Abstract
E‐cigarette or vaping product use–associated lung injury was recognized in the United States in the summer of 2019 and is typified by acute respiratory distress, shortness of breath, chest pain, cough, and fever, associated with vaping. It can mimic many of the manifestations of coronavirus disease 2019 (COVID‐19). Some investigators have suggested that E‐cigarette or vaping product use–associated lung injury was due to tetrahydrocannabinol or vitamin E acetate oil mixed with the electronic cigarette liquid. In experimental rodent studies initially designed to study the effect of electronic cigarette use on the cardiovascular system, we observed an E‐cigarette or vaping product use–associated lung injury‐like condition that occurred acutely after use of a nichrome heating element at high power, without the use of tetrahydrocannabinol, vitamin E, or nicotine. Lung lesions included thickening of the alveolar wall with foci of inflammation, red blood cell congestion, obliteration of alveolar spaces, and pneumonitis in some cases; bronchi showed accumulation of fibrin, inflammatory cells, and mucus plugs. Electronic cigarette users should be cautioned about the potential danger of operating electronic cigarette units at high settings; the possibility that certain heating elements may be deleterious; and that E‐cigarette or vaping product use–associated lung injury may not be dependent upon tetrahydrocannabinol, vitamin E, or nicotine.
Keywords: E‐cigarette, lung, lung injury, pneumonitis, respiratory distress, vaping
Subject Categories: Animal Models of Human Disease, Inflammation, Basic Science Research
Nonstandard Abbreviations and Acronyms
- eC
electronic cigarette
- NC
nickel‐chromium alloy (nichrome)
- THC
tetrahydrocannabinol
Studies of the cardiovascular consequences of electronic cigarettes (eC) are relevant to the American Heart Association's commitment to research and harm reduction; vaping can increase blood pressure, endothelial dysfunction, and the risk of myocardial infarction and stroke. A condition, which was dubbed “E‐cigarette or vaping product use–associated lung injury” (EVALI) was recognized in the United States in June 2019 and peaked in September 2019; new cases continue to be reported. In March 2020 there were 2800 US cases and 68 deaths reported. Typical victims are young males who recently have been using eC (vaping) and present with acute respiratory distress including shortness of breath, chest discomfort, cough, fever, and fatigue. Chest X‐rays have shown bilateral pulmonary infiltrates, computed tomography scans have shown “ground glass” appearing lung infiltrates, and histology has shown pneumonitis, bronchiolitis, and alveolar damage. 1 , 2 , 3 , 4 A total of 82% of reported cases included use of tetrahydrocannabinol (THC), leading the Centers for Disease Control and Prevention to suggest that THC or cannabidiol and/or the vitamin E acetate oil, which is used to dilute these additives, were possible culprits. In addition, 57% of EVALI cases reported using nicotine. While THC and perhaps cannabidiol, vitamin E acetate, or nicotine may be contributing factors, we are reporting that an experimental form of EVALI was induced in a rodent model without these additives, under controlled laboratory conditions, which reproduces many of the clinical findings in humans with EVALI. Animal use protocols were approved by both the University of California at Irvine's and Huntington Medical Research Institutes' Institutional Animal Care and Use Committees. The data that support the findings of this study are available from the corresponding author upon reasonable request.
The basic goals of our studies were to compare the effects of eC vapor (with and without nicotine), versus tobacco smoke, versus air on rat cardiovascular physiology and pathology. Prior to September 2019, we were studying eC vapor generated from propylene glycol/vegetable glycerin (50% each) plus tobacco flavor (classic tobacco flavoring, “single shot,” which is the lowest dose available, from VaporFi.com), plus nicotine, using a stainless‐steel atomizer (SS) heating element. None of these rats (>30 experiments) developed respiratory distress. The eC device we were using went off market and a substitute device was offered as an alternative. The new device was physically compatible with our exposure system, but the heating element changed from SS to a nickel‐chromium alloy (NC). Heating elements in commercially available eC are usually made of stainless steel, nickel‐chromium or nichrome, Kanthal nickel, or titanium. We resumed our experiments using the same eC liquid (propylene glycol/vegetable glycerin) plus tobacco flavoring, in the no‐nicotine group, during the months of September to November 2019. Eighteen rats received a single, nose‐only, acute eC exposure for 2 hours (NC heating element, 60 or 70 W); five rats received one exposure of eC vapor using the older SS heating element for 2 hours (60 or 70 W); seven rats received a single, nose‐only, 2‐hour exposure to air (Table). None of the air‐exposed rats developed respiratory distress. None of the 5 rats exposed using SS heating elements developed respiratory distress, whereas 14 of 18 rats exposed using NC heating units developed clinical acute respiratory distress (all of these rats had been exposed to the 70‐W power setting; P<0.05 Fisher's exact test), characterized by labored mouth breathing (highly unusual in rats, which are obligatory nose breathers), lack of activity, and audible wheezing. One rat became moribund and had to be euthanized as per the veterinary staff.
Table 1.
Groups Studied
| Group | Air (n=7) | NC Heating Element (n=18) | SS Heating Element (n=5) |
|---|---|---|---|
| 60‐W | N/A | 2 | 2 |
| 70‐W | N/A | 16 | 3 |
N=number of rats in each group. NC indicates nickel‐chromium alloy (nichrome); and SS, stainless steel atomizer.
Histology was performed for 19 rats euthanized after the first few days of exposure. Histology was available in 5 rats exposed to air, in 9 eC rats exposed using NC heating element (7 rats at 70 W and 2 at 60 W), and 5 eC rats exposed using the SS heating element (3 at 70 and 2 at 60 W). Semiquantitative histologic analysis was performed using a scoring system derived in part from a previous study. 5 A score of 0=no lesion; 1<10% of the lung and bronchi involved; 2=11% to 25% involvement; 3=26% to 50% involvement; 4=51% to 75% involvement; and 5=76% to 100% involvement. An estimate of the extent of the lung and bronchial lesion involvement in hematoxylin and eosin–stained sections was assessed in a blinded fashion. Histology of the 5 rats exposed to air showed normal bronchial and alveolar structure (Score=0; Figure—Panels A and B). Two of 7 rats exposed to the NC heating unit at 70 W showed pneumonitis, with scores of 5 and 4 (Figure—Panels C through F); 4 showed multiple foci of pulmonary inflammatory cells, with a score of 1 (Figure—Panel G). Histology of the moribund rat exposed to eC using NC/70 had a score of 2 with accumulation of fibrin and inflammatory cells in the lumen of the trachea. One of 2 rats in the NC heating unit and 60‐W group showed small foci of inflammation with a score of 1; the other NC/60 rat appeared normal (score=0). Two of 2 rats in the SS/60‐W group showed normal histology (score=0); 2 of 3 rats in the SS/70‐W group showed normal histology (score=0; Figure—Panel H); one showed a small area of inflammation with a score of 1.
Figure 1. Hematoxylin and eosin staining of respiratory system sections of rats.
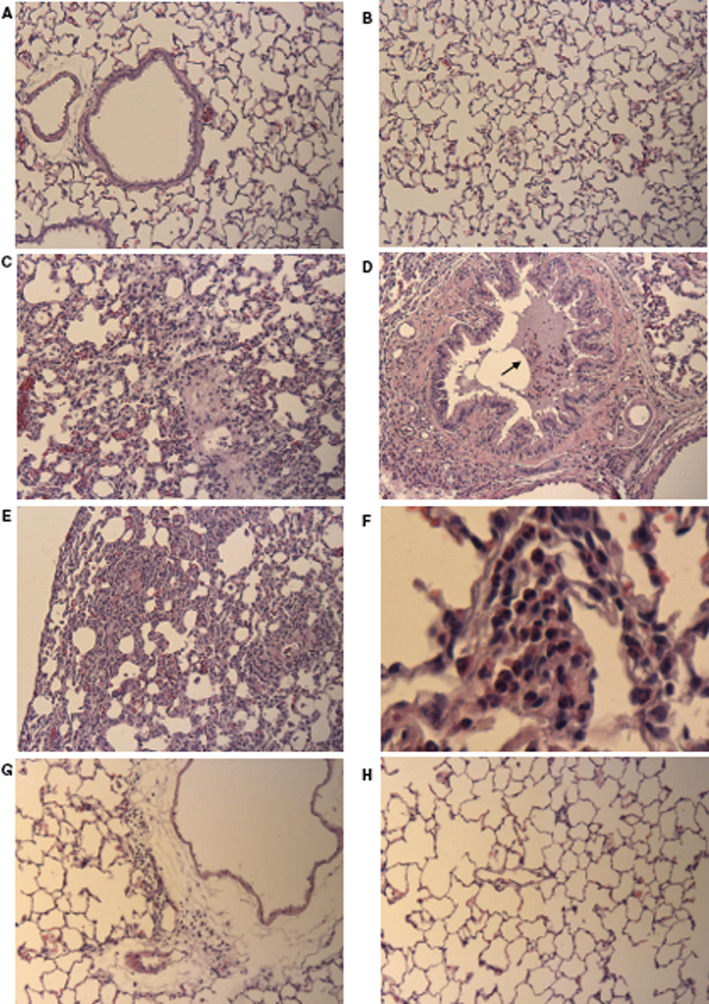
A, Bronchus and alveoli from control air rat, showing intact epithelial cells and thin alveolar walls (original magnification ×10). B, Alveoli of another air‐exposed control rat. Note the thin alveolar walls and minimal cellularity in the interstitial areas (original magnification ×10). C, Alveolar region of an eC‐exposed rat using the NC/70‐W heating element. Obliteration of alveolar spaces, thickened alveolar walls, infiltration of inflammatory cells, red cell congestion (original magnification ×10). D, Bronchus from rat shown in (C). Lumen of bronchus shows mucus plug with inflammatory cells (arrow). Bronchial epithelium shows wavy or folded pattern that may suggest bronchospasm (original magnification ×10). E, eC NC/70‐W rat showing very thickened alveolar septum and prominent inflammatory cellular infiltration (original magnification ×10). F, eC NC/70‐W from another rat showing infiltration of inflammatory cells in the alveoli, including neutrophils (original magnification ×50). G, Another eC NC/70‐W rat showing perivascular inflammation in the adventitia (original magnification ×10). H, Alveolar region of an eC exposed rat using the SS heating element. Alveoli appear normal (original magnification ×10). NC indicates nickel‐chromium alloy (nichrome); and SS, stainless steel atomizer.
An EVALI‐like condition can be induced in an animal model after exposure to tobacco‐flavored propylene glycol/vegetable glycerin, operated at high power setting and heated with a nichrome heating coil, without the addition of THC, vitamin E acetate, or nicotine. Chemical composition of vapor, reactive oxygen species, 6 and particle size may all play a role. Vaporization of propylene glycol/vegetable glycerin at a higher temperature may also have contributed, but was not measured in our study.
We are reporting these initial findings now, even though our studies are continuing, because of their public health significance in light of the life‐threatening potential of the EVALI epidemic as well as concern over vaping in the coronavirus disease 2019 (COVID‐19) era. eC users should be cautioned about the potential danger of operating units at higher than recommended settings and be aware that adverse effects can occur without THC, vitamin E, or nicotine. A limitation of this study is that we did not assess the effects of eC without flavoring; however, the fact that the e‐liquid and flavoring was the same for both heating elements, but the EVALI‐like syndrome only occurred in the NC group, makes it unlikely that the tobacco flavoring used in the eC was the sole culprit. However, this does not rule out that possibility that other flavorings that we did not test could contribute to lung injury.
Sources of Funding
This work was supported by NHLBI (R01HL144258 and 3RO1HL‐144258‐02S1). The effect of electronic cigarettes on young versus old normal hearts and pathologic hearts; Tobacco‐Related Disease Research Program (TRDRP; University of California). Effects of Cigarette Smoking and Vaping on Heart Attack. Proposal Number (pC ID): 587712; Award Number; 28IR‐0057.
Disclosures
None.
(J Am Heart Assoc. 2020;9:e017368 DOI: 10.1161/JAHA.120.017368.)
For Sources of Funding and Disclosures, see page 4.
Contributor Information
Michael T. Kleinman, Email: mtkleinm@uci.edu.
Robert A. Kloner, Email: robert.kloner@hmri.org.
References
- 1. Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde MW, Navon L, Hoots B, Salvatore PP, Elderbrook M, et al. Pulmonary illness related to E‐cigarette use in Illinois and Wisconsin—preliminary report. N Engl J Med. 2020;382:903–916. [DOI] [PubMed] [Google Scholar]
- 2. Butt YM, Smith ML, Tazelaar HD, Vaszar LT, Swanson KL, Cecchini MJ, Boland JM, Bois MC, Boyum JH, Froemming AT, et al. Pathology of vaping‐associated lung injury. N Engl J Med. 2019;381:1780–1781. [DOI] [PubMed] [Google Scholar]
- 3. Henry TS, Kanne JP, Kligerman SJ. Imaging of vaping‐associated lung disease. N Engl J Med. 2019;381:1486–1487. [DOI] [PubMed] [Google Scholar]
- 4. Hswen Y, Brownstein JS. Real‐time digital surveillance of vaping‐induced pulmonary disease. N Engl J Med. 2019;381:1778–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borensztajn K, Bresser P, van der Loos C, Bot I, van den Blink B, den Bakker MA, Daalhuisen J, Groot AP, Peppelenbosch MP, von der Thüsen JH, et al. Protease‐activated receptor‐2 induces myofibroblast differentiation and tissue factor up‐regulation during bleomycin‐induced lung injury. Am J Pathol. 2010;177:2753–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haddad C, Salman R, El‐Hellani A, Talih S, Shihadeh A, Saliba NA. Reactive oxygen species emissions from Supra‐and Sub‐Ohm electronic cigarettes. J Anal Toxicol. 2019;43:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


