Abstract
Background
Outcomes data in patients with cardiac amyloidosis after implantable cardioverter‐defibrillator (ICD) implantation are limited. We compared outcomes of patients with ICDs implanted for cardiac amyloidosis versus nonischemic cardiomyopathies (NICMs) and evaluated factors associated with mortality among patients with cardiac amyloidosis.
Methods and Results
Using National Cardiovascular Data Registry’s ICD Registry data between April 1, 2010 and December 31, 2015, we created a 1:5 propensity‐matched cohort of patients implanted with ICDs with cardiac amyloidosis and NICM. We compared mortality between those with cardiac amyloidosis and matched patients with NICM using Kaplan‐Meier survival curves and Cox proportional hazards models. We also evaluated risk factors associated with 1‐year mortality in patients with cardiac amyloidosis using multivariable Cox proportional hazards regression models. Among 472 patients with cardiac amyloidosis and 2360 patients with propensity‐matched NICMs, 1‐year mortality was significantly higher in patients with cardiac amyloidosis compared with patients with NICMs (26.9% versus 11.3%, P<0.001). After adjustment for covariates, cardiac amyloidosis was associated with a significantly higher risk of all‐cause mortality (hazard ratio [HR], 1.80; 95% CI, 1.56–2.08). In a multivariable analysis of patients with cardiac amyloidosis, several factors were significantly associated with mortality: syncope (HR, 1.78; 95% CI, 1.22–2.59), ventricular tachycardia (HR, 1.65; 95% CI, 1.15–2.38), cerebrovascular disease (HR, 2.03; 95% CI, 1.28–3.23), diabetes mellitus (HR, 1.55; 95% CI, 1.05–2.27), creatinine = 1.6 to 2.5 g/dL (HR, 1.99; 95% CI, 1.32–3.02), and creatinine >2.5 (HR, 4.34; 95% CI, 2.72–6.93).
Conclusions
Mortality after ICD implantation is significantly higher in patients with cardiac amyloidosis than in patients with propensity‐matched NICMs. Factors associated with death among patients with cardiac amyloidosis include prior syncope, ventricular tachycardia, cerebrovascular disease, diabetes mellitus, and impaired renal function.
Keywords: amyloid, cardiomyopathy, nonischemic cardiomyopathy, implantable cardioverter‐defibrillator, mortality
Subject Categories: Mortality/Survival, Cardiomyopathy, Catheter Ablation and Implantable Cardioverter-Defibrillator
Nonstandard Abbreviations and Acronyms
- AL
light‐chain amyloidosis
- aTTR
transthyretin amyloidosis
- DM
diabetes mellitus
- HR
hazard ratio
- ICD
implantable cardioverterdefibrillator
- IQR
interquartile range
- NICM
nonischemic cardiomyopathy
- RR
relative risk
Clinical Perspective
What Is New?
Patients with cardiac amyloidosis who have an implantable cardioverter‐defibrillator implanted have a 26.9% 1‐year mortality rate, which is significantly higher than patients with propensity‐matched nonischemic cardiomyopathy.
Impaired renal function, syncope, ventricular tachycardia, cerebrovascular disease, and diabetes mellitus are risk factors for death after implantable cardioverter‐defibrillator implantation in patients with cardiac amyloidosis.
What Are the Clinical Implications?
Patients with cardiac amyloidosis who have implantable cardioverter‐defibrillators implanted are at a high risk of mortality; thus, careful patient selection and shared decision‐making surrounding implantable cardioverter‐defibrillator implantation are important.
Amyloidosis, an infiltrative multisystem disease, is complicated by cardiac involvement in 50% to 60% of patients and can result in progressive heart failure, arrhythmias, and conduction abnormalities. 1 Immunoglobulin light‐chain amyloidosis (AL) and transthyretin amyloidosis (aTTR) account for the vast majority of cardiac amyloidosis cases, with the remaining 5% caused by rare forms such as heavy chain and apolipoprotein amyloid. 2 Amyloidosis fibril deposition is the common pathophysiologic mechanism; however, the natural history of these diseases is significantly different with AL being more rapidly progressive and fatal. Electromechanical dissociation is thought to be the most common cause of sudden cardiac death in patients with cardiac amyloidosis, but ventricular arrhythmias are also common. 3 , 4 Implantable cardioverter‐defibrillators (ICDs) are safe and effective in treating sudden cardiac death caused by fatal arrhythmias in cardiomyopathy; however, data regarding the use of ICDs in patients with cardiac amyloidosis are limited. Previous single‐center and multicenter retrospective studies evaluating mortality in patients with cardiac amyloidosis receiving ICDs have been small, methodologically limited, and inconclusive regarding the outcomes. 5 , 6 , 7 , 8 Current guidelines suggest individualized decision‐making regarding ICD implantation in patients with cardiac amyloidosis based on limited evidence and unclear benefit in this population. 9 Furthermore, current guidelines recommend against ICDs in patients with less than 1‐ year expected survival, 9 but predictors of survival in patients with cardiac amyloidosis and ICDs are poorly understood.
In this study, we evaluated the risk of mortality after ICD implantation in patients with cardiac amyloidosis compared with patients with other nonischemic cardiomyopathies (NICMs). We also investigated factors associated with 1‐year mortality exclusively among patients with cardiac amyloidosis. This information may offer patients with cardiac amyloidosis and healthcare providers vital information regarding risk and prognosis after ICD implantation.
METHODS
The data that support the findings of this study are available from the corresponding author on reasonable request. The Yale University Human Investigation Committee approved the present analysis with a waiver of informed consent.
Data Sources
The National Cardiovascular Data Registry’s ICD Registry was established in 2005 by the American College of Cardiology and the Heart Rhythm Society as a centralized registry for patients receiving ICD implantations. The goal of the registry was to improve safety, treatment, and patterns of care for patients receiving an ICD. 10 The registry met the Centers for Medicare and Medicaid Services data collection requirements, and in early 2006 they mandated that all Medicare patients receiving primary prevention ICDs be included in the registry, although this requirement was recently terminated in February 2018. Though hospitals were mandated to report only on Medicare beneficiaries receiving ICDs for primary prevention between 2006 and 2018, 90% of participating hospitals reported on other patient populations as well, such as those receiving ICDs for secondary prevention and those insured by other payers. 10 The ICD Registry data collection methods have previously been described and validated. 11 , 12 , 13 After initial training of the centers, data are subjected to random audits, with 10% of the centers randomly audited every year. 11
Vital status was obtained using the National Death Index (https://www.cdc.gov/nchs/ndi/index.htm). The National Death Index is a centralized database of death record information that is maintained by the Centers for Disease Control and Prevention’s National Center for Health Statistics. The National Death Index is available for a per‐case fee to epidemiologists and other health and medical investigators. Records are obtained from state and local‐government vital records offices; they contain identifiable information including names, social security numbers, demographic data, and date and cause of death. The accuracy of the National Death Index has been described previously. 14 , 15 , 16
Study Population
All patients in the ICD Registry from April 1, 2010 through December 31, 2015 were included in the study (Figure 1). Patients with cardiac amyloidosis were identified, and then a propensity‐matched cohort of patients with other patients with NICMs enrolled in the registry during the same period was created as a comparator group. Patients with NICMs were chosen as a comparator group to provide a large cohort of patients and a frame of reference for comparison with which the cardiology community is familiar. Diagnoses of cardiac amyloidosis and NICMs were established using site‐reported data from version 2.1 of the ICD Registry data collection form. According to the data dictionary, cardiac amyloidosis is defined as patients with a structural abnormality of the heart other than nonischemic dilated cardiomyopathy, ischemic heart disease, valvular heart disease, prior heart transplant, and a diagnosis of cardiac amyloidosis. The specific type of amyloid cardiomyopathy is not collected in the ICD Registry. A patient with a NICM is defined as one who has a history of nonischemic dilated cardiomyopathy documented by heart failure and reduced systolic function (ejection fraction <40%). Patients with other infiltrative cardiomyopathies, such as cardiac sarcoidosis, were excluded from the study. Patients with a previous ICD or pacemaker and patients with epicardial lead placement were excluded.
Figure 1. Study population selection flow diagram.
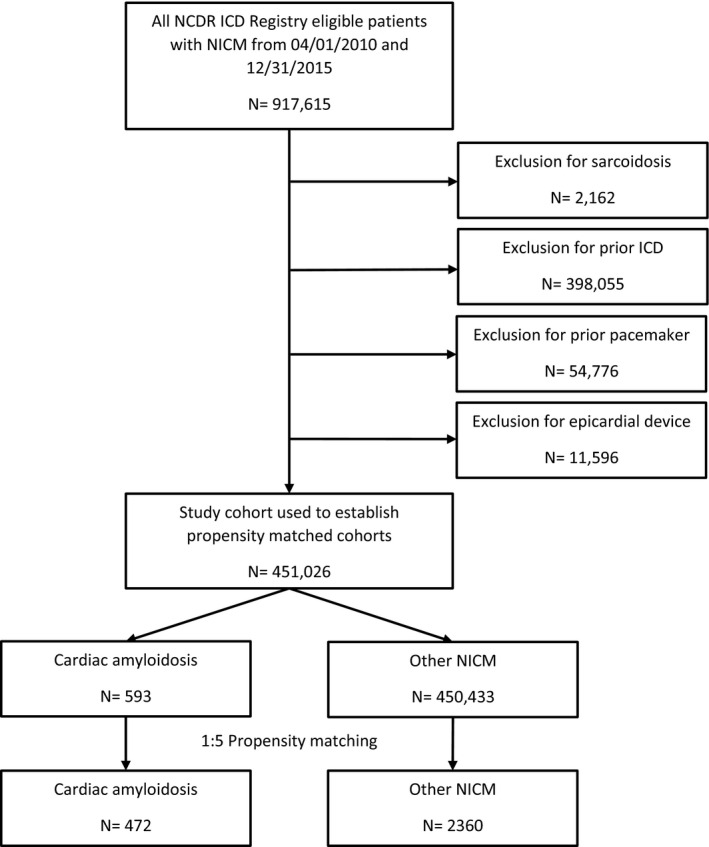
All patients in the NCDR ICD Registry with a diagnosis of cardiac amyloid or nonischemic cardiomyopathy who had ICDs (with or without CRT), implanted from April 1, 2010 through December 31, 2015, were included in the study. Patients with sarcoidosis, prior ICDs, prior pacemakers, or epicardial devices were excluded. A 1:5 propensity‐matched cohort of patients with cardiac amyloidosis and NICMs was created. CRT indicates cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; NCDR, National Cardiovascular Data Registry; and NICM, nonischemic cardiomyopathy
Propensity Score Matching and Outcomes
Patients were matched on sex, age (±1 year), and propensity score using a nearest‐neighbor matching technique 17 , 18 with 1 amyloid cardiomyopathy and up to 5 patients with NICMs. The propensity score was calculated using the logistic regression model with patients with cardiac amyloidosis as the dependent variable. The following variables were included in the model: congestive heart failure, New York Heart Association class, syncope, family history of sudden death, ventricular tachycardia, cerebrovascular disease, diabetes mellitus (DM), dialysis, chronic lung disease, hypertension, left ventricular ejection fraction (grouped by ≤30%, 30%–40%, >40%), creatinine, and ICD indication (primary or secondary prevention). A greedy matching technique was employed to match patients using the logit of the propensity‐score matching with a caliper width of 0.001 difference of the logit of the propensity score. The success of matching was assessed by calculating the standardized difference in the unmatched and matched cohort; a standardized difference of <10% has been proposed as a reasonable level balance between baseline covariates. 19 The primary outcome was all‐cause death.
Statistical Analysis
Baseline characteristics between patients with cardiac amyloidosis and patients with NICMs were compared using the chi‐square test for categorical variables and the T test for continuous variables. McNemar’s test was used for paired proportions or paired t tests for continuous variables in the matched cohort. Median and interquartile range (IQR) or number and percent with associated P values and standardized difference are reported. For the primary outcome of all‐cause mortality, the Kaplan‐Meier method was used to calculate the event‐free survival rates, and differences were compared using the stratified log‐rank test to account for the matched nature of our sample. 20 , 21 Event‐free rates were compared using a Cox‐proportional hazards regression model with a robust variance estimator. 21 Subgroup analysis was performed for the following groups: age (<60 years of age, ≥60 years of age), sex, history of syncope, left ventricular function (<30%, 30%–40%, >40%), inducible ventricular arrhythmia at electrophysiology study, pacing indication (combined second‐ and third‐degree heart block), abnormal intraventricular conduction, and indication (primary versus secondary). These subgroups represent important demographic and patient characteristics that are indications for ICD implantation in infiltrative cardiomyopathies in recent guidelines. 22 , 23 To select variables significantly associated with death among patients with cardiac amyloidosis and patients with NICMs, multivariable Cox‐proportional hazards regression models censored at 2 years with the stepwise method were used. A separate multivariable Cox‐proportional hazards regression model using only patients with cardiac amyloidosis and censored at 1 year from date of ICD implantation was done to evaluate patient factors associated with mortality. Medications were excluded from this analysis. Variables were selected for the models if they had a P value <0.05. The statistical analyses were completed using SAS version 9.4 (SAS, Cary, NC).
RESULTS
Baseline Patient Population and Propensity Matched Cohort
Between April 1, 2010 and December 31, 2015, there were 917 615 patients in the ICD Registry. After excluding patients with a prior ICD (n=398 055) or pacemaker (n=54 776), patients with an epicardial system (n=11 596), and patients with sarcoidosis (n=2162), 451 026 patients were available for analysis (Figure 1). The baseline characteristics of the entire cohort prior to propensity matching are shown in Table S1. Of these patients, 593 had cardiac amyloidosis and 450 433 had another form of NICM. After establishing a 1:5 propensity‐score‐matched cohort, 472 patients with cardiac amyloidosis were successfully matched with 2360 patients with NICMs (Figure 1). The average age of the patients in the overall cohort was 68 years; 22.7% of patients were female. The most common indication for ICD implantation was primary prevention (76.1%), and 26.3% of patients received a cardiac resynchronization therapy defibrillator device. The baseline characteristics of the propensity‐matched cohort are displayed in Table 1. Patients with cardiac amyloidosis were overall very similar to those with NICMs in the propensity‐matched cohort. However, patients with cardiac amyloidosis were more likely to have a history of prior ventricular tachycardia and third‐degree heart block.
Table 1.
Baseline Characteristics of the Propensity‐Matched Cohort Overall and Among Those With Cardiac Amyloidosis Compared With Nonischemic Cardiomyopathy After ICD Implantation
| Description |
Total n (%) |
Amyloidosis n (%) |
NICM n (%) |
P Value |
|---|---|---|---|---|
| All | 2832 (100) | 472 (100) | 2360 (100) | |
| Demographics | ||||
| Age, mean (SD), y | 68.2 (11.8) | 68.2 (11.8) | 68.2 (11.8) | 0.98 |
| Sex female | 642 (22.7) | 107 (22.7) | 535 (22.7) | 1.00 |
| Race | 0.67 | |||
| White (non‐Hispanic) | 1853 (65.4) | 299 (63.3) | 1554 (65.8) | |
| Black (non‐Hispanic) | 816 (28.8) | 146 (30.9) | 670 (28.4) | |
| Hispanic | 110 (3.9) | 17 (3.6) | 93 (3.9) | |
| Other | 53 (1.87) | 10 (2.1) | 43 (1.8) | |
| Clinical history | ||||
| Heart failure | 2388 (84.3) | 392 (83.1) | 1996 (84.6) | 0.41 |
| NYHA Class | 0.30 | |||
| Class I | 370 (13.1) | 69 (14.6) | 301 (12.8) | |
| Class II | 989 (34.9) | 159 (33.7) | 830 (35.2) | |
| Class III | 1327 (46.9) | 219 (46.4) | 1108 (46.9) | |
| Class IV | 131 (4.6) | 25 (5.3) | 106 (4.5) | |
| Syncope | 681 (24.1) | 116 (24.6) | 565 (23.9) | 0.79 |
| Family history of sudden death | 102 (3.6) | 17 (3.6) | 85 (3.6) | 0.74 |
| Atrial fibrillation/flutter | 1299 (45.9) | 221 (46.8) | 1078 (45.7) | 0.67 |
| VT | 1101 (38.9) | 224 (47.5) | 877 (37.2) | <0.001 |
| VT type | 0.24 | |||
| Nonsustained VT | 628 (57.0) | 143 (63.8) | 485 (55.3) | |
| Sustained monomorphic VT | 289 (26.3) | 49 (21.9) | 240 (27.4) | |
| Sustained polymorphic VT | 74 (6.7) | 10 (4.5) | 64 (7.3) | |
| Sustained monomorphic and polymorphic VT | 33 (3.0) | 7 (3.1) | 26 (3.0) | |
| Unknown | 77 (7.0) | 15 (6.7) | 62 (7.0) | |
| Cerebrovascular disease | 316 (11.2) | 50 (10.6) | 266 (11.3) | 0.77 |
| Lung disease | 429 (15.2) | 67 (14.2) | 362 (15.3) | 0.36 |
| Diabetes mellitus | 643 (22.7) | 112 (23.7) | 531 (22.5) | 0.47 |
| Sleep apnea | 361 (12.8) | 57 (12.1) | 304 (12.9) | 0.82 |
| Dialysis | 132 (4.7) | 19 (4.0) | 113 (4.8) | 0.38 |
| Hypertension | 1922 (67.9) | 325 (68.9) | 1597 (67.7) | 0.37 |
| Patient life expectancy of ≥1 y | 0.70 | |||
| No | 69 (2.4) | 10 (2.1) | 59 (2.5) | |
| Yes | 1176 (41.5) | 204 (43.2) | 972 (41.2) | |
| Not documented | 1552 (54.8) | 254 (53.8) | 1298 (55.0) | |
| Diagnostic studies | ||||
| Left ventricular ejection fraction | 0.75 | |||
| ≤30 | 1413 (49.9) | 236 (50.0) | 1177 (49.9) | |
|
>30– 40 |
630 (22.3) | 99 (21.0) | 531 (22.5) | |
| >40 | 699 (24.7) | 119 (25.2) | 580 (24.6) | |
| QRS duration | 0.84 | |||
| ≤140 | 2115 (74.7) | 353 (74.8) | 1762 (74.7) | |
| >140 | 677 (23.9) | 111 (23.5) | 566 (24.0) | |
| Creatinine, mean (SD) | 1.57 (1.7) | 1.63 (1.4) | 1.56 (1.7) | 0.38 |
| Inducible ventricular arrhythmia on EP study | 310 (11.0) | 28 (5.9) | 282 (11.9) | 0.00 |
| Abnormal intraventricular conduction | 1357 (47.9) | 244 (51.7) | 1113 (47.2) | 0.19 |
| Cardiac rhythm paced | 44 (1.6) | 7 (1.5) | 37 (1.6) | 0.89 |
| Cardiac rhythm third‐degree heart block | 53 (1.9) | 20 (4.2) | 33 (1.4) | 0.00 |
| Brain natriuretic peptide, mean (SD) | 1111 (1468) | 1244 (1617) | 1072 (1421) | 0.24 |
| Medications | ||||
| ACE inhibitor | 979 (34.6) | 165 (35.0) | 814 (34.5) | 0.85 |
| Angiotensin receptor blocker | 457 (16.1) | 77 (16.3) | 380 (16.1) | 0.91 |
| Aspirin | 1544 (54.5) | 252 (53.4) | 1292 (54.7) | 0.59 |
| Beta‐blocker | 1996 (70.5) | 339 (71.8) | 1657 (70.2) | 0.48 |
| Statin | 1379 (48.7) | 223 (47.2) | 1156 (49.0) | 0.49 |
| Nonstatin lipid medication | 105 (3.7) | 19 (4.0) | 86 (3.6) | 0.69 |
| Clopidogrel | 260 (9.2) | 39 (8.3) | 221 (9.4) | 0.45 |
| Ticlopidine | 9 (0.3) | 3 (0.6) | 6 (0.3) | 0.18 |
| Prasugrel | 9 (0.3) | 1 (0.2) | 8 (0.3) | 0.65 |
| Antiarrhythmic agent | 546 (19.3) | 90 (19.1) | 456 (19.3) | 0.73 |
| Diltiazem | 37 (1.3) | 7 (1.5) | 30 (1.3) | 0.71 |
| Verapamil | 14 (0.5) | 2 (0.4) | 12 (0.5) | 0.81 |
| Other calcium channel blocker | 128 (4.5) | 24 (5.1) | 104 (4.4) | 0.52 |
| Digoxin | 166 (5.9) | 29 (6.1) | 137 (5.8) | 0.77 |
| Diuretic | 2073 (73.2) | 341 (72.2) | 1732 (73.4) | 0.61 |
| Hydralazine | 146 (5.2) | 26 (5.5) | 120 (5.1) | 0.70 |
| Long‐acting nitroglycerin | 148 (5.2) | 28 (5.9) | 120 (5.1) | 0.45 |
| Warfarin | 973 (34.4) | 169 (35.8) | 804 (34.1) | 0.47 |
| Procedural factors | ||||
| ICD indication | 0.69 | |||
| Primary prevention | 2156 (76.1) | 356 (75.4) | 1800 (76.3) | |
| Secondary prevention | 676 (23.9) | 116 (24.6) | 560 (23.7) | |
| Device type | 0.95 | |||
| Single chamber | 722 (25.5) | 124 (26.3) | 598 (25.3) | |
| Dual chamber | 1355 (47.9) | 224 (47.5) | 1131 (47.9) | |
| CRT‐D | 746 (26.3) | 123 (26.1) | 623 (26.4) | |
ACE indicates angiotensin converting enzyme; CRT‐D, cardiac resynchronization therapy defibrillator; EP, electrophysiology; ICD, implantable cardioverter‐defibrillator; NICM, nonischemic cardiomyopathy; NYHA, New York Heart Association; and VT, ventricular tachycardia.
Mortality for Patients With Amyloid Cardiomyopathy Compared With NICMs
The 1‐year mortality rate of patients with cardiac amyloidosis was 26.9%, which was significantly higher than the propensity‐matched patients with NICMs at 11.3% (P<0.001). The difference in survival began immediately after implant and continued to progressively widen over time (Figure 2). The median follow‐up for patients with amyloid cardiomyopathy was 42 months (IQR, 25–62 months) and 46 months (IQR, 27–64 months) for patients with NICMs. The median time from ICD implantation to death was 12 months (IQR, 4–26 months) for patients with amyloidosis and 19 months (IQR, 5–35 months) for those with NICMs.
Figure 2. Probability of survival.
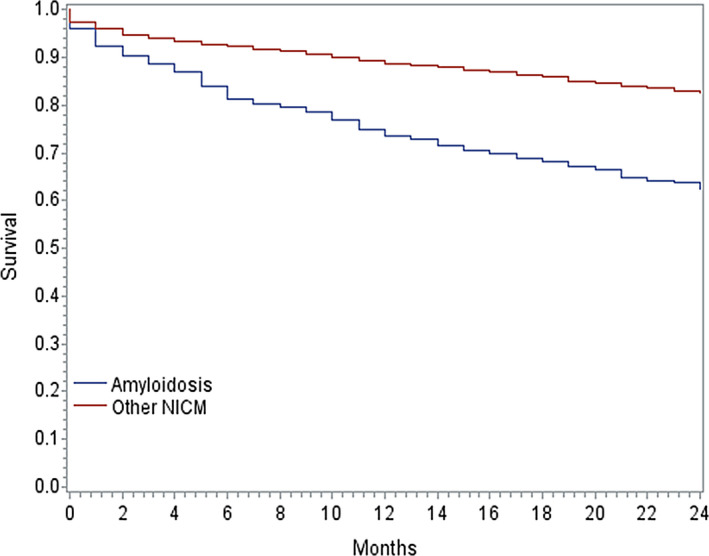
Kaplan‐Meier curves for survival following implantable cardioverter‐defibrillator implantation stratified by type of cardiomyopathy. Survival in patients with cardiac amyloidosis was significantly lower than the propensity‐matched cohort of patients with NICMs. NICM indicates nonischemic cardiomyopathy.
After adjusting for factors significantly associated with death in multivariate analysis, cardiac amyloidosis was associated with a significantly increased risk of death compared with NICMs (hazard ratio [HR], 1.80; 95% CI, 1.56–2.08; Table 2). This finding was consistent in nearly all subgroups evaluated including age (<60 years of age, ≥60 years of age); sex; syncope; left ventricular ejection fraction (≤30%, 30%–40%, >40%), inducible ventricular arrhythmia at electrophysiology study, abnormal intraventricular conduction, and ICD indication (primary versus secondary prevention). The only subgroup tested in which amyloid cardiomyopathy was not associated with increased mortality was the presence of advanced (2nd or 3rd degree) heart block (relative risk [RR], 2.32; 95% CI, 0.66–8.16).
Table 2.
Relative Risk of Death for Patients With Cardiac Amyloidosis Compared With Nonischemic Cardiomyopathy and an ICD Through 2 Years of Follow‐Up Overall and in Subgroups
| Description | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| Relative Risk | 95% CI | P Value | Relative Risk | 95% CI | P Value | |
| Overall | 1.85 | 1.61–2.13 | <0.001 | 1.80 | 1.56–2.08 | <0.001 |
| Age, y | ||||||
| Age<60 | 2.54 | 1.78–3.62 | <0.001 | 2.67 | 1.85 –3.86 | <0.001 |
| Age≥60 | 1.74 | 1.49–2.03 | <0.001 | 1.71 | 1.46–2.01 | <0.001 |
| Sex | ||||||
| Male | 1.85 | 1.58–2.17 | <0.001 | 1.84 | 1.56–2.16 | <0.001 |
| Female | 1.85 | 1.35–2.52 | <0.001 | 1.67 | 1.21–2.31 | 0.002 |
| Syncope | ||||||
| No | 1.72 | 1.46–2.02 | <0.001 | 1.73 | 1.47–2.04 | <0.001 |
| Yes | 2.34 | 1.77–3.09 | <0.001 | 2.00 | 1.48–2.70 | <0.001 |
| Left ventricular ejection fraction (%) | ||||||
| Unknown | 2.14 | 0.93–4.92 | 0.08 | 2.20 | 0.84–5.78 | 0.11 |
| ≤30 | 1.73 | 1.42–2.11 | <0.001 | 1.67 | 1.37–2.04 | <0.001 |
| >30–40 | 1.91 | 1.42–2.57 | <0.001 | 1.74 | 1.27–2.38 | 0.001 |
| >40 | 2.07 | 1.54–2.78 | <0.001 | 1.95 | 1.43–2.65 | <0.001 |
| Abnormal intraventricular conduction | ||||||
| No | 2.24 | 1.84–2.73 | <0.001 | 2.19 | 1.79–2.68 | <0.001 |
| Yes | 1.52 | 1.24–1.87 | <0.001 | 1.51 | 1.23–1.86 | <0.001 |
| Second‐ or third‐degree heart block | ||||||
| No | 1.85 | 1.60–2.14 | <0.001 | 1.81 | 1.56–2.09 | <0.001 |
| Yes | 1.49 | 0.71–3.13 | 0.29 | 2.32 | 0.66–8.16 | 0.192 |
| ICD indication | ||||||
| Primary prevention | 1.69 | 1.44–2.00 | <0.001 | 1.70 | 1.44–2.01 | <0.001 |
| Secondary prevention | 2.39 | 1.82–3.15 | <0.001 | 2.16 | 1.63–2.87 | <0.001 |
ICD indicates implantable cardioverter‐defibrillator.
Univariate and Multivariable Predictors of Mortality Among Patients With Cardiac Amyloidosis
Among patients with cardiac amyloidosis and an ICD, several variables were associated with an increased risk of mortality within 1 year after ICD implantation in univariate analysis including heart failure duration <3 months (HR, 1.68; 95% CI 1.03–2.76), syncope (HR, 1.84, 95% CI, 1.28–2.65), ventricular tachycardia (HR, 1.66; 95% CI, 1.17–2.36), cerebrovascular disease (HR, 1.98; 95% CI, 1.25–3.14), dialysis (HR, 3.37; 95% CI, 1.86–6.12), creatinine = 1.5 to 2.5 g/dL (HR, 2.01; 95% CI, 1.33–3.03), creatinine >2.5 (HR, 3.60; 95% CI, 2.27–5.70), and secondary prevention ICD indication (HR, 1.96; 95% CI, 1.36–2.81) (Table S2). In multivariable analysis, the following risk factors remained significantly associated with mortality: syncope (HR, 1.78; 95% CI, 1.22–2.59), ventricular tachycardia (HR, 1.65; 95% CI, 1.15–2.38), cerebrovascular disease (HR, 2.03; 95% CI, 1.28–3.23), DM (HR, 1.55; 95% CI, 1.05–2.27), creatinine = 1.6 to 2.5 g/dL (HR, 1.99; 95% CI, 1.32–3.02), and creatinine >2.5 g/dL (HR, 4.34; 95% CI, 2.72–6.93) (Table 3). Definitions for variables significant in multivariable analysis are provided in Table S3.
Table 3.
Patient Risk Factors Significantly Associated With 1‐Year Survival in Multivariable Analysis Among Those With Cardiac Amyloidosis
| Parameter | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Syncope | 1.78 | 1.22–2.59 | 0.003 |
| Ventricular tachycardia | 1.65 | 1.15–2.38 | 0.01 |
| Prior cerebrovascular disease | 2.03 | 1.28–3.23 | 0.003 |
| Diabetes mellitus | 1.55 | 1.05–2.27 | 0.03 |
| Creatinine = 1.6–2.5 g/dL | 1.99 | 1.32–3.02 | 0.001 |
| Creatinine >2.5 g/dL | 4.34 | 2.72–6.93 | <0.001 |
DISCUSSION
This study of 472 patients with cardiac amyloidosis and ICDs is the largest study of this population to date and found 2 key findings. First, cardiac amyloidosis was associated with a mortality rate of 26.9% at 1 year after ICD implantation compared with 11.3% among a propensity‐matched cohort of patients with other NICMs. After adjustment for covariates, cardiac amyloidosis was associated with a significantly higher risk of all‐cause mortality compared with NICMs (HR, 1.80; 95% CI, 1.56–2.08), and these findings were consistent in essentially all subgroups we evaluated. Second, we identified 6 variables independently associated with 1‐year mortality in patients with cardiac amyloidosis who underwent ICD implantation: syncope (HR, 1.78; 95% CI, 1.22–2.59), ventricular tachycardia (HR, 1.65; 95% CI, 1.15–2.38), cerebrovascular disease (HR, 2.03; 95% CI, 1.28–3.23), DM (HR, 1.55; 95% CI, 1.05–2.27), creatinine = 1.6 to 2.5 g/dL (HR, 1.99; 95% CI, 1.32–3.02), and creatinine >2.5 (HR, 4.34; 95% CI, 2.72–6.93).
Our finding that 26.9% of patients with amyloid cardiomyopathy die within 1 year of ICD implantation is fairly consistent with previous studies that were smaller and less definitive. 5 , 6 The largest prior study to date in this population included 53 patients with cardiac amyloidosis and an ICD implanted at the Mayo Clinic, and 60% of these patients died over a mean 1.9±1.8 years of follow‐up. 7 In another study of 45 patients with cardiac amyloidosis, 26.7% died over 1.4±1.2 years of follow‐up. 8 Although we cannot account for the competing risk of death from progression of underlying disease, our study confirms the high mortality of patients with cardiac amyloidosis despite ICD implantation in a much larger cohort treated at multiple centers around the United States, making our results broadly generalizable.
We also found that patients with cardiac amyloidosis were at a significantly increased risk of mortality compared with propensity‐matched patients with patients with NICMs. In subgroup analysis, we found that the risk of death was significantly higher in all subgroups tested except for advanced heart block. We suspect that patients who present with heart block and thus require a pacing device may receive an ICD earlier in their disease course; this likely represents a length time bias rather than an actual difference. It is possible that some patients within the NICM cohort had undiagnosed cardiac amyloidosis; recent studies have shown a prevalence of aTTR in 13% of patients with heart failure with preserved ejection fraction 24 and 16% among patients with patients with severe aortic stenosis. 25 This may result in an underestimation of the true difference in survival. Overall, these findings highlight the progressive nature of the disease and further confirm that the natural history of patients with cardiac amyloidosis differs substantially from other NICMs despite ICD implantation.
The 2 primary types of cardiac amyloidosis, AL and aTTR, have markedly different prognoses. AL has traditionally been associated with survival of less than 1 year; however, advances in the treatment of AL 26 and new therapies targeted at aTTR 27 , 28 may change the landscape for these patients. The NCDR ICD Registry does not collect data on the type of amyloid cardiomyopathy; however, age can be used as a proxy for the type of amyloid cardiomyopathy as most patients with AL will be captured in the <60 years‐of‐age group and aTTT in the ≥60 years‐of‐age group. 29 , 30 , 31 , 32 Age was not predictive of mortality in the multivariable analysis; however, patients <60 years of age had a relative risk of 2.7 and patients ≥60 years of age had a relative risk of 1.7 compared with patients with NICMs in subgroup analysis, suggesting that the risk of death in younger patients with AL may be higher despite ICD implantation.
We identified a number of risk factors for mortality in patients with amyloid cardiomyopathy and ICDs, which have been poorly assessed in prior studies because of their limited size and patient data. Syncope and ventricular tachycardia are well‐established risk factors for mortality in patients with cardiovascular disease. Although previous studies have identified ventricular arrhythmias 3 , 33 as a risk factor for mortality in patients with cardiac amyloidosis, the 2 studies on patients with cardiac amyloidosis with ICDs discussed above found that ventricular arrhythmias were not associated with differences in survival. 7 , 8 In our study of a much larger cohort, ventricular tachycardia was associated with increased mortality despite ICD presence. It is likely that ventricular tachycardia is a manifestation of progressive infiltration and scar burden and represents progression towards end‐stage disease.
We also found that patients with impaired renal function were at significantly increased risk of mortality, and that there was a dose‐response relationship between kidney disease and worse prognosis. Importantly, among patients with creatinine >2.5 the risk of death was more than 4‐fold higher compared with those without kidney dysfunction (HR, 4.34; 95% CI, 2.72–6.93). Over 50% of patients with AL have renal involvement and many patients progress to dialysis. 34 , 35 , 36 In an earlier study of 145 patients with AL, those with renal AL had significantly better outcomes compared with patients without renal involvement; however, patients without renal AL had a disproportionally higher prevalence of cardiac amyloidosis, which likely accounted for the unexpected outcomes. 34 When patients with renal AL were compared with patients without concomitant renal and cardiac AL, survival was not significantly different. 34 Although renal impairment is not a hallmark of aTTR, renal function, using estimated glomerular filtration rate, has been identified as an independent prognostic factor in cardiac aTTR and has been incorporated into a new staging system. 37 We cannot discern the type of amyloidosis or the etiology of the kidney disease, but we clearly demonstrate that the combination of cardiac amyloidosis and kidney dysfunction portends a markedly worse prognosis compared with those with normal renal function.
We also identified DM as a risk factor significantly associated with increased mortality among those with cardiac amyloidosis receiving an ICD. To our knowledge, DM has not previously been associated with mortality in patients with cardiac amyloidosis; our data suggest that DM should be taken into account when considering an ICD and patients with DM should be followed closely.
In summary, our findings suggest that patients with a history of syncope, ventricular tachycardia, DM, cerebrovascular disease, or renal dysfunction may be at particularly high risk for death within 1 year of ICD implantation. Despite ICD implantation, mortality remains relatively high in patients with cardiac amyloidosis. These findings underscore the importance of careful patient selection for ICD implantation, shared decision‐making with these patients, and close follow‐up after implantation.
Limitations
There are several limitations to this study. First, as a retrospective observational study, we were not able to account for all potential confounding factors, and our sample size was limited for subgroup analyses. However, the ICD Registry collects a large number of data elements, including patient characteristics, which were utilized in our analysis to limit the chances of significant residual confounding, and this data source was complete relative to prior studies of this topic. Second, the diagnosis of cardiac amyloidosis or other forms of cardiomyopathy was site‐reported. It is possible that some patients with amyloid cardiomyopathy or other specific cardiomyopathies are included in the NICM cohort; therefore, our data may underestimate any differences between the 2 groups. Third, the ICD Registry does not collect data on the type of amyloidosis. Different types of amyloid cardiomyopathy are treated differently and carry disparate prognoses, but as discussed above, a cut‐off of 60 years of age roughly correlates with the 2 most common types of amyloid cardiomyopathy, which allowed us to indirectly evaluate for differential outcomes. This study also does not have a control group of patients who did not receive an ICD or have ICD therapy data; thus, our data does not reflect the disease course or prognosis of patients with amyloid cardiomyopathy without ICDs; hence, we were not able to make direct inferences about the effectiveness of ICD implantation/therapy in prolonging survival. However, our comparison with matched patients with other NICMs allowed us to evaluate for overall prognosis despite ICD implantation. The ICD Registry is not linked to manufacturer‐collected device programming and therapy data, which could provide more information on appropriate and inappropriate shocks and that could be the subject of future studies. Lastly, we did not report cause of death. Although we had vital statistics data on cause of death through the National Death Index, prior reports have suggested that this is an inaccurate method of assessing cause of death. 38
Conclusions
In this study, we showed that, among patients with cardiac amyloidosis, mortality after ICD implantation was over 25% within 1 year, which is more than double that of propensity‐ matched patients with other NICMs. After controlling for other comorbidities, cardiac amyloidosis remained strongly associated with a higher risk of death compared with other NICMs. Risk factors for death at 1 year among those with cardiac amyloidosis and an ICD included syncope, ventricular tachycardia, cerebrovascular disease, DM, and impaired renal function. These data offer important information for physicians and patients when deciding whether to place an ICD in those with cardiac amyloidosis and a clearer sense of prognosis in the years after implantation.
Sources of Funding
This research was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry. The views expressed herein represent those of the author(s) and do not necessarily represent the official views of the National Cardiovascular Data Registry or its associated professional societies (www.ncdr.com).
Disclosures
Dr Minges and Yongfei Wang receive salary support for analytic services provided to the American College of Cardiology. Dr Lampert reports research grants from Medtronic and Abbott Laboratories/St. Jude Medical and serves on the Medtronic Advisory Board and receives moderate honoraria. Dr Rosenfeld reports fellowship support and stock ownership for Abbott Laboratories and fellowship support from Boston Scientific and Medtronic. Dr Jacoby reports being on the speaker’s bureau and an advisory board for Alnylam and participating in ongoing funded research with Alnylam; receiving a research grant from Myokardia; serving on an advisory board and steering committee for Myokardia; and receiving consulting fees from Abbott. Dr Curtis has a contract with the American College of Cardiology for his role as senior medical officer, National Cardiovascular Data Registry; receives salary support from the American College of Cardiology, National Cardiovascular Data Registry; receives funding from the Centers for Medicare & Medicaid Services to develop and maintain performance measures that are used for public reporting; and holds equity interest in Medtronic. Dr Miller reports grants from Bracco and Eidos, consulting for General Electric, Alnylam, and Pfizer, outside the submitted work. Dr Freeman reports consulting/advisory board fees from Janssen Pharmaceuticals, Medtronic, Boston Scientific, and Biosense Webster. He receives salary/research support from the American College of Cardiology, National Cardiovascular Data Registry, and the National Heart Lung and Blood Institute. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
(J Am Heart Assoc. 2020;9:e016038 DOI: 10.1161/JAHA.120.016038.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.016038
For Sources of Funding and Disclosures, see pages 9 and 10.
References
- 1. Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;2047–2060. [DOI] [PubMed] [Google Scholar]
- 2. Kyle RA, Linos A, Beard CM, Linke RP, Gertz MA, O'Fallon WM, Kurland LT. Incidence and natural history of primary systemic amyloidosis in olmsted county, minnesota, 1950 through 1989. Blood. 1992;1817–1822. [PubMed] [Google Scholar]
- 3. Palladini G, Malamani G, Co F, Pistorio A, Recusani F, Anesi E, Garini P, Merlini G. Holter monitoring in al amyloidosis: Prognostic implications. Pacing Clin Electrophysiol. 2001;1228–1233. [DOI] [PubMed] [Google Scholar]
- 4. Dubrey SW, Cha K, Anderson J, Chamarthi B, Reisinger J, Skinner M, Falk RH. The clinical features of immunoglobulin light‐chain (al) amyloidosis with heart involvement. QJM : monthly journal of the Association of Physicians. 1998;141–157. [DOI] [PubMed] [Google Scholar]
- 5. Varr BC, Zarafshar S, Coakley T, Liedtke M, Lafayette RA, Arai S, Schrier SL, Witteles RM. Implantable cardioverter‐defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm. 2014;158–162. [DOI] [PubMed] [Google Scholar]
- 6. Kristen AV, Dengler TJ, Hegenbart U, Schonland SO, Goldschmidt H, Sack FU, Voss F, Becker R, Katus HA, Bauer A. Prophylactic implantation of cardioverter‐defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm. 2008;235–240. [DOI] [PubMed] [Google Scholar]
- 7. Lin G, Dispenzieri A, Kyle R, Grogan M, Brady PA. Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol. 2013;793–798. [DOI] [PubMed] [Google Scholar]
- 8. Hamon D, Algalarrondo V, Gandjbakhch E, Extramiana F, Marijon E, Elbaz N, Selhane D, Dubois‐Rande JL, Teiger E, Plante‐Bordeneuve V, et al. Outcome and incidence of appropriate implantable cardioverter‐defibrillator therapy in patients with cardiac amyloidosis. Int J Cardiol. 2016;562–568. [DOI] [PubMed] [Google Scholar]
- 9. Al‐Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, et al. 2017 aha/acc/hrs guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. Circulation. 2018;e272–e391. [DOI] [PubMed] [Google Scholar]
- 10. Masoudi FA, Ponirakis A, de Lemos JA, Jollis JG, Kremers M, Messenger JC, Moore JW, Moussa I, Oetgen WJ, Varosy PD, et al. report from 4 acc national cardiovascular data registries. J Am Coll Cardiol. 2016;1427–1450. [DOI] [PubMed] [Google Scholar]
- 11. Messenger JC, Ho KK, Young CH, Slattery LE, Draoui JC, Curtis JP, Dehmer GJ, Grover FL, Mirro MJ, Reynolds MR, et al. Quality Oversight Committee Data Quality W. The national cardiovascular data registry (ncdr) data quality brief: The ncdr data quality program in 2012. J Am Coll Cardiol. 2012;1484–1488. [DOI] [PubMed] [Google Scholar]
- 12. Hammill S, Phurrough S, Brindis R. The national icd registry: Now and into the future. Heart Rhythm. 2006;470–473. [DOI] [PubMed] [Google Scholar]
- 13. Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to medicare claims data using indirect identifiers. Am Heart J. 2009;995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major us mortality databases. Ann Epidemiol. 2002;462–468. [DOI] [PubMed] [Google Scholar]
- 15. Curb JD, Ford CE, Pressel S, Palmer M, Babcock C, Hawkins CM. Ascertainment of vital status through the national death index and the social security administration. Am J Epidemiol. 1985;754–766. [DOI] [PubMed] [Google Scholar]
- 16. Hanna DB, Pfeiffer MR, Sackoff JE, Selik RM, Begier EM, Torian LV. Comparing the national death index and the social security administration's death master file to ascertain death in HIV surveillance. Public Health Rep. 2009;850–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bergstralh EJ, Kosanke JL. Computerized matching of cases and controls. Technical Report Series No. 56 Department of Health Sciences Research, Mayo Clinic, Rochester, Minnesota. 1995.
- 18. P.R. R. Optimal matching for observational studies . Journal of the American Statistical Association. 1989;1024–1032. [Google Scholar]
- 19. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Austin PC. Primer on statistical interpretation or methods report card on propensity‐score matching in the cardiology literature from 2004 to 2006: a systematic review. Circ Cardiovasc Qual Outcomes. 2008;62–67. [DOI] [PubMed] [Google Scholar]
- 21. Austin PC. The use of propensity score methods with survival or time‐to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;1242–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Russo AM, Stainback RF, Bailey SR, Epstein AE, Heidenreich PA, Jessup M, Kapa S, Kremers MS, Lindsay BD, Stevenson LW. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter‐defibrillators and cardiac resynchronization therapy: a report of the american college of cardiology foundation appropriate use criteria task force, heart rhythm society, american heart association, american society of echocardiography, heart failure society of America, society for cardiovascular angiography and interventions, society of cardiovascular computed tomography, and society for cardiovascular magnetic resonance. Heart Rhythm. 2013;e11–e58. [DOI] [PubMed] [Google Scholar]
- 23. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, et al.American College of Cardiology/American Heart Association Task Force on Practice G, American Association for Thoracic S, Society of Thoracic S . ACC/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities: a report of the American college of cardiology/American heart association task force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): Developed in collaboration with the American association for thoracic surgery and society of thoracic surgeons. Circulation. 2008;e350–e408. [DOI] [PubMed] [Google Scholar]
- 24. Gonzalez‐Lopez E, Gallego‐Delgado M, Guzzo‐Merello G, de Haro‐Del Moral FJ, Cobo‐Marcos M, Robles C, Bornstein B, Salas C, Lara‐Pezzi E, Alonso‐Pulpon L, et al. Wild‐type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;2585–2594. [DOI] [PubMed] [Google Scholar]
- 25. Castano A, Narotsky DL, Hamid N, Khalique OK, Morgenstern R, DeLuca A, Rubin J, Chiuzan C, Nazif T, Vahl T, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;2879–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sperry BW, Ikram A, Hachamovitch R, Valent J, Vranian MN, Phelan D, Hanna M. Efficacy of chemotherapy for light‐chain amyloidosis in patients presenting with symptomatic heart failure. J Am Coll Cardiol. 2016;2941–2948. [DOI] [PubMed] [Google Scholar]
- 27. Rosenblum H, Castano A, Alvarez J, Goldsmith J, Helmke S, Maurer MS. TTR (transthyretin) stabilizers are associated with improved survival in patients with TTR cardiac amyloidosis. Circ Heart Fail. 2018;e004769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington‐Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;1007–1016. [DOI] [PubMed] [Google Scholar]
- 29. Coelho T, Maurer MS, Suhr OB. Thaos ‐ the transthyretin amyloidosis outcomes survey: initial report on clinical manifestations in patients with hereditary and wild‐type transthyretin amyloidosis. Curr Med Res Opin. 2013;63–76. [DOI] [PubMed] [Google Scholar]
- 30. Connors LH, Sam F, Skinner M, Salinaro F, Sun F, Ruberg FL, Berk JL, Seldin DC. Heart failure resulting from age‐related cardiac amyloid disease associated with wild‐type transthyretin: a prospective, observational cohort study. Circulation. 2016;282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of al amyloidosis: a real‐world study using us claims data. Blood Adv. 2018;1046–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;1286–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldsmith YB, Liu J, Chou J, Hoffman J, Comenzo RL, Steingart RM. Frequencies and types of arrhythmias in patients with systemic light‐chain amyloidosis with cardiac involvement undergoing stem cell transplantation on telemetry monitoring. Am J Cardiol. 2009;990–994. [DOI] [PubMed] [Google Scholar]
- 34. Gertz MA, Leung N, Lacy MQ, Dispenzieri A, Zeldenrust SR, Hayman SR, Buadi FK, Dingli D, Greipp PR, Kumar SK, et al. Clinical outcome of immunoglobulin light chain amyloidosis affecting the kidney. Nephrol Dial Transplant. 2009;3132–3137. [DOI] [PubMed] [Google Scholar]
- 35. Obici L, Perfetti V, Palladini G, Moratti R, Merlini G. Clinical aspects of systemic amyloid diseases. Biochim Biophys Acta. 2005;11–22. [DOI] [PubMed] [Google Scholar]
- 36. Pinney JH, Lachmann HJ, Bansi L, Wechalekar AD, Gilbertson JA, Rowczenio D, Sattianayagam PT, Gibbs SD, Orlandi E, Wassef NL, et al. Outcome in renal al amyloidosis after chemotherapy. J Clin Oncol. 2011;674–681. [DOI] [PubMed] [Google Scholar]
- 37. Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez‐Naharro A, Quarta CC, Rezk T, Whelan CJ, Gonzalez‐Lopez E, et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;2799–2806. [DOI] [PubMed] [Google Scholar]
- 38. Olubowale OT, Safford MM, Brown TM, Durant RW, Howard VJ, Gamboa C, Glasser SP, Rhodes JD, Levitan EB. Comparison of expert adjudicated coronary heart disease and cardiovascular disease mortality with the national death index: results from the reasons for geographic and racial differences in stroke (regards) study. J Am Heart Assoc. 2017;6 DOI: 10.1161/JAHA.116.004966 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


