Abstract
Background
A vegetarian diet (VD) may reduce future cardiovascular risk in patients with ischemic heart disease.
Methods and Results
A randomized crossover study was conducted in subjects with ischemic heart disease, assigned to 4‐week intervention periods of isocaloric VD and meat diet (MD) with individually designed diet plans, separated by a 4‐week washout period. The primary outcome was difference in oxidized low‐density lipoprotein cholesterol (LDL‐C) between diets. Secondary outcomes were differences in cardiometabolic risk factors, quality of life, gut microbiota, fecal short‐chain and branched‐chain fatty acids, and plasma metabolome. Of 150 eligible patients, 31 (21%) agreed to participate, and 27 (87%) participants completed the study. Mean oxidized LDL‐C (−2.73 U/L), total cholesterol (−5.03 mg/dL), LDL‐C (−3.87 mg/dL), and body weight (−0.67 kg) were significantly lower with the VD than with the MD. Differences between VD and MD were observed in the relative abundance of several microbe genera within the families Ruminococcaceae, Lachnospiraceae, and Akkermansiaceae. Plasma metabolites, including l‐carnitine, acylcarnitine metabolites, and phospholipids, differed in subjects consuming VD and MD. The effect on oxidized LDL‐C in response to the VD was associated with a baseline gut microbiota composition dominated by several genera of Ruminococcaceae.
Conclusions
The VD in conjunction with optimal medical therapy reduced levels of oxidized LDL‐C, improved cardiometabolic risk factors, and altered the relative abundance of gut microbes and plasma metabolites in patients with ischemic heart disease. Our results suggest that composition of the gut microbiota at baseline may be related to the reduction of oxidized LDL‐C observed with the VD.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02942628.
Keywords: coronary artery disease, gut microbiota, plasma metabolome, randomized controlled trial, trimethylamine N-oxide, vegetarian diet
Subject Categories: Lifestyle, Secondary Prevention, Diet and Nutrition
Nonstandard Abbreviations and Acronyms
- APOB
apolipoprotein B
- BCFA
branched‐chain fatty acid
- BMI
body mass index
- CVD
cardiovascular disease
- HbA1c
hemoglobin A1c
- hs‐CRP
high‐sensitivity C‐reactive protein
- IHD
ischemic heart disease
- LDL‐C
low‐density lipoprotein cholesterol
- MD
meat diet
- PCI
percutaneous coronary intervention
- SCFA
short‐chain fatty acid
- TC
total cholesterol
- TMAO
trimethylamine N‐oxide
- VD
vegetarian diet
Clinical Perspective
What Is New?
Compared with a ready‐made meat diet, an isocaloric ready‐made vegetarian diet (VD) within an individually adapted diet plan showed secondary prevention potential in patients with ischemic heart disease receiving optimal medical treatment.
After a 4‐week intervention, subjects consuming a VD showed significantly lower oxidized low‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, total cholesterol, and body mass index than those on a meat diet.
Subjects on the VD exhibited reduced relative abundance of fecal microbial taxa and plasma metabolites associated with metabolic disease, including cardiovascular disease, and with increased taxa and metabolites associated with lower cardiometabolic risk than those on a meat diet.
What Are the Clinical Implications?
A VD in conjunction with optimal medical therapy improves levels of oxidized low‐density lipoprotein cholesterol, cardiometabolic risk factors, and phospholipids associated with an elevated risk of coronary events.
A ready‐made VD could be easily implemented in individuals with a history of ischemic heart disease to improve secondary prevention.
Assessment of gut microbiota in follow‐up of patients with ischemic heart disease could help to identify individuals potentially showing a favorable response to a VD.
A western diet, characterized by high consumption of red and processed meat, refined carbohydrates, and high calorie intake, has been associated with increased risk of cardiovascular disease (CVD), including ischemic heart disease (IHD). 1 A global change to an environmentally sustainable healthy diet, with considerable reduction of red meat consumption and increased consumption of plant‐based foods, may save ≈11 million premature deaths each year. 1
Epidemiological studies have shown that a vegetarian diet (VD), primarily based on vegetables, legumes, fruit, grains, nuts, and occasionally eggs or dairy products, is associated with reduced incidence of, and mortality in, IHD as well as all‐cause mortality. 2 , 3 Evidence from some randomized controlled trials supports the effectiveness of a plant‐based diet in the prevention of CVD 4 and reduction in CVD risk factors. 5 , 6 , 7 A VD as part of an intensive lifestyle change has been shown to reverse coronary atherosclerosis in patients with IHD. 8 Although mechanisms remain unclear, the effect of a VD in counteracting development of CVD might be attributed to reduced oxidative stress 9 , 10 and to beneficial effects on factors such as blood lipids, glucose tolerance, and body weight. 4 , 10 , 11 Most studies investigating the role of a VD in CVD prevention have comprised healthy participants and not consisted of a homogeneous group of patients on optimal medical therapy (eg, lipid‐ or blood pressure–lowering medication). The main barriers to adopting a VD have been reported to be enjoyment of eating meat and an unwillingness to alter eating habits. 12
Analysis of gut microbiota and the plasma metabolome before and after adoption of a VD offers the potential to gain mechanistic insight into nutritional influences on disease‐related metabolic processes. 13 , 14 Research has shown impact of a VD on microbial taxa linked to CVD risk, 14 and plant‐based diets have been demonstrated to alter circulating metabolites, such as short‐chain fatty acids (SCFAs) produced by gut fermentation of dietary fiber and phosphatidylcholines in multiple biological pathways 15 , 16 , 17 linked to CVD risk. 18 , 19 Carnitine, produced by ingestion of animal products, and its gut microbiota‐derived metabolite, trimethylamine N‐oxide (TMAO), have been associated with CVD. 20 , 21 A recent study reported increased risk of coronary heart disease with higher TMAO concentrations. Regular consumption of plant‐based foods could hypothetically lower such risk. 22 Individuals may respond differently to a given diet, and prediction models are being developed to determine the importance of anthropometrics, metabolomics, and microbiota to the outcomes of dietary intervention and to the design and implementation of personalized nutrition regimens. 23 , 24 Individual variation may contribute to inconsistency in results of dietary intervention studies. 25 , 26 Recent reports have suggested that responses to dietary intervention might depend on the gut microbiota composition at baseline, 23 , 24 , 27 as well as on metabotype. 28 However, little is known of whether individual baseline microbiota and/or metabolome are associated with the effect of a VD on metabolic CVD risk factors.
We conducted a 4‐week randomized crossover study, using subject‐specific dietary plans, to investigate effects of a VD on CVD risk factors in subjects with a history of IHD treated by percutaneous coronary intervention (PCI), compared with an isocaloric meat diet (MD). We aimed to determine the effect on oxidized low‐density lipoprotein cholesterol (LDL‐C) as the primary outcome and the secondary outcomes selected cardiometabolic risk factors, gut microbiota, and plasma metabolome, including TMAO, choline, l‐carnitine, and acetyl‐carnitine. We also explored whether gut microbiota or plasma metabolome at baseline could predict the level of response to a VD.
METHODS
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Participants
Patients with IHD who were treated with PCI and receiving optimal medical therapy were recruited from the outpatient clinic at the Department of Cardiology, Örebro University Hospital, Örebro, Sweden. Participant eligibility criteria were age >18 years, stable IHD, PCI conducted >1 month before study initiation, and optimal medical therapy, including aspirin and cholesterol‐lowering drugs. Exclusion criteria included age <18 years, unstable coronary disease, PCI treatment during the preceding 30 days, inability to provide informed consent, already following a VD or vegan diet, vitamin B deficiency, known food allergy, previous bariatric surgery, or life expectancy <1 year.
All participants provided written informed consent. The study was performed in compliance with the Declaration of Helsinki, and the regional ethical review board in Uppsala, Sweden, approved the study (Dnr 2016/456). The study is registered at ClinicalTrials.gov (NCT02942628).
Study Design
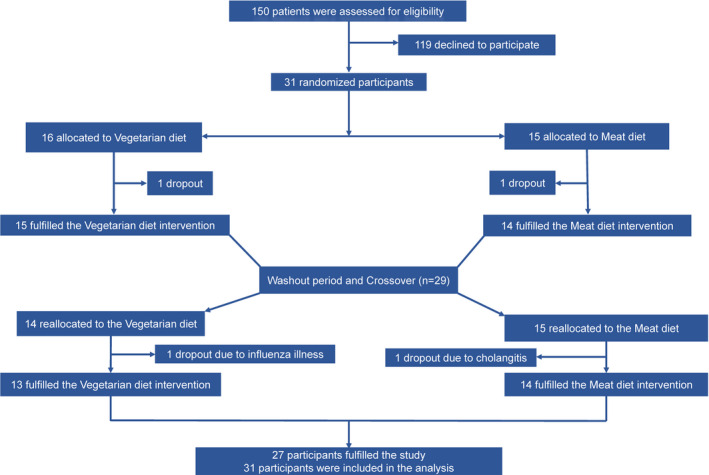
This was a prospective, open‐label, randomized, controlled crossover clinical trial. Subjects consumed isocaloric interventional diets, VD and MD, during 4‐week intervention periods separated by a 4‐week washout period (Figure 1). The study was performed from September 2017 through June 2018. Subjects were randomly allocated to a preselected intervention sequence, VD‐washout‐MD or MD‐washout‐VD, at a 1:1 ratio to ensure balance of sequences. Clinical follow‐up was performed on 4 occasions during the study, before and after each intervention period. Follow‐up visits were scheduled between 7 am and 10 am, and blood sampling was performed after overnight fasting. Patients were asked to collect stool samples in special sealed plastic containers on the day preceding each follow‐up visit.
Figure 1. Schedule of study visits and participant flow.
Diets
Dietary interventions were designed on the basis of eating habits in Sweden. They included food items available in standard grocery stores and were in agreement with the Nordic Nutrition Recommendations. 29 The VD was a lacto‐ovo‐vegetarian diet allowing intake of eggs and dairy products. The MD refers to a conventional diet that was based on the average meat consumption in Sweden and corresponded to a daily intake of 145 g of meat, including red, white, and processed meats. 30 All subjects received a meal plan to follow throughout the study. Lunches and dinners were provided as ready‐made frozen meals (Tables S1 and S2). These meals were based on traditional Swedish recipes and produced and supplied by Dafgård, Källby, Sweden. Subjects visited the clinic on a weekly basis to collect meals. At the first study visit, subjects met with a research dietitian who provided information on how to follow the individually energy‐adjusted meal plans (Data S1). In addition to the 2 meals provided, subjects were asked to have breakfast, 2 snacks, and a side dish for the main course, every day. The meal plans included 5 to 6 options for breakfast, light meals, and side dishes. The nutrient composition of the diets was calculated using nutrition calculation software (Dietist Net Pro; Kost och Näringsdata AB, Bromma, Sweden) (Table 1).
Table 1.
Macronutrient Profile of Prescribed Diet
| Variable | Energy, kcal | Protein, g | Carbohydrates, g | Fat, g | Saturated Fat, g | Dietary Fiber, g |
|---|---|---|---|---|---|---|
| Vegetarian diet | ||||||
| According to meal plan* | 1394 | 51.2 | 169.8 | 51 | 20.5 | 19.5 |
| Intervention food† | 999 | 38.4 | 104.8 | 45.7 | 17 | 15 |
| Total‡ | 2393 | 89.6 | 274.6 | 96.7 | 37.5 | 34.5 |
| Meat diet | ||||||
| According to meal plan* | 1318 | 48.9 | 168.7 | 43.8 | 15.2 | 22.4 |
| Intervention food† | 1076 | 41.8 | 102.4 | 55.9 | 22.2 | 10.7 |
| Total‡ | 2394 | 90.3 | 275.2 | 97.5 | 37.4 | 33.1 |
Bread with topping, side dish, breakfast, and 0 to 3 snacks/light meals.
Provided frozen dishes, including lunch and dinner.
Complete diet.
Adherence to Dietary Intervention
The subjects completed a 3‐day weighed food record before intervention, in the final week of each of the interventions, and at the end of the washout period (Table S3). During the intervention, patients were asked to complete a daily diary, recording whether they had consumed the provided lunch and dinner, which options they had chosen for breakfast and light meals, and if there were any deviations from the meal plan.
Primary and Secondary Outcomes
Difference in change in plasma oxidized LDL‐C between diets was the primary outcome measure. Secondary outcomes included differences in change of cardiometabolic risk factors (lipids, hemoglobin A1c [HbA1c], hs‐CRP [high‐sensitivity C‐reactive protein], weight, body mass index [BMI], blood pressure, heart rate, quality of life, gut microbiota in fecal samples, fecal SCFAs and branched‐chain fatty acids [BCFAs], plasma metabolome, and plasma levels of TMAO, choline, l‐carnitine, and acetyl‐carnitine).
Oxidized LDL‐C and Cardiometabolic Risk Factors
Venous blood samples were collected at the 4 study visits in evacuated plastic tubes (VACUETTE TUBE; Greiner Bio‐One GmbH, Kremsmunster, Austria) and centrifuged in a cooling system at 1560g for 10 minutes at −40°C and stored at −80°C in aliquots until analyses. An ELISA kit (Mercodia, Uppsala, Sweden) was used for quantitative measure of plasma oxidized LDL‐C levels, as described by Holvoet et al, 31 with an intra‐assay coefficient of variation <10% (mean, 3.74%) for most samples. Five samples showed a coefficient of variation >10%. Total cholesterol (TC), LDL‐C, high‐density lipoprotein cholesterol, triglycerides, apolipoprotein A1, apolipoprotein B (APOB), hs‐CRP, and HbA1c at each study visit were measured at the Clinical Chemistry Laboratory, Örebro University Hospital, according to a standardized protocol (Data S1). Cutoff values of clinical markers routinely monitored after a cardiac event were based on European guidelines on CVD prevention in clinical practice 32 : LDL‐C <70 mg/dL (<1.8 mmol/L), systolic blood pressure <130 mm Hg, diastolic blood pressure <80 mm Hg, and BMI <25 kg/m2. For LDL‐C, we used the cutoff according to European guidelines during the study period, <70 mg/dL. A digital automatic sphygmomanometer (Omron m6 ac; Omron Healthcare Co, Ltd, Kyoto, Japan) was used for blood pressure and heart rate measurements. Body height was measured at baseline, and body weight was measured at the 4 study visits. Quality of life was assessed by using the EuroQoL 5‐dimension questionnaire at all study visits, including a visual analogue scale and measures of mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression. 33 The Lund‐Malmö equation was used to determine the estimated glomerular filtration rate.
Gut Microbiota, Fecal Fatty Acids, and Plasma Metabolome
Details of instrumental analysis and preprocessing of raw reads for 16S rRNA gene sequencing analysis, SCFA and BCFA, plasma metabolome, and concentrations of plasma TMAO, choline, l‐carnitine, and acetyl‐carnitine are described in Data S1.
Fecal samples collected in a sterile stool tube by the participant on the day before each follow‐up visit and stored in the home freezer (≈−20°C) were brought to the clinic and stored at −80°C until extraction. DNA was extracted from samples by repeated bead beating and subjected to 16S rRNA gene sequencing in an Illumina Miseq instrument (2×250 bp paired‐end reads, V2 kit; Illumina, San Diego, CA) after PCR amplification of the V4 region with the 515F and 806R primers. A total of 1264 zero‐radius operational taxonomic units (abundance ≥0.002%) in 102 samples was obtained (Figure S1A), primarily represented by the phyla Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria (Figure S1B).
Concentrations of the SCFA acetate, propionate, and butyrate; BCFA isobutyrate and isovalerate; succinate; and lactate in fecal samples were determined using a gas chromatograph mass spectrometer (Agilent Technologies), as previously described. 34
For untargeted metabolomics, plasma samples were deproteinized using ultracentrifugation and analyzed by high‐performance liquid chromatography– quadrupole time‐of‐flight mass spectrometry (Agilent Technologies). 23 In total, 1882 metabolite features (a molecular entity with a unique mass/charge ratio and retention time, as measured by an instrument) with the coefficient of variation in quality control samples ≤30% were subjected to further analysis. Metabolite identification was based on accurate mass (mass tolerance ≤5 ppm) and tandem mass spectrometry fragmentation (mass tolerance ≤10 ppm) matched against online databases or the literature.
The concentrations of plasma TMAO, choline, l‐carnitine, and acetyl‐carnitine were analyzed by high‐performance liquid chromatography–mass spectrometry on an Exion UHPLC coupled to a QTRAP 6500+ tandem mass spectrometry system, both from AB Sciex LLC (Framingham, MA).
Statistical Analysis
The sample‐size calculation was based on previous studies in which a VD or food supplements (nuts, soy‐based cereal, or cranberry juice) were shown to reduce oxidized LDL‐C by 10% compared with no intervention. 35 , 36 Considering similar effects in our study and a mean reduction of oxidized LDL‐C of 9%, we needed to include 27 patients in a crossover design to be able to reject the null hypothesis that the experimental and control treatments were identical with a probability (power) of 0.80 and a type I error probability of 0.05. On the basis of an estimated 10% dropout rate, we therefore enrolled 31 subjects.
The effects of diets on oxidized LDL‐C and cardiometabolic outcomes were evaluated using a generalized linear mixed model that included a fixed effect of the diet, sequence of diet allocation, and their interaction. Missing values were imputed in an intention‐to‐treat analysis using the last observation carried forward for the subjects (n=2) who were randomized but did not receive intervention and for the subjects who dropped out after the first intervention period (n=2). In addition, we performed on‐treatment analysis. A 2‐sided P<0.05 was considered significant.
A Kruskal‐Wallis test was applied to the observed number of microbial species, and the Faith phylogenetic diversity index was used to examine potential differences in α diversity between results of the 2 diets. Principal coordinate analysis of the weighted and unweighted UniFrac distances or the Bray‐Curtis dissimilarity was used to analyze the overall composition of gut microbiota. A permutational multivariate ANOVA (Adonis) (n=9999) and analysis of similarities were used to assess the effect of the dietary intervention on principal coordinate analysis scores of β diversity metrics. To identify microbial taxa or plasma metabolites discriminating VD from MD, a random forest modeling approach based on multilevel data analysis was applied 37 , 38 for pair‐wise comparison of zero‐radius operational taxonomic unit or metabolite levels of VD and MD (Figure S2, Data S1). The multilevel analysis deals with dependent data structures and has been successfully used to exploit differences specific to diet in crossover intervention studies. Significance of multivariate models was assessed by permutation tests (n=100). A common baseline effect was assumed for both interventions, because no differences in bacterial genera or plasma metabolome were observed between baseline and the end of the washout period (Figures S3 and S4).
We further assessed the effect of VD versus MD on each selected optimally discriminating zero‐radius operational taxonomic unit or metabolite using generalized linear mixed models (R package "lme4"). Fixed factors included diet, sequence of diet allocation, and their interaction with baseline value as covariate and subject as random factor. The same analysis was applied to the concentrations of fecal SCFAs and BCFAs. Spearman correlation coefficients were calculated for all correlation analyses. The P values were adjusted for multiple comparisons using the Benjamini‐Hochberg false discovery rate, and a value of P<0.05 was considered significant.
In an exploratory analysis, we investigated whether gut microbiota configuration or plasma metabolome at baseline was associated with the influence of VD on metabolic risk factors, including levels of oxidized LDL‐C, LDL‐C, TC, and BMI. Random forest modeling 37 was used to identify a panel of microbial taxa or plasma metabolites that could enable discrimination of potential responders (subjects who benefitted from VD compared with MD and showed within‐individual difference in metabolic risk factors between VD and MD <0) from nonresponders (subjects in whom VD did not improve metabolic risk factors compared with MD and had within‐individual difference in metabolic risk factors between VD and MD >0).
Results
Study Population and Diet Adherence
Of 150 patients with a history of IHD treated with PCI and receiving optimal medical therapy who were invited, 31 (21%) agreed to participate and were randomized. Twenty‐nine were men (94%), with a median age of 67 years (range, 63–70 years) and a median BMI of 27.5 kg/m2 (Table 2). Two subjects dropped out because of difficulties adhering to the diet, one because of influenza and one because of cholangitis. Twenty‐seven subjects completed the study (Figure 1). Before enrollment, 12 (39%) subjects had experienced an ST‐segment–elevation myocardial infarction; 12 (39%) had experienced a non–ST‐segment–elevation myocardial infarction; 3 (10%) had unstable; and 5 (16%) had stable angina pectoris. All subjects were receiving statin therapy, 29 (94%) were treated with aspirin, and 20 (65%) received P2Y12 inhibitors (clopidogrel or ticagrelor). During the study, the only change in medical therapy was addition of calcium channel blockers in 2 subjects. Both dietary interventions were well tolerated, and overall adherence based on the self‐reported diaries was 88% for both interventions; however, there was a difference in adherence with respect to snacks (Table S4). On the basis of the 3‐day food records, there was no significant difference in the intake of macronutrients; however, there was a difference in intake of fiber (Table S3).
Table 2.
Baseline Characteristics of the Study Population at First Randomization Intervention
| Characteristics | All (n=31) | VD (n=16) | MD (n=15) |
|---|---|---|---|
| Age, median (range), y | 67 (63–70) | 67 (65–70) | 68 (61–70) |
| Sex, men, n (%) | 29 (94) | 15 (94) | 14 (93) |
| History before enrollment | |||
| STEMI, n (%) | 12 (39) | 6 (35) | 6 (40) |
| NSTEMI, n (%) | 12 (39) | 4 (25) | 8 (53) |
| Instable angina, n (%) | 3 (10) | 3 (19) | 0 (0) |
| Angina, n (%) | 5 (16) | 4 (25) | 1 (7) |
| Type 2 diabetes mellitus, n (%) | 2 (7) | 2 (13) | 0 (0) |
| Hypertension, n (%) | 17 (55) | 10 (63) | 7 (47) |
| Drug treatment | |||
| Statins, n (%) | 31 (100) | 16 (100) | 15 (100) |
| Ezetimibe, n (%) | 7 (23) | 4 (25) | 3 (20) |
| ASA, n (%) | 29 (94) | 15 (94) | 14 (93) |
| P2Y12 inhibitors, n (%) | 20 (65) | 8 (50) | 12 (80) |
| β Blockers, n (%) | 28 (90) | 14 (88) | 14 (93) |
| ACE inhibitors/ARBs, n (%) | 27 (87) | 13 (81) | 14 (93) |
| CCBs, n (%) | 11 (36) | 6 (38) | 5 (33) |
| Cardiometabolic risk factors and life quality | |||
| Weight, mean±SD, kg | 84±11.0 | 86±13.6 | 83±8.6 |
| BMI, mean±SD, kg/m2 | 28±2.9 | 28±3.3 | 27±2.5 |
| Systolic BP, mean±SD, mm Hg | 139±17.4 | 140±17.4 | 138±18.0 |
| Diastolic BP, mean±SD, mm Hg | 87±9.6 | 88±10.6 | 87±8.7 |
| Heart rate, mean±SD, bpm | 65.8±9.2 | 65.1±9.2 | 66.5±9.5 |
| EQ‐5D VAS, mean±SD | 80±10.7 | 78±11.2 | 82±10.2 |
| Oxidized LDL‐C, mean±SD, U/L | 40.9±11.7 | 39.4±11.7 | 42.1±11.8 |
| Total cholesterol, mean±SD, mg/dL | 133.4±23.2 | 135.7±28.2 | 130.7±17.0 |
| LDL‐C, mean±SD, mg/dL | 62.3±16.8 | 62.3±19.1 | 62.3±14.7 |
| HDL‐C, mean±SD, mg/dL | 48.7±13.0 | 50.6±15.9 | 46.5±9.0 |
| Triglycerides, mean±SD, mg/dL | 94.0±29.8 | 93.7±32.3 | 94.2±28.0 |
| APOB, mean±SD, g/L | 0.7±0.1 | 0.7±0.1 | 0.7±0.1 |
| APOA1, mean±SD, g/L | 1.4±0.2 | 1.4±0.2 | 1.4±0.1 |
| APOB/APOA1 ratio, mean±SD | 0.5±0.1 | 0.5±0.1 | 0.5±0.1 |
| HbA1c, median (range), mmol/mol | 39 (36–40) | 39 (36–42) | 39 (36–40) |
| hs‐CRP, median (range), mg/L | 0.7 (0.5–1.7) | 0.8 (0.4–1.7) | 0.7 (0.4–1.7) |
| eGFR, mean±SD, mL/min per 1.73 m2 | 76.4±9.7 | 75.1±7.6 | 77.7±11.7 |
Data are presented as median (interquartile range), number (percentage), or mean±SD. To convert cholesterol markers to millimoles per liter, multiply by 0.02586. To convert triglycerides to millimoles per liter, multiply by 0.01129. ACE indicates angiotensin‐converting enzyme; APOA1, apolipoprotein A1; APOB, apolipoprotein B; ARB, angiotensin II receptor blocker; ASA, acetylsalicylic acid; BMI, body mass index; BP, blood pressure; bpm, beats per minute; CCB, calcium channel blocker; eGFR, estimated glomerular filtration rate; EQ‐5D, EuroQoL 5‐dimension questionnaire (self‐reported quality of life); HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; MD, meat diet; NSTEMI, non–ST‐segment–elevation myocardial infarction; P2Y12 inhibitor, clopidogrel or ticagrelor; STEMI, ST‐segment–elevation myocardial infarction; VAS, visual analogue scale; and VD, vegetarian diet.
Effects on Oxidized LDL‐C and Cardiometabolic Risk Factors
Subjects consuming the VD showed significantly lower mean oxidized LDL‐C compared with MD (−2.73 U/L) (P=0.02) (Figure 2, Table 3). A significant decrease from baseline of oxidized LDL‐C after VD intervention was observed, whereas no difference was found after MD (Figure 2, Figure S4).
Figure 2. Changes in oxidized low‐density lipoprotein cholesterol (LDL‐C) and cardiometabolic risk factors according to dietary intervention.
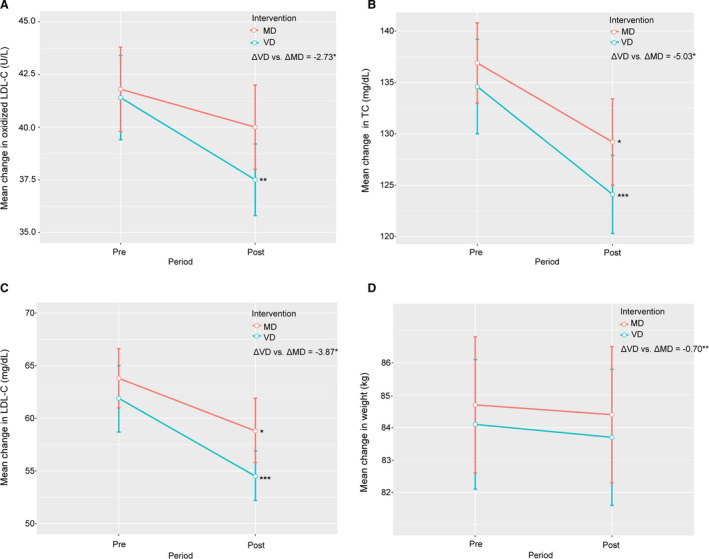
Mean change in oxidized LDL‐C (A), total cholesterol (TC) (B), LDL‐C (C), and weight (D) before and after each intervention. Error bars indicate SEM. ΔVD vs ΔMD indicates differences in risk factors between vegetarian diet (VD) and meat diet (MD) obtained using linear mixed‐effects models adjusted for sequence of diet randomization and intervention period. *P<0.05, **P<0.01, ***P<0.001. Post, 4 weeks after the dietary intervention; Pre, baseline.
Table 3.
Effect of Dietary Intervention on Clinical Parameters
| Clinical Parameters | Pre‐VD | Post‐VD | Pre‐MD | Post‐MD | Post‐VD vs Post‐MD* | P Value* |
|---|---|---|---|---|---|---|
| Oxidized LDL‐C, U/L |
41.4 (37.2–45.5) |
37.5 (33.8–40.7) † |
41.8 (37.7–46.0) |
40.0 (35.9–44.2) |
−2.73 (−4.9 to −0.6) |
0.02 |
| TC, mg/dL |
134.6 (124.9–144.2) |
124.1 (116.00–131.9) ‡ |
136.9 (129.9–145.0) |
129.2 (120.6–137.6) § |
−5.03 (−8.89 to −1.16) |
0.01 |
| LDL‐C, mg/dL |
61.9 (55.7–68.4) |
54.5 (49.5–59.6) ‡ |
63.8 (58.0–69.6) |
58.8 (52.6–65.0) § |
−3.87 (−7.35 to −0.77) |
0.02 |
| HDL‐C, mg/dL |
47.6 [42.9–53.0] |
44.5 [39.8–49.9] † |
49.1 [44.5–54.1] |
46.1 [41.4–51.43] § |
−1.16 [−2.71 to 0.39] |
0.2 |
| Triglycerides, mg/dL |
86.8 [76.2–98.3] |
92.1 [83.3–102.7] |
87.7 [77.1–99.2] |
86.8 [77.1–98.3] |
5.31 [−1.77 to 13.3] |
0.1 |
| APOB, g/L |
0.65 (0.60–0.70) |
0.59 (0.55–0.63) ‡ |
0.66 (0.62–0.71) |
0.61 (0.56–0.65) ‡ |
−0.021 (−0.044 to 0.001) |
0.06 |
| APOA1, g/L |
1.40 (1.35–1.49) |
1.41 (1.34–1.48) |
1.44 (1.37–1.51) |
1.42 (1.35–1.50) |
−0.019 (−0.049 to 0.011) |
0.2 |
| APOB/APOA1 ratio |
0.45 [0.42–0.48] |
0.41 [0.38–0.45] ‡ |
0.46 [0.42–0.5] |
0.42 [0.39–0.46] ‡ |
−0.021 [−0.07 to 0.03] |
0.4 |
| HbA1c, mmol/mol |
38.5 [37.1–40.0] |
38.7 [37.2–40.3] |
38.6 [37.0–40.4] |
38.8 [37.2–40.6] |
−0.003 [−0.023 to 0.017] |
0.8 |
| Weight, kg |
84.1 (80.1–88.2) |
83.7 (79.5–87.9) |
84.7 (80.5–88.9) |
84.4 (80.1–88.6) |
−0.7 (−1.1 to −0.2) |
0.008 |
| BMI, kg/m2 |
27.4 (26.4–28.5) |
27.3 (26.2–28.4) |
27.6 (26.5–28.7) |
27.5 (26.4–28.6) |
−0.2 (−0.36 to −0.06) |
0.009 |
| hs‐CRP, mg/L |
0.73 [0.51–1.03] |
0.74 [0.50–1.09] |
0.81 [0.60–1.09] |
0.81 [0.55–1.18] |
−0.09 [−0.42 to 0.23] |
0.6 |
| Systolic BP, mm Hg |
136 (129–143) |
133 (127–140) |
140 (133–146) |
136 (129–142) |
−2.3 (−5.4 to 0.8) |
0.1 |
| Diastolic BP, mm Hg |
86 (82–89) |
86 (83–89) |
87 (84–91) |
87 (83–91) |
−1.1 (−3.8 to –1.6) |
0.4 |
| HR, bpm |
62.7 [59.9–65.7] |
63.4 [60.6–66.3] |
64.3 [60.9–67.9] |
63.5 [60.1–67.1] |
−0.001 [−0.04 to 0.04] |
0.9 |
Data are presented as mean (95% CI) or as geometric mean [95% CI]. Within‐group change P value was calculated with paired t test. APOA1 indicates apolipoprotein A1; APOB, apolipoprotein B; BMI, body mass index; BP, blood pressure; bpm, beats per minute; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; HR, heart rate; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; MD, meat diet; TC, total cholesterol; and VD, vegetarian diet.
Differences in clinical parameters between VD and MD were examined using linear mixed‐effects models adjusted for sequence of diet randomization and period of interventions.
P<0.01.
P<0.001.
P<0.05.
Subjects on the VD showed lower mean TC (−5.03 mg/dL/−0.13 mmol/L) (P=0.01), LDL‐C (−3.87 mg/dL/−0.10 mmol/L) (P=0.02), body weight (−0.67 kg) (P=0.008), and BMI (−0.21 kg/m2) (P=0.009) compared with subjects on the MD (Figure 2, Table 3). No difference between diets was observed for high‐density lipoprotein cholesterol, triglycerides, APOB, apolipoprotein, APOB/apolipoprotein A1 ratio, HbA1c, hs‐CRP, blood pressure, heart rate, quality of life, or the number of subjects reaching guideline values of clinical markers LDL‐C, blood pressure, and BMI (Table 3, Tables S5 and S6). Similar results were obtained by the on‐treatment analysis (Table S7).
Compared with baseline, both the VD and MD led to significantly lower mean values of TC (−7.8% and −5.7%, respectively), LDL‐C (−11.9% and −7.9%, respectively), high‐density lipoprotein cholesterol (−6.5% and −6.3%, respectively), APOB (−9.0% and −3.8%, respectively), and APOB/apolipoprotein A1 ratio (−8.0% and −7.9%, respectively) (Table 3, Figure S5). There were no differences from baseline in triglycerides, apolipoprotein A1, HbA1c, body weight, BMI, hs‐CRP, blood pressure, heart rate, quality of life, or number of subjects reaching clinical marker guideline values after the 2 diet interventions (Table 3 and Tables S5 and S6).
Effects on Gut Microbiota, Fecal SCFAs and BCFAs, and Plasma Metabolome
The diets did not alter either richness or overall composition of gut microbiota at the phylum level (Figures S6 and S7) but differed with respect to the relative abundance of several microbial genera (Figure S8, Table S8). Multilevel predictive modeling revealed 46 microbial genera with the potential to distinguish VD from MD (Figure 3A), most belonging to the families Ruminococcaceae (n=13), Lachnospiraceae (n=11), and Eggerthellaceae (n=4). Among them, 12 genera differed in VD and MD when individually assessed by univariate analysis (Figure 3A, Table S8).
Figure 3. Gut microbiota and plasma metabolites discriminating the vegetarian and meat diets, and selected by multilevel random forest modeling.
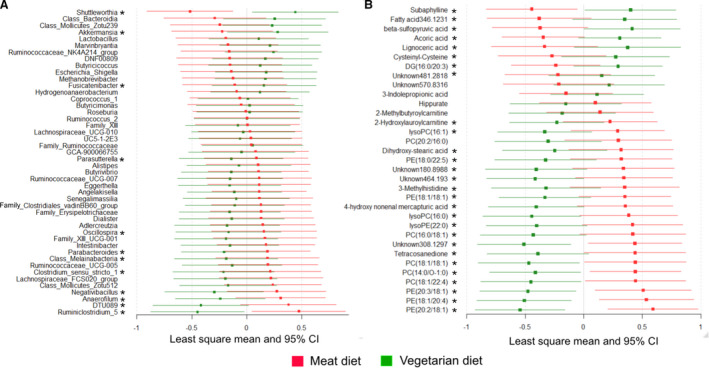
Least‐squares means and 95% CIs of abundance of zero‐radius operational taxonomic units (A) and levels of metabolites (B) after 4‐week intervention of the vegetarian and isocaloric meat diet obtained from random forest multivariate modeling. Standardized values are presented for comparison. *Denotes microbial genera or metabolites significantly differing between meat and vegetarian diet when assessed using generalized linear mixed models. DG indicates diacylglycerol; PC, phosphatidylcholine; and PE, phosphatidylethanolamine.
The fecal concentrations of acetate, propionate, butyrate, isobutyrate, and isovalerate were 4% 10%, 5%, 3%, and 6% higher, respectively, after 4 weeks of a VD than after MD. These results did not reach significance (Table S9).
The plasma metabolome differed significantly with diet (Figure S9). Thirty‐three plasma metabolites distinguished VD from MD with a predictive accuracy of 95%, among them acylcarnitine metabolites and several phosphatidylcholines and phosphatidylethanolamines (Figure 3B, Table S10). When assessed individually using univariate statistics, 28 of 33 metabolites were significantly different from MD in VD (Figure 3B).
We found a significant difference in plasma l‐carnitine (−14.77 μmol/L) (95% CI, −21.13 to −8.71 μmol/L; P<0.001), but not in TMAO, acyl‐carnitine, or choline, between the MD and VD (Figure 4).
Figure 4. Changes in plasma concentration of trimethylamine N‐oxide (TMAO), choline, l‐carnitine, and acetyl‐carnitine according to dietary intervention.
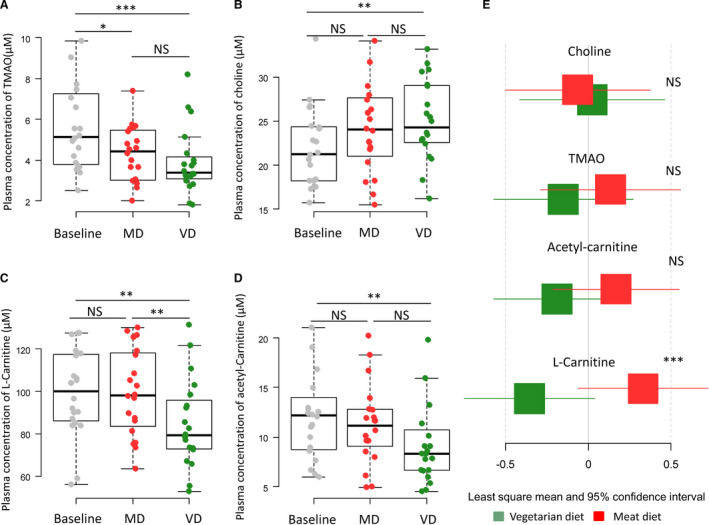
Boxplots (A through D) show the concentrations of the metabolites measured at baseline, after the vegetarian diet (VD) and the isocaloric meat diet (MD). Differences were assessed by paired t test. Least‐squares means and 95% CIs of levels of metabolites (E) after 4‐week intervention of VD and MD assessed by generalized linear modeling. Standardized values are presented for comparison. *P<0.05, **P<0.01, ***P<0.001. NS indicates not significant.
The plasma concentration of TMAO and l‐carnitine was lower after VD compared with baseline (−1.90 μmol/L [95% CI, −2.87 to −0.93 μmol/L; P<0.001] and −14.46 μmol/L [95% CI, −24.75 to −4.17 μmol/L; P<0.01]). The concentration of choline increased with the VD (3.09 μmol/L; 95% CI, 1.06–5.12 μmol/L; P=0.001) (Figure 4, Figure S10).
We observed multiple correlations of changes in microbiota, metabolites, and cardiometabolic risk factors with diet (Table 4, 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , Table S11, Figure S11). However, no correlation remained significant after correction for multiple testing. No correlations were observed between fecal SCFAs or BCFAs and assessed clinical risk factors.
Table 4.
Bacterial Genera Discriminating the VD From the MD and Their Correlation With Cardiometabolic Risk Factors and Metabolites as Well as Previously Reported Effects
| Genus | Description | VD* | MD | SEM | r † | r ‡ | Previous Findings |
|---|---|---|---|---|---|---|---|
| Fusicatenibacter | Class Clostridia, family Lachnospiraceae | 898.3 | 744.0 | 90.6 | 3‐Indolepropionic acid (0.32), 4‐hydroxy nonenal mercapturic acid (0.33), tetracosanedione (0.37) | ||
| Akkermansia | Class Verrucomicrobiae, family Akkermansiaceae | 811.5 | 426.3 | 203.6 |
3‐Indolepropionic acid (0.37), 2‐methylbutyroylcarnitine (−0.32) |
Anaerobic genus with health‐promoting effect, 14 reported to increase after intake of high fermentable oligosaccharides, disaccharides, 39 and monosaccharides and polyols diets or the dietary‐resistant starch. 40 | |
| Clostridium sensu stricto 1 | Class Clostridia, family Clostridiaceae_1 | 353.0 | 567.8 | 106.9 | BMI (0.29), weight (0.30) |
3‐Indolepropionic acid (−0.32), cysteinyl‐cysteine (0.35), lysophosphatidylethanolamine (22:0) (0.31), phosphatidylethanolamine (18:1/20:4) (0.34) |
Pathogenic genus, 41 reported to decrease after a 3‐mo VD, and was positively associated with inflammatory markers and LDL‐C. 42 |
| Parabacteroides | Class Bacteroidia, family Tannerellaceae | 216.7 | 309.6 | 33.5 | TC (0.35), LDL‐C (0.27) | Lysophosphatidylcholine (16:0) (0.31) | Reported to be a microbial marker for hypertension, 43 and was directly associated with weight gain. 44 |
| Ruminiclostridium 5 | Class Clostridia, family Ruminococcaceae | 200.7 | 390.1 | 38.4 |
Lignoceric acid (−0.37), phosphatidylethanolamine (18:1/20:4) (0.33), phosphatidylcholine (20:2/16:0) (0.50), phosphatidylethanolamine (18:0/22:5) (0.32), lysophosphatidylcholine (16:0) (0.31) |
Reported to be inversely associated with plant‐based diets and several beneficial nutrients (eg, vitamins and magnesium). 15 | |
| Parasutterella | Class Gammaproteobacteria, family Burkholderiaceae | 33.7 | 46.5 | 4.4 |
phosphatidylethanolamine (18:1/18:1) (0.32), phosphatidylcholine (18:1/22:4) (0.34) |
Reported to be associated with sodium and processed foods. 15 | |
| Negativibacillus | Class Clostridia, family Ruminococcaceae | 13.1 | 24.9 | 3.9 |
Phosphatidylcholine (20:2/16:0) (0.32), 4‐hydroxy nonenal mercapturic acid (0.40), N‐acetylanonaine (0.32) |
Reported to be correlated with body weight and obesity‐related parameters. 45 | |
| Oscillospira | Class Clostridia, family Ruminococcaceae | 11.5 | 16.4 | 2.4 | LDL‐C (−0.28) |
3‐Indolepropionic acid (−0.48), 2‐methylbutyroylcarnitine (0.41), tetracosanedione (−0.36) |
Reported to be increased after a 1‐y Mediterranean diet in obese population. 46 Ruminococcaceae was positively correlated with plasma indolepropionic acid, whereas was negatively correlated with atherosclerotic disease burden in an apolipoprotein E knockout mice model. 47 |
| Melainabacteria | Phylum Cyanobacteria | 8.3 | 19.4 | 4.4 | Diacylglycerol (16:0/20:3) (0.40) | ||
| Shuttleworthia | Class Clostridia, family Lachnospiraceae | 7.0 | 0.6 | 1.2 | Oxidized LDL‐C (−0.41), TC (−0.32), LDL (−0.28) |
Phosphatidylcholine (14:0/O‐1:0) (−0.35), lysophosphatidylcholine (16:1) (−0.49), lysophosphatidylethanolamine (22:0) (−0.39), diacylglycerol (16:0/20:3) (0.34), phosphatidylcholine (18:1/18:1) (−0.32) |
|
| DTU089 | Class Clostridia, family Ruminococcaceae | 7.2 | 15.4 | 2.0 | TC (0.29) |
Lignoceric acid (−0.32), phosphatidylethanolamine (18:1/20:4) (0.32), lysophosphatidylcholine (16:0) (0.32) |
|
| Anaerofilum | Clostridium cluster IV and family Ruminococcaceae | 2.0 | 4.3 | 0.7 | Oxidized LDL‐C (0.26), TC (0.27), LDL (0.27) | Phosphatidylethanolamine (18:0/22:5) (0.33) | Reported to decrease after supplements with prebiotic potential based on anaerobic human fecal cultivation study. 48 |
BMI indicates body mass index; LDL, low‐density lipoprotein; LDL‐C, LDL cholesterol; MD, meat diet; TC, total cholesterol; and VD, vegetarian diet.
The least square mean and SE of genera abundance or metabolite level were obtained from mixed modeling (n=20). Only genera that significantly differed between diets are presented (P<0.05). The effect of diet was evaluated using a generalized linear mixed model that included a fixed effect of diet, sequence of allocation, and their interaction.
Significant Spearman correlations of differences in genera with clinical parameters improved by VD (P<0.1).
Significant Spearman correlations of differences in genera with plasma metabolites discriminated between the diets (P<0.05).
Baseline Gut Microbiota and Plasma Metabolites Associated With Clinical Outcome Response to the VD
Although we found significantly lower mean oxidized LDL‐C and BMI after VD compared with MD, we observed substantial interindividual difference in response to dietary intervention (Figure 5, Figure S12). Oxidized LDL‐C and BMI were lower in 14 and 13 responders (subjects who benefitted from VD compared with MD and showed within‐individual difference in metabolic risk factors between VD and MD <0), respectively, after VD than after MD, whereas 6 and 7 nonresponders exhibited higher oxidized LDL‐C and BMI, respectively, with MD than with VD. In an exploratory analysis, we found that baseline relative abundance of 14 genera could discriminate responders from nonresponders: oxidized LDL‐C decreased with the VD in individuals with higher fecal relative abundance of genera of the Ruminococcaceae family, Ruminococcaceae UCG.010, Ruminococcaceae UCG.002, Ruminococcus 1, Ruminococcaceae UCG.007, Hydrogenoanaerobacterium, and Barnesiella and with low abundance of GCA.900066575 and Flavonifractor. The response of BMI to the VD was not associated with a specific baseline gut microbiota configuration (Figure S8). Plasma metabolites at baseline were not associated with any response to intervention (Figure S13).
Figure 5. Baseline gut microbiota associated with response to diets in reduction of oxidized low‐density lipoprotein cholesterol (LDL‐C).
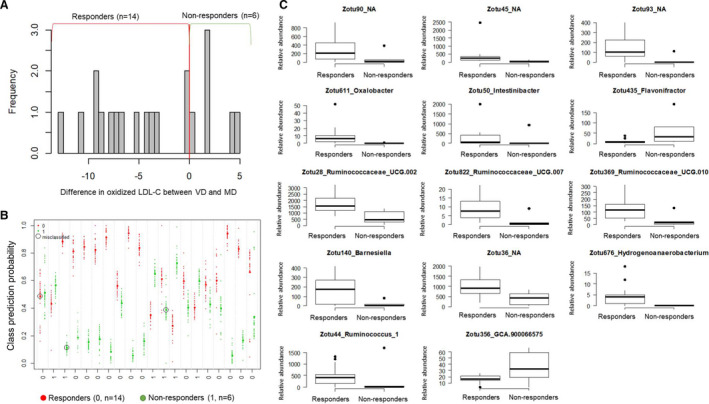
A, Intraindividual difference in oxidized LDL‐C between vegetarian diet (VD) and meat diet (MD) is presented. Responders were defined as participants who showed lower oxidized LDL‐C after VD than after MD. Patients who had higher oxidized LDL‐C after VD than after MD were considered as nonresponders. B, Discrimination of responders from nonresponders based on microbial genera at baseline. We applied random forest modeling on relative abundance of zero‐radius operational taxonomic units (ZOTUs) at baseline. Of 20 individuals, 17 could be successfully classified as responders or nonresponders. C, The optimal set of microbial genera for the successful classification (n=14). Relative abundance of ZOTUs for responders and nonresponders are presented. Boxes represent the interquartile range, and the line within represents the median. Whiskers denote the lowest and highest values within 1.5× interquartile range.
DISCUSSION
In this randomized, controlled, crossover study in subjects with IHD, a 4‐week VD showed lower oxidized LDL‐C and improved cardiometabolic risk factors compared with an isocaloric MD. The VD also influenced the relative abundance of microbial genera and plasma metabolites that have shown links to metabolic disease. 49 , 50 , 51 , 52 The change in oxidized LDL‐C with the VD occurred in people with a specific baseline gut microbiota showing higher abundance of several genera in the families Ruminococcaceae and Barnesiella, a gut microbe that might play an important role in clearance of intestinal infections and immunomodulation. 53 , 54
Diet Effects on Oxidized LDL‐C and Cardiometabolic Risk Factors
Conversion of LDL‐C to oxidized LDL‐C plays a central role in the development and progression of fatty streaks and atherosclerotic plaques. 55 Untreated individuals with IHD have significantly higher levels of oxidized LDL‐C compared with people free of IHD. 31 Independent of traditional cardiovascular risk factors, elevated oxidized LDL‐C has been shown to be a strong predictor of future IHD events. 6 It has recently been suggested that oxidized LDL‐C leads to unstable coronary plaques via complex mechanisms of lipid mediators. 56 Our study indicates that, in subjects with IHD on optimal medical therapy, change in diet was accompanied by a decrease in oxidized LDL‐C; hence, adoption of a VD in such patients could be of clinical importance. Studies of the link between diet and oxidized LDL‐C are scarce; however, a clinical trial of healthy subjects with no diagnosed CVD showed oxidized LDL‐C 5.4 U/L lower after 3 months of a gluten‐free vegan diet than seen in a nonvegan diet. 35 We found that 4 weeks on a VD resulted in significantly lower oxidized LDL‐C (−2.7 U/L) than with the MD in subjects with IHD treated with PCI, suggesting benefits of implementing VD intervention in addition to optimal medical therapy.
A recent meta‐analysis of 11 randomized controlled trials reported a lipid‐lowering effect of VD in healthy subjects free of CVD. 7 Most of the included trials comprised subjects not receiving lipid‐lowering drugs. The pooled estimated changes in TC and LDL‐C were −13.9 and −13.1 mg/dL, respectively, but no significant effects were observed for triglycerides. These effects were greater than those found in the current study. Interventions in the trials included in the meta‐analysis were of longer duration, and our subjects had low TC and LDL‐C levels at baseline. More important, our results suggest an additive effect of VD on TC and LDL‐C in subjects receiving lipid‐lowering medication. A 4% decrease in LDL‐C may result in a meaningful reduction of coronary events. In agreement with previous studies, we observed a reduction in body weight with the tested VD, supporting a role for a VD on weight control in patients with IHD. The observed effects of VD on oxidized LDL‐C and lipid profile may be partly attributed to weight loss. 57 On the other hand, we observed the greatest change in oxidized LDL‐C and lipid profile compared with baseline after the VD, despite no significant change in weight in this group.
Previous studies have shown benefit of a VD with respect to blood pressure, HbA1c, and hs‐CRP compared with an omnivore diet, 6 , 58 , 59 which was not supported by our study. The source of the lack of reduction in hs‐CRP with the VD may be the fact that all study participants were treated with statins, which show anti‐inflammatory properties, or the lack of power to detect changes in hs‐CRP.
The baseline treatment did not influence the results, because of the crossover design of the study. Moreover, because no alterations in cholesterol‐lowering drugs (statins or ezetimibe) were made during the study period, it is unlikely that medication had an impact on oxidized LDL‐C or cholesterol measures. On the other hand, a change in antihypertensive therapy (calcium channel blockers) of 2 subjects may partly explain the lack of effect of VD on blood pressure compared with MD.
Diet Effects on Gut Microbiota and Plasma Metabolome
The 4‐week dietary intervention did not alter either the richness or the overall composition of the gut microbiota, in line with previous findings. 60 However, we observed altered relative abundance of bacterial genera that have been associated with human metabolic health status. 13 , 15 , 39 , 40 , 46 , 61 , 62 For example, compared with MD, subjects consuming the VD exhibited higher relative abundance of the genus Akkermansia, shown to be enriched after intervention with prebiotic inulin and in polyphenol‐rich diets. 39 Akkermansia was also linked to beneficial effects on body fat distribution as well as fasting plasma glucose and triglyceride levels in a 6‐week interventional trial of caloric restriction in obese subjects. 49
The levels of fecal SCFAs were measured to quantify microbiota fiber fermentation capacity. Previous studies have shown effects of a VD or vegan diet on enrichment of SCFA‐producing bacteria (eg, Roseburia, Ruminococcus, and Blautia) and subsequent increase in fecal SCFA levels, which may contribute to improved metabolic health. 63 , 64 We found a trend of increased fecal SCFAs with the VD, consistent with the slightly higher increase of fiber intake compared with the MD. Fecal SCFA level is influenced by the quantity of ingested fiber as well as individual characteristics, including composition of gut microbiota, intestinal gut transit, and rate of intestinal absorption. 65 Therefore, a larger sample size and a greater difference in the ingested fiber content of the diets might have been required to show significant changes in SCFA levels. In the present study, we adjusted the MD meal plans to include higher fiber content of the side dishes, breakfast, and snacks to obtain daily dietary fiber intake similar to that of the VD compared with MD.
We also observed differences in plasma metabolites after VD in subjects with IHD. Subjects consuming the VD exhibited lower levels of the acylcarnitine metabolites 2‐hydroxylauroylcarnitine and 2‐methylbutyroylcarnitine, as well as of several phospholipids containing fatty acids C14:0, C16:0, C16:1, and C18:1. In addition to traditional risk factors, these metabolites may improve risk prediction for recurrent coronary events. 66
The VD compared with MD resulted in a reduction of plasma l‐carnitine, a metabolite found predominately in red meat, findings that support that most of the subjects were adherent to both interventions and verify the accuracy of the analysis. The conversion of l‐carnitine to trimethylamine is gut microbiota dependent, 67 and trimethylamine is absorbed by the portal system and transformed by the liver to TMAO, a potential proatherogenic compound. 21 , 68 Although no significant difference was observed between diets in TMAO, both VD and MD were shown to reduce its plasma level compared with baseline. These changes may have been caused by the reduced energy intake designed from individually adapted meal plans rather than dietary composition. However, the results should be interpreted with caution because others have showed a difference in TMAO levels between vegans and omnivores, 18 and there might have been a lack of power in our study to detect significant changes. Moreover, the metabolic control and the renal function may interfere with TMAO levels, 69 although our study subjects had normal HbA1c and estimated glomerular filtration rate at baseline and the crossover design minimizes the likelihood of bias from confounding.
Our results support previously reported correlation of TMAO with genus Bifidobacterium (r=−0.31; P=0.05), genera belonging to the family Ruminococcaceae (eg, Butyricicoccus: r=−0.42, P=0.01; and Intestinimonas: r=−0.40, P=0.02), and several unannotated species of Lachnospiraceae and Ruminococcaceae. 18 , 51 , 68 These findings indicate that a short‐term VD intervention might have influenced the activity of the gut microbiota in people who are omnivorous.
We observed an effect of VD on potential links among plasma metabolites, bacterial genera, and CVD risk factors. The correlations did not reach significance after false discovery rate correction for multiple testing, possibly because of the small number of participants and the similarity of microbial species in the gut microbiota. Our findings are consistent with previous studies 52 , 66 , 70 and indicate that mechanisms underlying the benefits of a short‐term VD intervention on CVD risk factors may be explained by modulation of the abundance and metabolism of gut microbes. 39
Baseline Gut Microbiota Associated With Oxidized LDL‐C Response to Diets
Our results underscore the role of individual gut microbiota in specific cardiometabolic risk factor response to a diet, 25 , 26 , 27 such as that of oxidized LDL‐C. We observed no significant association of relative abundance of gut bacteria at baseline with change in BMI during the study, in agreement with a recent meta‐analysis indicating a weak relationship between gut microbiota and BMI. 71 However, we observed that several genera of the Ruminococcaceae, as well as the genus Barnesiella, were more abundant in individuals in whom oxidized LDL‐C was reduced to a greater extent (responders) after a 4‐week VD; whereas GCA900066575 in the Lachnospiraceae family was less abundant relative to levels in nonresponders. Accumulating evidence supports a role of inflammation and the immune response in development of atherosclereosis. 72 , 73 Our results may suggest an interaction between specific gut bacteria and a VD in reduction of oxidized LDL‐C, a lipoprotein that has been found to contribute to atherosclerosis‐associated inflammation, activating both innate and adaptive immunity. 54 , 74
Strengths and Limitations
The major strengths of the reported study include its crossover design, well‐characterized subjects receiving optimal medical therapy, and a high rate of study completion. For future implementation, it is also a strength that the dietary interventions included ready‐made main meals, because people often state that a VD is inconvenient and that they are unfamiliar with preparing vegetarian food. The availability of acceptable ready‐made plant‐based foods could facilitate secondary prevention. 75 In our crossover study, effects were only attributed to differences in diet, we found no significant impact in the order of the 2 dietary interventions, and there were no carryover effects.
The study has several limitations. First, the small sample size might have affected results with respect to clinical parameters, such as blood pressure, lipid and apolipoprotein biomarkers, and low‐grade inflammation. Second, most of our study participants were men, decreasing generalizability. Third, a short‐term intervention period allows only limited conclusions on adherence and clinical impact of diet. Measures of oxidized LDL‐C levels in plasma ex vivo may not precisely reflect levels in vivo, as highly oxidized particles are rapidly cleared by scavenger receptors in the liver and antioxidants in blood. 44 We used a sandwich ELISA with a murine monoclonal antibody (mAb‐4E6) directed against the oxidized antigenic determinants on the oxidized APOB molecule. This antibody may react with oxidized particles other than LDL‐C, such as oxidized phospholipids and lipoproteins. 76 The untargeted metabolomics approach did not include a comprehensive analysis of bile acids, which precluded further investigation into the potential mechanistic role of gut microbiota regulation of bile acid metabolism in the cardiometabolic effects of the VD. We found that bacterial genera in the families Ruminococcaceae and Lachnospiraceae, known to modulate bile acid profile, 77 , 78 correlated with TC. The association did not remain significant after correction for multiple testing. Finally, information on the micronutrient content of the ready‐made dishes was lacking, and a potential difference in the diets might have influenced the study results.
Conclusions
Our study suggests cardiometabolic benefits of a 4‐week VD compared with an isocaloric MD in subjects with ischemic heart diease on optimal medical treatment. The VD reduced levels of oxidized LDL‐C, LDL‐C, TC, and body weight compared with MD. The VD intervention also influenced levels of several microbial genera and plasma metabolites known to be linked to metabolic health status, suggesting the role of host‐microbiota cometabolism for benefits of VD in people with ischemic heart diease. The composition of gut microbiota at baseline may have been associated with the lower oxidized LDL‐C seen with the VD, reinforcing the importance of implementing personalized approaches to nutrition in addition to medical treatment, for effective management of cardiovascular disease.
Sources of Funding
Djekic received support from Region Örebro County through Funding for Medical Training. Landberg and Shi were supported by grants from The Swedish Research Council, the Swedish Research Council Formas, and the Chalmers Foundation. The computations for 16S rRNA gene analyses were performed on resources provided by Swedish National Infrastructure for Computing through Uppsala Multidisciplinary Center for Advanced Computational Science under Project SNIC 2018‐3‐350.
Disclosures
None.
Supporting information
Data S1
Tables S1–S11
Figures S1–S13
Acknowledgments
We are grateful to all the participants in the study and the staff at the Department of Cardiology, Örebro University Hospital. We thank Dafgård of Källby, Sweden, for providing frozen meals. We thank Ingela Östman, Annika Eriksson, Johan Josefsson, and Kristina Karlström at Department of Cardiology, Örebro University Hospital, for recruiting subjects and practical help with planning the study, blood sampling, and storage and distribution of the ready‐made meals. We thank Manuela Krämer, Marcus Ståhlman, and Per‐Olof Bergh for assistance in analysis of the fecal microbiota profiles and short‐chain fatty acids/branched‐chain fatty acids. We thank Marina Armeni and Nafisa M. Yusuf for assistance in untargeted metabolomics analysis.
Author Contributions: Djekic, Särnqvist, Bäckhed, Landberg, and Frøbert conceived and planned the clinical trial; Djekic was the principal investigator and performed clinical evaluations, sample collection and analysis, and statistical analyses of the clinical data, interpreted the data, and drafted and revised the manuscript; Shi conducted plasma metabolome analysis, performed statistical analyses on omics data, interpreted the data, and drafted and revised the manuscript; Savolainen performed analysis of plasma trimethylamine N‐oxide, choline, l‐carnitine, and acetyl‐carnitine. Cao supervised data management and performed statistical analyses of the clinical data; Brolin and Tremaroli performed 16s RNA sequencing and participated in data analyses and interpretation; Carlsson revised the meal plan, provided instructions on following the diet plans, and performed dietary data processing; Cao, Bäckhed, Tremaroli, Landberg, and Frøbert supervised data interpretation and revised the manuscript. Frøbert assumed overall responsibility for the project. All authors read and approved the final article.
(J Am Heart Assoc. 2020;9:e016518 DOI: 10.1161/JAHA.120.016518.)
Supplementary materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.016518
For Sources of Funding and Disclosures, see page 15.
References
- 1. Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, Garnett T, Tilman D, DeClerck F, Wood A, et al. Food in the Anthropocene: the EAT‐Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393:447–492. [DOI] [PubMed] [Google Scholar]
- 2. Huang T, Yang B, Zheng J, Li G, Wahlqvist ML, Li D. Cardiovascular disease mortality and cancer incidence in vegetarians: a meta‐analysis and systematic review. Ann Nutr Metab. 2012;60:233–240. [DOI] [PubMed] [Google Scholar]
- 3. Orlich MJ, Singh PN, Sabaté J, Jaceldo‐Siegl K, Fan J, Knutsen S, Beeson WL, Fraser GE. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med. 2013;173:1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kahleova H, Levin S, Barnard ND. Vegetarian dietary patterns and cardiovascular disease. Prog Cardiovasc Dis. 2018;61:54–61. [DOI] [PubMed] [Google Scholar]
- 5. Yokoyama Y, Nishimura K, Barnard ND, Takegami M, Watanabe M, Sekikawa A, Okamura T, Miyamoto Y. Vegetarian diets and blood pressure: a meta‐analysis. JAMA Intern Med. 2014;174:577–587. [DOI] [PubMed] [Google Scholar]
- 6. Yokoyama Y, Barnard ND, Levin SM, Watanabe M. Vegetarian diets and glycemic control in diabetes: a systematic review and meta‐analysis. Cardiovasc Diagn Ther. 2014;4:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang F, Zheng J, Yang B, Jiang J, Fu Y, Li D. Effects of vegetarian diets on blood lipids: a systematic review and meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2015;4:e002408 DOI: 10.1161/JAHA.115.002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, Sparler S, Armstrong WT, Ports TA, Kirkeeide RL, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280:2001–2007. [DOI] [PubMed] [Google Scholar]
- 9. Lin YH, Luck H, Khan S, Schneeberger PHH, Tsai S, Clemente‐Casares X, Lei H, Leu YL, Chan YT, Chen HY, et al. Aryl hydrocarbon receptor agonist indigo protects against obesity‐related insulin resistance through modulation of intestinal and metabolic tissue immunity. Int J Obes (Lond). 2019;43:2407–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Viguiliouk E, Kendall CW, Kahleova H, Rahelic D, Salas‐Salvado J, Choo VL, Mejia SB, Stewart SE, Leiter LA, Jenkins DJ, et al. Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: a systematic review and meta‐analysis of randomized controlled trials. Clin Nutr. 2019;38:1133–1145. [DOI] [PubMed] [Google Scholar]
- 11. Sofi F, Dinu M, Pagliai G, Cesari F, Gori AM, Sereni A, Becatti M, Fiorillo C, Marcucci R, Casini A. Low‐calorie vegetarian versus Mediterranean diets for reducing body weight and improving cardiovascular risk profile: CARDIVEG Study (Cardiovascular Prevention With Vegetarian Diet). Circulation. 2018;137:1103–1113. [DOI] [PubMed] [Google Scholar]
- 12. Lea E, Worsley A. Benefits and barriers to the consumption of a vegetarian diet in Australia. Public Health Nutr. 2003;6:505–511. [DOI] [PubMed] [Google Scholar]
- 13. Jin Q, Black A, Kales SN, Vattem D, Ruiz‐Canela M, Sotos‐Prieto M. Metabolomics and microbiomes as potential tools to evaluate the effects of the Mediterranean diet. Nutrients. 2019;11:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hills RD Jr, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut microbiome: profound implications for diet and disease. Nutrients. 2019;11:1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tang ZZ, Chen G, Hong Q, Huang S, Smith HM, Shah RD, Scholz M, Ferguson JF. Multi‐omic analysis of the microbiome and metabolome in healthy subjects reveals microbiome‐dependent relationships between diet and metabolites. Front Genet. 2019;10:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puertollano E, Kolida S, Yaqoob P. Biological significance of short‐chain fatty acid metabolism by the intestinal microbiome. Curr Opin Clin Nutr Metab Care. 2014;17:139–144. [DOI] [PubMed] [Google Scholar]
- 17. Brial F, Le Lay A, Dumas ME, Gauguier D. Implication of gut microbiota metabolites in cardiovascular and metabolic diseases. Cell Mol Life Sci. 2018;75:3977–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al. Intestinal microbiota metabolism of L‐carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dragsted LO. Biomarkers of meat intake and the application of nutrigenomics. Meat Sci. 2010;84:301–307. [DOI] [PubMed] [Google Scholar]
- 20. Wu W‐K, Chen C‐C, Liu P‐Y, Panyod S, Liao B‐Y, Chen P‐C, Kao H‐L, Kuo H‐C, Kuo C‐H, Chiu THT, et al. Identification of TMAO‐producer phenotype and host–diet–gut dysbiosis by carnitine challenge test in human and germ‐free mice. Gut. 2019;68:1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heianza Y, Ma W, DiDonato JA, Sun Q, Rimm EB, Hu FB, Rexrode KM, Manson JE, Qi L. Long‐term changes in gut microbial metabolite trimethylamine N‐oxide and coronary heart disease risk. J Am Coll Cardiol. 2020;75:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mendes‐Soares H, Raveh‐Sadka T, Azulay S, Edens K, Ben‐Shlomo Y, Cohen Y, Ofek T, Bachrach D, Stevens J, Colibaseanu D, et al. Assessment of a personalized approach to predicting postprandial glycemic responses to food among individuals without diabetes. JAMA Netw Open. 2019;2:e188102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben‐Yacov O, Lador D, Avnit‐Sagi T, Lotan‐Pompan M, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163:1079–1094. [DOI] [PubMed] [Google Scholar]
- 25. Kolodziejczyk AA, Zheng D, Elinav E. Diet‐microbiota interactions and personalized nutrition. Nat Rev Microbiol. 2019;17:742–753. [DOI] [PubMed] [Google Scholar]
- 26. Sonnenburg JL, Backhed F. Diet‐microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Christensen L, Roager HM, Astrup A, Hjorth MF. Microbial enterotypes in personalized nutrition and obesity management. Am J Clin Nutr. 2018;108:645–651. [DOI] [PubMed] [Google Scholar]
- 28. Palmnas M, Brunius C, Shi L, Rostgaard‐Hansen A, Torres NE, Gonzalez‐Dominguez R, Zamora‐Ros R, Ye YL, Halkjaer J, Tjonneland A, et al. Perspective: metabotyping‐a potential personalized nutrition strategy for precision prevention of cardiometabolic disease. Adv Nutr. 2020;11:524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fogelholm M. New Nordic nutrition recommendations are here. Food Nutr Res. 2013;57:22903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Wildlife Foundation . Köttguiden. 2015. Available at: http://www.wwf.se/source.php/1595845/14-8929-WWF-Kottguiden_150608.pdf. Accessed June 8, 2015.
- 31. Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D. Oxidized LDL and malondialdehyde‐modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98:1487–1494. [DOI] [PubMed] [Google Scholar]
- 32. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, et al. European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol. 2016;23:NP1–NP96. [DOI] [PubMed] [Google Scholar]
- 33. Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, Swinburn P, Busschbach J. Measurement properties of the EQ‐5D‐5L compared to the EQ‐5D‐3L across eight patient groups: a multi‐country study. Qual Life Res. 2013;22:1717–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tremaroli V, Karlsson F, Werling M, Stahlman M, Kovatcheva‐Datchary P, Olbers T, Fandriks L, le Roux CW, Nielsen J, Backhed F. Roux‐en‐Y gastric bypass and vertical banded gastroplasty induce long‐term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22:228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Elkan AC, Sjöberg B, Kolsrud B, Ringertz B, Hafström I, Frostegård J. Gluten‐free vegan diet induces decreased LDL and oxidized LDL levels and raised atheroprotective natural antibodies against phosphorylcholine in patients with rheumatoid arthritis: a randomized study. Arthritis Res Ther. 2008;10:R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kay CD, Gebauer SK, West SG, Kris‐Etherton PM. Pistachios increase serum antioxidants and lower serum oxidized‐LDL in hypercholesterolemic adults. J Nutr. 2010;140:1093–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shi L, Westerhuis JA, Rosen J, Landberg R, Brunius C. Variable selection and validation in multivariate modelling. Bioinformatics. 2019;35:972–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Westerhuis JA, van Velzen EJ, Hoefsloot HC, Smilde AK. Multivariate paired data analysis: multilevel PLSDA versus OPLSDA. Metabolomics. 2010;6:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McIntosh K, Reed DE, Schneider T, Dang F, Keshteli AH, De Palma G, Madsen K, Bercik P, Vanner S. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut. 2017;66:1241–1251. [DOI] [PubMed] [Google Scholar]
- 40. Maier TV, Lucio M, Lee LH, VerBerkmoes NC, Brislawn CJ, Bernhardt J, Lamendella R, McDermott JE, Bergeron N, Heinzmann SS, et al. Impact of dietary resistant starch on the human gut microbiome, metaproteome, and metabolome. mBio. 2017;8:e01343 ‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Valdes L, Cuervo A, Salazar N, Ruas‐Madiedo P, Gueimonde M, Gonzalez S. The relationship between phenolic compounds from diet and microbiota: impact on human health. Food Funct. 2015;6:2424–2439. [DOI] [PubMed] [Google Scholar]
- 42. Pagliai G, Russo E, Niccolai E, Dinu M, Di Pilato V, Magrini A, Bartolucci G, Baldi S, Menicatti M, Giusti B, et al. Influence of a 3‐month low‐calorie Mediterranean diet compared to the vegetarian diet on human gut microbiota and SCFA: the CARDIVEG Study. Eur J Nutr. 2020;59:2011–2024. [DOI] [PubMed] [Google Scholar]
- 43. Yan QGY, Li X, Yang W, Jia L, Chen C, Han X, Huang Y, Zhao L, Li P, Fang Z, et al. Alterations of the gut microbiome in hypertension. Front Cell Infect Microbiol. 2017;24:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Piening BD, Zhou W, Contrepois K, Röst H, Gu Urban GJ, Mishra T, Hanson BM, Bautista EJ, Leopold S, Yeh CY, et al. Integrative personal omics profiles during periods of weight gain and loss. Cell Syst. 2018;6:157–170.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang W, Pan Y, Wang L, Zhou H, Song G, Wang Y, Liu J, Li A. Optimal dietary ferulic acid for suppressing the obesity‐related disorders in leptin‐deficient obese C57BL/6J ‐ob/ob mice. J Agric Food Chem. 2019;67:4250–4258. [DOI] [PubMed] [Google Scholar]
- 46. Haro C, Montes‐Borrego M, Rangel‐Zuniga OA, Alcala‐Diaz JF, Gomez‐Delgado F, Perez‐Martinez P, Delgado‐Lista J, Quintana‐Navarro GM, Tinahones FJ, Landa BB, et al. Two healthy diets modulate gut microbial community improving insulin sensitivity in a human obese population. J Clin Endocrinol Metab. 2016;101:233–242. [DOI] [PubMed] [Google Scholar]
- 47. Kappel BA, De Angelis L, Heiser M, Ballanti M, Stoehr R, Goettsch C, Mavilio M, Artati A, Paoluzi OA, Adamski J, et al. Cross‐omics analysis revealed gut microbiome‐related metabolic pathways underlying atherosclerosis development after antibiotics treatment. Mol Metab. 2020;36:100976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Peterson CT, Sharma V, Uchitel S, Denniston K, Chopra D, Mills PJ, Peterson SN. Prebiotic potential of herbal medicines used in digestive health and disease. J Altern Complement Med. 2018;24:656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dao MC, Everard A, Aron‐Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426–436. [DOI] [PubMed] [Google Scholar]
- 50. Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Org E, Blum Y, Kasela S, Mehrabian M, Kuusisto J, Kangas AJ, Soininen P, Wang Z, Ala‐Korpela M, Hazen SL, et al. Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort. Genome Biol. 2017;18:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. de Mello VD, Paananen J, Lindstrom J, Lankinen MA, Shi L, Kuusisto J, Pihlajamaki J, Auriola S, Lehtonen M, Rolandsson O, et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci Rep. 2017;7:46337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Daillère R, Vétizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier‐Colame V, Duong CPM, Flament C, Lepage P, Roberti MP, et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide‐induced therapeutic immunomodulatory effects. Immunity. 2016;45:931–943. [DOI] [PubMed] [Google Scholar]
- 55. Steinberg D. Lewis A. Conner Memorial Lecture: oxidative modification of LDL and atherogenesis. Circulation. 1997;95:1062–1071. [DOI] [PubMed] [Google Scholar]
- 56. Chatterjee M, Rath D, Schlotterbeck J, Rheinlaender J, Walker‐Allgaier B, Alnaggar N, Zdanyte M, Müller I, Borst O, Geisler T, et al. Regulation of oxidized platelet lipidome: implications for coronary artery disease. Eur Heart J. 2017;38:1993–2005. [DOI] [PubMed] [Google Scholar]
- 57. Duggan C, Tapsoba JD, Wang CY, Campbell KL, Foster‐Schubert K, Gross MD, McTiernan A. Dietary weight loss, exercise, and oxidative stress in postmenopausal women: a randomized controlled trial. Cancer Prev Res (Phila). 2016;9:835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Walter DH, Fichtlscherer S, Sellwig M, Auch‐Schwelk W, Schächinger V, Zeiher AM. Preprocedural C‐reactive protein levels and cardiovascular events after coronary stent implantation. J Am Coll Cardiol. 2001;37:839–846. [DOI] [PubMed] [Google Scholar]
- 59. Haghighatdoost F, Bellissimo N, Totosy de Zepetnek JO, Rouhani MH. Association of vegetarian diet with inflammatory biomarkers: a systematic review and meta‐analysis of observational studies. Public Health Nutr. 2017;20:2713–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rajilic‐Stojanovic M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev. 2014;38:996–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liu JP, Zou WL, Chen SJ, Wei HY, Yin YN, Zou YY, Lu FG. Effects of different diets on intestinal microbiota and nonalcoholic fatty liver disease development. World J Gastroenterol. 2016;22:7353–7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Byrne CS, Chambers ES, Morrison DJ, Frost G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int J Obes (Lond). 2015;39:1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Vosa U, Mujagic Z, Masclee AAM, Jonkers D, Oosting M, et al. Causal relationships among the gut microbiome, short‐chain fatty acids and metabolic diseases. Nat Genet. 2019;51:600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Flint HJ. Obesity and the gut microbiota. J Clin Gastroenterol. 2011;45(suppl):S128–S132. [DOI] [PubMed] [Google Scholar]
- 66. Hilvo M, Meikle PJ, Pedersen ER, Tell GS, Dhar I, Brenner H, Schottker B, Laaperi M, Kauhanen D, Koistinen KM, et al. Development and validation of a ceramide‐ and phospholipid‐based cardiovascular risk estimation score for coronary artery disease patients. Eur Heart J. 2020;41:371–380. [DOI] [PubMed] [Google Scholar]
- 67. Koeth RA, Lam‐Galvez BR, Kirsop J, Wang Z, Levison BS, Gu X, Copeland MF, Bartlett D, Cody DB, Dai HJ, et al. L‐carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J Clin Invest. 2019;129:373–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Manor O, Zubair N, Conomos MP, Xu X, Rohwer JE, Krafft CE, Lovejoy JC, Magis AT. A multi‐omic association study of trimethylamine N‐oxide. Cell Rep. 2018;24:935–946. [DOI] [PubMed] [Google Scholar]
- 69. Mueller DM, Allenspach M, Othman A, Saely CH, Muendlein A, Vonbank A, Drexel H, von Eckardstein A. Plasma levels of trimethylamine‐N‐oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis. 2015;243:638–644. [DOI] [PubMed] [Google Scholar]
- 70. Tuomainen M, Lindstrom J, Lehtonen M, Auriola S, Pihlajamaki J, Peltonen M, Tuomilehto J, Uusitupa M, de Mello VD, Hanhineva K. Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low‐grade inflammation in high‐risk individuals. Nutr Diabetes. 2018;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sze MA, Schloss PD. Looking for a signal in the noise: revisiting obesity and the microbiome. mBio. 2016;7:e01018 ‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke. 2006;37:291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hansson GK. Inflammation and immune response in atherosclerosis. Curr Atheroscler Rep. 1999;1:150–155. [DOI] [PubMed] [Google Scholar]
- 74. Rhoads JP, Major AS. How oxidized low‐density lipoprotein activates inflammatory responses. Crit Rev Immunol. 2018;38:333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lea EJ, Crawford D, Worsley A. Public views of the benefits and barriers to the consumption of a plant‐based diet. Eur J Clin Nutr. 2006;60:828–837. [DOI] [PubMed] [Google Scholar]
- 76. Holvoet P, De Keyzer D, Jacobs DR. Oxidized LDL and the metabolic syndrome. Future Lipidol. 2008;3:637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Staley C, Weingarden AR, Khoruts A, Sadowsky MJ. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl Microbiol Biotechnol. 2017;101:47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mullish BH, Pechlivanis A, Barker GF, Thursz MR, Marchesi JR, McDonald JAK. Functional microbiomics: evaluation of gut microbiota‐bile acid metabolism interactions in health and disease. Methods. 2018;149:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S11
Figures S1–S13


