Abstract
BACKGROUND
Internationally, most atrial fibrillation (AF) management guidelines recommend opportunistic screening for AF in people ≥65 years of age and oral anticoagulant treatment for those at high stroke risk (CHA₂DS₂‐VA≥2). However, gaps remain in screening and treatment.
METHODS AND RESULTS
General practitioners/nurses at practices in rural Australia (n=8) screened eligible patients (≥65 years of age without AF) using a smartphone ECG during practice visits. eHealth tools included electronic prompts, guideline‐based electronic decision support, and regular data reports. Clinical audit tools extracted de‐identified data. Results were compared with an earlier study in metropolitan practices (n=8) and nonrandomized control practices (n=69). Cost‐effectiveness analysis compared population‐based screening with no screening and included screening, treatment, and hospitalization costs for stroke and serious bleeding events. Patients (n=3103, 34%) were screened (mean age, 75.1±6.8 years; 47% men) and 36 (1.2%) new AF cases were confirmed (mean age, 77.0 years; 64% men; mean CHA₂DS₂‐VA, 3.2). Oral anticoagulant treatment rates for patients with CHA₂DS₂‐VA≥2 were 82% (screen detected) versus 74% (preexisting AF)(P=NS), similar to metropolitan and nonrandomized control practices. The incremental cost‐effectiveness ratio for population‐based screening was AU$16 578 per quality‐adjusted life year gained and AU$84 383 per stroke prevented compared with no screening. National implementation would prevent 147 strokes per year. Increasing the proportion screened to 75% would prevent 177 additional strokes per year.
CONCLUSIONS
An AF screening program in rural practices, supported by eHealth tools, screened 34% of eligible patients and was cost‐effective. Oral anticoagulant treatment rates were relatively high at baseline, trending upward during the study. Increasing the proportion screened would prevent many more strokes with minimal incremental cost‐effectiveness ratio change. eHealth tools, including data reports, may be a valuable addition to future programs.
REGISTRATION
URL: https://www.anzctr.org.au. Unique identifier: ACTRN12618000004268.
Keywords: cost‐effectiveness, digital health, general practice, primary care, rural, stroke prevention
Subject Categories: Atrial Fibrillation, Ischemic Stroke, Primary Prevention, Cost-Effectiveness, Treatment
Nonstandard Abbreviations and Acronyms
- AF
atrial fibrillation
- EDS
electronic decision support
- GP
general practitioner
- iECG
handheld single‐lead smartphone ECG
- ICER
incremental cost‐effectiveness ratio
- NOAC
non–vitamin K dependent oral anticoagulant
- OAC
oral anticoagulant
- QALY
quality‐adjusted life year
- QI
quality improvement
CLINICAL PERSPECTIVE
What Is New?
This study extends the evidence base in rural areas by demonstrating that a screening program using eHealth tools in a rural general practice setting can successfully screen 34% of eligible patients with atrial fibrillation with guideline‐indicated treatment rates >80% for screen‐detected atrial fibrillation cases.
Economic modeling showed that the program was cost‐effective compared with no screening.
Oral anticoagulant treatment rates for eligible patients were higher than previous studies at baseline (>70%) and were trending upward during the study (around 80%).
What Are the Clinical Implications?
eHealth tools, particularly customized data reports as part of an audit and feedback system, may be a valuable addition to screening programs.
Half of the practices screened 40% to 50% of eligible patients, suggesting that this may represent a ceiling of patients captured by opportunistic atrial fibrillation screening programs in the general practice setting.
Increasing the proportion screened would prevent many more strokes with minimal change to the incremental cost‐effectiveness ratio.
Internationally, opportunistic screening for atrial fibrillation (AF) in people ≥65 years of age is now recommended by most guidelines. 1 , 2 Single timepoint screening detects undiagnosed AF, which is often asymptomatic, in approximately 1.4% of people in this age group. 3 Guidelines generally recommend treatment with oral anticoagulants (OACs), 1 , 2 which can reduce the risk of AF‐related stroke by 64% for those at high risk (“sexless” CHA₂DS₂‐VA [C = congestive heart failure/left ventricular dysfunction, H = high blood pressure, A2 = 75 years of age and older, D = diabetes mellitus, S2 = stroke/transient ischemic attack/thromboembolism, V = vascular disease (coronary artery disease, myocardial infarction, peripheral artery disease, aortic plaque), A = 65–74 years of age] risk score≥2). 4
Large gaps in screening and treatment exist in practice. A survey conducted by The Economist in 2017 reported that only 11% of people ≥65 years of age were screened in Australian general practices in the previous fortnight. 5 Our previous 2018 study using eHealth tools conducted in metropolitan general practices increased screening to 16% of eligible patients. 6 In terms of treatment, rates have historically been 50% to 60%. However, since non–vitamin K dependent OAC (NOAC) medicines were introduced, an increase in treatment rates has been reported in Europe (>77% in England 7 and >65% in Denmark 8 ). This trend was also reflected in our 2018 metropolitan study, which reported a treatment rate of 71% for those diagnosed with AF before the study, increasing to >80% for those diagnosed during the study period. 6
Australians living in rural areas have more limited access to health services and worse cardiovascular outcomes. 9 The ratios of general practitioners (GPs), specialists, and nurses per capita of population are significantly lower in rural areas than in metropolitan areas, and access to specialist cardiac care is more limited. 10 , 11 Approximately 25% of the rural population suffers from cardiovascular diseases compared with 20% in metropolitan areas, and the likelihood of hospitalization and death resulting from cardiac events increases with the distance from metropolitan areas. 12 General practices play a key role in supporting cardiac health in rural areas as they tend to provide a broader range of community services compared with metropolitan practices. 13
Several of our previous studies showed that opportunistic screening in primary care by GPs and nurses was feasible. 6 , 14 , 15 A suite of customized eHealth tools, including an automated prompt and electronic decision support (EDS), were found to be promising. 6 These tools have been refined and enhanced with a quality improvement (QI) focus 16 , 17 and are designed to support all stages of screening.
This study aims to improve the proportion of patients screened and treated for AF using the refined eHealth tools and to inform strategies on AF screening implementation in the rural setting. In addition, this study provides the first cost‐effectiveness analysis in Australian general practice.
METHODS
This study was conducted in a convenience sample of 8 rural general practices from September 2018 to July 2019 in rural New South Wales, Australia. Practices were required to be located outside a major city (generally categorized under the Australian Statistical Geography Standard–Remoteness Area 2016 18 code 2 “inner regional Australia”) and were recruited by advertisements in primary health network newsletters and by word of mouth. Participating practices provided written informed consent, and patients provided oral consent for screening. This study was approved by the University of Sydney Human Research Ethics Committee (Project No. 2017/1017; Clinical Trial Registration No. ACTRN12618000004268). The data and materials will not be made available to other researchers as data sharing is not permitted by our ethics committee approval. Researchers interested in the data, methods, or analysis can contact the corresponding author for more information.
The methods for this study have been previously described in detail. 17 Briefly, GPs and/or practice nurses offered screening for AF with smartphone handheld single‐lead ECGs (iECGs) (KardiaMobile) to eligible patients attending the practice for any reason. Eligible patients were those ≥65 years of age without an existing AF diagnosis who had not already been screened with the iECG within the past 12 months. All follow‐up for those with abnormal screening results according to the iECG app (“possible AF” or “unclassified”) and treatment decisions were at the discretion of the GP.
To support screening, practices were provided with eHealth tools (Figure 1).
Screening prompt: An app located in a third‐party hosting platform automatically extracted information from patients’ electronic medical records. Using this information in real time, a prompt appeared when an eligible patient’s file was opened. The iECG automated screening result was also recorded in this app.
EDS: For those diagnosed with AF (either by screening or otherwise), the EDS app (also located on the third‐party hosting platform) calculated their CHA₂DS₂‐VA stroke risk score and made guideline recommendations regarding treatment. This app was part of the HealthTracker suite of cardiovascular QI tools.
Tailored clinical audit data for QI reporting: Customized, de‐identified clinical audit data extracts were obtained monthly from participating practices. These data were used to report back to practices and included data on the number and proportion screened, the number of patients with new AF, and the proportion treated according to guidelines.
Figure 1. Screening process and eHealth tools adapted from our 2018 metropolitan study. 6 .
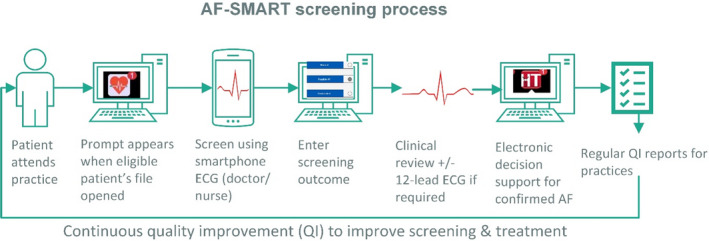
AF indicates atrial fibrillation; QI, quality improvement.
Reimbursement
Practices were paid $1000 to cover study setup time and data extraction costs plus $10 per patient screened (paid per 100 patients to encourage greater numbers). This was intended to cover the costs of screening in the Australian fee‐for‐service context and to replicate a real‐world fee if screening was covered by Medicare. Screening was free for patients, although any usual consultation fees applied.
Data Collection and Analysis
De‐identified data extracts included demographic, iECG screening, medication, and diagnostic information from the practices’ electronic patient records. The data extracts were designed to collect data for all active patients of the practices, that is, patients who had attended at least 3 times in the past 2 years and once in the past 6 months.
To provide additional context about broader screening and treatment trends, data from this study were compared with 2 other de‐identified data sets: the metropolitan group and the nonrandomized control group. These comparator data sets were collected from other Australian studies also using the HealthTracker app, with prospectively collected data using the same data extraction tool and data fields. The metropolitan group was from our 2018 AF screening study, 6 which included 8 metropolitan general practices. The nonrandomized control group was composed of 69 practices (64 metropolitan and 5 rural) that were using HealthTracker for general cardiovascular QI studies that did not involve AF screening. For the purposes of comparisons of treatment rates before and during the study period, the nonrandomized control group data were split into AF diagnoses before January 1, 2018 (baseline treatment rate) and AF diagnosed on or later than January 1, 2018 (AF diagnosed during the study period).
Descriptive analyses for the rural practices were carried out using Microsoft Excel. Descriptive analyses of nonrandomized control data were performed using R Statistical Programming, version 3.6.1. 19 Comparisons of treatment rates between groups were calculated using the Fisher exact test (2‐sided P values) performed using 2×2 contingency tables (GraphPad Prism version 7.04) with significance set a priori at P<0.05. Although our protocol paper specified a chi‐square test, the Fisher exact test was used as it was more accurate with the small numbers involved.
A detailed process evaluation was carried out using mixed methods, including semistructured interviews with selected practice staff. This evaluation examined outcomes related to implementation success and the acceptability/competing demands of the screening program. Methods and results of this evaluation have been described elsewhere. 16
Cost‐Effectiveness Analysis
The iECG screening program was evaluated by comparing population‐based AF screening with no screening from an Australian health funder perspective. The economic model developed in the SEARCH‐AF (Screening Education and Recognition in Community Pharmacies of Atrial Fibrillation) 20 pharmacy screening study was adapted to evaluate iECG screening in general practice. The model has previously been explained in detail. 20 Briefly, the model compares the cost of iECG screening, diagnosis, and treatment in general practice to diagnosed AF in the unscreened population of Australian men and women 65 to 84 years of age. That is, it compares population‐based AF screening to no screening. It assumes a base rate of AF (both diagnosed and unknown) and follows a cohort of the population 65 to 84 years of age for 10 years with annual stroke events and all‐cause mortality.
Stroke costs included hospitalization, rehabilitation, and other ongoing medical costs. For this study, the model was updated to include the cost of an echocardiogram for those diagnosed and the costs of major bleeding episodes for those on OAC treatment and a treatment regimen consistent with current trends (that is, including NOACs prescribed at rates observed in the current study).
The model included several key assumptions (full list included as Table S1).
The proportion screened was that observed in this study.
The prevalence of diagnosed AF in the population ≥65 years of age was 4.4%. 3
The prevalence of unknown AF in the population ≥65 years of age was 1.4%. 3
OAC and antiplatelet treatment rates were as observed for all patients diagnosed during the study period (both screen detected and otherwise detected).
The iECG test sensitivity was 97%, and specificity was 92%.
The cost per screen was $20.
For those diagnosed with AF, annual treatment and monitoring costs for those on OACs were $1063.78 (warfarin) and $1401.73 (mean cost for NOACs) and included annual costs of medication, pathology, GP, and specialist visits.
Costs for hospitalization for stroke were obtained from Cadilhac et al 21 and were updated to 2019 prices using the Australian Health Price Deflator Index. In addition, a present value of 5.09 quality‐adjusted life years (QALYs) (gained over a lifetime) was used for each ischemic stroke prevented by screening. 21
Results are presented in Australian dollars as an incremental cost‐effectiveness ratio (ICER) per stroke avoided and per QALY gained for population‐based screening compared with no screening. Sensitivity analyses were also performed for different proportions of patients screened, price reductions in NOAC medicines, differences in iECG test sensitivity and specificity, differences in OAC treatment rates, and differences in rates of major bleeding episodes.
Outcomes
Key study outcomes were the following: 17
the proportion of screened patients with confirmed new AF,
the proportion of AF and screened patients where the EDS was accessed,
the proportion of patients with AF diagnosed during the study period in the OAC recommended category (CHA₂DS₂‐VA risk score≥2) 1 who were prescribed an OAC according to guidelines,
baseline AF prevalence in patients ≥65 years of age compared with metropolitan and nonrandomized control groups,
new screen‐detected AF incidence at the end of the study period in patients ≥65 years of age compared with the metropolitan and nonrandomized control groups, and
rates of OAC and antiplatelet treatment at baseline and completion for patients in the OAC recommended category compared with the metropolitan and nonrandomized control groups.
RESULTS
Screening, Diagnosis, and Treatment
A total of 8 general practices were recruited and screened a total of 3103 eligible patients (mean age, 75.1±6.8 years; 47% men) during the study period. The median screening period was 4.6 months (range, 1.7–7.5 months). Practices screened a mean of 34% (median 35%) of eligible patients (range, 9%–51% per practice), with 4 of 8 practices screening >40% of eligible patients (Figure 2). In general, screening was highest in the first 1 to 2 months and declined thereafter. The mean proportion of all eligible patients who attended the practices during the study period was 94%.
Figure 2. Screening flowchart.
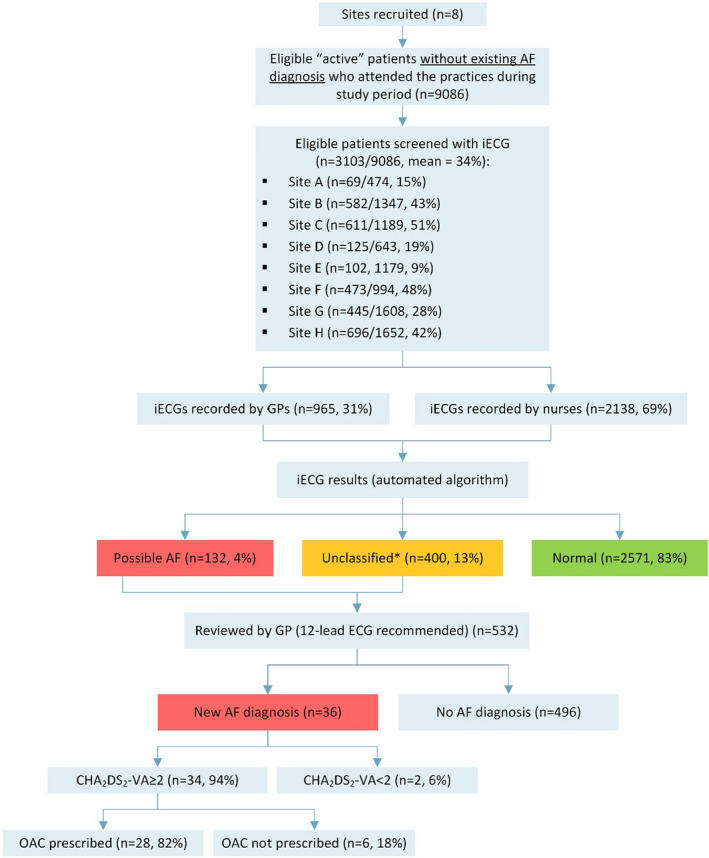
*Unclassified results may be attributed to sinus bradycardia, sinus tachycardia, left or right bundle branch block, multiple ectopic beats, or other arrythmias. AF indicates atrial fibrillation; CHA₂DS₂‐VA, C = congestive heart failure/left ventricular dysfunction, H = high blood pressure, A2 = 75 years of age and older, D = diabetes mellitus, S2 = stroke/transient ischemic attack/thromboembolism, V = vascular disease (coronary artery disease, myocardial infarction, peripheral artery disease, aortic plaque), A = 65 to 74 years of age; GP, general practitioner; iECG, handheld single‐lead smartphone ECG; and OAC, oral anticoagulant.
GPs (n=22) screened 31% (range, 1–182 per GP) of patients and nurses (n=40) screened 69% (range, 1–192 per nurse). According to the iECG automated algorithm (as entered into the app by GPs/nurses), 83% of screenings were normal, 13% were unclassified, and 4% were possible AF.
In total, 36 (1.2%) of new cases of screen‐detected AF were confirmed (mean age, 77.0 years; 64% men; mean CHA₂DS₂‐VA, 3.2) (Table 1). The proportion of screen‐detected patients with AF with at least 1 nonage or sex risk factor was 83%, and the proportion in the OAC recommended category (CHA₂DS₂‐VA≥2) was 94%. Characteristics and CHA₂DS₂‐VA groups for those with screen‐detected AF, otherwise‐detected AF (during the study period), and AF detected before the study are presented in Table 1.
Table 1.
Characteristics and Stroke Risk of Those ≥65 Years of Age With AF
| Screen‐Detected AF | Otherwise‐Detected AF During Study Period | Baseline: AF Diagnosed Before Study | |
|---|---|---|---|
| (n=36) | (n=58) | (n=1243) | |
| Age, y, mean±SD | 77.0±6.1 | 77.0±8.4 | 79.2±7.8 |
| Male, n (%) | 23 (64) | 32 (55) | 662 (53) |
| Mean CHA₂DS₂‐VA | 3.2 | 3.3 | 3.7 |
| CHA₂DS₂‐VA≥2, n (% of total) | 34 (94) | 55 (95) | 1223 (98) |
| CHA₂DS₂‐VA≥2 and prescribed OACs, n (% of those with CHA₂DS₂‐VA≥2) | 28 (82) | 41 (75) P=0.444* | 908 (74) P=0.326* |
| ≥1 nonage or sex risk factors, n (% of total) | 30 (83) | 54 (93) | 1178 (95) |
AF indicates atrial fibrillation; and CHA₂DS₂‐VA, C = congestive heart failure/left ventricular dysfunction, H = high blood pressure, A2 = 75 years of age and older, D = diabetes mellitus, S2 = stroke/transient ischemic attack/thromboembolism, V = vascular disease (coronary artery disease, myocardial infarction, peripheral artery disease, aortic plaque), A = 65 to 74 years of age; and OAC, oral anticoagulant.
P value for comparison to screen‐detected AF.
OAC treatment rates of patients with AF with CHA₂DS₂‐VA≥2 were 82% (screen‐detected), 75% (otherwise‐detected during study period), and 74% (preexisting AF), with no significant differences between treatment rates in the screen‐detected group and other groups (Table 1). The EDS was accessed for 54 of 1337 (4%) patients 65 years of age and older with AF and for 4 of 36 (11%) patients with new screen‐detected AF.
AF Prevalence and Treatment Rates Compared With Metropolitan and Nonrandomized Control Groups
The baseline prevalence of AF in the rural and metropolitan practices and nonrandomized control groups ranged from 9% to 12% (Table 2).
Table 2.
Treatment Rates and Comparisons Between Groups: Patients ≥65 Years of Age With AF
|
Rural Practices (n=8) |
Metropolitan Practices (n=8) |
Nonrandomized Control Practices (n=69) | |
|---|---|---|---|
| Total active* patients ≥65 years of age | 10 896 | 13 679 | 30 116 |
| Baseline AF prevalence | 12% | 11% | 9% |
| Baseline: AF detected before study with CHA2DS₂‐VA≥2 | |||
| Total, n | 1223 | 1306 | 1875 |
| Prescribed OAC, n (%) | 908 (74) | 933 (71) P=0.118† | 1450 (77) P=0.052† |
| Prescribed antiplatelet alone, n (%) | 178 (15) | 213 (16) | 248 (13) |
| Not prescribed OAC or antiplatelet, n (%) | 137 (11) | 160 (12) | 177 (9) |
| Screen‐detected AF during study period with CHA₂DS₂‐VA≥2 | |||
| Total, n | 34 | 18 | N/A |
| Prescribed OAC, n (%) | 28 (82) | 15 (83) P>0.999† | N/A |
| Prescribed antiplatelet alone, n (%) | 1 (3) | 1 (6) | N/A |
| Not prescribed OAC or antiplatelet, n (%) | 5 (15) | 2 (11) | N/A |
| All AF detected during study period (screen detected+otherwise detected) with CHA₂DS₂‐VA≥2 | |||
| Total, n | 89 | 64 | 399 |
| Prescribed OAC, n (%) | 69 (78) | 54 (84) P=0.312† | 333 (83) P=0.218† |
| Prescribed antiplatelet alone, n (%) | 7 (8) | 3 (5) | 29 (7) |
| Not prescribed OAC or antiplatelet, n (%) | 13 (15) | 7 (11) | 37 (9) |
AF indicates atrial fibrillation; CHA₂DS₂‐VA, C = congestive heart failure/left ventricular dysfunction, H = high blood pressure, A2 = 75 years of age and older, D = diabetes mellitus, S2 = stroke/transient ischemic attack/thromboembolism, V = vascular disease (coronary artery disease, myocardial infarction, peripheral artery disease, aortic plaque), A = 65 to 74 years of age; and OAC, oral anticoagulant.
Active patients are those who attended the practice at least 3 times in the past 2 years and once in the past 6 months.
P value for comparison to rural practices.
There were no significant differences between the rural and metropolitan practices’ treatment rates of those with AF detected before the study or during the study (screen‐detected and otherwise‐detected) (Table 2). Likewise, the treatment rates in the rural practices were similar to those in the nonrandomized control practices at baseline and during the study period (Table 2). The OAC treatment rates in all 3 cohorts tended to increase from baseline (Table 2), in contrast to antiplatelets.
Cost‐Effectiveness Analysis
Our cost‐effectiveness modeling showed that for population‐based AF screening for Australian men and women 65 to 84 years of age, assuming a 34% screening participation rate with a treatment rate of 82% in the screened population and a test sensitivity of 97% and specificity of 92%, the ICER per QALY gained was AU$16 578 and the ICER per stroke avoided was ≥≥84 383 compared with no screening.
Increasing the screening participation rate has a negligible effect on the ICER, but substantially increases the number of strokes prevented, that is, effectiveness (Table 3). Increasing the screening participation rate from 34% to 50% raises the number of strokes prevented from the base case of 147 per year to 216 per year (or 1467 to 2157 over 10 years). With a 75% screening participation rate, a total of 324 strokes are prevented each year (or 3235 strokes over 10 years) when compared with the no screening scenario.
Table 3.
Cost‐Effectiveness of Population‐Based AF Screening Compared With No Screening and Sensitivity Analyses Over 10 Years
| Base Case | |||||
|---|---|---|---|---|---|
| Screening participation rate, % | 34 | 50 | 60 | 70 | 75 |
| Number of strokes prevented | 1467 | 2157 | 2588 | 3020 | 3235 |
| Net cost [ICER] per stroke prevented compared with no screening | $84 383 | $83 304 | $82 922 | $82 649 | $82 540 |
| Net cost [ICER] per QALY gained compared with no screening | $16 578 | $16 366 | $16 291 | $16 238 | $16 216 |
| NOAC price reduction | ‐ | 12.5% | 25% | ||
| Number of strokes prevented | 1467 | 1467 | 1467 | ||
| Net cost [ICER] per stroke prevented compared with no screening | $84 383 | $76 336 | $68 289 | ||
| Net cost [ICER] per QALY gained compared with no screening | $16 578 | $14 997 | $13 416 | ||
| iECG test sensitivity | 97% | 92% | 100% | ||
| Number of strokes prevented | 1467 | 1391 | 1512 | ||
| Net cost [ICER] per stroke prevented compared with no screening | $84 383 | $85 940 | $83 524 | ||
| Net cost [ICER] per QALY gained compared with no screening | $16 578 | $16 884 | $16 409 | ||
| iECG test specificity | 92% | 89% | 93% | ||
| Number of strokes prevented | 1467 | 1467 | 1467 | ||
| Net cost [ICER] per stroke prevented compared with no screening | $84 383 | $86 818 | $83 571 | ||
| Net cost [ICER] per QALY gained compared with no screening | $16 578 | $17 057 | $16 419 | ||
| OAC treatment rate | 74%*/82%a, a | 55% | 90% | ||
| Number of strokes prevented | 1467 | 984 | 1610 | ||
| Net cost [ICER] per stroke prevented compared with no screening | $84 383 | $97 731 | $82 397 | ||
| Net cost [ICER] per QALY gained compared with no screening | $16 578 | $19 201 | $16 188 | ||
| Major bleeds–crude excess incidence rate per 1000 person‐years of major bleeds for those 65–74 years of age | 4.8 | 2.2 | 7.4 | ||
| Number of strokes prevented | 1467 | 1467 | 1467 | ||
| Net cost [ICER] per stroke prevented compared with no screening | $84 383 | $83 409 | $85 358 | ||
| Net cost [ICER] per QALY gained compared with no screening | $16 578 | $16 387 | $16 770 | ||
| Major bleeds–crude excess incidence rate per 1000 person‐years of major bleeds for those 75–84 years of age | 4.2 | 1.3 | 7.2 | ||
| Number of strokes prevented | 1467 | 1467 | 1467 | ||
| Net cost [ICER] per stroke prevented compared with no screening | $84 383 | $83 753 | $85 035 | ||
| Net cost [ICER] per QALY gained compared with no screening | $16 578 | $16 454 | $16 706 |
AF indicates atrial fibrillation; ICER, incremental cost effectiveness ratio; QALY, quality‐adjusted life year; NOAC, non‐vitamin K dependent anticoagulant; OAC, oral anticoagulant; and $ = Australian dollars (AUD).
Unscreened population.
Screened population.
Raising the OAC treatment rate also prevents more strokes, with a relatively small impact on the ICER. An OAC treatment rate of 90% (in both the screened and unscreened populations) would prevent a total of 1610 strokes over 10 years, with an ICER per QALY gained of $16 188 compared with no screening.
For population‐based screening, lowering the cost of NOAC treatment decreases the ICER per QALY gained to AU$14 997 (12.5% price reduction) or AU$13 416 (25% price reduction) compared with no screening.
Furthermore, changes to the iECG test sensitivity and specificity did not have material impacts on the ICER per stroke prevented nor the ICER per QALY gained compared with no screening. However, increasing the test sensitivity to 100% did prevent an additional 45 strokes over 10 years. Similarly, sensitivity analyses regarding major bleeding rates did not have a material impact on the ICER per stroke prevented nor the ICER per QALY gained compared with no screening.
DISCUSSION
This study investigated the impact of an AF screening program in rural general practices using an iECG together with a suite of custom‐designed eHealth tools designed to increase the proportion screened and treated for AF in accordance with guidelines. GPs and nurses at participating practices screened a total of 3103 eligible patients, and 36 (1.2%) new cases of AF were confirmed, with 82% prescribed OAC according to guidelines.
This study featured a unique suite of integrated, customized eHealth tools to support all stages of AF screening and treatment in general practice. These tools were refined following our metropolitan study 6 and included an automated screening prompt (with improved visibility and reliability), an EDS app to guide treatment, and de‐identified data extracts and with regular QI audit and feedback reporting to practices. We are not aware of any other studies that include tools to cover all stages of AF screening and treatment, including customized feedback. In particular, the refined screening prompt and the improved QI reporting were useful and motivating for participating GPs and nurses. 16
Proportion Screened and Treated
Practices screened 34% of eligible patients who attended during the study period, which is substantially higher than the 16% achieved in our metropolitan study. 6 Half of the study practices were able to screen >40% of eligible patients, although 51% was the maximum reached. It appeared that even practices with broad uptake and high motivation across staff were not able to capture more than 50% of eligible patients, which GPs and nurses indicated was largely attributed to time constraints and technical issues (eg, difficulty taking a reading on some patients). 16 Key features of the most successful practices included leadership from a senior GP “screening champion,” clear protocols for follow‐up of abnormal results for nurse‐led screening, and sufficient staff time allocation for screening. These are discussed in detail in our qualitative realist evaluation. 16
A recent study of AF screening in 184 Canadian practices was able to screen 42% of eligible patients. 22 In addition, a study from the Netherlands where patients ≥65 years of age were screened in 10 general practices during influenza vaccination sessions captured 35% of eligible patients, which is almost identical to our study. 23 These results suggest that 40% to 50% may be a “ceiling” of eligible patients captured by an opportunistic screening program in general practice.
As with the metropolitan study, treatment rates were high at baseline (>70%) compared with historical Australian data and increased during the study. The treatment rates were highest for screen‐detected AF (>80%). These treatment rates and trends were similar to those in the nonrandomized control practices. These rates are higher than previously reported in Australia, which were about 55% to 60% 24 before the introduction of NOACs (preferred by the Australian guidelines). 1 Our results show a similar trend to recent European treatment rates of around 65% to 80% 8 , 25 , 26 since the introduction of NOACs.
Our results also show a decline in antiplatelet prescription for those not on OACs. Of the patients diagnosed during the study period (≥65 years of age with CHA₂DS₂‐VA≥2) who were not prescribed OACs (n=20), only a minority were prescribed antiplatelets alone (n=7) with the remainder on no therapy (n=13). Of the 7 patients prescribed antiplatelets alone, 2 of these patients were prescribed antiplatelets before being diagnosed with AF (1 of whom had cardiovascular disease) and another 3 of these patients also had cardiovascular disease, which may be the reason antiplatelets were prescribed. This suggests that the prescription of antiplatelets alone for AF may be declining, as was recently reported in a US study 27 and that effectively the prescribing decision is becoming “OAC or no treatment.”
Rural Setting
This study extends the evidence base in rural areas and shows that a screening program in the rural general practice setting can successfully screen a large number of eligible patients with AF with guideline‐indicated treatment rates >80% for screen‐detected AF cases. A screening program using pulse palpation in rural general practice in Ireland achieved a similar reach to our study (30% of the general practice population ≥65 years of age screened), although OAC treatment rates were lower (65%). 28 The authors noted important differences regarding the density of population in rural studies compared with metropolitan studies, with implications for rural patients’ access to primary and secondary care.
Prevention programs suitable for rural areas are particularly important given that people living in these areas tend to have worse cardiovascular outcomes and less access to specialist medical services. 9 Rural general practice is potentially an ideal setting for implementation of innovative primary care‐based cardiac programs, such as ours, which contribute to upskilling GPs in cardiac care, training nurses to provide cardiac education/screening, and using novel technology.
Cost‐Effectiveness
Our cost‐effectiveness modeling showed that for population‐based AF screening in general practice for Australian men and women 65 to 84 years of age, the ICER per QALY gained was AU$16 578 and the ICER per stroke avoided was AU$84 383 compared with no screening. Increasing the proportion screened from 34% to 75% would prevent an additional 177 strokes per year (or 1768 strokes over 10 years) with a negligible effect on the ICER. These figures are higher than for SEARCH‐AF, 20 largely driven by an increased uptake of OAC treatment rates and in particular the higher prescription rates of NOACs. The increased proportion of people treated with OAC reduces the ICER, although this is offset by the higher cost of treatment with NOACs. These figures are well within the accepted thresholds of Australian government health expenditures. 29 This is consistent with several other studies, which found AF screening to be cost‐effective 30 or even cost‐saving. 31
Importantly, although we were able to screen 34% of eligible people with these tools (and have suggested that 40%–50% may be a “ceiling” of patients captured with opportunistic screening programs), these analyses highlight the impact of increasing the proportion screened in terms of stroke prevention and the need to consider new approaches to break the 40% to 50% barrier.
Limitations
The proportion of non‐normal results according to the iECG device algorithm was relatively high at 17% (possible AF, 4%; unclassified, 13%). This added to the workload substantially for practices, as was also noted in a recent Canadian study, 22 as all of these patients require some degree of follow‐up. In relation to the possible AF readings, it is likely that some were paroxysmal AF (AF not present on a subsequent 12‐lead ECG) or false positives (eg, attributed to sinus arrhythmia, multiple atrial ectopics, or a poor quality trace). It is also possible that some AF diagnoses were not recorded in the clinical system (see Limitations below). In relation to the unclassified results, previous studies have usually reported lower rates closer to 10%. 6 , 14 Improvements in the device algorithm (eg to identify sinus tachycardia/bradycardia) and training staff in techniques to take clearer readings will reduce this burden. We note that the research team was not able to review the iECGs and relied on GPs/nurses to manually enter the device’s interpretation into the AF app. The iECG automated algorithm has been reported to have a sensitivity of 97% and specificity of 92%. 20
The EDS was only used for a low proportion of patients. This is probably because it was in a separate app and was not accessed by GPs as it required extra clicks. Ideally, an EDS would need to be a more integral part of the electronic medical record system. Alternatively, an automatic calculation of patients’ CHA₂DS₂‐VA scores in the electronic medical record would assist, particularly if it included an alert to review treatment when the score changed (especially when it exceeds a treatment‐recommendation threshold).
The study relied on de‐identified data collected from practices. This was routinely collected general practice data with all its inherent limitations. For example, if GPs recorded a diagnosis of AF in the free‐text notes section instead of adding it as a condition from a drop‐down list, this would not be caught in our data, meaning our figures may underestimate the true rate of AF detected during the study. In addition, these data were limited to active patients because of the definition in the data collection tool. “Active patients” were defined as those who had attended the practice at least 3 times in the past 2 years and once in the past 6 months. Therefore, our data may be biased toward people with more chronic conditions requiring more frequent attendance at the practice.
There were some limitations in relation to the cost‐effectiveness analysis. A key methodological limitation is that a probabilistic sensitivity analysis was not undertaken to show model uncertainty, and therefore the model only reports point estimates on cost‐effectiveness. However, this is consistent with the main purpose of the model, which was to provide an estimate of the cost‐effectiveness and number of strokes prevented if the AF‐SMART system was implemented nationally. In addition, the model has not been validated or calibrated to test whether predicted events are consistent with observed data.
CONCLUSIONS
An AF screening program in rural general practices, supported by eHealth tools, screened 34% of eligible patients, with 82% of new screen‐detected cases treated according to guideline (Figure 3). Half of the practices screened 40% to 50% of eligible patients, suggesting that this may represent a “ceiling” of patients captured by opportunistic AF screening programs. OAC treatment rates were higher than previous studies at baseline and were trending upward during the study. Increasing the proportion screened would prevent many more strokes with minimal changes to the ICER. This may require new methods to break through the ceiling captured by numerous opportunistic programs. eHealth tools, particularly customized data reports as part of an audit and feedback system, may be a valuable addition to future screening programs.
Figure 3. Summary of findings.
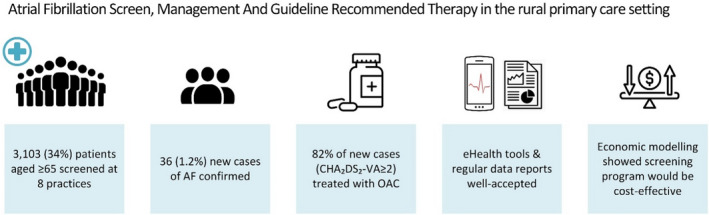
AF indicates atrial fibrillation; CHA₂DS₂‐VA, C = congestive heart failure/left ventricular dysfunction, H = high blood pressure, A2 = 75 years of age and older, D = diabetes mellitus, S2 = stroke/transient ischemic attack/thromboembolism, V = vascular disease (coronary artery disease, myocardial infarction, peripheral artery disease, aortic plaque), A = 65 to 74 years of age; and OAC, oral anticoagulant.
Sources of Funding
This work is supported by a Heart Foundation/New South Wales Health Cardiovascular Research Network Research Development Project Grant (101133) with top up funding from an investigator‐initiated grant from Pfizer/Bristol‐Myers Squibb. AliveCor have provided free smartphone ECG covers for study purposes. J. Orchard is supported by an Australian Government Research Training Program Scholarship. N. Lowres is funded by a New South Wales Health Early Career Fellowship (H16/52168). R. Webster is supported by a National Health and Medical Research Council Early Career Fellowship (APP1125044). A. Patel is supported by a National Health and Medical Research Council Principal Research Fellowship (APP1136898).
Disclosures
JL, GS, CH and RG have no disclosures. JO and NL report grants to the institution for investigator‐initiated grants from Pfizer/BMS. BF reports prior fees and advisory board honoraria from Bayer Pharma AG, Boehringer Ingelheim, Daiichi‐Sankyo, Omron and Pfizer/BMS, and grants to the institution for investigator‐initiated studies from BMS and Pfizer. LN reports speaker fees from Daiichi‐Sankyo, grants and honoraria from Pfizer/BMS, Bayer and Boehringer Ingelheim. RG, BK and AP report that the George Institute for Global Health has ownership of a social enterprise (George Health Enterprises) that may seek to commercialise some components of the tools used in this study.
Supporting information
Acknowledgments
The authors would like to acknowledge J. Canalese and all the practices that participated in the study.
(J Am Heart Assoc. 2020;9:e017080 DOI: 10.1161/JAHA.120.017080.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017080
For Sources of Funding and Disclosures, see page 11.
References
- 1. Brieger D, Amerena J, Attia J, Bajorek B, Chan KH, Connell C, Freedman B, Ferguson C, Hall T, Haqqani H, et al. National heart foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian Clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Heart Lung Circ. 2018;1209–1266. [DOI] [PubMed] [Google Scholar]
- 2. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;2893–2962. [DOI] [PubMed] [Google Scholar]
- 3. Lowres N, Olivier J, Chao TF, Chen SA, Chen Y, Diederichsen A, Fitzmaurice DA, Gomez‐Doblas JJ, Harbison J, Healey JS, et al. Estimated stroke risk, yield, and number needed to screen for atrial fibrillation detected through single time screening: a multicountry patient‐level meta‐analysis of 141,220 screened individuals. PLoS Medicine. 2019;e1002903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;857–867. [DOI] [PubMed] [Google Scholar]
- 5. The Economist Intelligence Unit . Preventing stroke: uneven progress. A global policy research programme. Economist. 2017;1‐28. [Google Scholar]
- 6. Orchard J, Neubeck L, Freedman B, Li J, Webster R, Zwar N, Gallagher R, Ferguson C, Lowres N. eHealth tools to provide structured assistance for atrial fibrillation screening, management, and guideline‐recommended therapy in metropolitan general practice: The AF ‐ SMART Study. J Am Heart Assoc. 2019; 10.1161/JAHA.118.010959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Public Health England . CVD: Primary Care Intelligence Packs, NHS South Norfolk CCG. 2017. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/622956/NHS_East_Riding_of_Yorkshire_CCG_CVD_intelligence_pack.pdf. Accessed October 23, 2019.
- 8. Gadsbøll K, Staerk L, Fosbøl EL, Sindet‐Pedersen C, Gundlund A, Lip GYH, Gislason GH, Olesen JB. Increased use of oral anticoagulants in patients with atrial fibrillation: temporal trends from 2005 to 2015 in Denmark. Eur Heart J. 2017;899–906. [DOI] [PubMed] [Google Scholar]
- 9. Australian Institute of Health and Welfare (AIHW) . Cardiovascular Medicines and Primary Health Care: A Regional Analysis. 2010.
- 10. Australian Bureau of Statistics . Australian Social Trends: Doctors and Nurses (Report No. 4102.0), 2013. Available at https://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/4102.0Main+Features20April+2013#p6. Accessed August 1, 2020.
- 11. Hamilton S, Mills B, McRae S, Thompson S. Evidence to service gap: cardiac rehabilitation and secondary prevention in rural and remote Western Australia. BMC Health Serv Res. 2018;64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Rural Health Alliance . Cardiovascular disease in rural Australia, 2015. Available at: https://www.ruralhealth.org.au/sites/default/files/publications/cardiovascular-disease-fact-sheet-may-2015.pdf. Accessed August 1, 2020.
- 13. Australian Department of Health . National Strategic Framework for Rural and Remote Health, 2016. Available at: https://www1.health.gov.au/internet/main/publishing.nsf/Content/national-strategic-framework-rural-remote-health. Accessed August 1, 2020.
- 14. Orchard J, Lowres N, Freedman SB, Ladak L, Lee W, Zwar N, Peiris D, Kamaladasa Y, Li J, Neubeck L. Screening for atrial fibrillation during influenza vaccinations by primary care nurses using a smartphone electrocardiograph (iECG): A feasibility study. Eur J Prev Cardiol. 2016;13–20. [DOI] [PubMed] [Google Scholar]
- 15. Orchard J, Freedman SB, Lowres N, Peiris D, Neubeck L. iPhone ECG screening by practice nurses and receptionists for atrial fibrillation in general practice: the GP‐SEARCH qualitative pilot study. Aust Fam Physician. 2014;315–319. [PubMed] [Google Scholar]
- 16. Orchard J, Li J, Gallagher R, Freedman B, Lowres N, Neubeck L. Uptake of a primary care atrial fibrillation screening program (AF‐SMART): a realist evaluation of implementation in metropolitan and rural general practice. BMC Fam Pract. 2019;170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Orchard JJ, Neubeck L, Freedman B, Webster R, Patel A, Gallagher R, Li J, Hespe CM, Ferguson C, Zwar N, et al. Atrial Fibrillation Screen, Management And Guideline Recommended Therapy (AF SMART II) in the rural primary care setting: an implementation study protocol. BMJ Open. 2018;e023130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Australian Government . Australian Statistical Geography Standard ‐ Remoteness Area. 2019. Available at: https://www.health.gov.au/health-workforce/health-workforce-classifications/australian-statistical-geography-standard-remoteness-area. Accessed December 11, 2019.
- 19. R Core Team . A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. Available at: https://www.R-project.org/. Accessed August 1, 2020.
- 20. Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, Bennett AA, Briffa T, Bauman A, Martinez C, et al. Feasibility and cost‐effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH‐AF study. Thromb Haemost. 2014;1167–1176. [DOI] [PubMed] [Google Scholar]
- 21. Cadilhac DA, Dewey HM, Vos T, Carter R, Thrift AG. The health loss from ischemic stroke and intracerebral hemorrhage: evidence from the North East Melbourne Stroke Incidence Study (NEMESIS). Health Qual Life Outcomes. 2010;49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Godin R, Yeung C, Baranchuk A, Guerra P, Healey JS. Screening for Atrial Fibrillation Using a Mobile, Single‐Lead Electrocardiogram in Canadian Primary Care Clinics. Can J Cardiol. 2019;840–845. [DOI] [PubMed] [Google Scholar]
- 23. Kaasenbrood F, Hollander M, Rutten FH, Gerhards LJ, Hoes AW, Tieleman RG. Yield of screening for atrial fibrillation in primary care with a hand‐held, single‐lead electrocardiogram device during influenza vaccination. Europace. 2016;1514–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alamneh EA, Chalmers L, Bereznicki LR. The Tasmanian atrial fibrillation study: transition to direct oral anticoagulants 2011–2015. Cardiovasc Ther. 2017;e12254. [DOI] [PubMed] [Google Scholar]
- 25. Cowan JC, Wu J, Hall M, Orlowski A, West RM, Gale CP. A 10 year study of hospitalized atrial fibrillation‐related stroke in England and its association with uptake of oral anticoagulation. Eur Heart J. 2018;2975–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodríguez‐Bernal CL, Hurtado I, García‐Sempere A, Peiró S, Sanfélix‐Gimeno G. Oral anticoagulants initiation in patients with atrial fibrillation: real‐world data from a population‐based cohort. Front Pharmacol. 2017;63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maggioni AP, Dondi L, Andreotti F, Pedrini A, Calabria S, Ronconi G, Piccinni C, Martini N. Four‐year trends in oral anticoagulant use and declining rates of ischemic stroke among 194,030 atrial fibrillation patients drawn from a sample of 12 million people. Am Heart J. 2020;12–19. [DOI] [PubMed] [Google Scholar]
- 28. Smyth B, Marsden P, Corcoran R, Walsh R, Brennan C, McSharry K, Clarke J, Kelly PJ, Harbison J. Opportunistic screening for atrial fibrillation in a rural area. QJM. 2016;539–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Edney LC, Haji Ali Afzali H, Cheng TC, Karnon J. Estimating the reference incremental cost‐effectiveness ratio for the Australian Health System. Pharmacoeconomics. 2018;239–252. [DOI] [PubMed] [Google Scholar]
- 30. Freedman B, Camm J, Calkins H, Healey JS, Rosenqvist M, Wang J, Albert CM, Anderson CS, Antoniou S, Benjamin EJ, et al. Screening for atrial fibrillation: a report of the AF‐SCREEN international collaboration. Circulation. 2017;1851–1867. [DOI] [PubMed] [Google Scholar]
- 31. Jacobs MS, Kaasenbrood F, Postma MJ, van Hulst M, Tieleman RG. Cost‐effectiveness of screening for atrial fibrillation in primary care with a handheld, single‐lead electrocardiogram device in the Netherlands. Europace. 2018;12–18. [DOI] [PubMed] [Google Scholar]
- 32. Lowres N, Neubeck L, Redfern J, Freedman SB. Screening to identify unknown atrial fibrillation. A systematic review. Thromb Haemost. 2013;213–222. [DOI] [PubMed] [Google Scholar]
- 33. Australian Bureau of Statistics . Australian Demographic Statistics, Jun 2019. Available at: https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3101.0Jun%202019?OpenDocument. Accessed February 7, 2020.
- 34. Medicare Benefit Schedule Online . 2019. Available at: http://www9.health.gov.au/mbs/search.cfm. Accessed January 24, 2020.
- 35. Pharmaceutical Benefits Scheme . 2019. Available at: http://www.pbs.gov.au/pbs/home. Accessed January 24, 2020.
- 36. Cadilhac DA, Carter RC, Thrift AG, Dewey HM. Why invest in a national public health program for stroke? An example using Australian data to estimate the potential benefits and cost implications. Health Policy. 2007;287–294. [DOI] [PubMed] [Google Scholar]
- 37. Martinez C, Katholing A, Freedman SB. Adverse prognosis of incidentally detected ambulatory atrial fibrillation. A cohort study. Thromb Haemost. 2014;276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Australian Institute of Health and Welfare . Australian refined diagnosis‐related groups (AR‐DRG) data cubes. 2019. Available at: https://www.aihw.gov.au/reports/hospitals/ar-drg-data-cubes/contents/data-cubes. Accessed February 7, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


