Abstract
Background
Recent investigations suggest that inflammation and autoimmunity might have a role in the pathophysiology of atrial fibrillation (AF). Given that abnormal ventriculovascular coupling often coexists with AF, we hypothesize that autoimmune vasculitis plays a significant role in the pathogenetic mechanism of AF.
Methods and Results
A standardized retrospective population‐based case–control study was conducted to evaluate the association between autoimmune vasculitis and AF, and all‐cause mortality. The study included 8459 patients with a new diagnosis of AF and 8459 age‐, sex‐, and registration calendar year–matched controls in Olmsted County, Minnesota, between January 1, 1980 and December 31, 2010. The association of each clinical characteristic, diagnosis, and treatment was assessed using conditional logistic regression to account for the matched case–control study design. Cox proportional hazards regression models and Kaplan‐Meier curves were used to detect independent predictors of mortality and examine cumulative survival. Of a total of 16 918 patients (mean age 72.3+14.4 years; 48.7% women), 320 (1.9%) were diagnosed with autoimmune vasculitis before the index date during the 30‐year period. Among the cases, the prevalence of any autoimmune vasculitis was 2.3%, whereas the frequency of autoimmune vasculitis in controls was 1.5% (P<0.001). After adjusting for potential confounders, the odds of autoimmune vasculitis in AF cases was 1.5 times higher than in controls (odds ratio, 1.47; 95% CI, 1.04–2.01; P=0.03). Patients with AF and autoimmune vasculitis had worse 5‐year survival than those without autoimmune vasculitis or AF (44.7% versus 77.2%; log‐rank P<0.001).
Conclusions
Autoimmune vasculitis is significantly associated with AF and independently confers worse survival. These observations may represent one mechanism linking autoimmunity and inflammation to the pathogenesis and prognosis of AF.
Keywords: arrhythmia, atrial fibrillation, outcome, survival, vasculitis
Subject Categories: Atrial Fibrillation, Inflammation, Mechanisms, Pathophysiology, Mortality/Survival
Clinical Perspective
What Is New?
This large‐scale population‐based study is the first to analyze the role of autoimmune vasculitis in the development and prognosis of atrial fibrillation (AF).
In this study, autoimmune vasculitis was significantly associated with incident AF cases compared with non‐AF controls.
AF was more strongly associated with overall mortality in the presence than the absence of autoimmune vasculitis.
What Are the Clinical Implications?
Our findings suggest that a preexisting autoimmune process may be a potential mechanistic link to the development of new‐onset AF.
Further research is needed to elucidate the underlying pathogenetic mechanisms that link these conditions.
Atrial fibrillation (AF) is an alarmingly growing epidemic and contributes to substantial morbidity and mortality. 1 , 2 Although many risk factors for AF have been identified, its underlying pathogenic mechanisms remain elusive. The public health burden of AF, combined with significant gaps in our knowledge about its pathogenesis and the lack of effective treatment, underscores the critical need for new research.
Emerging evidence increasingly suggests an association between autoimmune disorders and AF. 3 , 4 Anti–M2 receptor, 5 anti–B‐adrenergic receptor, 6 anti–heat shock protein, 7 anti–Na+/K+ pump, 8 and antimyosin autoantibodies 9 have been shown to be implicated in AF. 10 , 11 , 12 These autoantibodies can induce inflammation and atrial fibrosis, 3 , 13 predisposing the atrial myocardium to the development of AF 14 and suggesting that autoimmunity may be an important mechanism in AF pathogenesis. However, data on the association of autoimmune diseases with AF remain limited.
Although the pathogenesis and maintenance of AF may be multifactorial, abnormal ventriculovascular homeostatic mechanisms may also play a primary role in the development of AF by increasing ventricular filling pressures and, consequently, left atrial pressure and stretch. 15 , 16 , 17 A previous study from the Framingham Heart Study Offspring cohort examined the relationship between new‐onset AF and tonometry measures of arterial stiffness. Results showed that higher pulsatile load assessed by central pulse pressure and greater apparent wave reflection were associated with increased risk of incident AF. These noninvasive measures, which are known to predispose to left ventricular hypertrophy, diastolic heart failure, and left atrial enlargement, may be intermediate phenotypes of underlying cardiovascular disease associated with increased risk of incident AF. 16 , 18 , 19 In this study, we sought to determine the role of autoimmune vasculitis in the development and prognosis of AF using a population‐based approach. We hypothesize that autoimmune vasculitis, a systemic disease characterized by inflammation and fibrinous necrosis of blood vessel walls, 20 is significantly associated with incident AF.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request. The study was approved by the Mayo Clinic and Olmsted Medical Center institutional review boards. The requirement of informed consent was waived for the retrospective review of medical records.
Study Design
A case–control study of risk factors for incident AF was conducted to determine the prevalence of autoimmune vasculitis and the associated impact on all‐cause mortality in patients with AF in the community compared with age‐ and sex‐matched controls.
Data Source
The study was conducted in Olmsted County, Minnesota, which had an estimated population of 144 248 in 2010, 85.7% of which was White. Extensive details about the Olmsted County population have been reported elsewhere. 21
Study Cohort
From the Rochester Epidemiology Project database of residents of Olmsted County, Minnesota, we identified all incident cases of AF through linkage of study‐cohort data from January 1, 1980 to December 31, 2010. For each of the 8459 identified residents with AF (cases), we randomly selected 1 resident without AF (control) on the basis of age ±2 years, sex, and calendar year (±2 years) of Olmsted County residency at the index date (ie, AF diagnosis date) to ensure similar length of medical history for cases and controls.
Covariate and Exposure Data Collection
Trained abstractors collected clinical and laboratory data before the index date from the medical record of each patient for both cases and controls. All incident AF cases were verified through direct examination of the ECG, Holter monitor, event monitor, or cardiac implantable electronic devices. Patients were not included if they had transient new‐onset postoperative or postprocedure AF without recurrence of AF after 30 days from the index operation or procedure and if they had atrial flutter alone without evidence of AF.
Definition of Vasculitis
All cases of prevalent autoimmune vasculitis were confirmed through chart review of clinical data, laboratory, histologic, and radiologic reports based on established diagnostic criteria. 22 The following classification of primary vasculitis was defined on the basis of size of the affected blood vessel: 22 , 23
Large‐vessel vasculitis
Giant cell arteritis
Takayasu arteritis
Medium‐vessel vasculitis
Polyarteritis nodosa
Kawasaki disease
Small‐vessel vasculitis
Antineutrophil cytoplasmic antibody –associated vasculitis (granulomatosis with polyangiitis [Wegener granulomatosis], eosinophilic granulomatosis with polyangiitis [Churg‐Strauss syndrome], microscopic polyangiitis)
Henoch‐Schönlein purpura
Cutaneous leukocytoclastic angiitis
Cryoglobulinemic vasculitis
Echocardiography
Baseline echocardiographic parameters from cases and controls were analyzed. Echocardiographic measurements analyzed included conventional measures of systolic function (left ventricular ejection fraction and cardiac index) and diastolic function (left atrial volume index, mitral early diastolic velocity [E wave], mitral peak late diastolic velocity [A wave], E‐wave deceleration time by pulsed Doppler, mitral annular diastolic velocity (e′), E/e′ ratio, left ventricular mass index, and right ventricular systolic pressure).
Statistical Analysis
We examined study demographics for both cases and controls using descriptive statistics. Continuous data were expressed as mean±SD or median (interquartile range) for skewed data and compared using the Student t test following testing for normality of distribution, as appropriate, using the Shapiro‐Wilk test or Wilcoxon rank sum test for nonnormally distributed variables. Categorical variables were presented as counts with percentages and compared using the χ2 or Fisher exact test, as indicated. The association between autoimmune vasculitis diagnosed before the index date and incident AF was evaluated using conditional logistic regression models and summarized with odds ratios (OR) and 95% CIs. Adjustment was made to control for simultaneous and interactive effects of potential confounders (covariates) not used in matching if their effects modified the OR associated with AF by at least 5% on univariate analysis. 24 Cox proportional hazards regression models were used to test for the association of autoimmune vasculitis with the hazard of all‐cause mortality and to estimate the corresponding relative hazard ratio (HR) and 95% CI for each variable. Because the relationships between age and AF and death may be nonlinear, we also included a quadratic term for age in the models, with adjustment for other potential confounders. Because the quadratic term was not statistically significant (P>0.05), we used simpler models with the original scale of age in our analyses. Kaplan‐Meier survival analyses with log‐rank tests were used to compare time‐to‐event event‐free survival on the basis of all available follow‐up data among patients with autoimmune vasculitis and concurrent AF, autoimmune vasculitis and no AF, and no autoimmune vasculitis and coexisting AF, versus those without autoimmune vasculitis or AF. For associations, we used a 2‐sided test of significance at the P<0.05 level. All statistical analyses were performed using SAS v9.4 (SAS Institute).
Results
Baseline Characteristics
In total, 16 918 participants were identified for the study, including 8459 patients with AF and 8459 controls without AF. The baseline demographics and comorbidities of the case and control cohort were compared and demonstrated some differences, which are summarized in Table 1. Participants with AF had a significantly higher burden of cardiovascular disease risk factors, including higher body mass index and greater frequency of smoking, hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, myocardial infarction, congestive heart failure, stroke, sleep apnea, chronic lung disease, and chronic kidney disease. They also had higher prevalence of autoimmune vasculitis (2.3% versus 1.5%; P<0.001) and baseline levels of systemic inflammatory markers (white blood cell count, 9.2±5.7 versus 7.4±3.4×10(9)/L [P<0.001]; median CRP (C‐reactive protein), 20.8 versus 9.5, mg/L [P=0.01]) than control participants without AF.
Table 1.
Baseline Characteristics of the Study Population
| Baseline Variable | Cases (AF) | Controls (Non‐AF) | P Value |
|---|---|---|---|
| Participants, n | 8459 | 8459 | |
| Demographics | |||
| Age, y, mean±SD | 72.4±14.4 | 72.3±14.4 | 0.75 |
| Female sex, n (%) | 4122 (48.7) | 4120 (48.7) | 0.99 |
| Body mass index, kg/m2, mean±SD | 28.0±6.7 | 27.0±5.4 | <0.001 |
| Hemodynamics, mean±SD | |||
| Heart rate, bpm | 111.8±32.2 | 73.3±13.9 | <0.001 |
| Pulse pressure, mm Hg | 59.3±18.4 | 62.4±18.7 | <0.001 |
| Mean arterial pressure, mm Hg | 94.3±14.8 | 93.9±14.1 | 0.13 |
| History variables, n (%) | |||
| Hypertension | 5896 (69.7) | 3813 (45.1) | <0.001 |
| Diabetes mellitus | 1883 (22.3) | 1039 (12.3) | <0.001 |
| Dyslipidemia | 3895 (46.1) | 2756 (32.6) | <0.001 |
| Congestive heart failure | 2249 (26.6) | 486 (5.8) | <0.001 |
| Cardiomyopathy | 890 (10.5) | 208 (2.3) | <0.001 |
| Coronary artery disease | 3191 (37.7) | 1552 (18.4) | <0.001 |
| Previous myocardial infarction | 2061 (24.4) | 572 (6.8) | <0.001 |
| Stroke or transient ischemic attack | 1835 (21.7) | 876 (10.4) | <0.001 |
| Obstructive sleep apnea | 1411 (16.7) | 807 (9.6) | <0.001 |
| Smoking status (past or present) | 3177 (37.6) | 588 (7.0) | <0.001 |
| Chronic lung disease | 2780 (32.9) | 1545 (18.3) | <0.001 |
| Peripheral arterial disease | 1460 (17.3) | 736 (8.7) | <0.001 |
| Chronic renal disease | 2130 (25.2) | 705 (8.3) | <0.001 |
| Thyroid disease | 899 (10.6) | 749 (8.9) | <0.001 |
| Autoimmune vasculitis, n (%) | |||
| Any vasculitis | 191 (2.3) | 129 (1.5) | <0.001 |
| Small vessel | 50 (0.59) | 17 (0.20) | <0.001 |
| Medium vessel | 5 (0.06) | 4 (0.05) | 1.00 |
| Large vessel | 99 (1.2) | 83 (0.98) | 0.26 |
| Unclassified | 37 (0.44) | 25 (0.35) | 0.16 |
| Laboratory data | |||
| Thyroid‐stimulating hormone, MIU/L, mean±SD | 2.7±3.6 | 3.0±8.7 | 0.14 |
| White blood cell count, ×10(9)/L, mean±SD | 9.2±5.7 | 7.4±3.4 | <0.001 |
| Sedimentation rate, mm/h, mean±SD | 21.3±9.3 | 23.6±13.8 | 0.73 |
| CRP, mg/L, median (IQR) | 20.8 (7.8–90) | 9.5 (4.9–45.4) | 0.01 |
| Creatinine, mg/dL, mean±SD | 1.3±0.9 | 1.1±0.5 | <0.001 |
| BNP, pg/mL, median (IQR) | 394 (192–736) | 159 (59–403) | <0.001 |
| NT‐pro‐BNP, pg/mL, median (IQR) | 2078 (699–4840) | 871 (228–5976) | 0.76 |
| Echocardiography, mean±SD | |||
| LA volume index, mL/m2 | 43.4±16.3 | 35.9±12.7 | <0.001 |
| Mitral E/e’ ratio | 14.2±7.3 | 13.3±6.5 | <0.001 |
| Cardiac index, L/min/m2 | 2.9±0.9 | 3.1±0.9 | <0.001 |
| Right ventricular systolic pressure, mm Hg | 41.6±13.8 | 38.1±12.1 | <0.001 |
| Right atrial pressure, mm Hg | 9.3±4.7 | 7.3±3.6 | <0.001 |
| LV ejection fraction, % | 54.4±14.7 | 58.5±12.3 | <0.001 |
| LV mass index, g/m2 | 103.6±32.3 | 99.1±28.6 | <0.001 |
AF indicates atrial fibrillation; BNP, brain natriuretic peptide; CRP, C‐reactive protein; IQR, interquartile range; LA, left atrial; LV, left ventricular; and NT‐pro‐BNP, N‐terminal pro‐B‐type natriuretic peptide.
Association of Autoimmune Vasculitis and Incident AF
Results of the univariate and multivariable conditional logistic regression analyses of factors predictive of incident AF after autoimmune vasculitis exposure are presented in Table 2. The odds of an AF diagnosis after autoimmune vasculitis exposure were greatest for small‐vessel vasculitis (unadjusted OR, 2.94; 95% CI, 1.70–5.01) and lowest for large‐vessel vasculitis (unadjusted OR, 1.20; 95% CI, 0.89–1.60). The results of multivariable logistic regression analysis showed that autoimmune vasculitis was independently predictive of the development of AF (OR, 1.47; 95% CI, 1.04–1.94) (Table 3, model 1). When stratified by vasculitis subtype, the impact of autoimmune vasculitis on the development of incident AF appears to be driven predominantly by small‐vessel vasculitis (OR, 2.54; 95% CI, 1.15–5.57) (Table 3, model 2). For these analyses, all factors shown in the table with P<0.05 were initially included in the model as potential risk factors. Factors for which data were missing (eg, white blood cell count and creatinine and echocardiographic data) and that were not selected by the stepwise procedure were subsequently omitted, and the stepwise procedure was repeated to obtain the final model. The C statistics, a measure of concordance between the model‐based risk estimates and observed AF events, for models 1 and 2 were 0.808 and 0.808, respectively, indicating very good discriminating ability for predicting AF.
Table 2.
Univariate Regression Analysis for the Prediction of Atrial Fibrillation (Logistic) and Death (Cox)
| Baseline Variable | AF | P Value | Death | P Value |
|---|---|---|---|---|
| OR (95% CI) | HR (95% CI) | |||
| Demographics | ||||
| Age (per 5‐y increment) | 1.02 (1.01–1.03) | 0.004 | 1.46 (1.44–1.47) | <0.001 |
| Female sex | 1.04 (0.98–1.11) | 0.17 | 1.35 (1.30–1.40) | <0.001 |
| Body mass index (per 5‐U increment) | 1.15 (1.12–1.19) | <0.001 | 0.87 (0.85–0.89) | <0.001 |
| Hemodynamics | ||||
| Heart rate (per 5‐U increment) | 1.39 (1.37–1.41) | <0.001 | 1.03 (1.02–1.04) | <0.001 |
| Mean arterial pressure (per 5‐U increment) | 1.01 (1.00–1.02) | 0.14 | 1.00 (0.99–1.01) | 0.83 |
| Pulse pressure (per 5‐U increment) | 0.96 (0.95–0.97) | <0.001 | 1.06 (1.05–1.07) | <0.001 |
| Arterial elasticity (per 5‐U increment) | 0.98 (0.97–0.99) | <0.001 | 1.03 (1.02–1.04) | <0.001 |
| History variables | ||||
| Incident atrial fibrillation | … | … | 1.76 (1.69–1.82) | <0.001 |
| Hypertension | 3.45 (3.19–3.72) | <0.001 | 1.72 (1.65–1.78) | <0.001 |
| Diabetes mellitus | 1.87 (1.70–2.04) | <0.001 | 1.54 (1.47–1.61) | <0.001 |
| Dyslipidemia | 2.12 (1.97–2.29) | <0.001 | 0.90 (0.86–0.93) | <0.001 |
| Congestive heart failure | 6.77 (6.01–7.64) | <0.001 | 3.02 (2.89–3.16) | <0.001 |
| Cardiomyopathy | 4.67 (4.00–5.49) | <0.001 | 1.91 (1.78–2.04) | <0.001 |
| Coronary artery disease | 2.96 (2.73–3.21) | <0.001 | 1.78 (1.71–1.86) | <0.001 |
| Previous myocardial infarction | 4.75 (4.25–5.27) | <0.001 | 2.10 (2.01–2.20) | <0.001 |
| Stroke or transient ischemic attack | 2.45 (2.23–2.69) | <0.001 | 2.30 (2.20–2.41) | <0.001 |
| Obstructive sleep apnea | 2.06 (1.86–2.28) | <0.001 | 1.06 (1.00–1.12) | 0.04 |
| Smoking status (past or present) | 5.44 (4.95–5.97) | <0.001 | 1.24 (1.21–1.28) | <0.001 |
| Chronic lung disease | 2.22 (2.06–2.40) | <0.001 | 1.74 (1.67–1.81) | <0.001 |
| Peripheral arterial disease | 2.19 (1.97–2.42) | <0.001 | 2.12 (2.01–2.23) | <0.001 |
| Chronic renal disease | 4.12 (3.71–4.58) | <0.001 | 2.34 (2.23–2.45) | <0.001 |
| Thyroid disease | 1.25 (1.12–1.40) | <0.001 | 1.28 (1.21–1.37) | <0.001 |
| Autoimmune vasculitis | ||||
| Any vasculitis | 1.52 (1.20–1.90) | <0.001 | 1.72 (1.54–2.03) | <0.001 |
| Small vessel | 2.94 (1.70–5.01) | <0.001 | 1.71 (1.32–2.21) | <0.001 |
| Medium vessel | 1.25 (0.34–4.66) | 0.74 | 1.18 (0.56–2.47) | 0.67 |
| Large vessel | 1.20 (0.89–1.60) | 0.23 | 1.62 (1.39–1.89) | <0.001 |
| Unclassified | 1.48 (0.89–2.46) | 0.13 | 2.69 (1.69–2.80) | <0.001 |
| Laboratory data | ||||
| Thyroid stimulating hormone | 0.99 (0.98–1.01) | 0.33 | 1.00 (0.99–1.00) | 0.18 |
| White blood cell count | 1.14 (1.13–1.16) | <0.001 | 1.03 (1.02–1.04) | <0.001 |
| Sedimentation rate | 0.98 (0.87–1.10) | 0.71 | 0.99 (0.93–1.06) | 0.80 |
| CRP | 1.01 (0.99–1.03) | 0.33 | 1.00 (0.99–1.02) | 0.07 |
| Creatinine | 1.63 (1.48–1.80) | <0.001 | 1.29 (1.27–1.32) | <0.001 |
| BNP | 1.01 (1.01–1.03) | <0.001 | 1.06 (1.05–1.07) | <0.001 |
| NT‐pro‐BNP | 1.00 (1.00–1.01) | 0.54 | 1.05 (1.03–1.06) | <0.001 |
| Echocardiography | ||||
| LA volume index | 1.05 (1.04–1.06) | <0.001 | 1.02 1.02–1.03) | <0.001 |
| Mitral E/e’ ratio | 1.02 (1.01–1.03) | <0.001 | 1.06 (1.05–1.07) | <0.001 |
| Cardiac index | 0.80 (0.75–0.85) | <0.001 | 1.01 (0.98–1.05) | 0.46 |
| Right ventricular systolic pressure | 1.03 (1.03–1.04) | <0.001 | 1.04 (1.03–1.04) | <0.001 |
| Right atrial pressure, mm Hg | 1.18 (1.15–1.20) | <0.001 | 1.10 (1.10–1.11) | <0.001 |
| LV ejection fraction (per 5‐U increment) | 0.89 (0.87–0.90) | <0.001 | 0.91 (0.90–0.92) | <0.001 |
| LV mass index (per 5‐U increment) | 1.03 (1.02–1.04) | <0.001 | 1.04 (1.03–1.05) | <0.001 |
AF indicates atrial fibrillation; BNP, brain natriuretic peptide; CRP, C‐reactive protein; HR, hazard ratio; LA, left atrial; LV, left ventricular; NT‐pro‐BNP, N‐terminal pro‐B‐type natriuretic peptide; and OR, odds ratio.
Table 3.
Multivariable Conditional Logistic Regression Analysis for the Association Between Autoimmune Vasculitis and AF
| Covariates | AF, Model 1 | AF, Model 2 | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Participants, n | 16 912 | 16 912 | ||
| Any vasculitis | 1.47 (1.04–1.94) | 0.03 | ||
| Small vessel | 2.54 (1.15–5.57) | 0.02 | ||
| Medium vessel | 1.47 (0.23–4.32) | 0.68 | ||
| Large vessel | 1.20 (0.78–1.85) | 0.41 | ||
| Unclassified | 1.39 (0.68–2.83) | 0.37 | ||
| Age (per 5‐y increment) | 1.25 (1.15–1.35) | <0.001 | 1.25 (1.15–1.35) | <0.001 |
| Hypertension | 2.25 (2.04–2.49) | <0.001 | 2.25 (2.04–2.49) | <0.001 |
| Dyslipidemia | 1.14 (1.04–1.26) | 0.008 | 1.14 (1.04–1.26) | 0.008 |
| Congestive heart failure | 3.67 (3.17–4.25) | <0.001 | 3.67 (3.16–4.24) | <0.001 |
| Previous myocardial infarction | 2.12 (1.84–2.43) | <0.001 | 2.11 (1.84–2.43) | <0.001 |
| Stroke or transient ischemic attack | 1.57 (1.39–1.77) | <0.001 | 1.57 (1.39–1.77) | <0.001 |
| Obstructive sleep apnea | 1.27 (1.11–1.44) | <0.001 | 1.27 (1.12–1.44) | <0.001 |
| Smoking status (past or present) | 4.71 (4.25–5.22) | <0.001 | 4.71 (4.26–5.23) | <0.001 |
| Chronic lung disease | 1.17 (1.06–1.30) | 0.003 | 1.17 (1.06–1.30) | 0.003 |
| Chronic renal disease | 2.06 (1.82–2.34) | <0.001 | 2.05 (1.81–2.33) | <0.001 |
| C‐statistic | 0.808 | 0.808 | ||
AF indicates atrial fibrillation; and OR, odds ratio.
Association of Autoimmune Vasculitis and Overall Survival
During a median follow‐up period of 7.4 years, 11 450 (72.3%) patients died.
Kaplan–Meier survival analysis showed that individuals with autoimmune vasculitis had lower overall survival than those without autoimmune vasculitis, regardless of associated AF status (log‐rank P<0.001). Those with autoimmune vasculitis diagnosed before the index date who subsequently developed AF had worse 5‐year survival rates than those without autoimmune vasculitis who did not develop AF (44.7% versus 77.2%, log‐rank P<0.001) (Figure 1).
Figure 1.
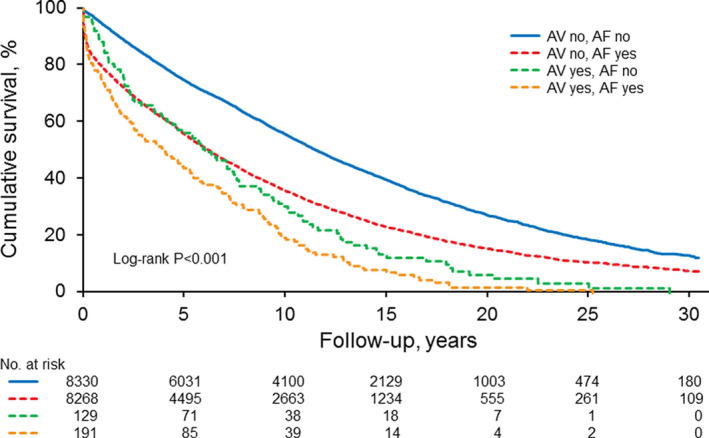
Kaplan‐Meier survival analysis stratified by autoimmune vasculitis and concurrent AF, autoimmune vasculitis and no AF, no autoimmune vasculitis and coexisting AF, and no autoimmune vasculitis or AF.
AF indicates atrial fibrillation; AV, autoimmune vasculitis; and LV, left ventricular.
In multivariable Cox regression analysis, autoimmune vasculitis was an independent predictor of shorter survival (HR, 1.20; 95% CI, 1.06–1.32) (Table 4, model 1). When stratified by vasculitis subtypes, the association of autoimmune vasculitis and overall risk of mortality was worse among patients with small‐vessel vasculitis (HR, 1.38; 95% CI, 1.08–1.75) and unclassified vasculitis (HR, 1.55; 95% CI, 1.22–1.97), respectively, than among those with other subtypes (Table 4, model 2). Other independent risk factors associated with increased mortality were incident AF, age, male sex, and prior history of hypertension, diabetes mellitus, dyslipidemia, congestive heart failure, myocardial infarction, stroke, smoking, chronic lung disease, peripheral arterial disease, or chronic renal disease.
Table 4.
Multivariable Cox Regression Analysis for the Association Between Autoimmune Vasculitis and All‐cause Mortality
| Baseline Variable | Death, Model 1 | P Value | Death, Model 2 | P Value |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Participants, n | 16 912 | 16 912 | ||
| Any vasculitis | 1.20 (1.06–1.32) | .003 | ||
| Small vessel | 1.38 (1.08–1.75) | 0.009 | ||
| Medium vessel | 1.41 (0.73–2.72) | 0.31 | ||
| Large vessel | 1.04 (0.89–1.22) | 0.95 | ||
| Unclassified | 1.55 (1.22–1.97) | <0.001 | ||
| Incident AF | 1.46 (1.40–1.53) | <0.001 | 1.46 (1.40–1.53) | <0.001 |
| Age (per 5‐y increment) | 1.52 (1.51–1.54) | <0.001 | 1.52 (1.51–1.54) | <0.001 |
| Male sex | 1.16 (1.11–1.20) | <0.001 | 1.16 (1.11–1.20) | <0.001 |
| Hypertension | 1.04 (1.02–1.10) | 0.04 | 1.04 (1.03–1.10) | 0.04 |
| Diabetes mellitus | 1.36 (1.30–1.43) | <0.001 | 1.36 (1.30–1.43) | <0.001 |
| Dyslipidemia | 0.71 (0.70–0.74) | <0.001 | 0.71 (0.70–0.74) | <0.001 |
| Congestive heart failure | 1.48 (1.41–1.56) | <0.001 | 1.48 (1.41–1.56) | <0.001 |
| Previous myocardial infarction | 1.26 (1.20–1.32) | <0.001 | 1.26 (1.20–1.32) | <0.001 |
| Stroke or transient ischemic attack | 1.31 (1.25–1.37) | <0.001 | 1.31 (1.25–1.37) | <0.001 |
| Smoking status (past or present) | 1.14 (1.11–1.17) | <0.001 | 1.14 (1.11–1.17) | <0.001 |
| Chronic lung disease | 1.32 (1.27–1.37) | <0.001 | 1.32 (1.27–1.37) | <0.001 |
| Peripheral arterial disease | 1.26 (1.20–1.33) | <0.001 | 1.26 (1.20–1.33) | <0.001 |
| Chronic renal disease | 1.44 (1.37–1.51) | <0.001 | 1.44 (1.37–1.51) | <0.001 |
AF indicates atrial fibrillation; and HR, hazard ratio.
Discussion
Principal Findings
In this population‐based case–control study of patients with incident AF compared with non‐AF controls, we observed that autoimmune vasculitis was significantly associated with incident AF cases compared with non‐AF controls. The odds of AF development were 50% greater in patients with a prior diagnosis of autoimmune vasculitis, suggesting that autoimmunity may confer an increased risk of incident AF that is independent of age, sex, and cardiovascular disease risk factors. We demonstrated that a preexisting inflammatory and autoimmune process contributes to the development of new‐onset AF and a potential mechanistic link between autoimmune disease and AF development. Finally, AF was more strongly associated with overall mortality in the presence than the absence of autoimmune vasculitis.
Comparison With Previous Studies and Potential Mechanisms
Role of Inflammation
To our knowledge, our study is the first to examine the association between autoimmune vasculitis and AF development. It is known that both conditions share many risk factors and are associated with increased levels of proinflammatory markers. 14 , 25 Autoimmune vasculitis is an autoimmune disease characterized by the presence of inflammation within blood vessel walls, with subsequent vascular damage and end‐organ ischemia. Several experimental and clinical studies have shown evidence of increased vascular inflammation in patients with AF. In atrial biopsies of patients with lone AF, Frustaci et al 13 reported infiltration of lymphomononuclear cells with concomitant focal necrosis of adjacent myocytes compatible with atrial myocarditis in 66% of patients; this infiltration was absent from the atria of patients in sinus rhythm. A number of case‐controlled and large population‐based studies have reported that chronic inflammation based on elevated baseline CRP levels, which can alter electrophysiologic and structural substrates in the atrial myocardium, 26 , 27 , 28 , 29 , 30 can predict the development and persistence of new‐onset AF. 31 In agreement with these prior studies, our study also found that patients with AF had significantly higher baseline systemic inflammatory markers, including white blood cell count and CRP, and larger left atrial dimension than control participants without AF in sinus rhythm. This finding is consistent with prior evidence that a preexisting persistent inflammatory state can promote the development of AF.
Role of Autoimmunity
We and others have postulated that the inflammation in AF may be associated with an autoimmune response. 32 , 33 , 34 , 35 Our findings support the hypothesis that autoimmune disease may precede AF development, which is likely mediated by inflammation.
Current epidemiologic evidence also suggests that autoimmunity is an important mediator in AF development. Several population‐based studies have shown that the risk of AF is significantly increased in autoimmune‐mediated diseases such as celiac disease 36 and psoriasis, 37 indicating a role for antibody‐mediated immune response in promoting the development of AF. To date, at least 4 types of autoantibodies to key mediators of atrial electrophysiology—antimyosin heavy chain, 12 antimuscarinic acetylcholine receptor, 10 anti–β‐1‐adrenergic receptor, 6 and anti–heat shock protein 7 —have been identified as significantly more prevalent in the sera of patients with established AF compared with those without AF, regardless of underlying heart disease. 5 , 6 , 7 , 9 These autoantibodies can trigger second‐messenger production and induce inflammation and atrial fibrosis, predisposing the atrial myocardium to the development of AF.
Role of Microvascular Disease
Alteration in microvascular circulation is another possible hemodynamic mechanism linking autoimmune vasculitis to AF. In our study, small‐vessel vasculitis accounted for most of the association of autoimmune vasculitis with AF. Arterial stiffness has been shown to be significantly related to small‐vessel diseases, 38 which usually present the greater resistance to blood flow. We postulate that inflammatory changes of the vessel wall due to vasculitis result in increased arterial stiffness and decreased vascular distensibility. Consequently, ventricular adaptive responses to the associated increases in afterload may lead to impairment of ventricular diastolic function. These hemodynamic alterations can also increase the duration of systole and, in turn, delay the onset of relaxation and increase filling pressure. 39 Our data confirm that potential contributions of hemodynamic alterations may be implicated in promoting the development of AF, including higher left ventricular filling pressure and more prevalent cardiac dysfunction in cases than controls. Delay in atrial emptying due to impaired diastolic distensibility that increases pressure and wall stretch within the atria and pulmonary veins is also an important mechanism for altered atrial electrical properties that promote AF development.
Association of Autoimmune Vasculitis With Mortality
In this study, we observed an increased risk of mortality in patients with autoimmune vasculitis above and beyond that seen with AF alone. Patients with autoimmune vasculitis and AF had a survival rate of 44.7% at 5 years compared with 77.2% for those without autoimmune vasculitis or AF. Our findings agree with those of prior studies showing a risk of mortality of 45% to 75% at 5 years in patients with small‐vessel vasculitis. 40 , 41 , 42 The biological mechanisms underlying the increased risk of death cannot by fully determined from our data. We speculate that inflammation, oxidative stress, and immune activation in the smaller arteries may be of importance for the increased mortality observed. In addition, comorbidities of the cardiovascular system or possibly complications of small‐vessel vasculitis such as thrombosis and organ failure may also play a role. 40 , 41 , 42 , 43 , 44 Other predictors of mortality observed in this analysis were well‐established cardiovascular risk factors including increasing age, male sex, preexisting hypertension, diabetes mellitus, dyslipidemia, congestive heart failure, myocardial infarction, stroke, smoking, chronic lung disease, peripheral arterial disease, and chronic renal disease.
Clinical Implications
Although we offer several plausible explanations for the association between autoimmune vasculitis and AF, both conditions share many risk factors and are associated with increased levels of proinflammatory markers. A clearer understanding of the pathogenesis of AF will probably lead to more highly targeted therapies and to greater reduction in AF‐related morbidity and mortality than can be achieved with current empirical treatment, particularly for those most at risk of death. Further research is needed to elucidate the underlying mechanisms that link these conditions.
Limitations
Despite the avoidance of referral or selection biases in our population‐based case–control study design, several limitations are inherent to the observational study design. Because this study was retrospective, we could not exclude unknown confounders or determine potential causality. Data on autoantibodies directed to cardiac structures (ie, antimyosin heavy chain, antimuscarinic acetylcholine receptor, anti–β‐1‐adrenergic receptor, and anti–heat shock protein) and electrophysiologic testing for the inducibility of atrial arrhythmias in patients with autoantibodies, which could elucidate a causal relationship, were not available. Consequently, the results could only support an association between autoimmune vasculitis and AF and, separately, mortality. Although we could not exclude unknown confounders, this shortcoming was minimized by standardizing retrospective case selection. It is possible that the control group may have developed AF in the follow‐up period. However, such occurrence would bias toward the null hypothesis and against an association of autoimmune vasculitis with AF. As with all retrospective studies, our observations were limited to the details documented in the health record and could have been affected by human error in the data‐abstraction process. In addition, our retrospective study design did not allow us to accurately ascertain the initial date of onset of autoimmune vasculitis in many patients; therefore, we were unable to perform statistical analyses assessing the interval between autoimmune vasculitis and AF. It is possible that part of the risk increase for AF might be due to ascertainment bias (ie, around the time of autoimmune vasculitis diagnosis). We measured the time intervals between the time of autoimmune vasculitis diagnosis and the index date (case or control), and the risk ratio was similar for recently and later diagnosed AF.
Conclusions
Our study demonstrated that autoimmune vasculitis is significantly associated with increased risk of AF development and independently confers worse survival in the presence than the absence of AF. Our findings support the hypothesis that autoimmune‐mediated disorders (eg, autoimmune vasculitis) increase the risk of AF. Because the study is restricted to retrospective and cross‐sectional data and cannot determine causality, further investigative studies are needed to elucidate the complex interaction between immune‐mediated disorders and the development of AF.
Sources of Funding
Dr Melduni is supported by National Institutes of Health (NIH) K01 (HL 135288).
Disclosures
None.
(J Am Heart Assoc. 2020;9:e015977 DOI: 10.1161/JAHA.120.015977.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Braunwald E. Shattuck lecture–cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. New Engl J Med. 1997;337:1360–1369. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 3. Ahlehoff O, Gislason GH, Jorgensen CH, Lindhardsen J, Charlot M, Olesen JB, Abildstrom SZ, Skov L, Torp‐Pedersen C, Hansen PR. Psoriasis and risk of atrial fibrillation and ischaemic stroke: a Danish Nationwide Cohort Study. Eur Heart J. 2012;33:2054–2064. [DOI] [PubMed] [Google Scholar]
- 4. Emilsson L, Smith JG, West J, Melander O, Ludvigsson JF. Increased risk of atrial fibrillation in patients with coeliac disease: a nationwide cohort study. Eur Heart J. 2011;32:2430–2437. [DOI] [PubMed] [Google Scholar]
- 5. Baba A, Yoshikawa T, Fukuda Y, Sugiyama T, Shimada M, Akaishi M, Tsuchimoto K, Ogawa S, Fu M. Autoantibodies against M2‐muscarinic acetylcholine receptors: new upstream targets in atrial fibrillation in patients with dilated cardiomyopathy. Eur Heart J. 2004;25:1108–1115. [DOI] [PubMed] [Google Scholar]
- 6. Stavrakis S, Yu X, Patterson E, Huang S, Hamlett SR, Chalmers L, Pappy R, Cunningham MW, Morshed SA, Davies TF, et al. Activating autoantibodies to the beta‐1 adrenergic and m2 muscarinic receptors facilitate atrial fibrillation in patients with Graves' hyperthyroidism. J Am Coll Cardiol. 2009;54:1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mandal K, Jahangiri M, Mukhin M, Poloniecki J, Camm AJ, Xu Q. Association of anti‐heat shock protein 65 antibodies with development of postoperative atrial fibrillation. Circulation. 2004;110:2588–2590. [DOI] [PubMed] [Google Scholar]
- 8. Bagrov AY, Fedorova OV, Roukoyatkina NI, Zhabko EP. Effect of endogenous digoxin‐like factor and digoxin antibody on myocardial Na+, K(+)‐pump activity and ventricular arrhythmias in acute myocardial ischaemia in rats. Cardiovasc Res. 1993;27:1045–1050. [DOI] [PubMed] [Google Scholar]
- 9. Maixent JM, Paganelli F, Scaglione J, Levy S. Antibodies against myosin in sera of patients with idiopathic paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 1998;9:612–617. [DOI] [PubMed] [Google Scholar]
- 10. Baba A, Yoshikawa T, Fukuda Y, Sugiyama T, Shimada M, Akaishi M, Tsuchimoto K, Ogawa S, Fu M. Autoantibodies against M2‐muscarinic acetylcholine receptors: new upstream targets in atrial fibrillation in patients with dilated cardiomyopathy. Eur Heart J. 2004;25:1108–1115. [DOI] [PubMed] [Google Scholar]
- 11. Fu M. Autoantibodies in atrial fibrillation: actors, biomarkers or bystanders? How far have we come? Cardiology. 2009;112:178–179. [DOI] [PubMed] [Google Scholar]
- 12. Maixent JM, Paganelli F, Scaglione J, Levy S. Antibodies against myosin in sera of patients with idiopathic paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 1998;9:612–617. [DOI] [PubMed] [Google Scholar]
- 13. Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. [DOI] [PubMed] [Google Scholar]
- 14. Bruins P, te Velthuis H, Yazdanbakhsh AP, Jansen PG, van Hardevelt FW, de Beaumont EM, Wildevuur CR, Eijsman L, Trouwborst A, Hack CE. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C‐reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96:3542–3548. [DOI] [PubMed] [Google Scholar]
- 15. Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic‐ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;32:1221–1227. [DOI] [PubMed] [Google Scholar]
- 16. Leite‐Moreira AF, Correia‐Pinto J, Gillebert TC. Afterload induced changes in myocardial relaxation: a mechanism for diastolic dysfunction. Cardiovasc Res. 1999;43:344–353. [DOI] [PubMed] [Google Scholar]
- 17. Shaikh AY, Wang N, Yin X, Larson MG, Vasan RS, Hamburg NM, Magnani JW, Ellinor PT, Lubitz SA, Mitchell GF, et al. Relations of arterial stiffness and brachial flow‐mediated dilation with new‐onset atrial fibrillation: The framingham heart study. Hypertension. 2016;68:590–596. [DOI] [PubMed] [Google Scholar]
- 18. Okin PM, Wachtell K, Devereux RB, Harris KE, Jern S, Kjeldsen SE, Julius S, Lindholm LH, Nieminen MS, Edelman JM, et al. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new‐onset atrial fibrillation in patients with hypertension. JAMA. 2006;296:1242–1248. [DOI] [PubMed] [Google Scholar]
- 19. Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC. Cardiac cycle‐dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. [DOI] [PubMed] [Google Scholar]
- 20. Weyand CM, Goronzy JJ. Medium‐ and large‐vessel vasculitis. N Engl J Med. 2003;349:160–169. [DOI] [PubMed] [Google Scholar]
- 21. St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores‐Suarez LF, Gross WL, Guillevin L, Hagen EC, et al. 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 23. Savage CO, Harper L, Adu D. Primary systemic vasculitis. Lancet. 1997;349:553–558. [DOI] [PubMed] [Google Scholar]
- 24. Kleinbaum DG, Kupper LL, Chambless LE. Logistic‐regression analysis of epidemiologic data ‐ theory and practice. Commun Stat a‐Theor. 1982;11:485–547. [Google Scholar]
- 25. Deng J, Younge BR, Olshen RA, Goronzy JJ, Weyand CM. Th17 and Th1 T‐cell responses in giant cell arteritis. Circulation. 2010;121:906–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zuo S, Li LL, Ruan YF, Jiang L, Li X, Li SN, Wen SN, Bai R, Liu N, Du X, et al. Acute administration of tumour necrosis factor‐alpha induces spontaneous calcium release via the reactive oxygen species pathway in atrial myocytes. Europace. 2018;20:1367–1374. [DOI] [PubMed] [Google Scholar]
- 27. Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, Bauer JA, Tchou PJ, Niebauer MJ, Natale A, et al. C‐reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. [DOI] [PubMed] [Google Scholar]
- 28. Li J, Solus J, Chen Q, Rho YH, Milne G, Stein CM, Darbar D. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7:438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Psychari SN, Apostolou TS, Sinos L, Hamodraka E, Liakos G, Kremastinos DT. Relation of elevated C‐reactive protein and interleukin‐6 levels to left atrial size and duration of episodes in patients with atrial fibrillation. Am J Cardiol. 2005;95:764–767. [DOI] [PubMed] [Google Scholar]
- 30. Verheule S, Wilson E, Everett T, Shanbhag S, Golden C, Olgin J. Alterations in atrial electrophysiology and tissue structure in a canine model of chronic atrial dilatation due to mitral regurgitation. Circulation. 2003;107:2615–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aviles RJ, Martin DO, Apperson‐Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. [DOI] [PubMed] [Google Scholar]
- 32. Lee HC, Huang KT, Wang XL, Shen WK. Autoantibodies and cardiac arrhythmias. Heart Rhythm. 2011;8:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee HC, Melduni RM. Autoimmunity and cardiac arrhythmias in endemic pemphigus foliaceus‐Association, correlation, or causation? Heart Rhythm. 2018;15:732–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li H, Scherlag BJ, Kem DC, Benbrook A, Shen X, Cunningham MW, Lazzara R, Aston CE, Yu X. Inducible cardiac arrhythmias caused by enhanced beta1‐adrenergic autoantibody expression in the rabbit. Am J Physiol Heart Circ Physiol. 2014;306:H422–H428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee HC, Huang KT, Wang XL, Shen WK. Autoantibodies and cardiac arrhythmias. Heart Rhythm. 2011;8:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Emilsson L, Smith JG, West J, Melander O, Ludvigsson JF. Increased risk of atrial fibrillation in patients with coeliac disease: a nationwide cohort study. Eur Heart J. 2011;32:2430–2437. [DOI] [PubMed] [Google Scholar]
- 37. Ahlehoff O, Gislason GH, Jorgensen CH, Lindhardsen J, Charlot M, Olesen JB, Abildstrom SZ, Skov L, Torp‐Pedersen C, Hansen PR. Psoriasis and risk of atrial fibrillation and ischaemic stroke: a Danish Nationwide Cohort Study. Eur Heart J. 2012;33:2054–2064. [DOI] [PubMed] [Google Scholar]
- 38. Henskens LH, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Fuss‐Lejeune MM, Hofman PA, Lodder J, de Leeuw PW. Increased aortic pulse wave velocity is associated with silent cerebral small‐vessel disease in hypertensive patients. Hypertension. 2008;52:1120–1126. [DOI] [PubMed] [Google Scholar]
- 39. Weber T, Auer J, O'Rourke MF, Punzengruber C, Kvas E, Eber B. Prolonged mechanical systole and increased arterial wave reflections in diastolic dysfunction. Heart. 2006;92:1616–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lane SE, Watts RA, Shepstone L, Scott DG. Primary systemic vasculitis: clinical features and mortality. QJM. 2005;98:97–111. [DOI] [PubMed] [Google Scholar]
- 41. Guillevin L, Durand‐Gasselin B, Cevallos R, Gayraud M, Lhote F, Callard P, Amouroux J, Casassus P, Jarrousse B. Microscopic polyangiitis: clinical and laboratory findings in eighty‐five patients. Arthritis Rheum. 1999;42:421–430. [DOI] [PubMed] [Google Scholar]
- 42. Hattori N, Mori K, Misu K, Koike H, Ichimura M, Sobue G. Mortality and morbidity in peripheral neuropathy associated Churg‐Strauss syndrome and microscopic polyangiitis. J Rheumatol. 2002;29:1408–1414. [PubMed] [Google Scholar]
- 43. Bourgarit A, Le Toumelin P, Pagnoux C, Cohen P, Mahr A. Le Guern V, Mouthon L, Guillevin L and French Vasculitis Study G. Deaths occurring during the first year after treatment onset for polyarteritis nodosa, microscopic polyangiitis, and Churg‐Strauss syndrome: a retrospective analysis of causes and factors predictive of mortality based on 595 patients. Medicine (Baltimore). 2005;84:323–330. [DOI] [PubMed] [Google Scholar]
- 44. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart disease and stroke statistics‐2019 update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]


