Abstract
Background
Acute penetrating aortic ulcers (PAUs) are reported to dynamically evolve into different clinical outcomes ranging from regression to aortic rupture, but no practice guidelines are available in China.
Methods and Results
All 109 patients with acute PAUs were monitored clinically. At 30 days follow‐up, 31 patients (28.44%) suffered from aortic‐related adverse events, a composite of aortic‐related mortality, aortic dissection, or an enlarged ulcer. In addition, 7 (6.42%) patients had clinically related adverse events, including all‐cause mortality, cerebral stroke, nonfatal myocardial infarction, acute heart failure alone or acute exacerbation of chronic heart failure, acute renal failure, arrhythmia, and bleeding events. In the present study, the intervention criteria for the Chinese PAU population included a PAU diameter of 12.5 mm and depth of 9.5 mm. The multivariate analysis showed that an ulcer diameter >12.5 mm (hazard ratio [HR], 3.846; 95% CI, 1.561–9.476; P=0.003) and an ulcer depth >9.5 mm (HR, 3.359; 95% CI, 1.505–7.494; P=0.003) were each independent predictors of aortic‐related events.
Conclusions
Patients with acute PAUs were at high risk for aortic‐related adverse events and clinically related adverse events within 30 days after onset. Patients with an ulcer diameter >12.5 mm or an ulcer depth >9.5 mm have a higher risk for disease progression, and early intervention may be recommended.
Keywords: acute aortic syndrome, aortic‐related adverse events, endovascular repair, penetrating aortic ulcer
Subject Categories: Aortic Dissection, Vascular Disease, Computerized Tomography (CT)
Nonstandard Abbreviations and Acronyms
- AAS
acute aortic syndrome
- AD
aortic dissection
- EVAR
endovascular repair
- PAUs
penetrating aortic ulcers
Clinical Perspective
What Is New?
The first penetrating aortic ulcer intervention criteria for the Chinese patients were 12.5 mm for ulcer diameter and 9.5 mm for ulcer depth.
What Are the Clinical Implications?
Patients with acute penetrating aortic ulcers were at high risk for aortic‐related adverse events and clinically related adverse events within 30 days after onset.
Patients with an ulcer diameter >12.5 mm or an ulcer depth >9.5 mm have a higher risk for disease progression, and early intervention may be recommended.
In aortic diseases, both penetrating aortic ulcers (PAUs) and aortic dissection (AD) are classified as acute aortic syndrome (AAS). 1 PAUs account for 2% to 7% of AAS cases and may deteriorate to AD, aneurysm formation, and aortic rupture. 2 The etiology of the PAU is not clear and may be related to an atheromatous plaque that disrupts the internal elastic lamina without a false lumen. 3
The PAU, a special type of AAS 4 that is similar to AD and intramural hematoma in terms of its clinical manifestation, has unique pathological features and clinical outcomes that are different from those of other types of AAS. 5 Furthermore, the long‐term evolution of the PAU is unclear. The American guidelines recommend endovascular repair (EVAR) for asymptomatic PAU (Ⅲ C) and symptomatic PAU (Ⅱ b C). 6 The European guidelines recognize PAU as a type of AAS with a surgical indication (Ⅱ b C). For type B PAU, the European guidelines suggest medical treatment (I C), but complicated type B PAU may be considered for EVAR (Ⅱ a C) or surgery (Ⅱ b C). 7
There are only a few case reports of PAUs, and the amount of detailed, large‐scale, real‐world clinical data reported for Chinese patients with PAUs is small. Thus, the present study aims to examine the imaging characteristics and clinical prognoses of PAUs and to explore the predictors of aortic‐related adverse events in cases of acute PAUs.
METHODS
This study was a non‐interventional retrospective and observational clinical data analysis study. It has been reviewed and approved by the ethical review committee of General Hospital of Northern Theater Command. The institutional review board of our hospital approved this study. Because the data are anonymous, the requirement for informed consent was waived.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Patients and Enrollment Criteria
From April 2002 to May 2018, according to the results of examinations by helical computed tomographic angiography of the chest and abdomen, 8 165 consecutive patients diagnosed with PAUs at the General Hospital of Northern Theater Command were enrolled. The main exclusion criteria were chronic PAUs, a lack of imaging review, incomplete clinical data, or an acute PAU that received EVAR immediately during hospitalization. A total of 109 patients were analyzed retrospectively (Figure 1). The lesions were characterized using the following clinical and radiological criteria: (1) PAU, defined as the presence of ≥1 focal, contrast‐filled, craterlike outpouching of the endoluminal border of the aortic wall 9 ; (2) aortic intramural hematoma; and (3) mediastinal hematoma, pericardial effusion, or pleural effusion.
Figure 1. Acute penetrating aortic ulcer: flow chart of research target.
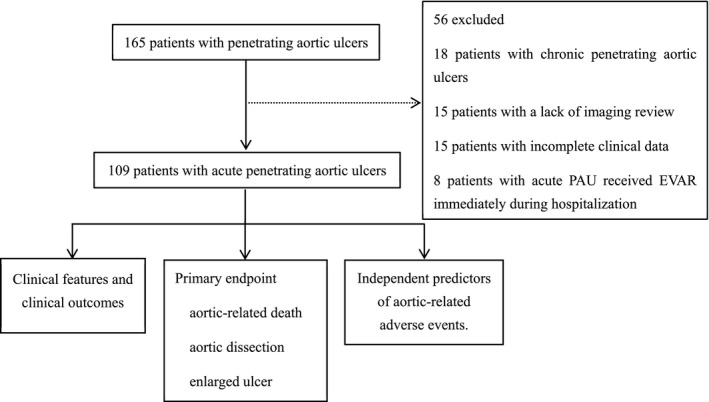
EVAR indicates endovascular repair; and PAU, penetrating aortic ulcer.
End Points and Definitions
The primary end point was 30‐day aortic‐related adverse events, a composite of aortic‐related mortality (death because of aortic rupture and other unexplained sudden death), AD, or an enlarged ulcer (ulcer diameter or ulcer depth). Progression of the observed pathologies was classified as follows: (1) “resolution” referred to a decrease in the size of the ulceration diameter or depth; (2) “worsening” was defined as a deterioration in the aortic conditions, including a significant increase in the diameter or depth of the lesion (according to the computed tomographic angiography, the diameter or depth of the ulcer increased more than 1 mm); (3) “stable” was defined as lesions that did not improved or worsened significantly; (4) rupture was defined as the presence of extraaortic blood confirmed by radiology (after the contrast medium is injected into the vascular cavity, the paraaortic hematoma was formation), surgical examination, or postmortem examination or the finding of an impending rupture during surgery 10 ; and (5) progression to AD was considered when an intimal flap appeared. 11
Major secondary end points were clinically related adverse events, including all‐cause mortality; cerebral stroke; nonfatal myocardial infarction; acute heart failure alone or acute exacerbation of chronic heart failure; acute renal failure; arrhythmia; or any bleeding as defined by the Bleeding Academic Research Consortium definition (grades 1–5). 12 Bleeding was considered medically actionable for Bleeding Academic Research Consortium types 2 through 5 and major for Bleeding Academic Research Consortium types 3 through 5.
Based on symptom onset, acute PAU was <15 days, and chronic PAU was >15 days. 7
Imaging Techniques
All patients in the acute phase underwent computed tomography examinations. 13 The location, diameter, and depth of the ulcer and maximum hematoma thickening were assessed by computed tomography as reported previously. Computed tomography was performed using a 64‐detector Siemens Sensation and intravenous boluses with 80 to 150 mL of nonionic contrast medium. Imaging data were the average value obtained by radiology technicians through three measurements. The average value of the other hospitals' imaging data was obtained by the same individual through three measurements.
Follow‐Up
After discharge, all cases were followed up promptly at 1, 6, and 12 months and annually thereafter. The final follow‐up date was October 2018. During the follow‐up period, patients who experienced recurrent, refractory pain or rupture or whose condition progressed to classical AD were immediately referred for EVAR. 14 For asymptomatic small (diameter <20 mm or depth <10 mm) PAUs, 7 conservative, expectant management was recommended, including strict blood pressure monitoring and vigilant follow‐up with imaging techniques annually. If complications or ulcer progression occurred, EVAR treatment or surgical treatment was recommended.
Statistical Analysis
Continuous variables were reported as the mean±SD or median and interquartile range (25th percentile, 75th percentile), and categorical variables were expressed as percentages. A chi‐square test or Fisher exact test was used to compare proportions, and an unpaired Student t test, 1‐way ANOVA, or Mann–Whitney U test was used to compare means or medians. Survival curves were generated via the Kaplan–Meier method with significant differences assessed for time‐to‐event data using log‐rank tests. The Pearson correlation coefficient was used for bivariate normally distributed data. Multivariable Cox regression models were constructed to identify factors associated with the primary end point. To evaluate whether the diameter or depth of the ulcer could be an effective predictor of the primary end point, a receiver operating characteristic curve analysis was performed. 15 The optimal cutoff level was calculated by the Youden index (sensitivity+specificity−1). A P<0.05 was considered significant. The analyses were performed using SPSS version 22.0 (SPSS, Chicago, IL) (Figure S1).
RESULTS
Study Patients
All 109 patients were diagnosed with acute PAUs by imaging examination. Among the 109 patients, 9 died and 100 survived. Data on patient demographics, risk factors, and outcomes were collected from the 109 patients with acute PAUs (Figure 1). Mean follow‐up was 27.11±22.61 months, and median follow‐up was 23.13 (11.1, 37.33) months.
Baseline Characteristics and Adverse Events of the Entire Cohort
The baseline characteristics of the entire cohort (109 cases) are shown in Table 1. The average age was 65.21±10.09 years. Of the patients, 87 were men. The incidence of systemic hypertension was 80.73%. More than half of the patients were smokers (71.56%). A total of 33.03% and 9.17% of the patients had hyperlipidemia and diabetes mellitus, respectively. The baseline imaging data are provided in Table 1. A total of 72 descending thoracic aortas were observed (66.06%). The diameter and depth of the ulcers were 11.31±5.44 and 8.19±4.50 mm, respectively. The maximum ascending and descending aortic diameters were 43.08±4.84 and 30.37±5.62 mm, respectively. We found 33 cases with pleural effusion and 1 case with pericardial effusion. The medication and laboratory examination data are provided in Table 1.
Table 1.
Clinical Characteristics, Aortic Segments With Penetrating Aortic Ulcers, Baseline Morphological Findings, and Laboratory Examinations (n=109)
| Characteristic | |
|---|---|
| Demographic and clinical data | |
| Age, y | 65.21±10.09 |
| Men | 87 (79.82) |
| BMI, kg/m2 | 24.96±3.31 |
| Systemic hypertension | 88 (80.73) |
| Smoking | 78 (71.56) |
| Hyperlipidemia | 36 (33.03) |
| Diabetes mellitus | 10 (9.17) |
| Coronary heart disease | 37 (33.94) |
| Imaging | |
| Mean number of penetrating atherosclerotic ulcers | 1.15±0.40 |
| Aortic arch | 33 (30.28) |
| Descending thoracic aorta | 72 (66.06) |
| Abdominal aorta | 4 (3.67) |
| Mean ulcer diameter, mm | 11.31±5.44 |
| Mean ulcer depth, mm | 8.19±4.50 |
| Maximum ascending aorta diameter, mm | 43.08±4.84 |
| Maximum descending aorta diameter, mm | 30.37±5.62 |
| Pleural effusion | 33 (30.28) |
| Pericardial effusion | 1 (0.92) |
| Medical | |
| Antiplatelet agents | 5 (4.59) |
| Beta‐blockers | 8 (7.34) |
| Calcium antagonists | 35 (32.11) |
| ACE inhibitors | 5 (4.59) |
| ARB inhibitors | 16 (14.68) |
| Nitrates | 4 (3.67) |
| Statins | 5 (4.59) |
| Systolic BP, mm Hg | 150.07±24.18 |
| Diastolic BP, mm Hg | 85.74±13.24 |
| Heart rate, BPM | 79.01±10.46 |
| Laboratory examinations | |
| CKMB, ng/mL | 11.00 (8.50, 15.50) |
| TNT exception | 33 (30.28) |
| ALT, U/L | 16.20 (12.00, 23.30) |
| AST, U/L | 18.00 (14.54, 24.01) |
| WBC, 109/L | 9.97±2.72 |
| PLT, 109/L | 206.00 (178.50, 253.00) |
| HG, g/L | 133.09±17.43 |
| BUN, mmol/L | 6.36 (4.97, 9.34) |
| CR, μmol/L | 81.10 (68.60, 103.00) |
| C‐reactive protein, mg/dL | 49.30 (19.50, 62.90) |
| D‐dimer, ng/mL | 1.60 (0.80, 2.85) |
Data are expressed as mean±SD, medians (25th percentiles, 75th percentiles), or number (percentage). ACE indicates angiotensin‐converting enzyme; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; BPM, beat per minute; BUN, blood urea nitrogen; CKMB, creatine phosphokinase‐myocardial band; CR, creatinine; HG, hemoglobin; PLT, platelet count; TNT, troponin‐T; and WBC, white blood cell.
The baseline differences characteristics between with and without aortic‐related adverse events are shown in Table S1. Patients without an aortic‐related adverse event had PAUs with a smaller diameter (13.27±5.18 mm versus 10.42±5.35 mm; P=0.010) and depth (9.91±5.13 mm versus 7.42±3.99 mm; P=0.007) than those with an aortic‐related adverse event. There were no significant differences in sex ratio, systolic and diastolic blood pressure, or laboratory examinations between the 2 groups (P>0.05) (Table S1).
Associations Between the Morphological Findings
A total of 109 patients with acute PAUs were reexamined by computed tomographic angiography within 30 days after onset. Among them, 7 (6.42%) patients progressed to typical AD, 23 (21.10%) patients worsened, and 79 (72.48%) patients were stable.
Table 2 shows the associations between the morphological findings and evolution of the PAUs. Patients with stable PAUs had PAUs with a smaller diameter (10.05±5.05 mm versus 14.23±4.71 mm; P<0.05) and depth (7.20±3.78 mm versus 10.60±4.94 mm; P<0.05) than those with PAUs that had worsened. There were no significant differences in maximum ascending aortic diameter between 3 groups and maximum descending aortic diameters between 2 groups (P>0.05).
Table 2.
Relationship Between Penetrating Aortic Ulcer Baseline Morphological Findings and Final Morphological Outcomes
| Morphologic Finding |
Stable or Regression (n=79) |
Worsened (n=23) |
Aortic Dissection (n=7) |
P Value |
|---|---|---|---|---|
| Mean ulcer diameter, mm | 10.05±5.05 | 14.23±4.71 | … | 0.001 |
| Mean ulcer depth, mm | 7.20±3.78 | 10.60±5.45 | … | 0.001 |
| Maximum ascending aorta diameter, mm | 43.32±4.92 | 42.57±4.94 | 41.97±3.75 | 0.624 |
| Maximum descending aorta diameter, mm | 30.49±5.09 | 31.66±6.54 | … | 0.367 |
Data are expressed as mean±SD.
The End Point Analysis During Follow‐Up
At 30 days of follow‐up, a total of 31 cases (28.44%) suffered from aortic‐related adverse events: 3 patients died from aortic‐related causes, 7 patients progressed to AD, and 21 patients experienced ulcer worsening. Until the actual longest follow‐up period, 34 patients (31.19%) experienced aortic‐related adverse events, 5 patients died from aortic‐related causes, 8 patients progressed to AD, and 21 patients experienced ulcer worsening (Table 3).
Table 3.
End Points During Follow‐Up (n=109)
| Events | N |
|---|---|
| After 30 days of follow‐up | |
| Primary end point | 31 |
| Aortic‐related deaths | 3 |
| Worsening | 21 |
| AD | 7 |
| Secondary end point | 7 |
| All‐cause mortality | 4 |
| Aortic‐related deaths | 3 |
| Non‐aortic‐related deaths | 1 |
| Cerebral stroke | 2 |
| Hemorrhagic stroke | 1 |
| Ischemic stroke | 1 |
| Arrhythmia | 1 |
| All bleeding | 2 |
| BARC 2–5 | 2 |
| BARC 3–5 | 2 |
| Long‐term follow‐up | |
| Long‐term aortic‐related adverse events | 34 |
| Aortic‐related deaths | 5 |
| Worsening | 21 |
| AD | 8 |
| Total adverse clinical events | 13 |
| All‐cause mortality | 9 |
| Aortic‐related deaths | 5 |
| Non‐aortic‐related deaths | 4 |
| Cerebral stroke | 2 |
| Hemorrhagic stroke | 1 |
| Ischemic stroke | 1 |
| Arrhythmia | 1 |
| All bleeding | 5 |
| BARC 2–5 | 4 |
| BARC 3–5 | 4 |
AD indicates aortic dissection; and BARC, Bleeding Academic Research Consortium.
BARC, bleeding is graded on a scale of 1 to 5, ranging from minor bleeding that is not actionable (type 1) to fatal bleeding (type 5).
At 30 days of follow‐up, 7 cases suffered from clinically related adverse events: 4 patients died and were classified as all‐cause mortality, 1 patient suffered from arrhythmia, and 2 patients suffered from cerebral stroke. Until the actual longest follow‐up period, 13 cases suffered from clinically related adverse events, 9 patients died and were classified as all‐cause mortality, 1 patient suffered from arrhythmia, and 2 patients suffered from cerebral stroke (Table 3 and Figure 2).
Figure 2. Survival curve of the clinical outcomes of all patients during follow‐up.
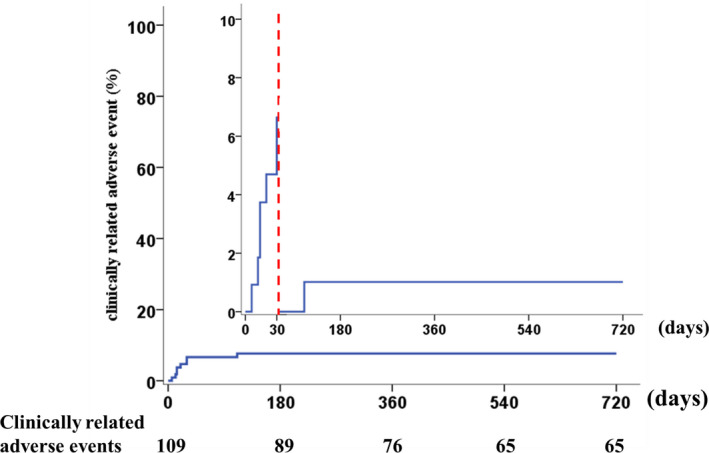
The data show that 7 adverse clinical events occurred within 30 days.
Predictive Value of the PAU Diameter and Depth for the Prognosis of Chinese Patients
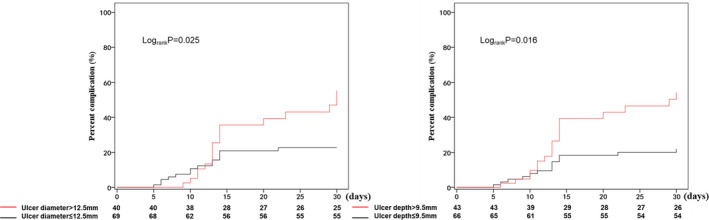
The receiver operating characteristic curve analysis demonstrated that the diameter and depth of the PAU could distinguish patients who met the primary end points from those who did not meet the primary end points, including aortic‐related death, AD, and ulcer progression (Figure 3). The optimal cutoff values for the PAU diameter and depth in Chinese patients were 12.5 and 9.5 mm, with areas under the curve of 0.636 and 0.646, respectively. The Kaplan–Meier survival curve analysis showed that patients with an ulcer diameter ≤12.5 mm had a significantly lower aortic‐related risk than those with an ulcer diameter >12.5 mm (42.50% [17/40] versus 20.29% [14/69]; P=0.025) (Figure 4). Moreover, patients with an ulcer depth ≤9.5 mm had a significantly lower aortic‐related risk than those with an ulcer diameter >9.5 mm (41.86% [18/43] versus 19.70% [13/66]; P=0.016) (Figure 4). Thus, an ulcer diameter >12.5 mm and an ulcer depth >9.5 mm may be risk factors for aortic‐related adverse events (primary end points) of acute PAU.
Figure 3. Receiver operating characteristic curve analysis for prediction of penetrating aortic ulcer based on the ulcer diameter and depth.
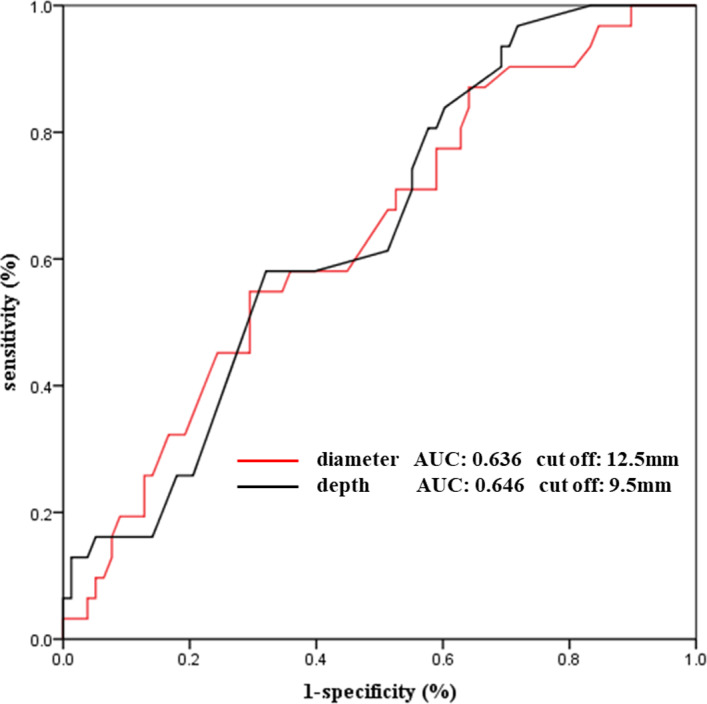
AUC indicates area under the curve.
Figure 4. An unadjusted Kaplan–Meier curve stratified by in‐hospital and follow‐up complications.
Multivariate Predictor Analysis
Linear regression analysis showed that there was a positive correlation between ulcer diameter and ulcer depth (R 2 linear, 0.467; P<0.001). Bivariate correlation analysis also confirmed that there was a significant positive correlation between ulcer diameter and ulcer depth (Pearson correlation coefficient, 0.683; P<0.001). Because of the significant positive correlation between ulcer diameter and ulcer depth, an ulcer diameter >12.5 mm and ulcer depth >9.5 mm were separately included in multivariate regression analysis. The influencing factors included sex, hypertension, diabetes mellitus, coronary heart disease, and dyslipidemia. The multivariate analysis showed that an ulcer diameter >12.5 mm (hazard ratio [HR], 3.846; 95% CI, 1.561–9.476; P=0.003) and ulcer depth >9.5 mm (HR, 3.359; 95% CI, 1.505–7.494; P=0.003) were independent predictors of aortic‐related adverse events (primary end points) (Tables 4 and 5).
Table 4.
Multivariate Predictor Analysis of Adverse Aortic‐Related Events in Patients With Penetrating Aortic Ulcer
| HR | 95% CI | P Value | |
|---|---|---|---|
| Ulcer diameter >12.5 mm | 3.846 | 1.561–9.476 | 0.003 |
| Male | 0.779 | 0.303–2.003 | 0.604 |
| Hypertension | 1.087 | 0.429–2.749 | 0.861 |
| Diabetes mellitus | 1.226 | 0.402–3.740 | 0.720 |
| Coronary heart disease | 0.580 | 0.258–1.304 | 0.188 |
| Dyslipidemia | 0.409 | 0.160–1.042 | 0.061 |
HR indicates hazard ratio.
Table 5.
Multivariate Predictor Analysis of Adverse Aortic‐Related Events in Patients With Penetrating Aortic Ulcer
| HR | 95% CI | P Value | |
|---|---|---|---|
| Ulcer depth >9.5 mm | 3.359 | 1.505–7.494 | 0.003 |
| Male | 0.941 | 0.372–2.379 | 0.898 |
| Hypertension | 0.945 | 0.378–2.362 | 0.904 |
| Diabetes mellitus | 1.866 | 0.612–5.693 | 0.273 |
| Coronary heart disease | 0.525 | 0.231–1.196 | 0.125 |
| Dyslipidemia | 0.535 | 0.230–1.242 | 0.146 |
HR indicates hazard ratio.
Morphological Evolution of Acute PAUs
Typical morphological multidetector computed tomography imaging data obtained during the acute phase of PAU evolution to a dissection are shown in Figure 5. Typical imaging data showing acute PAU stability and enlargement are also shown in Figure 5.
Figure 5. Typical morphological multidetector computed tomography imaging data.
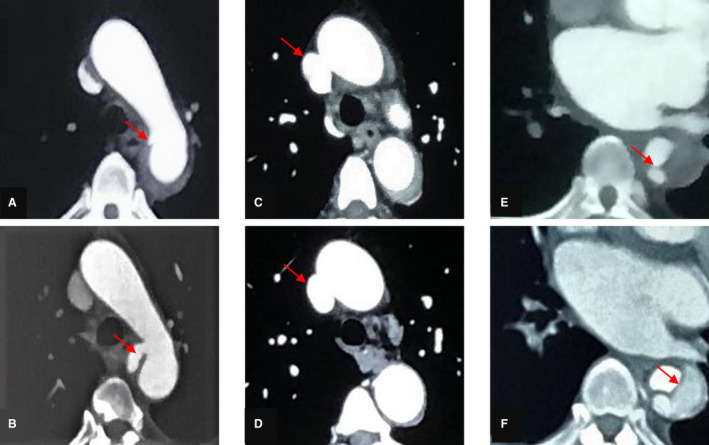
A, C, and E, During the acute phase, a computed tomographic angiography scan demonstrates the presence of a penetrating aortic ulcer and a periaortic hematoma. B, The ulcer is enlarged. D, Computed tomographic angiography scan shows that the ulcer is stable and that the periaortic hematoma has almost completely disappeared. F, Computed tomographic angiography scan reveals a typical dissection.
DISCUSSION
To our knowledge, the present study is the largest retrospective analysis to observe acute PAUs in the Chinese population. In addition, we evaluated the clinical features, evolution in imaging, clinical outcomes, and multivariate predictors of acute PAUs. There were several main findings. First, within 30 days after onset, the incidences of aortic‐related adverse events and clinically related adverse events in acute PAUs were 28.44% and 6.42%, respectively. Clinically related adverse events mainly occurred 30 days after onset, accounting for 53.85% of the total number of clinically related adverse events, which suggested that close medical surveillance during the first month might contribute a good prognosis. Second, the receiver operating characteristic curves for predicting the primary end points were plotted, and the first cutoff values for the Chinese patients were 12.5 mm for ulcer diameter and 9.5 mm for ulcer depth. Third, an ulcer diameter >12.5 mm and ulcer depth >9.5 mm were independent risk factors for aortic‐related adverse events (primary end points), which increased the incidence of events by 3.8 times and 3.3 times, respectively. Fourth, the Kaplan–Meier survival curve analysis showed that patients with an ulcer diameter ≤12.5 mm had a significantly lower aortic‐related risk than those with an ulcer diameter >12.5 mm (P=0.025); patients with an ulcer depth ≤9.5 mm had a significantly lower aortic‐related risk than those with an ulcer depth >9.5 mm (P=0.016). Finally, we analyzed the first evidence of PAU morphological evolution in the Chinese population, including stability, progression, and classical AD. The findings of and evidence from this study may be conducive to providing a reference for the treatment of PAUs in the Chinese population. Moreover, these findings may have a large influence on intervention standards and the primary prevention of PAUs in Chinese patients.
In the present study, within 30 days, the incidences of aortic‐related adverse events in and aortic‐related deaths of patients with acute PAUs were 28.44% and 6.42%, respectively. In addition, the incidence of long‐term aortic‐related adverse events was 31.19%. Botta et al 16 reported that the 30‐day mortality rate of 18 patients with PAUs was 11.1%. An average‐3‐year follow‐up study 17 reported the incidence of aortic‐related adverse events (including PAU enlargement and progression to AD) to be 30.00% for 20 patients who received imaging follow‐up. Furthermore, Tittle et al 11 reported that the incidence of aortic‐related adverse events (including PAU enlargement and progression to AD) was 77.33% for 15 patients who received imaging follow‐up. The incidence of aortic‐related adverse events was similar to that in the study by Botta et al, but significantly lower than that in the study by Tittle et al. In our center, all patients were under medical guidance after diagnosis, which may contribute to reducing the incidence of aortic‐related adverse events. To our knowledge, there were no computed tomography images of acute PAU evolution. The present study showed 3 types of clinical progression of PAU, which enriched the imaging data for PAU treatment.
The present study provides the first intervention criteria in a Chinese population, namely, a cutoff value of 12.5 mm for the ulcer diameter or 9.5 mm for the ulcer depth. In the 2014 European Society of Cardiology AD guidelines, 7 early intervention treatment may be recommended for patients with asymptomatic PAUs with diameters >20 mm or depths >10 mm. The differences between the present study and the Western guidelines suggest that race, bodily form, and habits may influence treatment strategies for PAUs. 18 Notably, the ulcer diameter is significantly smaller in this study than that in the European guidelines, but the ulcer depth is slightly smaller in this study than that in the European guidelines. The authors made a bold guess that the tolerance of Chinese people in terms of the ulcer depth was similar to that of European people but that the tolerance of Chinese people in terms of the ulcer diameter was poor. Thus, further verification is needed with a study with a larger sample size. Moreover, an ulcer diameter >12.5 mm and an ulcer depth >9.5 mm were independent risk factors for aortic‐related adverse events within 30 days. Nevertheless, in the study by Janosi et al, 9 a PAU depth >15 mm was an independent predictor of mortality. Moreover, in the Chinese population, an ulcer diameter >12.5 mm predicted a poor prognosis. The reasons may be as follows: (1) the present study enrolled patients acute PAUs; (2) the included patients in the present study were mainly from the Chinese population, in contrast to the study by Janosi et al; and (3) there were differences in the primary end points between the 2 studies, namely, the present study included aortic‐related deaths and PAU deterioration. Combining the findings of the aforementioned 2 studies may produce significant influences on the prognostic evaluations of patients with PAUs. The intervention criteria of the present study may greatly contribute to improving treatment strategies for PAUs in the Chinese population.
In 1986, PAU was first characterized by Stanson et al. 19 A PAU is formed by atheromatous plaque disruption in the internal elastic lamina. Previous studies have shown that hypertension, hyperlipidemia, and severe atherosclerosis are risk factors for PAUs. 7 , 20 , 21 , 22 In a study by Cho et al, 23 92.38% and 45.71% of the patients had hypertension and coronary artery disease, respectively. Botta et al 16 reported that the incidence of hypertension was 84.2%. In a study by Chou et al, 17 the incidences of hypertension, dyslipidemia, and coronary artery diseases were 86.11%, 55.56%, and 33.33%, respectively. Consistently, the results from the present study showed that the incidences of systemic hypertension, dyslipidemia, and coronary artery diseases were 80.73%, 33.03%, and 33.94%, respectively. Based on this analysis, for patients with acute PAUs with hypertension and coronary heart disease, we suggest that monitoring blood pressure and the levels of blood lipids and providing accurate medication guidance and health education may reduce the incidence of aortic‐related adverse events.
In the present study, early intervention treatment may be recommended for patients with acute PAUs with diameters >12.5 mm or depths >9.5 mm. Conversely, for patients with acute PAUs, controlling blood pressure and blood lipid levels while undergoing regular imaging reexaminations in accordance with the follow‐up time is suggested. To date, the treatment strategy for PAUs are ambiguous in the European and American guidelines, which do not have a criterion for interventional therapy. In the 2014 ESC guidelines7, medical treatment is recommended for all type B PAUs (Ⅰ C), and repetitive imaging is indicated for uncomplicated type B PAU (Ⅰ C). EVAR (Ⅱ a C) or surgery (Ⅱ b C) may be considered for complicated type B PAUs. In the 2010 ACCF guidelines6, EVAR is recommended for symptomatic PAU (II b C). Thus, the findings of and evidence from the present study may provide a reference for the diagnosis, treatment and long‐term prognosis of acute PAU in the Chinese population.
Limitations
The present study was a retrospective observational study with inherent shortcomings. Because most of the patients with acute PAUs were asymptomatic during long‐term follow‐up, patient compliance was poor, which may have led to an underestimation of the incidence of aortic‐related adverse events in acute PAUs. Most of the patients in the present study had thoracic PAUs, so we have not studied whether the location of ulcer affects the prognosis. In a future study, we will expand the sample size to specifically explore the effect of ulcer location on prognosis. The cutoff value in the present study needs to be validated in large samples. Therefore, a large‐scale, randomized control trial is required.
CONCLUSIONS
In the context of acute PAU, patients with aortic‐related adverse events and clinically related adverse events are at high risk within 30 days after onset. Patients with an ulcer diameter >12.5 mm or an ulcer depth >9.5 mm have a higher risk for disease progression, and early intervention may be recommended.
Sources of Funding
None.
Disclosures
The authors declare that there is no conflict of interest.
Supporting information
Table S1
Figure S1
Acknowledgments
We are grateful to the subjects who participated in the study and for the physician assistance in this study.
(J Am Heart Assoc. 2020;9:e014505 DOI: 10.1161/JAHA.119.014505.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.119.014505
For Sources of Funding and Disclosures, see page 9.
References
- 1. Wada H, Sakata N, Tashiro T. Clinicopathological study on penetrating atherosclerotic ulcers and aortic dissection: distinct pattern of development of initial event. Heart Vessels. 2016;1855–1861. [DOI] [PubMed] [Google Scholar]
- 2. Nathan DP, Boonn W, Lai E, Grace JW, Nimesh D, Edward YW, Ronald MF, Benjamin MJ. Presentation, complications, and natural history of penetrating atherosclerotic ulcer disease. J Vasc Surg. 2012;10–15. [DOI] [PubMed] [Google Scholar]
- 3. Mousa AY, Bozzay J, AbuRahma AF. Natural history and outcome of patients with intramural hematomas and penetrating aortic ulcers. Vascular. 2015;305–309. [DOI] [PubMed] [Google Scholar]
- 4. Patatas K, Shrivastava V, Ettles DF. Penetrating atherosclerotic ulcer of the aorta: a continuing debate. Clin Radiol. 2013;753–759. [DOI] [PubMed] [Google Scholar]
- 5. Movsowitz HD, Lampert C, Jacobs LE, Kotler MN. Penetrating atherosclerotic aortic ulcers. Am Heart J. 1994;1210–1217. [DOI] [PubMed] [Google Scholar]
- 6. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;e27–e129. [DOI] [PubMed] [Google Scholar]
- 7. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;2873–2926. [DOI] [PubMed] [Google Scholar]
- 8. Bonaca MP, O'Gara PT. Diagnosis and management of acute aortic syndromes: dissection, intramural hematoma, and penetrating aortic ulcer. Curr Cardiol Rep. 2014;536. [DOI] [PubMed] [Google Scholar]
- 9. Janosi RA, Gorla R, Tsagakis K, Kahlert P, Horacek M, Bruckschen F, Dohle DS, Jakob H, Schlosser T, Eggebrecht H, et al. Thoracic endovascular repair of complicated penetrating aortic ulcer: an 11‐year single‐center experience. J Endovasc Ther. 2016;150–159. [DOI] [PubMed] [Google Scholar]
- 10. Ganaha F, Miller DC, Sugimoto K, Do YS, Minamiguchi H, Saito H, Mitchell RS, Dake MD. Prognosis of aortic intramural hematoma with and without penetrating atherosclerotic ulcer: a clinical and radiological analysis. Circulation. 2002;342–348. [DOI] [PubMed] [Google Scholar]
- 11. Tittle SL, Lynch RJ, Cole PE, Singh HS, Rizzo JA, Kopf GS, Elefteriades JA. Midterm follow‐up of penetrating ulcer and intramural hematoma of the aorta. J Thorac Cardiovasc Surg. 2002;1051–1059. [DOI] [PubMed] [Google Scholar]
- 12. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;2736–2747. [DOI] [PubMed] [Google Scholar]
- 13. Lovy AJ, Rosenblum JK, Levsky JM, Godelman A, Zalta B, Jain VR, Haramati LB. Acute aortic syndromes: a second look at dual‐phase CT. AJR Am J Roentgenol. 2013;805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El Hassani I, Van Damme H, Creemers E, Boesmans E, Defraigne JO. Penetrating atherosclerosis aortic ulcer: a re‐appraisal. Acta Chir Belg. 2017;1–7. [DOI] [PubMed] [Google Scholar]
- 15. Han YL, Zhang QY, Li Y, Guan SY, Jing QM, Wang ZL, Zhao X, Wang XZ, Ma YY, Wang B, et al. Clinical presentations, antiplatelet strategies and prognosis of patients with stent thrombosis: an observational study of 140 patients. PLoS One. 2012;e48520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Botta L, Buttazzi K, Russo V, Parlapiano M, Gostoli V, Di Bartolomeo R, Fattori R. Endovascular repair for penetrating atherosclerotic ulcers of the descending thoracic aorta: early and mid‐term results. Ann Thorac Surg. 2008;987–992. [DOI] [PubMed] [Google Scholar]
- 17. Chou AS, Ziganshin BA, Charilaou P, Tranquilli M, Rizzo JA, Elefteriades JA. Long‐term behavior of aortic intramural hematomas and penetrating ulcers. J Thorac Cardiovasc Surg. 2016;361–373. [DOI] [PubMed] [Google Scholar]
- 18. Risch N, Burchard E, Ziv E, Tang H. Categorization of humans in biomedical research: genes, race and disease. Genome Biol. 2002;comment2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stanson AW, Kazmier FJ, Hollier LH, Edwards WD, Pairolero PC, Sheedy PF, Joyce JW, Johnson MC. Penetrating atherosclerotic ulcers of the thoracic aorta: natural history and clinicopathologic correlations. Ann Vasc Surg. 1986;15–23. [DOI] [PubMed] [Google Scholar]
- 20. Taniguchi I, Morimoto K, Miyasaka S, Marumoto A, Aoki T. Penetrating atherosclerotic ulcer in the juxtarenal abdominal aorta and coronary artery disease: emergency one‐stage repair with off‐pump coronary surgery. Jpn J Thorac Cardiovasc Surg. 2005;505–509. [DOI] [PubMed] [Google Scholar]
- 21. Harris JA, Bis KG, Glover JL, Bendick PJ, Shetty A, Brown OW. Penetrating atherosclerotic ulcers of the aorta. J Vasc Surg. 1994;90–99. [DOI] [PubMed] [Google Scholar]
- 22. Coady MA, Rizzo JA, Hammond GL, Pierce JG, Kopf GS, Elefteriades JA. Penetrating ulcer of the thoracic aorta: what is it? How do we recognize it? How do we manage it? J Vasc Surg. 1998;1006–1015; discussion 1015–1016. [DOI] [PubMed] [Google Scholar]
- 23. Cho KR, Stanson AW, Potter DD, Cherry KJ, Schaff HV, Sundt TM. Penetrating atherosclerotic ulcer of the descending thoracic aorta and arch. J Thorac Cardiovasc Surg. 2004;1393–1399; discussion 1399–1401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1


