. PLoS One 2012, 7, e40208 10.1371/journal.pone.0040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. B.; Xu J. Better adjuvants for better vaccines: Progress in adjuvant delivery systems, modifications, and adjuvant–antigen codelivery. Vaccines. 2020, 8, 128. 10.3390/vaccines8010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle P. M. Biotechnology approaches to produce potent, self-adjuvanting antigen-adjuvant fusion protein subunit vaccines. Biotechnol. Adv. 2017, 5, 375–389. 10.1016/j.biotechadv.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Coler R. N.; Day T. A.; Ellis R.; Piazza F. M.; Beckmann A. M.; Vergara J.; Rolf T.; Lu L.; Alter G.; Hokey D.; Jayashankar L.; Walker R.; Snowden M. A.; Evans T.; Ginsberg A.; Reed S. G. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: first-in-human trial. npj Vaccines. 2018, 3, 34. 10.1038/s41541-018-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholet S.; Ireton G. C.; Kahn M.; Guderian J.; Mohamath R.; Stride N.; Laughlin E. M.; Baldwin S. L.; Vedvick T. S.; Coler R. N.; Reed S. G. Identification of Human T Cell Antigens for the Development of Vaccines against Mycobacterium tuberculosis. J. Immunol. 2008, 181, 7948–7957. 10.4049/jimmunol.181.11.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr M. T.; Fox C. B.; Baldwin S. L.; Sivananthana S. J.; Lucas E.; Lina S.; Phan T.; Moon J. J.; Vedvicka T. S.; Reed S. G.; Coler R. N. Adjuvant formulation structure and composition are critical for the development of an effective vaccine against tuberculosis. J. Controlled Release 2013, 172, 190–200. 10.1016/j.jconrel.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaria P. V.; Chen B. B.; Rowe C. G.; Alani N.; Muratova O. V.; Barnafo E. K.; Lambert L. E.; Zaidi I. U.; Lees A.; Rausch K. M.; Naruma D. L.; Duffy P. E.; Scaria P. V.; Chen B. B.; Rowe C. G. Comparison of carrier proteins to conjugate malaria transmission blocking vaccine antigens, Pfs25 and Pfs230. Vaccine. 2020, 38, 5480–5489. 10.1016/j.vaccine.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimi J.; Azizi A.; Ausar S. F.; Todryk S. M.; Rahman N.; Brookes R. H. An adjuvant-modulated vaccine response in human whole blood. Hum Vaccines Immunother. 2017, 13, 2130–2134. 10.1080/21645515.2017.1337616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin S. L.; Ching L. K.; Pine S. O.; Moutaftsi M.; Lucas E.; Vallur A.; Orr M. T.; Bertholet S.; Reed S. G.; Coler R. N. Protection against Tuberculosis with Homologous or Heterologous Protein/Vector Vaccine Approaches Is Not Dependent on CD8 + T Cells. J. Immunol. 2013, 13, 2130–2134. 10.4049/jimmunol.1301161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coler R. N.; Bertholet S.; Pine S. O.; Orr M. T.; Reese V.; Windish H. P.; Davis C.; Kahn M.; Baldwin S. L.; Reed S. G. Therapeutic immunization against mycobacterium tuberculosis is an effective adjunct to antibiotic treatment. J Infect Dis. 2013, 207, 1242–1252. 10.1093/infdis/jis425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.; Moyle P. M. Bioconjugation Approaches to Producing Subunit Vaccines Composed of Protein or Peptide Antigens and Covalently Attached Toll-Like Receptor Ligands. Bioconjugate Chem. 2018, 29, 572–586. 10.1021/acs.bioconjchem.7b00478. [DOI] [PubMed] [Google Scholar]
- Van Den Brink-Van Der Laan E.; Antoinette Killian J.; De Kruijff B. Nonbilayer lipids affect peripheral and integral membrane proteins via changes in the lateral pressure profile. Biochim. Biophys. Acta, Biomembr. 2004, 1666, 275–288. 10.1016/j.bbamem.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Zegers N.; Gerritse K.; Deen C.; Boersma W.; Claassen E. An improved conjugation method for controlled covalent coupling of synthetic peptides to proteins using glutaraldehyde in a dialysis method. J. Immunol. Methods 1990, 130, 195–200. 10.1016/0022-1759(90)90048-Z. [DOI] [PubMed] [Google Scholar]
- Deen C.; Claassen E.; Gerritse K.; Zegers N. D.; Boersma W. J. A. A novel carbodiimide coupling method for synthetic peptides. Enhanced anti-peptide antibody responses. J. Immunol. Methods 1990, 129, 119–125. 10.1016/0022-1759(90)90428-X. [DOI] [PubMed] [Google Scholar]
- Cammarata C. R.; Hughes M. E.; Ofner C. M. Carbodiimide induced cross-linking, ligand addition, and degradation in gelatin. Mol. Pharmaceutics 2015, 12, 783–793. 10.1021/mp5006118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaria P. V.; Chen B. B.; Rowe C. G.; Jones D. S.; Barnafo E. K.; Fischer E. R.; Anderson C.; MacDonald N. J.; Lambert L.; Rausch K. M.; Narum D. L.; Duffy P. E. Protein-protein conjugate nanoparticles for malaria antigen delivery and enhanced immunogenicity. PLoS One 2017, 12, e0190312 10.1371/journal.pone.0190312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White W. I.; Cassatt D. R.; Madsen J.; Burke S. J.; Woods R. M.; Wassef N. M.; Alving C. F.; Koenig S. Antibody and cytotoxic T-lymphocyte responses to a single liposome-associated peptide antigen. Vaccine. 1995, 13, 1111–1122. 10.1016/0264-410X(94)00058-U. [DOI] [PubMed] [Google Scholar]
- An S. J.; Scaria P. V.; Chen B.; Barnafo E. K.; Muratovaa O.; Anderson C.; Lambert L.; Chae M. H.; Yang J. S.; Duffy E. Development of a bivalent conjugate vaccine candidate against malaria transmission and typhoid fever. Vaccine. 2018, 36, 2978–2984. 10.1016/j.vaccine.2018.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneichi M.; Tanaka Y.; Kakiuchi T.; Uchida T. Liposome-Coupled Peptides Induce Long-Lived Memory CD8+ T Cells Without CD4+ T Cells. PLoS One 2010, 5, e15091 10.1371/journal.pone.0015091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y.; Taneichi M.; Kasai M.; Kakiuchi T.; Uchida T. Liposome-Coupled Antigens Are Internalized by Antigen-Presenting Cells via Pinocytosis and Cross- Presented to CD8+ T Cells. PLoS One 2010, 5, e15225 10.1371/journal.pone.0015225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosino D. M.; Sood S. K.; Lee M. C.; et al. IgG1, IgG2 and IgM responses to two haemophilus influenzae type b conjugate vaccines in young infants. Pediatr Infect Dis J. 1992, 11, 855–859. 10.1097/00006454-199210000-00010. [DOI] [PubMed] [Google Scholar]
- Bartczak D.; Kanaras A. G. Preparation of peptide-functionalized gold nanoparticles using one pot EDC/Sulfo-NHS coupling. Langmuir 2011, 27, 10119–10123. 10.1021/la2022177. [DOI] [PubMed] [Google Scholar]
- Kramer R. M.; Archer M. C.; Orr M. T.; Cauwelaert N. D.; Beebe E. A.; Huang P. D.; Dowling Q. M.; Schwartz A. M.; Fedor D. M.; Vedvick T. S.; Fox C. B. Development of a thermostable nanoemulsion adjuvanted vaccine against tuberculosis using a design-of-experiments approach. Int. J. Nanomed. 2018, Volume 13, 3689–3711. 10.2147/IJN.S159839. [DOI] [PMC free article] [PubMed] [Google Scholar]