Abstract
Background
Psoriasis is a frequent chronic inflammatory cytokine‐mediated skin disease and was identified to be an independent risk factor for the occurrence of myocardial infarction (MI). However, data about the impact of psoriasis on mortality and other in‐hospital adverse events in the setting of MI are sparse and inconsistent.
Methods and Results
The nationwide German inpatient sample of the years 2005 to 2016 was used for statistical analysis. Hospitalized patients with MI were stratified for the presence of psoriasis and the impact of psoriasis on in‐hospital events was investigated. Overall, 3 307 703 patients with MI (37.6% females, 56.8% aged ≥70 years) were treated in Germany (2005–2016); among them 9028 (0.3%) were diagnosed with psoriasis. Patients with MI with psoriasis were significantly younger (68.0 [58.0–76.0] versus 73.0 [62.0–81.0] years; P<0.001) and showed significant lower in‐hospital case‐fatality rate (7.1% versus 12.4%; P<0.001), confirmed in the regression (odds ratio, 0.68; 95% CI, 0.63–0.74; P<0.001) adjusted for age, sex, and comorbidities. They more frequently revealed cardiovascular risk factors such as arterial hypertension (58.9% versus 55.0%; P<0.001), hyperlipidemia (44.4% versus 38.6%; P<0.001), smoking (14.3% versus 7.4%; P<0.001), diabetes mellitus (34.8% versus 30.4%; P<0.001) or obesity (17.9% versus 9.3%; P<0.001). While the rate of percutaneous coronary intervention (41.4 versus 42.0%; P=0.223) was comparable between both groups, coronary bypass surgery was more often performed in patients with MI with psoriasis (7.7% versus 4.7%; P<0.001).
Conclusions
Overall, only 0.3% of all MI cases were diagnosed with psoriasis, and patients with MI with psoriasis were in median 5 years younger than patients with MI without psoriasis. Psoriasis seems to enhance the prevalence of classical cardiovascular risk factors and might therefore explain the earlier time point for MI. Our data also showed in turn a lower in‐hospital mortality rate in patients with MI with psoriasis, presumably driven by younger age.
Keywords: mortality, myocardial infarction, psoriasis
Subject Categories: Cardiovascular Disease, Risk Factors, Myocardial Infarction, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- ICD‐10‐GM
International Classification of Diseases, Tenth Revision with German Modification
Clinical Perspective
What Is New?
Patients with myocardial infarction with psoriasis were younger and had more often cardiovascular risk factors.
Psoriasis and cardiovascular risk factors might boost coronary atherosclerosis.
Patients with myocardial infarction with psoriasis showed lower in‐hospital mortality rate than in those without psoriasis.
What Are the Clinical Implications?
Patients with psoriasis must be made aware of their increased cardiovascular risk profile.
Increased efforts have to be targeted on reduction of the inflammatory burden in psoriasis.
Psoriasis is a frequent chronic skin disease that is mediated by inflammatory cytokines and affects around 2% to 3% of the general population. 1 , 2 , 3 Meanwhile, there is a growing awareness that psoriasis goes “more than skin deep” and growing evidence supporting the concept that psoriasis is a complex systemic disease impacting several organ systems. Most important are cardiovascular comorbidities leading to life‐threatening cardiovascular manifestations such as myocardial infarction (MI). 1
A first association between psoriasis and vascular events was shown in 1973. 4 Nevertheless, the link between psoriasis and the associated cardiovascular comorbidity received little attention for many years; increased risk for poor outcome was attributed to the increased prevalence of classical cardiovascular risk factors in patients afflicted by psoriasis. In 2006, Gelfand et al 5 reported in a large population‐based cohort study including 127 139 patients with mild psoriasis and 3837 patients with severe psoriasis in comparison with 556 995 control patients without psoriasis (aged between 20 and 90 years) that patients with psoriasis had an increased adjusted relative risk for MI, which was greatest in young patients, especially in patients with severe psoriasis. The finding that psoriasis is independently associated with an increased risk of developing MI was also confirmed in other studies. 6 , 7 , 8 , 9 In line with these results, psoriasis may increase the prevalence as well as the severity of coronary artery calcification, 10 most likely attributable to the chronic systemic inflammation process. 10
Despite the higher prevalence of typical cardiovascular risk factors in patients with psoriasis, 11 , 12 , 13 , 14 , 15 severe psoriasis was identified as an independent risk factor for cardiovascular death, and established as a so‐called new cardiovascular risk factor. 16 The systemic nature of the interleukin‐17A, interleukin‐23, interleukin‐12, tumor necrosis factor‐α, and myeloid cell–driven inflammatory processes in psoriasis seems to be critical for the development of the associated cardiovascular disease and the independently increased risk of MI accompanied by a severe systemic disease. 10 , 17 , 18 In a recent double‐blinded randomized clinical trial, psoriasis was associated with impairment in endothelial function. 19 The authors speculated that anti–interleukin‐17A treatment might be beneficial for cardiovascular health in psoriasis patients. 19 The group around Mehta showed that biologic therapy in severe psoriasis (among others, also interleukin‐17A inhibitors such as secukinumab and ixekizumab) resulted in a beneficial modulation of coronary plaque morphology (evaluated by coronary computed tomography angiography) compared with psoriasis patients not treated with biologic therapy, indicating the cardiovascular importance of the systemic chronic ( interleukin‐17A–driven) inflammation in psoriasis. 20
Data on the impact of psoriasis on mortality and other in‐hospital adverse events in the setting of MI are sparse and inconsistent. 15 , 21 , 22 While some studies reported an impaired prognosis in psoriasis patients with MI, 15 , 21 others did not. 22 Thus, the objective of our study was to compare patients with MI with and without psoriasis and to analyze the impact of psoriasis on the in‐hospital outcome of acute MI in a large nationwide inpatient sample.
Methods
Data Source
We analyzed the large German nationwide inpatient sample for this study (source: Research Data Centre of the Federal Statistical Office and the Statistical Offices of the federal states, Diagnosis‐Related Groups Statistics 2005–2016, own calculations). In Germany, diagnoses are coded according to International Classification of Diseases, Tenth Revision with German Modification (ICD‐10‐GM) and diagnostic, surgical, and interventional procedures with diagnostic, surgery, and procedures codes (OPS codes). The Federal Statistical Office of Germany (Statistisches Bundesamt, Wiesbaden, Germany) gathers the treatment data from all inpatient cases in Germany (processed according to the diagnosis‐related groups system). The aggregated data that support the findings of this study are available from the corresponding author upon reasonable request.
Within our analysis, we included all patients with a MI hospitalized in Germany between the years 2005 and 2016. We identified these patients by the diagnostic codes of MI (ICD‐10 codes I21 and I22). Additionally, we stratified them for the presence of psoriasis (ICD‐10 code L40) (Figure S1). This made it possible to compare patients with MI with and without psoriasis regarding baseline parameters, especially cardiovascular risk factors and cardiovascular comorbidities, usage of revascularization treatments, age, and sex—and especially to focus on adverse in‐hospital events and in‐hospital case‐fatality rate. Additionally, we analyzed trends on hospitalization rate, in‐hospital case‐fatality rate, in‐hospital adverse events, and treatments.
Study End Points and In‐Hospital Adverse Events
The primary study outcome was death of all‐causes during in‐hospital stay (in‐hospital death). In addition, we analyzed the prevalence of adverse in‐hospital events such as pneumonia (ICD‐10 codes J12‐J18), deep venous thrombosis or thrombophlebitis of the leg veins (DVT, ICD‐10 code I80), pulmonary embolism (ICD‐10 code I26), acute kidney injury (ICD‐10 code N17), recurrent MI (ICD‐10 code I22), stroke (ischemic and hemorrhagic, ICD‐10 codes I61–64) intracerebral bleeding events (ICD‐10 code I61), gastro intestinal bleeding (ICD‐10 codes K920‐922), and transfusion of blood components (diagnostic, surgery, and procedures code ‐ OPS code 8‐800). Furthermore, particular attention was given to the differences regarding sex, age, classical cardiovascular risk factors, and atherosclerotic diseases of patients with MI with and without psoriasis. A further study end point was a prolonged length of in‐hospital stay (≥10 days, ≥14 days).
Ethical Aspects
Since this study did not involve direct access by the investigators to data of individual patients, approval by an ethical committee and informed consent were not required, in accordance with the German law.
Statistical Analysis
Descriptive statistics for relevant baseline comparisons of patients with MI with and without psoriasis are provided as median and interquartile range or absolute numbers and corresponding percentages. We tested the continuous variables using the Mann–Whitney U test and categorical variables with the Fisher's exact or the chi‐square test, as appropriate.
Total hospitalization rate for MI with psoriasis related to all hospitalized patients with MI (with and without psoriasis) and relative mortality rate (case‐fatality rate), the usage of interventional treatments, and rate of adverse in‐hospital events, were calculated on an annual basis, and linear regression was used to assess trends over time. The results are presented as β and corresponding 95% CIs.
Univariate and multivariate logistic regression models were analyzed to investigate the impact of psoriasis on in‐hospital events and on in‐hospital death in patients with MI. Results are presented as odds ratios (OR) and 95% CIs. The multivariate regression models were adjusted with different adjustments:
Adjustment 1: age and sex.
Adjustment 2: age, sex, Charlson Index, and treatment year.
Adjustment 3: age, sex, cancer, coronary artery disease, chronic obstructive pulmonary disease, essential arterial hypertension, renal insufficiency (glomerular filtration rate <60 mL/min per 1.73 m2), diabetes mellitus, atrial fibrillation/flutter, hyperlipidemia, and smoking.
We selected this epidemiological approach for the adjustment to test the widespread independence of these predictors on the case‐fatality rate during hospitalization. The software SPSS (version 20.0; SPSS Inc., Chicago, IL) was used for computerized analysis. P values of <0.05 (two‐sided) were considered to be statistically significant.
Results
In total, 3 307 703 hospitalizations of patients with acute MI (37.6% females, 56.8% aged ≥70 years), who were treated between 2005 and 2016 in German hospitals, were included in the present analysis. Among these patients, 410 737 patients with MI died during their stay in the hospital (12.4% in‐hospital mortality rate). Overall, 9028 (0.3%) patients with MI had psoriasis (Figure S1).
Patient Characteristics of Patients With MI With and Without Psoriasis
Characteristics of patients with MI with and without psoriasis are provided in Table 1: In brief, patients with MI with additional psoriasis were younger (68.0 [58.0–76.0] years versus 73.0 [62.0–81.0] years; P<0.001) and more frequently of male sex (69.0% versus 62.4%, P<0.001). All investigated cardiovascular risk factors such as essential arterial hypertension (58.9% versus 55.0%; P<0.001), hyperlipidemia (44.4% versus 38.6%; P<0.001), diabetes mellitus (34.8% versus 30.4%; P<0.001), smoking (14.3% versus 7.4%; P<0.001), as well as obesity (17.9% versus 9.3%; P<0.001) and the comorbidities peripheral artery disease and chronic obstructive pulmonary disease were more prevalent in patients with MI with psoriasis than in patients with MI without this chronic inflammatory skin disease (Table 1).
Table 1.
Baseline Characteristics, Medical History, Presentation, and Outcomes of the Included 3 307 703 Patients With MI Stratified According the Presence of Psoriasis
| Parameters |
Patients With MI With Psoriasis (n=9028; 0.3%) |
Patients With MI Without Psoriasis (n=3 298 675; 99.7%) |
P Value |
|---|---|---|---|
| Age | 68.0 (58.0–76.0) | 73.0 (62.0–81.0) | <0.001* |
| Age >70 y, n (%) | 3791 (42.0) | 1 875 095 (56.8) | <0.001* |
| Female sex,† n (%) | 2795 (31.0) | 1 240 241 (37.6) | <0.001* |
| In‐hospital stay, d | 9 (5–15) | 7 (4–13) | <0.001* |
| Traditional cardiovascular risk factors, n (%) | |||
| Obesity | 1618 (17.9) | 305 473 (9.3) | <0.001* |
| Smoking | 1290 (14.3) | 242 814 (7.4) | <0.001* |
| Essential arterial hypertension | 5316 (58.9) | 1 813 227 (55.0) | <0.001* |
| Hyperlipidemia | 4005 (44.4) | 1 273 037 (38.6) | <0.001* |
| Diabetes mellitus | 3143 (34.8) | 1 004 183 (30.4) | <0.001* |
| Myocardial infarction subtype, n (%) | |||
| STEMI | 3047 (33.6) | 1 146 793 (34.7) | 0.037 |
| NSTEMI | 5539 (61.4) | 2 003 161 (60.7) | 0.223 |
| Myocardial infarction without coded STEMI/NSTEMI subclassification | 442 (5.0) | 148 721 (4.6) | |
| Comorbidities | |||
| Charlson Index | 2 (1–5) | 2 (1–4) | <0.001* |
| Peripheral artery disease, n (%) | 818 (9.1) | 212 415 (6.4) | <0.001* |
| Cancer, n (%) | 345 (3.8) | 123 175 (3.7) | 0.662 |
| Atrial fibrillation/flutter, n (%) | 2060 (22.8) | 717 177 (21.7) | 0.013* |
| Chronic obstructive pulmonary disease, n (%) | 1169 (12.9) | 293 956 (8.9) | <0.001* |
| Sleep apnea, n (%) | 229 (2.5) | 38 945 (1.2) | <0.001* |
| Renal insufficiency (glomerular filtration rate <60 mL/min per 1.73 m2), n (%) | 1423 (15.8) | 497 139 (15.1) | 0.067 |
| Interventional treatments, n (%) | |||
| Cardiac catheter | 4960 (54.9) | 1 821 679 (55.2) | 0.587 |
| Percutaneous coronary intervention | 3737 (41.4) | 1 386 343 (42.0) | 0.223 |
| Bare metal stent | 1501 (16.6) | 577 806 (17.5) | 0.026* |
| Drug eluting stent | 2041 (22.6) | 730 316 (22.1) | 0.285 |
| Bioresorbable vascular scaffold | 27 (0.3) | 8295 (0.3) | 0.367 |
| Coronary artery bypass graft | 697 (7.7) | 153 419 (4.7) | <0.001* |
| Adverse events during hospitalization, n (%) | |||
| In‐hospital death | 640 (7.1) | 410 097 (12.4) | <0.001* |
| Recurrent myocardial infarction | 72 (0.80) | 21 522 (0.65) | 0.087 |
| Pneumonia | 1143 (12.7) | 383 522 (11.6) | 0.002* |
| Deep venous thrombosis or thrombophlebitis | 86 (1.0) | 22 399 (0.7) | 0.002* |
| Pulmonary embolism | 68 (0.8) | 22 588 (0.7) | 0.431 |
| Acute kidney injury | 554 (6.1) | 206 296 (6.3) | 0.645 |
| Shock | 495 (5.5) | 227 615 (6.9) | <0.001* |
| Stroke (ischemic or hemorrhagic) | 259 (2.9) | 95 105 (2.9) | 0.935 |
| Intracerebral bleeding | 37 (0.4) | 9373 (0.3) | 0.025* |
| Subarachnoid bleeding | 6 (0.1) | 2824 (0.1) | 0.717 |
| Gastrointestinal bleeding | 132 (1.5) | 47 754 (1.4) | 0.909 |
| Transfusion of blood constituents | 1383 (15.3) | 419 208 (12.7) | <0.001* |
| Pericardial effusion | 76 (0.8) | 21 262 (0.6) | 0.019* |
NSTEMI indicates non–ST‐segment–elevation myocardial infarction; and STEMI, ST‐segment–elevation myocardial infarction.
P values of <0.05 (two‐sided) were considered to be statistically significant.
Information available for 3 307 574 patients.
As expected, the prevalence of additional comorbidities and cardiovascular risk factors increased substantially with age in both groups (Figure S1). During the observation period, patient age ≥70 years as well as the prevalence of the cardiovascular risk factors arterial hypertension and hyperlipidemia increased in patients with MI with psoriasis and so did the comorbidities atrial fibrillation, chronic obstructive pulmonary disease, and renal insufficiency. In contrast, the frequency of obesity and smoking in patients with MI with psoriasis declined during the observation period (Table S1 and Figure S2).
Temporal Trends on Hospitalization Rate, Case‐Fatality Rate, and In‐Hospital Events in Patients With MI With and Without Psoriasis
Whereas the total number of patients with MI with psoriasis increased slightly over time from 754 patients in 2005 up to 814 patients in the year 2016 (β, 8.6; 95% CI, 3.4–13.8; P=0.004) (Figure 1A), in‐hospital mortality did not change significantly (7.6% in 2005 and 4.9% in 2016; P=0.894) during the observation period (Table S2). The in‐hospital mortality rate of patients with MI with psoriasis was consistently lower than that of patients with MI without psoriasis throughout the years (Figure 1B).
Figure 1. Temporal trends on hospitalization for MI and mortality rate in patients with psoriasis between 2005 and 2016.
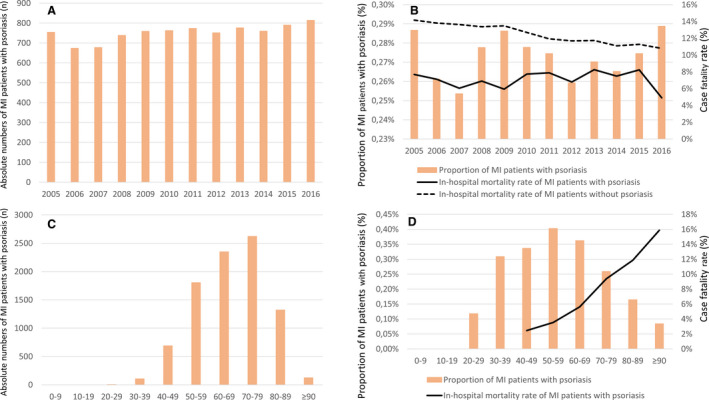
A, Temporal trends on absolute numbers of patients with MI with psoriasis (orange bars) from 2005 to 2016 in Germany. B, Temporal trends on the proportion of patients with MI with psoriasis related to all patients with MI hospitalized between 2005 and 2016 (orange bars) and the in‐hospital case‐fatality rate of patients with MI with psoriasis (black solid line) and those without psoriasis (black dashed line). C, Absolute numbers of patients with MI with psoriasis (orange bars) stratified for age‐decades (cumulative 2005–2016). D, Proportion of patients with MI with psoriasis (orange bars) related to all hospitalized patients with MI and the in‐hospital mortality rate of patients with MI with psoriasis stratified for age‐decades (cumulative 2005–2016). MI indicates myocardial infarction
The proportion of patients with MI with recurrent MI events decreased from 2.2% in the year 2005 to 0.4% in the year 2016 (β, −1.85; 95% CI, −2.65 to −1.05; P<0.001) in the subgroup of patients with MI with psoriasis (Figure 2A,and Table S2), whereas the proportion of MI events with non–ST‐segment–elevation MI increased in patients with MI both with and without psoriasis (β, 1.05; 95% CI, 0.90–1.19; P<0.001) (Figure 2B and Table S1).
Figure 2. Temporal trends in recurrent MI and NSTEMI in patients with and without psoriasis.
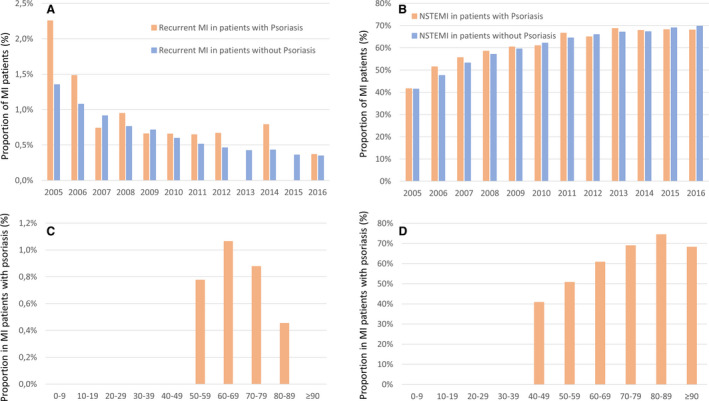
A, Temporal trends on the rate of recurrent MI events in patients with MI with psoriasis (orange bars) and without psoriasis (blue bars) from 2005 to 2016 in Germany. B, Temporal trends on the rate of NSTEMI events in patients with MI with psoriasis (orange bars) and without psoriasis (blue bars) from 2005 to 2016 in Germany. C, Proportion of recurrent MI events in patients with MI with psoriasis (orange bars) stratified for age‐decades (cumulative 2005–2016). D, Proportion of NSTEMI events in patients with MI with psoriasis (orange bars) stratified for age‐decades (cumulative 2005–2016). MI indicates myocardial infarction; and NSTEMI non–ST‐segment–elevation myocardial infarction.
The absolute numbers of patients with MI with psoriasis in relation to all hospitalized patients with MI inclined with age. While the absolute number of patients with MI with psoriasis was highest in the eighth decade of life (Figure 1C), the highest percentage of patients with MI with psoriasis was found in the sixth decade of life. The in‐hospital mortality rate increased steadily with age (Figure 1D). In line, the rate of non–ST‐segment–elevation MI events also increased with age (Figure 2D). The proportion of recurrent MI events was highest in the seventh decade of life (Figure 2C).
Treatment Differences in Patients With MI With and Without Psoriasis
Frequency of non–ST‐segment–elevation MI did not differ between groups (61.4% versus 60.7%; P=0.223). Nevertheless, patients with MI with psoriasis had longer hospital stays (9 [interquartile range, 5–15] versus 7 [4–13] days; P<0.001) (Table 1).
Patients with MI with psoriasis more often underwent coronary artery bypass graft (CABG) surgery (7.7% versus 4.7%; P<0.001). There was no difference in the use of coronary angiography (54.9% versus 55.2%; P=0.587) or percutaneous coronary intervention (41.4% versus 42.0%; P=0.223) (Table 1). Bare metal stents more often were implanted in patients without psoriasis (17.5% versus 16.6%; P=0.026).
We found a progressive increase in the use of interventional procedures in patients with MI with psoriasis (from 47.1% in 2005 to 64.7% of the patients in 2015; β, 0.75; 95% CI, 0.61–0.89; P<0.001). In parallel, revascularization therapy with percutaneous coronary intervention (36.2% in 2005 and 52.3% in 2016) as well as with CABG (7.0% in 2005 and 9.7% in 2016) increased significantly within the observation period (Table S3 and Figure 3A). More in detail, the implantation rate of bare metal stents decreased from 27.3% in 2005 to 1.8% in 2016, whereas the frequency of the use of drug eluting stents increased from 6.8% to 47.7% in the same period (Table S3 and Figure 3B).
Figure 3. Temporal trends in interventional/operative treatment in patients with MI with and without psoriasis between the years 2005 and 2016.
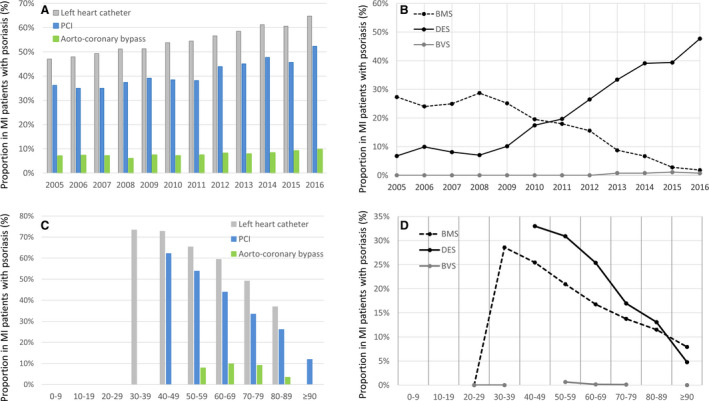
A, Temporal trends on left heart catheter (gray bars), PCI (blue bars) and aorto‐coronary bypass surgery (green bars) in patients with MI with psoriasis from 2005 to 2016 in Germany. B, Temporal trends on implantation of drug‐eluting stent (DES, solid black line), bare metal stent (BMS, dashed black line) and bioresorbable vascular scaffold (BVS, solid gray line) in patients with MI with psoriasis from 2005 to 2016 in Germany. C, Temporal trends on left heart catheter (gray bars), PCI (blue bars) and aorto‐coronary bypass surgery (green bars) in patients with MI with psoriasis stratified for age‐decades (cumulative 2005–2016). D, Temporal trends on implantation of drug eluting stent (DES, solid black line), bare metal stent (BMS, dashed black line) and bioresorbable vascular scaffold (BVS, solid gray line) in patients with MI with psoriasis stratified for age‐decades (cumulative 2005–2016). MI, myocardial infarction; and PCI, percutaneous coronary intervention.
Notably, the highest revascularization rates were found in patients between the fifth and eighth decade of life in patients with MI with psoriasis (Figure 3C and 3D).
Adverse In‐Hospital Events of Patients With MI Stratified for Presence of Psoriasis
While patients with MI with psoriasis showed a significantly lower in‐hospital case‐fatality rate (7.1% versus 12.4%; P<0.001) and were less often in cardiac shock (5.5% versus 6.9%; P<0.001) than patients with MI without psoriasis, the frequency of recurrent MI did not differ between groups (0.80% versus 0.65%; P=0.087) (Table 1). The in‐hospital incidence of pneumonia (12.7% versus 11.6%; P=0.002), deep vein thrombosis or thrombophlebitis (DVT, 1.0% versus 0.7%; P=0.002) and intracranial bleeding (0.4% versus 0.3%; P=0.025), but not pulmonary embolism (0.8% versus 0.7%; P=0.431), stroke (2.9% versus 2.9%; P=0.935) and acute kidney injury (6.1% versus 6.3%; P=0.645), were higher in patients with MI with psoriasis. Since the differences regarding prevalence of pneumonia, DVT, and intracranial bleeding were only marginally different between both groups, these differences might not be clinically relevant. While the prevalence of pneumonia and acute kidney injury increased over time, that of DVT, pulmonary embolism, stroke, and bleeding events remained unchanged (Figure S3 and Table S1).
The multivariate regression models adjusted for age, sex, and comorbidities supported the crude statistical results (Table 2): Psoriasis in patients with MI was an independent predictor for pneumonia (OR, 1.14; 95% CI, 1.07–1.21; P<0.001), DVT (OR, 1.45; 95% CI, 1.18–1.80; P=0.001), and intracerebral bleeding (OR, 1.53; 95% CI, 1.10–2.11; P=0.010) (Table 2). Nevertheless, it was associated with a lower risk for in‐hospital death (OR, 0.68; 95% CI, 0.63–0.74; P<0.001) as well as development of shock (OR, 0.80; 95% CI, 0.73–0.88; P<0.001) (Table 2).
Table 2.
Impact of Psoriasis on the Different Adverse In‐Hospital Events in Patients Hospitalized for MI (Univariate and Multivariate Logistic Regression Model)
| Univariate Regression Model | Multivariate Regression Model (Adjustment I)* | Multivariate Regression Model (Adjustment II)† | Multivariate Regression Model (Adjustment III)‡ | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| In‐hospital death | 0.54 (0.50–0.58) | <0.001§ | 0.65 (0.60–0.71) | <0.001§ | 0.59 (0.55–0.64) | <0.001§ | 0.68 (0.63–0.74) | <0.001§ |
| Recurrent myocardial infarction | 1.22 (0.97–1.54) | 0.088 | 1.23 (0.98–1.55) | 0.081 | 1.18 (0.93–1.49) | 0.165 | 1.18 (0.93–1.48) | 0.172 |
| Pneumonia | 1.10 (1.04–1.17) | 0.002§ | 1.26 (1.19–1.35) | <0.001§ | 1.12 (1.05–1.20) | <0.001§ | 1.14 (1.07–1.21) | <0.001§ |
| Deep venous thrombosis or thrombophlebitis | 1.41 (1.14–1.74) | 0.002§ | 1.25 (0.99–1.58) | 0.061 | 1.41 (1.14–1.74) | 0.001§ | 1.45 (1.18–1.80) | 0.001§ |
| Pulmonary embolism | 1.10 (0.87–1.40) | 0.431 | 1.52 (1.23–1.87) | <0.001§ | 1.16 (0.92–1.46) | 0.221 | 1.23 (0.97–1.56) | 0.093 |
| Acute kidney injury | 0.98 (0.90–1.07) | 0.645 | 1.12 (1.03–1.22) | 0.008§ | 0.88 (0.80–0.97) | 0.008§ | 1.02 (0.94–1.12) | 0.599 |
| Shock | 0.78 (0.72–0.86) | <0.001§ | 0.79 (0.73–0.87) | <0.001§ | 0.73 (0.66–0.80) | <0.001§ | 0.80 (0.73–0.88) | <0.001§ |
| Stroke (ischemic or hemorrhagic) | 1.00 (0.88–1.13) | 0.935 | 1.12 (0.99–1.27) | 0.077 | 0.94 (0.82–1.06) | 0.936 | 1.11 (0.98–1.26) | 0.098 |
| Intracerebral bleeding | 1.44 (1.05–2.00) | 0.026§ | 1.46 (1.06–2.02) | 0.021§ | 1.27 (0.91–1.75) | 0.156 | 1.53 (1.10–2.11) | 0.010§ |
| Gastrointestinal bleeding | 1.01 (0.85–1.20) | 0.909 | 1.16 (0.98–1.38) | 0.089 | 1.05 (0.88–1.24) | 0.605 | 1.11 (0.94–1.32) | 0.233 |
| Transfusion of blood constituents | 1.24 (1.17–1.32) | <0.001§ | 1.35 (1.27–1.43) | <0.001§ | 1.20 (1.13–1.28) | <0.001§ | 1.24 (1.17–1.32) | <0.001§ |
| Prolonged in‐hospital stay ≥10 d | 1.53 (1.47–1.59) | <0.001§ | 1.78 (1.70–1.85) | <0.001§ | 1.64 (1.57–1.71) | <0.001§ | 1.63 (1.56–1.71) | <0.001§ |
| Prolonged in‐hospital stay ≥14 d | 1.54 (1.47–1.61) | <0.001§ | 1.79 (1.71–1.88) | <0.001§ | 1.63 (1.56–1.71) | <0.001§ | 1.64 (1.56–1.72) | <0.001§ |
OR indicates odds ratio.
Adjustment I: age and sex.
Adjustment II: age, sex, Charlson Index, and treatment year.
Adjustment III: age, sex, cancer, coronary artery disease, chronic obstructive pulmonary disease, essential arterial hypertension, renal insufficiency (glomerular filtration rate <60 mL/min per 1.73 m2), diabetes mellitus, atrial fibrillation/flutter, hyperlipidemia, and smoking.
P values of <0.05 were considered to be statistically significant.
Lower in‐hospital mortality in patients with psoriatic MI in comparison with patients with MI without psoriasis was confirmed in the subgroups of both non–ST‐segment–elevation MI (OR, 0.75; 95% CI, 0.67–0.83; P<0.001) and patients with ST‐segment–elevation MI (OR, 0.47; 95% CI, 0.41–0.55; P<0.001) (regression models respectively adjusted with adjustment III).
Length of In‐Hospital Stay
The length of in‐hospital stay was longer in patients with MI with psoriasis (9 [5–15] versus 7 [4–13] days; P<0.001) than in those patients with MI without psoriasis. Patients with MI with psoriasis had more often to stay longer than ≥10 (46.2% versus 36.0%; P<0.001) as well as ≥14 days (30.7% versus 22.4%; P<0.001) in the hospital (Table 1). Psoriasis was accompanied by an elevated risk for a prolonged length of in‐hospital stay of ≥10 days (OR, 1.63; 95% CI, 1.56–1.71; P<0.001) and ≥14 days (OR, 1.64; 95% CI, 1.56–1.72; P<0.001) (Table 2).
Discussion
Our present study demonstrated substantial differences between patients with MI with and without psoriasis. As meanwhile accepted, severe psoriasis presents a new cardiovascular risk factor in addition to the classical cardiovascular risk factors. 16 Cardiovascular mortality was shown to be significantly higher in patients with severe psoriasis compared with patients without psoriasis, and highest in young individuals. 16 Prevalence of MI was described to be higher in mild and severe psoriasis than in patients without psoriasis. 8 , 9
Little is known about the prognosis of acute MI in patients with psoriasis,although this knowledge would be relevant for future secondary prevention strategies in this patient collective and might be also important for adequate management of public health as well as healthcare service planning.
In accordance with previous studies, 11 , 12 , 13 , 14 , 15 , 16 our data demonstrate that psoriasis is associated with increased prevalence of the cardiovascular risk factors arterial hypertension, hyperlipidemia, diabetes mellitus, smoking, and obesity. Nevertheless, studies have shown that patients with severe psoriasis have an increased risk of cardiovascular mortality that is independent of traditional cardiovascular risk factors. 16 , 23 , 24 In particular, the typical proinflammatory cytokines like interleukin‐17A and tumor necrosis factor‐α have been shown to also contribute to the development of metabolic syndrome, obesity, and type 2 diabetes mellitus as well as depression. 24 , 25
In line with this evidence, patients with MI with psoriasis in our database were significantly younger than patients with MI without psoriasis (68 versus 73 years). It has been previously described that the risk of MI associated with psoriasis is greatest in young patients with severe psoriasis, 5 possibly because of the systemic tumor necrosis factor‐α, interleukin‐17 and interleukin‐23 (interleukin‐12), and myeloid cell–driven inflammatory burden, 26 , 27 , 28 , 29 which adds to the general vascular inflammation associated with the classical cardiovascular risk factors. It has to be hypothesized that psoriasis with its specific concomitant vascular inflammation on top of the general vascular inflammation, which is triggered by classical cardiovascular risk factors, acts like a multiplier regarding the atherosclerotic process and the development of stenotic coronary artery disease and MI earlier in life.
Despite the medical progress, in‐hospital mortality of patients with MI in Germany remained high with 12.4% of the hospitalizations from 2005 to 2016, which is higher than the reported 30‐day mortality rate in the United States (10.5% in 1999 and 7.8% in 2008). 30 Interestingly and in contrast to other published studies, 15 , 16 , 21 , 31 there was a substantially lower in‐hospital mortality rate in patients with MI with psoriasis than in those without (7.1% versus 12.4%). This was confirmed in the multivariate regression model adjusted for age, sex, and comorbidities. Of note, we cannot exclude that this finding might be attributable to undercoding/underreporting of comorbidities (including psoriasis) in severe MI cases, for instance, those who died before or immediately after admission, leading to a bias toward better in‐hospital survival of patients with MI with psoriasis in comparison with those without.
Nonetheless, patients with MI with psoriasis were in median 5 years younger, and, as shown in Figure 1D, in‐hospital mortality rate increased substantially with age. The presentation in patients with MI with psoriasis at a younger age might be explained by the more frequently revealed cardiovascular risk factors such as arterial hypertension, hyperlipidemia, smoking, diabetes mellitus, or obesity. On the other hand, a younger age might be an important cause and an explanation for the lower in‐hospital mortality rate of patients with MI with psoriasis in comparison with the older patients without psoriasis. In this context, we have to keep in mind that, although we adjusted the multivariate regression model for age, sex, and several important comorbidities, we cannot be totally aware of every age‐dependent change in risk factors and comorbidities, especially in those risk factors and comorbidities that are not included in the multivariate regression model. Thus, age‐dependent differences of these risk factors and comorbidities might impact the mortality rate.
It has to be mentioned that within the observational period of our study, new biologic therapies for psoriasis targeting the cytokine network (eg, interleukin‐17A, interleukin‐17R, interleukin‐23/12, and interleukin‐23 antibodies and anti–tumor necrosis factor‐α) were introduced into clinical practice and therefore could also contribute to a decreased mortality and recurrent MI rates by dampening the general systemic inflammation in psoriasis. 19 , 24 , 32 , 33 These new and causal and extremely efficient treatment strategies seem to have an important impact on the development and the outcome of coronary artery disease and its critical manifestation MI. Studies have shown that new biologic therapies attacking systemic inflammation indeed also provide beneficial effects on the vascular system of psoriasis mice and patients, 19 , 34 and the reduction in inflammatory burden might be beneficial for MI development and outcome. 35 , 36
In addition, we identified interesting differences regarding the reperfusion treatments between patients with MI with and without psoriasis. While reperfusion treatment with percutaneous coronary intervention did not differ between MI with and without psoriasis, patients with MI with psoriasis were treated more often with CABG surgery. This may indicate more complex coronary lesions with higher SYNTAX Score in patients with psoriasis, which prompted the treating physicians to opt for CABG according to coronary revascularization guidelines. 37 , 38 , 39 This finding can easily be set in the context of the chronic persisting systemic inflammation in psoriasis impacting the coronary arteries. 40 , 41 The use of drug‐eluting stent implantations in patients with MI with psoriasis substantially increased throughout the observational period from 6.8% in 2005 to 47.7% in 2016, which was accompanied by a decrease of recurrent MI events. Consequently, the outcome benefit received by the usage of modern drug‐eluting stents, as recommended in the current guidelines, 42 , 43 seems to be of substantial impact on the outcome in patients with MI and especially of patients with MI with psoriasis presumably because of their anti‐inflammatory effect on coronary lesions. 38 , 44 However, the proportion of patients with MI treated with CABG, bare metal stents, and drug‐eluting stents in Germany was comparable with published numbers of the United States (revascularizations within 30 days after MI: 40.7% in 1999 and 47.2% in 2008), 30 , 45 and higher than in England, where only every fifth patient with MI received a percutaneous transluminal coronary angioplasty and only 0.8% a CABG. 45 , 46
Psoriasis was an independent predictor for pneumonia possibly because of the chronic inflammation itself or the concomitant use of anti‐inflammatory therapies, especially with immunosuppressants or biologicals. Additionally, in line with previous literature, 47 , 48 psoriasis was associated with increased in‐hospital occurrence of DVT and intracerebral bleeding in patients with MI, and the rate of atrial fibrillation/flutter was higher in patients with MI with psoriasis than without.
In sum, our data show that MI events may occur earlier in life in patients with psoriasis and that they are associated with higher in‐hospital complication (but not mortality) rates. Patients with psoriasis must be made aware of their increased cardiovascular risk and further attention has to be undertaken to reduce the inflammatory burden in psoriasis for better cardiovascular risk management.
Limitations
Some limitations require consideration: First, the study results are based on ICD‐10 and diagnostic, surgery, and procedures discharge codes (OPS codes) of hospitalized patients, which might lead to an underreporting/undercoding, particularly in more severe MI cases with early mortality. Second, data from later follow‐up were not available. Third, in only 0.3% of MI cases the skin disease psoriasis was coded additionally, which lies under the known incidence for psoriasis in Europe (2%–3%). Nevertheless, other studies reported even smaller prevalence. 21 Besides, no exact classification of disease severity is given.
Conclusions and Clinical Impact
Overall, only 0.3% of all MI cases were coded additionally with the skin disease psoriasis. MI events in patients with psoriasis occurred in median 5 years earlier in life than in those patients with MI without psoriasis. Psoriasis was associated with increased prevalence of classical cardiovascular risk factors, and both might boost coronary atherosclerosis. Our data show a substantially lower in‐hospital mortality rate in patients with MI with psoriasis than in those without, which might be mainly driven by the younger age of patients with MI with psoriasis.
Sources of Funding
This study was supported by the German Federal Ministry of Education and Research (BMBF 01EO1503); institutional grant of the Center for Thrombosis and Hemostasis Mainz. Dr Karbach is funded by the German Research Foundation (DFG KA 4035/1‐1) and by the CRC/Transregio 156 (“The Skin as Sensor and Effector Organ Orchestrating Local and Systemic Immune Responses”). Drs Karbach and Wenzel receive funding from the Boehringer Ingelheim Foundation “Novel and Neglected Cardiovascular Risk Factors: Molecular Mechanisms and Therapeutic Implications” and the Federal Ministry of Education and Research (BMBF 01EO1503). Thomas Münzel is principal investigator of the DZHK (German Center for Cardiovascular Research), Partner Site Rhine‐Main, Mainz, Germany. All authors are responsible for the content of this publication. The funders had no role in study design, data collection and analysis, the decision to publish, nor in preparation of the manuscript.
Disclosures
Dr Hobohm reports having received lecture honoraria from MSD. Prof Dr Steinbrink reports having received consultancy and lecture honoraria from Actelion, Pfizer and Novarti. Prof Dr Gori has received grant support (CARIMA study) and speaker's honoraria from Novartis. Prof Dr Wenzel reports having received consultancy and lecture honoraria from Abbot Vascular, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi‐Sankyo, and Novartis. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
Figures S1–S3
Acknowledgments
We thank the Federal Statistical Office of Germany (Statistisches Bundesamt, DEStatis) for providing the data/results and the kind permission to publish these data/results (source: Research Data Centre of the Federal Statistical Office and the Statistical Offices of the federal states, Diagnosis‐Related Groups Statistics 2005–2016, own calculations). Open access funding enabled and organized by Projekt DEAL.
[Correction added on September 30, 2020, after first online publication: Projekt DEAL funding statement has been added.]
Author contributions: All author(s) were involved in the conception and design of the study and analysis and interpretation of the data; all authors contributed in drafting and revising the article critically for intellectual content and gave final approval of the version to be published and agree to be accountable for all aspects of the work.
(J Am Heart Assoc. 2020;9:e016956 DOI: 10.1161/JAHA.120.016956.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.016956
For Sources of Funding and Disclosures, see page 10.
References
- 1. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. [DOI] [PubMed] [Google Scholar]
- 2. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM; Identification and Management of Psoriasis and Associated ComorbidiTy project team . Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–385. [DOI] [PubMed] [Google Scholar]
- 3. Boehncke WH, Schon MP. Psoriasis. Lancet. 2015;386:983–994. [DOI] [PubMed] [Google Scholar]
- 4. McDonald CJ, Calabresi P. Thromboembolic disorders associated with psoriasis. Arch Dermatol. 1973;107:918. [PubMed] [Google Scholar]
- 5. Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–1741. [DOI] [PubMed] [Google Scholar]
- 6. Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: a systematic review and meta‐analysis of observational studies. J Am Heart Assoc. 2013;2:e000062 DOI: 10.1161/JAHA.113.000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin HW, Wang KH, Lin HC, Lin HC. Increased risk of acute myocardial infarction in patients with psoriasis: a 5-year population‐based study in Taiwan. J Am Acad Dermatol. 2011;64:495–501. [DOI] [PubMed] [Google Scholar]
- 8. Xu T, Zhang YH. Association of psoriasis with stroke and myocardial infarction: meta‐analysis of cohort studies. Br J Dermatol. 2012;167:1345–1350. [DOI] [PubMed] [Google Scholar]
- 9. Xiao J, Chen LH, Tu YT, Deng XH, Tao J. Prevalence of myocardial infarction in patients with psoriasis in central China. J Eur Acad Dermatol Venereol. 2009;23:1311–1315. [DOI] [PubMed] [Google Scholar]
- 10. Ludwig RJ, Herzog C, Rostock A, Ochsendorf FR, Zollner TM, Thaci D, Kaufmann R, Vogl TJ, Boehncke WH. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271–276. [DOI] [PubMed] [Google Scholar]
- 11. Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895–902. [DOI] [PubMed] [Google Scholar]
- 12. Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55:829–835. [DOI] [PubMed] [Google Scholar]
- 13. Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321–328. [DOI] [PubMed] [Google Scholar]
- 14. Wakkee M, Thio HB, Prens EP, Sijbrands EJ, Neumann HA. Unfavorable cardiovascular risk profiles in untreated and treated psoriasis patients. Atherosclerosis. 2007;190:1–9. [DOI] [PubMed] [Google Scholar]
- 15. Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700–703. [DOI] [PubMed] [Google Scholar]
- 16. Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brauchli YB, Jick SS, Miret M, Meier CR. Psoriasis and risk of incident myocardial infarction, stroke or transient ischaemic attack: an inception cohort study with a nested case‐control analysis. Br J Dermatol. 2009;160:1048–1056. [DOI] [PubMed] [Google Scholar]
- 18. Abuabara K, Lee H, Kimball AB. The effect of systemic psoriasis therapies on the incidence of myocardial infarction: a cohort study. Br J Dermatol. 2011;165:1066–1073. [DOI] [PubMed] [Google Scholar]
- 19. von Stebut E, Reich K, Thaci D, Koenig W, Pinter A, Korber A, Rassaf T, Waisman A, Mani V, Yates D, et al. Impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. J Invest Dermatol. 2019;139:1054–1062. [DOI] [PubMed] [Google Scholar]
- 20. Elnabawi YA, Dey AK, Goyal A, Groenendyk JW, Chung JH, Belur AD, Rodante J, Harrington CL, Teague HL, Baumer Y, et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res. 2019;115:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ahlehoff O, Gislason GH, Lindhardsen J, Olesen JB, Charlot M, Skov L, Torp‐Pedersen C, Hansen PR. Prognosis following first‐time myocardial infarction in patients with psoriasis: a Danish nationwide cohort study. J Intern Med. 2011;270:237–244. [DOI] [PubMed] [Google Scholar]
- 22. Desai R, Patel U, Bhuva R, Kumar G. Psoriasis does not increase the mortality in acute myocardial infarction patients: a nationwide analysis. Circulation. 2017;136:A18814. [Google Scholar]
- 23. Mehta NN, Yu Y, Pinnelas R, Krishnamoorthy P, Shin DB, Troxel AB, Gelfand JM. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med. 2011;124:775.e1–775.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. von Stebut E, Boehncke WH, Ghoreschi K, Gori T, Kaya Z, Thaci D, Schaffler A. IL-17A in psoriasis and beyond: cardiovascular and metabolic implications. Front Immunol. 2019;10:3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gisondi P, Tessari G, Conti A, Piaserico S, Schianchi S, Peserico A, Giannetti A, Girolomoni G. Prevalence of metabolic syndrome in patients with psoriasis: a hospital‐based case‐control study. Br J Dermatol. 2007;157:68–73. [DOI] [PubMed] [Google Scholar]
- 26. Mehta NN, Yu Y, Saboury B, Foroughi N, Krishnamoorthy P, Raper A, Baer A, Antigua J, Van Voorhees AS, Torigian DA, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography‐computed tomography (FDG‐PET/CT): a pilot study. Arch Dermatol. 2011;147:1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joshi AA, Lerman JB, Dey AK, Sajja AP, Belur AD, Elnabawi YA, Rodante JA, Aberra TM, Chung J, Salahuddin T, et al. Association between aortic vascular inflammation and coronary artery plaque characteristics in psoriasis. JAMA Cardiol. 2018;3:949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karbach S, Croxford AL, Oelze M, Schuler R, Minwegen D, Wegner J, Koukes L, Yogev N, Nikolaev A, Reissig S, et al. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis‐like skin disease. Arterioscler Thromb Vasc Biol. 2014;34:2658–2668. [DOI] [PubMed] [Google Scholar]
- 29. Boehncke WH, Boehncke S, Tobin AM, Kirby B. The 'psoriatic march': a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol. 2011;20:303–307. [DOI] [PubMed] [Google Scholar]
- 30. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. [DOI] [PubMed] [Google Scholar]
- 31. Freisinger E, Fuerstenberg T, Malyar NM, Wellmann J, Keil U, Breithardt G, Reinecke H. German nationwide data on current trends and management of acute myocardial infarction: discrepancies between trials and real‐life. Eur Heart J. 2014;35:979–988. [DOI] [PubMed] [Google Scholar]
- 32. Nast A, Amelunxen L, Augustin M, Boehncke WH, Dressler C, Gaskins M, Harle P, Hoffstadt B, Klaus J, Koza J, et al. S3 guideline for the treatment of psoriasis vulgaris, update—short version part 1—systemic treatment. J Dtsch Dermatol Ges. 2018;16:645–669. [DOI] [PubMed] [Google Scholar]
- 33. Nast A, Amelunxen L, Augustin M, Boehncke WH, Dressler C, Gaskins M, Harle P, Hoffstadt B, Klaus J, Koza J, et al. S3 guideline for the treatment of psoriasis vulgaris, update—short version part 2—special patient populations and treatment situations. J Dtsch Dermatol Ges. 2018;16:806–813. [DOI] [PubMed] [Google Scholar]
- 34. Schuler R, Brand A, Klebow S, Wild J, Veras FP, Ullmann E, Roohani S, Kolbinger F, Kossmann S, Wohn C, et al. Antagonization of IL-17A attenuates skin inflammation and vascular dysfunction in mouse models of psoriasis. J Invest Dermatol. 2019;139:638–647. [DOI] [PubMed] [Google Scholar]
- 35. Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernan MA, Ridker PM, Mozaffarian D. Systematic review and meta‐analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boehncke S, Salgo R, Garbaraviciene J, Beschmann H, Hardt K, Diehl S, Fichtlscherer S, Thaci D, Boehncke WH. Effective continuous systemic therapy of severe plaque‐type psoriasis is accompanied by amelioration of biomarkers of cardiovascular risk: results of a prospective longitudinal observational study. J Eur Acad Dermatol Venereol. 2011;25:1187–1193. [DOI] [PubMed] [Google Scholar]
- 37. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST‐elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2016;133:1135–1147. [DOI] [PubMed] [Google Scholar]
- 38. Neumann FJ, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 39. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–e651. [DOI] [PubMed] [Google Scholar]
- 40. Daghem M, Newby D. Psoriasis and inflammation more than skin deep. Circ Cardiovasc Imaging. 2018;11:e007849. [DOI] [PubMed] [Google Scholar]
- 41. Mehta NN, Shin DB, Joshi AA, Dey AK, Armstrong AW, Duffin KC, Fuxench ZC, Harrington CL, Hubbard RA, Kalb RE, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo‐controlled trial. Circ Cardiovasc Imaging. 2018;11:e007394 DOI: 10.1161/CIRCIMAGING.117.007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 43. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 44. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Rev Esp Cardiol (Engl Ed). 2015;68:1125. [DOI] [PubMed] [Google Scholar]
- 45. Keller K, Hobohm L, Munzel T, Ostad MA. Sex‐specific differences regarding seasonal variations of incidence and mortality in patients with myocardial infarction in Germany. Int J Cardiol. 2019;287:132–138. [DOI] [PubMed] [Google Scholar]
- 46. Smolina K, Wright FL, Rayner M, Goldacre MJ. Long‐term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circ Cardiovasc Qual Outcomes. 2012;5:532–540. [DOI] [PubMed] [Google Scholar]
- 47. Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375:657–663. [DOI] [PubMed] [Google Scholar]
- 48. Fox EA, Kahn SR. The relationship between inflammation and venous thrombosis. A systematic review of clinical studies. Thromb Haemost. 2005;94:362–365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S3


