Abstract
Background
Carotid plaques with expansive arterial remodeling are closely related to cerebral ischemic events. Although S100A4 (S100 calcium‐binding protein A4) is expressed in atherosclerotic lesions, its role in atherosclerotic plaque progression remains unknown. In this study, we examined the association between carotid arterial expansive remodeling and S100A4 expression.
Methods and Results
Preoperative high‐resolution magnetic resonance imaging was used to assess luminal stenosis and vascular remodeling in patients undergoing carotid endarterectomy. To examine murine carotid atherosclerosis, we induced experimental lesions by flow cessation in apolipoprotein E‐deficient mice fed a high‐fat diet. The role of S100A4 in plaque formation and smooth muscle cell proliferation was investigated in vivo and in vitro, respectively. Human carotid arterial expansive remodeling showed positive correlations with the expression of S100A4, MMP2, and MMP9. S100A4 mRNA levels were positively correlated with those of MMP2, MMP9, and MMP13. S100A4 was expressed in vascular smooth muscle cells (VSMCs) and VSMC‐derived foam cells in the plaque shoulder and marginal areas. S100A4 expression increased concomitantly with plaque formation in our animal model. Exogenous recombinant S100A4 protein enhanced the levels of Mmp2, Mmp9, and Mmp13 and the cell proliferation ability in VSMCs. A chemotaxis assay indicated that extracellular S100A4 functions as a chemoattractant for VSMCs.
Conclusions
S100A4 expression was elevated in human carotid plaques and showed a positive correlation with the degree of expansive remodeling. S100A4‐positive VSMC‐derived cells are considered to play an important role in carotid expansive remodeling.
Keywords: atherosclerotic plaque, carotid stenosis, S100A4, vascular remodeling, vascular smooth muscle cell
Subject Categories: Cerebrovascular Disease/Stroke, Atherosclerosis, Vascular Biology, Smooth Muscle Proliferation and Differentiation
Nonstandard Abbreviations and Acronyms
- CA
carotid artery
- LSS
low shear stress
- MMP
matrix metalloproteinase
- S100A4
S100 calcium‐binding protein A4
- VSMC
vascular smooth muscle cell
Clinical Perspective
What Is New?
The expression of S100A4 (S100 calcium‐binding protein A4) was elevated in both human and mouse carotid plaque lesions and showed a positive correlation with the degree of expansive remodeling.
Extracellular S100A4 enhances proliferation and migration of vascular smooth muscle cells.
What Are the Clinical Implications?
This study provides novel evidence suggesting that increased expression of S100A4 in dedifferentiated vascular smooth muscle cells and the foam cells derived from these cells contributes to vascular remodeling.
Our findings indicate a contribution of S100A4 to atherosclerotic plaque formation and instability. S100A4 may serve as a biomarker and therapeutic target for carotid artery stenosis.
Carotid artery (CA) stenosis contributes to ≈20% of the incidence of cerebral infarction. 1 Currently, luminal stenosis is accepted as the standard indicator of future ischemic stroke and is used in patient selection for reconstructive surgery based on the findings of several randomized clinical trials published in the 1990s. 2 , 3 However, an increasing number of findings relating to the pathophysiology of atherosclerosis have indicated that the qualitative and morphological characteristics of plaque are associated with the risk of future ischemic events. 4
Expansive remodeling is characterized by an enlargement of atherosclerotic arteries with outward plaque growth. It was first described in a postmortem histological study of coronary circulation and was subsequently proven to be associated with plaque instability and a higher risk of myocardial infarction. 5 , 6 , 7 We previously performed a retrospective study of patients scheduled for reconstructive surgery and reported the significant association between carotid expansive remodeling and cerebral ischemic events. 8 , 9 Nevertheless, although expansive remodeling is considered a potential marker indicating plaque vulnerability, the underlying molecular mechanisms and their pathological significance remain to be elucidated.
Previous animal model‐based studies have demonstrated that matrix metalloproteinases (MMPs) secreted by macrophages facilitate expansive remodeling by promoting increased matrix degradation. 10 , 11 Although it has long been accepted that MMPs are secreted mainly by macrophages in atherosclerosis, recent studies have shown that substantial amounts of foam cells expressing macrophage markers such as CD68 are derived from vascular smooth muscle cells (VSMCs), 12 , 13 , 14 indicating that VSMCs may also play a pivotal role in MMP secretion and subsequent vascular morphological changes in expansive remodeling. 15 , 16 , 17
S100A4 (S100 calcium‐binding protein A4), which is upregulated in dedifferentiated VSMCs, 18 has been extensively studied in various types of tumor cells. S100A4 has both intracellular and extracellular functions. The S100 proteins, lacking a leader sequence, are not secreted via the classical Golgi pathway; thus, the underlaying mechanism of their secretion remains unknown. 19 Intracellular S100A4 promotes the expression of multiple growth factors and confers migration ability by interacting with cytoskeletal proteins such as nonmuscle myosin heavy‐chain IIA, whereas extracellular S100A4 upregulates the expression of MMPs in the surrounding cells in a paracrine manner, thereby contributing to an increase in cell migration, proliferation, and metastasis. 20 , 21 Although S100A4 has also been reported to be expressed in human carotid plaques, 22 its role in carotid atherosclerosis is yet to be determined.
We hypothesized that S100A4 is expressed mainly in VSMCs in atherosclerotic lesions, and contributes to expansive remodeling by enhancing MMP expression. To verify our hypothesis, we examined the localization of S100A4 in human CA plaques and its association with MMP expression and expansive remodeling. The role of S100A4 in plaque formation and VSMC proliferation was further investigated using an in vivo animal model and in vitro experiments, respectively.
Methods
Supporting data are available from the corresponding author upon a reasonable request.
Subjects and Human Carotid Plaque Sampling
From patients who underwent carotid endarterectomy from June 2012 to June 2016 at Kyoto University Hospital, we collected samples from the plaque region of 27 consecutive patients immediately following endarterectomy for quantitative polymerase chain reaction (PCR) analysis. Where possible, we dissected the plaque region, including the adjacent intact area, as a single specimen. After washing off the adhered blood, all samples were preserved at −80°C until mRNA extraction. The collection of human tissue samples was approved by the institutional review board of Kyoto University Hospital (approval no.: R1208), and informed consent was obtained from all study participants.
Quantitative Polymerase Chain Reaction
We extracted total RNA from the thickened portions of the plaques and resection stumps of carotid endarterectomy specimens using an RNeasy Micro kit (QIAGEN, Hilden, Germany). The resection stumps that did not exhibit atherosclerotic lesions were used as normal controls. Subsequently, we generated first‐strand cDNA using high‐capacity cDNA reverse transcription kits (Applied Biosystems, Foster City, CA).
As a murine partial carotid ligation model, the left common CA was excised 0 weeks (n=6), 1 week (n=8), and 6 weeks (n=9) after surgery. Total RNAs were isolated and the corresponding first‐strand cDNA was generated as described above.
We performed quantitative polymerase chain reaction (qPCR) using an ABI PRISM 7700 Sequence Detection System (Applied Biosystems). mRNA levels of the human EEF1A1 or mouse RplI13 genes served as internal controls for normalization among samples. The findings were analyzed using the ΔΔCT method. The PCR primers used for amplification are described in Table.
Table 1.
Polymerase Chain Reaction Primers Used for Amplification
| Species | Gene | Primers |
|---|---|---|
| Human | EEF1A1 |
5ʹ‐CTTTGGGTCGCTTTGCTGTT‐3ʹ, 5ʹ‐CCGTTCTTCCACCACTGATT‐3ʹ |
| S100A4 |
5ʹ‐TCAGAACTAAAGGAGCTGCTGACC‐3ʹ, 5ʹ‐TTTCTTCCTGGGCTGCTTATCTGG‐3ʹ |
|
| MMP2 |
5ʹ‐AGATACCCTCAAGAAGATGC‐3ʹ, 5ʹ‐AGCATCATCCACGGTTTCAG‐3ʹ |
|
| MMP9 |
5ʹ‐AGTCTGGATAAGTTGGGTCT‐3ʹ, 5ʹ‐AGATGTCGTGTGAGTTCCAG‐3ʹ |
|
| MMP13 |
5ʹ‐CTATCCTGGCCACCTTCTTC‐3ʹ, 5ʹ‐GGGACCATTTGAGTGTTCTAGG‐3ʹ |
|
| Mouse | Rpl13a |
5ʹ‐TTCGGCTGAAGCCTACCAGAAAGT‐3ʹ, 5ʹ‐GCATCTTGGCCTTTTTCCGTT‐3ʹ |
| α‐Smooth muscle actin (α‐Sma) |
5ʹ‐CCACCGCAAATGCTTCTAAGT‐3ʹ, 5ʹ‐GGCAGGAATGATTTGGAAAGG‐3ʹ |
|
| Mmp2 |
5ʹ‐CACCTCCTACAACAGCTGTAC‐3ʹ, 5ʹ‐AGGAAAGTGAAGGGGAAGACA‐3ʹ |
|
| Mmp9 |
5ʹ‐TCAGAAGGTGGATCCCCAGA‐3ʹ, 5ʹ‐TTCACCTCATGGTCCACCTTG‐3ʹ |
|
| Mmp13 |
5ʹ‐TCCCTTGATGCCATTACCAGTC‐3ʹ, 5ʹ‐AAAAAGAGCTCAGCCTCAACCTG‐3ʹ |
|
| S100a4 | Mm_S100a4_1_SG QuantiTect primer assay (QT00107632*) |
MMP indicates matrix metalloproteinase.
QIAGEN, Hilden, Germany.
Histological Studies
For immunohistochemistry, formalin‐fixed, paraffin‐embedded samples were cut into 5‐μm‐thick sections. The sections were deparaffinized, rehydrated, and incubated in boiling citrate buffer (pH 9.0) for 10 minutes. After blocking, sections were incubated overnight at 4℃ with primary antibodies. Following the addition of horseradish peroxidase‐conjugated secondary antibodies (EnVision labeled polymer; Dako, Glostrup, Denmark), sections were incubated at room temperature for 1 hour, and then visualized using 3,3ʹ‐diaminobenzidine with hematoxylin counterstaining. For immunofluorescence analysis, we prepared serial frozen sections of 7‐μm thickness. Prior to blocking, autofluorescence was reduced using lipofuscin quencher (Biotium, Fremont, CA), after which the sections were incubated with primary antibodies followed by incubation with Alexa Fluor 488‐ or 594‐conjugated secondary antibodies (Invitrogen, Carlsbad, CA). The following primary antibodies were used: rabbit polyclonal anti‐S100A4 (ab27957; Abcam, Cambridge, UK), mouse monoclonal anti‐non‐muscle myosin IIB/embryonic form of smooth muscle myosin heavy chain (ab684; Abcam), goat polyclonal anti‐CD68 (sc‐7082; Santa Cruz Biotechnology, Dallas, TX), and mouse monoclonal anti‐CD45 (sc‐1178; Santa Cruz Biotechnology). 4′,6‐diamidino‐2‐phenylindole (Invitrogen, Waltham, MA) was used as a nuclear counterstain.
Carotid Magnetic Resonance Imaging and Evaluation of Expansive Remodeling
Patients underwent carotid plaque imaging using a 3‐Tesla magnetic resonance imaging system (MAGNETOM Prisma; Siemens Healthcare GmbH, Erlangen, Germany) incorporating a 20‐channel coil. The imaging parameters were as previously described. 8 Expansive remodeling of carotid atherosclerotic lesions was evaluated in T1‐weighted SPACE (sampling perfection with application optimized contrasts using different flip angle evolution) images of sagittal sections parallel to the long axis of the CA. The expansive remodeling ratio for CA was calculated as previously described. 8 Briefly, the maximum distance between the lumen and the outer borders of the plaque is divided by the maximal luminal diameter of the distal internal CA. We also examined the correlation between the expansive remodeling ratio and the levels of S100A4 and MMPs.
Animal Model
Atherosclerosis‐prone apolipoprotein E‐deficient mice were fed a normal diet until 8 weeks of age. Thereafter, the mice were fed a Western diet (0.15% cholesterol and 21% fat) until 10 weeks of age, when the left CA was partially ligated as previously described. 23 Briefly, a ventral midline incision was made in the neck portion under general anesthesia by intraperitoneal injection of pentobarbital sodium (50 mg/kg). The left common CA was exposed and 3 of the 4 caudal branches (the external carotid, internal carotid, and occipital arteries) were ligated with a 6.0 silk suture. In this study, we only used male animals to avoid the effects of estrogen, one of the major modulators in atherosclerosis. All animal experiments were approved by the institutional animal care and use committee and the ethics committee of Kyoto University and were conducted in accordance with the guidelines of Japan's Act on Welfare and Management of Animals.
Preparation of Paraffin Sections and Histopathology
At 0, 1, and 6 weeks after partial carotid ligation, the left and right CAs, trachea, and esophagus were embedded in paraffin, and tissue‐embedded blocks were prepared. Tissue sections (5 μm) of the left CA were prepared at 500‐μm interval using a tissue processor. To determine plaque area, cross sections were stained using a protocol for modified Elastica van Gieson staining 24 and viewed under a bright field microscope. Immunohistochemical analysis was performed as described above.
Morphometric Analysis
Each prepared section contained the intimal area, medial area, and perimeter of the external elastic lamina. The length of the external elastic lamina was used as an index of expansive remodeling. For the 1‐ and 6‐week groups, we obtained mean values of 3 consecutive sections where plaque formation was observed in the midportion of the left common CA (2500–4500 μm proximal from the bifurcation of the CA). In the 0‐week group, we obtained average values of 3 consecutive sections of the same portion. For each group, we calculated the average values for 5 mice and evaluated changes over time.
Cell Culture
VSMCs derived from mouse aorta (BBASE7001; DS Pharma Biomedical, Suita, Osaka, Japan) were passaged using DMEM and the cells of the 4th to 10th passages were used in the experiment.
Recombinant S100A4 Treatment in Mouse VSMCs
Mouse VSMCs were seeded in 24‐well plates and cultured in DMEM containing 10% FCS until subconfluent, and were subsequently incubated with different concentrations (0–0.1 μg/mL) of recombinant mouse S100A4 protein (ProSpec‐Tany TechnoGene Ltd, Ness‐Ziona, Israel) for 16 hours. Total RNA was extracted from stripped cells using an RNeasy Mini kit (QIAGEN) according to the manufacturer's instructions. Subsequent steps and PCR primers were the same as described above. The expression levels of Mmp2, Mmp9, and Mmp13 were measured by qPCR.
Cell Proliferation
To determine the effects of extracellular S100A4 on cell proliferation, we performed a water‐soluble tetrazolium salt‐1 colorimetric assay (Roche Applied Science, Penzberg, Upper Bavaria, Germany). The assays were performed with the addition of different concentrations (0–1.0 μg/mL) of recombinant mouse S100A4 protein. Following incubation of mouse VSMCs with recombinant mouse S100A4 protein, absorbance was subsequently measured using a microplate ELISA reader (VERSAmax; Molecular Devices, Sunnyvale, CA) at wavelengths of 450 nm for measurements and 650 nm as reference; we performed analysis using SoftMax Pro 5 software (Molecular Devices, San Jose, CA). In all experiments, each condition was assessed in 8 microplate wells. Cell proliferation was calculated by comparing the absorbance values of samples after background subtraction.
Chemotaxis Assay
To investigate whether extracellular S100A4 acts as a chemoattractant for VSMCs, we performed chemotaxis assays using a Boyden Chamber Migration Assay kit (Cell Biolabs Inc, San Diego, CA). Mouse VSMCs suspended in serum‐free medium were plated in the upper part of a 24‐well, 8‐μm pore, cell‐migration chamber at a concentration of 3×105/mL according to the manufacturer's protocol. Medium with or without recombinant S100A4 at a concentration of 1 μg/mL was placed in the lower wells as a chemotactic stimulus and control, respectively. Cells were allowed to migrate for 24 hours in an incubator. Subsequently, the migratory cells were stained with crystal violet, and the dye was eluted. Optical densities were read at 560 nm using a microplate absorbance spectrophotometer (VERSAmax; Molecular Devices), and the data were statistically analyzed.
Statistical Analysis
The data are presented as the mean±SEM. Statistical analysis was performed using a paired t test, and a linear regression analysis was conducted using Ekuseru‐Toukei 2015 (Social Survey Research Information Co, Ltd, Shinjuku‐ku, Tokyo, Japan). A P value of <0.05 was considered to indicate statistical significance.
Results
Increased Expression of S100A4 mRNA in Human Carotid Atherosclerotic Lesions
We found that expression of the S100A4 gene in atheromatous plaque areas was significantly (2.1 times) higher than that in the resection stumps (the normal controls; P=0.016; Figure 1A).
Figure 1. Relationship among the mRNA expression levels of S100A4, MMP, and the degree of expansive remodeling in human carotid atheromatous plaques.
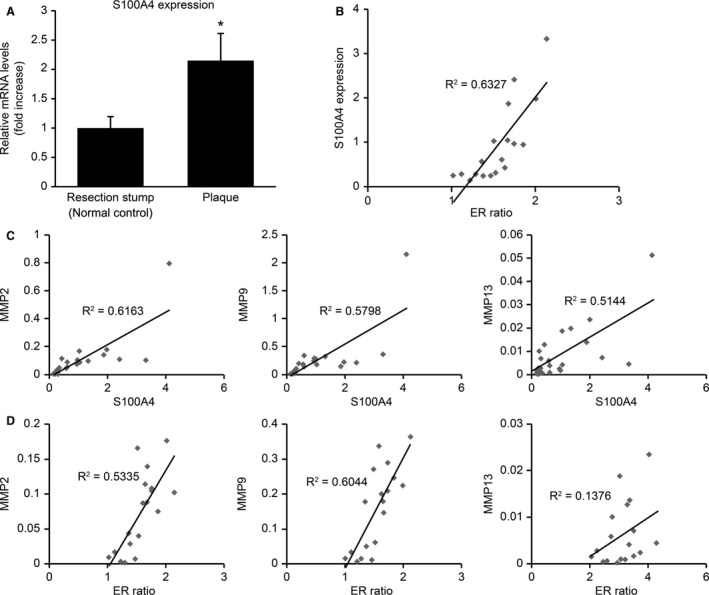
A, S100A4 expression increased significantly in the atheromatous plaque lesions compared with the resection stumps of human carotid endarterectomy specimens. The results are presented as the mean±SEM; *P<0.05, paired t test. B, Statistically significant correlations were detected between expansive remodeling ratio (ER ratios) and S100A4 mRNA levels in carotid atherosclerotic plaques. C, Statistically significant correlations were detected between S100A4 mRNA levels and those of MMP2, MMP9, and MMP13 in carotid atherosclerotic plaques. D, ER ratios were positively correlated with the mRNA levels of MMP2 and MMP9, but not with those of MMP13 in carotid atherosclerotic plaques. ER indicates expansive remodeling; MMP, matrix metalloproteinase; and S100A4, S100 calcium‐binding protein A4.
Associations Between S100A4 and MMP Expression Levels
Levels of S100A4 mRNA in the atheromatous plaque areas were positively correlated with those of MMP2 (r 2=0.6163, P<0.001), MMP9 (r 2=0.5798, P<0.001), and MMP13 (r 2=0.5144, P<0.001; Figure 1C), indicating that the expression of S100A4 reflects the degradation of the extracellular matrix in atheromatous plaques.
Degree of Expansive Remodeling Is Correlated With the Levels of S100A4, MMP2, and MMP9 mRNA in Carotid Atheromatous Plaques
Expansive remodeling ratios were found to be positively correlated with the mRNA levels of S100A4 (r 2=0.6327, P<0.001; Figure 1B), MMP2 (r 2=0.5335, P<0.001), and MMP9 (r 2=0.6044, P<0.001), but not with those of MMP13 (r 2=0.1376, P=0.13; Figure 1D).
S100A4 Is Expressed in Both the Intima and Media of the Human Carotid Atheroma
Immunohistochemical analysis revealed that S100A4 is expressed in both the intima and media of the human carotid atheroma (Figure 2A). Moreover, S100A4 was observed to colocalize with the embryonic form of smooth muscle myosin heavy chain, a marker of dedifferentiated VSMCs (Figure 2C). S100A4 was also found to colocalize with CD68, but not with CD45, the latter of which known as a common leukocyte antigen (Figure 2C). Among S100A4‐positive cells, the copositive rates of the embryonic form of smooth muscle myosin heavy chain, CD68, and CD45 were 100%, 95.8%, and 6.1%, respectively. Furthermore, S100A4‐positive foam cells were observed to have accumulated in the plaque shoulder and marginal areas where elastic fibers are disrupted (Figure 2B). Double‐immunofluorescence also revealed that S100A4‐positive cells express MMP9 (Figure 2D).
Figure 2. S100A4 exists in both the intima and media of the human carotid atheroma.
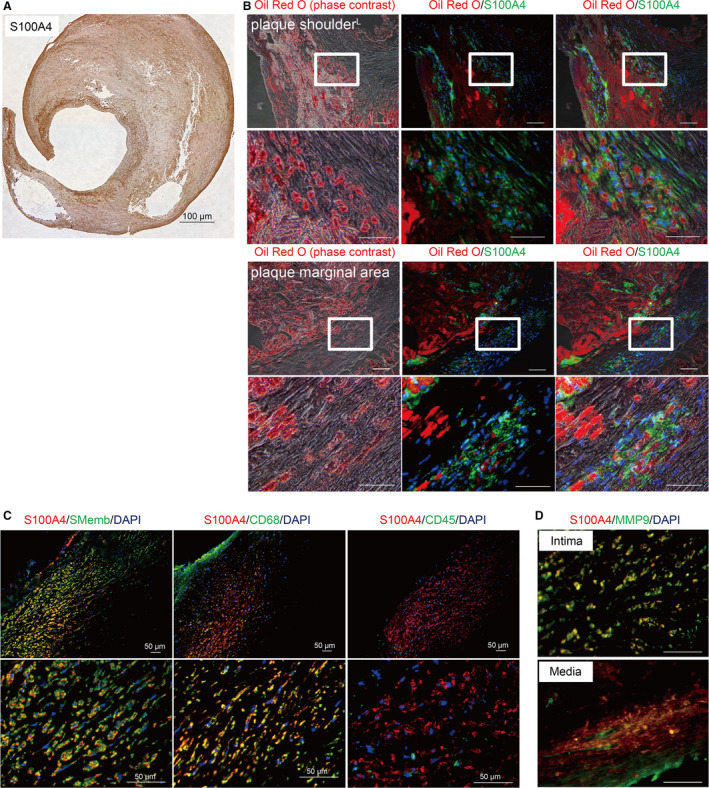
A, A human carotid endarterectomyspecimen immunostained with anti‐S100A4 (S100 calcium‐binding protein A4) antibody. S100A4 was expressed in both the intima and media of the human carotid atheroma. S100A4‐positive cells were mainly expressed in the plaque shoulder and marginal areas. Scale bar: 1000 μm. B, Human carotid endarterectomy specimens stained with Oil Red O and anti‐S100A4 antibody. The left image was obtained using a phase‐contrast microscope; the center image was obtained using a fluorescence microscope (red: Oil Red O, green: S100A4, blue: nuclei); and the right image is a merged image of the phase‐contrast and fluorescence images. Images of the plaque shoulder and marginal areas are shown in the upper and lower sections, respectively. Foam cells accumulated around the necrotic core of the plaque shoulder, most of which were positive for S100A4. Similarly, in the plaque marginal area, accumulation of S100A4‐positive foam cells was observed between the expanded elastic laminae. Scale bars: 100 μm (low‐power field) and 50 μm (high‐power field). C, Human carotid endarterectomy specimens stained with anti‐S100A4 and anti‐SMemb antibodies (left: red, S100A4; green, SMemb; blue, nuclei); anti‐S100A4 and anti‐CD68 antibodies (center: red, S100A4; green, CD68; blue, nuclei); and anti‐S100A4 and anti‐CD45 antibodies (right: red, S100A4; green, CD45; blue, nuclei). S100A4 was found to colocalize with SMemb (left) and CD68 (center), but not CD45 (right). Scale bars: 50 μm. D, Samples stained with anti‐S100A4 and anti‐MMP9 antibodies (red, S100A4; green, MMP9). The upper image shows a plaque neointima, and the lower image, a plaque marginal area. In both these areas, S100A4‐positive cells were found to express MMP9. Scale bars: 50 μm. DAPI indicates 4′,6‐diamidino‐2‐phenylindole; MMP, matrix metalloproteinase; and SMemb, embryonic form of smooth muscle myosin heavy chain.
S100A4 is Upregulated in the Plaques of a Mouse Partial Carotid Ligation Model
The left CAs of mice at 0, 1, and 6 weeks after partial ligation were stained with Elastica van Gieson. Representative images of the arteries at these time points are shown in Figure 3A. Atherosclerotic lesions of left CAs at 6 weeks after ligation showed expansive arterial remodeling with the proliferation of both intimal and medial lesions. The intimal area had already expanded 1 week after ligation and had expanded further after 6 weeks (1 week: P=0.02, 6 weeks versus 1 week: P=0.006). The medial area also showed a statistically significant increase at 1 week after ligation and showed a tendency to further expand at 6 weeks after ligation (1 week: P=0.02, 6 weeks versus 1 week: P=0.06). Expansive remodeling was detected at 6 weeks after ligation (1 week: P=0.95, 6 weeks: P=0.03; Figure 3B).
Figure 3. Expression of S100A4 (S100 calcium‐binding protein A4) in the plaques of a mouse partial carotid ligation model.
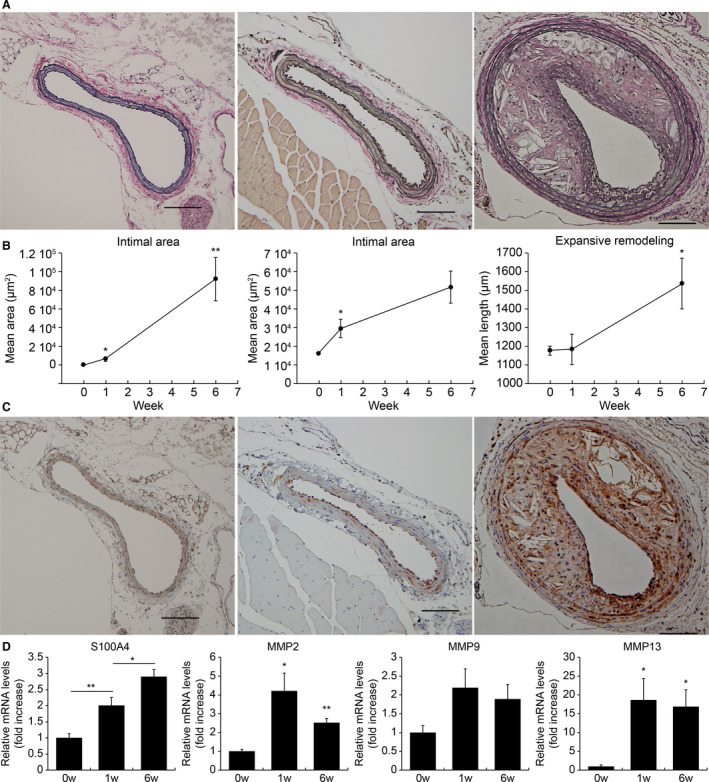
A, Mouse left carotid arteries (CAs) stained with Elastica van Gieson at 0, 1, and 6 weeks after partial ligation. Representative images are as follows: e left=0 weeks, center=1 week), and right=6 weeks. Atherosclerotic lesions of the left CAs at 6 weeks after ligation exhibited expansive arterial remodeling with the proliferation of both intimal and medial lesions. B, Intimal area, medial area, and perimeter of the external elastic lamina of partially ligated CAs. For each group, the average values of 5 mice were calculated, and changes over time were evaluated. The intimal area had already expanded 1 week after ligation and then expanded further after 6 weeks. The medial area also showed a significant increase at 1 week after ligation and showed a tendency to further expand at 6 weeks after ligation. Expansive remodeling was observed at 6 weeks after ligation. C, Mouse left CAs stained with anti‐S100A4 antibody at 0, 1, and 6 weeks after partial ligation. Representative images are as follows: left=0 weeks, center=1 week, and right=6 weeks. S100A4 was expressed in the medial area at 1 week after ligation, and extensive expression was observed in both the medial and neointimal areas 6 weeks after ligation. D, The expression levels of S100a4, Mmp2, Mmp9, and Mmp13 at 0, 1, and 6 weeks after ligation (n=6–9 each time point). The expression of S100a4 showed a statistically significant increase over time. The expression levels of Mmp2 and Mmp13 increased from 1 week after ligation and showed a significant increase even after 6 weeks. In contrast, there was no significant difference in the expression levels of Mmp9. The gene expression levels were evaluated using quantitative PCR. *P<0.05, **P<0.01 for the indicated comparisons. MMP indicates matrix metalloproteinase.
S100a4 expression was significantly higher in the left CAs at 1 week after ligation than before ligation (P=0.006, Figure 3C, 3D). It was also significantly higher in arteries at 6 weeks after ligation than at 1 week after ligation (P=0.019; Figure 3C, 3D). In contrast, the levels of αSMA decreased at 1 week after ligation (P=0.006), although the expression was recovered at 6 weeks after ligation (P=0.026; data not shown). Furthermore, we found that the expression levels of Mmp2 and Mmp13 increased at 1 week after ligation (P=0.014 and P=0.023, respectively) and increased significantly at 6 weeks (P=0.0003 and P=0.014, respectively) after ligation. In contrast, we detected no significant difference in the expression of Mmp9 (1 week: P=0.27, 6 weeks: P=0.25; Figure 3D).
Extracellular S100A4 Enhances the Expression of Mmp2, Mmp9, and Mmp13 in Mouse VSMCs
The mRNA level of Mmp2 in VSMCs increased in response to treatment with 0.02 μg/mL (P=0.049) and 0.1 μg/mL (P=0.0008) S100A4, whereas the mRNA levels of Mmp9 and Mmp13 increased after treatment with 0.1 μg/mL S100A4 (P=0.003 and P=0.013, respectively; Figure 4A).
Figure 4. Extracellular S100A4 (S100 calcium‐binding protein A4) enhances MMPs expression in mouse vascular smooth muscle cells (VSMCs) and regulates VSMC proliferation and migration in vitro.
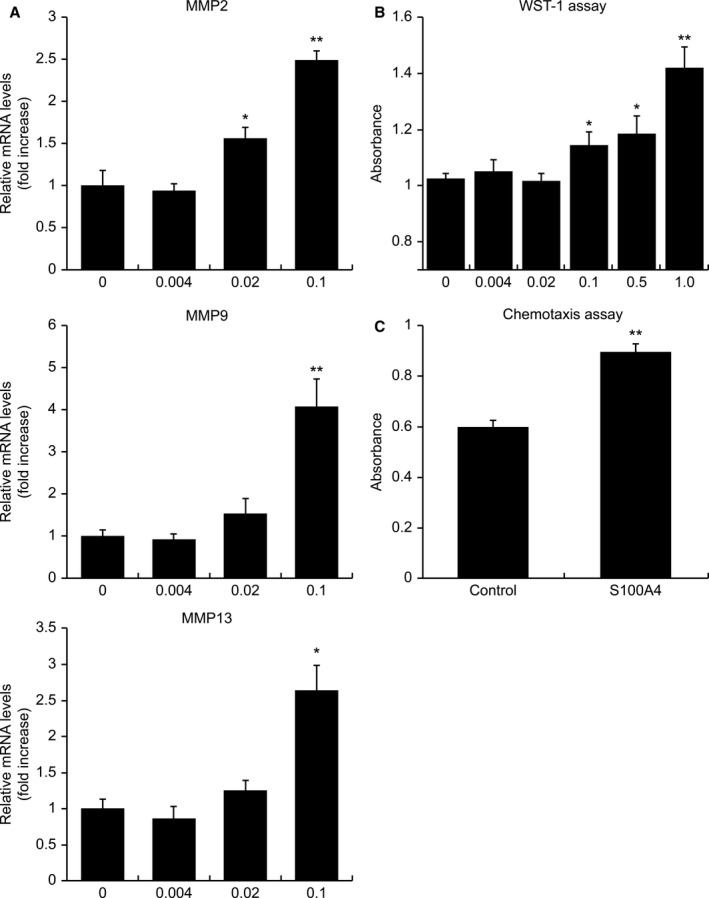
A, Bar graphs showing the levels of Mmp2, Mmp9, and Mmp13 mRNA in mouse VSMCs incubated with different concentrations (0–0.1 μg/mL) of recombinant mouse S100A4 protein for 16 hours. The expression level of Mmp2 increased at concentrations between 0.02 and 0.1 μg/mL, whereas the expression levels of Mmp9 and Mmp13 increased at a concentration of 0.1 μg/mL. B, Bar graph showing the proliferation of mouse VSMCs treated for 48 hours with different concentrations (0–1.0 μg/mL) of recombinant mouse S100A4 protein. Cell proliferation was enhanced by treatment with S100A4 at concentrations higher than 0.1 μg/mL. C, Bar graph showing the chemoattractant activity of S100A4 for mouse VSMCs using a chemotaxis assay. VSMCs' migration to the opposite compartment containing 1 μg/mL recombinant S100A4 was greater than VSMCs' migration to the compartment containing the control medium. *P<0.05, **P<0.01. MMP indicates matrix metalloproteinase; and WST, water‐soluble tetrazolium salt.
Extracellular S100A4 Enhances Proliferation of VSMCs
The water‐soluble tetrazolium salt‐1 assay revealed that VSMC proliferation was enhanced by treatment with S100A4 at concentrations higher than 0.1 μg/mL (P=0.03 at 0.1 μg/mL, P=0.03 at 0.5 μg/mL, and P=0.0002 at 1.0 μg/mL; Figure 4B).
Extracellular S100A4 Functions as a Chemoattractant for VSMCs
VSMCs were subjected to 24‐hour incubation with S100A4 or a control medium in the opposite compartment of a migration chamber. VSMC migration to the opposite compartment containing S100A4 was higher than that to the compartment containing the control medium (P<0.0001; Figure 4C), thereby indicating that extracellular S100A4 functions as a chemoattractant for VSMCs.
Discussion
In the present study, we report the increased expression of S100A4 in carotid atherosclerotic lesions. We further evaluated whether the expression of S100A4 is enhanced concomitantly with the growth of carotid plaques using a mouse model of partial carotid ligation. It has been determined that atherosclerosis is accelerated by the occurrence of low shear stress (LSS) during vessel bifurcation. 25 , 26 In the partial carotid ligation model, LSS occurs in the left common CA, and plaques are generated in this lesion. 23
Six weeks after ligation, most samples showed large plaques in the left CAs and exhibited expansive remodeling, and S100A4 expression in the left CA increased. S100A4 expression was enhanced as early as 1 week after ligation, at which time only a few neointima had developed. Immunohistochemical analyses revealed that the expression of S100A4 was enhanced at an early stage in the medial SMCs. We thus suspect that certain factors that promote S100A4 expression in VSMCs may be secreted by endothelial cells in response to LSS. One candidate in this regard is platelet‐derived growth factor‐BB (PDGF‐BB), the expression of which was found to be enhanced in the left CA at 1 week after ligation (data not shown). PDGF‐BB is known to be a potent dedifferentiation‐promoting factor of VSMCs in vitro, and has been reported to be an important factor in the cross talk between endothelial and smooth muscle cells in ex vivo LSS experiments. 27 It had been reported that extracellular S100A4 induces the phenotypic transition of porcine VSMCs, 28 indicating that increased expression of PDGF‐BB in endothelial cells and the subsequent expression of S100A4 in the medial VSMCs play important roles in the early stage of atherosclerosis.
Yoshida et al reported that the internal CA angle was significantly larger in groups with extensive expansive remodeling than in those with slight expansive remodeling, and that it is associated with the symptomatology of patients with CA stenosis who were scheduled to undergo carotid endarterectomy or CA stenting. 29 Sitzer et al found that the angle of the internal CA bifurcation might be an independent risk factor for early atherosclerosis in a cross‐sectional ultrasound study of 1300 healthy individuals. 30 Simulation studies have shown that as the angle of the internal CA bifurcation increases, so does the oscillating shear index, but the wall shear stress decreases. 31 The results of our study suggest that LSS on human carotid artery is involved in expansive remodeling.
The expression of the marker of dedifferentiated VSMCs, but not the common leukocyte antigen CD45, in S100A4‐positive foam cells indicates that these cells are probably derived from VSMCs, rather than from macrophages in human carotid plaques. Accordingly, we believe that the present study is the first to demonstrate that foam cells derived from VSMCs express S100A4.
Because of the positive correlation between S100A4 and MMP expression in carotid endarterectomy specimens and the coexpression of S100A4 and MMP9 in immunohistochemical analysis, we assumed that S100A4‐positive cells derived from VSMCs express MMPs in human carotid plaques. It has previously been reported that plaque rupture tends to occur in the shoulder region, 32 and that rupture of the elastic lamina in the marginal area of plaques is associated with expansive remodeling. Our results indicate that S100A4 expressed in the plaque shoulder and marginal areas may promote plaque destabilization and expansive remodeling by promoting the expression of MMPs.
We further investigated whether S100A4 promotes the expression of MMP and enhances cell proliferation in VSMCs in vitro. It was previously shown that extracellular S100A4 upregulates the expression of MMPs in dedifferentiated porcine VSMCs in a receptor for advanced glycation end product‐dependent manner. 28 In myocardium, S100A4 promotes smooth muscle cell motility and proliferation with extracellular signal‐regulated kinase 1/2 activation. 33 The proliferation‐inducing property of S100A4 may promote cell proliferation in the neointima, such that foam cells develop in the necrotic core, which may play a role in increasing plaque volume. It has been assumed that VSMC migration from the media to the neointima is mainly attributable to the activity of growth factors secreted primarily by macrophages. 34 Our results, however, provide evidence that S100A4 secreted by VSMC‐derived cells in the neointima may also stimulate VSMC migration as a chemoattractant factor. We observed a disturbed layer structure of the elastic lamina in the plaque marginal area where S100A4‐positive cells accumulate (Figure 2B). These VSMCs that migrate to the plaque margins further secrete S100A4, which may enhance MMP activity in both the cells of origin and surrounding cells, thereby degrading the elastic lamina and resulting in expansive remodeling.
Our findings indicate that S100A4 can serve as a biomarker and therapeutic target of CA stenosis. It has been reported that serum S100A4 levels in patients with acute myocardial infarction are significantly higher than those in healthy volunteers. 35 It is conceivable that serum S100A4 levels also increase in response to the rupture of carotid plaques. S100A4 is a unique molecule involved in inflammation, cell migration, and cell proliferation both intracellularly and extracellularly, and may play an important role in the early‐to‐advanced stages of arteriosclerosis. S100A4 may offer a novel therapeutic target/strategy that differs from what is currently available, such as lipid, blood glucose, and blood pressure management. The receptor for advanced glycation end products is an established receptor for extracellular S100A4. In recent years, various findings have emerged outlining the association between the receptor for advanced glycation end products and arteriosclerosis, 36 supporting our consideration. It has previously been reported that an S100A4‐blocking antibody reduced tumor growth and metastasis in a model of spontaneous breast cancer, 37 and we suspect that such an antibody might be efficient for the treatment of unstable carotid atherosclerotic lesions.
This study has certain limitations. First, it is not clear whether S100A4 actually promotes plaque progression and expansive remodeling in arteriosclerotic lesions. Accordingly, it will be necessary to investigate the plaque changes occurring in response to the suppression or overexpression of S100A4 in animal models. Second, although we show that S100A4 promotes the expression of MMPs in VSMCs and enhances proliferative capacity in vitro, its function in the foam cells derived from VSMCs has not been clarified. Further experiments are warranted to investigate the role of S100A4 in VSMC transformation into foam cells.
Sources of Funding
This work was supported in part by grants from the Japan Society for the Promotion of Science JSPS KAKENHI (Grants No. 26460338 and 17K08592 to M.M., and 15K10299 to K.Y.).
Disclosures
None.
(J Am Heart Assoc. 2020;9:e016128 DOI: 10.1161/JAHA.120.016128.)
For Sources of Funding and Disclosures, see page 11.
Contributor Information
Manabu Minami, Email: mminami@kuhp.kyoto-u.ac.jp.
Kazumichi Yoshida, Email: kazuy@kuhp.kyoto-u.ac.jp.
References
- 1. Petty GW, Brown RD Jr, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population‐based study of incidence and risk factors. Stroke. 1999;2513–2516. [DOI] [PubMed] [Google Scholar]
- 2. Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, Fox AJ, Rankin RN, Hachinski VC, Wiebers DO, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high‐grade carotid stenosis. N Engl J Med. 1991;445–453. [DOI] [PubMed] [Google Scholar]
- 3. Walker MD, Marler JR, Goldstein M, Grady PA, Toole JF, Baker WH, Castaldo JE, Chambless LE, Moore WS, Robertson JT, et al. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;1421–1428. [Google Scholar]
- 4. Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003;1664–1672. [DOI] [PubMed] [Google Scholar]
- 5. Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;1371–1375. [DOI] [PubMed] [Google Scholar]
- 6. Alfonso F, Macaya C, Goicolea J, Iniguez A, Hernandez R, Zamorano J, Perez‐Vizcayne MJ, Zarco P. Intravascular ultrasound imaging of angiographically normal coronary segments in patients with coronary artery disease. Am Heart J. 1994;536–544. [DOI] [PubMed] [Google Scholar]
- 7. Nakamura M, Nishikawa H, Mukai S, Setsuda M, Nakajima K, Tamada H, Suzuki H, Ohnishi T, Kakuta Y, Nakano T, et al. Impact of coronary artery remodeling on clinical presentation of coronary artery disease: an intravascular ultrasound study. J Am Coll Cardiol. 2001;63–69. [DOI] [PubMed] [Google Scholar]
- 8. Yoshida K, Fukumitsu R, Kurosaki Y, Funaki T, Kikuchi T, Takahashi JC, Takagi Y, Yamagata S, Miyamoto S. The association between expansive arterial remodeling detected by high‐resolution MRI in carotid artery stenosis and clinical presentation. J Neurosurg. 2015;434–440. [DOI] [PubMed] [Google Scholar]
- 9. Kurosaki Y, Yoshida K, Fukumitsu R, Sadamasa N, Handa A, Chin M, Yamagata S. Carotid artery plaque assessment using quantitative expansive remodeling evaluation and MRI plaque signal intensity. J Neurosurg. 2016;736–742. [DOI] [PubMed] [Google Scholar]
- 10. Ivan E, Khatri JJ, Johnson C, Magid R, Godin D, Nandi S, Lessner S, Galis ZS. Expansive arterial remodeling is associated with increased neointimal macrophage foam cell content: the murine model of macrophage‐rich carotid artery lesions. Circulation. 2002;2686–2691. [DOI] [PubMed] [Google Scholar]
- 11. Ota R, Kurihara C, Tsou TL, Young WL, Yeghiazarians Y, Chang M, Mobashery S, Sakamoto A, Hashimoto T. Roles of matrix metalloproteinases in flow‐induced outward vascular remodeling. J Cereb Blood Flow Metab. 2009;1547–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage‐like cells in human atherosclerosis. Circulation. 2014;1551–1559. [DOI] [PubMed] [Google Scholar]
- 13. Vengrenyuk Y, Nishi H, Long X, Ouimet M, Savji N, Martinez FO, Cassella CP, Moore KJ, Ramsey SA, Miano JM, et al. Cholesterol loading reprograms the microRNA‐143/145–myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage‐like phenotype. Arterioscler Thromb Vasc Biol. 2015;535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenfeld ME. Converting smooth muscle cells to macrophage‐like cells with KLF4 in atherosclerotic plaques. Nat Med. 2015;549–551. [DOI] [PubMed] [Google Scholar]
- 15. Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grootaert MO, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, De Meyer GR. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res. 2018;622–634. [DOI] [PubMed] [Google Scholar]
- 17. Chappell J, Harman JL, Narasimhan VM, Yu H, Foote K, Simons BD, Bennett MR, Jørgensen HF. Extensive proliferation of a subset of differentiated, yet plastic, medial vascular smooth muscle cells contributes to neointimal formation in mouse injury and atherosclerosis models. Circ Res. 2016;1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaabane C, Coen M, Bochaton‐Piallat ML. Smooth muscle cell phenotypic switch: implications for foam cell formation. Curr Opin Lipidol. 2014;374–379. [DOI] [PubMed] [Google Scholar]
- 19. Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, Geczy CL. Functions of S100 proteins. Curr Mol Med. 2013;24–57. [PMC free article] [PubMed] [Google Scholar]
- 20. Bjørnland K, Winberg JO, Ødegaard OT, Hovig E, Loennechen T, Aasen AO, Fodstad Ø, Mælandsmo GM. S100A4 involvement in metastasis: deregulation of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in osteosarcoma cells transfected with an anti‐S100A4 ribozyme. Cancer Res. 1999;4702–4708. [PubMed] [Google Scholar]
- 21. Stewart RL, Carpenter BL, West DS, Knifley T, Liu L, Wang C, Weiss HL, Gal TS, Durbin EB, Arnold SM, et al. S100A4 drives non‐small cell lung cancer invasion, associates with poor prognosis, and is effectively targeted by the FDA‐approved anti‐helminthic agent niclosamide. Oncotarget. 2016;34630–34642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coen M, Marchetti G, Palagi PM, Zerbinati C, Guastella G, Gagliano T, Bernardi F, Mascoli F, Bochaton‐Piallat ML. Calmodulin expression distinguishes the smooth muscle cell population of human carotid plaque. Am J Pathol. 2013;996–1009. [DOI] [PubMed] [Google Scholar]
- 23. Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;H1535–H1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Puchtler H, Waldrop FS. On the mechanism of Verhoeff’s elastica stain: a convenient stain for myelin sheaths. Histochemistry. 1979;233–247. [DOI] [PubMed] [Google Scholar]
- 25. Gibson CM, Diaz L, Kandarpa K, Sacks FM, Pasternak RC, Sandor T, Feldman C, Stone PH. Relation of vessel wall shear stress to atherosclerosis progression in human coronary arteries. Arterioscler Thromb. 1993;310–315. [DOI] [PubMed] [Google Scholar]
- 26. Koskinas KC, Chatzizisis YS, Baker AB, Edelman ER, Stone PH, Feldman CL. The role of low endothelial shear stress in the conversion of atherosclerotic lesions from stable to unstable plaque. Curr Opin Cardiol. 2009;580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qi YX, Jiang J, Jiang XH, Wang XD, Ji SY, Han Y, Long DK, Shen BR, Yan ZQ, Chien S, et al. PDGF‐BB and TGF‐β1 on cross‐talk between endothelial and smooth muscle cells in vascular remodeling induced by low shear stress. Proc Natl Acad Sci USA. 2011;1908–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chaabane C, Heizmann CW, Bochaton‐Piallat ML. Extracellular S100A4 induces smooth muscle cell phenotypic transition mediated by RAGE. Biochim Biophys Acta. 2015;2144–2157. [DOI] [PubMed] [Google Scholar]
- 29. Yoshida K, Yang T, Yamamoto Y, Kurosaki Y, Funaki T, Kikuchi T, Ishii A, Kataoka H, Miyamoto S. Expansive carotid artery remodeling: possible marker of vulnerable plaque. J Neurosurg. 2019:1–6. 10.3171/2019.7.JNS19727. [DOI] [PubMed] [Google Scholar]
- 30. Sitzer M, Puac D, Buehler A, Steckel DA, von Kegler S, Markus HS, Steinmetz H. Internal carotid artery angle of origin: a novel risk factor for early carotid atherosclerosis. Stroke. 2003;950–955. [DOI] [PubMed] [Google Scholar]
- 31. Nguyen KT, Clark CD, Chancellor TJ, Papavassiliou DV. Carotid geometry effects on blood flow and on risk for vascular disease. J Biomech. 2008;11–19. [DOI] [PubMed] [Google Scholar]
- 32. Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993;377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schneider M, Kostin S, Strom CC, Aplin M, Lyngbaek S, Theilade J, Grigorian M, Andersen CB, Lukanidin E, Lerche Hansen J, et al. S100A4 is upregulated in injured myocardium and promotes growth and survival of cardiac myocytes. Cardiovasc Res. 2007;40–50. [DOI] [PubMed] [Google Scholar]
- 34. Waltenberger J. Modulation of growth factor action: implications for the treatment of cardiovascular diseases. Circulation. 1997;4083–4094. [DOI] [PubMed] [Google Scholar]
- 35. Gong XJ, Song XY, Wei H, Wang J, Niu M. Serum S100A4 levels as a novel biomarker for detection of acute myocardial infarction. Eur Rev Med Pharmacol Sci. 2015;2221–2225. [PubMed] [Google Scholar]
- 36. Olejarz W, Lacheta D, Gluszko A, Migacz E, Kukwa W, Szczepanski MJ, Tomaszewski P, Nowicka G. Rage and TLRs as key targets for antiatherosclerotic therapy. Biomed Res Int. 2018;7675286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grum‐Schwensen B, Klingelhöfer J, Beck M, Bonefeld CM, Hamerlik P, Guldberg P, Grigorian M, Lukanidin E, Ambartsumian N. S100A4‐neutralizing antibody suppresses spontaneous tumor progression, pre‐metastatic niche formation and alters T‐cell polarization balance. BMC Cancer. 2015;44. [DOI] [PMC free article] [PubMed] [Google Scholar]


