Abstract
Background
Elevated lipoprotein(a) is a well‐established risk factor for atherosclerotic vascular disease but is not measured in routine clinical care. Screening of high lipoprotein(a) in individuals with moderate elevations of low‐density lipoprotein cholesterol (LDL‐C) may identify individuals at high risk of cardiovascular disease.
Methods and Results
We examined 2606 Framingham Offspring participants (median age, 54 years; 45% men) prospectively with a median follow‐up of 15 years (n=392 incident cardiovascular events). Individuals with higher (≥100 nmol/L) versus lower lipoprotein(a) were divided into groups based on LDL‐C <135 mg/dL versus ≥135 mg/dL. In Cox models, after adjustment for known risk factors, high lipoprotein(a) (≥100 nmol/L) and LDL‐C ≥135 mg/dL were each significant predictors of cardiovascular disease (LDL‐C ≥135 mg/dL: hazard ratio [HR], 1.34; 95% CI, 1.09–1.64; P=0.006; high lipoprotein (a): HR, 1.31; 95% CI, 1.03–1.66; P=0.026). Across the groups of high/low lipoprotein (a) and LDL‐C ≥135 mg/dL or <135 mg/dL, the absolute cardiovascular disease risks at 15 years were 22.6% (high lipoprotein(a)/LDL‐C ≥135 mg/dL, n=248), 17.3% (low lipoprotein(a)/LDL‐C ≥135 mg/dL, n=758), 12.7% (high lipoprotein(a)/LDL‐C <135 mg/dL, n=275) and 11.5% (low lipoprotein(a)/LDL‐C <135 mg/dL, n=1328, reference group). Among individuals with LDL‐C ≥135 mg/dL, those with high lipoprotein(a) had a 43% higher risk (HR, 1.43; 95% CI, 1.05–1.97; P=0.02). Presence of high lipoprotein(a) with moderate LDL‐C levels (135–159 mg/dL) yielded absolute risks equivalent to those with LDL‐C ≥160 mg/dL (23.5%, 95% CI, 17.4%–31.3%; and 20.7%, 95% CI, 16.8%–25.3%, respectively).
Conclusions
Concomitant elevation of LDL‐C ≥135 mg/dL and lipoprotein(a) ≥100 nmol/L is associated with a high absolute risk of incident cardiovascular disease. lipoprotein(a) measurement in individuals with moderate elevations in LDL‐C, who do not otherwise meet criteria for statins, may identify individuals at high cardiovascular risk.
Keywords: cardiovascular disease, dyslipidemia, Framingham, lipoprotein(a)
Subject Categories: Cardiovascular Disease, Epidemiology, Primary Prevention, Risk Factors
Nonstandard Abbreviations and Acronyms
- CARDIoGRAMplusC4D
Coronary Artery Disease Genome wide Replication and Meta analysis plus the Coronary Artery Disease Genetics
- CHARGE
Cohorts for Heart and Aging Research in Genomic Epidemiology
- CVD
cardiovascular disease
- FATS
Familial Atherosclerosis Treatment Study
- FHS
Framingham Heart Study
- LDL‐C
low‐density lipoprotein cholesterol
- NRI
net reclassification index
Clinical Perspective
What Is New?
Individuals with both lipoprotein(a) ≥100 nmol/L and low‐density lipoprotein cholesterol ≥135 mg/dL represent a group at higher risk of developing cardiovascular disease.
What Are the Clinical Implications?
Measuring lipoprotein(a) may help stratify cardiovascular disease risk associated with moderate low‐density lipoprotein cholesterol, identifying individuals who may benefit from statin treatment.
Elevated lipoprotein(a) is among the most common genetic dyslipidemias worldwide, affecting 1 in 5 individuals, and is an independent risk factor for cardiovascular disease (CVD). 1 , 2 Although evidence from Mendelian randomization supports a causal role of lipoprotein(a) in CVD, 3 , 4 limited therapeutic options exist to directly lower lipoprotein(a). 5 Current recommended strategies for management of individuals with high lipoprotein(a) emphasize the importance of managing other risk factors, including lowering elevated low‐density lipoprotein cholesterol (LDL‐C) concentrations, without much supporting evidence for such an approach. 6 , 7 , 8
Among individuals with premature acute coronary syndromes, elevations of LDL‐C >135 mg/dL were more frequent among patients with high lipoprotein(a), suggesting greater absolute risk when both lipoproteins were elevated. 9 Accordingly, we sought to evaluate the absolute and relative risks of incident CVD associated with elevated lipoprotein(a) (≥100 nmol/L, approximately equivalent to 50 mg/dL) in the presence of LDL‐C values above and below 135 mg/dL, prospectively in the community‐based Framingham Offspring Cohort. Given that current American College of Cardiology/American Heart Association lipids guidelines 10 also consider an LDL‐C ≥160 mg/dL as an optional indication for early lipid‐lowering therapy, we also evaluated whether the concomitant presence of high lipoprotein(a) with LDL‐C levels between 135 and <160 mg/dL conferred similar CVD risk.
METHODS
Study Sample
The FHS (Framingham Heart Study) Offspring Cohort is a prospective community‐based study that enrolled the children of the original FHS and their spouses starting in 1971. The design and selection criteria of the study have been detailed previously. 11 , 12 For the present investigation, we focused on FHS Offspring Cohort participants attending their fifth examination cycle (1991–1995; referred to as baseline for the present investigation). Informed consent was provided by all participants at the beginning of examination. Permission to analyze the Framingham Offspring database was obtained from the National Heart, Lung, and Blood Institute according to a research proposal approved by our institutional review board. All participants underwent a standardized cardiovascular‐targeted medical history and physical examination including measurement of resting blood pressure, electrocardiographic evaluation; they had a fasting blood specimen drawn for measurement of plasma lipids and lipoprotein levels. The original number of participants included 3799 individuals, of which 833 were excluded due to unavailable lipoprotein(a) values, 5 were excluded for missing covariate data, 284 had missing information on CVD, and 71 had missing LDL levels or were on lipid‐lowering therapy. The final study sample included 2606 participants (median age of 54 years, 45% of whom are men) who were free of CVD and who had their plasma lipoprotein(a) levels measured at the baseline examination.
Lipoprotein(a), Apolipoprotein(B), and Lipid Measurement
Fasting plasma samples were collected and plasma concentrations of total cholesterol, triglycerides, and high‐density lipoprotein cholesterol were measured using established automated methods in an ABA‐200 analyzer using Abbott A‐Gent enzymatic reagents (Abbott Diagnostics, Lake Forest, IL). Plasma LDL‐C concentration was calculated using the Friedewald equation. 13 We excluded individuals with triglyceride level >400 mg/dL. Plasma lipoprotein(a) concentration (in nmol/L) was measured via ELISA. For this assay, a monoclonal antibody against the apolipoprotein(a) moiety was used that was independent of the apolipoprotein(a) isoform size. 14 Lipoprotein(a) measurements were performed at the Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington, Seattle, Washington. We defined high LDL‐C as ≥135 mg/dL and high lipoprotein(a) as ≥100 nmol/L (≈80th percentile), which were the median levels among individuals who developed CVD. This level of lipoprotein(a) also corresponded to the 80th percentile in FHS participants. Apolipoprotein B levels were measured using immunoturbidimetric assays in plasma. 15 High apolipoprotein B was defined as ≥120 mg/dL.
Other Covariates
Resting blood pressure was defined as the average of two measurements made on the left arm of each seated participant using a mercury column sphygmomanometer and a cuff of appropriate size. Hypertension was defined as a systolic blood pressure ≥140 mm Hg, or a diastolic blood pressure ≥90 mm Hg, or the use of antihypertensive medications. Diabetes mellitus was defined as having a fasting blood glucose level of ≥126 mg/dL, or the use of hypoglycemic medications including insulin. Current smoking status was obtained via a standardized self‐reported questionnaire. In the FHS, participants were considered as current smokers if they had smoked at least 1 cigarette per day within the 12 months preceding the Heart Study examination.
Outcome Events
We defined the composite end point of incident “major cardiovascular event” as: (1) coronary heart disease that includes coronary death, fatal or nonfatal myocardial infarction, coronary insufficiency, and unstable angina with documented dynamic ST‐segment changes; (2) cerebrovascular events including ischemic or hemorrhagic stroke or transient ischemic attack; (3) peripheral artery disease or intermittent claudication; and (4) new diagnosis of heart failure. 16 Complete medical records for hospitalizations and physician office visits related to the cardiovascular events were obtained and reviewed by an end‐points adjudication committee composed of 3 experienced physician investigators who evaluated all pertinent medical records. All events were adjudicated by following previously established criteria. 17 , 18
Statistical Analysis
Descriptive statistics are presented as means (with standard deviations) for continuous variables and as percentages for dichotomous variables. All participants were classified into high/low–LDL‐C and high/low‐lipoprotein(a) groups for analysis. Crude risks of CVD incidence at 15 years, not accounting for competing risk of death, were calculated as 1‐(Kaplan–Meier estimator) with censoring at the earliest of 15 years or last contact. Estimates were made for each of the 4 groups (LDL‐C <135 mg/dL/low lipoprotein(a); LDL‐C <135 mg/dL/high lipoprotein(a); LDL‐C ≥135 mg/dL/low lipoprotein(a); and high LDL‐C ≥135 mg/dL/high lipoprotein[a]) using SAS 9.4 PROC LIFETEST SAS Institute, Cary, NC). After confirmation of the proportionality of hazards using SAS 9.4 PHREG procedure with the SAS default parameters, we used Cox regression models to estimate the association between CVD with high lipoprotein(a) ≥100 nmol/L and LDL‐C ≥135 mg/dL first individually to confirm that each was associated with incident CVD, and evaluated for statistical interaction by modeling an interaction term “high lipoprotein(a)×high LDL‐C.” The proportional hazards assumption was tested for each variable in our multivariable Cox model using SAS 9.4 PHREG procedure, ASSESS PH statement, with 1000 resampled data sets. The proportionality assumption holds for each variable. Results are presented as hazards ratios with 95% CIs.
We estimated the associations for each of the 4 categories of LDL‐C and lipoprotein(a), using the highest risk group as the reference (high lipoprotein[a]/LDL‐C ≥135 mg/dL). All models were adjusted for age, sex, hypertension, cigarette smoking, and diabetes mellitus. To evaluate whether high lipoprotein(a) and LDL‐C ≥135 mg/dL was a risk‐equivalent to high LDL‐C ≥160 mg/dL (an optional treatment threshold for statins), we estimated the absolute risks for individuals across the following mutually exclusive groups: (1) LDL‐C ≥160 mg/dL; (2) LDL‐C 135 to <160 mg/dL and high ipoprotein(a); (3) LDL‐C 135 to <160 mg/dL without high lipoprotein(a) and (4) LDL‐C <135 mg/dL.
To assess whether the addition of lipoprotein(a) improved risk reclassification, we calculated the net reclassification index (NRI) for adding lipoprotein(a) to a regression model including age, sex, LDL‐C, diabetes mellitus, hypertension, and smoking using a risk threshold of 10% CVD event rates over 15 years. We also present the NRI stratified for individuals with and without LDL‐C >135 mg/dL. In secondary analyses, we (1) replaced LDL‐C ≥135 mg/dL with apolipoprotein B ≥120 mg/dL and (2) corrected the cholesterol content in LDL‐C for lipoprotein(a) cholesterol using Dahlen’s formula. We also performed sensitivity analyses by using different cutoff values for low, moderate, and high LDL‐C (LDL <130, LDL‐C ≥130, and LDL‐C ≥160) as well as moderate (and high lipoprotein[a]).
We estimated adjusted survival functions using SAS PHREG, using the full multivariable model with the BASELINE statement and DIRADJ option, with censoring at the earliest of 15 years or last contact. To display cumulative risk, we plotted 1—survival function for the cross‐classification levels of binary lipoprotein(a) and LDL‐C variates.
All analyses were performed in SAS version 9.4. Using 2‐sided tests, a P<0.05 was considered statistically significant.
Anonymized data and materials from the Framingham’s Original Cohort, Offspring, and Third Generation exam data are available through the National Heart, Lung, and Blood Institute’s BioLINCC and can be accessed by qualified investigators with approval. Requests for Framingham data should follow the process outlined in BioLINCC (https://biolincc.nhlbi.nih.gov/home/).
RESULTS
Baseline Characteristics
Table 1 summarizes the baseline characteristics of study participants. Median age was 54 years (SD, 10 years), and 55% were women. The mean LDL‐C in our sample was 126.6 mg/dL and the mean lipoprotein(a) was 51.9 nmol/L (SD, 70.4 nmol/L). During a median follow‐up period of 15 years, 394 individuals experienced a first CVD event.
Table 1.
Baseline Characteristics Based on the Groups (N=2606)
| All N=2606 |
Low LDL‐C/Low Lipoprotein(a) N=1325 |
Low LDL‐C/High Lipoprotein(a) N=275 |
High LDL‐C/Low Lipoprotein(a) N=758 |
High LDL‐C/High Lipoprotein(a) N=248 |
|
|---|---|---|---|---|---|
| Age, y | 54.6±9.8 | 53.4±9.8 | 53.2±9.4 | 56.5±9.5 | 56.6±9.6 |
| Women, % | 55.5 | 57.4 | 58.9 | 51.1 | 55.7 |
| Total cholesterol, mg/dL | 205±36 | 184±25 | 188±21 | 235±25 | 239±29 |
| Current smokers, % | 9.5 | 19.3 | 17.1 | 21.1 | 18.6 |
| Diabetes mellitus, % | 5.4 | 5.9 | 4.7 | 5.3 | 4.0 |
| Systolic blood pressure, mm Hg | 126±19 | 124±18 | 124±19 | 128±19 | 129±20 |
| Hypertension, % | 30.2 | 28.1 | 29.1 | 32.8 | 35.1 |
| HDL‐C, mg/dL | 51±15 | 53±16 | 52±16 | 48±12 | 50±14 |
| LDL‐C, mg/dL | 127±33 | 106±20 | 109±17 | 158±21 | 160±23 |
| LDL‐C corrected, mg/dL | 19±33 | 102±20 | 87±18 | 155±21 | 134±25 |
| lipoprotein(a), nmol/L | 52±71 | 21±23 | 162±57 | 23±24 | 183±77 |
HDL‐C indicates high‐density lipoprotein cholesterol; and LDL‐C, low‐density lipoprotein cholesterol.
Relative Risks of CVD Attributable to High Lipoprotein(a) or High LDL‐C
Table 2 summarizes the hazard ratios (HR) associated with each of the known risk factors for CVD. Compared with participants with lower lipoprotein(a) levels, those with higher plasma levels had significantly increased cardiovascular risk after adjustments for other known risk factors including high LDL‐C (lipoprotein[a] ≥100 nmol/L, HR, 1.31; 95% CI, 1.03–1.66; P=0.026; LDL‐C ≥135 mg/dL, HR, 1.34; 95% CI, 1.09–1.64; P=0.006). Multiplicative interactions between lipoprotein(a) and LDL‐C were nonsignificant but had limited statistical power. Results were similar and remained significant after we corrected for the cholesterol content carried by lipoprotein(a) (see Table S1).
Table 2.
High Lipoprotein(a) and LDL‐C Are Significant Predictors of CVD
| Hazard Ratio | 95% CI | P Value | |
|---|---|---|---|
| Age | 1.06 | 1.05–1.07 | <0.0001 |
| Women | 0.60 | 0.49–0.74 | <0.0001 |
| Systolic blood pressure | 1.02 | 1.01–1.02 | <0.0001 |
| Current smoking | 1.66 | 1.29–2.12 | <0.0001 |
| Diabetes mellitus | 1.96 | 1.43–2.68 | <0.0001 |
| LDL‐C >135 mg/dL | 1.34 | 1.09–1.64 | 0.0057 |
| Lipoprotein(a) >100 nmol/L | 1.31 | 1.03–1.66 | 0.0258 |
CVD indicates cardiovascular disease; and LDL‐C, low‐density lipoprotein cholesterol.
Absolute and Relative Risks of CVD Among Individuals With High Lipoprotein(a) ≥100 nmol/L With and Without LDL‐C ≥135 mg/dL
Across the 4 groups of high/low lipoprotein(a) and LDL‐C ≥135 mg/dL, the group with both high lipoprotein(a) and LDL‐C ≥135 mg/dL had the highest absolute risk at 15 years (Table 3). The group with the lowest absolute risk included participants with both lipoprotein(a) <100 nmol/L and LDL‐C <135 mg/dL. Those with high lipoprotein(a) ≥100 nmol/L, but LDL‐C <135 mg/dL had a modestly higher absolute risk that than the lowest‐risk group (Figure). Results were similar when we used apolipoprotein B ≥100 mg/dL or apolipoprotein B ≥120 mg/dL instead of LDL‐C ≥135 mg/dL (Figures S1 and S2).
Table 3.
CVD Event Rates and Kaplan–Meier Risk Estimates for LDL‐C and Lipoprotein(a) Groups at 15 Years
| N | Events | Events (%) | Risk (%) | 95% CI | |
|---|---|---|---|---|---|
| Lipoprotein(a) ≥100 nmol/L | |||||
| +LDL‐C ≥135 mg/dL | 248 | 56 | 22.6 | 23.4 | 18.5–29.3 |
| +LDL‐C <135 mg/dL | 275 | 35 | 12.7 | 13.2 | 9.6–17.8 |
| Lipoprotein(a) <100 nmol/L | |||||
| +LDL‐C ≥135 mg/dL | 758 | 131 | 17.3 | 18.1 | 15.5–21.1 |
| +LDL‐C <135 mg/dL | 1325 | 152 | 11.5 | 12.0 | 10.3–13.9 |
CVD indicates cardiovascular disease; and LDL‐C, low‐density lipoprotein cholesterol.
Figure 1. Multivariable adjusted cumulative risks across lipoprotein(a) and LDL‐C categories.
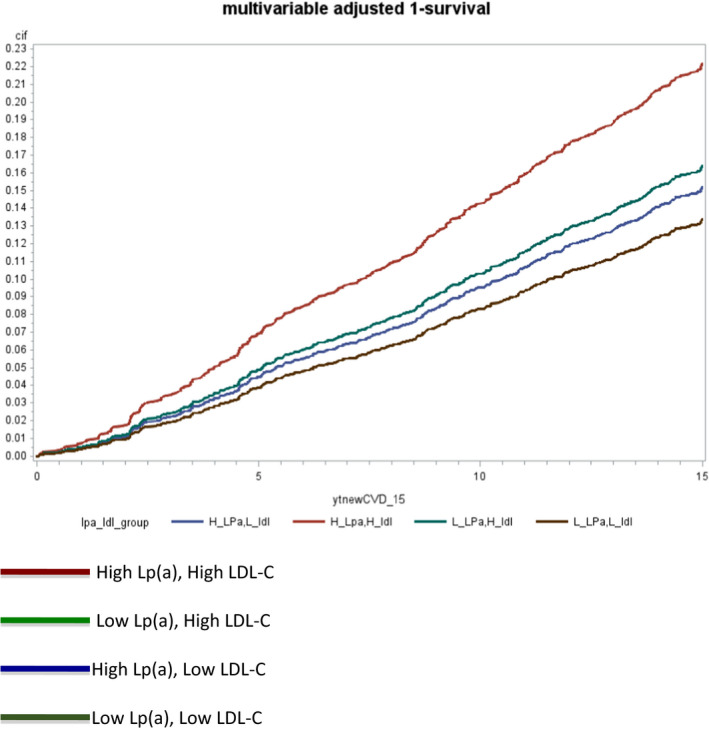
LDL‐C indicates low density lipoprotein cholesterol; and Lp(a), lipoprotein(a).
Net Reclassification Index for the Addition of Lipoprotein(a) for Predicting CVD in Individuals With and Without LDL‐C >135 mg/dL
The addition of lipoprotein(a) ≥100 nmol/L led to no improvements in NRI for the full sample. Among individuals with LDL‐C ≥135 mg/dL, presence of lipoprotein(a) ≥100 nmol/L was associated with a 43% higher risk (HR, 1.43; 95% CI, 1.05–1.97; P=0.02). In individuals with LDL‐C ≥135 mg/dL, the addition of lipoprotein(a) ≥100 nmol/L led to marginal improvements in risk reclassification (NRI, 0.02).
High Lipoprotein(a) in the Presence of Moderate LDL‐C Levels
Current guidelines consider plasma LDL‐C levels ≥160 mg/dL as a risk enhancer and an optional indication for treatment. To better evaluate the clinical utility of screening for high lipoprotein(a), we calculated the observed cardiovascular risks for high LDL‐C ≥160 mg/dL and for LDL‐C 135 to <160 mg/dL+lipoprotein(a) ≥100 nmol/L. The absolute risks at 15 years for those with LDL‐C ≥160 mg/dL was 21.0%. Among participants who had LDL‐C levels between 135 to <160 mg/dL, risk was estimated at 23.5% when lipoprotein(a) ≥100 nmol/L and 17.3% when lipoprotein(a) <100 nmol/L. Risk for individuals with LDL‐C <135 mg/dL was 13.7% (Table 4). In sensitivity analysis, we calculated the observed cardiovascular risk using different cutoff values for low, moderate, and high LDL‐C (LDL <130, LDL‐C ≥130, and LDL‐C ≥160) and moderate (70 nmol/L) and high lipoprotein(a) (100 nmol/L) and results were largely consistent with the main analysis (see Tables S2 and S3).
Table 4.
Kaplan–Meier Risk Estimates for LDL‐C ≥160 mg/dL, LDL‐C 135 to <160 mg/dL With or Without High Lipoprotein(a) and LDL‐C <135 mg/dL
| Events | Participants | Events, % | Risk, % | 95% CI | |
|---|---|---|---|---|---|
| LDL‐C ≥160 mg/dL | 73 | 369 | 19.8 | 20.7 | 16.8–25.3 |
| LDL‐C ≥135 to <160 mg/dL | |||||
| + Lipoprotein(a) ≥100 nmol/L | 34 | 151 | 22.5 | 23.5 | 17.4–31.3 |
| + Lipoprotein(a) <100 nmol/L | 80 | 482 | 16.6 | 17.3 | 14.2–21.1 |
| LDL‐C <135 mg/dL | 187 | 1604 | 11.7 | 12.2 | 10.6–13.9 |
LDL‐C indicates low density lipoprotein cholesterol.
DISCUSSION
In our prospective investigation of over 2600 FHS participants, we confirm that both lipoprotein(a) ≥100 nmol/L and LDL‐C ≥135 mg/dL, were each significantly associated with an increased incidence of CVD over 15 years. However, there were marked differences in the absolute risks of CVD across the 4 groups based on elevations in either lipoprotein(a) or LDL‐C. Absolute cardiovascular event rates over a 15‐year period were highest among individuals with both high lipoprotein(a) (≥100 nmol/L) and LDL‐C ≥135 mg/dL reaching 22.6%. Our results confirm that high lipoprotein(a) confers higher cardiovascular risk, especially in the presence of elevated LDL‐C. Conversely, when LDL‐C was <135 mg/dL, we found that high lipoprotein(a) was associated with a modest increase in cardiovascular risk. We found similar results when stratified by apolipoprotein B ≥100 mg/dL. In multivariable‐adjusted models, we show that among individuals with LDL‐C ≥135 mg/dL, the added presence of lipoprotein(a) ≥100 nmol/L was associated with a 43% increase in cardiovascular risk. Most importantly, we show that in individuals with only moderate elevations of LDL‐C (135–159 mg/dL), the presence of high lipoprotein(a) identifies individuals at high risk, equivalent to that seen for individuals with LDL‐C ≥160 mg/dL, which is considered a risk enhancer in recent lipid guidelines and a possible indication for earlier therapy in recent lipid guidelines.
We have previously reported in over 900 patients with premature acute coronary syndrome, that the presence of high LDL‐C was more frequent than expected among individuals with high lipoprotein(a), suggesting that LDL‐C and lipoprotein(a) may interact in the development of premature acute coronary syndrome. 9 Similarly, Kronenberg and colleagues using prospective data in 500 individuals demonstrated that lipoprotein(a) was a predictor of accelerated progression of carotid atherosclerosis only when LDL‐C levels were >127.6 mg/dL. 19 Similarly, the Women’s Health Study reported an interaction between lipoprotein(a) above 44 mg/dL (≈75th percentile, or ≈90 nmol/L) and LDL‐C levels above 120 mg/dL for cardiovascular events. 20 Our analysis adds to the evidence that the presence of both high LDL‐C and high lipoprotein(a) leads to a marked increase in the risk of cardiovascular events. Although, recent genetic evidence 21 and a large meta‐analysis of lipid lowering 22 suggest that lipoprotein(a) remains a risk factor even when LDL‐C is lowered with statins, our results demonstrate that the absolute risk from lipoprotein(a) is greatest when LDL‐C is also elevated.
Recent evidence suggests that elevated lipoprotein(a) could help with reclassification of individuals at intermediate CVD risk. 23 , 24 , 25 However, a recent large meta‐analysis of European cohorts did not find any significant reclassification when adding lipoprotein(a) to standard risk prediction algorithms. 26 Despite the moderately strong statistical associations between lipoprotein(a) and cardiovascular events in FHS offspring, we found that the addition of lipoprotein(a) did not lead to meaningful improvements in risk prediction using the NRI metric. Nonetheless, among individuals with moderate elevations of LDL‐C (135–159 mg/dL), the addition of high lipoprotein(a) was shown to marginally improve risk reclassification with a 2% increase in the NRI. More importantly, we estimate that the associated risk of incident CVD among individuals with moderate elevations in LDL‐C and high lipoprotein(a) is of similar magnitude to that observed for LDL‐C ≥160 mg/dL, which is an optional treatment indication according to the 2013 American College of Cardiology/American Heart Association guidelines. 10 Therefore, our results suggest that lipoprotein(a) measurement may have greatest immediate clinical relevance in individuals with moderate elevations in LDL‐C (who would otherwise not meet criteria for lipid lowering) because of the unrecognized high cardiovascular risk conferred from the additional atherogenic lipoprotein burden from high lipoprotein(a). Our results are in agreement with Verbeek and colleagues, 27 who reported that the CVD risk associations with lipoprotein(a) are attenuated with low LDL‐C. However, recent work by Stiekema and colleagues 28 studying the effects of evolocumab on arterial wall inflammation in those with elevated lipoprotein(a) (median lipoprotein[a] of 203 nmol/L), suggests that in such individuals, LDL‐C lowering may be insufficient to fully diminish the increased risk of CVD.
Although additional studies are needed to confirm the value of this approach, our findings that the presence of high lipoprotein(a) is associated with high cardiovascular risk among individuals with moderate LDL‐C elevations (135–159 mg/dL) support recent recommendations from European 7 and Canadian 8 societies for lipoprotein(a) screening in specific patient populations such as those with intermediate CVD risk or moderate elevations in LDL‐C. Langsted et al 29 have also shown that among individuals with extreme elevations in LDL‐C attributable to familial hypercholesterolemia, the presence of high lipoprotein(a) markedly increases the risk of myocardial infarction. Our work extends these findings to individuals with moderate LDL‐C levels, more commonly seen in the general community, and provides new evidence that concomitant elevation of lipoprotein(a) in individuals with moderate elevations in LDL‐C adds to the atherogenic lipoprotein burden in such individuals.
The importance of elevated lipoprotein(a) as a strong independent genetic risk factor of CVD, including aortic stenosis, has been clearly demonstrated by work from the Emerging Risk Factors Collaboration, CARDIoGRAMplusC4D (Coronary Artery Disease Genome‐wide Replication and Meta‐analysis plus the Coronary Artery Disease Genetics) consortium and the CHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology) consortium. 1 , 2 , 30 Results from Mendelian randomization studies have also highlighted the causal role of lipoprotein(a) in these cardiovascular outcomes. 3 , 31 , 32 Despite these associations and the evidence for causality, limited therapeutic approaches exist for the management of high lipoprotein(a) levels. The recent development of apolipoprotein(a) antisense molecules, which have shown a >90% reduction in lipoprotein(a), 33 may eventually provide a targeted approach to managing high lipoprotein(a). Although no randomized trials have been performed that confirm that aggressive control of LDL‐C (or other risk factors) is beneficial among individuals with high lipoprotein(a), in a post hoc analysis of participants with high lipoprotein(a) enrolled in the FATS (Familial Atherosclerosis Treatment Study) trial, atherosclerosis regression and cardiovascular event reduction was observed only among individuals with marked LDL‐C reduction. 34 These results provide supportive evidence for aggressive LDL‐C lowering in individuals with high lipoprotein(a). Taken in the context of these data, our results add evidence that among individuals with high lipoprotein(a), a lower LDL‐C could partially mitigate the risk associated with high lipoprotein(a). In addition, a healthier lifestyle, as defined by the American Heart Association’s ideal cardiovascular health score, has been shown to be associated with reduced risk among individuals with high lipoprotein(a). 35 Nonetheless, we acknowledge the need for randomized trials of LDL‐C lowering and other specific preventive approaches among individuals with high lipoprotein(a) to fully evaluate the benefits of these approaches.
Strengths and Limitations
Our study has several strengths including the use of a well‐characterized sample from a community cohort with follow‐up to 15 years and adjudicated cardiovascular events. Lipoprotein(a) measurements were also performed using an isoform independent, highly accurate, and well‐validated method. However, there are several limitations. First, we had a low event rate in our sample; therefore, we had limited power to test for statistical interactions between lipoprotein(a) and LDL‐C. However, our goal was not to assess for departure from the multiplicative model for LDL‐C and lipoprotein(a), which had low statistical power, but to estimate whether the presence of having elevations of both lipoproteins is associated with higher absolute risks than having either one elevated, which may have important clinical implications. Second, although we adjusted for standard cardiovascular risk factors, it remains possible that other unmeasured confounders could be responsible for the higher cardiovascular risks observed when both lipoprotein(a) and LDL‐C were elevated. Third, participants were predominantly of white European descent, which may limit the generalizability of our findings to other ethnicities. Fourth, we did not adjust LDL‐C levels for the cholesterol content conferred by lipoprotein(a), and this would suggest that our thresholds for LDL‐C are higher than the true plasma LDL‐C concentration. However, LDL‐C levels are not normally corrected clinically, and therefore thresholds described here are most relevant to practicing physicians.
CONCLUSIONS
The presence of both high lipoprotein(a) and LDL‐C ≥135 mg/dL is associated with a high absolute risk of incident CVD in the community. Our results indicate that individuals with both high lipoprotein(a) and LDL‐C ≥135 mg/dL represent a high‐risk subgroup, and that lipoprotein(a) concentrations may help stratify CVD risk associated with moderate LDL‐C levels. Our results, if confirmed, raise the possibility that lipoprotein(a) measurements could be considered in individuals with moderate elevations of LDL‐C, who do not otherwise meet criteria for lipid lowering, to better quantify the burden of atherogenic lipoproteins and cardiovascular risk.
Sources of Funding
This work was partially supported by the National Heart, Lung, and Blood Institute’s Framingham Heart Study (Contracts N01‐HC‐25195 and HHSN268201500001I). Dr Vasan is supported by an Evans Scholar award and the Jay and Louis Coffman Foundation from the Department of Medicine, Boston University School of Medicine.
Disclosures
Dr Thanassoulis has received speaker fees and participated in advisory boards for Amgen, Sanofi, and Servier Canada, and is a consultant to Ionis Pharmaceuticals. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
Figures S1–S2
Acknowledgments
The authors thank the Framingham investigators, the staff, and participants for their valuable contributions.
(J Am Heart Assoc. 2020;9:e014711 DOI: 10.1161/JAHA.119.014711.)
For Sources of Funding and Disclosures, see page 8.
References
- 1. Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, et al.; CARDIoGRAMplusC4D Consortium . Large‐scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nordestgaard BG, Chapman MJ, Ray K, Boren J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, et al.; European Atherosclerosis Society Consensus Panel . Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kamstrup PR, Tybjaerg‐Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(A) and increased risk of myocardial infarction. JAMA. 2009;2331–2339. [DOI] [PubMed] [Google Scholar]
- 4. Thanassoulis G, Campbell CY, Owens DS, Gustav Smith J, Smith AV, Peloso GM, Kerr KF, Pechlivanis S, Budoff MJ, Harris TB, et al.; CHARGE Extracoronary Calcium Working Group . Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Afshar M, Thanassoulis G. Lipoprotein(a): new insights from modern genomics. Curr Opin Lipidol. 2017;170–176. [DOI] [PubMed] [Google Scholar]
- 6. Davidson MH, Ballantyne CM, Jacobson TA, Bittner VA, Braun LT, Brown AS, Brown WV, Cromwell WC, Goldberg RB, McKenney JM, et al. Clinical utility of inflammatory markers and advanced lipoprotein testing: advice from an expert panel of lipid specialists. J Clin Lipidol. 2011;338–367. [DOI] [PubMed] [Google Scholar]
- 7. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) developed with the special contribution of the European Association for Cardiovascular Prevention and Rehabilitation (EACPR). Eur Heart J. 2016;2999–3058.27567407 [Google Scholar]
- 8. Anderson TJ, Gregoire J, Pearson GJ, Barry AR, Couture P, Dawes M, Francis GA, Genest J Jr, Grover S, Gupta M, et al. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;1263–1282. [DOI] [PubMed] [Google Scholar]
- 9. Afshar M, Pilote L, Dufresne L, Engert JC, Thanassoulis G. Lipoprotein(a) interactions with low‐density lipoprotein cholesterol and other cardiovascular risk factors in premature acute coronary syndromes (ACS). J Am Heart Assoc. 2016;e003012 DOI: 10.1161/JAHA.115.003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;S1–S45. [DOI] [PubMed] [Google Scholar]
- 11. Dawber TR, Meadors GF, Moore FE Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham Offspring Study. Am J Epidemiol. 1979;281–290. [DOI] [PubMed] [Google Scholar]
- 13. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;499–502. [PubMed] [Google Scholar]
- 14. Marcovina SM, Albers JJ, Gabel B, Koschinsky ML, Gaur VP. Effect of the number of apolipoprotein(a) kringle 4 domains on immunochemical measurements of lipoprotein(a). Clin Chem. 1995;246–255. [PubMed] [Google Scholar]
- 15. Contois JH, McNamara JR, Lammi‐Keefe CJ, Wilson PW, Massov T, Schaefer EJ. Reference intervals for plasma apolipoprotein B determined with a standardized commercial immunoturbidimetric assay: results from the Framingham Offspring Study. Clin Chem. 1996;515–523. [PubMed] [Google Scholar]
- 16. Cupples LA, D’Agostino RB, Kiely D; The Framingham Study . An Epidemiological Investigation of Cardiovascular Disease, Section 34. Some Risk Factors Related to the Annual Incidence of Cardiovascular Disease and Death Using Pooled Repeated Biennial Measurements Framingham Heart Study, 30‐Year Follow‐Up. Bethesda, MD: National Heart, Lung, and Blood Institute; 1987. [Google Scholar]
- 17. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham Study. N Engl J Med. 1971;1441–1446. [DOI] [PubMed] [Google Scholar]
- 18. Frankel DS, Vasan RS, D’Agostino RB Sr, Benjamin EJ, Levy D, Wang TJ, Meigs JB. Resistin, adiponectin, and risk of heart failure the Framingham Offspring Study. J Am Coll Cardiol. 2009;754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kronenberg F, Kronenberg MF, Kiechl S, Trenkwalder E, Santer P, Oberhollenzer F, Egger G, Utermann G, Willeit J. Role of lipoprotein(a) and apolipoprotein(a) phenotype in atherogenesis: perspective results from the Bruneck study. Circulation. 1999;1154–1160. [DOI] [PubMed] [Google Scholar]
- 20. Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially health women. JAMA. 2006;1363–1370. [DOI] [PubMed] [Google Scholar]
- 21. Burgess S, Ference BA, Staley JR, Freitag DF, Mason AM, Nielsen SF, Willeit P, Young R, Surendran P, Karthikeyan S, et al. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)‐lowering therapies: a Mendelian randomization analysis. JAMA Cardiol. 2018;619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Willeit P, Ridker PM, Nestel PJ, Simes J, Tonkin AM, Pedersen TR, Schwartz GG, Olsson AG, Colhoun HM, Kronenberg F, et al. Baseline and on‐statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient‐data meta‐analysis of statin outcome trials. Lancet. 2018;1311–1320. [DOI] [PubMed] [Google Scholar]
- 23. Kamstrup PR, Tybjaerg‐Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and improved cardiovascular risk prediction. J Am Coll Cardiol. 2013;1146–1156. [DOI] [PubMed] [Google Scholar]
- 24. Willeit P, Kiechl S, Kronenberg F, Witztum JL, Santer P, Mayr M, Xu Q, Mayr A, Willeit J, Tsimikas S. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15‐year outcomes in the Bruneck Study. J Am Coll Cardiol. 2014;851–860. [DOI] [PubMed] [Google Scholar]
- 25. Verbeek R, Sandhu MS, Hovingh GK, Sjouke B, Wareham NJ, Zwinderman AH, Kastelein JJP, Khaw KT, Tsimikas S, Boekholdt SM. Lipoprotein(a) improves cardiovascular risk prediction based on established risk algorithms. J Am Coll Cardiol. 2017;1513–1515. [DOI] [PubMed] [Google Scholar]
- 26. Waldeyer C, Makarova N, Zeller T, Schnabel RB, Brunner FJ, Jorgensen T, Linneberg A, Niiranen T, Salomaa V, Jousilahti P, et al. Lipoprotein(a) and the risk of cardiovascular disease in the European population: results from the BiomarCaRE consortium. Eur Heart J. 2017;2490–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verbeek R, Hoogeveen RM, Langsted A, Stiekema LCA, Verweij SL, Hovingh GK, Wareham NJ, Khaw KT, Boekhodlt SM, Nordestgaard BJ, et al. Cardiovascular disease risk associated with elevated lipoprotein(a) attenuates at low low‐density lipoprotein cholesterol levels in a primary prevention setting. Eur Heart J. 2018;2589–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stiekema LCA, Stroes ESG, Verweij SL, Kassahun H, Chen L, Wasserman SM, Sabatine MS, Mani V, Fayad ZA. Persistent arterial wall inflammation in patient with elevated lipoprotein(a) despite strong low‐density lipoprotein cholesterol reduction by proprotein convertase subtilisin/kexin type 9 antibody treatment. Eur Heart J. 2019;2775–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Langsted A, Kamstrup PR, Benn M, Tybjaerg‐Hansen A, Nordestgaard BG. High lipoprotein(a) as a possible cause of clinical familial hypercholesterolaemia: a prospective cohort study. Lancet Diabetes Endocrinol. 2016;577–587. [DOI] [PubMed] [Google Scholar]
- 30. Eroquo S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kamstrup PR, Tybjaerg‐Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;470–477. [DOI] [PubMed] [Google Scholar]
- 32. Arsenault BJ, Boekholdt SM, Dube MP, Rheaume E, Wareham NJ, Khaw KT, Sandhu MS, Tardiff JC. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: a prospective Mendelian randomization study and replication in a case‐control cohort. Circ Cardiovasc Genet. 2014;304–310. [DOI] [PubMed] [Google Scholar]
- 33. Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, Marcovina SM, Hughes SG, Graham MJ, Crooke RM, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raise lipoprotein(a): two randomised, double‐blind, placebo‐controlled, dose‐ranging trials. Lancet. 2016;2239–2253. [DOI] [PubMed] [Google Scholar]
- 34. Maher VM, Brown BG, Marcovina SM, Hillger LA, Zhao XQ, Albers JJ. Effects of lowering elevated LDL cholesterol on the cardiovascular risk of lipoprotein(a). JAMA. 1995;1771–1774. [PubMed] [Google Scholar]
- 35. Perrot N, Verbeek R, Sandhu M, Boekholdt SM, Hovingh GK, Wareham NJ, Khaw KT, Arsenault BJ. Ideal cardiovascular health influences cardiovascular disease risk associated with high lipoprotein(a) levels and genotype: the EPIC‐Norfolk prospective population study. Atherosclerosis. 2017;47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S2


